Abstract
Scleral stiffening has been proposed as a therapy for glaucoma and myopia. Previous in vivo studies have evaluated the efficacy of scleral stiffening after multiple treatments with a natural collagen crosslinker, genipin. However, multiple injections limit clinical translatability. Here, we examined whether scleral stiffening was maintained after four weeks following a single genipin treatment. Eyes from brown Norway rats were treated in vivo with a single 15 mM genipin retrobulbar injection, sham retrobulbar injection, or were left naive. Eyes were enucleated either 1 day or four weeks post-injection and underwent whole globe inflation testing. We assessed first principal Lagrange strain of the posterior sclera using digital image correlation as a proxy for scleral stiffness. Four weeks post-injection, genipin treatment resulted in a 58% reduction in scleral strain as compared to controls (p = 0.005). We conclude that a single in vivo injection of genipin effectively stiffened rat sclera for at least four weeks which motivates further functional studies and possible clinical translation of genipin-induced scleral stiffening.
Keywords: glaucoma, myopia, stiffening, genipin, collagen-crosslinking, sclera
1. Background
Scleral biomechanical properties are thought to play a role in two major ocular pathologies: glaucoma and myopia [1,2]. Glaucoma, the leading cause of irreversible blindness worldwide [3], is characterized by the loss of retinal ganglion cells (RGCs) at the optic nerve head (ONH). Although the exact aetiology remains unknown, a major risk factor for glaucoma is elevated intraocular pressure (IOP) [4]. Elevated IOP leads to deformation of the ONH, which may negatively affect RGCs via several mechanisms, including changes in blood flow and chronic cellular mechanostimulation [5,6]. Previous work has shown that chemical cross-linking of the peripapillary sclera decreased IOP-induced ONH deformation [7], making scleral cross-linking a potential therapeutic strategy for glaucoma patients.
Scleral cross-linking has also been proposed as a treatment for patients suffering from myopia (nearsightedness). Myopia is the most common refractive error and is expected to affect nearly half of the world's population by the year 2050 [8]. In myopia, the eye elongates and the sclera becomes weaker and thinner due to active remodelling of collagen [9]. Therefore, it is hypothesized that scleral cross-linking could slow or reverse the effects of the scleral remodelling to prevent late stage complications, more common in cases of high myopia [10].
Chemical cross-linking has been widely used to stiffen hydrogels and biological tissues [11–13]. Recently, photochemical cross-linking of corneal collagen has been approved as an effective clinical treatment for keratoconus, in which the cornea becomes weaker and misshapen, resulting in impaired visual acuity [14]. This treatment uses ultraviolet-A (UVA) irradiated riboflavin to induce cross-linking; however, this method has been shown to be cytotoxic to the retina when applied to stiffen the sclera [15,16]. Therefore, alternative biocompatible treatments that avoid retinal UVA exposure are better suited for scleral stiffening [17].
Previous research in our laboratory has investigated the efficacy of three crosslinkers (methylglyoxal, glyceraldehyde and genipin) to stiffen the rat sclera ex vivo [18]. Of these agents, genipin demonstrated effective stiffening at low concentrations and has been reported to be a biocompatible collagen crosslinker [19,20]. Further, Avila et al. [21] evaluated genipin and riboflavin/UVA cross-linking in porcine corneas and found that both treatments yielded similar amounts of stiffening and minimal toxicity. Genipin has also been used to effectively stiffen tree shrew [1] and porcine [22,23] sclera ex vivo, as well as rabbit [24] and guinea pig [25] sclera in vivo. A widely demonstrated scleral stiffening effect and high biocompatibility of make genipin an excellent candidate for eventual clinical translation.
The current study presents new support for the use of genipin as a scleral crosslinker by demonstrating the ability of a single in vivo genipin injection to produce sustained stiffening in the rat (a useful model of ocular disease), with improved mechanical testing methods. All previously published in vivo studies have implemented multiple injections of genipin over time to stiffen the sclera [24,25]. To assess translational potential, we tested whether scleral stiffness modifications were sustained for four weeks after a single injection of genipin. Additionally, these previous tests of in vivo genipin treatment have been evaluated by uniaxial strip testing. While these biomechanical tests measure intrinsic scleral stiffness, they are known to have significant limitations [26], including the inability to test the sclera under physiological modes of deformation. Thus, we used whole globe inflation testing, which more closely mimics in vivo scleral loading conditions [18]. Since glaucoma is a disease that progresses over decades, we were primarily interested in assessing the effects of our cross-linking treatment on the mechanical stiffness of the sclera at steady state than on assessing scleral viscoelastic properties, although the viscoelastic properties of the sclera may also be important in glaucoma due to fluctuations in IOP [27]. To focus on steady state properties, we chose a creep test rather than a ramp-hold test to assess the effects of our treatment on the sclera. Further, there were technical advantages to using a creep test (see Discussion). Finally, no previously published in vivo scleral stiffening studies have been conducted using rat eyes. The rat is a widely used model for vision research, and has several advantages for use as a glaucoma therapy test bed [28], including the fact that there are established methods for inducing rat ocular hypertension that result in glaucomatous damage [29,30]. Furthermore, evidence indicates that the rat sclera is structurally similar to that of the human, being composed mainly of collagen fibres [31,32] that are organized in a circumferential pattern near the ONH and are less organized further from ONH [33]. In addition, the rat affords advantages over other glaucoma models. Namely, rat eyes are larger and much easier to test biomechanically than mouse eyes, and rats are more amenable for high subject studies compared to monkeys, due to their low cost, ease of animal husbandry and low genetic variability between individuals. Rat models of myopia have also been reported [34,35]. Therefore, this study represents the successful development of methods to investigate genipin-induced scleral stiffening as a therapy for common ocular pathologies in an affordable animal model.
2. Methods
2.1. Overview of experimental design
In brief, rat eyes were treated in vivo with either a genipin or sham retrobulbar injection or were left naive (figure 1). Eyes were enucleated 1 day or four weeks post-injection and prepared for whole globe inflation testing to determine scleral stiffness. Whole globes were attached cornea-side down to a mounting block and IOP was controlled by raising and lowering a hydrostatic pressure reservoir in fluidic connection with the eye. Images were taken of the posterior sclera throughout the inflation test and analysed by digital image correlation (DIC) software to compute scleral surface displacement and strains. Pressure–strain data were fit with a constitutive equation that was then used to quantify scleral stiffening.
Figure 1.
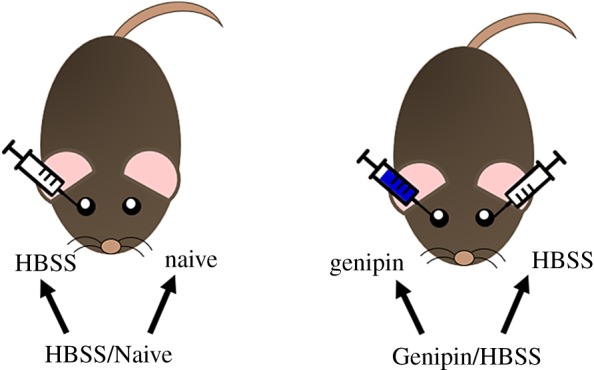
Experimental groups: HBSS/Naive rats received a single (unilateral) retrobulbar injection of HBSS, while Genipin/HBSS rats received a unilateral retrobulbar injection of genipin and a unilateral retrobulbar injection of HBSS contralaterally. (Online version in colour.)
2.2. Animals
This study used 28 retired breeder (male, 6–13 months old) brown Norway rats (Charles River Laboratories, Inc., Wilmington, MA). All procedures were approved by the Institutional Animal Care and Use Committee at the Georgia Institute of Technology. Some rats from the starting cohort were removed from this study according to exclusion criteria, as described below.
2.3. Scleral stiffening procedure
Rats were anaesthetized systemically with ketamine (60 mg kg−1) and xylazine (7.5 mg kg−1), and a drop of topical tetracaine (1%) was applied to the eye. Rats were randomly assigned to one of two groups for stiffening treatment (figure 1). The first group (HBSS/Naive) received a single (unilateral) retrobulbar injection of Hank's balanced salt solution (HBSS; 150 µl), while the contralateral eye was left as a naive control. The second group (Genipin/HBSS) received a single retrobulbar injection of genipin (Wako Pure Chemical Industries, Ltd, Richmond, VA) mixed in HBSS (15 mM, 150 µl), while the contralateral eye received a single retrobulbar injection of HBSS (150 µl). This genipin concentration was motivated by our previous ex vivo study [18] and recent in vivo work (table 1) [24,25]. Eyes were randomized to each treatment. After injections, rats were given topical antibiotic (Certi-sporyn, Kansas City, MO) to prevent infection and antisedan (1 mg kg−1) to reverse anaesthesia [36]. Genipin/HBSS rats were divided into two subgroups and euthanized (via CO2 overdose) either 1 day or four weeks post-injection. All Naive/HBSS rats were euthanized 1 day post-injection. Eyes were immediately enucleated and refrigerated until testing, which always occurred within 6 h of euthanasia.
Table 1.
Comparison of in vivo genipin studies.
| study | animal | genipin concentration (mM) | injection type | volume injected (µl) | number of injections | time between injections | minimum duration of stiffening | mechanical testing method |
|---|---|---|---|---|---|---|---|---|
| Liu & Wang [24] | rabbit | 0.5 | sub-Tenon | 500 | four | 2–3 days | two weeks | uniaxial strip |
| Wang & Corpuz [25] | guinea pig | 22 | sub-Tenon | 100 | three | 7 days | three weeks | uniaxial strip |
| current study | rat | 15 | retrobulbar | 150 | one | n.a. | four weeks | whole globe inflation |
2.4. Whole globe inflation testing
2.4.1. Eye mounting procedure
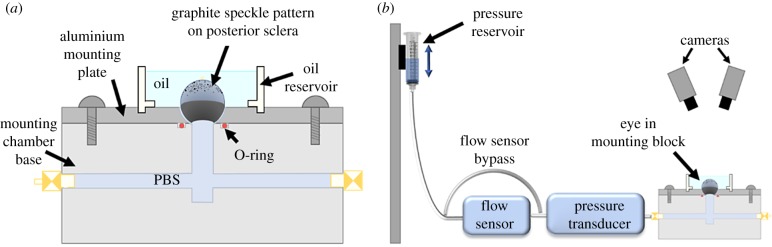
Eyes were carefully cleaned under a dissecting microscope with micro-scissors to remove all fat, episclera and extraocular muscles from the sclera. Since genipin-induced collagen cross-links are known to autofluoresce [37], we then imaged whole globes using an epifluorescent scope (Leica DM6 B, Leica Microsystems, Wetzlar, Germany) to confirm posterior scleral coverage of genipin cross-links (figure 2). After imaging, a hole, approximately 4 mm in diameter, was cut in the cornea using micro-scissors. A thin ring of ethyl 2-cyanoacrylate adhesive (CAS#7085-85-0, Henkel, Rocky Hill, CT) was applied around the interior edge of a hemispherical well on an aluminium mounting plate (figure 3a) and the eye was glued cornea-side down into the well. Care was taken to ensure that the limbus of the eye was in full contact with the glue and the ONH was centred upwards. The glue was cured with phosphate-buffered saline (PBS) to create a seal between the plate and limbus. After the eye was securely attached, the lens and vitreous humour were removed through the 4 mm hole in the cornea by gently pressing on the posterior sclera, as previously described [38]. The mounting plate was then attached with screws to the base of the empty mounting chamber (figure 3a) and securely fastened to create a watertight seal. The sealed chamber was flooded with PBS and purged of air bubbles using two ports on opposite sides of the chamber which were re-sealed once all air bubbles had been cleared. Throughout the cleaning and mounting process, the eye was kept hydrated by frequent applications of PBS.
Figure 2.

Whole globe autofluorescence of HBSS (a) and genipin (b) injected paired eyes from Genipin/HBSS rat at four weeks post-injection. The arrow and corresponding outline indicate the approximate optic nerve (ON) location on each eye, while the outer dashed outline indicates the approximate size of the eye. Asterisks: vessel locations on posterior sclera. Photos taken using Y5 filter cube ((590–650 nm)/(660–740 nm) excitation/emission). The fluorescence induced by genipin-induced cross-links was evident and suggested a relatively even distribution of genipin over much of the posterior sclera. Exposure level and gain settings were identical in both images. (Online version in colour.)
Figure 3.
Inflation testing schematic. (a) Mounting chamber, including rat eye mounted cornea-side down. (b) Testing apparatus with hydrostatic pressure reservoir, flow sensor, pressure transducer, mounting block and DIC cameras. The flow sensor bypass was used to eliminate the lag in pressure experienced by the eye (e.g. during preconditioning cycles), as described more fully in the text. (Online version in colour.)
To provide a high-contrast pattern for DIC analysis, a uniform speckle pattern was applied to the posterior scleral surface using graphite powder (#970 PG, General Pencil Company, Inc., Redwood City, CA). Finally, a 3D printed oil reservoir (figure 3a) was attached to the top of the mounting block and filled with mineral oil (CAS# 8042-47-5, McMaster-Carr, Douglasville, GA) to prevent the eye from dehydrating during testing while allowing camera visibility to the posterior sclera.
2.4.2. Inflation testing experimental hardware
The experimental set-up used for inflation testing is shown in figure 3b. IOP was elevated by increasing the height of a hydrostatic pressure reservoir attached to a stepper motor. Flow rates into the eye were measured with an in-line flow sensor (SLG64-0075; Sensirion, Stafa, Switzerland), and IOP was measured downstream of the flow sensor with a gage pressure transducer (142PC01G; Honeywell, Charlotte, NC). The flow sensor, pressure transducer and eye mounting block were connected to a fluid manifold to enable modifications to the flow path as needed during calibration and inflation testing. While flow measurements were useful (e.g. to facilitate leak detection), the inclusion of a flow sensor in the system also introduced a high fluidic resistance between the pressure reservoir and eye. This resistance to flow, together with the compliance of the eye, led to a delay during pressurization/filling of the eye, as well as the ocular pressure being less than the reservoir pressure. Thus, we included tubing to bypass the flow sensor when rapid pressurization of the eye was desired (figure 3b). iPerfusion software was used to record pressure and flow data, and to initiate pressure stepping protocols [39]. Throughout inflation tests, scleral deformation was recorded with two stereo cameras (Dantec Dynamics, Holtsville, NY).
The measurement hardware was calibrated regularly before testing. The pressure transducer was zeroed to atmospheric pressure at the free surface of the mineral oil by using a custom-built chamber filled with PBS to simulate the hydrostatic pressure of mineral oil on the eye, which accounted for the difference in specific gravity between PBS and mineral oil. The pressure transducer was calibrated by setting the reservoir to a series of pressure (height) steps to correlate sensor voltage with known pressure steps. Weekly intrinsic and daily extrinsic calibrations of the stereo cameras were performed with a calibration target according to manufacturer protocols. For extrinsic calibrations, the calibration target was submerged in mineral oil in the custom calibration chamber to ensure compensation for the refractive index of mineral oil. The surface of the eye was imaged throughout the inflation test with a 100 ms exposure time.
2.4.3. Preconditioning and creep procedure
After attaching the mounting chamber to the system hardware, the flow sensor was bypassed, and the pressure was set to 2 mm Hg. With the flow sensor in-line, image recording was initiated (1 image per minute), and the pressure reservoir was set to 15 mm Hg to check for leaks. Based on preliminary testing, an eye was considered leak-free if the flow rate dropped below 600 nl min−1 and the eye pressure reached 11 mm Hg in less than 20 min. If a leak was present at this step, the eye (and fellow contralateral eye) was excluded from the study. The non-zero steady state flow rate was likely due to several possible outflow passages from the eye, including remnants of the trabecular meshwork in the anterior eye, vortex veins exposed by removal of the vitreous, and micro-leaks in the glue. In certain instances, outflow from the vortex veins created a fluid bubble in the oil that distorted the cameras' view of the speckle pattern, resulting in a large fraction of the scleral surface that could not be tracked by the DIC algorithm. Eyes (and fellow contralateral eyes) affected by either exclusion criteria: (1) a leak in the eye, or (2) a fluid bubble impeding sufficient DIC analysis, were excluded from the study (13 of 28 rats).
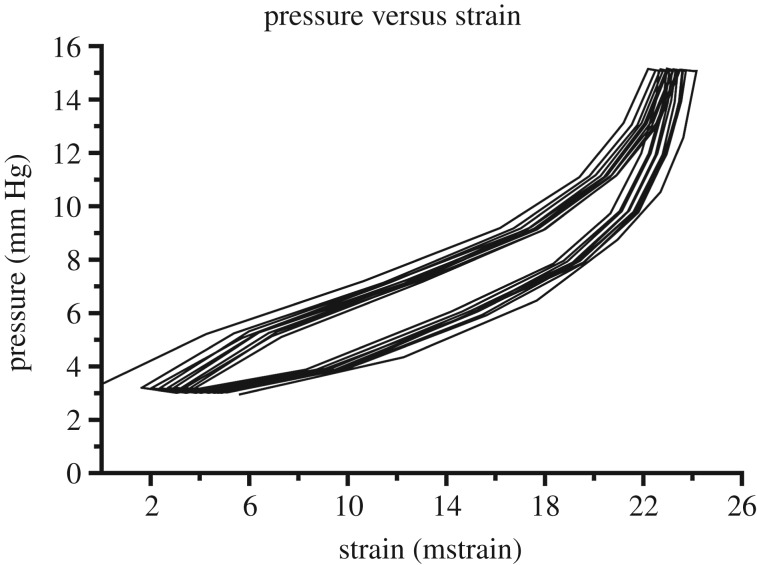
The flow sensor bypass was then opened, causing the pressure to immediately increase from the approximately 11 mm Hg reached during the leak check to the 15 mm Hg set pressure. The eye was then held at 15 mm Hg for 5 min as an acclimatization step. The eye was subsequently subjected to 10 load–unload cycles from 3 to 15 mm Hg at a rate of 0.5 mm Hg s−1 and images were taken every 4 s (figure 4). We determined empirically that 10 cycles were sufficient to ensure that variation in the mean peak scleral strain was no greater than 2% between cycles (figure 5). In preliminary testing, we found that the acclimatization step was very important to reach a preconditioned state within the 10 cycles. When the eye was subjected to load–unload cycles only, the number of cycles required was variable. After preconditioning, eye pressure was lowered to 3 mm Hg, and the flow sensor bypass was closed, which added the flow sensor back into the flow path.
Figure 4.

Overview of the reservoir pressure during inflation testing protocol. The preconditioning protocol consisted of an acclimatization step where the pressure reservoir was set to 15 mm Hg, followed by 10 preconditioning cycles from 3 to 15 mm Hg. The creep testing protocol included three pressure steps: 3–10, 10–20 and 20–30 mm Hg. The timing of pressure steps varied, with typical step timing shown here. Note that this figure represents the set reservoir pressure and not the eye pressure as measured by the pressure transducer. It is important to note that the actual measured eye pressure was used in data fitting. (Online version in colour.)
Figure 5.
Representative pressure–strain plot for cyclic loading from 3 to 15 mm Hg during preconditioning. The difference in maximum first principal Lagrange strain value between two consecutive cycles reached an equilibrium (less than 2% change) by 10 cycles.
The eye was then subjected to a creep testing protocol comprising three pressure steps of 10, 20 and 30 mm Hg (figure 4). Pressure steps were initiated only after the flow reading reached a designated stability criterion based on measured flow rate of ±2 nl min−1 s−1 of variation over a 10-min window. This flow stability criterion ensured that posterior ocular tissues had sufficient time to creep under the applied pressure, resulting in stabilized strain readings for each pressure step (figure 6). Note that since the flow sensor had a high fluid resistance, there was a small pressure drop, usually 0.5–2 mm Hg, between the pressure applied to the system and IOP. Throughout the creep testing, images were captured every 30 s.
Figure 6.
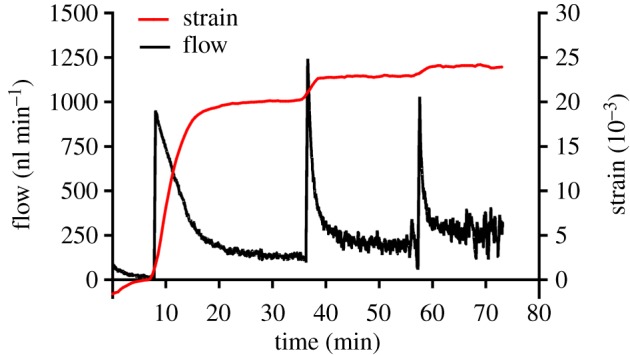
Representative flow sensor and strain measurements during a creep test. Flow sensor output is shown by the black line (left y-axis), where peaks correspond to pressure steps. Mean first principal strain averaged over the posterior sclera is shown by the red line (right y-axis). Strain values are referenced to the end of the first pressure step of 3 mm Hg. Time shown is relative to the start of the creep test. (Online version in colour.)
2.5. Data analysis
Dantec's Istra 4D software (v. 4.4.1, Dantec Dynamics, Holtsville, NY) was used for image analyses. The reference configuration for local surface strain calculations was chosen as the frame 1 min before the first pressure step, at which point the eye was exposed to a pressure of approximately 3 mm Hg. The following correlation settings were used: facet size of 45 pixels, grid spacing of 28 pixels, maximum permissible start point accuracy of 0.2 pixels, residuum of 30 grey values and 3D residuum of 1.1 pixels. In 3D space, the resulting grid spacing between facets was approximately 150 µm. Displacement data were smoothed prior to strain calculations using parameters recommended by Dantec Dynamics: a grid reduction factor (minimizing the difference between the data point and the spline function) of 2 for displacement and contours, and a smoothness factor (straightens filtered data) of −0.5 for displacement and 0 for contours.
A custom Matlab code (2018b; MathWorks, Natick, MA) was used for further analysis. First, facets that were correlated in fewer than 75% of the images were removed. Further, facets from each image whose first principal strain values were in the top and bottom 5th percentile ranges were excluded from that image as outliers. The mean first principal strain averaged over the entire posterior sclera, excluding the ONH region and the excluded facets, was then calculated in each frame by averaging the x, y and xy components of Lagrange strain from all facets, populating a 2 × 2 matrix with these averages and calculating the two eigenvalues. The more positive eigenvalue was taken as the average first principal strain magnitude. The mean strain for each pressure step was computed by averaging the first principal strain over the five frames prior to the increase in pressure. The mean pressure for each step was computed by averaging the recorded pressure values for the last 5 min of the step. Pressure–strain data from each eye was fit to an isotropic Fung-type model, similar to the approaches used in [22] and [40]:
where ε represents the first principal Lagrange strain, C represents the IOP reached at the reference pressure step (reservoir pressure of 3 mm Hg), and A and B are fitting coefficients. An R2 value was computed for the Fung model fit to the experimental data, and if either R2 value for a pair of eyes was lower than 0.9, that pair was excluded from the study according to this exclusion criteria (2 of 28 rats). This fit was then used to compute the strain at 22 mm Hg, which is the physiological IOP of awake normotensive brown Norway rats recorded in our laboratory, which is similar to that reported in other laboratories [41]. This procedure allowed us to compare scleral deformations in all eyes at an equal IOP.
We computed the difference in strain at 22 mm Hg by subtracting control strain from experimental strain. In the genipin/HBSS group, the experimental eye was the genipin-treated eye, and in the HBSS/naive group, the HBSS eye was the experimental eye. Negative values indicated that the experimental eye was stiffer. Relative difference in strain for each rat was computed using the equation:
where εexperimental and εcontrol are the strains at 22 mm Hg in the experimental eye and control eye, respectively.
2.5.1. Statistical analysis
Fifteen rats were removed from the study due to the exclusion criteria described above. Thus, 13 rats were used for statistical analysis, broken down as follows: Naive/HBSS rats 1 day post-injection (n = 4), Genipin/HBSS rats 1 day post-injection (n = 4) and Genipin/HBSS rats four weeks post-injection (n = 5).
Difference in strain for each group was compared using one-way ANOVA with a Holm–Sidak post hoc test, and per cent strain reduction for each group was compared using Kruskal–Wallis with a Dunn's post hoc test (GraphPad Software v8, San Diego, CA). Results are presented as mean ± s.d. unless otherwise noted.
3. Results
The Fung model provided an excellent fit to each set of pressure versus strain data, with an average R2 value of 0.99 ± 0.015 as seen in figure 7. All curves for genipin-treated eyes were clustered towards the left side of the graph, indicating a stiffer sclera in these eyes. In contrast, the curves for all HBSS and naive eyes were dispersed throughout the middle and right portions of the graph, indicating a softer sclera and higher variability in these eyes.
Figure 7.
Genipin treated eyes yielded visibly stiffer pressure–strain curves. Fit of isotropic Fung-type constitutive model to pressure–strain data from each eye (all R2 > 0.93). Naive eyes are shown in dashed black lines, HBSS eyes are shown in various shades of purple and genipin eyes are shown in various shades of blue. Dotted red line denotes 22 mm Hg, at which the difference in strain and per cent strain reduction were computed. Letters above curves indicate paired eyes from each rat (A–M). For overlapping curves, the letter corresponding to the leftmost curve is on top. (Online version in colour.)
As expected, the mean difference in strain in the HBSS/Naive rats was approximately zero (0.0002 ± 0.00022 strain), indicating that HBSS (sham) injection did not affect scleral stiffness 1 day post-injection. Genipin/HBSS rats showed a reduction in strain at both 1 day (−0.0098 ± 0.0052 strain) and four weeks (−0.0154 ± 0.006 strain) post-injection (figure 8), i.e. there was less scleral strain in the genipin-treated eyes. There was a significant scleral stiffening effect due to genipin treatment (one way ANOVA: F2,10 = 11.25, p = 0.0028) as evidenced by the greater difference in strain in the Genipin/HBSS groups compared with the HBSS/Naive group (Holm–Sidak post hoc: 1 day, p = 0.016; four weeks, p = 0.002). Relative difference in strain (figure 9) in Genipin/HBSS rats 1 day (−41.2 ± 14.1%) and four weeks (−58.3 ± 15.6%) post-injection were greater in magnitude than Naive/HBSS rats (1.6 ± 6.5%); however, this result was only significant at four weeks post-injection (Kruskal–Wallis, p < 0.001; Dunn's post hoc, HBSS/Naive versus Genipin/HBSS at 1 day: p = 0.170 and HBSS/Naive versus Genipin/HBSS at four weeks: p = 0.005).
Figure 8.

Genipin treatment resulted in reduced scleral strain. Difference in strain at an IOP of 22 mm Hg for HBSS/Naive rats (n = 4) 1 day post-injection and for Genipin/HBSS rats 1 day (n = 4) and four weeks (n = 5) post-injection. The plotted quantity is the average first principal scleral strain in the contralateral control eye minus the strain in the experimental eye for each rat, all evaluated at 22 mm Hg. There was a greater difference in strain in Genipin/HBSS rats compared to HBSS/Naive rats at 1 day and four weeks. Bars show mean ± s.d. (* indicates p ≤ 0.05, ** indicates p ≤ 0.01, both by one-way ANOVA, Holm–Sidak post hoc). (Online version in colour.)
Figure 9.
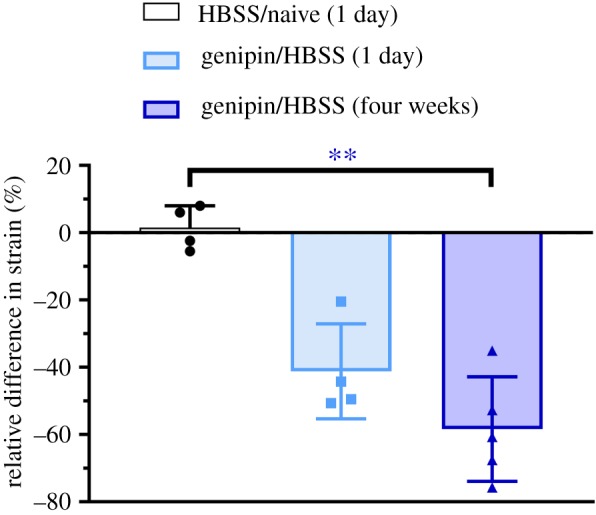
Genipin treatment resulted in greater relative difference in strain. Relative difference in strain at 22 mm Hg for HBSS/Naive rats (n = 4) 1 day post-injection and for Genipin/HBSS rats 1 day (n = 4) and four weeks (n = 5) post-injection. The plotted quantity is the difference in average first principal scleral strain relative to the contralateral control eye for each rat, all evaluated at 22 mm Hg, and provides an alternative way of presenting the data shown in figure 8. Relative difference in strain was significantly greater at four weeks after injection. Bars show mean ± s.d. (** indicates p ≤ 0.01 by Kruskal–Wallis test, Dunn's post hoc). (Online version in colour.)
4. Discussion
In this study, we show that a single retrobulbar in vivo injection of 15 mM genipin can effectively stiffen rat sclera and that the effect is sustained for at least four weeks post injection. This finding is significant because it justifies the use of a single injection in future studies evaluating the effects of scleral stiffness, whereas previous protocols used multiple injections (table 1). We expect that this improvement will greatly simplify future studies, since each injection introduces risk and variability, and may eventually increase the potential for translation of genipin stiffening treatments in the eye.
The degree of scleral stiffening that is required to provide an effective treatment for glaucoma is not known. However, we can predict minimum efficacious stiffening levels based on the stiffening magnitude needed to significantly reduce strains in the ONH. Coudrillier et al. evaluated the effects of posterior scleral stiffening via glutaraldehyde on ONH strain in ex vivo porcine eyes [7]. Specifically, a −40% relative difference in scleral strain (at an IOP of 22 mm Hg) led to a relative difference in ONH strain of −47% and −39% at 15 mm Hg and 30 mm Hg, respectively. In this study, we reduced scleral strain at 22 mm Hg by a similar amount at 1 day (−41.2%) and four weeks (−58.3%) post-injection, suggesting that our genipin treatment is likely to markedly reduce ONH strains in the rat eye. In the context of myopia, Wang et al. demonstrated a significant decrease in refractive error after genipin treatment [25]. However, it is challenging to directly compare our stiffening results due to differences in uniaxial loading regime [25].
More broadly, we note that the ultimate clinical benefits of scleral stiffening in glaucoma remain to be determined. A preliminary study suggested that increasing scleral stiffness provides neuroprotection in glaucoma [42], yet a larger study by Kimball et al. [43] reported that stiffening the posterior mouse scleral increased (rather than decreased, as hypothesized in this study) glaucomatous damage. While these results are important, the stiffening technique described in this study has significant differences compared to Kimball et al.'s study, including: animal type, number of injections, type of injection, volume of crosslinker, concentration of crosslinker and type of crosslinker. There are also the observations that the sclera becomes stiffer in glaucoma, and that African descent is associated with a higher peripapillary scleral stiffness and a higher incidence of glaucoma. These data could suggest that a stiffer sclera increases glaucoma risk, or alternatively they could mean that scleral stiffening is a beneficial adaptive response to mitigate glaucomatous damage/risk. Considering all of the above, the reality is that we do not know whether scleral stiffening will provide neuroprotection in glaucoma, and we expect that research such as described in this work will help to answer this fundamental question. We have confirmed a successful genipin retrobulbar injection by visualizing autofluorescent genipin-induced collagen cross-links (figure 2), observing a relatively uniform autofluorescence distribution posteriorly, with reduced autofluorescence as the limbus was approached. This reduced cross-linking in the more anterior portions of the sclera was expected, since the conjunctiva acts as an anatomical barrier for fluid transport from the retrobulbar space. Importantly, when considering the posterior sclera, there was no obvious correlation between the local variations in autofluorescence and local variations in strains as measured by DIC (data not shown). This is likely because the stiffness and thickness of the sclera naturally vary by location even before genipin treatment. Therefore, regional differences in strain magnitude as shown by DIC could be due to natural variations in structural stiffness that existed before genipin injection, to a localized effect of the stiffening agent, or both.
This study introduces several methodological improvements as compared to our previously published inflation testing procedure [18]. First, we included a preconditioning protocol to reduce variability between samples. Preconditioning was not required in our previous study because we used a partial incubation protocol that allowed us to determine a treatment effect using only a single eye. Since the present study compared strains between treated and contralateral eyes, reducing the testing variability increased the power of the study. Second, we incorporated a flow sensor into the testing set-up to determine when the eye reached equilibrium at each pressure step. The time to reach equilibrium is expected to vary with viscoelastic properties and outflow rates, both of which are variable in normal populations and may be altered with genipin treatment. Yet without a flow sensor, pressure steps could only be initiated at fixed time intervals, since the DIC software was unable to provide information about sample deformation while a test was in progress. The flow sensor also enabled accurate identification and exclusion of eyes with unstable leaking or creeping phenomena that would have biased the results. Finally, we developed an improved mounting procedure similar to that described by Bianco et al. [38]. Specifically, we removed the lens from each eye to prevent it from occluding the flow of PBS into the eye. We also used an eye bath of mineral oil rather than PBS to prevent evaporation of fluid during the test while maintaining tissue hydration.
These changes have also introduced some minor but notable limitations, were outweighed by their benefits, as follows. First, the addition of a flow sensor (with its inherent flow resistance) makes it difficult to precisely set the pressure in the eye. Since the flow rate entering each eye can differ, an eye-specific pressure drop occurs across the flow sensor and this difference becomes more significant as the IOP changes. However, eye pressure throughout the duration of the test is directly measured in the described set-up. This allows for all test data to be referenced to a common IOP through use of a Fung-type constitutive model which permits comparisons across eyes with different starting pressures. Also, by specifying a creep test rather than a ramp-hold test, we were able to minimize the potential importance of this effect. Second, since the eye was pressurized with PBS but was tested in a bath of mineral oil, leaking PBS from the eye, even at very low flow rates, occasionally resulted in a bubble of PBS that distorted the image of the underlying speckle pattern seen by the cameras. Finally, we chose to implement a preconditioning procedure which used cyclic loads between 3 and 15 mm Hg, whereas our creep pressure steps were higher than this range (20 and 30 mm Hg). Because of this, our results could potentially be affected by the Mullins effect, in which a material's softening effect is dependent on the maximum load the tissue is subjected to during its preconditioning loading history. We chose our preconditioning pressures to minimize leakage from eyes, which occurred more often when eyes were dynamically loaded to higher pressures during preconditioning. Additionally, while preconditioning is extremely important in uniaxial and biaxial strip testing, it has been shown that the sclera exhibits only modest preconditioning effects during inflation tests in other species [44–46]. These results are encouraging and motivate further testing with genipin.
Although this study did not explicitly evaluate any potential toxicity of genipin, animals were monitored after retrobulbar injections with either genipin or HBSS. In all rats, there was a small bleb of fluid visible in the inferior and nasal regions of the conjunctiva which resolved within 1–5 days post-injection. Adverse effects from the retrobulbar injections included a few eyes in which there was a small amount of bleeding from the periocular space immediately following the injection, presumably due to a blood vessel being accidentally damaged during the injection. Further, we do not expect genipin treatments to be cytotoxic, since Liu et al. demonstrated no cytotoxicity due to genipin treatment in rabbits [24]. However, Liu et al. used a lower concentration than that used in the present study (table 1), and future work must assess potential genipin-induced cytotoxicity. We are currently evaluating the safety of genipin injections and plan to describe this in a separate publication in due course. Future work should also determine the maximum duration of this stiffening effect.
5. Conclusion
We have demonstrated stiffening of rat sclerae after a single in vivo injection of genipin in rats. The stiffening effect was sustained for at least four weeks, longer than previously demonstrated. This is important for simplifying research studies and facilitating possible future clinical translation, where frequent injections are undesirable. These findings were enabled by development of an improved inflation testing protocol for measuring strain in genipin-treated eyes. Altogether, these results will be used to guide future in vivo studies aimed at evaluating the efficacy of scleral stiffening via genipin injection as a treatment for glaucoma, myopia or other applications.
Supplementary Material
Acknowledgements
The authors would like to thank Joseph M. Sherwood for help with the iPerfusion software used for the inflation tests.
Ethics
All procedures were approved by the Institutional Animal Care and Use Committee at the Georgia Institute of Technology, and all experiments were performed in compliance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
Data accessibility
The datasets supporting this article have been uploaded as part of the electronic supplementary material.
Authors' contributions
B.G.H. collected data, performed data analysis, drafted the manuscript and designed the study. S.A.S. developed the experimental testing set-up, wrote all data analysis code, performed data analysis and drafted the manuscript. E.M.B., B.G.G. and E.J.W. developed the experimental testing set-up and drafted the manuscript. M.R.P. and C.R.E. designed the study, coordinated the study and drafted the manuscript. All authors gave final approval for publication.
Competing interests
We declare we have no competing interests.
Funding
National Institutes of Health (grant nos. R01 EY025286 (C.R.E. and M.R.P.), 5T32 EY007092-32 (B.G.H.), F31 EY028832 (S.A.S.)) and Georgia Research Alliance (C.R.E.).
References
- 1.Levy AM, Fazio MA, Grytz R. 2018. Experimental myopia increases and scleral crosslinking using genipin inhibits cyclic softening in the tree shrew sclera. Ophthalmic Physiol. Opt. 38, 246–256. ( 10.1111/opo.12454) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Campbell IC, Coudrillier B, Ross Ethier C. 2014. Biomechanics of the posterior eye: a critical role in health and disease. J. Biomech. Eng. 136, 021005 ( 10.1115/1.4026286) [DOI] [PubMed] [Google Scholar]
- 3.Cook C, Foster P. 2012. Epidemiology of glaucoma: what's new? Can. J. Ophthalmol. 47, 223–226. ( 10.1016/j.jcjo.2012.02.003) [DOI] [PubMed] [Google Scholar]
- 4.Bengtsson B, Heijl A. 2005. A long-term prospective study of risk factors for glaucomatous visual field loss in patients with ocular hypertension. J. Glaucoma 14, 135–138. ( 10.1097/01.ijg.0000151683.04410.f3) [DOI] [PubMed] [Google Scholar]
- 5.Burgoyne CF. 2011. A biomechanical paradigm for axonal insult within the optic nerve head in aging and glaucoma. Exp. Eye Res. 93, 120–132. ( 10.1016/j.exer.2010.09.005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hernandez MR. 2000. The optic nerve head in glaucoma: role of astrocytes in tissue remodeling. Prog. Retin. Eye Res. 19, 297–321. ( 10.1016/S1350-9462(99)00017-8) [DOI] [PubMed] [Google Scholar]
- 7.Coudrillier B, et al. 2016. Effects of peripapillary scleral stiffening on the deformation of the lamina cribrosa. Invest. Ophthalmol. Vis. Sci. 57, 2666–2677. ( 10.1167/iovs.15-18193) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Holden BA, Fricke TR, Wilson DA, Jong M, Naidoo KS, Sankaridurg P, Wong TY, Naduvilath TJ, Resnikoff S. 2016. Global prevalence of myopia and high myopia and temporal trends from 2000 through 2050. Ophthalmology 123, 1036–1042. ( 10.1016/j.ophtha.2016.01.006) [DOI] [PubMed] [Google Scholar]
- 9.Rada JA, Shelton S, Norton TT. 2006. The sclera and myopia. Exp. Eye Res. 82, 185–200. ( 10.1016/j.exer.2005.08.009) [DOI] [PubMed] [Google Scholar]
- 10.Saw SM, Gazzard G, Shih-Yen EC, Chua WH. 2005. Myopia and associated pathological complications. Ophthalmic Physiol. Opt. 25, 381–391. ( 10.1111/j.1475-1313.2005.00298.x) [DOI] [PubMed] [Google Scholar]
- 11.Sung HW, Chen CN, Huang RN, Hsu JC, Chang WH. 2000. In vitro surface characterization of a biological patch fixed with a naturally occurring crosslinking agent. Biomaterials 21, 1353–1362. ( 10.1016/S0142-9612(00)00017-X) [DOI] [PubMed] [Google Scholar]
- 12.Yoo JS, Kim YJ, Kim SH, Choi SH. 2011. Study on genipin: a new alternative natural crosslinking agent for fixing heterograft tissue. Korean J. Thorac. Cardiovasc. Surg. 44, 197–207. ( 10.5090/kjtcs.2011.44.3.197) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rowland CR, Lennon DP, Caplan AI, Guilak F. 2013. The effects of crosslinking of scaffolds engineered from cartilage ECM on the chondrogenic differentiation of MSCs. Biomaterials 34, 5802–5812. ( 10.1016/j.biomaterials.2013.04.027) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hersh PS, Stulting RD, Muller D, Durrie DS, Rajpal RK, United States Crosslinking Study Group. 2017. United States multicenter clinical trial of corneal collagen crosslinking for keratoconus treatment. Ophthalmology 124, 1259–1270. ( 10.1016/j.ophtha.2017.03.052) [DOI] [PubMed] [Google Scholar]
- 15.Glickman RD. 2011. Ultraviolet phototoxicity to the retina. Eye Contact Lens 37, 196–205. ( 10.1097/ICL.0b013e31821e45a9) [DOI] [PubMed] [Google Scholar]
- 16.Wollensak G, Iomdina E, Dittert DD, Salamatina O, Stoltenburg G. 2005. Cross-linking of scleral collagen in the rabbit using riboflavin and UVA. Acta Ophthalmol. Scand. 83, 477–482. ( 10.1111/j.1600-0420.2005.00447.x) [DOI] [PubMed] [Google Scholar]
- 17.Backhouse S, Gentle A. 2018. Scleral remodelling in myopia and its manipulation: a review of recent advances in scleral strengthening and myopia control. Ann. Eye Sci. 3, 5 ( 10.21037/aes.2018.01.04) [DOI] [Google Scholar]
- 18.Campbell IC, Hannon BG, Read AT, Sherwood JM, Schwaner SA, Ethier CR. 2017. Quantification of the efficacy of collagen cross-linking agents to induce stiffening of rat sclera. J. R Soc. Interface 14, 20170014 ( 10.1098/rsif.2017.0014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Song W, Tang Y, Qiao J, Li H, Rong B, Yang S, Wu Y, Yan X. 2017. The comparative safety of genipin versus UVA-riboflavin crosslinking of rabbit corneas. Mol. Vis. 23, 504–513. [PMC free article] [PubMed] [Google Scholar]
- 20.Sung HW, Liang IL, Chen CN, Huang RN, Liang HF. 2001. Stability of a biological tissue fixed with a naturally occurring crosslinking agent (genipin). J. Biomed. Mater. Res. 55, 538–546. () [DOI] [PubMed] [Google Scholar]
- 21.Avila MY, Gerena VA, Navia JL. 2012. Corneal crosslinking with genipin, comparison with UV-riboflavin in ex-vivo model. Mol. Vis. 18, 1068–1073. [PMC free article] [PubMed] [Google Scholar]
- 22.Wong FF, Lari DR, Schultz DS, Stewart JM. 2012. Whole globe inflation testing of exogenously crosslinked sclera using genipin and methylglyoxal. Exp. Eye Res. 103, 17–21. ( 10.1016/j.exer.2012.06.010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu TX, Luo X, Gu YW, Yang B, Wang Z. 2014. Correlation of discoloration and biomechanical properties in porcine sclera induced by genipin. Int. J. Ophthalmol. 7, 621–625. ( 10.3980/j.issn.2222-3959.2014.04.06) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu TX, Wang Z. 2017. Biomechanics of sclera crosslinked using genipin in rabbit. Int. J. Ophthalmol. 10, 355–360. ( 10.18240/ijo.2017.03.05) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang M, Corpuz CC. 2015. Effects of scleral cross-linking using genipin on the process of form-deprivation myopia in the guinea pig: a randomized controlled experimental study. BMC Ophthalmol. 15, 89 ( 10.1186/s12886-015-0086-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lari DR, Schultz DS, Wang AS, Lee OT, Stewart JM. 2012. Scleral mechanics: comparing whole globe inflation and uniaxial testing. Exp. Eye Res. 94, 128–135. ( 10.1016/j.exer.2011.11.017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Coleman DJ, Trokel S. 1969. Direct-recorded intraocular pressure variations in a human subject. Arch. Ophthalmol. 82, 637–640. ( 10.1001/archopht.1969.00990020633011) [DOI] [PubMed] [Google Scholar]
- 28.Morrison JC, Guo WOCY, Johnson EC. 2011. Pathophysiology of human glaucomatous optic nerve damage: insights from rodent models of glaucoma. Exp. Eye Res. 93, 156–164. ( 10.1016/j.exer.2010.08.005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bunker S, Holeniewska J, Vijay S, Dahlmann-Noor A, Khaw P, Ng YS, Shima D, Foxton R. 2015. Experimental glaucoma induced by ocular injection of magnetic microspheres. J. Vis. Exp. 96, e52400 ( 10.3791/52400) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bouhenni RA, Dunmire J, Sewell A, Edward DP. 2012. Animal models of glaucoma. J. Biomed. Biotechnol. 2012, 692609 ( 10.1155/2012/692609) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cone-Kimball E, Nguyen C, Oglesby EN, Pease ME, Steinhart MR, Quigley HA. 2013. Scleral structural alterations associated with chronic experimental intraocular pressure elevation in mice. Mol. Vis. 19, 2023–2039. [PMC free article] [PubMed] [Google Scholar]
- 32.Coudrillier B, Pijanka J, Jefferys J, Sorensen T, Quigley HA, Boote C, Nguyen TD. 2015. Collagen structure and mechanical properties of the human sclera: analysis for the effects of age. J. Biomech. Eng. 137, 041006 ( 10.1115/1.4029430) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Girard MJ, Dahlmann-Noor A, Rayapureddi S, Bechara JA, Bertin BM, Jones H, Albon J, Khaw PT, Ethier CR. 2011. Quantitative mapping of scleral fiber orientation in normal rat eyes. Invest. Ophthalmol. Vis. Sci. 52, 9684–9693. ( 10.1167/iovs.11-7894) [DOI] [PubMed] [Google Scholar]
- 34.Shinohara K, et al. 2018. Establishment of novel therapy to reduce progression of myopia in rats with experimental myopia by fibroblast transplantation on sclera. J. Tissue Eng. Regen. Med. 12, e451–e461. ( 10.1002/term.2275) [DOI] [PubMed] [Google Scholar]
- 35.Zhang N, Favazza TL, Baglieri AM, Benador IY, Noonan ER, Fulton AB, Hansen RM, Iuvone PM, Akula JD. 2013. The rat with oxygen-induced retinopathy is myopic with low retinal dopamine. Invest. Ophthalmol. Vis. Sci. 54, 8275–8284. ( 10.1167/iovs.13-12544) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Turner PV, Albassam MA. 2005. Susceptibility of rats to corneal lesions after injectable anesthesia. Comp. Med. 55, 175–182. [PubMed] [Google Scholar]
- 37.Hwang YJ, Larsen J, Krasieva TB, Lyubovitsky JG. 2011. Effect of genipin crosslinking on the optical spectral properties and structures of collagen hydrogels. ACS Appl. Mater. Interfaces 3, 2579–2584. ( 10.1021/am200416h) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bianco G, Levy AM, Grytz R, Fazio MA. 2018. Substantial preconditioning effect observed in the inflation tests of juvenile tree shrew sclera. Invest. Ophthalmol. Vis. Sci. 59, 709. [Google Scholar]
- 39.Sherwood JM, Reina-Torres E, Bertrand JA, Rowe B, Overby DR. 2016. Measurement of outflow facility using iPerfusion. PLoS ONE 11, e0150694 ( 10.1371/journal.pone.0150694) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schultz DS, Lotz JC, Lee SM, Trinidad ML, Stewart JM. 2008. Structural factors that mediate scleral stiffness. Invest. Ophthalmol. Vis. Sci. 49, 4232–4236. ( 10.1167/iovs.08-1970) [DOI] [PubMed] [Google Scholar]
- 41.Jia L, Cepurna WO, Johnson EC, Morrison JC. 2000. Patterns of intraocular pressure elevation after aqueous humor outflow obstruction in rats. Invest. Ophthalmol. Vis. Sci. 41, 1380–1385. [PubMed] [Google Scholar]
- 42.Gonzalez P, Luna CC, Campbell IC, Ethier CR. 2015. Protective effects of adenoviral mediated subconjunctival delivery of BMP2 in an experimental glaucoma model. Invest. Ophthalmol. Vis. Sci. 56, 2006. [Google Scholar]
- 43.Kimball EC, Nguyen C, Steinhart MR, Nguyen TD, Pease ME, Oglesby EN, Oveson BC, Quigley HA. 2014. Experimental scleral cross-linking increases glaucoma damage in a mouse model. Exp. Eye Res. 128, 129–140. ( 10.1016/j.exer.2014.08.016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tonge TK, Murienne BJ, Coudrillier B, Alexander S, Rothkopf W, Nguyen TD. 2013. Minimal preconditioning effects observed for inflation tests of planar tissues. J. Biomech. Eng. 135, 114502 ( 10.1115/1.4025105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Coudrillier B, Tian J, Alexander S, Myers KM, Quigley HA, Nguyen TD. 2012. Biomechanics of the human posterior sclera: age- and glaucoma-related changes measured using inflation testing. Invest. Ophthalmol. Vis. Sci. 53, 1714–1728. ( 10.1167/iovs.11-8009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Myers KM, Coudrillier B, Boyce BL, Nguyen TD. 2010. The inflation response of the posterior bovine sclera. Acta Biomater. 6, 4327–4335. ( 10.1016/j.actbio.2010.06.007) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets supporting this article have been uploaded as part of the electronic supplementary material.