The current era of personalised medicine promises us medications tailored to the individual patient, minimising adverse effects, and maximising effectiveness. Yet, medication is only effective when taken as prescribed, which in the ‘real world’ turns out to be a major challenge. Indeed, WHO and OECD estimate that one-out-of-two patients with chronic diseases does not use their medication as prescribed [1], [2]. In Europe alone, non-adherence is estimated to annually contribute to the premature death of 200,000 patients and excess healthcare costs of €125 billion [2].
Contrary to what is often assumed, the non-adherence problem is not exclusive to ‘real-world’ patients, but it also influences the strictly regulated setting of clinical drug registration trials. Of every hundred trial participants, four do not initiate a study drug. Each study day, 10–12% does not take their medication while still on treatment. In long-term studies, after one year, almost 40% of trial participants have stopped taking their medication [3]. Novel digital adherence monitoring devices may offer a solution for patients who tend to forget their medication and for trial regulators to have granular data on the exact timing of medication use.
Recent publications highlight two important reasons why close monitoring of adherence in drug registration trials is warranted. First, ignoring non-adherence could lead to underestimated drug efficacy and safety. This may flaw regulators' benefit-risk assessment at time of drug registration [4]. Lower efficacy and artificially improved safety outcomes may result in approving higher doses than appropriate, increasing healthcare costs and potentially avoidable adverse reactions in the ‘real world’. The second issue is the loss of statistical power of clinical trials caused by non-adherence. Notably, a recent systematic review showed that undetected variations in adherence double the required number of participants of severe asthma drug trials [5]. This leads to prolonged recruitment, inefficient trials, longer time to market access, and higher costs.
In the last decades, trialists have been mostly dependent on indirect (e.g. pill counts) and subjective methods (e.g. patient self-report) to monitor medication adherence. Fortunately, the rise of advanced digital technologies currently enables more objective and granular adherence monitoring. Some examples of these advanced technologies include smart pill dispensers, electronic medication packaging, and smart inhaler add-ons. Some of these devices connect to a mobile app that records when medication is administered and send reminders or motivational messages. These stand-alone medical devices are usually developed independently of the drug they are monitoring and only assessed under medical device regulations by so-called notified bodies.
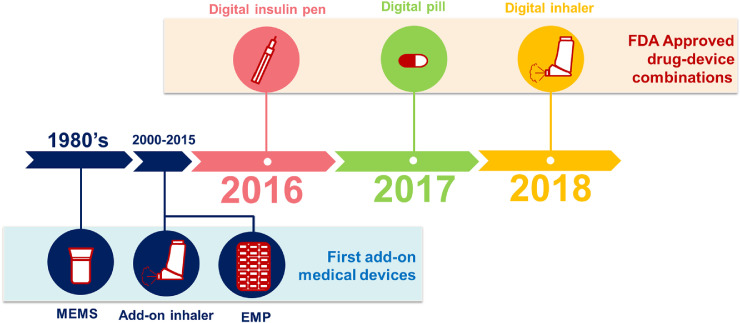
More recent developments combine a drug with a smart adherence monitoring tool, the so-called “drug-device combinations”. Newly Food and Drug Administration (FDA) approved drug-device combinations include a smart insulin pen, a smart pill, and a smart inhaler (Fig. 1). The first approved smart pill was a formula of a digital period-size sensor with aripiprazole, an antipsychotic agent. The same sensor is also combined in capsules with the oral chemotherapeutic agent capecitabine [6]. The first approved smart inhaler has a built-in sensor that measures inhaler adherence and the adequacy of inspiratory flow. These integrated devices may help personalised disease management and could also be relevant for doctors and payers to rule out non-adherence to first line therapies before more expensive second line therapies, such as biologicals, are considered [7]. On the other hand, two key barriers for wider smart device use in daily practice are their sustainability (e.g. battery waste, technical robustness) and system implementation (e.g. privacy regulations, doctor and patient acceptability, and affordability). Furthermore, it is important to define in which patient populations these drug-device combinations offer “value-for-money”.
Fig. 1.
Timeline of FDA approved drug-device combinations and the first add-on medical devices. MEMS: medication event monitoring system, EMP: electronic medication packages.
The aforementioned issues are typical real-world challenges, but what about clinical drug trials? Currently, the FDA and EMA do not enforce digital adherence monitoring in clinical trials. Notably, the International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use (ICH) has recently revised the ICH-E9 guidance, where the term ‘estimand’ was introduced [8]. Where previously the principal drug efficacy analysis was based on the intention-to-treat principle, this revised global regulatory guideline proposes a more precise estimate of treatment effect that prospectively defines how to deal with e.g. patients discontinuing treatment. There is however no mentioning of a distinction between non-initiators, poor adherers, or discontinued patients [9]. This also hampers proper distinction between a true ‘pharmacological’ non-responder and a simply non-adherent patient. In the assessment of a drug's efficacy, it is crucial to consider this distinction before concluding that a drug is ineffective based on a black box of actual medication use. We therefore believe formal guidance for clinical trials is needed on when, and especially how, objective, digital, adherence monitoring should be implemented. In our view, this would require joint efforts of regulators (to enforce guideline changes), healthcare professionals and trialists (to raise awareness on availability of adherence devices and utilise them when deemed appropriate), and payers (to reimburse these technologies).
While personalised medicine is slowly becoming reality, this cannot be implemented without proper insights into medication adherence. It seems time to embrace the digital opportunities and open up the adherence black box once and for all.
Author Contributions
JB conceived the comment. All authors discussed the outline. TZ and JB did the literature search. TZ wrote the first draft and designed the figure. All authors commented on- and approved- the final version.
Declaration of Competing Interest
No funding was received for this comment. JB reports being co-director of the Medication Adherence Expertise Center of the northern Netherlands (part of University of Groningen). This institute has received research grants to perform studies on medication adherence. DJT has received grants to study new methods of drug measurement in patients. Other authors report no conflicts of interest.
References
- 1.World Health Organization Adherence to long-term therapies: evidence for action. www.who.int/chp/knowledge/publications/adherence_full_report.pdf Available from.
- 2.Khan R., Socha-Dietrich K. OECD health working papers. OECD Publishing; Paris: 2018. Investing in medication adherence improves health outcomes and health system efficiency: adherence to medicines for diabetes, hypertension, and hyperlipidaemia. No. 105. [Google Scholar]
- 3.Blaschke T.F., Osterberg L., Vrijens B., Urquhart J. Adherence to medications: insights arising from studies on the unreliable link between prescribed and actual drug dosing histories. Annu Rev Pharmacol Toxicol. 2012;52:275–301. doi: 10.1146/annurev-pharmtox-011711-113247. [DOI] [PubMed] [Google Scholar]
- 4.Breckenridge A., Aronson J.K., Blaschke T.F., Hartman D., Peck C.C., Vrijens B. Poor medication adherence in clinical trials: consequences and solutions. Nat Rev Drug Discov. 2017;16(3):149–150. doi: 10.1038/nrd.2017.1. [DOI] [PubMed] [Google Scholar]
- 5.Mokoka M.C., McDonnell M.J., MacHale E. Inadequate assessment of adherence to maintenance medication leads to loss of power and increased costs in trials of severe asthma therapy: results from a systematic literature review and modelling study. Eur Respir J. 2019;53(5) doi: 10.1183/13993003.02161-2018. [pii: 1802161] [DOI] [PubMed] [Google Scholar]
- 6.Proteus Available from. https://www.proteus.com/press-releases/proteus-digital-health-launches-digital-oncology-medicines-to-improve-patient-outcomes/
- 7.Hew M., Reddel H.K. Integrated adherence monitoring for inhaler medications. JAMA. 2019;321(11):1045–1046. doi: 10.1001/jama.2019.1289. [DOI] [PubMed] [Google Scholar]
- 8.International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use Statistical principles for clinical trials. Addendum: Estimands and Sensitivity Analysis in Clinical Trials. E9(R1). https://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E9/E9-R1EWG_Step2_Guideline_2017_0616.pdf Available from.
- 9.Vrijens B., De Geest S., Hughes D.A. A new taxonomy for describing and defining adherence to medications. Br J Clin Pharmacol. 2012;73(5):691–705. doi: 10.1111/j.1365-2125.2012.04167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]