Short abstract
Objective
Enhanced recovery after surgery (ERAS) protocols help optimize inpatient care and minimize discomfort. This study was performed to explore the safety, feasibility, and clinical and social value of ERAS in pediatric gastrointestinal surgery.
Methods
This study included all children (n = 125) who underwent appendectomy, pyloromyotomy, transabdominal Soave’s procedure, Meckel’s diverticulum resection, or reduction of intussusception in our institution from January to September 2018. We compared surgical outcomes between children who underwent surgery under conventional perioperative regimens (control group, n = 57) and those who were treated with ERAS protocols (ERAS group, n = 68).
Results
There were no significant intergroup differences in demographic or surgical data. However, the bowel function recovery time, postoperative intravenous nutrition time, duration of postoperative hospital stay, and hospital costs were significantly lower in the ERAS group than control group. There was no significant intergroup difference in the complication rate.
Conclusions
Our results indicate that implementation of ERAS protocols is safe and feasible in pediatric gastrointestinal surgery. They can improve patient comfort, shorten the duration of the postoperative hospital stay, reduce hospital costs, and accelerate postoperative rehabilitation without increasing the risk of postoperative complications. Therefore, ERAS protocols deserve wider implementation and promotion.
Keywords: Enhanced recovery after surgery (ERAS), perioperative period, gastrointestinal surgery, children, surgical outcomes, safety
Introduction
Gastrointestinal disease is one of the more common and highly prevalent disorders in the pediatric population. Most children cannot accurately describe the symptoms of gastrointestinal disease, resulting in rapid aggravation of the situation and a high risk of mortality. Surgery is an effective treatment for this disease. However, surgery causes severe trauma and psychological pressure in both children and their parents. Furthermore, the conventional perioperative management approaches—including prolonged preoperative fasting, use of surgical drains and tubes, and long-term postoperative bed rest—cause pain, aggravate stress responses, and delay the recovery of normal bowel function, thus prolonging the patient’s hospital stay. Therefore, the question of how to help children recover quickly and shorten their hospital stay while improving postsurgical outcomes remains to be addressed.
The concept of enhanced recovery after surgery (ERAS) was first described by the Danish surgeon Henrik Kehlet in the 1990s to reduce the perioperative stress response and organ dysfunction in surgical patients.1 ERAS is not a completely new concept; it is based on evidence-based medical techniques and was derived by combining and optimizing various techniques used in conventional multidisciplinary perioperative management, including surgery, anesthesia, nursing, and nutrition. The central aspects of the optimized clinical pathway throughout the perioperative period include preoperative counseling, limited preoperative fasting, optimal anesthesia, minimally invasive techniques, immediate postoperative oral nutrition and mobilization, and nonroutine use of surgical drains and tubes. ERAS challenges conventional perioperative protocols to optimize inpatient care and minimize patient discomfort. Studies have demonstrated that implementation of ERAS protocols is associated with a decrease in the hospital stay duration and incidence of postoperative complications as well as rapid convalescence.2,3 Literature has also shown that ERAS can improve the 5-year survival rate, safety, and satisfaction of patients with colorectal cancer.4
ERAS has become a topic of intense discussion both in China and abroad. However, most studies on ERAS have focused on surgery in adults; there is a lack of high-quality literature on implementing ERAS protocols in the pediatric population. Children experience more complicated surgical stress responses than do adults. The physical stress response and internal environmental disturbances caused by conventional perioperative management are often more severe in children. Therefore, optimizing perioperative management for pediatric populations is even more important and urgent. After nearly a year of practice, we developed and implemented pediatric ERAS protocols in children undergoing gastrointestinal surgery in our institution. In the present study, we comprehensively evaluated the clinical and social value of ERAS protocols in pediatric gastrointestinal surgery.
Patients and methods
We retrospectively reviewed the data of all pediatric patients (age, 1 month to 14 years) who underwent gastrointestinal surgery at our hospital before (January–April 2018) and after (May–September 2018) the implementation of ERAS protocols. Patients who were managed in accordance with conventional perioperative pathways served as the control group. Five procedures, including both elective and emergency surgeries, were selected for analysis: appendectomy, pyloromyotomy, transabdominal Soave’s procedure, Meckel’s diverticulum resection, and reduction of intussusception. All surgeries were performed by the same group of doctors. Patients with severe infectious shock, hemorrhagic shock, multiple organ dysfunction, or cancer were excluded, as were those older than 14 years of age.
Based on the ERAS Society guidelines for adults as well as previous reports of limited success in the pediatric population,5–12 we developed ERAS protocols that would meet the special needs of children undergoing gastrointestinal surgery in our institution, including preoperative counseling, shortened mechanical bowel preparation, limited preoperative fasting and intravenous fluids, nonroutine use of surgical drains and tubes, immediate postoperative mobilization and feeding, and early removal of nasogastric tubes and urinary tubes (≤24 hours).
Before surgery, the patients or their parents were informed about the ERAS protocols, surgical procedure, and discharge criteria using multimodal health education aids (including cards and multimedia) to assure them that their early discharge would be safe. Appropriate preoperative bowel preparation is critical for patients with Hirschsprung’s disease. To this end, the children in the present study were administered a glycerin enema to induce defecation 3 to 7 days before surgery; they then underwent mechanical bowel preparation the day before surgery. No bowel preparation was needed in the other four procedures. The preoperative fasting time was also shortened; patients in the ERAS group were allowed to have high-carbohydrate beverages within 2 hours, breast milk within 4 hours, and formula milk or solids within 6 hours before surgery. Intraoperatively, all surgeries except pyloromyotomy and reduction of intussusception were performed by minimally invasive operative methods. Nasogastric tubes and urinary tubes were not routinely used in all types of surgery, but indwelling nasogastric tubes were placed preoperatively in children with gastrointestinal obstruction. Postoperatively, oral intake started with clear liquids in the post-anesthesia care unit until postoperative day 1 and advanced to a regular diet (carbohydrates were allowed from awakening to 2 hours after surgery). Nasogastric tubes and urinary tubes were removed within 24 hours after surgery. For children with gastrointestinal obstructions, the indwelling duration of the nasogastric tubes was extended as required based on the amount and color of gastric juice and abdominal signs such as the degree of bloating and bowel sounds. Children were encouraged to begin mobilization on the bed with the help of family members within 2 hours of surgery; they were then advanced to out-of-bed activity upon returning to the normal ward. Consequently, most patients in the ERAS group could tolerate an adequate volume of oral fluids by postoperative day 3, and we used this as the time point for discontinuing fluids and transitioning the patients onto oral medication. Table 1 shows the details of our ERAS protocols.
Table 1.
Components of ERAS protocols for perioperative care.
| Stage | Detailed information of ERAS |
|---|---|
| Preoperative | Detailed preoperative counseling |
| Nonroutine bowel preparation | |
| Avoidance of prolonged fasting | |
| Clear liquids allowed until 2 hours before operation; | |
| Preoperative carbohydrate loading: 5 mL/kg; | |
| Breast milk completed 4 hours before operation; | |
| Formula milk or general diet completed 6 hours before operation. | |
| Intraoperative | Minimally invasive surgery |
| Nonroutine use of surgical drains and tubes | |
| Maintenance of normothermia | |
| Maintenance of near-zero fluid balance: limit crystalloids to 3–5 mL/kg/h | |
| Postoperative | Early mobilization and oral nutrition were started 2 hours postoperatively |
| Oral intake starting with clear liquids in the PACU and advancement to regular diet | |
| Maintenance of near-zero fluid balance | |
| Early removal of nasogastric tubes and urinary tubes (≤24 hours) | |
| Stop intravenous nutrition as soon as possible |
ERAS, enhanced recovery after surgery; PACU, post-anesthesia care unit
The discharge criteria were a good general condition, complete oral nutrition and independent mobility, normal micturition and defecation, and parents’ satisfaction and consent. The discharge was scheduled by a consultant, and all patients’ parents could contact their attending physician online if they needed help after discharge.
The primary outcomes were the postoperative bowel function recovery time, postoperative intravenous nutrition time, duration of postoperative hospital stay, hospitalization expenses, and complications. The patient was required to meet all discharge criteria to be deemed safe for discharge. Two medical interns collected and documented all patient data, including demographic information, clinical characteristics, and primary outcomes. Complications included nausea, vomiting, abdominal distension, intestinal obstruction, urinary retention, infection, anastomotic leakage, and anastomotic stricture. Notably, we defined complications associated with ERAS as those medical problems attributable to a delay in diagnosis and treatment because of early discharge.
Statistical analysis
The data are expressed as percentage and mean ± standard deviation. Statistical significance was determined by Student’s t-test and one-way analysis of variance when comparing two groups. Associations between clinicopathological variables and ERAS were analyzed by Pearson’s chi-squared test. Odds ratios (ORs) were calculated by univariate logistic regression analysis. All statistical analyses were conducted by using SPSS software, version 21.0 (IBM Corp., Armonk, NY, USA). A p value of <0.05 was considered statistically significant.
Ethics
Written informed consent was obtained from the patients’ parents, and the study was approved by the ethics committee of the First Affiliated Hospital of Zhengzhou University. All procedures were in accordance with the ethical standards of the institutional ethics committee and with the 1964 Helsinki declaration and its later amendments. All applicable international, national, and/or institutional guidelines for the care were followed.
Results
We identified 125 children who underwent one of the five selected procedures during the study period. Of these children, 57 (45.6%) underwent surgery under conventional perioperative regimens from January to April 2018 (control group), and 68 (54.4%) were treated with our ERAS protocols from May to September 2018 (ERAS group). Table 2 shows the demographic and clinical data for both groups. There was no significant intergroup difference in age, sex, weight, or surgical data.
Table 2.
Association between clinicopathological variables and ERAS.
| Characteristics |
ERAS |
p | |
|---|---|---|---|
| No | Yes | ||
| Number of patients | 57 (45.6) | 68 (54.4) | |
| Sex | 0.097 | ||
| Male | 40 (32.0) | 39 (31.2) | |
| Female | 17 (13.6) | 29 (23.2) | |
| Age, years | 4.485 ± 0.576 | 5.164 ± 0.533 | 0.389 |
| Weight, kg | 19.949 ± 1.958 | 19.666 ± 1.479 | 0.907 |
| Operation | 0.849 | ||
| Appendectomy | 23 (18.4) 90 | 31 (24.8) | |
| Pyloromyotomy | 5 (4.0) | 8 (6.4) | |
| Transabdominal Soave’s procedure | 18 (14.4) | 17 (13.6) | |
| Meckel’s diverticulum resection | 5 (4.0) | 7 (5.6) | |
| Reduction of intussusception | 6 (4.8) | 5 (4.0) | |
| Operation time, minutes | 158.140 ± 10.551 | 131.040 ± 11.406 | 0.088 |
| Intraoperative hemorrhage, mL | 9.42 ± 1.115 | 13.410 ± 4.524 | 0.431 |
| Time to first exhaust, hours | 36.175 ± 1.775 | 13.618 ± 0.671 | <0.001* |
| Time to first defecation, hours | 44.386 ± 1.819 | 17.176 ± 0.639 | <0.001* |
| Time to removal of gastric tube, hours (hours) | 54.561 ± 2.953 | 14.132 ± 1.032 | <0.001* |
| Time to removal of catheter, hours | 56.211 ± 8.615 | 10.147 ± 0.847 | <0.001* |
| Time to removal of drainage tube, days | 2.386 ± 0.347 | 1.515 ± 0.216 | 0.029 |
| Time to first meal, hours | 39.754 ± 1.980 | 8.074 ± 0.460 | <0.001* |
| Postoperative intravenous nutrition time, days | 5.860 ± 0.201 | 3.412 ± 0.115 | <0.001* |
| Postoperative hospitalization time, days duration (days) | 7.737 ± 0.255 | 4.809 ± 0.276 | <0.001* |
| Hospitalization expenses, RMB | 29028.156 ± 671.130 | 26624.069 ± 640.336 | 0.011 |
| Complications | 3 (2.4) | 4 (3.2) | 1.000 |
Data are presented as n (%) or mean ± standard deviation. ERAS, enhanced recovery after surgery. *p < 0.05.
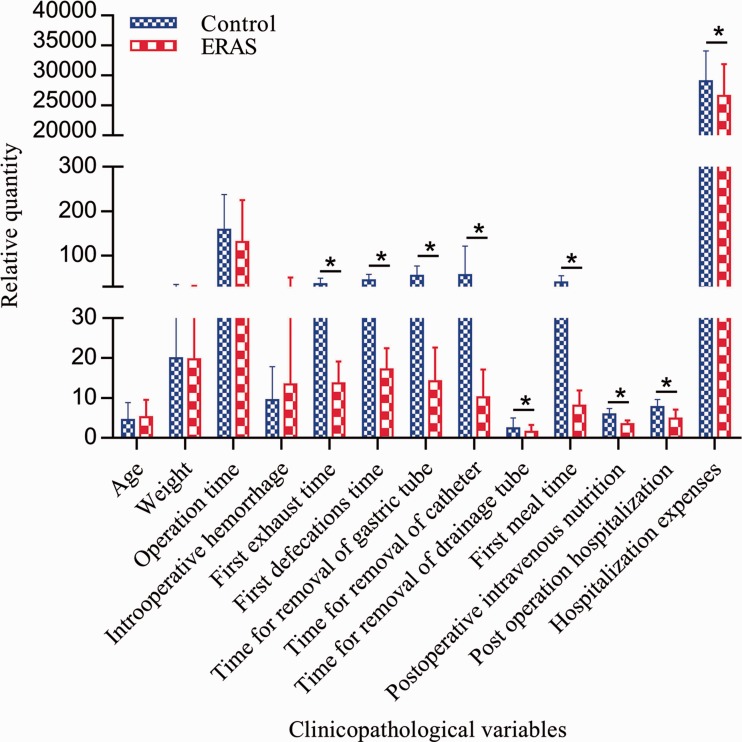
We implemented six ERAS protocols in the ERAS group (Table 1). Relative to the children in the control group, those in the ERAS group realized the benefits of the ERAS protocols in terms of a shorter time to bowel function recovery (p < 0.001), a shorter duration of postoperative hospital stay (p < 0.001), and lower hospitalization expenses (p = 0.11) without a statistically significant increase in the incidence of complications. The average length of postoperative hospital stay (4.809 vs. 7.737 days), time to first exhaust (13.618 vs. 36.175 hours), time to first defecation (17.176 vs. 44.386 hours), and postoperative intravenous nutrition time (3.412 vs. 5.860 days) were significantly shorter in the ERAS group than in the control group (all p < 0.001). Similarly, the hospitalization expenses in the ERAS group were significantly lower than those in the control group (RMB 26624.069 vs. RMB 29028.156, p = 0.11) (Table 2, Figure 1). There was no significant difference in the incidence of complications between the two groups (Table 2).
Figure 1.
Comparison of relative quantity of clinical varieties between ERAS group and control group. ERAS, enhanced recovery after surgery.
Table 3 shows the univariate ORs and 95% confidence intervals (95% CIs) of the clinicopathological variables in both groups. Relative to the control group, the OR for first exhaust time was 0.004 (95% CI, 0.001–0.019; p < 0.001) in the ERAS group. The OR for first defecation time was lower in the ERAS group (0.002; 95% CI, 0.000–0.020; p < 0.001) than in the control group. The analysis also showed that the children in the ERAS group had a shorter postoperative intravenous nutrition time (OR, 0.018; 95% CI, 0.006–0.054; p < 0.001) and duration of postoperative hospitalization (OR, 0.018; 95% CI, 0.005–0.066; p < 0.001) along with lower hospitalization expenses (OR, 0.454; 95% CI, 0.221–0.932; p = 0.031) compared with those in the control group (Table 3).
Table 3.
ERAS and its effects on clinicopathological variables by logistic regression analysis.
| Characteristics |
ERAS |
||
|---|---|---|---|
| No | Yes | ||
| Time to first exhaust | OR | 1 | 0.004 |
| 95% CI | 0.001–0.019 | ||
| p | <0.001* | ||
| Time to first defecation | OR | 1 | 0.002 |
| 95% CI | 0.000–0.020 | ||
| p | <0.001* | ||
| Postoperative intravenous nutrition time | OR | 1 | 0.018 |
| 95% CI | 0.006–0.054 | ||
| p | <0.001* | ||
| Postoperative hospitalization duration | OR | 1 | 0.018 |
| 95% CI | 0.005–0.066 | ||
| p | <0.001* | ||
| Hospitalization expenses | OR | 1 | 0.454 |
| 95% CI | 0.221–0.932 | ||
| p | 0.031* | ||
ERAS, enhanced recovery after surgery; OR, odds ratio; CI, confidence interval. *p < 0.05.
Four instances of postoperative complications occurred in the ERAS group (3.2%): vomiting (n = 2), urinary retention (n = 1), and incision infection (n = 1) (Table 4). The two cases of vomiting occurred after pyloromyotomy: one on day 1 after surgery, when the gastric tube had not yet been removed, and the other after removal of the gastric tube. In both instances, conservative treatment led to relief and full recovery. The lone case of urinary retention in the ERAS group occurred after appendectomy; the symptoms were resolved after insertion of an indwelling urinary catheter. The patient who developed a postoperative wound infection had undergone surgery for reduction of intussusception; the wound healed after debridement and dressing. No problems could be attributed to ERAS. In the control group, three patients developed postoperative complications (2.4%): vomiting (after pyloromyotomy), intestinal obstruction (after appendectomy), and abdominal incisional hernia (after transabdominal Soave’s procedure) (Table 4). The vomiting appeared on day 1 after surgery and resolved spontaneously. The intestinal obstruction required lysis of adhesions 1 month after surgery, while the abdominal incisional hernia required hernioplasty.
Table 4.
Comparison of postoperative complications.
| Control group (n = 57) | ERAS group (n = 68) | |
|---|---|---|
| Vomiting | 1 | 2 |
| Urinary retention | − | 1 |
| Incision infection | − | 1 |
| Intestinal obstruction | 1 | − |
| Incisional defect | 1 | − |
| Total | 3 | 4 |
ERAS, enhanced recovery after surgery
Discussion
The present study showed that ERAS protocols were successfully applied for children undergoing gastrointestinal surgery in our institution. Five types of procedures, including both elective and emergency surgeries, were selected for analysis. To limit the influence of confounding factors, we performed Student’s t-test or Pearson’s chi-squared test on the baseline data of the two groups. This analysis showed that the baseline confounding factors were comparable between the two groups in terms of age, sex, weight, type of procedure, operation time, and intraoperative hemorrhage.
Children’s fears and anxieties generally arise from unfamiliar environments and examinations rather than the severity of their disease. Appropriate psychological counseling and friendly communication with children can help alleviate their psychological stress, allowing them to better tolerate surgery and related treatments. In our study, parents in the ERAS group had a greater awareness of each stage of postoperative recovery and were better able to participate in the perioperative care of their children. A previous study suggested that parents usually overestimate the time it takes to achieve several key milestones in the ERAS process and that there is a need for preoperative education that will allow parents to be more involved in their child’s care.13
The shortened preoperative fasting time in the ERAS group improved patient comfort and helped maintain the stability of the internal environment without any relevant complications such as intraoperative or postoperative regurgitation, aspiration pneumonia, or postoperative gastric retention. In addition, the limited preoperative application of mechanical bowel preparation in children with Hirschsprung’s disease was not associated with a greater risk of wound infection or other complications, which corresponds well with the findings of reports on children undergoing intestinal surgery.14,15 The rapid recovery of gastrointestinal function in the ERAS group may have been due to their early enteral nutrition, mobilization, and receipt of appropriate intravenous fluids and was comparable with the results seen in other studies.16–20 Research has also indicated that exercise can relieve pain and reduce the risk of both cancer and disease recurrence.21,22 Excessive intravenous fluid administration can cause postoperative intestinal edema, which slows the recovery of gastrointestinal function. In the present study, the children in the ERAS group were started on full oral nutrition after a mean duration of 4.809 ± 0.276 days, and intravenous fluid administration was discontinued once the patients could tolerate a regular diet. Furthermore, reasonable use of various tubes, including drains, nasogastric tubes, and urinary catheters, can reduce the incidence of urinary tract infections and promote early mobilization, thereby accelerating the recovery of gastrointestinal function. Similarly, a study by Mattioli et al.23 in 2009 showed that good bowel movement, rapid mobilization, and early feeding can be achieved by avoiding the use of drains, nasogastric tubes, and urinary catheters and by achieving acceptable pain control and limiting the use of systemic opioid drugs. Therefore, a combination of multimodal perioperative interventions rather than a single intervention on its own might contribute to more rapid postoperative recovery.
Minimally invasive surgery is a very important part of ERAS. Compared with open surgery, laparoscopic techniques are associated with less blood loss and pain, better cosmesis, and an earlier discharge from the hospital. A randomized controlled trial of laparoscopy in combination with fast-track multimodal management from The Netherlands showed that laparoscopic surgery was associated with a significantly shorter length of hospital stay than open surgery,24 while the EnROL study from the UK achieved similar results.25 However, a prospective, multicenter, randomized clinical trial published in the New England Journal of Medicine showed that minimally invasive radical hysterectomy was associated with lower rates of disease-free survival and overall survival than open abdominal radical hysterectomy among women with early-stage cervical cancer.26 In our study, the same surgical procedure was used for the same disease, so it is worth investigating which surgical method is most advantageous in children. Actually, regardless of the type of surgery, as long as it can reduce trauma and intraoperative bleeding and shorten the operation time, it will accelerate postoperative rehabilitation. Meanwhile, appropriate narcotic administration and maintenance of normothermia intraoperatively are equally important. Narcotic administration was not standardized and was performed at the discretion of the anesthesia team in our study. Warm mattresses and heaters were used to maintain children’s body temperature at ≥36°C, which was already part of the routine practice for traditional perioperative care in the present study. Studies have shown that perioperative hypothermia is associated with clinical complications such as surgical site infection, delayed wound healing, increased bleeding, or cardiovascular events, and cutaneous warming with an underbody warming system is a feasible and effective method to prevent intraoperative hypothermia during gastrointestinal surgery.27,28
The rapid recovery of gastrointestinal function and administration of less intravenous fluid resulted in significant reductions in the postoperative hospital stay and hospitalization costs. A previous study showed that early discharge might result in economic disadvantages for the hospital,29 which is contrary to our findings. The implementation of ERAS protocols in our hospital not only improved the postsurgical outcomes but also decreased the hospitalization time and medical costs, thus speeding up the bed turnover rate and increasing the efficiency of use of health resources.
Moreover, our ERAS protocols in pediatric gastrointestinal surgery were associated with a tendency toward milder postoperative complications. Compared with the control group, the four complications in the ERAS group were successfully alleviated following conservative treatment. None of these complications was associated with the implementation of ERAS and early discharge. Notably, one patient with intestinal obstruction required lysis of adhesions 1 month after surgery in the control group, while no postoperative intestinal obstruction occurred in the ERAS group. We consider that this lack of intestinal obstruction in the ERAS group is closely related to early oral nutrition and mobilization. Likewise, a recent study showed that the increase in ERAS adherence appears to be associated with a decrease in postoperative complications.30
Compared with an average of 23.8 interventions typically included in the ERAS Society guidelines for adults,5–9 our ERAS protocols involved only 6 interventions. Narcotics administration was not standardized and was performed at the discretion of the anesthesia team. The establishment of a postoperative pain scoring system and standardized analgesia protocol would improve upon our findings. Furthermore, prospective studies with larger samples are necessary to investigate whether additional ERAS protocols are suitable for the pediatric population.
This study has several limitations. First, in clinical practice, it is difficult to accurately estimate the convergence time for consecutive operations. Consequently, the preoperative fasting time cannot be strictly controlled, which might result in prolonged preoperative fasting. Second, this study is limited by its small sample size. As a third-grade, class-A hospital, some of the patients who were transferred here from lower-level hospitals had an advanced stage of disease with serious complications. These patients did not meet the inclusion criteria of our study because in the pilot stage, we were not inclined to perform the ERAS protocols in patients in more severely critical condition and might therefore develop more severe postoperative complications, which could have resulted in performance bias. In the future, we will further improve the study design, collect a larger set of samples, consider more factors, and derive more detailed ERAS protocols for different patient characteristics.
Although the current evidence supports the implementation of ERAS protocols, there is still strong resistance to the application of such protocols for the following reasons. First, the conventional concept of perioperative management is deeply rooted and has become the largest obstacle to the implementation of ERAS protocols.31 Second, the ERAS concept has not been adequately promoted and popularized, and many medical professionals still do not understand it. Finally, implementation of ERAS protocols requires multidisciplinary collaboration. In clinical practice, however, each department is involved in its own operations. A previous study showed that the most important safeguards for successful implementation of ERAS protocols are good organization and coordination by hospital administrators, updating of management philosophy, and innovative management.32
Conclusions
The single-center study demonstrated that ERAS protocols are feasible and safe in children undergoing gastrointestinal surgery. They can improve patient comfort, resulting in a quicker return to gastrointestinal function, less intravenous fluid postoperatively, a shorter postoperative hospitalization duration and lower hospital costs without increasing the risk of complications. These protocols have considerable clinical and economic benefits and deserve further promotion and implementation. Further randomized controlled studies are needed to validate our findings and provide guidance for expanding existing ERAS protocols in terms of the scope of procedures and outcomes of interest.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1.Wilmore DW, Kehlet H. Management of patients in fast track surgery. BMJ 2001; 322: 473–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thiele RH, Rea KM, Turrentine FE, et al. Standardization of care: impact of an enhanced recovery protocol on length of stay, complications, and direct costs after colorectal surgery. J Am Coll Surg 2015; 220: 430–443. [DOI] [PubMed] [Google Scholar]
- 3.Miller TE, Thacker JK, White WD, et al. Reduced length of hospital stay in colorectal surgery after implementation of an enhanced recovery protocol. Anesth Analg 2014; 118: 1052–1061. [DOI] [PubMed] [Google Scholar]
- 4.Asklid D, Segelman J, Gedda C, et al. The impact of perioperative fluid therapy on short-term outcomes and 5-year survival among patients undergoing colorectal cancer surgery - A prospective cohort study within an ERAS protocol. Eur J Surg Oncol 2017; 43: 1433–1439. [DOI] [PubMed] [Google Scholar]
- 5.Gustafsson UO, Scott MJ, Hubner M, et al. Guidelines for Perioperative Care in Elective Colorectal Surgery: enhanced Recovery After Surgery (ERAS®) Society Recommendations: 2018. World J Surg 2019; 43: 659–695. [DOI] [PubMed] [Google Scholar]
- 6.Weimann A, Braga M, Carli F, et al. ESPEN guideline: clinical nutrition in surgery. Clin Nutr 2017; 36: 623–650. [DOI] [PubMed] [Google Scholar]
- 7.Mortensen K, Nilsson M, Slim K, et al. Consensus guidelines for enhanced recovery after gastrectomy: enhanced Recovery After Surgery (ERAS®) Society recommendations. Br J Surg 2014; 101: 1209–1229. [DOI] [PubMed] [Google Scholar]
- 8.Nygren J, Thacker J, Carli F, et al. Guidelines for perioperative care in elective rectal/pelvic surgery: enhanced Recovery After Surgery (ERAS®) Society recommendations. World J Surg 2013; 37: 285–305. [DOI] [PubMed] [Google Scholar]
- 9.Lassen K, Coolsen MM, Slim K, et al. Guidelines for perioperative care for pancreaticoduodenectomy: enhanced Recovery After Surgery (ERAS®) Society recommendations. World J Surg 2013; 37: 240–258. [DOI] [PubMed] [Google Scholar]
- 10.Short HL, Heiss KF, Burch K, et al. Implementation of an enhanced recovery protocol in pediatric colorectal surgery. J Pediatr Surg 2018; 53: 688–692. [DOI] [PubMed] [Google Scholar]
- 11.Vrecenak JD, Mattei P. Fast-track management is safe and effective after bowel resection in children with Crohn's disease. J Pediatr Surg 2014; 49: 99–102; discussion 102–103. [DOI] [PubMed] [Google Scholar]
- 12.Schukfeh N, Reismann M, Ludwikowski B, et al. Implementation of fast-track pediatric surgery in a German nonacademic institution without previous fast-track experience. Eur J Pediatr Surg 2014; 24: 419–425. [DOI] [PubMed] [Google Scholar]
- 13.Jawahar K, Scarisbrick AA. Parental perceptions in pediatric cardiac fast-track surgery. AORN J 2009; 89: 725–731. [DOI] [PubMed] [Google Scholar]
- 14.Serrurier K, Liu J, Breckler F, et al. A multicenter evaluation of the role of mechanical bowel preparation in pediatric colostomy takedown. J Pediatr Surg 2012; 47: 190–193. [DOI] [PubMed] [Google Scholar]
- 15.Leys CM, Austin MT, Pietsch JB, et al. Elective intestinal operations in infants and children without mechanical bowel preparation: a pilot study. J Pediatr Surg 2005; 40: 978–981; discussion 982. [DOI] [PubMed] [Google Scholar]
- 16.Lassen K, Kjaeve J, Fetveit T, et al. Allowing normal food at will after major upper gastrointestinal surgery does not increase morbidity: a randomized multicenter trial. Ann Surg 2008; 247: 721–729. [DOI] [PubMed] [Google Scholar]
- 17.van Barneveld KW, Smeets BJ, Heesakkers FF, et al. Beneficial effects of early enteral nutrition after major rectal surgery: a possible role for conditionally essential amino acids? Results of a randomized clinical trial. Crit Care Med 2016; 44: e353–e361. [DOI] [PubMed] [Google Scholar]
- 18.Adamina M, Kehlet H, Tomlinson GA, et al. Enhanced recovery pathways optimize health outcomes and resource utilization: a meta-analysis of randomized controlled trials in colorectal surgery. Surgery 2011; 149: 830–840. [DOI] [PubMed] [Google Scholar]
- 19.Sun Y, Chai F, Pan C, et al. Effect of perioperative goal-directed hemodynamic therapy on postoperative recovery following major abdominal surgery-a systematic review and meta-analysis of randomized controlled trials. Crit Care 2017; 21: 141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reismann M, Arar M, Hofmann A, et al. Feasibility of fast-track elements in pediatric surgery. Eur J Pediatr Surg 2012; 22: 40–44. [DOI] [PubMed] [Google Scholar]
- 21.Kehlet H, Jensen TS, Woolf CJ. Persistent postsurgical pain: risk factors and prevention. Lancet 2006; 367: 1618–1625. [DOI] [PubMed] [Google Scholar]
- 22.Pedersen L, Idorn M, Olofsson GH, et al. Voluntary running suppresses tumor growth through epinephrine and IL-6-dependent NK cell mobilization and redistribution. Cell Metab 2016; 23: 554–562. [DOI] [PubMed] [Google Scholar]
- 23.Mattioli G, Palomba L, Avanzini S, et al. Fast-track surgery of the colon in children. J Laparoendosc Adv Surg Tech A 2009; 19: S7–S9. [DOI] [PubMed] [Google Scholar]
- 24.Vlug MS, Wind J, Hollmann MW, et al. Laparoscopy in combination with fast track multimodal management is the best perioperative strategy in patients undergoing colonic surgery: a randomized clinical trial (LAFA-study). Ann Surg 2011; 254: 868–875. [DOI] [PubMed] [Google Scholar]
- 25.Kennedy RH, Francis EA, Wharton R, et al. Multicenter randomized controlled trial of conventional versus laparoscopic surgery for colorectal cancer within an enhanced recovery programme: EnROL. J Clin Oncol 2014; 32: 1804–1811. [DOI] [PubMed] [Google Scholar]
- 26.Ramirez PT, Frumovitz M, Pareja R, et al. Minimally invasive versus abdominal radical hysterectomy for cervical cancer. N Engl J Med 2018; 379: 1895–1904. [DOI] [PubMed] [Google Scholar]
- 27.Pu Y, Cen G, Sun J, et al. Warming with an underbody warming system reduces intraoperative hypothermia in patients undergoing laparoscopic gastrointestinal surgery: a randomized controlled study. Int J Nurs Stud 2014; 51: 181–189. [DOI] [PubMed] [Google Scholar]
- 28.Madrid E, Urrútia G, Roqué i Figuls M, et al. Active body surface warming systems for preventing complications caused by inadvertent perioperative hypothermia in adults. Cochrane Database Syst Rev 2016; 4: CD009016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ure BM, Dingemann J, vonWildenradt M, et al. Fast track in der Kinderchirurgie. Monatsschr Kinderheilkd 2013; 161: 131–134. [Google Scholar]
- 30.Ripollés-Melchor J, Ramírez-Rodríguez JM, Casans-Francés R, et al. Association between use of enhanced recovery after surgery protocol and postoperative complications in colorectal surgery: the Postoperative Outcomes Within Enhanced Recovery After Surgery Protocol (POWER) study. JAMA Surg 2019. doi: 10.1001/jamasurg.2019.0995. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Short HL, Taylor N, Thakore M, et al. A survey of pediatric surgeons' practices with enhanced recovery after children's surgery. J Pediatr Surg 2018; 53: 418–430. [DOI] [PubMed] [Google Scholar]
- 32.Li L, Jin J, Min S, et al. Compliance with the enhanced recovery after surgery protocol and prognosis after colorectal cancer surgery: a prospective cohort study. Oncotarget 2017; 8: 53531–53541. [DOI] [PMC free article] [PubMed] [Google Scholar]