Short abstract
Objective
No studies to date have focused on the safety of coloanal/ileoanal anastomosis (CAIAA) in cytoreductive surgery and hyperthermic intraperitoneal chemotherapy (CRS + HIPEC), which is associated with severe morbidity and mortality. We herein present the outcomes of patients with peritoneal carcinomatosis (PC) who underwent CAIAA.
Methods
We evaluated the prospectively collected data from 20 patients with PC who underwent CRS + HIPEC with respect to the primary disease, synchronous resections, intraoperative chemotherapy regimen, timing of protective ileostomy closure, and overall postoperative complications.
Results
Most patients underwent CRS + HIPEC and CAIAA for PC due to colorectal cancer. Coloanal anastomosis was performed in 15 (75%) patients, and J-pouch ileoanal anastomosis was performed in 5 (25%) patients. No anastomosis-related complications occurred in any patients who underwent CAIAA; however, one patient died of pulmonary embolism on postoperative day 7.
Conclusions
CAIAA is associated with serious complications even after performing benign colorectal surgery. However, it may be challenging for surgeons to simultaneously perform CAIAA in patients with PC who undergo CRS + HIPEC. We emphasize that this procedure can be safely performed with experienced surgical teams by using a multidisciplinary approach.
Keywords: Cytoreductive surgery, hyperthermic intraperitoneal chemotherapy, peritoneal carcinomatosis, coloanal/ileoanal anastomosis, colorectal cancer, safety
Introduction
Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy (CRS + HIPEC) is an effective method for the management of peritoneal carcinomatosis (PC) and provides a significant survival benefit. The first step in the CRS + HIPEC procedure is to achieve complete resection of all intra-abdominal tumors with an R0 status, such as those located in the omentum, pancreas, liver, colon, spleen, and gallbladder. During the entire procedure, anastomotic leakage has a significant effect on morbidity and mortality.
Anastomotic leakage continues to be a major concern for colorectal surgeons. Additionally, HIPEC increases the rate of anastomotic leakage. The intestinal anastomosis performed in the CRS and HIPEC procedures is controversial.1 Some studies have shown that HIPEC has adverse effects on the anastomosis, but others have shown that HIPEC does not increase the risk of anastomotic leakage.2 All of these studies evaluated intra-abdominal anastomosis; no studies have focused on the safety of coloanal/ileoanal anastomosis (CAIAA) in CRS + HIPEC, which is associated with severe morbidity and mortality.
CAIAA is a well-described method of sphincter preservation and avoids permanent colostomy for lower localized rectal cancer; however, it is associated with high complication rates.1–5 In this study, we evaluated CAIAA during the CRS + HIPEC procedure performed by two surgeons in a single institution. We herein present the outcomes of patients with PC who underwent CAIAA.
Patients and methods
Of all patients who underwent CRS + HIPEC for PC, patients whose data were prospectively recorded from May 2016 to November 2018 in the University of Health Sciences, Umraniye Training and Research Hospital were retrospectively analyzed. Of these, patients who underwent manual CAIAA were included in the study. The study was approved by the local ethics committee (150/2018). Written and verbal informed consent was obtained from all participating patients.
All patients who underwent CRS + HIPEC were evaluated based on age, sex, American Society of Anesthesiologists score, primary disease, synchronous resections, intraoperative chemotherapy regimen, concurrent synchronous resections, adjuvant chemotherapy/radiotherapy, timing of protective ileostomy closure, early and late complications of CAIAA, and overall postoperative complications.
Surgical procedure for CRS + HIPEC and CAIAA
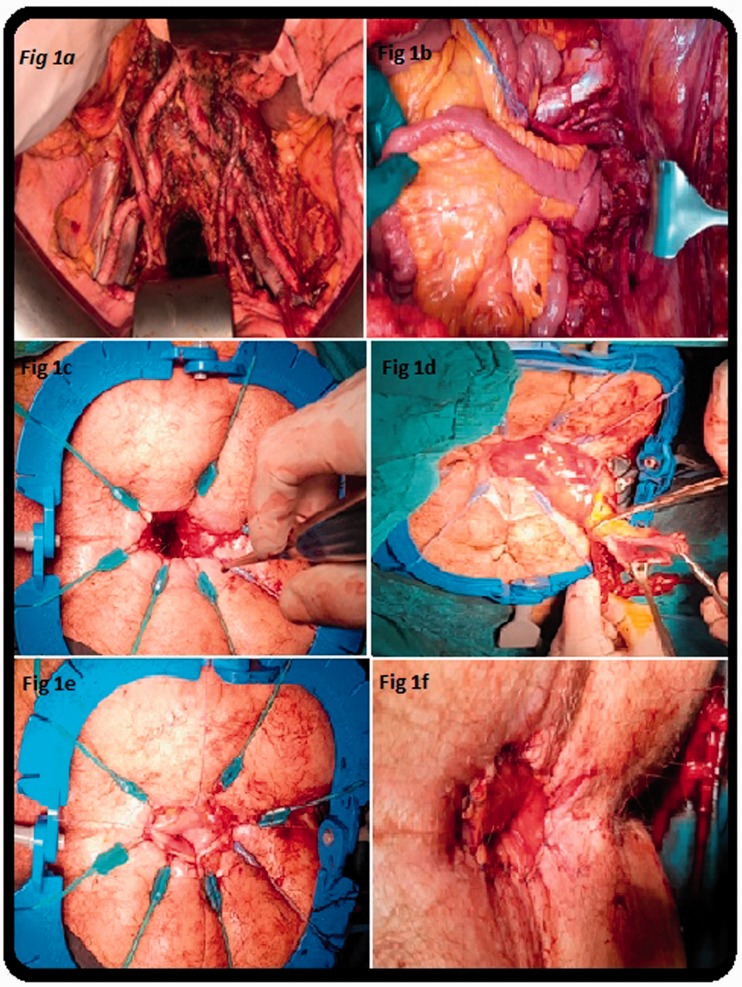
In all patients, a camera port was inserted at the umbilicus using an ultrasound or Hasson trocar technique, and the PC index (PCI) score was calculated. CRS was performed as previously described by Sugarbaker.6 Before CAIAA was initiated, the colon segment where the anastomosis to the distal part was planned was controlled by extending the segment of the colon to 10 cm below the pubic symphysis. A Lone Star retractor was placed in the anal region. The resection borders were determined at the level of the dentate line in the intersphincteric groove. In patients who underwent an ileoanal procedure, the J-pouch procedure was performed using staples and was secured with Lambert sutures. After ensuring that the intestine to be attached to the anal region had a good blood supply, sutures were placed on the mucosa and submucosa using 4-0 Vicryl (Ethicon Inc., Somerville, NJ, USA). The CAIAA procedure was performed using 4-0 Vicryl sutures. In all cases, a temporary loop ileostomy was formed 30 cm proximal to the ileocecal valve or approximately 50 cm proximal to the ileoanal anastomosis (Figure 1(a)–(f)).
Figure 1.
Surgical procedure of cytoreductive surgery + hyperthermic intraperitoneal chemotherapy and coloanal/ileoanal anastomosis. (a) Pelvic and para-aortic dissection. (b) Replacement of ileal pouch to the pelvis for anastomosis. (c) Preparation of anal canal for anastomosis. (d) Preparation of viable colonic segment for anastomosis. (e) Fixation of colonic mucosa with sutures. (f) Completed hand-sewn coloanal anastomosis.
HIPEC procedure
After the completion of CRS and CAIAA, four drains were placed: one outflow drain into each of two subdiaphragmatic areas and one outflow and inflow drain each into the pelvic and epigastric areas. Heat probes were placed in the neighboring region of the inflow drain in the epigastric region and around the pelvic outflow drain. As the abdominal closure was performed using PDS loop No. 1 (Ethicon Inc.), colorectal, appendiceal, and pseudomyxoma peritonei tumors were intravenously treated using 5-fluorouracil [400 mg/m2 body surface area (BSA)] + leucovorin (20 mg/m2 BSA). The skin was closed using staples or Prolene sutures (Ethicon Inc.), and an intraperitoneal HIPEC procedure was initiated. For colorectal, appendiceal, and pseudomyxoma peritonei, oxaliplatin (300 mg/m2 BSA) in 5% dextrose was administered at 42°C to 43°C for 30 minutes. The patients with sarcomatosis, mesothelioma, and rectal gastrointestinal stromal tumors underwent intraperitoneal injection of cisplatin (75 mg/m2 BSA) + doxorubicin (15 mg/m2 BSA) in 0.9% sodium chloride solution for 60 minutes. The patient with breast carcinoma underwent intraperitoneal injection of cisplatin (75 mg/m2 BSA) for 60 minutes. During this procedure, the intra-abdominal body temperature was measured using a probe placed in the esophagus. The patients who were administered oxaliplatin underwent monitoring of their blood glucose levels using the Belmont Hyperthermia Pump (Belmont Medical Technologies, Billerica, MA, USA).7 The temporary loop ileostomy was closed at approximately the sixth postoperative month. The patients performed pelvic floor muscle exercises before loop ileostomy closure.8
Statistical analyses
The raw data were recorded in IBM SPSS Statistics 22 (IBM Corp., Armonk, NY, USA). The average, mode, median, and percent distributions of the data were calculated as shown in the tables in the Results section. No statistical analysis was performed because no comparisons were assessed in our study.
Results
Of 108 patients who underwent CRS + HIPEC for PC from May 2016 to November 2018, the prospectively collected data of 20 consecutive patients who underwent manual CAIAA were retrospectively analyzed in the present study. The mean age of all patients was 56.7 (29–74) years. Thirteen (65%) patients were male and seven (35%) were female. In terms of comorbidities, two (10%) patients had diabetes mellitus, three (15%) had hypertension, one (5%) had chronic obstructive lung disease, and one (5%) had coronary artery disease. The demographic data of the patients are summarized in Table 1. In all patients, pelvic floor muscle exercises were started in the preoperative period. The most common diagnosis among all patients was rectal cancer [8 (40%) patients]. The other diagnoses were colon cancer in five (25%) patients, gynecological cancer in four (20%), sarcomatosis in one (5%), gastrointestinal stromal tumorosis in one (5%), and a breast cancer origin in one (5%) (Table 2).
Table 1.
Patient demographics.
| Features | |
|---|---|
| Age, years | 56.7 (29–82) |
| Sex | |
| Male | 13 |
| Female | 7 |
| ASA scores | |
| 1 | 6 |
| 2 | 9 |
| 3 | 5 |
| Body surface area, m2 | 1.65 (1.3–2.0) |
| Peritoneal carcinomatosis index score | 12 (5–20) |
| Completeness of cytoreduction score | |
| 0 | 14 |
| 1 | 6 |
| 2 | 0 |
| 3 | 0 |
| Hospital stay, days | 8.8 (7–22) |
| Number of anastomoses | 1.25 (1–3) |
| Proximal segment of coloanal/ileoanal anastomosis | |
| Ileum | 5 |
| Ascending colon | 8 |
| Transverse colon | 1 |
| Descending colon | 3 |
| Sigmoid colon | 3 |
| Temporary ileostomy closure | |
| Yes | 12 |
| No | 8 |
| Clavien–Dindo complication score | |
| 1 (wound infection, emesis) | 6 |
| 2 (pneumonia, UTI, DVT) | 9 |
| 3A (pleural effusion) | 2 |
| 3B (evisceration) | 2 |
| 4 (ARDS, DIC) | 0 |
| 5 (exitus) | 1 |
| Complications associated with coloanal/ileoanal anastomosis | 0 |
| Local recurrence | 0 |
| Transfusion requirement, units | |
| Erythrocyte suspension | 1.31 (0–2) |
| Fresh frozen plasma | 0.53 (0–1) |
| Intraoperative fluid requirement | |
| Crystalloids, mL | 2100 (800–8500) |
| Colloids, mL | 320 (100–200) |
Data are presented as n or mean (range).
ASA: American Society of Anesthesiologists, DVT: deep venous thrombosis, UTI: urinary tract infection, ARDS: acute respiratory distress syndrome, DIC: disseminated intravascular coagulation.
Table 2.
Histopathological diagnosis, HIPEC treatment protocol, recurrence rate, mortality, and surgical duration.
| Diagnosis | n = 20 (100%) | Recurrence | HIPEC treatment protocol | Mortality, n | Mean operation time, hours |
|---|---|---|---|---|---|
| Rectum Ca + PC | 8 (40%) | 6 (75%) | OXA + 5FU + LOC | 1 | 6.5 |
| Colon Ca + PC | 5 (25%) | 3 (60%) | OXA + 5FU + LOC | 0 | 6.4 |
| Ovary Ca + PC | 2 (10%) | 0 (0%) | CIS + DOXO | 0 | 7.1 |
| Cervix Ca + PC | 2 (10%) | 2 (100%) | CIS + DOXO | 0 | 7.6 |
| Rectal GISTosis (imatinib-resistant) | 1 (5%) | 1 (100%) | CIS + DOXO | 0 | 7.5 |
| Sarcomatosis | 1 (5%) | 1 (100%) | CIS + DOXO | 0 | 8.0 |
| Breast Ca + PC | 1 (5%) | 1 (100%) | CIS | 0 | 12.0 |
HIPEC: hyperthermic intraperitoneal chemotherapy, Ca: carcinoma, PC: peritoneal carcinomatosis, GISTosis: gastrointestinal stromal tumorosis, CIS: cisplatin, 5FU: 5-fluorouracil, OXA: oxaliplatin, LOC: leucovorin, DOXO: doxorubicin.
The mean preoperative PCI score of the patients was 12. Fourteen patients had a completeness of cytoreduction score of 0, and six patients had a score of 1. No early complications were noted in any of the patients; however, one (5%) patient had late pulmonary embolism-related mortality. The histopathological diagnoses, HIPEC treatment protocols, recurrence rates, mortality, and surgical duration (hours) of the patients in this study are shown in Table 2. Eight of the patients had undergone one previous recurrent intra-abdominal operation, four of the patients had undergone two, and two of the patients had undergone three. Therefore, the Douglas pouch reflection was previously impaired in all patients, and the pelvic floor was very close to malignancies. Technically, it was impossible to apply mechanical staples in this region; therefore, CAIAA was performed in all 20 patients.
In terms of CAIAA, coloanal anastomosis was performed in 15 (75%) patients, and J-pouch ileoanal anastomosis was performed in 5 (25%) patients. No complications were detected during an average follow-up period of 20 months (range, 3–30 months). In all 20 patients whose temporary ileostomy was opened during CRS, ileostomy closure was performed in 12 (60%) patients with no complications. Ileostomy closure could not be performed in the remaining eight (40%) patients because of current adjuvant chemotherapy. The continence of 12 patients who underwent temporary ileostomy closure was evaluated using the Cleveland continence score, and positive results were obtained in balloon expulsion tests. No recurrence or metastatic lesions were observed in these 12 patients. Upon the detection of new metastatic spots, one patient without closed ileostomy began receiving transarterial radioembolization treatment in the Nuclear Medicine Unit. Details of the patients who underwent CRS + HIPEC and synchronous resections are provided in Table 3. The final status of all patients is summarized in Figure 2.
Table 3.
Patients who underwent cytoreductive surgery + hyperthermic intraperitoneal chemotherapy with synchronous organ resections.
| Organ resections | Patients, n |
|---|---|
| Greater omentum | 20 |
| Diaphragm | 1 |
| Small bowel mesentery | 14 |
| Stomach | 1 |
| Small bowel | 10 |
| Liver | 5 |
| Colorectal area | 20 |
| Pancreas | 1 |
| Gallbladder | 11 |
| Spleen | 3 |
| Bilateral pelvic para-aortic lymph node dissection | 3 |
| Total abdominal hysterectomy + bilateral salpingo-oophorectomy | 3 |
Figure 2.
Recent status of patients.
CRS+HIPEC, cytoreductive surgery + hyperthermic intraperitoneal chemotherapy.
Discussion
PC can develop at an incidence of 25%, especially in patients with colorectal cancer, ovarian cancer, malignant mesothelioma, and sarcomas, and is identified either during diagnosis or after the initial surgery.6–8 In PC caused by intra-abdominal and especially gastrointestinal tract tumors, palliative surgery or medical oncological treatment does not result in satisfactory results; the survival in such patient groups ranges from 3 to 6 months. Sugarbaker3 introduced a new dimension of treatment with CRS + HIPEC, especially for carcinomatosis, and considerable improvements have been made in terms of survival. In patients with colorectal and ovarian malignant mesotheliomas and sarcomas and a PCI score of <20 (preferably <10), considerable improvements in survival rates have been made when HIPEC is simultaneously implemented with R0-R1 resection. The current treatment algorithm of PC has begun to be standardized by multidisciplinary approaches among medical oncology specialists, radiation oncology specialists, and colorectal surgeons in consensus and with common treatment decisions.9,10
One of the most serious controversies in the performance of CRS + HIPEC for PC is high-risk anastomoses. A leakage rate of approximately 20% after performing anastomoses using staples, even in patients with stage 2 rectal tumors without carcinomatosis, has been reported in studies conducted at large centers.11 CRS is considered an aggressive surgical technique, and the surgical procedure and high-pressure hyperthermic chemotherapy can be serious risk factors for anastomotic leakage. In such cases, the clinician must consider both patient-related factors (advanced age, hypoalbuminemia, performance status, and obesity) and operative factors (high PCI score, bowel resection, diaphragmatic involvement, distal pancreatectomy, hepatobiliary resections, and surgeon experience). In a study conducted by Piso et al.12 involving 2,149 patients who underwent CRS + HIPEC, the anastomotic leakage rate was 5.7%. Additionally, Von Breitenbuch et al.7 reported that the rectal anastomotic leakage rate was 5% in patients who underwent CRS + HIPEC and strongly recommended opening of a protective ileotomy to prevent morbidity and mortality that may occur after rectal anastomosis surgery. Whealon et al.13 investigated the necessity of fecal diversion in pelvic anastomosis during CRS + HIPEC and emphasized the need for protective ileostomy because the anastomotic leakage rate was >10%. A recent study showed that <30% of loop ileostomies that opened during CRS + HIPEC were suitable for closure.14 In light of these reported findings, one of the best examples of high-risk anastomoses is the Whipple procedure; this procedure involves the performance of a pancreaticojejunostomy anastomosis, which is associated with serious morbidity. Tentes et al.15 conducted a study of 21 patients and reported anastomotic leakage in 2 patients following CRS + HIPEC; one of these patients underwent choledochojejunal anastomosis, and the other underwent pancreaticojejunostomy anastomosis. In light of these reported findings, we used CAIAA, which has been scarcely reported in the literature, in patients who underwent CRS + HIPEC. Twelve of our patients underwent ileostomy closure with no complications; the remaining eight patients will be evaluated for ileostomy closure after the completion of their oncological treatments.
Sphincter-preserving surgery and recognition of the CAIAA technique in patients undergoing ultralow-radiation therapy have brought a new perspective to the treatment algorithm in rectal cancer. CAIAA techniques have become more important considering the provision of 1-cm and negative radial distal surgical margins for tumors in this region. However, complications such as anastomosis dehiscence with complete or partial leakage may occur in the early postoperative period after CAIAA, and related mortality and morbidity may occur secondary to pelvic sepsis.16 In the long term, patients may develop complications such as stricture, incontinence, stenosis, and pouchitis.17,18 Stenosis can occur in up to 30% of colorectal anastomoses using either using staples or handsewing.1 In terms of anastomotic leakage, De Cuba et al.19 reported leakage rates of up to 10% with CRS + HIPEC; however, the effect of HIPEC on anastomotic leakage is still controversial.
Based on the favorable results of the CAIAA technique, factors of great importance include lack of tension of the colon or ileal segment brought to the anal canal, good blood flow, effective preparation of the mucosa and submucosa for anastomosis in the anal canal, and careful suturing techniques.20,21 A literature review using PubMed and Google Scholar revealed no data regarding the early and late results of manual CAIAA during CRS + HIPEC. Considering CRS + HIPEC in the current treatment algorithm, we utilized sphincter-preserving surgery with CAIAA, which avoids permanent colostomy, and the same technique was used in all 20 patients. One of our patients exhibited long-term pulmonary mortality; however, none of our patients developed complications related to the anastomosis in the short or long term. In addition, we noted that the initial sutures in the colon placed from the mucosa toward the serosa or in the ileum were associated with favorable outcomes. Moreover, we assume that surgical experience significantly influences the success of the operation. However, recurrence of metastasis in the liver was detected in one patient, who thus began transarterial radioembolization treatment.
A well-described risk factor for anastomotic leakage is the localization of the anastomosis and its distance from the anal verge; the limit for the level of the anastomosis is approximately 6 cm from the dentate line.22 It is often challenging to manage mortality caused by leakage in anastomoses that are very close to the dentate line; such leakages can discourage surgeons from performing sphincter-preserving interventions. Considering the positive effects on patients’ quality of life, psychological consequences caused by permanent colostomy, and costs related to colostomy, we believe that a CAIAA performed with a sphincter-preserving surgery in appropriately selected patients may have positive effects, although some technical difficulties still exist.
In conclusion, we have herein presented our experience with CAIAA during CRS + HIPEC. We believe that our findings will contribute to the literature. However, the results of our study require additional prospective randomized trials to evaluate CAIAA during CRS + HIPEC.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1.Woo IT, Park JS, Choi GS, et al. Clinical outcomes of a redo for a failed colorectal or coloanal anastomosis. Ann Coloproctol 2018; 34: 259–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baratti D, Kusamura S, Iusco D, et al. Postoperative complications after cytoreductive surgery and hyperthermic intraperitoneal chemotherapy affect long-term outcome of patients with peritoneal metastases from colorectal cancer: a two-center study of 101 patients. Dis Colon Rectum 2014; 57: 858–868. [DOI] [PubMed] [Google Scholar]
- 3.Koppe MJ, Boerman OC, Oyen WJ, et al. Peritoneal carcinomatosis of colorectal origin: incidence and current treatment strategies. Ann Surg 2006; 243: 212–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hyman N, Manchester TL, Osler T, et al. Anastomotic leaks after intestinal anastomosis: it's later than you think. Ann Surg 2007; 245: 254–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Slesser AA, Pellino G, Shariq O, et al. Compression versus hand-sewn and stapled anastomosis in colorectal surgery: a systematic review and meta-analysis of randomized controlled trials. Tech Coloproctol 2016; 20: 667–676. [DOI] [PubMed] [Google Scholar]
- 6.Sugarbaker PH. Surgical responsibilities in the management of peritoneal carcinomatosis. J Surg Oncol 2010; 101: 713–724. [DOI] [PubMed] [Google Scholar]
- 7.Von Breitenbuch P, Piso P, Schlitt HJ. Safety of rectum anastomosis after cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. J Surg Oncol 2018; 118: 551–556. [DOI] [PubMed] [Google Scholar]
- 8.Van Eden WJ, Kok NF, Jozwiak K, et al. Timing of systemic chemotherapy in patients with colorectal peritoneal carcinomatosis treated with cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Dis Colon Rectum 2017; 60: 477–487. [DOI] [PubMed] [Google Scholar]
- 9.Jayne DG, Fook S, Loi C, et al. Peritoneal carcinomatosis from colorectal cancer. Br J Surg 2002; 89: 1545–1550. [DOI] [PubMed] [Google Scholar]
- 10.Li Y, Zhou YF, Liang H, et al. Chinese expert consensus on cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for peritoneal malignancies. World J Gastroenterol 2016; 22: 6906–6916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Westerduin E, Borstlap WAA, Musters GD, et al. Redo coloanal anastomosis for anastomotic leakage after low anterior resection for rectal cancer: an analysis of 59 cases. Colorectal Dis 2018; 20: 35–43. [DOI] [PubMed] [Google Scholar]
- 12.Piso P, Nedelcut SD, Rau B, et al. Morbidity and mortality following cytoreductive surgery and hyperthermic intraperitoneal chemotherapy: data from the DGAV StuDoQ registry with 2149 consecutive patients. Ann Surg Oncol 2019; 26: 148–154. [DOI] [PubMed] [Google Scholar]
- 13.Whealon MD, Gahagan JV, Sujatha-Bhaskar S, et al. Is fecal diversion needed in pelvic anastomoses during hyperthermic intraperitoneal chemotherapy (HIPEC)? Ann Surg Oncol 2017; 24: 2122–2128. [DOI] [PubMed] [Google Scholar]
- 14.Spratt JS, Adcock RA, Sherrill W, et al. Hyperthermic peritoneal perfusion system in canines. Cancer Res 1980; 40: 253–255. [PubMed] [Google Scholar]
- 15.Tentes AA, Kyziridis D, Kakolyris S, et al. Preliminary results of hyperthermic intraperitoneal intraoperative chemotherapy as an adjuvant in resectable pancreatic cancer. Gastroenterol Res Pract 2012; 2012: 506571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sciuto A, Merola G, De Palma GD, et al. Predictive factors for anastomotic leakage after laparoscopic colorectal surgery. World J Gastroenterol 2018; 24: 2247–2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kraenzler A, Maggiori L, Pittet O, et al. Anastomotic stenosis after coloanal, colorectal and ileoanal anastomosis: what is the best management? Colorectal Dis 2017; 19: O90–O96. [DOI] [PubMed] [Google Scholar]
- 18.De La Fuente SG, Mantyh CR. Reconstruction techniques after proctectomy: what's the best? Clin Colon Rectal Surg 2007; 20: 221–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.De Cuba EM, Verwaal VJ, De Hingh IH, et al. Morbidity associated with colostomy reversal after cytoreductive surgery and HIPEC. Ann Surg Oncol 2014; 21: 883–890. [DOI] [PubMed] [Google Scholar]
- 20.Brown S, Margolin DA, Altom LK, et al. Morbidity following coloanal anastomosis: a comparison of colonic J-pouch vs straight anastomosis. Dis Colon Rectum 2018; 61: 156–161. [DOI] [PubMed] [Google Scholar]
- 21.Pai VD, Desouza A, De Menezes JL, et al. Laparoscopic intersphincteric resection and hand-sewn coloanal anastomosis: a natural orifice specimen extraction technique. J Laparoendosc Adv Surg Tech A 2015; 25: 396–400. [DOI] [PubMed] [Google Scholar]
- 22.Rullier E, Laurent C, Garrelon JL, et al. Risk factors for anastomotic leakage after resection of rectal cancer. Br J Surg 1998; 85: 355–358. [DOI] [PubMed] [Google Scholar]