Short abstract
Objective
Helicobacter pylori commonly occurs in the stomach, but localizations outside the stomach and related diseases have also been investigated. However, the relationship between H. pylori and gallstones remains controversial. We aimed to investigate the relationships between H. pylori in the stomach and the gallbladder and gallstones.
Methods
This prospective case-control study included patients who underwent cholecystectomy because of gallstones, pancreatic head cancer, or hepatic resection. The patients were separated into two groups according to the detection of H. pylori in gallbladder samples using Giemsa staining. Stomach H. pylori status was based on previous gastroscopy.
Results
The study enrolled 60 patients, comprising 27 patients with gallstones and 33 without. There was no significant difference in the incidence of gallstones between patients with or without H. pylori in the stomach or gallbladder. Furthermore, the presence of H. pylori in the stomach was measured in 14 patients and was significantly correlated with H. pylori in the gallbladder.
Conclusion
The current study showed no relationship between the occurrence of gallstones and the presence of H. pylori in either the gallbladder or the stomach. In contrast to previous reports, this suggests that H. pylori does not play a role in the development of gallstones.
Keywords: Biliary stone, gallstone, gastritis, general surgery, hepato-pancreatic biliary surgery, Helicobacter pylori
Introduction
Gallstones occur in patients worldwide, but are especially common in western countries.1 Several factors have been reported to play roles in the formation of gallstones, including bacterial infections in the gallbladder and bile ducts.2 Gallstones also cause various clinical conditions, such as acute cholecystitis, chronic cholecystitis, jaundice related to bile duct obstruction, and acute pancreatitis.3
Helicobacter pylori is a Gram-negative bacillus thought to infect more than half the global population. H. pylori has primarily been identified in the stomach and has been related to several diseases, including duodenal ulcer, chronic gastritis, non-Hodgkin’s lymphoma of the stomach, and gastric adenocarcinoma.4–8 However, increasing numbers of studies have investigated localizations of H. pylori outside the stomach and diseases potentially related to such infections.9 H. pylori was first described in the gallbladder mucosa in patients with gallstones 1996, and a relationship between H. pylori and gallstone formation was reported, as in the stomach.10 However, despite subsequent reports of a relationship between H. pylori and chronic cholecystitis, no mechanism has yet been identified.11,12 Importantly in this regard, it remains unclear if H. pylori reaches the gallbladder and biliary tree via an ascending route from the duodenum, or via the portal venous system.13,14
Given the conflicting reports regarding the relationship between H. pylori infections and gallstone formation, this study aimed to investigate the relationships between H. pylori in the stomach and gallbladder and the occurrence of gallstones.
Methods
Patients
This prospective, case-control study reviewed a total of 732 patients who underwent cholecystectomy for any reason at Istanbul Training and Research Hospital between September 2015 and May 2018. Exclusion criteria were cholecystectomy performed because of acute cholecystitis, pregnancy, and patients who did not wish to participate in the study. Indications for cholecystectomy in the included patients were gallstones, pancreatic head cancer, or hepatic resection. Patients were ordered according to the date of cholecystectomy and 60 patients were chosen at random using the www.random.org website. Written informed consent was obtained from each patient and the study protocol was approved by the local Ethics Committee.
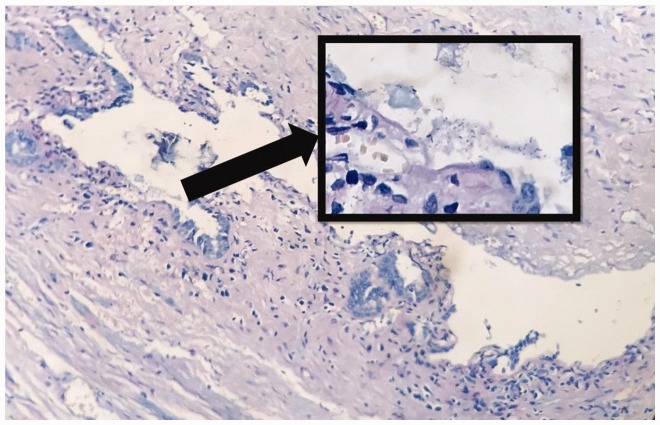
Gallbladders excised during cholecystectomy were placed in 10% buffered formaldehyde solution and sent to the pathology laboratory for routine pathology examination and investigation of H. pylori using Giemsa staining (Figure 1). The patients were then separated into two groups according to the presence or absence of H. pylori in the gallbladder. Patient records included information on age, sex, pathology report and H. pylori status in the stomach if gastroscopy had been applied preoperatively, gallbladder wall thickness, cholesterolosis, intestinal metaplasia, activation, and pyloric metaplasia.
Figure 1.
Gallbladder biopsy stained for H. pylori using Giemsa staining (magnification ×100). Box shows representative H. pylori staining (magnification ×400).
Statistical analysis
Data were analyzed using IBM SPSS Statistics for Windows, Version 22.0 software (IBM Corp., Armonk, NY, USA). Descriptive statistics were given as mean ± standard deviation (SD), median (maximum, minimum), number (n), and percentage (%). Conformity of the data to a normal distribution was assessed using the Kolmogorov–Smirnov test. Quantitative independent data were analyzed using the Mann–Whitney U-test. Qualitative independent data were analyzed by the χ2 test, or by Fisher’s test if the χ2 test conditions were not met. A value of P < 0.05 was accepted as statistically significant.
Results
A total of 60 patients were included in the final evaluation (34 women, 26 men, mean age 56.0 ± 11.7 years). The study was limited to 60 patients because of financial restrictions. Twenty-seven patients had gallstones and 33 did not. Group 1 comprised 52 patients without H. pylori in the gallbladder and Group 2 comprised eight patients with H. pylori in the gallbladder. There was no significant difference between the groups in terms of age, sex, wall thickness, presence of gallstones, rate of intestinal metaplasia, rate of activation, rate of previous stomach biopsy, or rate of gastritis in previous stomach biopsy (Table 1).
Table 1.
Characteristics of patients according to gallbladder H. pylori status.
|
Gallbladder H. pylori (−) |
Gallbladder H. pylori (+) |
P value | |||||
|---|---|---|---|---|---|---|---|
| Mean ± SD or n | Percentage (%) | Median | Mean ± SD or n | Percentage (%) | Median | ||
| Age | 55.8±12.1 | 58 | 57.4±9.6 | 60 | 0.524 | ||
| Sex | |||||||
| Female | 29 | 55.8% | 5 | 62.5% | 0.721 | ||
| Male | 23 | 44.2% | 3 | 37.5% | |||
| Wall thickness (mm) | 3.8±3.3 | 3 | 3.1±1.1 | 3 | 0.508 | ||
| Gallstones | |||||||
| Absent | 28 | 53.8% | 5 | 62.5% | 0.647 | ||
| Present | 24 | 46.2% | 3 | 37.5% | |||
| Cholesterolosis | |||||||
| Absent | 44 | 84.6% | 8 | 100.0% | 0.582 | ||
| Present | 8 | 15.4% | 0 | 0.0% | |||
| Intestinal metaplasia | |||||||
| Absent | 47 | 90.4% | 7 | 87.5% | >0.95 | ||
| Present | 5 | 9.6% | 1 | 12.5% | |||
| Activation | |||||||
| Absent | 38 | 73.1% | 6 | 75.0% | 0.909 | ||
| Present | 14 | 26.9% | 2 | 25.0% | |||
| Pyloric metaplasia | |||||||
| Absent | 39 | 75.0% | 8 | 100.0% | 0.182 | ||
| Present | 13 | 25.0% | 0 | 0.0% | |||
| Previous stomach biopsy | |||||||
| Absent | 40 | 76.9% | 6 | 75.0% | 0.222 | ||
| Present | 10 | 23.1% | 4 | 25.0% | |||
| Gastritis | |||||||
| Absent | 1 | 8.3% | 0 | 0.0% | >0.95 | ||
| Present | 9 | 91.7% | 4 | 100.0% | |||
Fourteen patients underwent preoperative gastroscopy and biopsy for H. pylori detection. There was no significant difference between patients with and without H. pylori in the stomach in relation to the detection of gallstones.
Out of five patients with H. pylori-positive gastritis (confirmed by biopsy), four were also positive for H. pylori on gallbladder biopsy, while all nine patients negative for H. pylori gastritis were also negative for gallbladder H. pylori, suggesting a significant correlation between stomach and gallbladder H. pylori status (P = 0.005) (Table 2)
Table 2.
Gallstones and H. pylori status according to presence of H. pylori in stomach biopsy
|
Stomach H. pylori (−) |
Stomach H. pylori (+) |
P value | ||||
|---|---|---|---|---|---|---|
| n | Percentage (%) | n | Percentage (%) | |||
| Gallstones | Absent | 4 | 44.4% | 3 | 60.0% | <0.95 |
| Present | 5 | 55.6% | 2 | 40.0% | ||
| Gallbladder H. pylori status | Absent | 9 | 100.0% | 1 | 20.0% | 0.005 |
| Present | 0 | 0.0% | 4 | 80.0% | ||
In relation to the possible route of spread, 24 of the 33 patients without gallstones, including four of the five patients with H. pylori in the gallbladder, had a history of preoperative endoscopic retrograde cholangiography (ERCP), compared with only 2 of the 27 patients who underwent cholecystectomy because of gallbladder stones.
Discussion
Many recent studies have investigated the occurrence of H. pylori outside the stomach, e.g., in the skin, nose, and gallbladder,9,15 while other studies have evaluated the statuses of other Helicobacter strains within the gallbladder.10,11
Fatemi et al. compared Helicobacter strains between patients without gallstones and those with acute cholecystitis and chronic cholecystitis associated with gallstones. H. pylori was observed at a significantly higher rate in cases with acute cholecystitis associated with gallstones compared with the other cases, and there was no relationship with other Helicobacter strains.16 A meta-analysis that reviewed studies of the relationship between H. pylori and gallstones and cholecystectomy reported that H. pylori was more common in patients with chronic cholecystitis and gallstones compared with the control group (24.98% vs. 8.28%, P<0.05).17 Another meta-analysis also demonstrated a relationship between H. pylori in the gallbladder and cholelithiasis.18 Zhou et al.14 found H. pylori in 20% of chronic cholecystitis cases, and also emphasized that gallbladder metaplasia and pre-malignant lesions such as adenomyomatosis were more frequent in patients positive for gallbladder H. pylori. In another study, Hassan et al.19 reported that H. pylori infection aggravated potentially precancerous mucosal lesions.
In contrast to many studies, a retrospective study15 of 45 cases including cholecystectomy samples detected H. pylori-positivity in the gallbladder in 40% of patients without gallstones and 20% of those with gallstones. However, this difference was not significant and no relationship could be established between H. pylori-positivity and the occurrence of gallstones. Moreover, no H. pylori gallbladder infection was noted in 10 patients with ulceration of the gallbladder.15 Similarly, the current study found no relationship between H. pylori-positivity in the gallbladder and gallstones, or the development of cholesterolosis, intestinal metaplasia, pyloric metaplasia, and activation.
Previous studies reported that H. pylori was more prevalent in eastern countries and showed no sex difference, but was related to age, with a particular increase in colonization rates above the age of 70 years.20–22 The mean age of the patients with gallbladder H. pylori-positivity in the current study was 57.4 years, which was not significantly different from that of the patients who were H. pylori-negative in the gallbladder.
There is currently insufficient evidence regarding the route whereby H. pylori settles in the gallbladder. It may reach the gallbladder via an ascending route from the duodenum or via the portal circulation system.23,24 A meta-analysis of patients undergoing ERCP reported that the procedure could result in contamination of the gallbladder with Helicobacter strains via the ascending pathway from the duodenum, leading to false-positive results.17 In the current study, although four out of the five patients with H. pylori in the gallbladder without gallstones underwent preoperative ERCP, thus supporting its possible spread via the ascending pathway, no conclusions can be drawn about the route of spread in patients with gallstones who did not undergo ERCP.
An extensive retrospective study25 that evaluated the relationship between the presence of H. pylori antibodies in the blood and gallstones found no relationship between the rates of gallstones in H. pylori-positive patients overall, but a significant negative correlation was observed in individuals aged <45 years. The same study identified a significant relationship between gallbladder polyps and H. pylori-positivity.25 Helaly et al.26 reported a significant relationship between H. pylori-positivity in the stomach and the gallbladder, while another study also found a strong association between gallbladder and stomach H. pylori-positivity.14 The current study found no significant relationship between the occurrence of gallstones and stomach H. pylori-positivity in patients with stomach biopsy results, but did confirm a significant relationship between stomach and gallbladder H. pylori-positivity.
This study had some limitations owing to its small size and limited variation, which meant that it was not possible to examine subgroups of patients with cancers of the hepatobiliary system and pre-malignant lesions because of the low patient numbers. In addition, H. pylori status was not measured in the bile ducts, thus limiting the information available in relation to the route of H. pylori spreading.
In contrast to several previous studies, the current results suggested that the rates of gallbladder H. pylori-positivity, as well as stomach H. pylori-positivity, were similar in patients with and without gallstones. However, there was a significant relationship between stomach and gallbladder H. pylori statuses. Although further studies with larger series are required, the present results indicate that H. pylori infection does not contribute to the development of gallstones.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1.Stinton LM, Shaffer EA. Epidemiology of gallbladder disease: cholelithiasis and cancer. Gut Liv 2012; 6: 172–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maurer KJ, Carey MC, Fox JG. Roles of infection, inflammation, and the immune system in cholesterol gallstone formation. Gastroenterol 2009; 136: 425–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leong RW, Sung JJ. Helicobacter species and hepatobiliary diseases. Aliment Pharmacol Ther 2002; 16: 1037–1045. [DOI] [PubMed] [Google Scholar]
- 4.Parsonnet J, Friedman GD, Vandersteen DP. Helicobacter pylori infection and the risk of gastric carcinoma. N Engl J Med 1991; 325: 1127–1131. [DOI] [PubMed] [Google Scholar]
- 5.Correa P, Houghton J. Carcinogenesis of Helicobacter pylori. Gastroenterol 2007; 133: 659–672. [DOI] [PubMed] [Google Scholar]
- 6.Marshall BJ, Warren JR. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet 1984; 1: 1311–1315. [DOI] [PubMed] [Google Scholar]
- 7.Veldhuyzen van Zanten SJ, Sherman PM. Helicobacter pylori infection as a cause of gastritis, duodenal ulcer, gastric cancer and nonulcer dyspepsia: a systematic over-view. Can Med Assoc J 1994; 150: 177–185. [PMC free article] [PubMed] [Google Scholar]
- 8.Parsonnet J, Hansen S, Rodriguez L, et al. Helicobacter pylori infection and gastric lymphoma. N Engl J Med 1994; 330: 1267–1271. [DOI] [PubMed] [Google Scholar]
- 9.Testerman TL, Morris J. Beyond the stomach: an updated view of Helicobacter pylori pathogenesis, diagnosis, and treatment. World J Gastroenterol 2014; 20: 12781–12808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kawaguchi M, Saito T, Ohno H, et al. Bacteria closely resembling Helicobacter pylori detected immunohistologically and genetically in resected gallbladder mucosa. J Gastroenterol 1996; 31: 294–298. [DOI] [PubMed] [Google Scholar]
- 11.Moricz AD, Melo M, Castro AM, et al. Prevalence of Helicobacter spp in chronic cholecystitis and correlation with changes on the histological pattern of the gallbladder. Acta Cir Bras 2010; 25: 218–224. [DOI] [PubMed] [Google Scholar]
- 12.Apostolov E, Al-Soud WA, Nilsson I, et al. Helicobacter pylori and other Helicobacter species in gallbladder and liver of patients with chronic cholecystitis detected by immunological and molecular methods. Scand J Gastroenterol 2005; 40: 96–102. [DOI] [PubMed] [Google Scholar]
- 13.Mishra RR, Tewari M, Shukla HS. Association of Helicobacter pylori infection with inflammatory cytokine expression in patients with gallbladder cancer. Indian J Gastroenterol 2013; 32: 232–235. [DOI] [PubMed] [Google Scholar]
- 14.Zhou D, Guan WB, Wang JD, et al. A comparative study of clinicopathological features between chronic cholecystitis patients with and without Helicobacter pylori infection in gallbladder mucosa. PLoS One 2013; 8: e70265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Patnayak R, Reddy V, Jena A, et al. Helicobacter pylori in cholecystectomy specimens-morphological and immunohistochemical assessment. Clin Diagn Res 2016; 10: EC01-3. doi: 10.7860/JCDR/2016/14802.7716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fatemi SM, Doosti A, Shokri D, et al. Is there a correlation between Helicobacter pylori and enterohepatic Helicobacter species and gallstone cholecystitis? Middle East J Dig Dis 2018; 10: 24–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cen L, Pan J, Zhou B, et al. Helicobacter Pylori infection of the gallbladder and the risk of chronic cholecystitis and cholelithiasis: a systematic review and meta-analysis. Helicobacter 2018; 23. doi: 10.1111/hel.12457. [DOI] [PubMed] [Google Scholar]
- 18.Zhou D, Zhang Y, Gong W, et al. Are Helicobacter pylori and other Helicobacter species infection associated with human biliary lithiasis? A meta-analysis PLoS ONE 2011; 6: e27390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hassan EH, Gerges SS, El-Atrebi KA, et al. The role of H. pylori infection in gall bladder cancer: clinicopathological study. Tumour Biol 2015; 36: 7093–7098. [DOI] [PubMed] [Google Scholar]
- 20.Bulajic M, Maisonneuve P, Schneider-Brachert W, et al. Helicobacter pylori and the risk of benign and malignant biliary tract disease. Cancer 2002; 95: 1946–1953. [DOI] [PubMed] [Google Scholar]
- 21.Leja M, Axon A, Brenner H. Epidemiology of Helicobacter pylori infection. Helicobacter 2016; 21: 3–7. [DOI] [PubMed] [Google Scholar]
- 22.Apostolopoulos P, Vafiadis-Zouboulis I, Tzivras M, et al. Helicobacter pylori (H pylori) infection in Greece: the changing prevalence during a ten-year period and its antigenic profile. BMC Gastroenterol 2002; 2: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pellicano R, Ménard A, Rizzetto M, et al. Helicobacter species and liver diseases: association or causation? Lancet Infect Dis 2008; 8: 254–260. [DOI] [PubMed] [Google Scholar]
- 24.Tiwari SK, Khan AA, Ibrahim M, et al. Helicobacter pylori and other Helicobacter species DNA in human bile samples from patients with various hepato-biliary diseases. World J Gastroenterol 2006; 12: 2181–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu MY, Ma JH, Yuan BS, et al. Association between Helicobacter pylori infection and gallbladder diseases: a retrospective study. J Gastroenterol Hepatol 2017; 27: 1207–1212. [DOI] [PubMed] [Google Scholar]
- 26.Helaly GF, El-Ghazzawi EF, Kazem AH, et al. Detection of Helicobacter pylori infection in Egyptian patients with chronic calcular cholecystitis. Br J Biomed Sci 2014; 71: 13–18. [DOI] [PubMed] [Google Scholar]