Short abstract
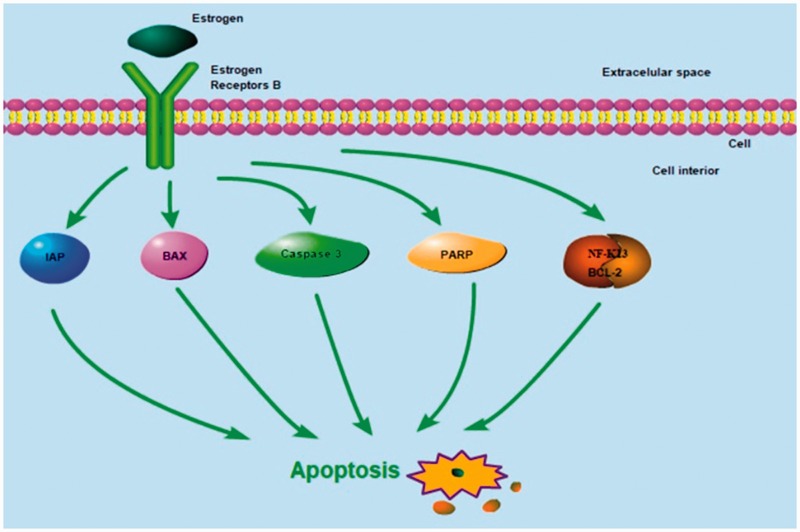
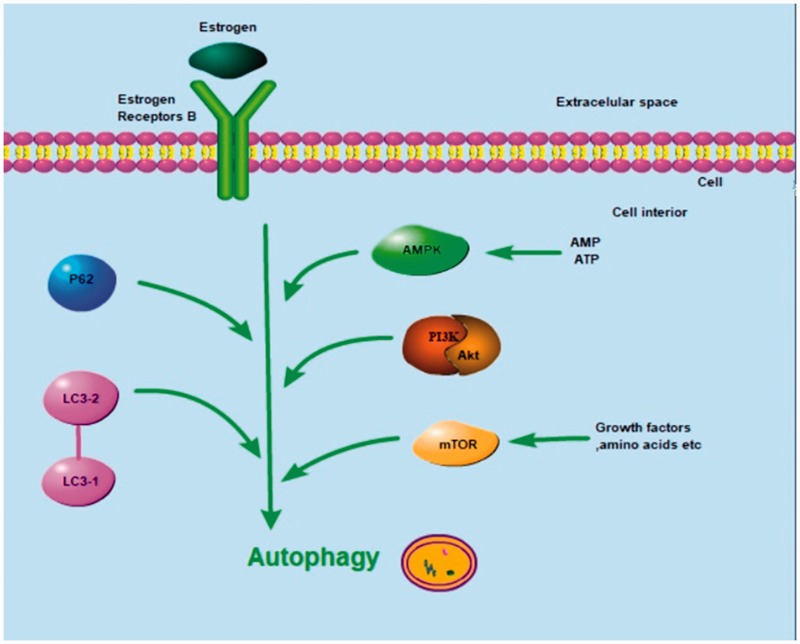
The estrogen receptors α (ERα) and β (ERβ) are located in the nucleus and bind to estrogen to initiate transcription of estrogen-responsive genes. In a variety of tumor cells, ERβ has been shown to be a tumor suppressor. In particular, ERβ has anti-proliferative effects in osteosarcoma cells. Additionally, ERβ has been proven to regulate the apoptosis-related molecules IAP, BAX, caspase-3, and PARP, and to act on the NF-κB/BCL-2 pathway to induce apoptosis in tumors. Moreover, ERβ can regulate the expression of the autophagy associated markers LC3-I/LC-3II and p62 and induce autophagy in tumors by inhibiting the PI3K/AKT/mTOR pathway and activating the AMPK pathway. Here, we review the molecular mechanisms by which ERβ induces apoptosis and autophagy in a variety of tumors to further delineate more specific molecular mechanisms underlying osteosarcoma tumorigenesis and pathogenesis. Considering the broad involvement of ERβ in apoptosis, autophagy, and their interaction, it is plausible that the critical role of ERβ in inhibiting the proliferation and metastasis of osteosarcoma cells is closely related to its regulation of apoptosis and autophagy.
Keywords: Estrogen, estrogen receptor, apoptosis, autophagy, signal transduction, anti-tumor effect, osteosarcoma
Introduction
Estrogen receptors are ligand-dependent receptors that are located in the nucleus and composed of two subtypes: estrogen receptor α (ERα) and estrogen receptor β (ERβ). These two subtypes have similar structures, and both consist of a DNA binding domain, a ligand binding domain, a ligand-independent transcriptional activation region (AF-1) at the N-terminus, and a ligand-dependent transcriptional activation region at the C-terminus (AF-2).1 Both estrogen receptor subtypes can initiate transcription of estrogen-responsive genes either in the form of a homodimer or a heterodimer after binding to estrogen. While the transcriptional activation function of AF-1 from ERβ is weaker compared with ERα, AF-2 from ERβ has comparable function to ERα.2 Additionally, ERβ contains a repressor domain at the N-terminus. When hormones are at sub-saturated levels, ERβ can inhibit the transcriptional activity of ERα, thereby reducing the sensitivity of cells to estrogen.3
ERβ has been proven to be a tumor suppressor in many tumor types. In prostate cancer cells, ERβ promotes apoptosis and inhibits cell proliferation, invasion, metastasis, and epithelial–mesenchymal transition (EMT).4,5 In ovarian cancer cells, ERβ inhibits cell growth and potentiates the antitumor activity of chemotherapy drugs, including cisplatin and taxol.6,7 ERβ overexpression inhibits the growth of ERα-expressing breast cancer cells and prevents the production of estrogen-induced breast cancer xenografts in nude mice.8,9 ERβ can influence downstream cell cycle progression by initiating the transcription of cell cycle-related target genes.10,11 For example, in breast cancer cells that endogenously express ERα, ERβ overexpression prevents proliferation by inhibiting cyclin D1 expression and activating p21 and p27 expression to induce G2 cell cycle arrest.8 In malignant pleural mesothelioma cells, ERβ functions as a tumor suppressor and its activation sensitizes tumor cells to cisplatin.12 Moreover, ERβ expression has been revealed to be regulated by the AKT1/SIRT1/FOXM1 axis, while activated ERβ can inhibit AKT1 signaling, thus demonstrating an inhibitory feedback loop for ERβ.13
ERα and ERβ have been identified in both healthy human bone cells and osteosarcoma cells,14–16 and ERα and ERβ are stably expressed at a 1:4 ratio in the osteosarcoma cell line U2-OS.17 However, in the osteosarcoma cell line 143B, which has high metastatic ability, only ERβ expression was detected.18 In recent years, estrogen and its nuclear receptors have attracted widespread attention as potential targets for treating osteosarcoma. In the highly metastatic osteosarcoma cell line 143B, inhibition of cell proliferation by the 17-e-diol derivative 2-ME was more prominent when higher doses were used, and the estrogen inhibitor fulvestrant inhibited cell growth at high concentrations. Intriguingly, fulvestrant down-regulated ERβ expression, while 2-ME enhanced ERβ expression.18 Therefore, the specific molecular mechanisms by which ERβ inhibits tumorigenesis in osteosarcoma cells are unclear. Recently, we reported that ERβ exerts antitumor effects in osteosarcoma U2-OS cells that are reliant on the roles of ERβ in regulating integrin, IAP, and the Nuclear factor-κB (NF-kB)/BCL-2 and phosphoinositide 3-kinase (PI3K)/AKT (protein kinase B) signaling pathways.19 Here, we review the molecular mechanisms by which ERβ induces apoptosis and autophagy in a variety of tumors to further delineate more specific molecular mechanisms underlying osteosarcoma tumorigenesis and pathogenesis, as this might help pave the way for targeting ERβ to treat osteosarcoma and reduce mortality rates.
Overview of the molecular mechanisms by which ERβ inhibits tumor cell proliferation and metastasis
Generally, cell death is predominantly induced by apoptosis and autophagy, but other processes like necrosis, aging, and karyokinesis also result in cell death. Studies have confirmed that ERβ plays a role in inducing apoptosis in various tissues. In estrogen-treated mouse mammary cells, ERα promotes cell proliferation, whereas ERβ inhibits cell growth and induces apoptosis.20 In human prostate cancer cells, ERβ induces apoptosis by enhancing the transcription of FOXO3a, which in turn elevates p53 upregulated modulator of apoptosis (PUMA) expression.21 In nude mice, the ERβ activator diosgenin can inhibit the growth of prostate cancer xenografts.22
Autophagy is another mechanism by which cell death occurs. Autophagy is usually activated in cells under stress conditions.23 During autophagy, redundant proteins and/or organelles that do not affect survival are phagocytosed in double- or multi-layered vesicles to form autophagosomes. Subsequently, lysosomes are fused with autophagosomes and release proteases to degrade the contents of the autophagosomes.24 Autophagy is a double-edged sword, such that it can be both beneficial and detrimental. Under normal environmental conditions, healthy cells maintain basal cellular activities and prevent malignant transformation through autophagy. In contrast, during stress, such as hypoxia or starvation, tumor cells can be controlled by autophagy pathways and are more likely to survive than healthy cells.25 This also applies to conditions related to cancer chemotherapy, in which tumor cells can evade anticancer drugs through autophagy and become drug resistant.25 It has been reported that autophagy suppresses tumors in most breast, uterine, and prostate cancers.26 Studies have shown that estrogen receptors also induce autophagy. For instance, in hormone-resistant breast cancer cells, ERβ agonists reduce Bcl-2 expression and activate autophagy.27 In Hodgkin's lymphoma, ERβ activation induces autophagy, inhibits proliferation, and causes cell cycle arrest.28
Recently, complex interactions between autophagy and apoptosis have been uncovered. Multiple stresses activate both autophagy and apoptosis, which share multiple upstream and downstream regulatory molecules, and thus can be mutually transformed.29 In osteosarcoma cells, lignin DPT can simultaneously induce apoptosis and autophagy. DPT induces autophagy by inhibiting activation of the PI3K/AKT/mammalian target of rapamycin (mTOR) pathway and blocking tumor cell apoptosis. In contrast, the autophagy inhibitor 3-methyladeine (3-MA) reverses this effect and promotes apoptosis.30 An in-depth study of the interactions between autophagy and apoptosis will reveal mechanisms underlying the pathogenesis of various diseases and tumorigenesis of various cancers, shedding new light on methods to treat these cancers and other diseases.
Molecular mechanisms of ERβ-induced apoptosis in tumor cells
ERβ induces apoptosis by regulating expression of the anti-apoptotic IAP proteins
The anti-apoptotic proteins inhibitors of apoptosis proteins (IAP) are a class of functional proteins that bind and inhibit caspases to prevent cell death.31 A mixture of the anticancer drugs mistletoe and triterpene inhibits IAP expression in osteosarcoma cells and synergistically induces apoptosis.32 Decreased expression of the IAP family protein X-linked inhibitor of apoptosis (X-IAP) inhibits proliferation and induces apoptosis in osteosarcoma cells.33 Microarray analysis showed that ERβ regulates expression of the IAP family protein SURVIVIN in human breast cancer cells.10 Moreover, ERβ regulates expression of the IAP family protein cIAP2 in epithelial colorectal cancer cells.34
ERβ induces apoptosis by regulating the NF-κB/BCL-2 pathway
NF-κB is a pro-inflammatory factor that is involved in a variety of cellular processes including proliferation, differentiation, apoptosis, and inflammation.35 It has been reported that estrogen receptors are associated with NF-κB signaling pathways in tumor cells. In bladder cancer cells, ERβ levels are negatively correlated with nuclear p65 levels.36 NF-κB directly regulates transcription of the anti-apoptotic factor BCL-2; thus, the NF-κB/BCL-2 pathway is thought to play an important role in tumorigenesis and apoptosis.37 Immunohistochemical analysis showed that the occurrence of endometriosis-associated tumors correlated with high BCL-2 expression and decreased expression of estrogen receptors.38 In hormone-resistant breast cancer cells, ERβ agonists reduce BCL-2 expression and activate autophagy.27
ERβ regulates expression of proapoptotic factor BAX
The pro-apoptotic protein BAX is a member of the BCL-2 gene family and forms a heterodimer with BCL-2 to function as a pro-apoptotic factor.39 After opening the mitochondrial voltage-dependent anion channel, BAX releases cytochrome C to force cells to enter the apoptotic program.40 In clinical studies of non-small cell lung cancer, high ERβ2 and BAX expression were positively correlated with patient survival.41 Moreover, artificial introduction of ERβ into prostate cancer cells that do not express estrogen receptors can upregulate BAX expression and induce apoptosis.42
ERβ regulates caspase-3 expression
Caspase-3 is a key regulator of apoptosis that specifically catalyzes the cleavage of many important cellular proteins.43,44 A biomarker of apoptosis, caspase-3 is essential for the chromatin condensation and DNA fragmentation during apoptosis.44 The phytoestrogens genistein and apigenin inhibit proliferation of prostate cancer and breast cancer cells by activating caspase-3 and promoting apoptosis. Luciferase reporter assays and knockdown experiments have indicated that apigenin specifically activates caspase-3 mRNA transcription through ERβ, while genistein activates caspase-3 transcription through both ERα and ERβ.45 In prostate cancer cells, diosgenin induces apoptosis by activating ERβ to regulate caspase-3 expression.22 Artificial expression of ERβ in prostate cancer cells that do not express nuclear estrogen receptors promote the expression of caspase-3 and induce apoptosis.42 In colon cancer cells, nitric oxide inhibits ERβ activity and down-regulates caspase-3 expression, preventing estrogen-induced apoptosis.46
ERβ regulates PARP expression
The primary role of Poly (ADP-ribose) polymerase 1 (PARP) is to detect breaks in single-stranded DNA and induce stress responses to repair DNA in cells.47 PARP uses nicotinamide adenine dinucleotide (NAD) as a donor to attach ADP-ribose to various nuclear proteins.48 As PARP is activated by binding to the ends of DNA strands or strand breaks, it is believed that PARP causes cell death by depleting NAD and ATP in cells.49 During apoptosis, caspase-3 is primarily responsible for cleaving PARP at a highly evolutionarily conserved cleavage site, indicating that PARP cleavage plays an important role in apoptosis.50 In breast cancer cells that express estrogen receptors, isoflavones induce apoptosis by increasing ERβ expression to initiate PARP cleavage.51 Furthermore, artificial expression of ERβ in estrogen receptor-deficient prostate cancer cells promote PARP expression and accelerate apoptosis.42
Molecular mechanisms of ERβ-induced autophagy in tumor cells
ERβ induces autophagy by regulating LC3-I/II expression
LC3 is a microtubule-associated protein that is constitutively expressed in mammalian tissues. During autophagy, LC3-I, the cytosolic form of LC3, binds to phosphatidylethanolamine to form LC3-II, which is transported to autophagosome membranes.52 When autophagosomes are fused to lysosomes to form autophagosomes, LC3-II in autophagosomes is degraded.52 Thus, the relative ratio of LC3-I/LC3-II expression can be used to monitor autophagy progression.53 In Hodgkin's lymphoma cells that are treated with lysosomal protease inhibitors, the ERβ agonist DPN enhances LC3-II expression. This suggests that ERβ induces autophagy to promote autophagosome formation and causes LC3-II formation even when the lysis function of lysosomes is inhibited.28
ERβ regulates p62 expression
P62 is a cytoskeletal protein with a ubiquitin-binding domain that has been found to co-localize with ubiquitinated protein aggregates in many neuropathic and liver diseases.54 LC3 and the GABA type A receptor-associated protein (GABARAP) family proteins recognize and bind to specific sequences in p62. During autophagy, p62 recognizes toxic cellular waste, which is engulfed by autophagosomes and degraded by lysosomes.55 When autophagy is inhibited, p62 and ubiquitinated protein aggregates in the cell accumulate, and when autophagy is activated, p62 levels continuously decrease.54,55 Thus, p62 is used as a marker to study autophagic flow in cells. In choriocarcinoma cells, reactive oxygen species regulate the transition of methotrexate-induced apoptosis to autophagy through the JNK/p62 pathway, which results in the resistance of choriocarcinoma to methotrexate.56 ERα overexpression in breast cancer cells with endogenous ERα expression has been reported to enhance p62 expression and activate autophagy.57 However, the role of ERβ and p62 in autophagy has not been reported in the literature so far, and more extensive investigations are needed to explore their possible link.
ERβ induces autophagy by inhibiting the PI3K/AKT/mTOR pathway
The protein kinase mTOR is the primary regulator of autophagy. mTOR receives signals from various pathways, especially those related to the cellular energy state and the initiation or arrest of protein synthesis.58 mTOR forms two complexes, mammalian target of rapamycin complex 1 (mTORC1) and complex 2 (mTORC2), which have different protein compositions.59 The PI3K/AKT signaling pathway is a major upstream regulator of mTORC1 and is normally activated by cell growth factors to promote cell survival and inhibit apoptosis in various cell types.60,61 mTORC2 is involved in AKT phosphorylation.59,60 Activation of AKT can lead to the phosphorylation of BAD,62 inactivation of caspase-9,63 inhibition of the nuclear transfer of the transcription factor FKHRL1 (which regulates the transcription of cell death genes),64 and enhancement of mTOR activity,65 thereby inhibiting apoptosis. In the case of starvation or environmental stress, inhibition of mTOR activity leads to the activation of the autophagy-activated kinase ULK1 and autophagosome formation.66 Additionally, immunosuppressive drugs and rapamycin, which inhibits mTOR, initiates autophagy and autophagosome formation.66 Overall, activation of the PI3K/AKT/mTOR pathway increases cell viability and prevents cell death caused by excessive autophagy, while inhibition of mTORC1 activity induces autophagy to clean up toxic waste in cells. Meanwhile, mTORC2 regulates AKT activity to increase cell viability.67 The PI3K/AKT/mTOR pathway is associated with the estrogen receptor signaling pathway, and the downstream target gene p70S6K of mTORC1 negatively regulates AKT and activates estrogen receptors through phosphorylation. Moreover, in breast cancer cells, p70S6K overexpression has been reported to activate estrogen receptors.68,69 In ERα-positive breast cancer, arctigenin, a member of the Asteraceae family, has been shown to inhibit mTOR pathway activation, resulting in decreased ER expression and increased autophagic cell death.69 Additionally, arctigenin has also been reported to function as a selective agonist of ERβ to restrict mTORC1 activation in T cell lines;70 thus, it is plausible that agonist-mediated activation of ERβ can inhibit mTORC1 to induce autophagic cell death in tumors.
ERβ induces autophagy by activating the AMPK pathway
In mammalian cells, the protein kinase AMPK senses ATP levels, thereby sensing the cellular energy status.24,71 When the ATP/AMP ratio in cells decreases, AMPK is activated by the upstream protein kinase LKB1.71 Activated AMPK phosphorylates and activates the TSC1/2 complex, which inhibits mTOR activity via Rheb, thereby initiating autophagy.72 Autophagy leads to the reuse of nutrients in cells, which enhances ATP production and restores a normal ATP/AMP ratio.71 Conversely, the LKB1–AMPK pathway leads to the phosphorylation and activation of the cyclin-dependent kinase inhibitor p27kip1.73 Under conditions of growth factor withdrawal and nutrient deprival, p27kip1 responds to stress by inducing autophagy to prevent cells from entering the apoptotic process, allowing survival.73 Several studies have shown that estradiol activates AMPK by enhancing phosphorylation of the alpha catalytic subunit (AMPKα) of AMPK.74–76 In breast cancer and cardiomyocytes, ERα directly binds to AMPKα, and both ERα and ERβ interact with LKB1, which is upstream of AMPK.77 In castrated male mice, testosterone activates the expression of the autophagosome-forming marker ALP and induces TSC2 expression to activate AMPKα, while ERα is downregulated and ERβ expression is enhanced in muscle cells.78 These findings demonstrate that ERβ induces autophagy by activating the AMPK pathway.
Conclusions
ERβ exhibits antitumor effects in different tumor types. ERβ regulates the apoptosis-related proteins IAP, BAX, caspase-3, and PARP, and influences NF-κB/BCL-2 signaling to induce apoptosis (Figure 1). ERβ is also involved in the induction of autophagy by inhibiting the PI3K/AKT/mTOR pathway and activating the AMPK pathway (Figure 2). Our previous report revealed that ERβ exerts antitumor effects in osteosarcoma U2-OS cells through the NF-κB/BCL-2 and PI3K/AKT/mTOR pathways.19 Considering the broad involvement of ERβ in the linked processes of apoptosis, autophagy, it is plausible that the critical role of ERβ in inhibiting the proliferation and metastasis of osteosarcoma cells is closely related to its regulation of apoptosis and autophagy.
Figure 1.
Possible mechanisms of ERβ-induced apoptosis in tumor cells. Estrogen/ERβ signaling has been shown to regulate the apoptosis-related proteins IAP, BAX, caspase-3, and PARP, and to act on the NF-κB/BCL-2 signaling pathway to induce apoptosis in tumor cells. ERβ, estrogen receptor β; IAP, inhibitors of apoptosis proteins; PARP, Poly (ADP-ribose) polymerase 1.
Figure 2.
Possible mechanisms of ERβ-induced autophagy in tumor cells. Estrogen/ERβ signaling regulates expression of the autophagy-associated markers LC3-I/LC-3II and p62, and is involved in autophagy induction in tumors cells by inhibiting the PI3K/AKT/mTOR pathway and activating the AMPK pathway. ERβ, estrogen receptor β; AMPK, 5' AMP-activated protein kinase; PI3K, phosphoinositide 3-kinase; AKT, protein kinase B; mTOR, mammalian target of rapamycin; NF-κB, nuclear factor κ-light-chain-enhancer of activated B cells; BCL-2, B-cell lymphoma 2.
Abbreviations
ERα (estrogen receptors α);
ERβ (estrogen receptors β);
EMT (epithelial–mesenchymal transition);
PI3K (phosphoinositide 3-kinase);
mTOR (mammalian target of rapamycin);
3-MA (3-methyladeine);
PUMA (p53 upregulated modulator of apoptosis);
IAP (inhibitors of apoptosis proteins);
X-IAP (X-linked inhibitor of apoptosis);
NF-κB (Nuclear factor-κB);
PARP (Poly (ADP-ribose) polymerase);
NAD (nicotinamide adenine dinucleotide);
GABARAP (GABA type A receptor-associated protein);
BCL-2 (B cell lymphoma/leukemia-2);
BAX (Bcl-2 associated X protein);
AMPK (adenosine monophosphate-activated protein kinase)
Authors' contributions
Yang Z wrote the manuscript. Tao H critically revised the manuscript. Yang M and Yu W sourced the literature and wrote the first draft of the manuscript. All authors have read and approved the final version of the manuscript.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1.Edwards D. The role of coactivators and corepressors in the biology and mechanism of action of steroid hormone receptors. J Mammary Gland Biol Neoplasia 2000; 5: 307–324. [DOI] [PubMed] [Google Scholar]
- 2.Delaunay F, Pettersson K, Tujague M, et al. Functional differences between the amino-terminal domains of estrogen receptors alpha and beta. Mol Pharmacol 2000; 58: 584–590. [DOI] [PubMed] [Google Scholar]
- 3.Hall J, McDonnell D. The estrogen receptor beta-isoform (ERbeta) of the human estrogen receptor modulates ERalpha transcriptional activity and is a key regulator of the cellular response to estrogens and antiestrogens. Endocrinology 1999; 140: 5566–5578. [DOI] [PubMed] [Google Scholar]
- 4.Mak P, Leav I, Pursell B, et al. ERβ impedes prostate cancer EMT by destabilizing HIF-1α and inhibiting VEGF-mediated snail nuclear localization: implications for Gleason grading. Cancer Cell 2010; 17: 319–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gustafsson JÅ, Ström A. Antiproliferative and pro-apoptotic actions of oestrogen receptor β in prostate cancer. Hamdan Med J 2014; 7: 403–410. [Google Scholar]
- 6.Schüler-Toprak S, Moehle C, Skrzypczak M, et al. Effect of estrogen receptor β agonists on proliferation and gene expression of ovarian cancer cells. BMC Cancer 2017; 17: 319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pinton G, Nilsson S, Moro L. Targeting estrogen receptor beta (ERβ) for treatment of ovarian cancer: importance of KDM6B and SIRT1 for ERβ expression and functionality. Oncogenesis 2018; 7: 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Paruthiyil S, Parmar H, Kerekatte V, et al. Estrogen receptor β inhibits human breast cancer cell proliferation and tumor formation by causing a G2 cell cycle arrest. Cancer Res 2004; 64: 423–428. [DOI] [PubMed] [Google Scholar]
- 9.Ström A, Hartman J, Foster JS, et al. Estrogen receptor β inhibits 17β-estradiol-stimulated proliferation of the breast cancer cell line T47D. Proc Natl Acad Sci U S A 2004; 101: 1566–1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chang EC, Frasor J, Komm B, et al. Impact of estrogen receptor β on gene networks regulated by estrogen receptor α in breast cancer cells. Endocrinology 2006; 147: 4831–4842. [DOI] [PubMed] [Google Scholar]
- 11.Vivar OI, Zhao X, Saunier EF, et al. Estrogen receptor [beta] binds to and regulates three distinct classes of target genes. J Biol Chem 2010; 285: 22059–22066. DOI: 10.1074/jbc.M110.114116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pinton G, Manente AG, Daga A, et al. Agonist activation of estrogen receptor beta (ERbeta) sensitizes malignant pleural mesothelioma cells to cisplatin cytotoxicity. Mol Cancer 2014; 13: 227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pinton G, Zonca S, Manente AG, et al. SIRT1 at the crossroads of AKT1 and ERbeta in malignant pleural mesothelioma cells. Oncotarget 2016; 7: 14366–14379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen FP, Hsu T, Hu CH, et al. Expression of estrogen receptors alpha and beta in human osteoblasts: identification of exon-2 deletion variant of estrogen receptor beta in postmenopausal women. Chang Gung Med J 2004; 27: 107–115. [PubMed] [Google Scholar]
- 15.Dohi O, Hatori M, Suzuki T, et al. Sex steroid receptors expression and hormone‐induced cell proliferation in human osteosarcoma. Cancer Sci 2008; 99: 518–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Manolagas SC, O'brien CA, Almeida M. The role of estrogen and androgen receptors in bone health and disease. Nat Rev Endocrinol 2013; 9: 699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Monroe DG, Secreto FJ, Subramaniam M, et al. Estrogen receptor α and β heterodimers exert unique effects on estrogen-and tamoxifen-dependent gene expression in human U2OS osteosarcoma cells. Mol Endocrinol 2005; 19: 1555–1568. [DOI] [PubMed] [Google Scholar]
- 18.Gorska M, Wyszkowska RM, Kuban-Jankowska A, et al. Impact of apparent antagonism of estrogen receptor β by fulvestrant on anticancer activity of 2-methoxyestradiol. Anticancer Res 2016; 36: 2217–2226. [PubMed] [Google Scholar]
- 19.Yang M, Liu B, Jin L, et al. Estrogen receptor beta exhibited anti-tumor effects on osteosarcoma cells by regulating integrin, IAP, NF-kB/BCL-2 and PI3K/Akt signal pathway. J Bone Oncol 2017; 9: 15–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Helguero L, Faulds M, Gustafsson J, et al. Estrogen receptors alfa (ERalpha) and beta (ERbeta) differentially regulate proliferation and apoptosis of the normal murine mammary epithelial cell line HC11. Oncogene 2005; 24: 6605–6616. [DOI] [PubMed] [Google Scholar]
- 21.Dey P, Ström A, Gustafsson J. Estrogen receptor β upregulates FOXO3a and causes induction of apoptosis through PUMA in prostate cancer. Oncogene 2014; 33: 4213–4225. [DOI] [PubMed] [Google Scholar]
- 22.Tao X, Xu L, Yin L, et al. Dioscin induces prostate cancer cell apoptosis through activation of estrogen receptor-β. Cell Death Dis 2017; 8: e2989. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 23.Yang Z, Klionsky D. Mammalian autophagy: core molecular machinery and signaling regulation. Curr Opin Cell Biol 2010; 22: 124–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tooze S, Yoshimori T. The origin of the autophagosomal membrane. Nat Cell Biol 2010; 12: 831–835. [DOI] [PubMed] [Google Scholar]
- 25.Doria A, Gatto M, Punzi L. Autophagy in human health and disease. N Engl J Med 2013; 368: 1845. [DOI] [PubMed] [Google Scholar]
- 26.Liang XH, Jackson S, Seaman M, et al. Induction of autophagy and inhibition of tumorigenesis by beclin 1. Nature 1999; 402: 672–676. [DOI] [PubMed] [Google Scholar]
- 27.Ruddy S, Lau R, Cabrita M, et al. Preferential estrogen receptor β ligands reduce Bcl-2 expression in hormone-resistant breast cancer cells to increase autophagy. Mol Cancer Ther 2014; 13: 1882–1893. [DOI] [PubMed] [Google Scholar]
- 28.Pierdominici M, Maselli A, Locatelli S, et al. Estrogen receptor β ligation inhibits Hodgkin lymphoma growth by inducing autophagy. Oncotarget 2017; 8: 8522–8535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bincoletto C, Bechara A, Pereira G, et al. Interplay between apoptosis and autophagy, a challenging puzzle: new perspectives on antitumor chemotherapies. Chem Biol Interact 2013; 206: 279–288. [DOI] [PubMed] [Google Scholar]
- 30.Kim S, Son K, Kim K, et al. Deoxypodophyllotoxin induces cytoprotective autophagy against apoptosis via inhibition of PI3K/AKT/mTOR pathway in osteosarcoma U2OS cells. Pharmacol Rep 2017; 69: 878–884. [DOI] [PubMed] [Google Scholar]
- 31.Wei Y, Fan T, Yu M. Inhibitor of apoptosis proteins and apoptosis. Acta Biochim Biophys Sin (Shanghai) 2008; 40: 278–288. [DOI] [PubMed] [Google Scholar]
- 32.Kleinsimon S, Kauczor G, Jaeger S, et al. ViscumTT induces apoptosis and alters IAP expression in osteosarcoma in vitro and has synergistic action when combined with different chemotherapeutic drugs. BMC Complement Altern Med 2017; 17: 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mirandola P, Sponzilli I, Gobbi G, et al. Anticancer agents sensitize osteosarcoma cells to TNF-related apoptosis-inducing ligand downmodulating IAP family proteins. Int J Oncol 2006; 28: 127–133. [PubMed] [Google Scholar]
- 34.Paruthiyil S, Cvoro A, Zhao X, et al. Drug and cell type-specific regulation of genes with different classes of estrogen receptor beta-selective agonists. PLoS One 2009; 4: e6271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xu M, Chen X, Han Y, et al. Clusterin silencing sensitizes pancreatic cancer MIA-PaCa-2 cells to gmcitabine via regulation of NF-kB/Bcl-2 signaling. Int J Clin Exp Med 2015; 8: 12476–12486. [PMC free article] [PubMed] [Google Scholar]
- 36.Kontos S, Kominea A, Melachrinou M, et al. Inverse expression of estrogen receptor-beta and nuclear factor-kappaB in urinary bladder carcinogenesis. Int J Urol 2010; 17: 801–809. [DOI] [PubMed] [Google Scholar]
- 37.Yan M, Xu Q, Zhang P, et al. Correlation of NF-kappaB signal pathway with tumor metastasis of human head and neck squamous cell carcinoma. BMC Cancer 2010; 10: 437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Haidarali E, Vahedi A, Mohajeri S, et al. Evaluation of the Pathogenesis of Tumor Development from Endometriosis by Estrogen Receptor, P53 and Bcl-2 Immunohistochemical Staining. Asian Pac J Cancer Prev 2016; 17: 5247–5250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Oltvai Z, Milliman C, Korsmeyer S. Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell 1993; 74: 609–619. [DOI] [PubMed] [Google Scholar]
- 40.Berens H, Tyler K. The proapoptotic Bcl-2 protein Bax plays an important role in the pathogenesis of reovirus encephalitis. J Virol 2011; 85: 3858–3871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu J, Nie Z, Lei Y, et al. The expression of ERβ2, Bcl-xl and Bax in non-small cell lung cancer and associated with prognosis. Int J Clin Exp Pathol 2017; 10: 10040–10046. [PMC free article] [PubMed] [Google Scholar]
- 42.Cheng J, Lee E, Madison L, et al. Expression of estrogen receptor beta in prostate carcinoma cells inhibits invasion and proliferation and triggers apoptosis. FEBS Lett 2004; 566: 169–172. [DOI] [PubMed] [Google Scholar]
- 43.Gervais F, Xu D, Robertson G, et al. Involvement of caspases in proteolytic cleavage of Alzheimer's amyloid-beta precursor protein and amyloidogenic A beta peptide formation. Cell 1999; 97: 395–406. [DOI] [PubMed] [Google Scholar]
- 44.Porter A, Jänicke R. Emerging roles of caspase-3 in apoptosis. Cell Death Differ 1999; 6: 99–104. [DOI] [PubMed] [Google Scholar]
- 45.Mak P, Leung Y, Tang W, et al. Apigenin suppresses cancer cell growth through ERbeta. Neoplasia 2006; 8: 896–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Marino M, Galluzzo P, Leone S, et al. Nitric oxide impairs the 17beta-estradiol-induced apoptosis in human colon adenocarcinoma cells. Endocr Relat Cancer 2006; 13: 559–569. [DOI] [PubMed] [Google Scholar]
- 47.Berger N, Petzold S. Identification of minimal size requirements of DNA for activation of poly(ADP-ribose) polymerase. Biochemistry 1985; 24: 4352–4355. [DOI] [PubMed] [Google Scholar]
- 48.Boulares A, Yakovlev A, Ivanova V, et al. Role of poly(ADP-ribose) polymerase (PARP) cleavage in apoptosis. Caspase 3-resistant PARP mutant increases rates of apoptosis in transfected cells. J Biol Chem 1999; 274: 22932–22940. [DOI] [PubMed] [Google Scholar]
- 49.Berger N. Poly(ADP-ribose) in the cellular response to DNA damage. Radiat Res 1985; 101: 4–15. [PubMed] [Google Scholar]
- 50.Le Rhun Y, Kirkland J, Shah G. Cellular responses to DNA damage in the absence of Poly(ADP-ribose) polymerase. Biochem Biophys Res Commun 1998; 245: 1–10. [DOI] [PubMed] [Google Scholar]
- 51.Chen J, Zhao X, Ye Y, et al. Estrogen receptor beta-mediated proliferative inhibition and apoptosis in human breast cancer by calycosin and formononetin. Cell Physiol Biochem 2013; 32: 1790–1797. [DOI] [PubMed] [Google Scholar]
- 52.Tanida I, Ueno T, Kominami E. LC3 and autophagy. Methods Mol Biol 2008; 445: 77–88. [DOI] [PubMed] [Google Scholar]
- 53.Klionsky D, Abdelmohsen K, Abe A, et al. Guidelines for the use and interpretation of assays for monitoring autophagy (3rd edition). Autophagy 2016; 12: 1–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bjørkøy G, Lamark T, Pankiv S, et al. Monitoring autophagic degradation of p62/SQSTM1. Meth Enzymol 2009; 452: 181–197. [DOI] [PubMed] [Google Scholar]
- 55.Rusten T, Stenmark H. p62, an autophagy hero or culprit? Nat Cell Biol 2010; 12: 207–209. [DOI] [PubMed] [Google Scholar]
- 56.Shen Y, Yang J, Zhao J, et al. The switch from ER stress-induced apoptosis to autophagy via ROS-mediated JNK/p62 signals: a survival mechanism in methotrexate-resistant choriocarcinoma cells. Exp Cell Res 2015; 334: 207–218. [DOI] [PubMed] [Google Scholar]
- 57.Felzen V, Hiebel C, Koziollek-Drechsler I, et al. Estrogen receptor alpha regulates non-canonical autophagy that provides stress resistance to neuroblastoma and breast cancer cells and involves BAG3 function. Cell Death Dis 2015; 6: e1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cuyàs E, Corominas-Faja B, Joven J, et al. Cell cycle regulation by the nutrient-sensing mammalian target of rapamycin (mTOR) pathway. Methods Mol Biol 2014; 1170: 113–144. [DOI] [PubMed] [Google Scholar]
- 59.Bhaskar P, Hay N. The two TORCs and Akt. Dev Cell 2007; 12: 487–502. [DOI] [PubMed] [Google Scholar]
- 60.Dudek H, Datta S, Franke T, et al. Regulation of neuronal survival by the serine-threonine protein kinase Akt. Science 1997; 275: 661–665. [DOI] [PubMed] [Google Scholar]
- 61.Hay N. The Akt-mTOR tango and its relevance to cancer. Cancer Cell 2005; 8: 179–183. [DOI] [PubMed] [Google Scholar]
- 62.Datta S, Dudek H, Tao X, et al. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell 1997; 91: 231–241. [DOI] [PubMed] [Google Scholar]
- 63.Zhou H, Li XM, Meinkoth J, et al. Akt regulates cell survival and apoptosis at a postmitochondrial level. J Cell Biol 2000; 151: 483–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Brunet A, Bonni A, Zigmond M, et al. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell 1999; 96: 857–868. [DOI] [PubMed] [Google Scholar]
- 65.Li Y, Inoki K, Yeung R, et al. Regulation of TSC2 by 14-3-3 binding. J Biol Chem 2002; 277: 44593–44596. [DOI] [PubMed] [Google Scholar]
- 66.Nazio F, Strappazzon F, Antonioli M, et al. mTOR inhibits autophagy by controlling ULK1 ubiquitylation, self-association and function through AMBRA1 and TRAF6. Nat Cell Biol 2013; 15: 406–416. [DOI] [PubMed] [Google Scholar]
- 67.Heras-Sandoval D, Pérez-Rojas J, Hernández-Damián J, et al. The role of PI3K/AKT/mTOR pathway in the modulation of autophagy and the clearance of protein aggregates in neurodegeneration. Cell Signal 2014; 26: 2694–2701. [DOI] [PubMed] [Google Scholar]
- 68.Shrivastav A, Murphy L. Interactions of PI3K/Akt/mTOR and estrogen receptor signaling in breast cancer. Breast Cancer Manag 2012; 1: 235–249. [Google Scholar]
- 69.Maxwell T, Lee KS, Kim S, et al. Arctigenin inhibits the activation of the mTOR pathway, resulting in autophagic cell death and decreased ER expression in ER-positive human breast cancer cells. Int J Oncol 2018; 52: 1339–1349. [DOI] [PubMed] [Google Scholar]
- 70.Wu X, Tong B, Yang Y, et al. Arctigenin functions as a selective agonist of estrogen receptor beta to restrict mTORC1 activation and consequent Th17 differentiation. Oncotarget 2016; 7: 83893–83906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.He C, Klionsky D. Regulation mechanisms and signaling pathways of autophagy. Annu Rev Genet 2009; 43: 67–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Inoki K, Zhu T, Guan K. TSC2 mediates cellular energy response to control cell growth and survival. Cell 2003; 115: 577–590. [DOI] [PubMed] [Google Scholar]
- 73.Liang J, Shao S, Xu Z, et al. The energy sensing LKB1-AMPK pathway regulates p27(kip1) phosphorylation mediating the decision to enter autophagy or apoptosis. Nat Cell Biol 2007; 9: 218–224. [DOI] [PubMed] [Google Scholar]
- 74.D'Eon T, Rogers N, Stancheva Z, et al. Estradiol and the estradiol metabolite, 2-hydroxyestradiol, activate AMP-activated protein kinase in C2C12 myotubes. Obesity (Silver Spring) 2008; 16: 1284–1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rogers N, Witczak C, Hirshman M, et al. Estradiol stimulates Akt, AMP-activated protein kinase (AMPK) and TBC1D1/4, but not glucose uptake in rat soleus. Biochem Biophys Res Commun 2009; 382: 646–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kim J, Jo K, Kim O, et al. Parenteral 17beta-estradiol decreases fasting blood glucose levels in non-obese mice with short-term ovariectomy. Life Sci 2010; 87: 358–366. [DOI] [PubMed] [Google Scholar]
- 77.Lipovka Y, Chen H, Vagner J, et al. Oestrogen receptors interact with the α-catalytic subunit of AMP-activated protein kinase. Biosci Rep 2015; 35: pii: e00264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Serra C, Sandor N, Jang H, et al. The effects of testosterone deprivation and supplementation on proteasomal and autophagy activity in the skeletal muscle of the male mouse: differential effects on high-androgen responder and low-androgen responder muscle groups. Endocrinology 2013; 154: 4594–4606. [DOI] [PMC free article] [PubMed] [Google Scholar]