Abstract
Background
Pressure ulcers (PUs) are complications of serious acute/chronic illness. Specialist mattresses used for prevention lack high quality effectiveness evidence. We aimed to compare clinical and cost effectiveness of 2 mattress types.
Methods
Multicentre, Phase III, open, prospective, parallel group, randomised controlled trial in 42 UK secondary/community in-patient facilities.
2029 high risk (acutely ill, bedfast/chairfast and/or Category 1 PU/pain at PU site) adult in-patients were randomised (1:1, allocation concealment, minimisation with random element) factors including: centre, PU status, facility and consent type. Interventions were alternating pressure mattresses (APMs) or high specification foam (HSF) for maximum treatment phase 60 days. Primary outcome was time to development of new PU Category ≥ 2 from randomisation to 30 day post-treatment follow-up in intention-to treat population. Trial registration: ISRCTN 01151335.
Findings
Between August 2013 and November 2016, we randomised 2029 patients (1016 APMs: 1013 HSF) who developed 160(7.9%) PUs. There was insufficient evidence of a difference between groups for time to new PU Category ≥ 2 Fine and Gray Model Hazard Ratio HR = 0.76, 95%CI0.56–1.04); exact P = 0.0890; absolute difference 2%). There was a statistically significant difference in the treatment phase time to event sensitivity analysis, Fine and Gray model HR = 0.66, 95%CI, 0.46–0.93; exact P = 0.0176); 2.6% absolute difference). Economic analyses indicate that APM are cost-effective.
There were no safety concerns.
Interpretation
In high risk (acutely ill, bedfast/chairfast/Category 1 PU/ pain on a PU site) in-patients, we found insufficient evidence of a difference in time to PU development at 30-day final follow-up, which may be related to a low event rate affecting trial power. APMs conferred a small treatment phase benefit. Patient preference, low PU incidence and small group differences suggests the need for improved targeting of APMs with decision making informed by patient preference/comfort/rehabilitation needs and the presence of potentially modifiable risk factors such as being completely immobile, nutritional deficits, lacking capacity and/or altered skin/Category1 PU.
Keywords: Pressure ulcer, Randomised controlled trial, Medical device, Prevention
Research in context
Evidence before this study
Pressure-relieving mattresses are a key component of pressure ulcer prevention practice and lack of evidence of comparative effectiveness may lead to widespread adoption of ‘high tech’ solutions (vs ‘low tech’) without demonstrated patient benefit. A Cochrane systematic review of support surfaces was available at trial inception and updated in 2015. The latter identifies 5 Randomised Controlled Trials (RCTs) demonstrating evidence that a ‘low-tech’ mattress type, High Specification Foam (HSF) confers benefit over ‘standard’ hospital foam mattresses (overall relative risk reduction of 60% (95%Confidence Interval (CI) 26% to 79%), hence they are recommended in national and international guidelines as a minimum prevention intervention for patients at risk of Pressure Ulcer development. The Cochrane review also identified 10 RCTs comparing a ‘high-tech’ group of interventions, (Alternating Pressure Mattresses (APMs)) versus HSF/other constant low pressure mattresses and a meta-analysis found no evidence of a difference (9 trials, overall relative risk of developing PU with APM 0.85 (95%CI 0.64 to 1.13)). However, only one RCT directly compared APMs and HSF plus 4 hourly turning and found no evidence of a difference. Despite the lack of evidence APMs are in common use for prevention of pressure ulcers.
Qualitative data from one large RCT and feedback from the Pressure Ulcer Research Service User Network (PURSUN) suggests some patients do not like APMs (due to pump noise/soft air cells impacting upon sleep, creating an unsafe feeling, restricting movement, exacerbating existing balance/mobility problems and increasing care burden/reducing ability to self-move).
A research recommendation from the National Institute of Health and Care Excellence (NICE), uncertainty of clinical and cost effectiveness, clinicians and patient preferences and a considerable difference in unit costs of both these most commonly used types of mattresses confirmed that an RCT was necessary.
Added value of this trial
PRESSURE 2 is the largest pragmatic RCT of pressure relieving mattresses undertaken world-wide and the only direct comparison of ‘high tech’ APMs and ‘low tech’ HSF. We report time to development of new Category ≥ 2 PUs and cost effectiveness to 30 days post treatment. Our trial provides approximately 80% of the data for the comparison of APMs and HSF, with 160 new Category 2 PU events in 2029 patients. There was insufficient evidence of a difference between APM and HSF in time to event at the end of trial follow-up: a pre-planned treatment phase sensitivity analysis identified early benefit of APM vs HSF, which diminished in the long-term/primary outcome.
This is the first trial to compare mattress safety and report detailed reasons for non-compliance which demonstrated no differences in the safety profile of APMs and HSF (i.e., ruled out harm), but compliance data highlighted that more patients requested a change from APM due to comfort or to aid movement compared to HSF.
Finally, this is the first study in the field to include an exploratory moderator analysis to assess potential modifiable factors by mattress group interactions in the primary model and these included altered and Category 1 skin status, complete immobility, nutritional deficits and lack of capacity.
Implications of all the available evidence
In patients who were at high risk of PU development: acutely ill in-patients, who were bedfast/chairfast and/or had an existing Category 1 PU (or pain on a PU site), we found insufficient evidence of a difference in time to PU development at the end of trial follow-up. However, APMs did confer a small treatment phase time to event benefit. Given the small absolute differences during treatment (2.6%) and long-term follow-up (2%), patient APM compliance and very low PU incidence rate observed (7.9%), there is a trade-off between using APMs and HSF. We recommend work on improving the personalisation of mattress type. This should take into account patient preferences, rehabilitation needs and the presence of risk factors which may be modifiable. Those gaining more potential benefit from APM are likely to be completely immobile, have nutritional deficits, lack capacity and/or have altered skin/Category1 PU.
Alt-text: Unlabelled Box
1. Introduction
Pressure ulcers (PUs) have detrimental impacts on patients' physical, social and psychological health including increased care burden, bedrest, prolonged rehabilitation and hospitalisation and distressing symptoms of pain, exudate and odour [1]. PUs are prevalent in the health-care sector [2] and as the elderly population increases and advances in medical care lead to increased long-term disability burden and complexity in patient management, improving the evidence base for improvements in their prevention is a priority. As well as high personal costs incurred by patients there are also high financial costs incurred by healthcare funders and providers in the treatment of PUs due to increased length of hospital stay, hospital admission, community nursing, treatments (reconstruction surgery/ mattresses/ dressings/ technical therapies) and complications (serious infection) [3].
They manifest when mechanical load applied to soft tissues causes cell deformation leading to cell membrane rupture and/or impairment of the blood supply and tissue ischaemia, both resulting in tissue damage [4].
A priority in clinical practice is prevention of PUs through repositioning (to intermittently completely off-load high risk skin areas) and provision of specialist mattresses/cushions to reduce mechanical load [5], [6]. In relation to specialist mattresses, systematic review evidence [7] supports guideline recommendations that high specification foam (HSF) mattresses are used as a minimum for high risk patients to prevent PUs [5], [6]. The McInnes et al. review [7] identified 11 RCTs comparing alternating pressure mattresses (APMs) with constant low pressure mattresses and a meta-analysis of 10 showed no evidence of a difference.
Despite the lack of evidence APMs are recommended in guidelines for patients where HSF is failing [5] or repositioning is not possible [6] and they are in widespread clinical use.
However, lived experience studies [1], secondary trial data [8] and feedback from the PU Research Service User Network (PURSUN) (http://medhealth.leeds.ac.uk/pursun accessed 06.08.18) suggests some patients do not like APMs (due to pump noise/soft air cells impacting upon sleep, creating an unsafe feeling, restricting movement, exacerbating existing balance/mobility problems and increasing care burden/reducing ability to self-move [8]. More recently a network meta-analysis reported that APMs had the lowest probability of being the most comfortable compared to other mattress types [9].
To address clinical uncertainty this pragmatic real world evaluation was designed to compare the clinical and cost effectiveness of two functionally distinct mattress types: ‘high tech’ alternating pressure mattresses (APMs) and ‘low tech’ high specification foam (HSF) [7].
The primary objective was to compare time to developing a new PU Category ≥ 2 by 30 days post end of treatment phase. Secondary objectives were to compare: time to developing a new PU Category ≥ 1; time to developing a new PU Category ≥ 3; time to healing of all pre-existing Category 2 PUs; mattress compliance; safety; impact on health related quality of life and incremental cost effectiveness.
2. Methods
2.1. Study Design
This was designed as a multicentre, Phase III, open, prospective, double triangular group sequential, parallel group, randomised controlled trial (RCT), with two planned interim analyses providing the possibility of early stopping for either futility or inferiority. The trial protocol is published [10] and summary methods detailed.
The trial, approved by Leeds West Research Ethics Committee (13/YH/0066), was monitored by an independent Trial Steering Committee (TSC) and Data Monitoring Committee (DMC).
2.2. Participants
The trial was promoted to centres through national networks and any organisation with sufficient access to trial eligible mattresses (see eDocument 1) and electric profiling beds were able to take part in the trial.
Patients were recruited from adult secondary care and community in-patient acute admission facilities in the UK (facilities are defined in eDocument 2). Prior to recruitment patients could be laying on any type of mattress. Consent was obtained though written/witnessed verbal consent or consultee agreement (see eDocument 3).
Patients were eligible if they were: in-patient with evidence of acute illness [10]; ≥ 8 years; expected stay ≥ 5 days; expected to comply with follow-up; on electric profiling bed-frame; high PU risk due to at least one of following:
Patients were excluded if they: had previously participated; current/previous PU Category ≥ 3; planned ICU admission; unable to receive intervention; outwith mattress weight limits (< 45 kg or > 180 Kg); ethically inappropriate e.g. thought to be in the last few days of their life.
Since a large proportion of patients suffering from or at risk of PUs have cognitive impairment and this impacts upon understanding and compliance with repositioning and self-care, including ability to reposition independently using the electric profiling beds, inclusion of patients who lacked capacity was necessary to ensure the study population was generalisable to a usual clinical population. Ethical approval was obtained to include patients who lacked capacity.
2.3. Randomisation and Masking
Participants were randomised centrally (24 h automated telephone system, ensuring allocation concealment) on a 1:1 basis using minimisation (with random element) and minimisation factors: centre, PU status, type of facility, and type of consent. Following randomisation the patient was expected to be transferred to the allocated mattress within 24 h. Full details of randomisation and allocation procedures are given in eDocument 4.
Blinding of the research and clinical staff or patients was not possible due to the appearance of the mattresses. Assessment of risk of bias of the primary endpoint was done with central blind review of photographs and a 10% sample of patients who had skin assessments by a practitioner blinded to previous assessments was performed. Details of methods are provided elsewhere [14].
2.4. Procedures
Patients were allocated to APM or HSF mattress for a maximum 60 day treatment phase (or until discharge, or no longer at risk), in conjunction with electric profiling bed frames. Treatment follow-up was twice weekly to day 30 and weekly from day 31–60 and there was a post treatment 30 day final follow-up.
Mattress specifications were defined (eDocument 1) and utilised from usual hospital supplies, maximising generalisability.
Participant recruitment and assessments were conducted by trained dedicated Clinical Research Nurse/Practitioners (CRN/P), employed by the local centre and independent of the ward teams. The assessment schedule was as follows:
Baseline only: risk factors were recorded using the PU Minimum Data Set [12], [15].
Baseline and follow-up visits: a) skin status, assessed on 14 anatomical sites including PU classification [6], additional descriptors of alterations to intact skin and skin site exclusions (Table 1 and eDocument 6) [12] and pain [13], b) PU prevention measures (e.g. repositioning frequency) and c) expected adverse events/serious adverse events (AEs/SAEs) including death, hospital re-admission, device-related ulcers and falls and ‘related’ and ‘unexpected’ SAEs (RUSAEs).
Table 1.
Additional skin classifications.
| Category | Description | |
|---|---|---|
|
Category A Alterations to intact skin |
Alterations to intact skin. Please specify with sub-category code: | |
| 001 = Blanching redness which persists | 011 = Papery thin | |
| 002 = Bruising – red hue | 012 = Cracks/calloused | |
| 003 = Bruising – purple hue | 013 = Spongy | |
| 004 = Scar | 014 = Macerated | |
| 005 = Oedema | 015 = Scratches | |
| 006 = Cellulitis | 016 = Rash | |
| 007 = Lymphodema | 017 = Scab | |
| 008 = Discoloration – ischaemia | 018 = Induration | |
| 009 = Discoloration – cyanosis | 019 = Heat | |
| 010 = Dry/flaky | 999 = None of the above, please describe | |
|
Category N/A Not applicable |
Specify with sub-category code: | |
| 001 = Amputation | 007 = Device-related ulcer | |
| 002 = Bandage in situ | 008 = Surgical wound/bruising | |
| 003 = Cast in situ | 009 = Traumatic wound/bruising | |
| 004 = Dressing in situ | 010 = Dermatological skin condition e.g. eczema | |
| 005 = Incontinence associated dermatitis | 011 = Unable to assess | |
| 006 = Other chronic wound | 999 = None of the above, please describe | |
Daily: mattress compliance (including which mattress the patient was on and whether, if it had dual function, it was in APM mode) was recorded during the treatment phase.
Baseline, week 1, week 3 and post treatment 30 day final follow-up: a generic QOL instrument EuroQol-5Dimension-5 Level (EQ-5D-5 L) [16] and condition specific utility measure Pressure Ulcer-Quality of Life-Utility Index (PU-QOL-UI) [17], [18] were researcher administered with a healthcare resource utilisation questionnaire (combining healthcare records and patient reported sources).
2.5. Outcomes
The primary outcome was time to developing a new PU Category ≥ 2 from randomisation to 30 days from the end of the treatment phase (maximum of 90 days).
Secondary outcomes (Clinical)
-
1.
Time to developing a new PU Category ≥ 3 from randomisation to trial completion
-
2.
Time to developing a new PU Category ≥ 1 from randomisation to trial completion
-
3.
Time to healing of all pre-existing Category 2 PUs from randomisation to trial completion
-
4.
Mattress change during the treatment phase
-
5.
Adverse events
Secondary objectives (Health Economic)
-
1.
Health related quality of life (QOL) using SF-12 and PU-QoL-P instruments
-
2.
Incremental cost-effectiveness of APM compared to HSF from the perspective of the health and social care sectors using EQ-5D-5 L and health and social care resource utilisation questionnaire
PUs were classified using the 2009 National Pressure Ulcer Advisory Panel/European Pressure Ulcer Advisory Panel (NPUAP/EPUAP) [19] system. In the absence of a PU, additional descriptors were recorded including ‘healthy intact skin’, alterations to intact skin (referred to as Category A for data recording purposes) and ‘not applicable’ as detailed in Table 1.
2.6. Sample Size and Statistical Analysis
588 events (~ 2954 patients) were required for 90% power, 5% difference (APM 18% vs HSF 23%, corresponding hazard ratio 0.759), 5% 2-sided significance level, 6% loss to follow-up [8], [13], [20] accounting for multiplicity in interim analyses using Lan-DeMets α and β spending functions [21]. Event driven interim analyses were planned (300 and 445 events) and a Value of Information (VOI) Analysis if futility boundary was crossed; futility boundaries were non-binding.
The trial recruited participants at a much slower rate than anticipated and an unplanned interim analysis and VOI Analysis using confidential trial data was requested by the funder and conducted in November/December 2015 on 909 participants. Unblind analyses were reviewed by the DMC and remained confidential. The DMC informed the Independent TSC that the event rate was much lower (9.9%) than originally estimated. The DMC and TSC asked the Trial Management Group (TMG) who remained blind to the event rate, to consider the minimum clinically relevant differences on varying centred event rates of 15%, 10% and 5%. The preferred TMG option was a funded extension to detect absolute differences of 3.75%, 3.3% and 2.5% (relative differences of 25%, 33.3% and 50%) respectively, which were considered to be the minimum clinically important differences. The TMG also noted that a no cost extension to detect absolute differences of 5%, 4% and 3% (relative difference of 33.3%, 40% and 60%) respectively were considered of clinical relevance. The funder agreed to a no cost recruitment extension with a final minimum recruitment target of 1996 patients, under a revised assumption of an overall event rate of 10%, absolute difference of 4% (corresponding HR of 0.652) with 80% power, requiring 172 events to be observed. This resulted in the trial design being modified to have one final analysis. Further details of the review process, analysis and recommendations can be found in eDocument 5.
Analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA) and pre-approved statistical analysis plan (eDocument 6). All participants recruited were included using ‘Intention-To-Treat’ (ITT) and analysed by randomised allocation.
The Fine and Gray [22] model (accounting for death and withdrawal due to clinical condition as competing risks) was fitted to the primary and secondary time to event endpoints and the planned treatment phase sensitivity analysis with adjustment for minimisation factors (except centre), presence of pain and presence of a condition affecting peripheral circulation; a likelihood ratio test was used to assess the effect of mattress group. Corresponding hazard ratios (HR), 95% confidence intervals (CI) and P-values are reported, and cumulative incidence of PU Category ≥ 2 development presented by mattress group.
AEs/SAEs and RUSAEs were summarised using descriptive statistics.
Analysis of data from the ‘Per Protocol’ population was undertaken.
An exploratory moderator analysis was also undertaken to assess potential risk factor by mattress group interactions in the primary model.
A mediator analysis was planned to identify potential mediators, such as mattress compliance and patient repositioning, however methods for competing risks data are currently under developed and therefore only descriptive summaries were produced.
To meet the health economic objectives, an ITT analysis used quality adjusted life years (QALYs) as the main outcome and adopted the perspective of the UK National Health Service (NHS) and Personal Social Services (PSS). The NICE £20,000 per QALY gained threshold was used to determined cost-effectiveness. Utility values were derived from the EQ-5D-5L [23], and costs were estimated using the UK tariff [23]. Multiple imputation was used to provide data for all patients and incremental cost effectiveness ratios (ICERs) reported. Costs and outcomes were adjusted for baseline imbalances using multiple regression analysis. Adjustment was made by utility at baseline (for outcomes only), PU status, setting, peripheral circulation and presence of pain. Sampling uncertainty was determined via a probabilistic sensitivity analysis (PSA) using a non-parametric bootstrap [24], [25], [26]. Additional sensitivity analyses were undertaken using QALYs estimated from the PUQOL-UI and complete cases only [18].
ISRCTN 01151335 URL: https://www.isrctn.com/search?q=01151335
2.7. Role of Funding Source
This project was funded by the National Institute for Health Research (NIHR) Health Technology Assessment (HTA) programme (project number 11/36/33). The funder monitored recruitment, requested an unplanned interim and VOI analysis, reviewed the VOI analysis and the recommendations of the TSC and DMC for trial continuation and made the final decision for an unfunded time extension to meet the revised minimum of 1996 participants.
3. Results
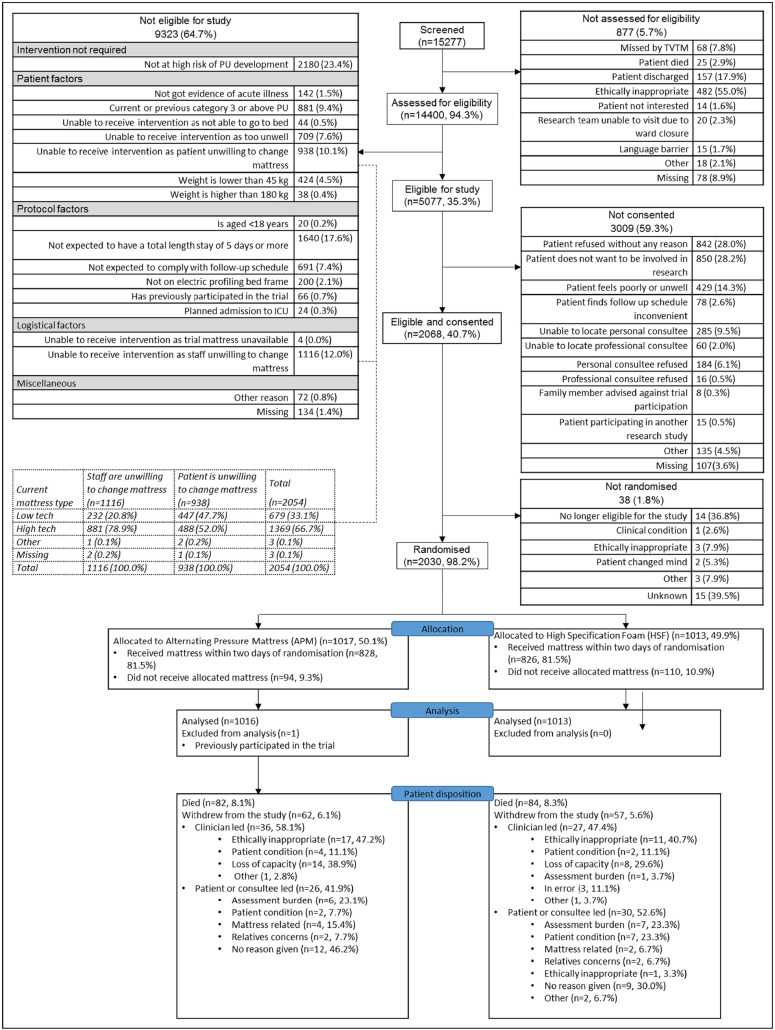
Between 1st August 2013 and 30th November 2016, 15,277 patients were screened (Fig. 1) from 39 English NHS Trusts/Scottish Health Boards (total 47 centres, comprising 25 teaching hospitals, 13 general hospitals and 9 community hospitals). Of those screened, 5077(33.2%) patients were eligible of whom 2068(40.7%) consented and 2030(40.0%) were randomised to APMs (1017, 50.1%) and HSF (1013, 49.9%), Fig. 1.
Fig. 1.
Consort diagram.
Screened and randomised populations were similar in age, gender and ethnicity but not by ward allocated mattress type. At screening, 7640(50.0%) patients were on APM or other ‘high tech’ and 7462(48.8%) patients were on HSF or other ‘low tech’, whereas, of those randomised 868(42.8%) were on APM and 1149(56.6%) were on HSF, reflecting greater staff unwillingness to randomise patients already provided with an APM (Table 3).
Table 3.
Baseline PU prevention interventions.
| Interventions | APM n = 1016 | HSF n = 1013 | Overall n = 2029 |
|---|---|---|---|
| Current mattress type | |||
| HSF or other ‘low tech’ | 575(56.6%) | 574(56.7%) | 1149 (56.6%) |
| APM or other ‘high tech’ | 435(42.8%) | 433(42.7%) | 868(42.8%) |
| Missing | 6(0.6%) | 6(0.6%) | 12(0.6%) |
| Frequency of repositioning in last 24 h | |||
| More frequently than 2 hourly | 148(14.6%) | 146(14.4%) | 294(14.5%) |
| 2–3 hourly | 473(46.6%) | 494(48.8%) | 967(47.7%) |
| 4–5 hourly | 333(32.8%) | 307(30.3%) | 640(31.5%) |
| 6–7 hourly | 36(3.5%) | 48(4.7%) | 84(4.1%) |
| Less frequently than 8 hourly | 20(2.0%) | 12(1.2%) | 32(1.6%) |
| Missing | 6(0.6%) | 6(0.6%) | 12(0.6%) |
| Time spent sat out of bed in last 24 h | |||
| N/A i.e. bedfast | 270(26.6%) | 271(26.8%) | 541(26.7%) |
| Less than 2 h | 91(8.9%) | 90(8.9%) | 181(8.9%) |
| 2–3 h | 134(13.2%) | 134(13.2%) | 268(13.2%) |
| 4–5 h | 178(17.5%) | 183(18.1%) | 361(17.8%) |
| 6–7 h | 125(12.3%) | 128(12.6%) | 253(12.5%) |
| More than 8 h | 207(20.4%) | 191(18.9%) | 398(19.6%) |
| Missing | 11(1.1%) | 16(1.6%) | 27(1.3%) |
| Type of cushion | |||
| Standard chair only | 203(27.3%) | 206(27.9%) | 409(27.6%) |
| High tech specialist cushion | 56(7.5%) | 52(7.0%) | 108(7.3%) |
| Low tech specialist cushion/ chair with integral pressure relief | 434(58.3%) | 429(58%) | 863(58.2%) |
| Pillow | 45(6.1%) | 47(6.4%) | 92(6.2%) |
| Missing | 6(0.8%) | 5(0.7%) | 11(0.7%) |
| Total (number of patients who sat out) | 744(100%) | 739(100%) | 1483(100%) |
| Participant on electronic profiling bedframe | |||
| Yes | 1012(99.6%) | 1008(99.5%) | 2020(99.6%) |
| No | 3(0.3%) | 3(0.3%) | 6(0.3%) |
| Missing | 1(0.1%) | 2(0.2%) | 3(0.1%) |
| Adjuvant devices and dressing | |||
| Yes | 143(14.1%) | 141(13.9%) | 284(14.0%) |
| No | 867(85.3%) | 865(85.4%) | 1732(85.4%) |
| Missing | 6(0.6%) | 7(0.7%) | 13(0.6%) |
Patient disposition was balanced across groups including, withdrawals (APM 6.1%, HSF 5.6%) and deaths (APM 8.1%, HSF 8.3%) (Fig. 1). One patient randomised twice, was withdrawn and the second randomisation excluded from the ITT population (n = 2029).
Patient characteristics and pre-randomisation preventative care interventions were balanced across groups (Table 2, Table 3 and Table 7a in eDocument 7).
Table 2.
Baseline characteristics of the intention-to-treat population.
| Attribute | APM n = 1016 | HSF n = 1013 | Overall n = 2029 |
|---|---|---|---|
| Gender | |||
| Male | 462(45.5%) | 445(43.9%) | 907(44.7%) |
| Female | 553(54.4%) | 566(55.9%) | 1119(55.2%) |
| Missing | 1(0.1%) | 2(0.2%) | 3(0.1%) |
| Age (years) | |||
| Mean (S.D.) | 77.8(13.42) | 78.2(12.87) | 78.0(13.1) |
| Median (range) | 81(21.1105) | 81(21.9101) | 81(21,105) |
| IQR | (71.3,87.0) | (71.9,87.2) | (71.6,87.1) |
| Missing | 0 | 0 | 0 |
| Ethnicity | |||
| White | 1000(98.4%) | 992(97.9%) | 1992(98.2%) |
| Mixed race/non-white | 15(1.5%) | 19(1.9%) | 34(1.7%) |
| Missing | 1(0.1%) | 2(0.2%) | 3(0.1%) |
| Medical speciality | |||
| Medical | 641(63.1%) | 669(66.1%) | 1310(64.6%) |
| Surgical | 83(8.2%) | 72(7.1%) | 155(7.6%) |
| Orthopaedics and trauma | 233(22.9%) | 220(21.7%) | 453(22.3%) |
| Oncology | 21(2.1%) | 16(1.6%) | 37(1.8%) |
| Critical care | 10(1.0%) | 6(0.6%) | 16(0.8%) |
| Neurosciences | 17(1.7%) | 15(1.5%) | 32(1.6%) |
| Spinal injury | 8(0.8%) | 9(0.9%) | 17(0.9%) |
| Other | 2(0.2%) | 2(0.2%) | 4(0.2%) |
| Missing | 1(0.0%) | 4(0.3%) | 5(0.2%) |
| Consent type | |||
| Written | 706(69.5%) | 696(68.7%) | 1402(69.1%) |
| Witnessed verbal | 151(14.9%) | 152(15.0%) | 303(14.9%) |
| Consultee agreement | 159(15.6%) | 163(16.1%) | 322(15.9%) |
| Missinga | 0(0.0%) | 2(0.2%) | 2(0.1%) |
| Healthcare setting | |||
| Secondary care hospital | 710(69.9%) | 704(69.5%) | 1414(69.7%) |
| Community hospital | 191(18.8%) | 188(18.6%) | 379(18.7%) |
| NHS intermediate care/ rehabilitation facility | 115(11.3%) | 119(11.7%) | 234(11.5%) |
| Missingb | 0(0.0%) | 2(0.2%) | 2(0.1%) |
| Days between admission to randomising | |||
| Mean (S.D.) | 12.7(20.27) | 13.3(21.23) | 13.0(20.8) |
| Median (range) | 6(0.0,306) | 7(0.0,388) | 7(0,388) |
| IQR | (3.0,15.0) | (3.0,17.0) | (3.0,16.0) |
| Missing | 1 | 2 | 3 |
Standard deviation (SD), Inter-Quartile Range (IQR).
These were entered on the 24 h system as written consent, and therefore included in the analyses.
These were entered on the 24 h system as Secondary care hospital, and therefore included in the analyses,
Table 7.
Time to development of new PU Category ≥ 2 by 30 day final follow-up.
| Covariate | Level of covariate | Incidence | Reference level | HR point Estimate | HR 95% Wald Confidence limits | Wald P-value | |
|---|---|---|---|---|---|---|---|
| Treatment | HSF | 90/1013 (8.9%) | – | – | – | – | 0.0890⁎ |
| APM | 70/1016 (6.9%) | vs HSF | 0.76 | 0.56 to | 1.04 | ||
| Skin status | No PU | 115/1648 (7.0%) | – | – | – | – | 0.0057 |
| PU Category 1 | 27/236 (11.4%) | vs No PU | 1.83 | 1.16 to | 2.87 | ||
| PU Category 2 | 18/145 (12.4%) | vs No PU | 1.83 | 1.09 to | 3.09 | ||
| Consent type | Written | 100/1404 (7.1%) | – | – | – | – | 0.3025 |
| Witnessed verbal | 32/303 (10.6%) | vs Written | 1.34 | 0.90 to | 1.99 | ||
| Consultee agreement | 28/322 (8.7%) | vs Written | 1.23 | 0.79 to | 1.91 | ||
| Setting | Secondary care hospital | 102/1416 (7.2%) | – | – | – | – | 0.6182 |
| Community hospital | 34/379 (9.0%) | vs Secondary care hospital | 1.06 | 0.71 to | 1.58 | ||
| NHS intermediate care/ rehabilitation facility | 24/234 (10.3%) | vs Secondary care hospital | 1.26 | 0.79 to | 1.99 | ||
| Pain on a healthy, altered or PU Category 1 skin site | No | 67/890 (7.5%) | – | – | – | – | 0.5070 |
| Yes | 90/1084 (8.3%) | vs No | 1.15 | 0.82 to | 1.61 | ||
| Unable to assess | 1/30 (3.3%) | vs No | 0.38 | 0.05 to | 2.94 | ||
| Missing | 2/25 (8.0%) | vs No | 2.02 | 0.43 to | 9.45 | ||
| Presence of condition affecting peripheral circulation | No | 120/1567 (7.7%) | – | – | – | – | 0.5688 |
| Yes | 39/455 (8.6%) | vs No | 1.09 | 0.75 to | 1.57 | ||
| Missing | 1/7 (14.3%) | vs No | 2.91 | 0.35 to | 24.51 | ||
P-values obtained from corresponding likelihood ratio tests for the effect of treatment is 0.0890.
The trial comprised largely of elderly patients (median 81 years, range 21–105), 1119 (55.2%) were female and 1992 (98.2%) of white ethnicity. Patients were in-patients for a median of 7 (range 0–388) days pre-randomisation.
Overall 322 (15.9%) patients lacked capacity, 909(44.8%) had a history of falls in the preceding month, 1961 (96.6%) had limitations to independent movement and 2003 (98.7%) and 1879 (92.6%) patients were classified as ‘at risk’ of PU development on the PURPOSE-T and Braden Scale respectively (Table 4). Subscales of Braden: Activity and Mobility have been included as they were eligibility criteria.
Table 4.
Baseline risk factors.
| Risk factor | APM n = 1016 | HSF n = 1013 | Overall n = 2029 |
|---|---|---|---|
| BMI | |||
| Underweight (< 18.5 kg/m2) | 52(5.1%) | 49(4.8%) | 101(5.0%) |
| Normal weight (18.5 to < 25 kg/m2) | 455(44.8%) | 392(38.7%) | 847(41.7%) |
| Overweight (25 to < 30 kg/m2) | 266(26.2%) | 336(33.2%) | 602(29.7%) |
| Obese (≥ 30 kg/m2) | 235(23.1%) | 217(21.4%) | 452(22.3%) |
| Missing | 8(0.8%) | 19(1.9%) | 27(1.3%) |
| History of falls in the past month | |||
| Yes | 458(45.1%) | 451(44.5%) | 909(44.8%) |
| No / not aware of any falls | 554(54.5%) | 559(55.2%) | 1113(54.9%) |
| Missing | 4(0.4%) | 3(0.3%) | 7(0.3%) |
| Analysis of independent movement | |||
| Moves frequently / Major position changes | 28(2.8%) | 32(3.2%) | 60(3.0%) |
| Moves frequently / Slight position changes | 141(13.9%) | 139(13.7%) | 280(13.8%) |
| Moves occasionally / Major position changes | 110(10.8%) | 110(10.9%) | 220(10.8%) |
| Moves occasionally / Slight position changes | 624(61.4%) | 621(61.3%) | 1245(61.4%) |
| Doesn't move | 109(10.7%) | 107(10.6%) | 216(10.6%) |
| Missing | 4(0.4%) | 4(0.4%) | 8(0.4%) |
| Risk status recorded on PURPOSE T | |||
| Not at risk | 12(1.2%) | 11(1.1%) | 23(1.1%) |
| No PU but at risk | 820(80.7%) | 816(80.6%) | 1636(80.6%) |
| PU Category ≥ 1 or scarring from previous PU | 183(18.0%) | 184(18.2%) | 367(18.1%) |
| Missing | 1(0.1%) | 2(0.2%) | 3(0.1%) |
| Braden Activity subscale | |||
| Walks Frequently | 13(1.3%) | 9(0.9%) | 22(1.1%) |
| Walks Occasionally | 108(10.6%) | 113(11.2%) | 221(10.9%) |
| Chairfast | 677(66.6%) | 667(65.8%) | 1344(66.2%) |
| Bedfast | 217(21.4%) | 222(21.9%) | 439(21.6%) |
| Missing | 1(0.1%) | 2(0.2%) | 3(0.1%) |
| Braden Mobility subscale | |||
| No Limitation | 22(2.2%) | 20(2.0%) | 42(2.1%) |
| Slightly Limited | 125(12.3%) | 115(11.4%) | 240(11.8%) |
| Very Limited | 790(77.8%) | 797(78.7%) | 1587(78.2%) |
| Completely Immobile | 78(7.7%) | 79(7.8%) | 157(7.7%) |
| Missing | 1(0.1%) | 2(0.2%) | 3(0.1%) |
| Overall Braden PU risk | |||
| Not at risk (> 18) | 78(7.7%) | 69(6.8%) | 147(7.2%) |
| At risk (≤ 18) | 937(92.2%) | 942(93.0%) | 1879(92.6%) |
| Missing | 1(0.1%) | 2(0.2%) | 3(0.1%) |
There were high levels of skin morbidity including ‘worst’ PU/skin status of 7.1%(n = 145) Category 2 PUs, 11.6%(n = 235) Category 1 and 66.4%(n = 1347) alterations to intact skin with 53.4%(n = 1084) reporting pressure area related pain (Table 5).
Table 5.
Skin status at baseline.
| Question | APM n = 1016 | HSF n = 1013 | Overall n = 2029 |
|---|---|---|---|
| Worst category of skin reported at baseline (patient level) | |||
| Normal Skin | 147(14.5%) | 152(15.0%) | 299(14.7%) |
| Category A | 673(66.2%) | 674(66.5%) | 1347(66.4%) |
| Category 1 | 125(12.3%) | 110(10.9%) | 235(11.6%) |
| Category 2 | 70(6.9%) | 75(7.4%) | 145(7.1%) |
| Missing | 1(0.1%) | 2(0.2%) | 3(0.1%) |
| Pressure related pain on any skin site | |||
| Yes | 577(56.8%) | 584(57.7%) | 1161(57.2%) |
| No | 393(38.7%) | 388(38.3%) | 781(38.5%) |
| Unable to assess | 15(1.5%) | 15(1.5%) | 30(1.5%) |
| Combination of ‘missing’ and ‘no’ | 6(0.6%) | 6(0.6%) | 12(0.6%) |
| Combination of ‘No’ and ‘unable to assess | 15(1.5%) | 13(1.3%) | 28(1.4%) |
| Missing | 10(1.0%) | 7(0.7%) | 17(0.8%) |
| Pressure related pain on a healthy, altered or Category 1 skin site? | |||
| Yes | 541(53.2%) | 543(53.6%) | 1084(53.4%) |
| No | 440(43.3%) | 439(43.3%) | 879(43.3%) |
| Unable to assess | 15(1.5%) | 15(1.5%) | 30(1.5%) |
| Combination of ‘missing’ and ‘no’a | 2(0.2%) | 1(0.1%) | 3(0.1%) |
| Combination of ‘No’ and ‘unable to assessa | 5(0.5%) | 3(0.3%) | 8(0.4%) |
| Missing | 9(0.9%) | 5(0.5%) | 14(0.7%) |
| No skin sites reported as healthy, altered or Category 1b | 4(0.4%) | 7(0.7%) | 11(0.5%) |
Classified as ‘no’ in the analysis.
Classified as ‘missing’ in the analysis.
Of 2029 ITT population, 160 (7.9%) patients developed at least one new PU Category ≥ 2 with an absolute difference of 2% (APM 70 (6.9%), HSF 90(8.9%)), see Table 6 with a total of 213 new PUs Category ≥ 2 observed (APM, N = 89, HSF, N = 124). Skin sites location of all new PUs can be found in eDocument 7b.
Table 6.
Number of patients developing a new PU Category ≥ 2 at post treatment 30 day final follow-up.
| Mattress | New PU Cat ≥ 2 |
No new PU Category ≥ 2 |
Baseline Assessment not eligible |
Total | ||
|---|---|---|---|---|---|---|
| No | Died | Withdrawn | ||||
| APM | 70(6.9%) | 825(81.2%) | 77(7.6%) | 40(3.9%) | 4(0.4%) | 1016 (100%) |
| HSF | 90(8.9%) | 812(80.2%) | 72(7.1%) | 31(3.1%) | 8(0.8%) | 1013 (100%) |
| Overall | 160(7.9%) | 1637(80.7%) | 149(7.3%) | 71(3.5%) | 12(0.6%) | 2029 (100%) |
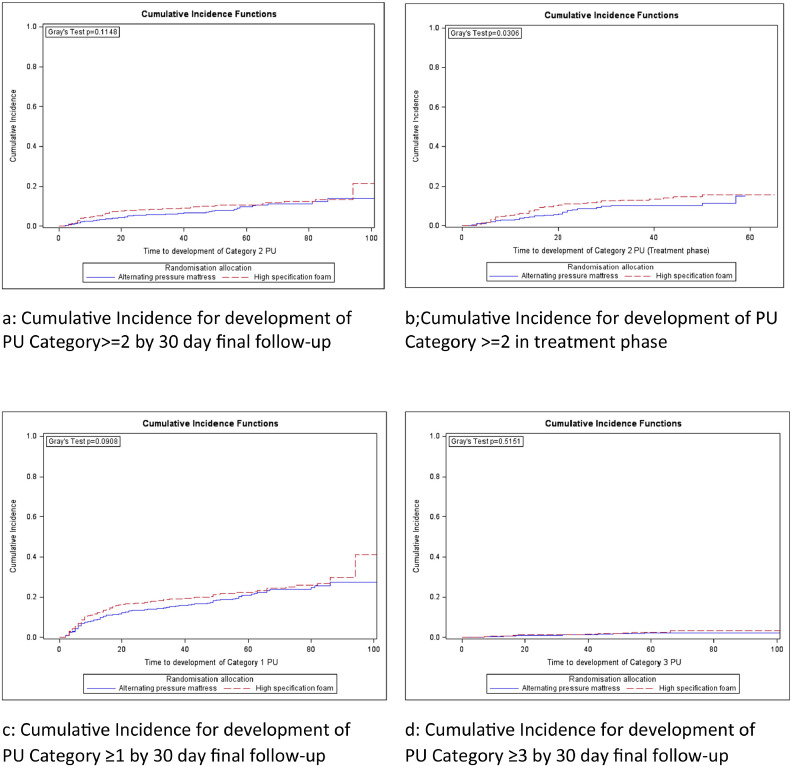
Where patients developed a PU Category ≥ 2, the median (range) time to first new PU Category ≥ 2 was APM 18 days (2–86) and HSF 12 days (2–94). There was no evidence of a difference between mattress groups for the primary endpoint (time to development of PU Category ≥ 2) in the adjusted analysis (Fine and Gray model (HR = 0.76 (95%CI, 0.56 to 1.04; exact P = 0.0890))). Only skin status was statistically significantly associated with the primary endpoint (Wald P = 0.0057); specifically, patients with a pre-existing Category 1 PU (HR = 1.83(95% CI, 1.17 to 2.87)) and pre-existing Category 2 PU (HR = 1.83(95% CI, 1.09 to 3.09)) were more likely to develop a new Category 2 PU than those who did not have a Category 1 or 2 PU at baseline (see Table 7). Fig. 2 represents the unadjusted cumulative incidence curves for the primary and secondary analyses.
Fig. 2.
a-d: Survival analysis (Cumulative incidence functions for time to development of PUs).
In the treatment phase sensitivity analysis, 132 (6.5%) developed new PU Category ≥ 2 between randomisation and end of treatment phase (APM 53(5.2%), HSF 79 (7.8%)) with a statistically significant difference observed in time to development of PU Category ≥ 2 in the Fine and Gray model (HR = 0.66(95% CI, 0.46 to 0.93; exact P = 0.0176)) (see eDocument 8a).
350 (17.2%) patients reached the secondary endpoint of developing a new PU Category ≥ 1 by 30 day final follow-up (APM 160(15.7%), HSF 190(18.8%)), with no evidence of a difference in the time to event (Fine and Gray model (HR = 0.83(95% CI, 0.67 to 1.02; exact P = 0.0733))) (see eDocument 8b).
32 (1.6%) patients reached the secondary endpoint of developing a new PU Category ≥ 3 by 30 day final follow-up (APM 14(1.4%), HSF 18(1.8%)) with no evidence of a difference in the time to event (Fine and Gray model (HR = 0.81(95% CI, 0.40 to 1.62; exact P = 0.5530)) (see eDocument 8c)). The number of Category ≥ 3 PUs were comparable by arm (APM N = 19 vs HSF N = 21).
Of 145 patients with a pre-existing PU Category 2 (APM 70(48.3%), HSF 75(51.7%)), healing was observed in 89 (APM 44(62.9%); HSF 45(60.0%)) (see eDocument 8d), with no evidence of a difference in the time to event (Fine and Gray model (HR = 1.12 (95% CI, 0.74 to 1.68; exact P = 0.6122))).
There were no ‘RUSAEs’ and only 3 mattress related AEs reported. Deaths (APM 8.1%, HSF 8.3%), re-admission rates (APM 8.1%, HSF 6.1%) and fall rates (APM 14.9%, HSF 15.7%) were similar in both groups. Of all 486 reported falls, 62.3% occurred after the treatment phase and 17.5% resulted in serious injury but none were mattress related.
Compliance with mattress allocation within 48 h of randomisation was reported for 81.5% of patients in each group (see eDocument 9). The median (range) proportion of time spent on the randomised mattress was 92% (0–100%) APM and 100% (0–100%) HSF. Only 94 (9.3%) APM and 110 (10.9%) HSF randomised patients never received their allocated mattress.
Where allocated mattress was received, 24.1%(222/922) APM and 24.4%(220/903) HSF had at least one mattress change (eDocument 9), with reasons for first change including mattress being uncomfortable (APM 90(40.5%), HSF 28(12.7%)); to aid rehabilitation or movement (APM 49 (22.1%), HSF 5(2.3%)); ward transfer (APM 40(18.0%), HSF 20(9.1%)) and clinical condition (APM 3(1.4%), HSF 130(59.1%)).
The blinded central photography sub-study [14] undertaken to establish systematic bias in endpoint assessment found high levels of agreement in both arms and a Prevalence and Bias Adjusted Kappa statistic of 0.93 demonstrating ‘very good agreement’ of blinded photograph assessments compared to un-blind clinical assessments.
3.1. Per Protocol Population
The per-protocol population consisted of 1352(66.6%) patients. Patients were excluded from the per-protocol analysis for compliance and eligibility reasons (not mutually exclusive) as follows; less than 60% compliance with allocated mattress (N = 545, 26.9%), not at high risk of PU development (N = 42, 2.1%), current or previous PU Category ≥ 3 (N = 8, 0.4%), outside weight limits (N = 6, 0.3%), consent form was not received or consent date was after randomisation (N = 13, 0.6%). Of the 1352 patients in the per-protocol population, 663(49.0%) were allocated to APM and 689(51.0%) were allocated to HSF. There was no evidence of a difference between mattress groups in time to development of new PU Category ≥ 2 for the primary endpoint (adjusted Fine and Gray model HR (95%CI) = 0.79 (0.54 to 1.16), P = 0.2249), absolute difference 1.2% (APM 7.2%, HSF 8.4%). In the treatment phase sensitivity analysis a marginally significant treatment effect in time to development of new PU Category ≥ 2 was observed indicating some evidence of a difference between mattress groups (adjusted Fine and Gray model HR (95%CI) of 0.76 (0.32 to 1.00), P = 0.0508), absolute difference 1.7% (APM 5.7%, HSF 7.4%).
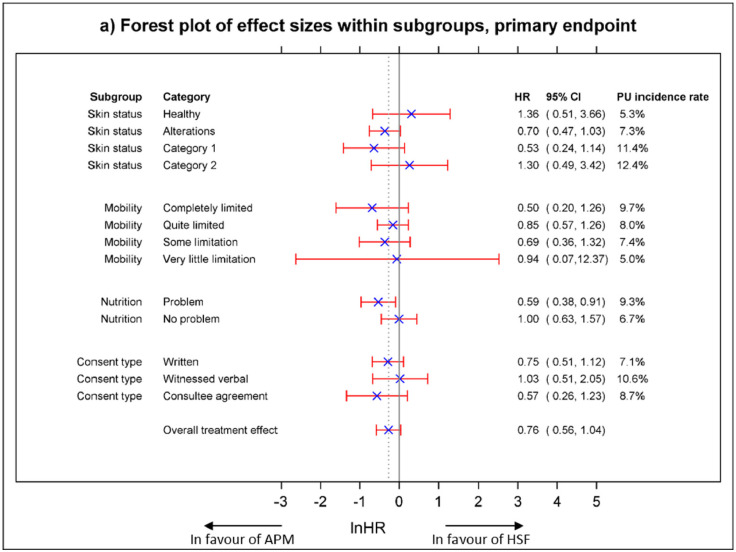
3.2. Moderator Analysis (Exploratory Analysis)
The results of the exploratory moderator analysis of the treatment effects for each level of each covariate (risk factor) for the primary endpoint are presented in Fig. 3. The Forest plots present the point estimate of the treatment effect, ln(HR), together with the corresponding 95% CI, alongside the plot, the corresponding HR and 95% CIs. Incidence rates observed within each level of risk factor are aligned with the PU conceptual framework [4] and whilst there is no evidence of differential treatment effects within risk factors at the 5% significance level, treatment effects from these exploratory analyses suggest that there may be a potential benefit of APM vs HSF in patients whose worst skin status was assessed as altered or PU Category 1; those with mobility limitations; those with a nutritional problem and; patients who participated via consultee agreement.
Fig. 3.
Moderator analysis (Forest plots of effect size within subgroups).
3.3. Mediator Analysis (Exploratory Analysis)
Throughout the treatment phase at least 50% of patients in both APM and HSF arms at each visit with complete data were repositioned 2–3 hourly or more frequently. However, the proportion of patients repositioned 2–3 hourly or more frequently appears to reduce during the treatment phase in both arms (eDocument 10).
3.4. Health Economics
The mean total health care costs of using APM was lower than HSF (£4482; 95% CI £4438–£4526 vs £4621; 95% CI £4577–£4665; P < 0.000), this despite the APM mattress being more costly as the biggest proportion of the costs (around 60%) correspond to in-patient care (APM £2810.08; HSF £2888.68) (eDocument 11 Health Economic Analysis). In terms of outcomes, the mean estimated QALYs were higher for APM than HSF (0.128; 95% CI 0.126–0.130 vs 0.127 95% CI 0.124–129; P = 0.47). Although the negligible difference in QALYs, the difference in costs between the two alternatives drive the cost-effectiveness towards APM.
The results of the PSA confirm those of the deterministic analysis as in 99% of the 10,000 Monte Carlo simulations APM is cost-saving, whilst in 77% APM it produces more QALYs than HSF. Although in some cases (33% iterations) HSF produces more QALYs, its higher cost overcome these gains. Therefore, the PSA estimates indicate that APM has a 99% probability of being cost effective at a threshold of £20,000 (eDocument 11 Health Economic Analysis).
The results of the sensitivity analyses using QALYs derived from the PUQOL-UI were in the same direction as when derived from the EQ-5D-5L (eDocument Health Economic Analysis).
4. Discussion
Overall, only 7.9% of patients recruited to the PRESSURE 2 trial developed one or more new Category ≥ 2 pressure ulcers. The point estimate of the hazard ratio suggests a benefit of APM over HSF but the trial was underpowered for the primary 30 day post treatment phase endpoint due to the low number of PU events and the time to event difference observed was not statistically significant different between mattress groups. All analyses were in the same direction as the primary endpoint and the treatment phase sensitivity analysis demonstrated small but significant early benefit of APM vs HSF. Importantly, all analyses ruled out harm.
The long-term outcome was considered the most important for the NHS and it appears that the early benefit of the APM in the delayed onset of new PUs during the treatment phase, is diminished following treatment phase cessation and can be explained by cross over, increased population heterogeneity post-acute illness and variation in post discharge prevention provision.
PRESSURE 2 is the largest randomised controlled mattress trial undertaken world-wide, and results are consistent with the study by Vanderwee [20] and colleagues who reported new Category 2 PU incidence rates of 15.3% for APM and 15.6% for HSF plus turning. Our trial provides approximately 80% of the data for the comparison of APMs and HSF, with 160 events in 2029 patients, with Vanderwee [20] providing data on 69 events in 447 patients.
Despite the negligible difference in QALYs equating to around half a quality adjusted life day, the difference in costs between the two alternatives drive the cost-effectiveness towards APM. Overall estimates suggest that APM has a 99% probability of being cost-effective at a £20,000 per QALY gained threshold.
Overall, mattress compliance was good and balanced across both groups for numbers not receiving randomised mattress, numbers receiving randomised mattress within 2 days and median time on randomised mattress. As such compliance was better than expected compared to the PRESSURE 1 trial [8] and importantly comparable across groups. Of note, however, is that the reason for first mattress change was imbalanced across groups with a higher proportion of patient and ward led changes from APMs due to comfort and to aid movement/rehabilitation and a higher proportion of ward led changes from HSF due to clinical condition, suggesting issues with equipoise amongst clinical staff who ‘upgraded’ from HSF where patient condition deteriorated, patient preference for HSF in relation to comfort and both ward and patient preference for HSF where patient rehabilitation was a therapy priority. Analyses of the Per Protocol Population which retained only patients who were compliant with their mattress allocation were consistent with the primary ITT analyses suggesting that these issues did not impact on the overall trial conclusions.
Risk factors found to be predictive of PU development in the adjusted analysis (Tables 8a–8c in eDocument 8: Additional results tables) are in line with previous work and adds to the growing body of evidence that a key risk factor in immobile patients is skin status including presence of Category 1 and Category 2 PU [6], [9], [12].
No safety concerns were indicated for either APMs or HSF. Concerns expressed in previous research by patients about feeling unsafe on APMs [8] were not reflected in falls which were balanced across groups and consistent with those reported in acutely ill hospital populations [27] and community dwelling settings.
The trial recruited more slowly than originally anticipated leading to a smaller sample size than the planned maximum sample size (2029 compared to 2954 patients), with fewer events (160 compared to 588) and was therefore underpowered under the original trial design assumptions. Under the revised design, the trial had 80% power for detecting a difference of 4% between mattress groups assuming an overall event rate of 10% with corresponding hazard ratio of 0.652. The overall event rate observed was lower at 7.9% with an absolute difference of 2.0% between mattress groups, and corresponding hazard ratio of 0.76. The a priori discussions with the TMG on the revised minimum clinically important difference for varying overall event rates (3.3% difference for 10% event rate, 2.5% difference for 5% event rate — see Methods and eDocument 5) suggest that the difference observed may not be considered to be clinically important. If APMs were allocated to patients fulfilling the trial inclusion criteria, the difference equates to a Number Needed to Treat (NNT) of 50. That means that for every 50 patients allocated an APM it will benefit only 1 patient.
The event rate (7.9%) was considerably lower than the sample size estimate of 20.5%, based upon contemporary studies [7], [8], [13]. A key question is whether the low rate was because: the patient population was ‘low risk’ due to issues around selection bias and/or; it reflects general improvements in clinical practice resulting from national level PU improvement targets.
Recruitment of 40.7% of eligible patients is in line with the contemporary studies and in terms of ‘low risk’, despite a lack of equipoise by ward staff (i.e., unwilling to change mattress) the randomised patient population were similar to the previous study populations [8], [13] and characterised by acute illness, old age, high levels of pre and post randomisation falls, and high levels of adverse skin status at baseline, with higher proportions of patients lacking capacity [8].
The impact of general improvements in practice resulting from national targets is difficult to elicit from national monitoring, due to problems of data accuracy [2] and difficult interpretation of AE data [28]. A key observation is the low proportion of patients with a Category 1 PU at baseline who subsequently developed a new Category 2 PU (11.4%) compared to other studies which report rates of circa 33% [8], [12], [13].
Overall, the conclusion drawn is that the patient population were high risk and that the low incidence observed reflected the prevailing improvements in PU prevention care in the participating centres.
A key issue raised by members of the Pressure Ulcer Research Service User Network during a results interpretation event was ‘given the low incidence and the disadvantages of APMs in terms of impact upon independent movement and comfort, who will benefit most from APMs’. As previously indicated the adjusted analysis indicates that a key predictor of new Category 2 PU development in the study population characterised by high levels of immobility, was the presence of Category 1 and Category 2 PUs at baseline. In addition, the moderator analysis was included in order to explore the potential benefit of each mattress on patients with known PU risk factors. Direct application of this analysis to practice must be undertaken with caution since it was exploratory and interactions with mattress allocation were non-significant, but the results suggest that the impact of altered and Category 1 PU skin status, complete immobility, nutritional deficits and the vulnerability afforded by lack of capacity may be modifiable as risk factors through use of the APMs. Given the low event rate this may help clinicians considering the trade-offs between risks and benefits of mattress allocation and frequency of repositioning decisions.
The trial was planned as a double-triangular group sequential trial, however, independent monitoring of the event rate and a slower than planned recruitment rate led to a funder requested unplanned interim analysis and trial modification with one final analysis.
Due to clinical difficulties in concealing the mattress type, a limitation was the lack of blind outcome assessment. However, the blinded central photograph review did not identify any systematic bias concerns as a result of the lack of blinding. This work will underpin the design of blinded endpoint assessment in future trials.
Interpretation of the trial results is based on the primary endpoint at post treatment 30 days final follow-up with the treatment phase sensitivity analysis used to support these findings. This is the first study to include a longer-term perspective post-treatment phase follow-up [7]. The treatment phase endpoint could be considered more clinically meaningful due to the majority of Category ≥ 2 PUs developing within this phase (83%), the relevance of the outcome to the institution providing the mattress intervention and the different patient pathways following the treatment phase and associated discharge. However, the longer term outcome provides a realistic estimate of effectiveness within current in-patient and community services.
In high risk patients (acutely ill in-patients who were bedfast/chairfast and/or had an existing Category 1 PU or pain on a PU skin site), we found insufficient evidence of a difference in PU development at the end of trial follow-up. However, APMs did confer a small treatment phase benefit. Overall the APM compliance and very low PU incidence rate observed (7.9%) and small differences between mattresses indicates the need for improved indicators for targeting of APMs and individualised decision making taking into account patient preferences (comfort/movement ability), rehabilitation needs and the presence of risk factors which may be modifiable through APM allocation.
Future research should consider the primacy given to treatment phase endpoint, an updated estimate of the event rate, and focusing on groups of patients identified as potentially benefitting more from APM compared to HSF to confirm whether there is a true treatment effect in these groups. However it is recognised that recruitment to a trial in these patient groups is likely to be challenging based on the experiences of this trial.
The findings provide the RCT evidence to underpin current guidelines which recommend the use of HSF for patients at risk of PU and consideration of ‘high tech’ mattresses where HSF is failing. This study will inform recommendation revisions and the grade of evidence on which recommendations are based. Current device regulations fall short in the requirement for evidence of clinical effectiveness and can result in widespread adoption of ‘high tech’ solutions prior to demonstrated clinical or patient benefit.
Funding
The National Institute for Health Research Health Technology Assessment programme. The views expressed in this presentation are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health.
Acknowledgements
The authors wish to thank the advisory committees, staff at recruiting centres and the patients for their contributions to this study.
The authors have no conflicts of interest to declare.
Data Sharing Statement: The datasets during and/or analysed during the current study will be available from the corresponding author on reasonable request.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.eclinm.2019.07.018.
Appendix A. Supplementary Data
Supplementary material
References
- 1.Gorecki C., Brown J.M., Nelson E.A. Impact of pressure ulcers on quality of life in older patients: a systematic review. J Am Geriatr Soc. 2009;57(7):1175–1183. doi: 10.1111/j.1532-5415.2009.02307.x. [DOI] [PubMed] [Google Scholar]
- 2.Smith I.L., Nixon J., Brown S., Wilson L., Coleman S. Pressure ulcer and wounds reporting in NHS hospitals in England part 1: audit of monitoring systems. J Tissue Viability. 2016;25(1):3–15. doi: 10.1016/j.jtv.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 3.(AHRQ) AfHRaQ Healthcare cost and utilization project (HCUP) 2014. www.ahrq.gov/research/data/hcup/index.html Accessed 9 September 2016. [DOI] [PubMed]
- 4.Coleman S., Nixon J., Keen J. A new pressure ulcer conceptual framework. J Adv Nurs. 2014;70(10):2222–2234. doi: 10.1111/jan.12405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.NICE Pressure ulcers: Prevention and management (CG179) 2014. https://www.nice.org.uk/guidance/cg179 Accessed 29 June 2017.
- 6.Haesler E., National Pressure Ulcer Advisory P, European Pressure Ulcer Advisory P, Pan Pacific Pressure Injury A . 2014. Prevention and treatment of pressure ulcers : Clinical practice guideline. [DOI] [PubMed] [Google Scholar]
- 7.McInnes E., Jammali-Blasi A., Bell-Syer S.E.M., Dumville J.C., Middleton V., Cullum N. Support surfaces for pressure ulcer prevention. Cochrane Database Syst Rev. 2015;9 doi: 10.1002/14651858.CD001735.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nixon J., Nelson E.A., Cranny G. Pressure relieving support surfaces: a randomised evaluation. Health Technol Assess. 2006;10(22):1–163. doi: 10.3310/hta10220. iii-iv, ix-x. [DOI] [PubMed] [Google Scholar]
- 9.Shi C., Dumville J.C., Cullum N. Support surfaces for pressure ulcer prevention: a network meta-analysis. PloS one. 2018;13(2) doi: 10.1371/journal.pone.0192707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brown S., Smith I.L., Brown J.M. Pressure RElieving support SUrfaces: a randomised evaluation 2 (PRESSURE 2): study protocol for a randomised controlled trial. Trials. 2016;17(1):604. doi: 10.1186/s13063-016-1703-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Braden B., Bergstrom N. A conceptual schema for the study of the etiology of pressure sores. Rehabil Nurs. 1987;12(1):8–12. doi: 10.1002/j.2048-7940.1987.tb00541.x. [DOI] [PubMed] [Google Scholar]
- 12.Coleman S., Gorecki C., Nelson E.A. Patient risk factors for pressure ulcer development: systematic review. Int J Nurs Stud. 2013;50(7):974–1003. doi: 10.1016/j.ijnurstu.2012.11.019. [DOI] [PubMed] [Google Scholar]
- 13.Smith I.L., Brown S., McGinnis E. Exploring the role of pain as an early predictor of category 2 pressure ulcers: a prospective cohort study. BMJ Open. 2017;7(1) doi: 10.1136/bmjopen-2016-013623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McGinnis E., Brown S., Collier H. Pressure RElieving Support SUrfaces: a randomised evaluation 2 (PRESSURE 2) photographic validation sub-study: study protocol for a randomised controlled trial. Trials. 2017;18(1):132. doi: 10.1186/s13063-017-1851-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coleman S., Nelson E.A., Keen J. Developing a pressure ulcer risk factor minimum data set and risk assessment framework. J Adv Nurs. 2014;70(10):2339–2352. doi: 10.1111/jan.12444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.EuroQol EQ-5D-5L. 2017. https://euroqol.org/eq-5d-instruments/eq-5d-5l-about/ Accessed 13 October 2017.
- 17.Czoski M.C., Meads D.M., McCabe C. A utility algorithm forthe pressure ulcer quality of life - utility instrument (Puqol-Ui) Value in health : the journal of the International Society for Pharmacoeconomics and Outcomes Research. 2014;17(7):A513. doi: 10.1016/j.jval.2014.08.1582. [DOI] [PubMed] [Google Scholar]
- 18.Nixon J., Nelson E.A., Rutherford C. Pressure UlceR Programme Of reSEarch (PURPOSE): using mixed methods (systematic reviews, prospective cohort, case study, consensus and psychometrics) to identify patient and organisational risk, develop a risk assessment tool and patient-reported outcome Quality of Life and Health Utility measures. Programme Grants for Applied Research. 2015;3 [PubMed] [Google Scholar]
- 19.EPUAP - NPUAP Prevention and Treaatment of pressure ulcers: Quick reference guides. 2009. http://www.epuap.org/guidelines/guidelines-old/ Accessed 28 June 2017.
- 20.Vanderwee K., Grypdonck M.H., Defloor T. Effectiveness of an alternating pressure air mattress for the prevention of pressure ulcers. Age Ageing. 2005;34(3):261–267. doi: 10.1093/ageing/afi057. [DOI] [PubMed] [Google Scholar]
- 21.Lan G.K.K., Demets D.L. Discrete sequential boundaries for clinical trials. Biometrika. 1983;70(3):659–663. [Google Scholar]
- 22.Fine J.P., Gray R.L. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94(446):496–509. [Google Scholar]
- 23.Devlin N.J., Shah K.K., Feng Y., Mulhern B., van Hout B. Valuing health-related quality of life: an EQ-5D-5L value set for England. Health Econ. 2017;27(1):7–22. doi: 10.1002/hec.3564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Glick H.A., Doshi J.A., Sonnad S.S., Polsky D. Oxford University Press; Oxford: 2014. Economic evaluation in clinical trials. [Google Scholar]
- 25.O'Brien B.J., Briggs A.H. Analysis of uncertainty in health care cost-effectiveness studies: an introduction to statistical issues and methods. Stat Methods Med Res. 2002;11(6):455–468. doi: 10.1191/0962280202sm304ra. [DOI] [PubMed] [Google Scholar]
- 26.Manca A., Hawkins N., Sculpher M.J. Estimating mean QALYs in trial-based cost-effectiveness analysis: the importance of controlling for baseline utility. Health Econ. 2005;14(5):487–496. doi: 10.1002/hec.944. [DOI] [PubMed] [Google Scholar]
- 27.Royal College of Physicians National Audit of in-patient falls. 2015. https://www.rcplondon.ac.uk/file/1545/download?token=ArMxSgx Accessed 11 March 2017.
- 28.Brennan T.A., Leape L.L., Laird N.M. Incidence of adverse events and negligence in hospitalized patients: results of the Harvard medical practice study I. 1991. Qual Saf Health Care. 2004;13(2):145–151. doi: 10.1136/qshc.2002.003822. [discussion 51-2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material