Highlights
-
•
An optimal and safe irradiation method for pregnant woman remains controversial.
-
•
Flattening-filter free VMAT could reduce the fetal exposure significantly.
-
•
As adjuvant CCRT, 66 Gy to the involved nodes was delivered over 33 fractions.
-
•
Without any shielding device, the fetal dose of 0.03 Gy in total was estimated.
-
•
A vigorous liveborn girl was born vaginally and she had no complications until now.
Keywords: Pregnancy, Tongue cancer, Fetal dose, Intensity-modulated radiation therapy, Flattening filter-free
Abstract
Optimizing irradiation protocols for pregnant women is challenging, because there are few cases and a dearth of fetal dosimetry data. We cared for a 36-year-old pregnant woman with tongue cancer. Prior to treatment, we compared three intensity-modulated radiation therapy (IMRT) techniques, including helical tomotherapy, volumetric arc therapy (VMAT), and flattening-filter free VMAT (FFF-VMAT) using treatment planning software. FFF-VMAT achieved the minimum fetal exposure and was selected as the optimal modality. We prescribed 66 Gy to the involved nodes, 60 Gy to the tumor bed and ipsilateral neck, and 54 Gy to the contralateral neck over 33 fractions. To confirm the out-of-field exposure per fraction, surface doses and the rectal dose were measured during FFF-VMAT delivery. Postoperative chemoradiotherapy was delivered using IMRT and a cisplatin regimen. Without any shielding, the total fetal dose was 0.03 Gy, within the limits established by the ICRP. A healthy girl was born vaginally at 37 weeks’ gestation.
1. Introduction
The rare coincidence of cancer and pregnancy is challenging to treat given the dearth of data on fetal dosimetry. Oral cancer accounts for <2% of all cancers during pregnancy, and there is no standard treatment approach [1]. For non-pregnant patients with negative prognostic factors, including surgical margin positivity and extracapsular lymph node extension, postoperative chemoradiotherapy (POCRT) is indicated as adjuvant treatment. Because POCRT may incur risk to the fetus [2], minimizing fetal dose is vital.
Several intensity-modulated radiotherapy (IMRT) techniques can reduce fetal dose. Although IMRT is excellent at sparing organs at risk (OARs) in head and neck cancers, it has scatter and leakage issues that must be dealt with [3]. Some studies have focused on the flattening-filter free mode (FFF) of a linear accelerator to reduce OAR dose [4], [5].
Herein, we present our phantom simulations and describe our treatment of a pregnant woman who underwent POCRT for tongue cancer.
2. Case report
A 36-year-old primipara presented with a nodule at the tongue tip, and reported increasing pain. Biopsy and multiple imaging tests revealed a squamous cell carcinoma with involvement of multiple ipsilateral cervical lymph nodes (Fig. 1). This cT2N2bM0 case was discussed in depth at a multidisciplinary tumor board, and it was decided that surgery should be performed during pregnancy. At the 17th week of pregnancy, partial glossectomy and lymph node dissection (bilateral levels I–III) were performed. There were no severe operative complications. Pathology confirmed extracapsular lymph node extension. To reduce the risk of locoregional failure, our tumor board decided that POCRT during pregnancy was justified. Written informed consent from the patient and her family was obtained before the initiation of POCRT.
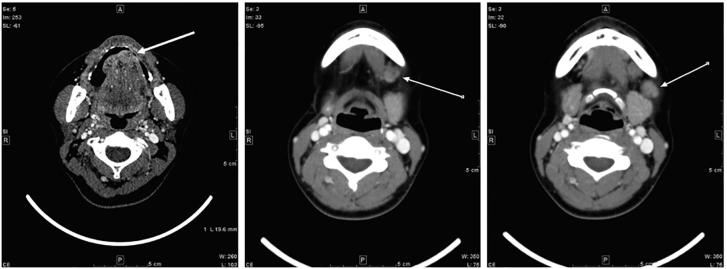
Fig. 1.
Pretreatment CT scan. Squamous cell carcinoma in the tongue with involvement of multiple ipsilateral cervical lymph nodes (arrows).
Concomitant with the radiotherapy, the patient was scheduled to receive intravenous tri-weekly cisplatin at 80 mg/m2 based on previous studies reporting its safety in the 2nd trimester of pregnancy [6].
2.1. RT planning and optimal modality selection
Using a thermoplastic mask for immobilization, a planning CT dataset was acquired using a 16-detector scanner (Toshiba Aquilion LB, Toshiba Medical Systems, Otawara, Japan). For IMRT planning, CT image data were reconstructed as 2 mm sections and were then sent to our treatment planning systems (TPS).
To find an optimal modality for fetal dose reduction during IMRT of the pregnant patient, a fetal dosimetric comparison was performed among helical tomotherapy (HT), single-arc volumetric arc therapy (VMAT), and FFF-VMAT.
Using 6 MV photon beams modulated by binary multileaf collimation, the HT plan was simulated on the Tomotherapy Planning Station software (v5.1.1.6, Accuray Inc. Sunnyvale, CA, USA). The VMAT and FFF-VMAT plans were created on the Pinnacle3 TPS (v9.10, Philips Medical Systems, Fitchburg WI, USA), with maximum dose rates of 600 and 1,500 MU/min, respectively. One complete arc was used for both VMAT techniques using the Agility MLC, achieving <0.5% leakage.
Using a simultaneous integrated boost method, we prescribed 66 Gy to the involved nodes, 60 Gy to the tumor bed and ipsilateral nodal levels I–V, and 54 Gy to the contralateral levels I–V over 33 fractions. The planning target volume (PTV) was defined by adding a 3-mm margin in all directions. Dose constraints for the PTV and OARs were: median values of D2% (maximum dose received by 2% of the PTV), D10% and D50% were <115%, 110%, and 105% of the prescribed dose, respectively. The three rotational IMRT plans (HT, VMAT, FFF-VMAT) had a mean PTV coverage of 96%. The maximum dose to the brainstem was <54 Gy; the maximum dose to the spinal cord was <50 Gy; the mean and median doses to the contralateral parotid gland were <26 Gy and <20 Gy.
Before FFF-VMAT was performed, we compared the respective absorbed out-of-field doses. Glass dosimeters (Chiyoda Technol. Co., Ltd., Tokyo, Japan) were placed on the umbilicus and pubic symphysis of an anthropomorphic phantom (The Phantom Laboratory, Salem, NY, USA). During actual beam delivery, surface doses at the umbilicus and pubic symphysis and rectal doses were measured.
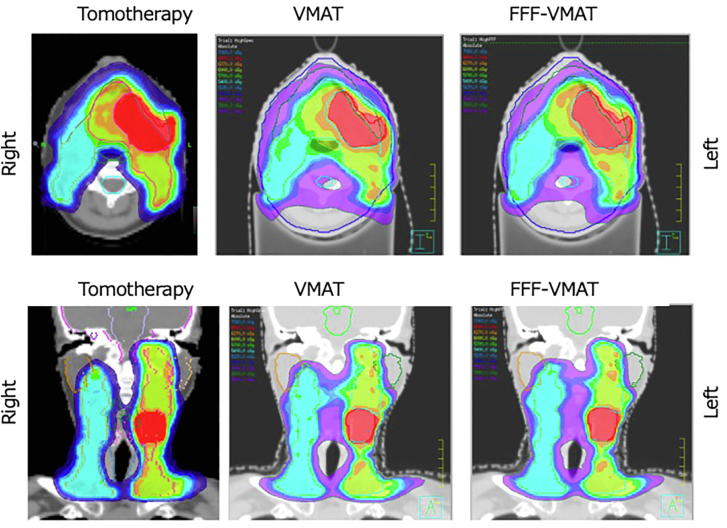
Previous reports have suggested that some patient-specific lead shielding devices could reduce the fetal dose in the setting of conventional radiotherapy [7], [8]. However, in this IMRT case using rotational beams, a patient-specific shield above the patient was not used because there were safety concerns (it might collapse due to its own weight) and no general-purpose properties despite the major expense involved. All three IMRT modalities satisfied the PTV and OAR constraints. Fig. 2 presents the dose distributions.
Fig. 2.
Treatment plans for helical tomotherapy (HT), volumetric arc therapy (VMAT), and flattening filter-free VMAT (FFF-VMAT). Representative dose distribution with 66 Gy (red), 60 Gy (yellow), and 54 Gy (blue) isodoses. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
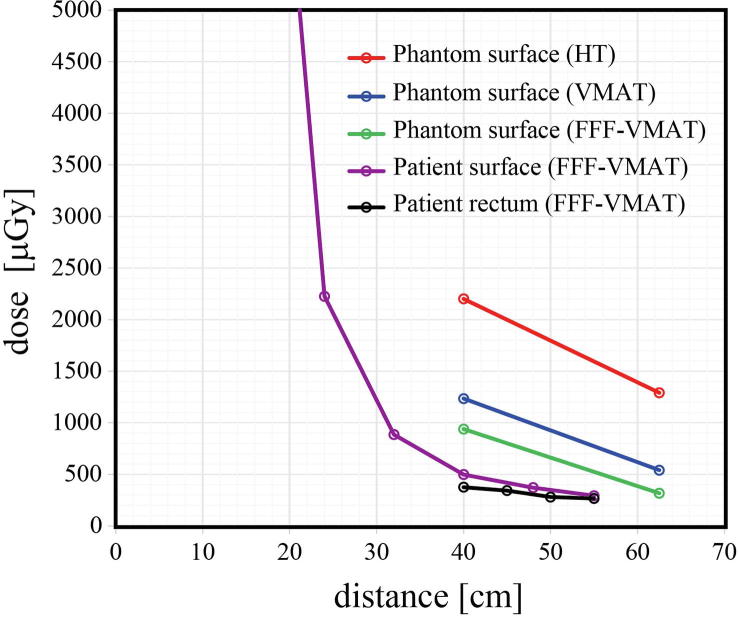
The results of the phantom study are summarized in Fig. 3. VMAT showed large out-of-field doses due to scattered photons from the flattening filter. HT, even with an FFF beam, produced high abdominal and pelvic doses, mainly due to a comparably larger number of MUs [9]. Depending on reduced scatter from the flattening filter and less head leakage associated with more efficient x-ray generation than conventional VMAT, FFF-VMAT achieved the lowest fetal exposure during beam delivery and was selected as the optimal modality [10]. The total fetal dose from FFF-VMAT was estimated to be approximately 0.05 Gy [11], which did not exceed the 0.1 Gy limit set by the ICRP [12]. The patient and her family consented to the treatment.
Fig. 3.
Out-of-field dose per fraction for helical tomotherapy (HT), volumetric arc therapy (VMAT), and flattening filter-free VMAT (FFF-VMAT). Horizontal axis shows the distance [cm] from the edge of the planning target volume. Rectal doses are shown as “patient rectum (FFF-VMAT).” Distances of 40 cm and 62.5 cm correspond to the umbilicus and pubic symphysis, respectively, on the phantom. Distances of 32 cm and 55 cm correspond to the umbilicus and pubic symphysis, respectively, of the patient.
2.2. Treatment and course
Glass dosimeters on the patient’s ventral surface and in the rectum revealed that the observed doses were lower than the phantom doses (Fig. 3). At this time, the patient’s weight was 53 kg and height was 160 cm. The final fetal dose of 0.03 Gy (886 μGy per fraction) was below the threshold value associated with cognitive abnormalities and physical malformations [11], [13], [14].
Although a second course of cisplatin was cancelled due to grade 4 neutropenia, the planned course of radiotherapy was completed with only two days’ interruption. Non-hematologic acute toxicities included grade 2 mucositis and dysgeusia.
A healthy female infant was born vaginally at 37 weeks’ gestation. Unfortunately, the tumor recurred in the patient’s lung and pleura 3 months after POCRT (1 week after vaginal delivery) without any locoregional recurrence. Despite pleural drainage and salvage chemotherapy, the patient died from lung metastases 6 months after radiotherapy. Her child was vigorous and had no complications at the last follow-up at 2 years old. To assess for possible cognitive or growth impairment and the risk of developing a malignancy, long-term follow-up is ongoing.
3. Discussion and conclusions
Obviously, pregnant women should avoid radiation therapy until post parturition since radiation could cause harm the fetus. Given the urgency of the high risk of recurrence, and the patient’s being in the second trimester of pregnancy, the risk of inducing radiation damage to the fetus was considered to be low and the need for immediate radiotherapy to be uppermost. With respect to cisplatin, the second trimester also poses less risk than the first trimester [12]. According to a previous report on peripartum complications and immediate fetal outcome, chemotherapy can be safely administered to women during the second trimester of pregnancy [15]. After the multidisciplinary meeting, we decide to deliver POCRT to her.
In this pre-treatment assessment, we did not test 3-dimensional conformal radiotherapy or opposing fields with/without wedges for less scattering due to less primary fluence. We wished to administer IMRT to the patient because of its reduced rate of side effects such as xerostomia. If an unacceptable fetal dose had been calculated for IMRT, we would have had to consider 2- or 3-dimensional RT techniques. However, in our assessment, FFF-VMAT would deliver a low fetal dose without unacceptable doses to the organs at risk. In FFF-VMAT, the removal of the flattening filter reduced the out-of-field dose near the treatment field because of decreased collimator scatter, relative to the usual VMAT technique with the filter. In FFF-VMAT, the removal of the flattening filter reduced the out-of-field dose because of decreased collimator scatter, relative to the usual VMAT technique with the filter. As for HT, regardless of using FFF beams, the more-than-expected scattered radiation were detected. This could be due to the shielding in its accelerator design, the small-bore size of HT, and its longer beam-on time than VMAT.
In summary, the fetal exposure to radiation during FFF-VMAT was exceedingly low, compared to the other rotational IMRT techniques. Therefore, we identified FFF-VMAT as a good choice for pregnant patients needing radiation therapy, at least in the head and neck region. However, the accuracy of the findings might not be representative of the entire pregnant population, and it is therefore still necessary to perform accurate model experiments in each case. To our knowledge, this is the first report of a successful live birth in a patient with locally advanced tongue cancer who successfully underwent postoperative FFF-VMAT as part of POCRT.
Declarations
Availability of data and materials
All data generated or analyzed during this study are included in this published article. The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Consent for publication
Written informed consent was obtained from the patient for publication of this case report and accompanying images.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Authors’ contributions
WT participated in the radiation management of the patient, collected patient’s data, reviewed literature and drafted the manuscript. KN, AH, TI, KO CK performed the radiation treatment, and dosimetrical measurement and calculations for this patient. HY, OA, KN revised the article. All authors read and approved the final manuscript.
Ethics approval and consent to participate
This report has been performed in accordance with the Declaration of Helsinki.
We would like to thank Dr. Mizuo Ando, Dr. Masafumi Yoshida, and Dr. Yuki Saito for establishing the treatment strategy and for helpful discussions. We thank Libby Cone, MD, MA, from DMC Corp. (www.dmed.co.jp <http://www.dmed.co.jp/>) for editing drafts of this manuscript.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper
Footnotes
Portions of this study were presented at the 54th Autumn Assembly of the Japan Radiological Society in Fukuoka, Japan in October 2018.
References
- 1.Layton S.A., Rintoul M., Avery B.S. Oral carcinoma in pregnancy. Br J Oral Maxillofac Surg. 1992;30:161–164. doi: 10.1016/0266-4356(92)90148-c. [DOI] [PubMed] [Google Scholar]
- 2.Bernier J., Cooper J.S., Pajak T.F., Van Glabbeke M., Bourhis J., Forastiere A. Defining risk levels in locally advanced head and neck cancers: A comparative analysis of concurrent postoperative radiation plus chemotherapy trials of the EORTC (#22931) and RTOG (#9501) Head Neck. 2005;27:843–850. doi: 10.1002/hed.20279. [DOI] [PubMed] [Google Scholar]
- 3.Öğretici A., Akbaş U., Köksal C., Bilge H. Investigation of conformal and intensity-modulated radiation therapy techniques to determine the absorbed fetal dose in pregnant patients with breast cancer. Med Dosim. 2016;41:95–99. doi: 10.1016/j.meddos.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 4.Alvarez Moret J., Obermeier T., Pohl F., Loeschel R., Koelbl O., Dobler B. Second cancer risk after radiation therapy of ependymoma using the flattening filter free irradiation mode of a linear accelerator. J Appl Clin Med Phys. 2018;19:632–639. doi: 10.1002/acm2.12438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dobler B., Maier J., Knott B., Maerz M., Loeschel R., Koelbl O. Second Cancer Risk after simultaneous integrated boost radiation therapy of right sided breast cancer with and without flattening filter. Strahlentherapie Und Onkol. 2016;192:687–695. doi: 10.1007/s00066-016-1025-5. [DOI] [PubMed] [Google Scholar]
- 6.Mir O., Berveiller P., Ropert S., Goffinet F., Goldwasser F. Use of platinum derivatives during pregnancy. Cancer. 2008;113:3069–3074. doi: 10.1002/cncr.23935. [DOI] [PubMed] [Google Scholar]
- 7.Orlandi E., Zonca G., Pignoli E., Stucchi C., Borroni M., Collini P. Postoperative radiotherapy for synovial sarcoma of the head and neck during pregnancy: Clinical and technical management and fetal dose estimates. Tumori. 2007;93:45–52. doi: 10.1177/030089160709300109. [DOI] [PubMed] [Google Scholar]
- 8.da Costa E.C., da Rosa L.A.R., Batista D.V.S. Fetus absorbed dose evaluation in head and neck radiotherapy procedures of pregnant patients. Appl Radiat Isot. 2015;100:11–15. doi: 10.1016/j.apradiso.2015.01.013. [DOI] [PubMed] [Google Scholar]
- 9.Kim D.W., Chung K., Chung W.K., Bae S.H., Shin D.O., Hong S. Risk of secondary cancers from scattered radiation during intensity-modulated radiotherapies for hepatocellular carcinoma. Radiat Oncol. 2014;9:109. doi: 10.1186/1748-717X-9-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sutlief S.G. Protection and measurement in radiation therapy. Health Phys. 2015;108:224–241. doi: 10.1097/HP.0000000000000241. [DOI] [PubMed] [Google Scholar]
- 11.Nakagawa K., Aoki Y., Kusama T., Ban N., Nakagawa S., Sasaki Y. Radiotherapy during pregnancy: Effects on fetuses and neonates. Clin Ther. 1997;19:770–777. doi: 10.1016/s0149-2918(97)80101-4. [DOI] [PubMed] [Google Scholar]
- 12.Van Calsteren K., Amant F. Cancer during pregnancy. Acta Obstet Gynecol Scand. 2014;93:443–446. doi: 10.1111/aogs.12380. [DOI] [PubMed] [Google Scholar]
- 13.Kal H.B., Struikmans H. Radiotherapy during pregnancy: Fact and fiction. Lancet Oncol. 2005;6:328–333. doi: 10.1016/S1470-2045(05)70169-8. [DOI] [PubMed] [Google Scholar]
- 14.Stovall M., Blackwell C.R., Cundiff J., Novack D.H., Palta J.R., Wagner L.K. Fetal dose from radiotherapy with photon beams: report of AAPM Radiation Therapy Committee Task Group No. 36. Med Phys. 1995;22:63–82. doi: 10.1118/1.597525. [DOI] [PubMed] [Google Scholar]
- 15.Amant F., Vandenbroucke T., Verheecke M., Fumagalli M., Halaska M.J., Boere I. Pediatric Outcome after Maternal Cancer Diagnosed during Pregnancy. N Engl J Med. 2015;373:1824–1834. doi: 10.1056/NEJMoa1508913. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article. The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.