Abstract
Background
Sex can be an important biological variable in the immune response to infections and the response to vaccines. The magnitude and consistency in age-specific sex differences in the incidence of viral infections remain unclear.
Methods
We obtained data from national official agencies on cases of viral meningitis by sex and age group over a period of 6–16 years from five countries: Canada, Czech Republic, Germany, Israel, and Poland. Male to female incidence rate ratios (RR) were computed for each year, by country, and age group. For each age group, we used meta-analysis methodology to combine the incidence RRs. Meta-regression was conducted to the estimate the effects of age, country, and time period on the RR.
Findings
In the age groups < 1, 1–4, 5–9, 10–14, there were consistently higher incidence rates in males, over countries and time. The pooled incidence RRs (with 95% CI) were 1.38 (1.30–1.47), 1.94 (1.85–2.03), 1.98 (1.88–2.07), and 1.58 (1.47–1.71) respectively. In young and middle-age adults there were no differences with pooled incidence RRs of 1.00 (0.97–1.03), and 0.97 (0.94–1.00), respectively. Sensitivity analysis confirms that the results are stable and robust. Meta-regression showed that almost all the variations in the incidence RRs were contributed by age group.
Interpretation
The higher incidence rates from viral meningitis in males under the age of 15 are remarkably consistent across countries and time-periods. These findings emphasize the importance of sex as a biological variable in infectious diseases. This could provide keys to the mechanisms of infection and lead to more personalized treatment and vaccine doses and schedules.
Funding
There was no funding source for this article.
Keywords: Viral meningitis, Sex differences, Incidence rates, Metaanalysis, Male excess
1. Introduction
Individual characteristics affect the immune response to infectious diseases [1] and vaccines [2]. Sex differences in the incidence of infectious diseases have long been recognized [3], [4]. The study of sex differences in infectious diseases can provide keys to the mechanism of infection and lead to improved treatment and vaccine development. Recently, Poland et al. [2] pointed out that gaps in understanding how vaccines stimulate an immune response has inhibited our understanding of the variability in the responses of individuals and sub-groups.
Viral meningitis can result from a number of infections such as enteroviruses, Herpes simplex, West Nile virus, mumps, and measles. In children in highly vaccinated communities, infection is usually attributed to enteroviruses such as Coxsackie, ECHO, and enterovirus 71 [5], [6], [7], [8]. In such cases, the transmission is usually feco–oral and occurs mainly in the summer and autumn months in temperate climates. In isolated reports, the incidence rates of viral meningitis have been reported to be higher in males [3], [9], [10]. However, the subject has not been rigorously evaluated and the reports on sex differences in the incidence rates are usually based on single data sources from individual countries and for varying age groups [3], [9], [10]. The possible mechanism underlying this sex difference is not clear. Behavioral differences may play a part, although that seems unlikely in very young children. X chromosome related sex differences in immunodeficiency could also play a role [11], [12] and sex hormones may be important [13].The aim of this study was to evaluate the magnitude and consistency of the sex differences in the incidence rates of viral meningitis at different ages, in different countries, and over a number of years.
Research in context
Evidence Before This Study
The importance of evaluating sex differences in disease is an increasing area of interest in the understanding of the mechanisms of disease. Sex differences in the incidence rates for viral meningitis have been reported from studies in single countries and over defined time periods. It is not clear to what extent these differences vary by age and are consistent over countries and over time.
Added Value of This Study
To the best of our knowledge, this is the first comprehensive study of magnitude of the sex differences in the incidence rates for viral meningitis at different ages for a number of countries over different time periods. Our study provides stable estimates of the extent of the excess male incidence rates in viral meningitis in infants and children up to age 15.
Implications of All The Available Evidence
Our findings should stimulate investigations on sex-related factors impacting on the clinical manifestation of viral meningitis, which can have important implications for targeted treatment and the development and dose schedules of current and new vaccines.
Alt-text: Unlabelled Box
2. Methods
2.1. Search Strategy
We restricted our analyses to published national data on viral meningitis incidence rates by age and sex. Published studies based on hospital or local data, usually do not have population denominators, and thus age and sex specific incidence rates cannot be calculated. This could be an important source of selection bias. We identified five countries with reliable reporting and diagnostic systems for which published national data by age, sex and year were available. In most of the databases, the actually causative organism is not specified and is generalized for all causes of viral meningitis. The surveillance and the methods of viral meningitis diagnosis may not be identical over countries and time, but are unlikely to differ between males and females.
2.2. Sources of Data
For Canada, data were available from the Public Health Agency of Canada, for the period 1991 to 1999 (latest available data) [14]. For the Czech Republic, data for the years 2008–2013 were obtained from the Institute of Health Information and Statistics [15]. For Germany, data for the years 2000–2015, were obtained from the German Federal Health Monitoring System [16]. In Israel, data were available for 2001–2016, from the archives of the Department of Epidemiology in the Ministry of Health. For Poland, data for the years 2006–2016 were obtained from the National Institute of Public Health [17]. Information on the population size by age, sex and year was obtained via the official web site of each country - for Canada, from the Statistics, CANSIM database [18], for the Czech Republic from the Czech Statistical Office [19], for Germany, from the German Federal Health Monitoring System [20], for Israel from the Central Bureau of Statistics [21], and for Poland from official web site Statistics Poland [22].
2.3. Ethics
Since, national, open access, aggregative and anonymous data were used, there was no need for ethics committee approval.
2.4. Statistical analyses
2.4.1. Calculation of incidence rates
We calculated viral meningitis incidence rates (IR) by sex and age group, for each country and calendar year. Incidence rates per 100,000 were calculated as the number of reported cases divided by the respective population size and multiplied by 100,000. The age groups considered were < 1 (infants), 1–4 (early childhood), 5–9 (late childhood), 10–14 (puberty), 15–44 (young adulthood), and 45–64 (middle adulthood) years. The Canadian surveillance system used similar age-groups except for the ages 15–39 and 40–59. The male:female incidence rate ratio (RR) was calculated by dividing the incidence rate in males by that of females.
2.5. Meta-analyses
We performed the statistical analyses using meta-analysis methodology where country and calendar year within each age group were considered as “studies”. The outcome variable was the male:female incidence RR. We combined the incidence RR's using the meta–analysis package in Stata version 12.1 (Stata Corp., College Station, TX), separately for each age group, by country and time period (years). Pooled incidence RRs were obtained for each age group, combining countries and calendar years. The results are presented in forest plots. Heterogeneity was evaluated using Cochran's Q statistic. Tau2 and I2 were used to estimate the between-study variance [23]. If I2 ≥ 50% and/or the Q test yielded a p-value < 0.1, the random effects model (DerSimonian and Laird) was used to estimate pooled RRs and 95% confidence intervals (CI). Otherwise the fixed effects model was used.
2.6. Sensitivity analyses
To evaluate the effect of individual county on the pooled male:female incidence RR's, we performed leave-one-out sensitivity analysis and recomputed the pooled RRs for each age group separately.
2.7. Meta-regression analyses
In order to explore the main sources of variation in the incidence RRs, meta-regression analyses were performed including age-group, country and time periods as the possible explanatory variables.
2.8. Asymmetry analysis
We explored possible differences in the impact of selected countries or time periods using funnel plots with visual inspection and the Egger test for asymmetry.
3. Results
The summary of male and female incidence rates (per 100,000 populations) in different countries for each age group is presented in Table 1. In every country (Czech Republic data is missing for age < 1) the incidence rate of viral meningitis was higher in males compared to females at ages < 1, 1–4, 5–9 and 10–14. Viral meningitis incidence rates tend to decline with age.
Table 1.
Details of the countries included in the meta-analysis, by sex and age group - descriptive data (IR per 100,000 male or female population). IR = incidence ratio.
| Males |
Females |
|||||
|---|---|---|---|---|---|---|
| Age group | Country | Years | n/N | IR | n/N | IR females |
| < 1 | Canada | 1991–1999 | 342/1737028 | 19.7 | 260/1650180 | 15.8 |
| Czech Republic | 2008–2013 | No data | No data | No data | No data | |
| Germany | 2000–2015 | 1466/5742020 | 25.5 | 997/5449156 | 18.3 | |
| Israel | 2001–2016 | 546/1284600 | 42.5 | 362/1219600 | 29.7 | |
| Poland | 2006–2016 | 44/2177523 | 2.0 | 31/2055764 | 1.5 | |
| 1–4 | Canada | 1991–1999 | 134/7248136 | 1.8 | 84/6903656 | 1.2 |
| Czech Republic | 2008–2013 | 67/1410748 | 4.7 | 53/1343670 | 3.9 | |
| Germany | 2000–2015 | 4010/23640633 | 17 | 1904/22438113 | 8.5 | |
| Israel | 2001–2016 | 382/4965300 | 7.7 | 192/4717300 | 4.1 | |
| Poland | 2006–2016 | 339/8763810 | 3.9 | 183/8294052 | 2.2 | |
| 5–9 | Canada | 1991–1999 | 307/9204230 | 3.3 | 168/8766807 | 1.9 |
| Czech Republic | 2008–2013 | 261/1532669 | 17 | 138/1450621 | 9.5 | |
| Germany | 2000–2015 | 8155/31049959 | 26.3 | 3846/29467539 | 13.1 | |
| Israel | 2001–2016 | 380/5727300 | 6.6 | 193/5442900 | 3.5 | |
| Poland | 2006–2016 | 937/10753278 | 8.7 | 468/10206501 | 4.6 | |
| 10–14 | Canada | 1991–1999 | 216/9166633 | 2.4 | 141/8696455 | 1.6 |
| Czech Republic | 2008–2013 | 313/1416001 | 22.1 | 145/1339518 | 10.8 | |
| Germany | 2000–2015 | 3972/33984562 | 11.7 | 2496/32237008 | 7.7 | |
| Israel | 2001–2016 | 119/5252800 | 2.3 | 81/4995400 | 1.6 | |
| Poland | 2006–2016 | 954/11130177 | 8.6 | 506/10591951 | 4.8 | |
| 15–44 | Canada [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39] | 1991–1999 | 549/51767983 | 1.1 | 581/50452545 | 1.2 |
| Czech Republic | 2008–2013 | 987/13725818 | 7.2 | 837/12978912 | 6.4 | |
| Germany | 2000–2015 | 23,505/260599725 | 9 | 23,949/250238197 | 9.6 | |
| Israel | 2001–2016 | 386/25542600 | 1.5 | 343/25239300 | 1.4 | |
| Poland | 2006–2016 | 3649/92802239 | 3.9 | 3177/90097352 | 3.5 | |
| 45–64 | Canada (40–59) | 1991–1999 | 76/32668296 | 0.2 | 99/32789422 | 0.3 |
| Czech Republic | 2008–2013 | 202/8403729 | 2.4 | 238/8624880 | 2.8 | |
| Germany | 2000–2015 | 5829/179937846 | 3.2 | 6057/180119691 | 3.4 | |
| Israel | 2001–2016 | 25/10822000 | 0.2 | 45/11644800 | 0.4 | |
| Poland | 2006–2016 | 1039/55127862 | 1.9 | 1050/59180678 | 1.8 | |
3.1. Pooled incidence RRs and forest plots
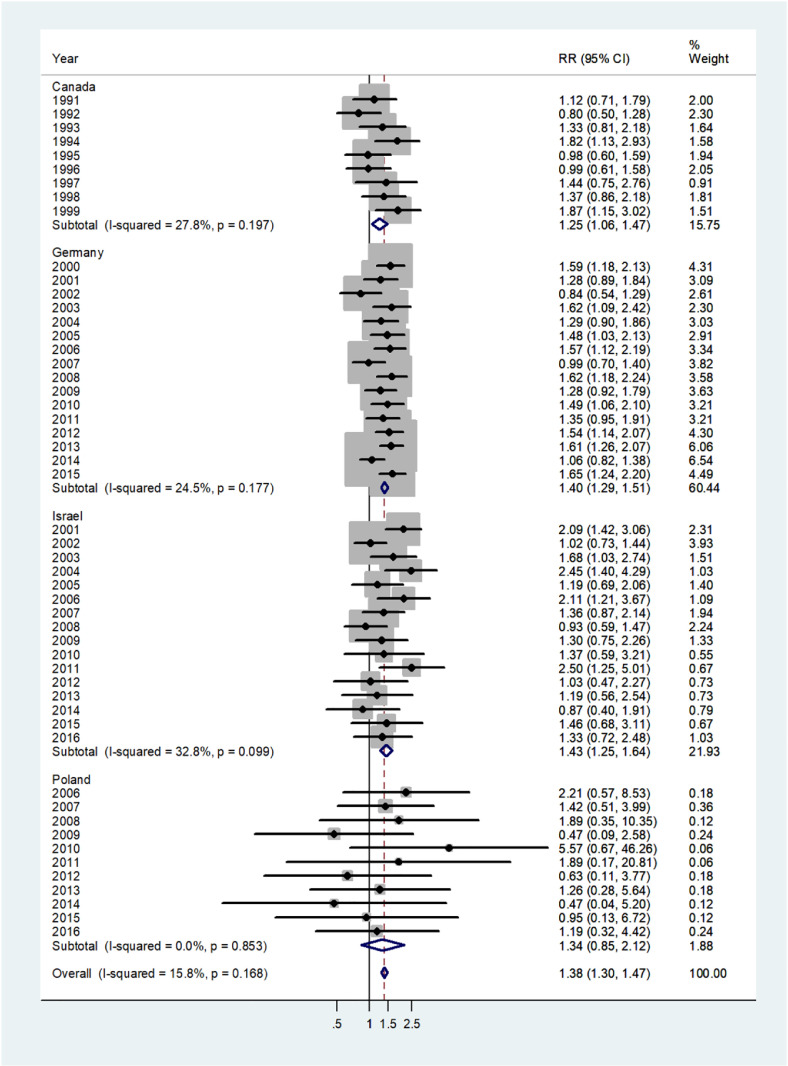
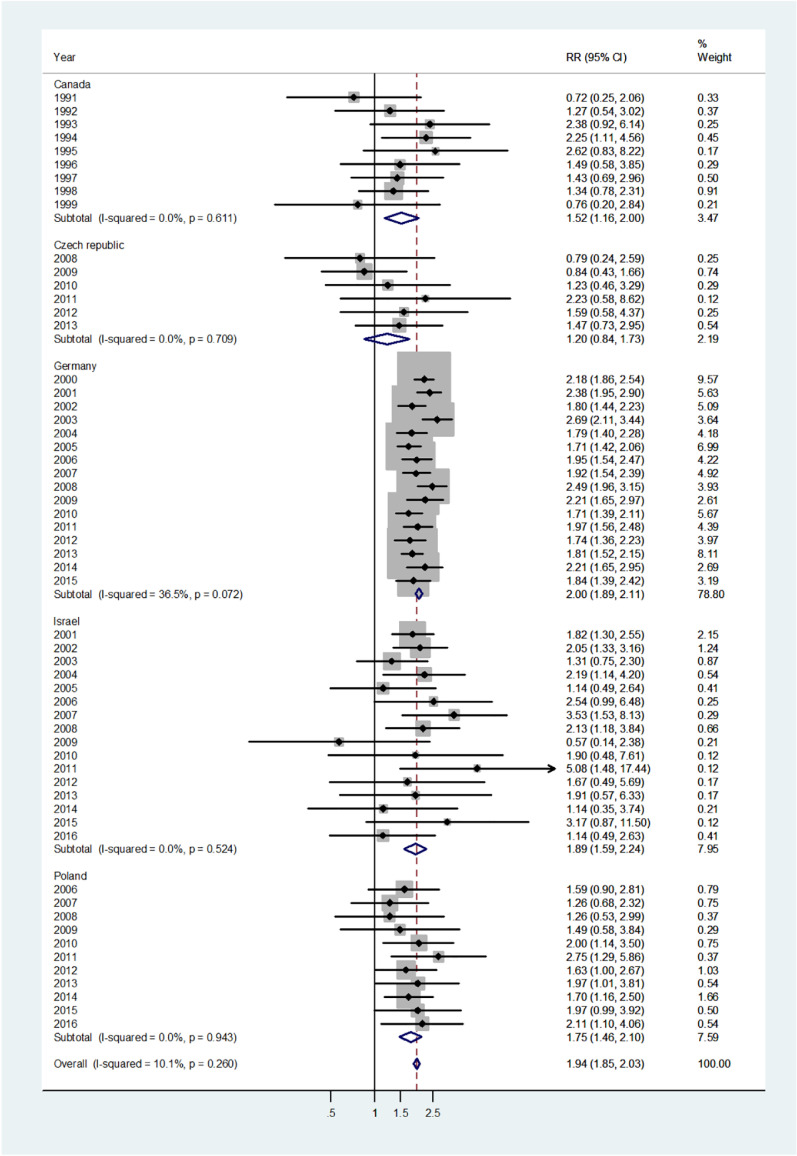
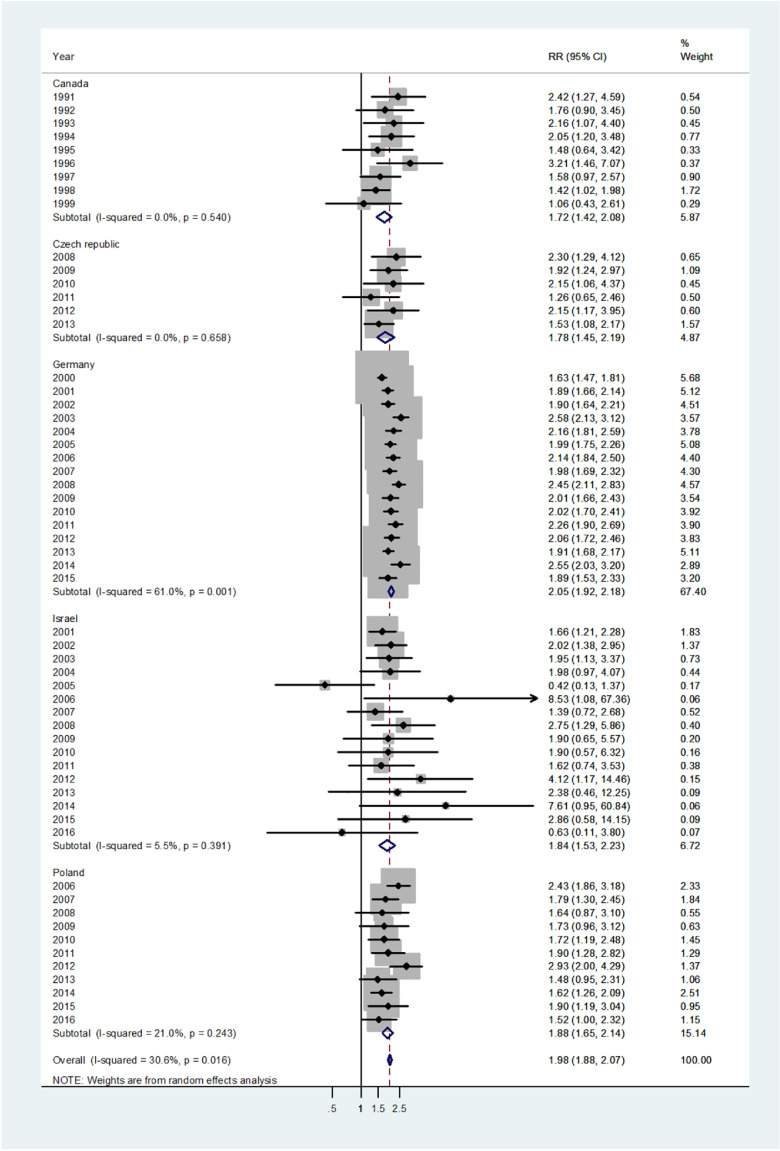
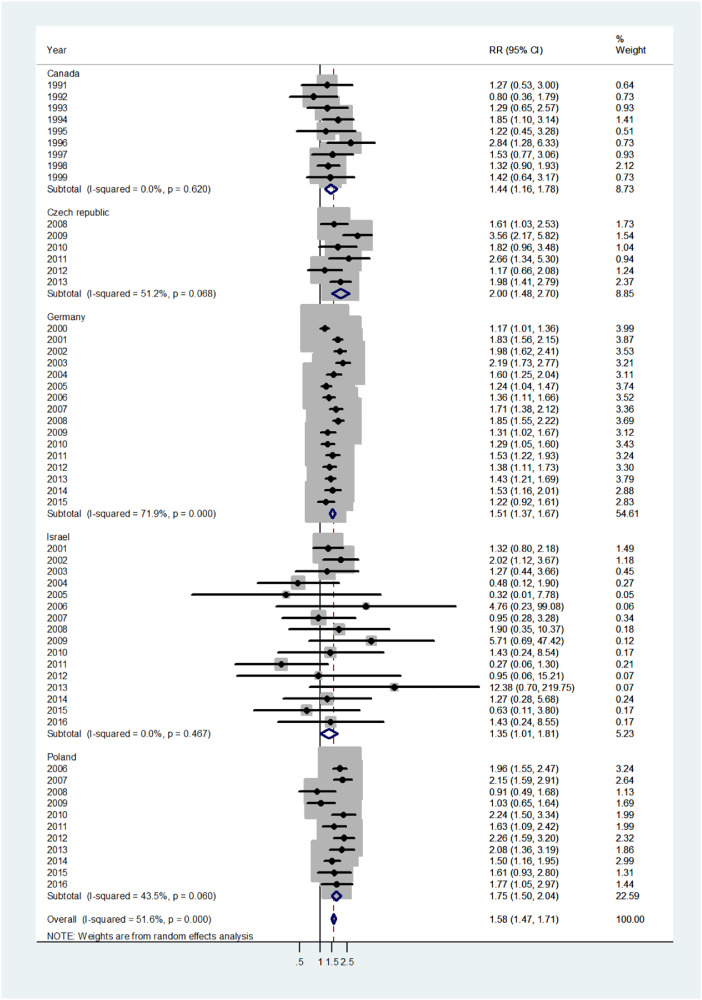
The forest plot for infants (age 0–1) is shown in Fig. 1. The overall pooled incidence RR was 1.38 (95% CI 1.30–1.47), indicating a 38% higher incidence of disease in males. By country, the pooled incidence RRs varied from 1.25 in Canada to 1.43 in Israel. Heterogeneity was low with I2 = 15.8%, and Tau2 = 0.0103. The forest plot for early childhood (ages 1–4) is shown in Fig. 2. The overall pooled incidence RR was1.94 (95% CI 1.85–2.03) indicating a 94% excess in incidence rates in males. By country the pooled incidence RRs varied from 1.20 for the Czech population to 2.0 for Germany with I2 = 10.1%, and Tau2 = 0.0042. The forest plot for late childhood [5], [6], [7], [8], [9] is shown in Fig. 3. The overall pooled RR was 1.98 (95% CI 1.88–2.07), indicating a 98% excess in males, and the pooled incidence RRs varied between Canada, incidence RR = 1.72 to Germany with incidence RR = 2.05. Heterogeneity was mild I2 = 30.6%, and Tau2 = 0.0082. The forest plot for puberty [10], [11], [12], [13], [14] is shown in Fig. 4. The overall pooled incidence RR was 1.58 (95% CI 1.47–1.71), indicating a 58% excess in males with I2 = 51.6%, and Tau2 = 0.0307. The pooled incidence RRs varied between 1.35 for Israel to 2.0 for the Czech Republic. The forest plots for young adults (15–44 or 15–39 years) and middle-age adults (45–64 or 40–59 years) are shown in Appendix A. For young adults (Fig. A1), there was no significant difference in incidence rates between males and females (overall incidence RR = 1.00, 95% CI 0.97–1.03, I2 = 60.2%, and Tau2 = 0.0063). Female predominance is significant only for the German population (incidence RR = 0.94). For middle-age adults (Fig. A2), females had borderline higher incidence rates compared with males (overall incidence RR = 0.97, 95% CI 0.94–1.00, I2 = 6.9%, and Tau2 = 0.0012). Although meta-analysis results by age groups showed the highest heterogeneity in Germany, the incidence rates over the time period were consistently higher in males.
Fig. 1.
Forest plot of the male to female viral meningitis incidence rate ratios (RR) for different years in Canada, Germany, Israel, and Poland in infants (< 1 years). (The shading indicates the weight of the study in the analysis). CI = confidence interval. RR = rate ratio.
Fig. 2.
Forest plot of the male to female viral meningitis incidence rate ratios (RR) for different years in Canada, Czech Republic, Germany, Israel, and Poland in early childhood (ages 1–4). (The shading indicates the weight of the study in the analysis). CI = confidence interval. RR = rate ratio.
Fig. 3.
Forest plot of the male to female viral meningitis incidence rate ratios (RR) for different years in Canada, Czech Republic, Germany, Israel, and Poland in late childhood (ages 5–9). (The shading indicates the weight of the study in the analysis). CI = confidence interval. RR = rate ratio.
Fig. 4.
Forest plot of the male to female viral meningitis incidence rate ratios (RR) for different years in Canada, Czech Republic, Germany, Israel, and Poland in puberty (ages 10–14). (The shading indicates the weight of the study in the analysis). CI = confidence interval. RR = rate ratio.
3.2. Sensitivity analysis
To evaluate the effect of individual county on the pooled RR, we performed leave-one-out sensitivity analysis and recomputed the pooled RRs (are represented in Table 2). After omitting each country (one country at a time), the pooled incidence RR's remained very similar. Thus, no single country substantially affected the pooled incidence RRs. This confirms that the results of this meta-analysis are stable and robust.
Table 2.
Sensitivity analysis, by age group and removing one country at a time. (RR = rate ratio; CI = confidence interval).
| RR's (CI) for one country removed | ||||||
|---|---|---|---|---|---|---|
| Country removed | Age group |
|||||
| < 1 | 1–4 | 5–9 | 10–14 | 15–44 (15–39 for Canada) |
45–64 (40–59 for Canada) |
|
| Canada | 1.40 (1.31–1.50) |
1.95 (1.86–2.05) |
2.00 (1.92–2.06) |
1.68 (1.44–1.94) |
1.06 (0.94–1.19) |
0.97 (0.94–1.004) |
| Czech Republic | – | 1.95 (1.86–2.05) |
1.99 (1.92–2.06) |
1.57 (1.40–1.76) |
1.02 (0.91–1.14) |
0.97 (0.94–1.004) |
| Germany | 1.35 (1.23–1.50) |
1.71 (1.53–1.53) |
1.85 (1.71–2.00) |
1.69 (1.45–1.97) |
1.07 (0.99–1.16) |
0.99 (0.92–1.07) |
| Israel | 1.36 (1.27–1.47) |
1.94 (1.85–2.04) |
1.98 (1.92–2.06) |
1.67 (1.45–1.92) |
1.02 (0.91–1.14) |
0.97 (0.94–1.003) |
| Poland | 1.38 (1.30–1.47) |
1.95 (1.86–2.05) |
1.99 (1.92–2.06) |
1.59 (1.37–1.84) |
1.01 (0.91–1.12) |
0.95 (0.92–0.99) |
3.3. Asymmetry analysis
Egger's test was not significant for infants, in late childhood, puberty and middle adulthood suggesting no asymmetry in the findings (Egger test for asymmetry p = 0.618, p = 0.716, p = 0.873, p = 0.102 respectively). Some evidence of asymmetry was observed for the early childhood (p = 0.016) and young adulthood (p = 0.010). Data are presented in Appendix B, Fig. B1 (A–F).
3.4. Meta-regression analysis
Meta-regression results revealed that age group (p < 0.0001) contributed almost all the source of heterogeneity (for country p = 0.292 and for reported year p = 0.294). In the age-groups, there was no significant difference between the infancy and puberty, early and late childhood and between young and middle adulthood (p > 0.05). The heterogeneity for Germany resulted from differences in the sex ratio from year to year. However, the male to female ratio was consistently greater than unity, and the heterogeneity over time is likely to be due to random variation. The heterogeneity in late childhood, puberty and young adulthood resulted are alos likely to be due at least partly from random variation in the sex ratios from year to year and country to country.
4. Discussion
Using national data on viral meningitis from five countries, over a period of 6–16 years, we used meta-analyses and meta-regression to evaluate the male to female, incidence rate ratios by age group. Our analyses revealed that the incidence rates were 38%, 94%, 98% and 58% higher in males in infancy, young and late childhood and puberty, respectively. The findings are remarkably consistent over countries and over a number of years. In young and older adults, there were no significant sex differences. We believe that the current study is the first to provide stable estimates of the excess male incidence rates for specific age groups from a number of countries in diverse geographic regions and over extended time periods.
In general, the excess male incidence rates for viral meningitis that we have demonstrated, using data with reliable, national denominators, considerably strengthen the findings reported from hospital-based studies in single countries, at specific time periods. For example, in the British Pediatric Surveillance Unit (BPSU) system, over an 18-month period, there was an excess of 20% in male incidence rates in newborn babies [24], and in a cohort study in Denmark between 1977 and 2001, the incidence rate was higher in males in the first 6 months of life [10]. In China, a multicenter prospective study of viral meningitis at five hospitals from 2009 to 2012 revealed a male to female incidence ratio of 2.24 in children with a mean age of 6.4 years [25]. In a retrospective cohort study in Spain [9] there was male predominance among children with a mean age of 5.9 years. In Poland, between 2011 and 2014,the male to female ratio of viral meningitis rates in children aged 5–9 years was 1.77 [26]. In Germany, the male to female incidence rate ratios for non-polio enteroviruses meningitis ratios were 2.8 at ages 6.8 ± 3.8 years and 2.0 at ages 7.1 ± 4.1 [27].
For Israel between 1971 and 1985, the average male to female incidence rate ratio was 2.0 for children under the age of 10 [3]. In addition, in children aged under 4 years, a higher incidence among males was observed for viral hepatitis, shigellosis, and salmonellosis [3]. Our study cannot provide clues to mechanisms. However, we can speculate on mechanism. As we mentioned earlier, the countries in the study do not have a culture of differentiating between the sexes in providing health care to infants and children. There may be sex differences in adults in the use of health services, and this could influence the results in adults. In very young children, it seems unlikely that there are behavioral differences between the sexes that could impact on the incidence rates for viral meningitis.
Sex differences in response to infections may be due to the imbalance in the expression of genes encoded on the X and Y-chromosomes of a host [28]. X chromosome associated biological processes and X-linked genes appear to be responsible for the immunological advantage of females due to the X-linked microRNAs related processes. It has been proposed that the phenomenon of X chromosome inheritance and expression is a cause of immune disadvantage of males and the enhanced survival of females following immunological challenges [28]. In females, two X chromosomes associate with plasmacytoid dendritic cells (pDCs) that are more activated and display a more anti-viral phenotype [11]. Actual sex-linked immunodeficiency seems to be too rare to explain the magnitude of the excess incidence rates observed in young males, but it may be partially explained by relative immunodeficiency in males [12].
Hormonal factors could be implicated. Females generally exhibit elevated humoral and cell-mediated immune responses to viral infections compared to males [4], [29], [30], [31]. The development and activity of both innate and adaptive immunity can be mediated through estrogen, androgen, and progesterone receptors [32]. Estradiol enhances production of pro-inflammatory cytokines and chemokines in response to TLR ligand stimulation of dendritic cells and macrophages, which could contribute to the superior immune response to viral infection often observed in females [30], [31]. Whereas estrogens are associated with proliferation and differentiation of lymphocytes and monocytes, androgens suppress the activity of immune cells by increasing the synthesis of anti-inflammatory cytokines [33], [34]. Progesterone regulates the development of myeloid cells and dendritic cells to promote immune tolerance [35], [36]. Some studies have demonstrated that progesterone modifies the activity of the T cell population, suppresses the type I interferon producing Th1cells and favors the IL-10 producing Th2 type cells [36].
In children, in highly-vaccinated populations such as in the current study, the most common cause of viral meningitis is usually enteroviruses, such as coxsackie, ECHO and enterovirus 71 [5], [6], [7], [8], [37]. The first line of defense is mediated through the innate immune system, with type I interferon (IFN) initialization at the first stage and subsequently induction of the expression of IFN-stimulated genes that lead to destruction of viruses [38]. In the adaptive response, following enterovirus infection, T cells play a critical role in infiltration of CD4 + and CD8 + lymphocytes within the central nervous system. Both innate and adaptive immune responses differ between males and females. Estradiol promotes innate immune signaling pathways, including macrophages, dendritic cells (DCs), granulocytes, and lymphocytes cell development and the production of type I interferon [39], the key factor in the immune response against viral infections. Female pDCs produce more type I IFN than male pDCs [40]. Progesterone inhibits the immune response to viruses via IFN-alpha production downregulation by pDCs and providing a potential explanation for innate antiviral immunity modulation in women [41].
In infancy, the transient rise in sex steroid levels (‘minipuberty’) could affect immune cells differently in boys and girls [42]. During this period of life, there is a post-natal hypothalamic–pituitary-gonadal surge, characterized by an activation of gonadal hormones in both, boys and girls [42], [43]. Testosterone levels predominate in boys at 1–3 months of age and decline at 6–9 months of age, whereas in girls, estradiol high levels remain elevated longer. This phenomenon of “minipuberty” with high gonadal hormone levels is able to stimulate the target tissues in both sexes [42], [43], [44]. It is possible that the physiological and immune differences that occur in mini-puberty may be “carried over” from infancy into the early childhood. Courant F et al. [45] demonstrated significant sex differences in late childhood (pre-pubertal) sex hormone levels. Plasmacytoid dendritic cells from healthy females produced more type 1 IFN than males, even before puberty, but puberty itself associated with increased production of type 1 IFN [11]. This was related to X chromosome number, and serum testosterone concentration, in a manner which differed depending on the number of X chromosomes present [11]. Sex hormones and genetic factors (X chromosome number) were associated individually and interactively with the type I interferon response [11], which could contribute to our understanding of why young males exhibit higher viral meningitis incidence rates.
In the present study, during the female reproductive age, the dominance of overall male incidence rates is no longer evident. This may partly be due to behavioral differences and partly because of viral meningitis etiology. As we mentioned, in young adults, females may be more likely to be exposed to viruses when caring for sick children and in the workplace such as hospitals, schools and kindergartens. Young adult females may be more likely to be with enteroviruses during the care of children excreting the virus. It should be noted that HSV-2 is a cause of viral meningitis in adult females and males [46], [47], [48], and is sexually transmitted. During middle adulthood, there is no significant overall differences in incidence rates between the sexes. With aging, there are profound changes in the immune and endocrine systems, reducing the sex differences in the susceptibility to infectious diseases [49].
4.1. Strengths and limitations of the study
This study has several strengths and limitations. One of the main strengths is that the study was based on national data with very large populations and consequently large numbers of cases. Selection bias has been minimized by using national data over different time periods, which should be representative of each country. The inclusion of five countries, each evaluated over a number of years, allowed us to evaluate the consistency of the findings over different populations and different time periods. As regards the potential for information bias, there does not appear to be any reason to believe that the reporting of viral meningitis in infants and children is different for males and females. The countries examined do not have a culture of giving preferable treatment to males, although in adults, there could be differences between males and females in the utilization of medical care. Although the surveillance and methods for the diagnosis of viral meningitis may not be identical over countries and time, it does not seem likely that this will differ between males and females. Any misdiagnosis is unlikely to be different in males and females and is likely to be non-differential. Thus, if anything, the excess incidence rates in males in infants and children may be higher than we found.
5. Conclusions
This study has demonstrated excess incidence rates from viral meningitis in male infants and children, which is remarkably consistent over different countries and time periods. This suggests that the finding is unlikely to be due entirely to behavioral differences between males and females, particularly in infancy. The mechanism underlying the excess incidence rates remains largely unexplained. The importance of evaluating sex differences in disease is an increasing area of interest in the understanding of the mechanisms of disease. Our findings should stimulate investigations on sex-related factors impacting on the clinical manifestation of viral meningitis and help in the development of new vaccines.
Acknowledgments
Acknowledgments
We would like to thank all the official institutions of all countries (Canada, Czech Republic, Germany, Israel, and Poland) for the publication of their national data on viral meningitis incidence and their willingness to share them with the world.
Contributors
VP participated in the conception and design of the study, acquisition of data, analysis and interpretation of the data and writing the manuscript. NS assisted in the data analysis and reviewed the manuscript. MSG designed and supervised the study, interpreted the data, helped in drafting the and revising the manuscript for important intellectual content. All authors approved the final version submitted.
Declaration of Competing Interests
We declare no competing interests.
Role of Funding Sources
There was no funding source for this study. Victoria Peer carried out this study as part of the requirements for the degree Doctor of Philosophy in the University of Haifa.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.eclinm.2019.08.006.
Appendix A. Supplementary data
Supplementary material
References
- 1.Chapman S.J., Hill A.V. Human genetic susceptibility to infectious disease. Nat Rev Genet. 2012;13:175–188. doi: 10.1038/nrg3114. [DOI] [PubMed] [Google Scholar]
- 2.Poland G.A., Ovsyannikova I.G., Kennedy R.B. Personalized vaccinology: a review. Vaccine. 2018;36:5350–5357. doi: 10.1016/j.vaccine.2017.07.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Green M.S. The male predominance in the incidence of infectious diseases in children: a postulated explanation for disparities in the literature. Int J Epidemiol. 1992;21:381–386. doi: 10.1093/ije/21.2.381. [DOI] [PubMed] [Google Scholar]
- 4.Klein S.L. The effects of hormones on sex differences in infection: from genes to behavior. Neurosci Biobehav Rev. 2000;24:627–638. doi: 10.1016/s0149-7634(00)00027-0. [DOI] [PubMed] [Google Scholar]
- 5.Shukla B., Aguilera E.A., Salazar L., Wootton S.H., Kaewpoowat Q., Hasbun R. Aseptic meningitis in adults and children: diagnostic and management challenges. J Clin Virol. 2017;94:110–114. doi: 10.1016/j.jcv.2017.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ben Abid F., Abukhattab M., Ghazouani H. Epidemiology and clinical outcomes of viral central nervous system infections. Int J Infect Dis. 2018;73:85–90. doi: 10.1016/j.ijid.2018.06.008. [DOI] [PubMed] [Google Scholar]
- 7.Harvala H., Jasir A., Penttinen P., Pastore Celentano L., Greco D., Broberg E. Surveillance and laboratory detection for non-polio enteroviruses in the European Union/European economic area, 2016. Euro Surveill. 2017;22(45) doi: 10.2807/1560-7917.ES.2017.22.45.16-00807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Irani D.N. Aseptic meningitis and viral myelitis. Neurol Clin. 2008;26:635–655. doi: 10.1016/j.ncl.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jiménez Caballero P.E., Muñoz Escudero F., Murcia Carretero S., Verdú Pérez A. Descriptive analysis of viral meningitis in a general hospital: differences in the characteristics between children and adults. Neurologia. 2011;26:468–473. doi: 10.1016/j.nrl.2010.12.010. [DOI] [PubMed] [Google Scholar]
- 10.Hviid A., Melbye M. The epidemiology of viral meningitis hospitalization in childhood. Epidemiology. 2007;18:695–701. doi: 10.1097/ede.0b013e3181567d31. [DOI] [PubMed] [Google Scholar]
- 11.Webb K., Peckham H., Radziszewska A. Sex and pubertal differences in the type 1 interferon pathway associate with both X chromosome number and serum sex hormone concentration. Front Immunol. 2019;9:3167. doi: 10.3389/fimmu.2018.03167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Washburn T.C., Medearis D.N., Jr., Childs B. Sex differences in susceptibility to infections. Pediatrics. 1965;35:57–64. [PubMed] [Google Scholar]
- 13.Fish E.N. The X-files in immunity: sex-based differences predispose immune responses. Nat Rev Immunol. 2008;8:737–744. doi: 10.1038/nri2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Public Health Agency of Canada. https://www.canada.ca/en/public-health.html. Accessed on June 1, 2018.
- 15.Institute of Health Information and Statistics. https://www.uzis.cz/en/catalogue/infectious-diseases. Accessed on March 1, 2018.
- 16.German Federal Health Monitoring System http://www.gbe-bund.de/gbe10/pkg_isgbe5.prc_isgbe?p_uid=gast&p_aid=0&p_sprache=D; Accessed on February 1, 2018.
- 17.National Institute of Public Health http://wwwold.pzh.gov.pl/oldpage/epimeld/index_a.html; Accessed on December 1, 2018.
- 18.Statistics, Canada CANSIM database. http://www5.statcan.gc.ca/cansim/a26?lang=eng&retrLang=eng&id=0510038&pattern=&csid=#customizeTab; Accessed on June 1, 2018.
- 19.Czech Statistical Office https://www.czso.cz/csu/czso/population; Accessed on March 1, 2018.
- 20.German Federal Health Monitoring System http://www.gbe-bund.de/oowa921-install/servlet/oowa/aw92/WS0100/_XWD_FORMPROC?TARGET=&PAGE=_XWD_106&OPINDEX=20&HANDLER=_XWD_CUBE.SETPGS&DATACUBE=_XWD_134&D.000=3732&D.003=42&D.004=1000006&D.100=10103; Accessed on February 1, 2018.
- 21.Central Bureau of Statistics http://www.cbs.gov.il/reader/shnatonhnew_site.htm?sss=%E4%EE%F9%EA&shnaton_scan=45; Accessed on March 1, 2018.
- 22.Statistics Poland https://stat.gov.pl/en/basic-data/
- 23.Higgins J.P., Thompson S.G. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 24.Holt D.E., Halket S., de Louvois J., Harvey D. Neonatal meningitis in England and Wales: 10 years on. Arch Dis Child Fetal Neonatal Ed. 2001;84:F85–F89. doi: 10.1136/fn.84.2.F85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ai J., Xie Z., Liu G. Etiology and prognosis of acute viral encephalitis and meningitis in Chinese children: a multicenter prospective study. BMC Infect Dis. 2017;17:494. doi: 10.1186/s12879-017-2572-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wieczorek M., Figas A., Krzysztoszek A. Enteroviruses associated with aseptic meningitis in Poland, 2011–2014. Pol J Microbiol. 2016;65:231–235. doi: 10.5604/17331331.1204485. [DOI] [PubMed] [Google Scholar]
- 27.Rudolph H., Prieto Dernbach R., Walka M. Comparison of clinical and laboratory characteristics during two major paediatric meningitis outbreaks of echovirus 30 and other non-polio enteroviruses in Germany in 2008 and 2013. Eur J Clin Microbiol Infect Dis. 2017;36:1651–1660. doi: 10.1007/s10096-017-2979-7. [DOI] [PubMed] [Google Scholar]
- 28.Pinheiro I., Dejager L., Libert C. X-chromosome-located microRNAs in immunity: might they explain male/female differences? The X chromosome-genomic context may affect X-located miRNAs and downstream signaling, thereby contributing to the enhanced immune response of females. BioEssays. 2011;33:791–802. doi: 10.1002/bies.201100047. [DOI] [PubMed] [Google Scholar]
- 29.Straub R.H. The complex role of estrogens in inflammation. Endocr Rev. 2007;28:521–574. doi: 10.1210/er.2007-0001. [DOI] [PubMed] [Google Scholar]
- 30.Seillet C., Rouquie N., Foulon E. Estradiol promotes functional responses in inflammatory and steady-state dendritic cells through differential requirement for activation function-1 of estrogen receptor alpha. J Immunol. 2013;190:5459–5470. doi: 10.4049/jimmunol.1203312. [DOI] [PubMed] [Google Scholar]
- 31.vom Steeg L.G., Klein S.L. SeXX matters in infectious disease pathogenesis. PLoS Pathog. 2016;12(2) doi: 10.1371/journal.ppat.1005374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bereshchenko O., Bruscoli S., Riccardi C. Glucocorticoids, sex hormones, and immunity. Front Immunol. 2018;9:1332. doi: 10.3389/fimmu.2018.01332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liva S.M., Voskuhl R.R. Testosterone acts directly on CD4 + T lymphocytes to increase IL-10 production. J Immunol. 2001;167:2060–2067. doi: 10.4049/jimmunol.167.4.2060. [DOI] [PubMed] [Google Scholar]
- 34.Klein S.L., Flanagan K.L. Sex differences in immune responses. Nat Rev Immunol. 2016;16:626–638. doi: 10.1038/nri.2016.90. [DOI] [PubMed] [Google Scholar]
- 35.Butts C.L., Shukair S.A., Duncan K.M. Progesterone inhibits mature rat dendritic cells in a receptor-mediated fashion. Int Immunol. 2007;19:287–296. doi: 10.1093/intimm/dxl145. [DOI] [PubMed] [Google Scholar]
- 36.Miyaura H., Iwata M. Direct and indirect inhibition of Th1 development by progesterone and glucocorticoids. J Immunol. 2002;168:1087–1094. doi: 10.4049/jimmunol.168.3.1087. [DOI] [PubMed] [Google Scholar]
- 37.Hasbun R., Wootton S.H., Rosenthal N. Epidemiology of meningitis and encephalitis in infants and children in the United States, 2011–2014. Pediatr Infect Dis J. 2019;38:37–41. doi: 10.1097/INF.0000000000002081. [DOI] [PubMed] [Google Scholar]
- 38.Devasthanam A.S. Mechanisms underlying the inhibition of interferon signaling by viruses. Virulence. 2014;5:270–277. doi: 10.4161/viru.27902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kovats S. Estrogen receptors regulate innate immune cells and signaling pathways. Cell Immunol. 2015;294:63–69. doi: 10.1016/j.cellimm.2015.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Seillet C., Laffont S., Trémollières F. The TLR-mediated response of plasmacytoid dendritic cells is positively regulated by estradiol in vivo through cell-intrinsic estrogen receptor α signaling. Blood. 2012;119:454–464. doi: 10.1182/blood-2011-08-371831. [DOI] [PubMed] [Google Scholar]
- 41.Hughes G.C., Thomas S., Li C., Kaja M.K., Clark E.A. Cutting edge: progesterone regulates IFN-alpha production by plasmacytoid dendritic cells. J Immunol. 2008;180:2029–2033. doi: 10.4049/jimmunol.180.4.2029. [DOI] [PubMed] [Google Scholar]
- 42.Kuiri-Hänninen T., Sankilampi U., Dunkel L. Activation of the hypothalamic-pituitary-gonadal axis in infancy: minipuberty. Horm Res Paediatr. 2014;82:73–80. doi: 10.1159/000362414. [DOI] [PubMed] [Google Scholar]
- 43.Winter J.S., Faiman C., Hobson W.C., Prasad A.V., Reyes F.I. Pituitary-gonadal relations in infancy. I. Patterns of serum gonadotropin concentrations from birth to four years of age in man and chimpanzee. J Clin Endocrinol Metab. 1975;40:545–551. doi: 10.1210/jcem-40-4-545. [DOI] [PubMed] [Google Scholar]
- 44.Kuiri-Hänninen T., Seuri R., Tyrväinen E. Increased activity of the hypothalamic-pituitary-testicular axis in infancy results in increased androgen action in premature boys. J Clin Endocrinol Metab. 2011;96:98–105. doi: 10.1210/jc.2010-1359. [DOI] [PubMed] [Google Scholar]
- 45.Courant F., Aksglaede L., Antignac J.P. Assessment of circulating sex steroid levels in prepubertal and pubertal boys and girls by a novel ultrasensitive gas chromatography-tandem mass spectrometry method. J Clin Endocrinol Metab. 2010;95:82–92. doi: 10.1210/jc.2009-1140. [DOI] [PubMed] [Google Scholar]
- 46.Miller S, Mateen FJ, Aksamit AJ Jr. Herpes simplex virus 2 meningitis: a retrospective cohort study. J Neurovirol 2013;19:166–71. [DOI] [PubMed]
- 47.Ihekwaba U.K., Kudesia G., McKendrick M.W. Clinical features of viral meningitis in adults: significant differences in cerebrospinal fluid findings among herpes simplex virus, varicella zoster virus, and enterovirus infections. Clin Infect Dis. 2008;47:783–789. doi: 10.1086/591129. [DOI] [PubMed] [Google Scholar]
- 48.Kadambari S., Okike I., Ribeiro S. Seven-fold increase in viral meningo-encephalitis reports in England and Wales during 2004-2013. J Infect. 2014;69:326–332. doi: 10.1016/j.jinf.2014.05.012. [DOI] [PubMed] [Google Scholar]
- 49.Giefing-Kroll C., Berger P., Lepperdinger G., Grubeck-Loebenstein B. How sex and age affect immune responses, susceptibility to infections, and response to vaccination. Aging Cell. 2015;14:309–321. doi: 10.1111/acel.12326. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material