Short abstract
Objective
The objective was to further investigate apoptosis induction by Babao Dan (BBD), which supports its anti-tumor mechanisms, using two human gastric cancer cell lines (AGS and MGC80-3).
Methods
After treatment with various BBD concentrations, cell viability and cytotoxic effects were investigated using methyl thiazolyl tetrazolium (MTT) and lactate dehydrogenase (LDH) assays, respectively. The following indicators of cell apoptosis were evaluated: Annexin V-APC staining, caspase-3/-8/-9 activation, and mitochondrial membrane potential loss. Apoptosis-related protein levels (including Bcl-2-associated X protein [Bax], B-cell CLL/lymphoma 2 [Bcl-2], factor associated suicide [Fas], and Fas ligand [FasL]) were determined by western blot. The following multi-pathway factors were also assessed: p-ERK1/2, p-JNK, p-p38, and p-NF-κB.
Results
The MTT and LDH assays both demonstrated increased BBD cytotoxicity. BBD induced cell apoptosis by stimulating caspase-3/-8/-9 activity and destroying the mitochondrial membrane potential. BBD also regulated key factor expression levels including Bcl-2, Bax, Fas, and FasL and down-regulated protein phosphorylation via the MAPK and NF-κB pathway.
Conclusions
The possible anti-tumor mechanism is that BBD induces apoptosis via the MAPK and NF-κB signaling pathways.
Keywords: Babao Dan, gastric cancer, apoptosis, MAPK pathway, NF-κB pathway, anti-tumor, cell viability, cytotoxic effect
Introduction
Gastric cancer (GC) is one of the top three causes of death worldwide, accounting for 12% of total deaths from cancer originating in the human digestive system.1 Currently, standard clinical treatment of GC consists of surgery with perioperative chemotherapy.2 This strategy, however, has several inadequacies. For example, many GC patients miss the optimal period for surgery, and they are diagnosed when the cancer is in advanced stages with distant metastases. Additionally, a short-term disease control study showed that many kinds of chemotherapy drugs intrinsically have potential cytotoxic effects on normal cells.3–6 Recent studies have reported that many natural products exhibit anticancer activity with low toxicity.7,8 Therefore, the 5-year survival rate associated with more conventional therapeutic agents could be increased.
Previous studies have shown that the pathologic mechanisms of tumors include apoptosis resistance, proliferative activity of tumor cells, angiogenesis, multidrug resistance, and cancer stem cells.9 Apoptosis resistance has recently become one of the most studied pro-cancer mechanisms. Inducing apoptosis, which is a major way to kill cancer cells with anticancer agents, mainly involves two types of signaling pathways: one involves activating intracellular caspases by extracellular signals, such as tumor necrosis factor-α (TNF-α) and FasL binding with tumor necrosis factor-R (TNF-R) and Fas, and the other is caspase activation by mitochondria-derived activators. The caspase-dependent pathway may be activated through either the extrinsic or intrinsic pathway.10,11 Multiple studies have shown that suppression of mitogen-activated protein kinase (MAPK) and nuclear factor-κB (NF-κB) activity is a critical element in tumor cell apoptosis. Developmental signals, such as growth factors and stress, are transduced into the programmed apoptosis process by MAPKs, including the extracellular signal-regulated protein kinases (ERK1/2), p38 MAPK, c-Jun N-terminal kinases (JNKs), and the NF-κB signaling pathway through activating the caspase cascade effect.12–14 Thus, to further investigate the effect and mechanism of BBD on GC cells, we focused on the MAPK pathway in this study.
Traditional Chinese medicines (TCMs) are widely accepted as useful in cancer treatment in China and east Asian countries.15,16 Some studies have shown that many preparations, such as Prunella vulgaris L,17 Hedyotis diffusa Willd,18 Patrina villosa,19 and Sophora flavescens,20 have anti-tumor effects involving apoptosis induction. To treat various kinds of cancers, including liver cancer, pancreatic cancer, and non-small cell lung cancer,21 BBD was approved by the Food and Drug Administration in China and it is widely used in a formulation as an alternative and complementary medicine. BBD is a mixed powder that consists of eight components, including natural antelope horn, calculus bovis, snake gall, pearl, musk, and radix notoginseng, and two unreported herbs. According to TCM theory, most tumors are caused by heat and toxins, qi deficiency, and phlegm stagnation. The clinical efficacy of BBD is mainly attributed to its capability to clear heat, remove blood stasis, resolve dampness, dissipate mass, and relieve pain. BBD has been reported to significantly inhibit gastric tumor growth, but the biological mechanism remains unclear. A better understanding of BBD’s anti-tumor mechanisms and its ability to induce apoptosis was obtained in this study by demonstrating that BBD can down-regulate MAPK and NF-κB pathways.
Materials and methods
Reagents
All cell culture consumables were obtained from Nest (Wuxi, China). BBD (No. 150930) was obtained and authenticated by Xiamen Traditional Chinese Medicine Factory Co., Ltd. (Xiamen, China). All experimental protocols were approved by the Fujian University of Traditional Chinese Medicine.
Cell culture
AGS and MGC80-3 cell lines were maintained at the Cell Bank of Chinese Academy of Sciences (Shanghai, China) and cultured in RPMI-1640 complete cell culture medium, supplemented with 10% (v/v) heat-inactivated FBS and 1% penicillin-streptomycin solutions (Thermo Fisher Scientific, Waltham, MA, USA). Cells were kept in a humidified incubator (37°C, 5% CO2) before the experiments and were then stored in liquid nitrogen at the medical laboratory center of Fujian University of Traditional Chinese Medicine.
Cell viability assay by MTT
Cell viability was assessed using the methyl thiazolyl tetrazolium (MTT) (Sigma, Darmstadt, Germany) assay, in accordance with the manufacturer’s instructions as described previously.22 Briefly, MGC80-3 and AGS cells in the logarithmic phase were collected and diluted with culture medium (1 × 105 cells/mL) and then seeded in 96-well plates (100 μL/well) in triplicate. The cells were cultured overnight to 50% to 60% confluence and treated with different concentrations of BBD (0, 0.25, 0.5, 0.75, or 1 mg/mL) for 12 hours, 24 hours, and 48 hours. MTT (0.5 mg/mL) was added into each well (100 μL/well), and then incubated for 4 hours. Subsequently, formazan crystals were solubilized in 100 μL dimethyl sulfoxide (DMSO) (Sigma, Darmstadt, Germany). Next, the absorbance of each well at 570 nm was recorded using an ELISA reader (Multiskan FC, Thermo Fisher Scientific).
Cytotoxicity by LDH release assay
An LDH release kit (Beyotime, Shanghai, China) was used to analyze the cytotoxic effect of BBD on both MGC80-3 and AGS cells. The MGC80-3 and AGS cells were harvested with the indicated doses of BBD (0, 0.25, 0.5, 0.75, and 1 mg/mL) and incubated for 12 hours, 24 hours, or 48 hours in 12-well plates. Then, the LDH release assay kit was used to determine LDH activity in culture medium, in accordance with the manufacturer’s instructions. Specifically, the cells were first inoculated into 12-well plates (1.5 × 105 cells/mL). After they were cultured for 11 hours, 23 hours, and 47 hours in an incubator, LDH-releasing agent was added in each well of LDH-releasing groups and cultured continuously for 1 hour in an incubator. The supernatant (120 μL/well) was collected and added to a 96-well plate. Then, 60 μL/well of LDH-detection working fluid was added to each well, mixed thoroughly, and incubated for 30 minutes at room temperature (RT) in the dark. Finally, the optical density (OD) value was immediately measured at 408 nm using an ELISA reader.
Assessment of apoptosis by flow cytometry
Apoptosis rates of AGS and MGC80-3 cells after treatment with BBD for 24 hours were assessed using flow cytometry and the Annexin V-APC/PI Binding Apoptosis Assay Kit (AAT Bioquest, Sunnyvale, CA, USA), in accordance with the manufacturer’s instructions. Annexin V specifically binds to phosphatidylserine on the surface of cells undergoing apoptosis and labels them with luciferin APC. The dyes can be used to detect cellular apoptosis. Cells that are stained in the late stage of apoptosis, while cells that are not stained are normal, live cells. Cells were resuspended in 1 mL of 1× binding buffer to a concentration of 5 × 105 cells/mL. Then, 5 μL of Annexin V-APC and PI solution was added to the appropriate centrifuge tubes. After blending, the tubes were incubated for 30 minutes in the dark. Samples were analyzed using a flow cytometer (BD Biosciences, San Diego, CA, USA). All experiments were performed three times.
Evaluation of mitochondrial membrane potentials (Δ ψm)
JC-1, a fluorescent lipophilic carbocyanine dye, was used as an indicator by which to measure the mitochondrial membrane potential. MGC80-3 and AGS cells (1.5 × 105 cells/mL) were seeded in 6-well plates. After treatment for 24 hours, AGS and MGC80-3 cells were incubated with 500 μL/well of JC-1 (10 μg/mL) at 37°C for 30 minutes using JC-1 Mitochondrial Membrane Potential Assay Kit (KeyGEN BioTECH, Nanjing, China). The cells were then resuspended twice in 500 μL 1× incubation buffer for 3 minutes each. Finally, they were suspended with 500 μL of 1× incubation buffer. Both red and green fluorescence emissions of mitochondrial membrane potential (Δ ψm) were measured using a flow cytometer (EX = 488 nm, EM = 530 nm).
Measurement of caspase activity
Caspase activity detection is another way to evaluate AGS and MGC80-3 cell apoptosis. A colorimetric assay kit (KeyGEN BioTECH) was used to detect the activities of caspase-3, caspase-8, and caspase-9, in accordance with the kit instructions. Briefly, the specific steps refer to the procedures that were previously described.11 Equal amounts of protein were isolated from the cells to be analyzed were incubated in 96-well plates with the supplied reaction buffer containing dithiothreitol and Asp-Glu-Val-Asp (DEAD)-p-nitroaniline (pNA), Ile-Glu-Thr-Asp (IETD)-pNA, or Leu-Glu-His-Asp (LEHD)-pNA as substrates for caspase-3, -8, and -9, respectively, at 37°C in the dark. The reactions were measured after 4 hours by changing the absorbance using an ELISA reader (Thermo, Multiskan FC, Rockford, IL, USA) at 405 nm.
Protein expression analyzed using a western blot assay
Approximately 50 μg of proteins were extracted from AGS and MGC80-3 cells. Collected proteins were determined by the bicinchoninic acid assay (BCA; Thermo Fisher Scientific). The targeted proteins were subjected to running sodium dodecyl sulfate-polyacrylamide gel electrophoresis on 12% gels and then transferred to nitrocellulose membranes (Pierce, Rockford, IL, USA). Primary antibodies diluted to the appropriate concentrations were incubated overnight with the blots at 4°C for 14 hours to 16 hours (rabbit anti-Bax, 1:1000 and rabbit anti-Bcl-2, 1:1000 from ProteinTech Group, Chicago, IL, USA; rabbit anti-Fas, 1:1000 and rabbit anti-FasL, 1:1000 from Abcam Technology, Cambridge, UK; and rabbit anti-β-actin, 1:20,000 from ProteinTech Group, Chicago, IL, USA). Then the blots were washed three times using TBST with Tween-20 for 10 minutes each. Subsequently, the second antibody was incubated with horseradish peroxidase at RT in a shaking bed for 1 hour. Following three TBST washes, protein bands were visualized and enhanced with SuperLumia ECL HRP Substrate Kit (Abbkine Inc., Redlands, CA, USA), and the two substrate components (Reagent A and Reagent B) were mixed at a 1:1 ratio to prepare the substrate working solution on the blot membrane. Then, the substrate was added, and the protein bands were visualized using Image Lab™ software (version 3.0; Beyotime Institute of Biotechnology, Jiangsu, China).
Multi-Plex phosphoprotein assay
To detect the activation of phosphoproteins in MAPK and NF-κB signaling pathways, we used ERK/MAP kinase1/2 (Thr185/Tyr187), NF-κB (Ser536), p38 (Thr180/Tyr182), and JNK (Thr183/Tyr185) in cell lysates using the Luminex® system. Cells (1.5 × 105) were seeded into 6-well plates in 2 mL medium and treated with BBD for 24 hours. Treated cells were lysed using a commercially available lysis kit and centrifuged at 14,000 × g for 15 minutes. The protein extracts were quantified using a BCA protein assay for the Multi-Plex Phosphoprotein Assay. The Multi-Plex Phosphoprotein Assay was implemented using a bead-based multiplex kit (EMD Millipore Corp., Billerica, MA, USA). Briefly, the suggested working range for the total protein concentration in the assay was 1 μg to 25 μg of cell lysates.23 In the present study, we added 25 μL of protein into 96-well solid plates, and added 1 × beads into each well (25 μL/well). After incubating at 4°C for 16 hours, 1 × Detection Antibody was added into each well (25 μL/well), followed by shaking for 1 hour at RT and the liquid was poured out. Then 1 × SAPE was added into each well (25 μL/well), followed by shaking for 15 minutes at RT and the liquid was poured out. Then, 1 × Amplification Buffer was added into each well (25 μL/well), followed by shaking for 15 minutes at RT, and the liquid was poured out. Assay buffer was then added into each well (150 μL/well) using the Bio-Plex 200 suspension array system (Bio-Rad, Hercules, CA, USA) to measure the levels in each well.
Statistical analysis
Data were expressed as the mean ± standard deviation (SD) for all experiments. Statistical analyses were performed using SPSS software (version 22.0; IBM Corp., Armonk, NY, USA). First, the normality of the data was checked. If the distribution was normal and the variance was homogeneous, we used a one-way ANOVA to determine significance among three or more groups. If the variance was not identical, the rank-sum test was used. If the data did not conform to the normal distribution, the non-parametric test was used. P < 0.05 and P < 0.01 were considered to be statistically significant.
Results
BBD suppressed growth of AGS and MGC80-3 cells
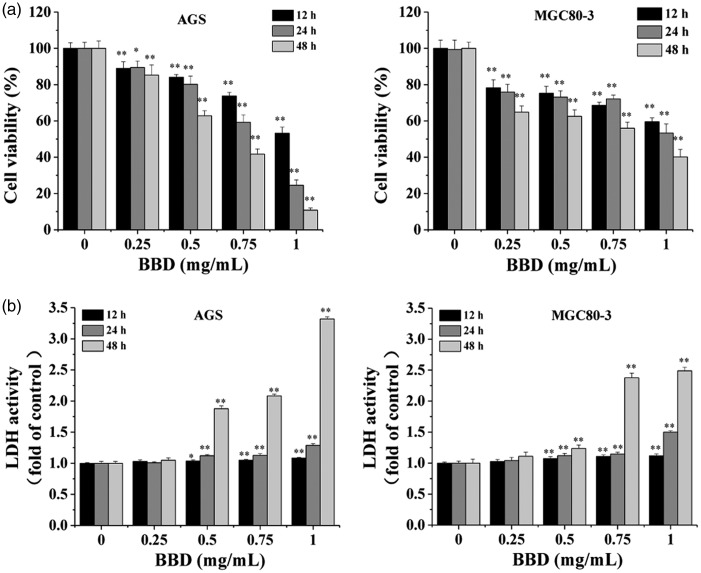
As shown in Figure 1a, BBD inhibited the viability of MGC80-3 and AGS cells in a dose- and time-dependent manner using the MTT assay (P < 0.05). The specific situation was as follows: BBD reduced cell viability by 11% to 46.8% (12 hours), 10.6% to 75.4% (24 hours), and 14.7% to 89.2% (48 hours) in AGS compared with the untreated control cells (0 mg/mL) from the 0.25 to 1 mg/mL BBD treatments. Similarly, BBD decreased cellular activity by 21.8% to 40.4% (12 hours), 24.1% to 46.7% (24 hours), and 35.2% to 59.8% (48 hours) in MGC80-3 cells (Figure 1a). The LDH activity assay also showed that the toxic effect of BBD increased fractionally (0.25 mg/mL to 1 mg/mL) in AGS and MGC80-3 after treatment for 12 hours, 24 hours, or 48 hours in the above-mentioned cell lines (Figure 1b, P < 0.05) compared with untreated cells (BBD 0 mg/mL). There was little BBD cytotoxicity observed at 12 hours and 24 hours, and the cytotoxicity was the most obvious at 48 hours. This caused a significant inhibition of gastric cancer cell growth and few surviving cells, so we chose 24 hours for follow-up cell experiments.
Figure 1.
Effect of BBD on proliferation of AGS and MGC80-3 cells. AGS and MGC80-3 cells were incubated at the indicated BBD concentrations (0, 0.25, 0.5, 0.75, and 1 mg/mL) for 12 hours, 24 hours, or 48 hours. (a) Cell viability was analyzed using the MTT assay. (b) BBD cytotoxicity in AGS and MGC80-3 cell lines was tested using an LDH release assay. Data are shown as the mean ± SD of three independent experiments. *P < 0.05 and **P < 0.01 versus control (0 mg/mL).
BBD promoted apoptosis in AGS and MGC80-3 cells
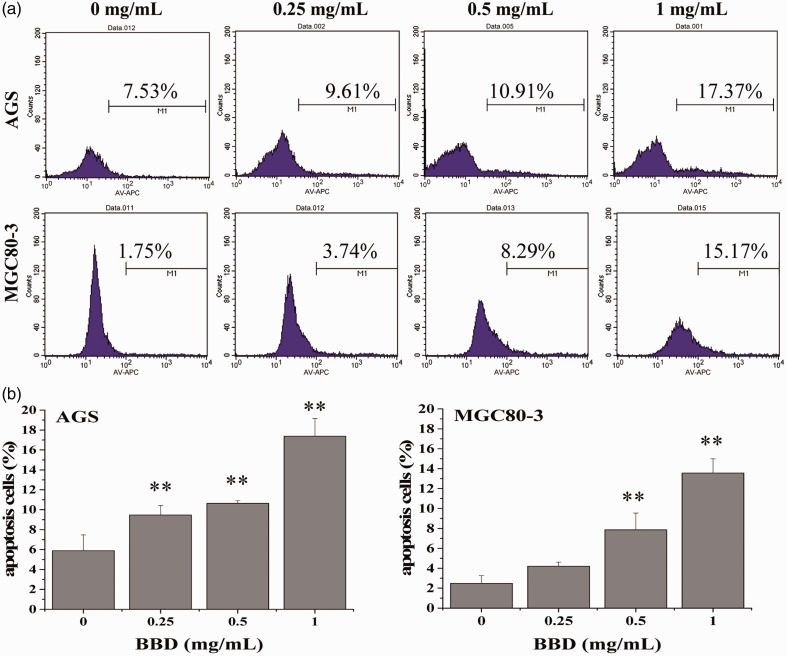
To help quantify the induction of cell apoptosis induced by BBD treatment, Annexin V-APC staining was used to detect the treated cells. We chose this method because Annexin V labeling by APC as a fluorescent probe has been shown to be one of the more sensitive methods for detecting cells.24–26 BBD increased the ratio of cells undergoing apoptosis in a dose-dependent manner (Figure 2a). More specifically, the proportion of AGS cells undergoing apoptosis in response to BBD treatment increased as the dose increased (0, 0.25, 0.5, and 1 mg/mL), from 7.53% to 9.61%, to 10.91%, and to 17.37%, respectively (Figure 2b, P < 0.05). Similarly, the percentages of MGC80-3 cells undergoing apoptosis after BBD treatment were 1.75%, 3.74%, 8.29%, and 15.17%, respectively (Figure 2b, P < 0.05). The results demonstrate that BBD effectively inhibited AGS and MGC80-3 cells in a dose-dependent manner with BBD treatment for 12 hours, 24 hours, and 48 hours.
Figure 2.
Effect of BBD on apoptosis in AGS and MGC80-3 cells. (a) The apoptosis rate of AGS and MGC80-3 cells was assessed using FACS with Annexin-APC staining. (b) Quantitative data are shown as the mean ± SD of three independent experiments. **P < 0.01 versus control cells (0 mg/mL).
BBD induced loss of mitochondrial membrane surface potential in AGS and MGC80-3 cells
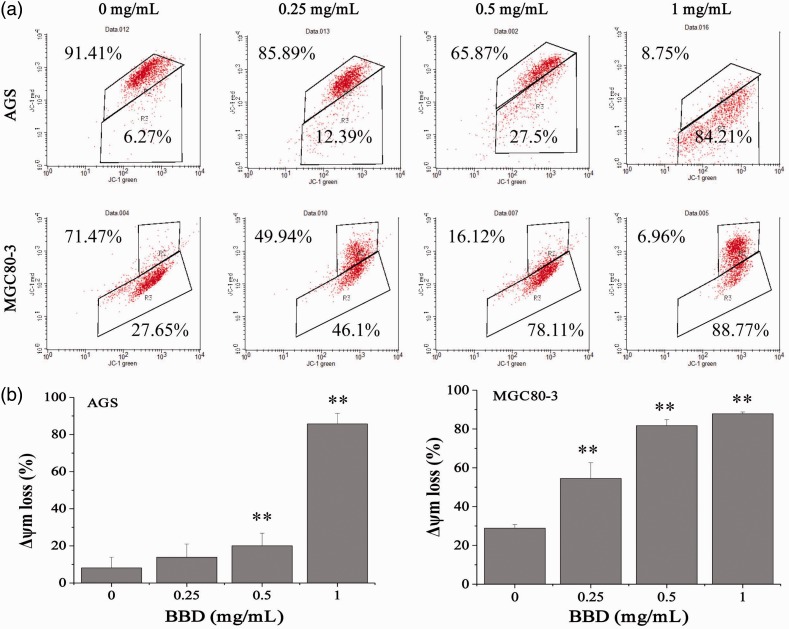
Mitochondrial membrane potential in AGS and MGC80-3 cells was examined by FACS analysis with JC-1 staining, a method that can be used to examine the changes in the mitochondrial membrane potential after apoptosis. These data showed that BBD reduced the fluorescence ratio of JC-1 red/green, which indicates mediation of the mitochondrial membrane potential (Δ ψm) in AGS and MGC80-3 cells (Figure 3, P < 0.01) had taken place. The loss of mitochondrial membrane potential was 6.27% to 84.21% in AGS cells and 27.65% to 88.77% in MGC80-3 cells. These data suggested that BBD effectively induced GC cell apoptosis via the mitochondrial-dependent pathway.
Figure 3.
Effect of BBD on the loss of mitochondrial membrane potential in AGS and MGC80-3 cells. (a) Images are representative FACS scattergrams. (b) Data are shown as the mean ± SD of three independent experiments. **P < 0.01 versus control cells (0 mg/mL).
BBD accelerated the activity of caspase-3, -8, and -9 in AGS and MGC80-3 cells
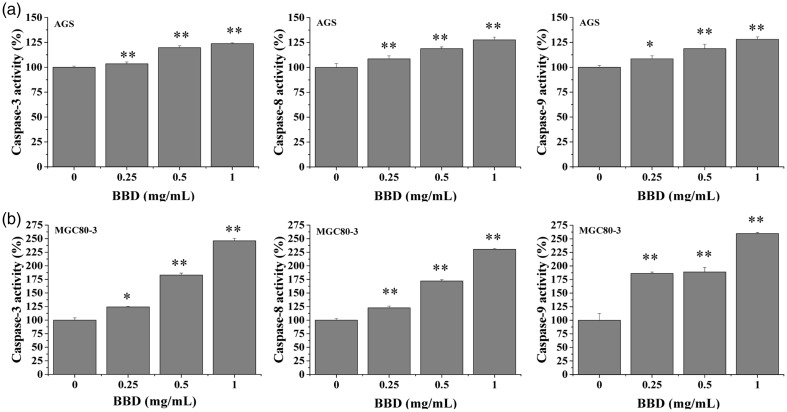
To clarify the underlying mechanism of BBD-induced apoptosis in AGS and MGC80-3 cells, we tested caspase-3, -8, and -9 activation via colorimetric assay using a specific chromophore. As shown in Figure 4, BBD activated caspase-3, -8, and -9 in the above cells (P < 0.05), and the activity of these caspases was slightly higher in MGC80-3 cells (Figure 4b) compared with AGS cells (Figure 4a).
Figure 4.
Effect of BBD on activation of caspase-3, caspase-8, and caspase-9 in AGS and MGC80-3 cells. (a) Cells were treated with the indicated concentrations of BBD for 24 hours. (b) Data were normalized to the caspase activities within control cells (0 mg/mL) and are presented as the fold of control. *P < 0.05 and **P < 0.01 versus control cells.
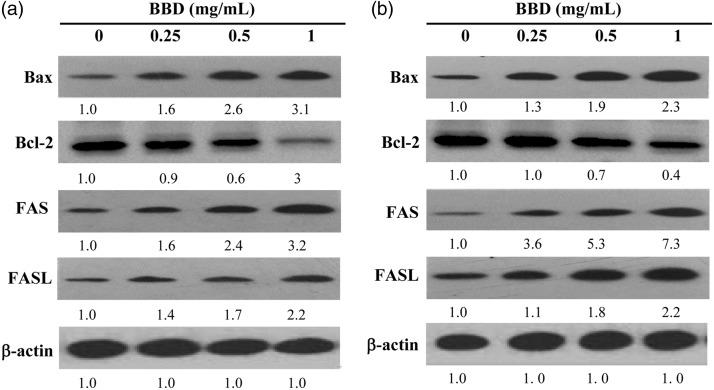
BBD treatment regulated the expression of proteins (Bax, Bcl-2, Fas, and FasL) that are involved in apoptosis in AGS and MGC80-3 cells
To investigate another mechanism of BBD-mediated apoptosis, we detected protein levels of Bax, Bcl-2, Fas, and FasL by western blot. Anti-apoptotic (Bcl-2) and pro-apoptotic (Bax) proteins are emerging as important agents in the regulation of apoptosis. Bcl-2 was investigated in apoptosis that was induced by BBD in AGS (Figure 5a) and MGC80-3 (Figure 5b) cells. Additionally, Bax was up-regulated after cell exposure to BBD. Consistently, Fas and FasL served significant roles in the external death receptor pathway. Western blot assay results also indicated that Fas and FasL expression increased with 0.25, 0.5, and 1 mg/mL of BBD treatment in AGS (Figure 5a) and MGC80-3 (Figure 5b) cells.
Figure 5.
Effect of BBD on Bax, Bcl-2, Fas, and FasL expression by western blot. (a) AGS and (b) MGC80-3 cells after treatment with the indicated concentration of BBD for 24 hours by western blot. β-actin was used as the internal control for western blot.
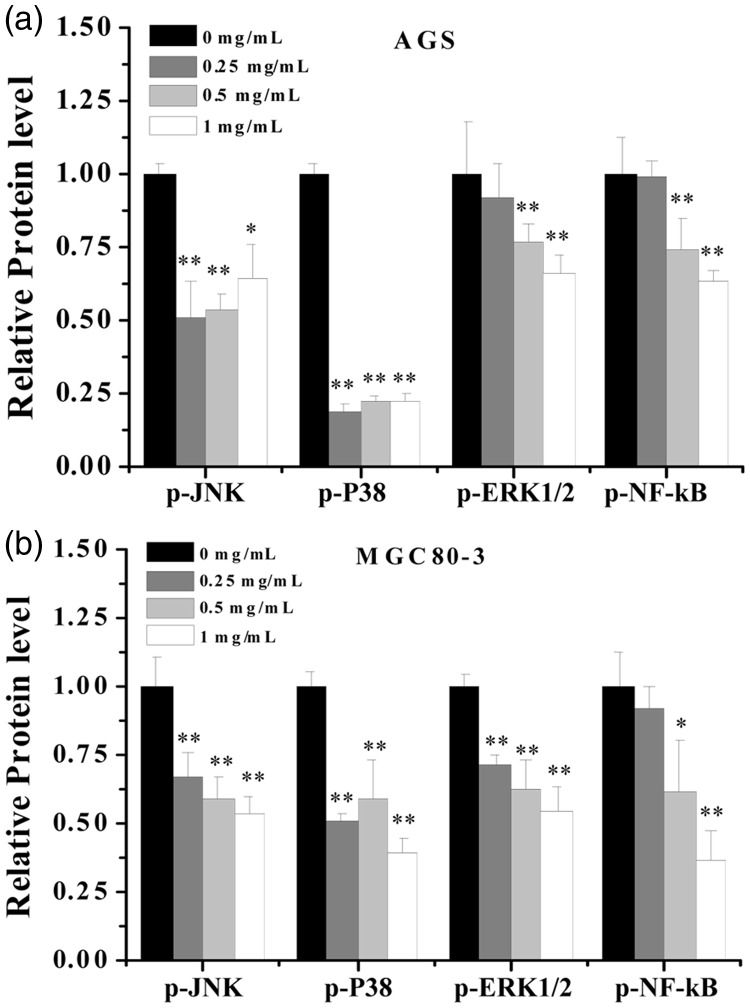
BBD suppressed the activation of MAPK and NF-κB signaling pathways
As described above, BBD has apparent anti-tumor activity via induction of apoptosis in AGS and MGC80-3. Additionally, we detected some important signaling pathway-related cell apoptosis factors, including MAPK and NF-κB, to elucidate the details of the molecular mechanism. Because protein phosphorylation is a key step in initiating cell apoptosis, we assayed the phosphorylation levels of important proteins (p-ERK1/2, p-JNK, p-p38, and p-NF-κB) that are involved in the signaling pathways of interest using a bead-based multiplex kit. As shown in Figure 6, the phosphorylation levels of NF-κB, ERK1/2, JNK, and p38 in two GC cell lines were decreased compared with the untreated group (0 mg/mL) after treatment with different BBD concentrations (P < 0.05), especially in AGS cells. The results suggested that BBD exerts anti-tumor activity, which likely occurs through influencing phosphorylation of multiple intracellular proteins.
Figure 6.
Detection of phosphorylated proteins (JNK, p38, ERK1/2, NF-κB). (a) AGS and (b) MGC80-3 cells were exposed to different concentrations of BBD for 24 hours. Whole cell extracts were obtained, and the phosphorylated protein levels were determined using a Bio-Plex assay. *P < 0.05 and **P < 0.01 versus control cells (0 mg/mL).
Discussion
Gastric cancer is primarily caused by infection with Helicobacter pylori, a bacterium that accounts for 60% of gastric cancer cases.27 Causes in the remaining 40% of gastric cancer cases are thought to be genetic and geographic factors.28 Gastric cancer can metastasize elsewhere in the body, including the liver, bones, and lymph nodes,28 which makes it difficult to cure in patients who are diagnosed with advanced tumors.29 Currently, the main clinical treatment for gastric cancer involves surgery with chemotherapy or radiation therapy, but the cumulative toxicity of these treatments to bone marrow and the digestive tract as well as their tendency to decrease appetite limit their usefulness. Therefore, the development of novel strategies to treat gastric cancer is desirable. Despite intense research, the specific mechanisms involved in BBD-induced apoptosis remain largely unexplained.
TCM has become a popular and promising alternative therapy in eastern Asia and especially in China.5,30 BBD is a mixture of eight traditional Chinese powdered herbal preparations, including components such as antelope horn, snake gall, natural calculus bovis, radix notoginseng, pearl, and musk, the functions of which are mainly detoxification, clearing heat, removing dampness and blood stasis, dissipating hard masses, and alleviating pain. Previous studies have reported BBD’s wide use in TCM clinical treatment, and it was also chosen for its anti-tumor activity in particularly aggressive types of cancers, such as gastric cancer, pancreatic cancer, liver cancer, and non-small cell lung cancer.20 However, the biologic mechanisms responsible for BBD’s efficacy in treating gastric cancer remain unclear.
In our previous studies, we detected the effect of BBD on four gastric cancer cell lines (AGS, MGC80-3, NCI-N87, and BGC-823) using the MTT assay. The results showed that two cell lines (NCI-N87 and BGC-823) were not sensitive to BBD and it had no obvious effect. However, there were relatively more studies involving AGS and MGC80-3. Therefore, we selected AGS and MGC80-3 cell lines for the follow-up experiments. Our previous studies showed that BBD can suppress cell growth in AGS and MGC80-3 cells in vitro.31 The inhibitory effect of BBD mainly relates to cell morphological changes in apoptosis and cell activity reduction, which led us to further investigate the BBD biologic apoptosis mechanism in AGS and MGC80-3 cells in vitro. We focused on two major apoptotic pathways (endogenous and exogenous intrinsic pathways) to elucidate the molecular mechanisms in the current study. The current results showed that BBD has significant anticancer effects by inducing AGS and MGC80-3 cell apoptosis. As demonstrated by the current study, BBD reduced AGS and MGC80-3 cell viability in a dose-dependent manner and increased cytotoxicity to these gastric cancer cells. Additionally, a flow cytometry assay confirmed that BBD treatment caused apoptosis in both AGS and MGC80-3 cells.
Apoptosis is triggered via different signaling pathways, mainly the death receptor pathway and the mitochondrial pathway. Between the two, the mitochondrial pathway is the crucial route toward cell apoptosis. The family of Bcl-2 proteins includes two kinds of proteins with opposite functions—an anti-apoptotic protein (Bcl-2) and a pro-apoptotic protein (Bax)—both of which participate in regulation of the apoptosis pathway by influencing mitochondrial membrane permeability. While Bcl-2 is a crucial anti-apoptotic protein, Bax is a pro-apoptotic agent. Additionally, Bax responds to an external signal and transduces it to the mitochondrial membrane, which causes the release of cytochrome c and stimulates caspase-9 activity. Caspase-3 will not be activated until caspase-9 is cleaved by a proteolytic enzyme to initiate the apoptotic cascade effect. In the present study, AGS and MGC80-3 cell death was observed in conjunction with a reduction in the mitochondrial membrane potential after treatment with BBD for 24 hours. This was further reinforced by the significant difference between Bax and Bcl-2 expression levels after cells were exposed to BBD. The loss of mitochondrial membrane potential often caused a mitochondrial malfunction, which induces cell apoptosis. Protein expression analysis also revealed that BBD caused considerable down-regulation of Bcl-2 and up-regulation of Bax.
Another cell apoptosis signaling pathway, the death receptor pathway, is mediated by Fas, which is a TNF-R family member and a cell surface death receptor. Fas can bind with FasL and then lead to subsequent activation of caspase-8 protein by Fas-associated death domain protein. Caspase-3 is then activated by the cleavage of caspase-8, and the cell death signal pathway is activated. Additionally, to clarify the initiation of the apoptosis signaling pathway in AGS and MGC80-3 cells treated by BBD, the Fas/FasL-mediated signal pathway was also detected. The data suggested that BBD treatment up-regulates Fas and FasL protein expression. All these results showed that BBD induces AGS and MGC80-3 cell apoptosis via the intrinsic and extrinsic apoptotic pathways.
Recent studies have reported that activating the MAPK signaling pathway can regulate death receptors and survival proteins to induce apoptosis.32,33 Newhouse34 also reported that rotenone-induced apoptosis is mediated by JNK MAP kinases and p38 in human dopaminergic SH-SY5Y cells. To illustrate the molecular mechanism whereby BBD induces apoptosis, such relevant signaling pathways as MAPK and NF-κB were detected. The levels of phosphorylated proteins JNK, p38, and ERK were regulated by BBD treatment. NF-κB and MAPK communicate the external signal from the membrane to the nucleus and govern a series of biological functions, including apoptosis, proliferation, and differentiation. Antitumor activities were extensively reported through the suppression of key protein activation or level of NF-κB and MAPK pathway. Our study results are consistent with publications on activation of the caspase cascade in tumor cells leading to apoptosis.35 Therefore, BBD plays a significant role in inhibiting the activation of NF-κB and MAPK signaling pathways in AGS and MGC80-3 cells.
Generally, our study indicates that the BBD-induced apoptosis of AGS and MGC80-3 cells is mediated via both intrinsic and extrinsic apoptotic pathways via down-regulating NF-κB and MAPK signaling pathways. The anti-cancer mechanism of BBD needs further exploration because it involves many possible signaling pathways, and further in vitro experiments are required. The current findings may help pave the way for future investigation into the potential therapeutic usefulness of BBD in the clinical treatment of human gastric cancer.
Acknowledgments
The authors thank Xiamen Traditional Chinese Medicine Factory Co., Ltd. for providing the BBD.
Consent
Because our report did not including human studies, a consent statement was not provided.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Ethics
The submitted MS is mainly an in vitro study (gastric cancer cell lines AGS and MGC80-3). There anywhere no studies in animals and humans in our report, so the ethics approval was not obtained
Funding
This research was supported by the Natural Science Foundation of Fujian Province, China (grant no. 2019J01355).
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin 2017; 67: 7–30. [DOI] [PubMed] [Google Scholar]
- 2.Jing X, Cheng W, Wang Set al. Resveratrol induces cell cycle arrest in human gastric cancer MGC803 cells via the PTEN-regulated PI3K/Akt signaling pathway. Oncol Rep 2016; 35: 472–478. [DOI] [PubMed] [Google Scholar]
- 3.Fan GF, Pan JJ, Fan PSet al. The clinical observation of verapamil in combination with interventional chemotherapy in advanced gastric cancer. Eur Rev Med Pharmacol Sci 2018; 22: 5508–5518. [DOI] [PubMed] [Google Scholar]
- 4.Oba K, Paoletti X, Bang YJet al. Role of chemotherapy for advanced/recurrent gastric cancer: an individual-patient-data meta-analysis. Eur J Cancer 2013; 49: 1565–1577. [DOI] [PubMed] [Google Scholar]
- 5.Ling CQ, Wang LN, Wang Yet al. The roles of traditional Chinese medicine in gene therapy. J Integr Med 2014; 12: 67–75. [DOI] [PubMed] [Google Scholar]
- 6.Ferlay J, Shin HR, Bray Fet al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 2010; 127: 2893–2917. [DOI] [PubMed] [Google Scholar]
- 7.Anand P, Kunnumakkara AB, Sundaram Cet al. Cancer is a preventable disease that requires major lifestyle changes. Pharm Res 2008; 25: 2097–2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Newman DJ, Cragg GM. Natural products as sources of new drugs from 1981 to 2014. J Nat Prod 2016; 79: 629–661. [DOI] [PubMed] [Google Scholar]
- 9.Yuan Y, Jiang YC, Sun CKet al. Role of the tumor microenvironment in tumor progression and the clinical applications (Review). Oncol Rep 2016; 35: 2499–2515. [DOI] [PubMed] [Google Scholar]
- 10.Liu XR, Li YQ, Hua Cet al. Oxidative stress inhibits growth and induces apoptotic cell death in human U251 glioma cells via the caspase-3-dependent pathway. Eur Rev Med Pharmacol Sci 2015; 19: 4068–4075. [PubMed] [Google Scholar]
- 11.Javadov S, Karmazyn M. Mitochondrial permeability transition pore opening as an endpoint to initiate cell death and as a putative target for cardio protection. Cell Physiol Biochem 2007; 20: 1–22. [DOI] [PubMed] [Google Scholar]
- 12.Chen TC, Chien CC, Wu MSet al. Evodiamine from Evodia rutaecarpa induces apoptosis via activation of JNK and PERK in human ovarian cancer cells. Phytomedicine 2016; 23: 68–78. [DOI] [PubMed] [Google Scholar]
- 13.Yang J, Wu LJ, Tashiro Set al. Nitric oxide activated by p38 and NF-κB facilitates apoptosis and cell cycle arrest under oxidative stress in evodiamine-treated human melanoma A375-S2 cells. Free Radic Res 2008; 42: 1–11. [DOI] [PubMed] [Google Scholar]
- 14.Wang C, Li S, Wang MW. Evodiamine-induced human melanoma A375-S2 cell death was mediated by PI3K/Akt/caspase and Fas-L/NF-kB signaling pathways and augmented by ubiquitin-proteasome inhibition. Toxicol In Vitro 2010; 24: 898–904. [DOI] [PubMed] [Google Scholar]
- 15.Chen KJ, Lu AP. Situation of integrative medicine in China: results from a national survey in 2004. Chin J Integr Med 2006; 12: 161–165. [DOI] [PubMed] [Google Scholar]
- 16.Wang L, Zhou GB, Liu Pet al. Dissection of mechanisms of Chinese medicinal formula Realgar-Indigo naturalis as an effective treatment for promyelocytic leukemia. Proc Natl Acad Sci USA 2008; 105: 4826–4831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jeong JC, Jang SW, Kim THet al. Mulberry fruit (Moris fructus) extracts induce human glioma cell death in vitro through ROS dependent mitochondrial pathway and inhibits glioma tumor growth in vivo. Nutr Cancer 2010; 62: 402–412. [DOI] [PubMed] [Google Scholar]
- 18.Sun GD, Wei LH, Feng JYet al. Inhibitory effects of Hedyotis diffusa Willd on colorectal cancer stem cells. Oncology Letters 2016; 11: 3875–3881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jeon J, Lee J, Kim Cet al. Aqueous extract of the medicinal plant Patrinia villosa Juss. induces angiogenesis via activation of focal adhesion Kinase. Microvasc Res 2010; 80: 303–309. [DOI] [PubMed] [Google Scholar]
- 20.Wei J, Zhu Y, Xu Get al. Oxymatrine extracted from Sophora flavescens inhibited cell growth and induced apoptosis in human osteosarcoma MG-63 cells in vitro. Cell Biochem Biophys 2014; 70: 1439–1444. [DOI] [PubMed] [Google Scholar]
- 21.Liang L, Yang X, Yu Yet al. Babao Dan attenuates hepatic fibrosis by inhibiting hepatic stellate cells activation and proliferation via TLR4 signaling pathway. Oncotarget 2016; 7: 82554–82566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gong W, Liu L, Li Met al. Evaluation of antiviral efficacy of Chinese traditional medicine Babao Dan in rabbits infected with hepatitis E virus. J Gen Virol 2018; 99: 1036–1043. [DOI] [PubMed] [Google Scholar]
- 23.Lin J, Chen Y, Wei Let al. Ursolic acid promotes colorectal cancer cell apoptosis and inhibits cell proliferation via modulation of multiple signaling pathways. Int J Oncol 2013; 43:1235–1243. [DOI] [PubMed] [Google Scholar]
- 24.Vakifahmetoglu-Norberg H, Ouchida AT, Norberg E. The role of mitochondria in metabolism and cell death. Biochem Biophys Res Commun 2017; 482: 426–431. [DOI] [PubMed] [Google Scholar]
- 25.Wlodkowic D, Telford W, Skommer Jet al. Apoptosis and beyond: cytometry in studies of programmed cell death. Methods Cell Biol 2011; 103: 55–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Demchenko AP. Beyond annexin V: fluorescence response of cellular membranes to apoptosis. Cytotechnology 2013; 65: 157–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hanahan D, Coussens LM. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell 2012; 21: 309–322. [DOI] [PubMed] [Google Scholar]
- 28.Sjomina O, Pavlova J, Niv Yet al. Epidemiology of Helicobacter pylori Infection. Helicobacter 2018; 23: 12514. [DOI] [PubMed] [Google Scholar]
- 29.Ruddon RW. Introduction to the molecular biology of cancer: translation to the clinic. Prog Mol Biol Transl Sci 2010; 95: 1–8. [DOI] [PubMed] [Google Scholar]
- 30.Alimbetov D, Askarova S, Umbayev Bet al. Pharmacological targeting of cell cycle, apoptotic and cell adhesion signaling. Int J Mol Sci 2018; 19: E1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang LN, Wang Y, Lu Yet al. Pristimerin enhances recombinant adeno-associated virus vector-mediated transgene expression in human cell lines in vitro and murine hepatocytes in vivo. J Integr Med 2014; 12: 20–34. [DOI] [PubMed] [Google Scholar]
- 32.Zang LL, Wu BN, Lin Yet al. Research progress of ursolic acid’s anti-tumor actions. Chin J Integr Med 2014; 20: 72–79. [DOI] [PubMed] [Google Scholar]
- 33.Lee KC, Chen YL, Lin PYet al. Ursolic acid-induced apoptosis via regulation of the PI3K/Akt and MAPK signaling pathways in Huh-7 cells. Molecules 2018; 23: pii: E2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Newhouse K, Hsuan SL, Chang SHet al. Rotenone-induced apoptosis is mediated by p38 and JNK MAP kinases in human dopaminergic SH-SY5Y cells. Toxicol Sci 2004; 79: 137–146. [DOI] [PubMed] [Google Scholar]
- 35.Zhou Q, Wu X, Wen Cet al. Toosendanin induces caspase-dependent apoptosis through the p38 MAPK pathway in human gastric cancer cells. Biochem Biophys Res Commun 2018; 505: 261–266. [DOI] [PubMed] [Google Scholar]