Short abstract
Objective
This study was performed to evaluate the clinical efficacy and safety of a novel surgical procedure in treating tracheal or bronchial compression related to severe congenital heart disease.
Methods
The clinical data of 28 patients with tracheal or bronchial compression related to severe congenital heart disease were retrospectively analyzed. In the control group, 12 patients underwent surgery for congenital cardiac malformations. In the treatment group, 16 patients underwent surgery for congenital cardiac malformations combined with partial resection of the pulmonary artery wall. The cardiothoracic ratio, pulmonary arterial pressure, left ventricular end-diastolic dimension, diameter of the pulmonary artery, and diameter of the trachea in the stenotic segment were quantitatively measured before and 9 days after the operation.
Results
The diameter of the pulmonary artery and diameter of the trachea in the stenotic segment were almost restored to the normal range in the treatment group. Patients in the treatment group recovered more rapidly and effectively than those in the control group.
Conclusion
Partial resection of the pulmonary artery wall is an efficacious and safe technique in the treatment of tracheal or bronchial compression related to severe congenital heart disease.
Keywords: Partial resection, bronchial compression, congenital heart disease, clinical efficacy, safety, pulmonary artery wall, cardiac malformation
Introduction
The diagnosis and treatment of bronchial compression related to congenital heart disease are challenging.1 Moreover, the clinical efficacy and safety of current interventions remain unsatisfactory. The diagnosis and effective treatment of severe congenital heart disease complicated with severe tracheal or bronchial compression are even more difficult.2 In some patients with severe congenital heart disease, tracheal or bronchial compression is caused by cardiac enlargement and an increased pressure and diameter of blood vessels that have a normal origin, course, and position3 For these patients, appropriate clinical treatment can yield high efficacy and a favorable prognosis. Unlike other types of tracheal stenosis, tracheal compression caused by pulmonary hypertension or an increased pulmonary blood volume can be associated with congenital heart disease. In this clinical trial, we performed a novel surgical procedure for the treatment of tracheal or bronchial compression related to severe congenital heart disease. The clinical efficacy and safety of this new intervention were evaluated.
Materials and methods
Baseline data
We retrospectively analyzed the clinical data of patients who were diagnosed with tracheal or bronchial compression related to severe congenital heart disease and admitted to the Second Hospital of Lanzhou University from June 2010 to June 2014. These patients were randomly divided into the control and treatment groups. Patients in the control group underwent conventional surgery for congenital cardiac malformation alone. Patients in the treatment group underwent conventional surgery combined with partial resection of the pulmonary artery wall to relieve the pressure from visceral organs adjacent to the trachea. The two groups showed no significant differences in age, sex, body weight, valvular regurgitation, or other parameters, as shown in Table 1. The patients underwent preliminarily screening according to their auscultation findings and clinical symptoms at the pediatric outpatient service or community outpatient service. The diagnostic methods included chest orthography, cardiac ultrasound, computed tomography (CT) angiography, and other clinical examinations. Written informed consent was obtained from all patients’ parents or caregivers. The study was approved by the Ethics Committee of the Second Hospital of Lanzhou University.
Table 1.
Comparison of baseline data between the control and treatment groups.
| Parameter | Control group | Treatment group | P-value |
|---|---|---|---|
| Number of patients | 12 | 16 | |
| Sex | |||
| Male | 7 | 9 | |
| Female | 5 | 7 | 0.912 |
| Age, months | 10.7 ± 4.6 | 11.5 ± 5.9 | 0.689 |
| Weight, kg | 11.5 ± 5.9 | 9.5 ± 3.6 | 0.986 |
| Mitral regurgitation | |||
| Mild | 5 | 6 | |
| Moderate | 3 | 4 | |
| Severe | 4 | 3 | 0.845 |
| Tricuspid regurgitation | |||
| Mild | 4 | 7 | |
| Moderate | 2 | 2 | |
| Severe | 2 | 3 | 0.893 |
| Pulmonary valve regurgitation | |||
| Mild | 5 | 6 | |
| Moderate | 3 | 4 | |
| Severe | 1 | 2 | 0.932 |
| Tracheal stenosis | |||
| Mild | 1 | 1 | |
| Moderate | 3 | 5 | |
| Severe | 8 | 10 | 0.926 |
| Preoperative respiratory status | |||
| Shortness of breath | 8 | 9 | 0.576 |
| Invasive ventilator | 2 | 4 | 0.595 |
| Noninvasive ventilator | 3 | 5 | 0.717 |
| Pulmonary infection | 6 | 12 | 0.172 |
| Atelectasis | 2 | 6 | 0.227 |
| Heart failure | 9 | 12 | 1.000 |
Data are presented as n or mean ± standard deviation.
Inclusion and exclusion criteria
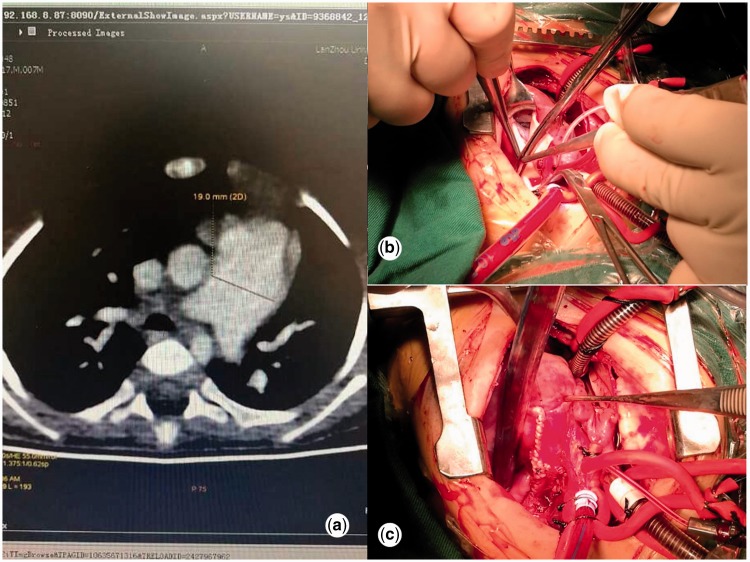
The inclusion criteria were the presence of a congenital cardiac malformation and severe pulmonary hypertension (Figure 1(a)); the absence of a double aortic arch, vascular ring, or pulmonary artery sling (Figure 1(b),(c)); severe tracheal compression with >70% obstruction of the bronchus in the narrow segment caused by the pulmonary artery; and parents’ or caregivers willingness for their child to undergo examination after treatment. The exclusion criteria were congenital tracheal or bronchial stenosis and complex congenital heart defects complicated with severe heart failure.
Figure 1.
(a) Computed tomography scan illustrating the preoperative status. (b) Photograph showing the intraoperative status. (c) Photograph showing the postoperative condition.
Surgical procedures and postoperative follow-up
Cardiac malformations were treated with conventional surgery. All arterial catheters were cut and then sutured to each vascular incision. Ventricular septal defects were continuously sutured with a 0.6% glutaraldehyde-treated pericardial sheet. Atrial septal defects were continuously sutured with a fresh pericardial patch or directly sutured. Moderate mitral and tricuspid regurgitation were treated with valvuloplasty. Patients diagnosed with severe tracheal compression in the control group were left untreated, whereas those in the treatment group underwent direct intervention of the heart and blood vessels and indirect intervention of the trachea or bronchus. Before extracorporeal circulation, the artery ligament or arterial catheter was cut and the left and right pulmonary arteries were separated from other tissues except for the posterior wall of the pulmonary artery; this was adhered to the trachea to avoid tracheomalacia after surgery. After the cardiac malformation had been corrected, the pulmonary artery wall and the left and right pulmonary arteries were partially cut along the long axis and then sutured to complete the pulmonary angioplasty. The extent of the resection depended on the pulmonary artery diameter of the age-matched patients in the control group to ensure that this diameter after angioplasty was equal to or slightly greater than the normal diameter. The pulmonary artery was resected from the point at which it protruded from the pericardium to the pulmonary artery bifurcation. Significant dilation of the main pulmonary arteries required resection of the anterior pulmonary artery wall, but resection failed to prevent the incidence of pulmonary artery stenosis. The bilateral diameter should be equivalent; thus, bilateral removal was performed to avoid an imbalance between the bilateral pulmonary blood vessels. All patients underwent conventional treatment in the cardiac intensive care unit after the operation and were administered cardiotonic and diuretic drugs for 1 month after discharge. The patients were followed up monthly for 6 consecutive months and then every 3 to 6 months thereafter.
Statistical analysis
The cardiothoracic ratio, pulmonary arterial pressure, left ventricular end-diastolic dimension, diameter of the pulmonary artery, diameter of the trachea in the stenotic segment, and diameter of the adjacent trachea were quantitatively measured before and 9 days after the operation. Data are presented as mean ± standard deviation for continuous variables and as frequency for categorical variables. Differences between the two groups were examined for statistical significance using the χ2 test for categorical variables and Student’s t-test for continuous variables. All statistical analyses were performed using SPSS 21.0 software (IBM Corp., Armonk, NY, USA). A P value of <0.05 was considered statistically significant.
Results
Patient grouping
Twenty-eight patients were included in the study and assigned to the control group (n = 12) and treatment group (n = 16). The patients’ mean age at diagnosis was 10.2 ± 4.3 months, and their mean age at surgery was 11.0 ± 5.2 months. The two groups showed no significant differences in the cardiothoracic ratio, pulmonary arterial pressure, left ventricular end-diastolic dimension, diameter of the pulmonary artery, or diameter of the trachea in the stenotic segment, as shown in Table 2.
Table 2.
Comparison of preoperative observation parameters between the control and treatment groups.
| Parameter | Control group | Treatment group | P-value |
|---|---|---|---|
| Cardiothoracic ratio | 0.65 ± 0.26 | 0.64 ± 0.21 | 0.867 |
| Pulmonary artery pressure, mmHg | 79.5 ± 2.9 | 80.0 ± 3.5 | 0.691 |
| Left ventricular end-diastolic dimension, mm | 21.9 ± 2.3 | 21.4 ± 2.7 | 0.625 |
| Main pulmonary artery diameter, mm | 15.9 ± 2.4 | 15.1 ± 2.6 | 0.412 |
| Left pulmonary artery diameter, mm | 10.3 ± 1.4 | 9.9 ± 1.5 | 0.502 |
| Right pulmonary artery diameter, mm | 9.9 ± 1.4 | 9.6 ± 1.5 | 0.529 |
| Diameter of tracheal stenosis, mm | 3.8 ± 1.8 | 4.1 ± 1.8 | 0.655 |
Data are presented as mean ± standard deviation.
Comparison of postoperative data
The cardiothoracic ratio, pulmonary arterial pressure, and left ventricular end-diastolic dimension were not significantly different between the two groups on postoperative day 9. The diameter of the main, left, and right pulmonary artery was significantly smaller and the diameter of the trachea in the stenotic segment was significantly larger in the treatment group than in the control group (P ≤ 0.004 and P = 0.000, respectively) (Table 3). Patients in the control group showed no statistically significant differences in the cardiothoracic ratio, diameter of the pulmonary artery, or diameter of the trachea in the stenotic segment before and 9 days after surgery. The pulmonary arterial pressure and left ventricular end-diastolic dimension were significantly lower on postoperative day 9 than preoperatively (P = 0.000 and P = 0.035, respectively) (Table 4). In the treatment group, chest X-ray examination revealed that the cardiothoracic ratio was lower 9 days after the operation than preoperatively, although the difference was not statistically significant. In addition, the left ventricular end-diastolic dimension and pulmonary artery pressure were significantly lower 9 days after the operation than preoperatively (P < 0.05 and P < 0.01, respectively). The velocity of the blood flow in the pulmonary artery was almost normal. CT angiography showed a significant reduction in the diameter of the pulmonary artery in all patients on postoperative day 9 (P < 0.01). The diameter of the trachea in the stenotic segment was also significantly larger on postoperative day 9 (P < 0.01), as shown in Table 5. As shown in Table 6, the extracorporeal circulation time in the control group was 69.6 ± 20.1 minutes, which was significantly shorter than that in the treatment group (73.2 ± 14.7 minutes; P = 0.000). The incidences of postoperative lung infection (n = 7), pleural effusion (n = 6), and local atelectasis (n = 6) in the control group were significantly higher than those (n = 2, 2, 1) in the treatment group (n = 2, 2, and 1, respectively) (P = 0.000, P = 0.030, and P = 0.008, respectively). In addition, the time of ventilator use, length of intensive care unit stay, and length of hospital stay were considerably longer in the control group than treatment group (all P = 0.000). The hospital cost in the control group was also significantly higher than that in the treatment group (P = 0.000).
Table 3.
Comparison of observational parameters at postoperative day 9 between the control and treatment groups.
| Parameter | Control group | Treatment group | P-value |
|---|---|---|---|
| Cardiothoracic ratio | 0.64 ± 0.21 | 0.63 ± 0.21 | 0.486 |
| Pulmonary artery pressure, mmHg | 34.1 ± 2.0 | 33.8 ± 2.4 | 0.753 |
| Left ventricular end-diastolic dimension, mm | 19.8 ± 3.7 | 19.6 ± 2.4 | 0.857 |
| Main pulmonary artery diameter, mm | 15.8 ± 2.0 | 11.9 ± 2.3 | 0.004 |
| Left pulmonary artery diameter, mm | 9.8 ± 1.6 | 6.9 ± 1.1 | 0.000 |
| Right pulmonary artery diameter, mm | 9.7 ± 1.6 | 7.2 ± 1.6 | 0.000 |
| Diameter of tracheal stenosis, mm | 4.7 ± 2.0 | 8.1 ± 1.4 | 0.000 |
Data are presented as mean ± standard deviation.
Table 4.
Comparison of observation indexes before and after the operation in the control group.
| Parameter | Preoperative | 9 days postoperative | P-value |
|---|---|---|---|
| Cardiothoracic ratio | 0.65 ± 0.26 | 0.64 ± 0.21 | 0.204 |
| Pulmonary artery pressure, mmHg | 79.5 ± 2.1 | 34.1 ± 2.0 | 0.000 |
| Left ventricular end-diastolic dimension, mm | 21.9 ± 2.3 | 19.8 ± 3.7 | 0.035 |
| Main pulmonary artery diameter, mm | 15.9 ± 2.4 | 15.8 ± 2.0 | 0.917 |
| Left pulmonary artery diameter, mm | 10.3 ± 1.4 | 9.8 ± 1.6 | 0.429 |
| Right pulmonary artery diameter, mm | 9.9 ± 1.4 | 9.7 ± 1.6 | 0.491 |
| Diameter of tracheal stenosis, mm | 3.8 ± 1.8 | 4.7 ± 2.0 | 0.366 |
Data are presented as mean ± standard deviation.
Table 5.
Comparison of observation indexes before and after the operation in the treatment group.
| Parameter | Preoperative | 9 days postoperative | P-value |
|---|---|---|---|
| Cardiothoracic ratio | 0.64 ± 0.26 | 0.63 ± 0.21 | 0.078 |
| Pulmonary artery pressure, mmHg | 80.0 ± 3.5 | 33.8 ± 2.4 | 0.000 |
| Left ventricular end-diastolic dimension, mm | 21.4 ± 2.7 | 19.6 ± 2.4 | 0.041 |
| Main pulmonary artery diameter, mm | 15.1 ± 2.6 | 11.9 ± 2.3 | 0.004 |
| Left pulmonary artery diameter, mm | 9.9 ± 1.5 | 6.9 ± 1.1 | 0.000 |
| Right pulmonary artery diameter, mm | 9.6 ± 1.5 | 7.2 ± 1.6 | 0.000 |
| Diameter of tracheal stenosis | 4.1 ± 1.8 | 8.1 ± 1.4 | 0.000 |
Data are presented as mean ± standard deviation.
Table 6.
Comparison of clinical outcomes between the two groups.
| Control group (n = 12) | Treatment group (n = 16) | P | |
|---|---|---|---|
| Aortic occlusion time, minutes | 43.3 ± 4.1 | 42.5 ± 3.4 | 0.120 |
| Extracorporeal circulation time, minutes | 69.6 ± 20.1 | 73.2 ± 14.7 | 0.000 |
| Postoperative lung infection | 7 | 2 | 0.010 |
| Postoperative local atelectasis | 6 | 2 | 0.030 |
| Time of ventilator use, hours | 66.4 ± 16.2 | 30.4 ± 6.4 | 0.000 |
| Length of ICU stay, days | 7.0 ± 3.6 | 2.8 ± 1.0 | 0.000 |
| Length of hospital stay, days | 15.5 ± 6.8 | 10.5 ± 3.0 | 0.000 |
| Hospital cost, ×10,000 Yuan | 11.2 ± 4.3 | 7.8 ± 2.9 | 0.000 |
| Residual shunt | 0 | 1 | 0.378 |
| 3rd-degree atrioventricular occlusion | 0 | 0 | – |
| Pleural effusion | 6 | 1 | 0.008 |
Data are presented as mean ± standard deviation or number of patients.
ICU, intensive care unit.
Postoperative follow-up
As shown in Table 7, postoperative follow-up ranged from 3 to 35 months in the treatment group and from 9 to 33 months in the control group. The overall bronchial compression rate was 58.3% in the control group, which was significantly higher than that in the treatment group (6.3%, P < 0.05). Mild bronchial compression was untreated in one patient in the treatment group, probably because of the excessively long-term compression. Therefore, the bronchus was slowly recovered during the first 6 months postoperatively. Pulmonary artery stenosis did not occur in the treatment group. No repeated operation was performed in either group. No death or severe complications were reported.
Table 7.
Follow-up results between the control and treatment groups.
| Parameter | Control group(n = 12) | Treatment group(n = 16) | Statistical significance |
|---|---|---|---|
| Pulmonary artery stenosis rate | 0 (0.0) | 0 (0.0) | NS |
| Bronchial compression rate | 7 (58.3) | 1 (6.3) | P < 0.05 |
| Follow-up period, months | 19.6 ± 9.2 | 21.3 ± 7.1 | NS |
Data are presented as n (%) of patients or mean ± standard deviation.
NS, not statistically significant (P > 0.05) by Student’s t-test or χ2 test.
Discussion
Airway compression can occur in conjunction with acyanotic or cyanotic congenital heart disease and can be accompanied by large left-to-right shunts, tetralogy of Fallot with absent pulmonary valves, tetralogy of Fallot with pulmonary atresia, congenital or ischemic mitral regurgitation, truncus arteriosus, or dilated cardiomyopathy. Patients with cardiac defects are at risk of developing significant pulmonary artery enlargement.1 Some patients with tracheal compression are prone to develop shortness of breath, wheezing, and recurrent respiratory tract infection. These symptoms are often attributed to heart failure, pulmonary congestion, and pneumonia. Therefore, tracheal compression is often ignored. Most cases are diagnosed when it is challenging to deliver airway intubation intraoperatively or when patients cannot be separated from the ventilator after the operation.2 The findings in the present study demonstrate that patients with bronchial compression obtain significant clinical benefits after undergoing pulmonary artery wall resection. No serious adverse events were reported. True extrinsic compression of the airway or lungs because of underlying heart disease is an important but often unrecognized source of pulmonary dysfunction. At present, most patients with tracheal stenosis can be identified before surgery because of the increased awareness and popularity of CT scans.
The association between cardiac defects and vascular tracheobronchial compression deserves special attention because delayed diagnosis and treatment significantly increase the morbidity and mortality rates.3 Concurrent treatment with cardiac and tracheal surgery has been accepted by most surgeons.4,5 Significant bronchial compression by hypertensive pulmonary arteries may result in respiratory symptoms. Sites of predilection are the left main bronchus and the left upper and right middle bronchi.6 The latter two sites correlate with the distribution of lobar emphysema in acyanotic congenital cardiac disease. Prolonged extrinsic compression probably leads to tracheomalacia and/or bronchomalacia, which can persist even after surgery. Pulmonary artery-induced bronchial compression has also been shown to be a primary cause of congenital lobar emphysema.7 The combination of congenital heart disease and congenital tracheal stenosis remains a vexing problem. Successful management of tracheal stenosis by means of slide tracheoplasty or resection and reconstruction has been reported.8
Previous studies have demonstrated that the incidence of tracheal compression among patients with congenital heart disease is higher than that among other populations.9 The tracheal or bronchial compression in some patients is caused by an increase in the pulmonary artery diameter. Therefore, the right pulmonary artery is placed in the forward position or the pulmonary artery wall is folded to relieve this compression.10 In the present investigation, the trachea or its affiliated structures that are adjacent to the heart, namely the descending aorta and the pulmonary artery, were compressed in patients with high pressure in the pulmonary artery, an increased pulmonary artery diameter, and an enlarged heart. These structures appear to “hold” the trachea, which may lead to tracheal compression. Partial tracheal stenosis that is mainly caused by high pressure in the pulmonary artery and a significantly increased pulmonary artery diameter without abnormalities in the organizational structure of the trachea itself can be termed “tracheal stenosis associated with high pressure in the pulmonary artery.” It can also be termed “tracheal compression associated with increased pulmonary blood volume and congenital heart disease” because of the involvement of an enlarged heart and increased pulmonary vein diameter. Direct surgical intervention is not required because of the lack of abnormalities in the trachea itself. In patients who are treated with cardiac surgery, the heart will gradually become normal and the pulmonary artery pressure will significantly decrease. For patients with a significantly increased diameter of the pulmonary artery, we can resect part of the pulmonary artery wall, thereby decreasing the diameter of the pulmonary artery. Compression from the heart and trachea will subsequently be reduced or even disappear, and the narrow trachea is likely to recover.
In the present study, the aortic occlusion time in the treatment group was slightly longer than that in the control group, but the difference was not statistically significant. The duration of extracorporeal circulation was significantly longer in the treatment than control group. The treatment group had shorter postoperative ventilator time, fewer pulmonary complications, a shorter length of hospital stay, and lower hospitalization costs. Although the operation method in the treatment group increased the extracorporeal circulation time, which is unfavorable to patients, the final result showed that the children in the treatment group recovered quickly after the operation and that the clinical effect was obviously better than that in the control group. The main reason for this difference is that the extracorporeal circulation time in the treatment group increased to a minimal degree and had a limited influence on the patients. However, the new operation method can relieve the pressure of the adjacent pulmonary artery on the trachea and bronchus more rapidly and effectively, which can promote the discharge of pulmonary secretions, improve pulmonary ventilation function, and reduce atelectasis and pulmonary infection. These benefits will in turn decrease the ventilator-assisted time, shorten the length of hospital stay, and reduce hospitalization expenses.
Based on the above findings, the present study suggests that patients with mild tracheal compression with <70% obstruction of the bronchus do not require special interventions for the trachea. However, patients with severe tracheal compression require pulmonary artery angioplasty, arterial catheter or arterial ligament transection, or other methods to increase the space around the trachea or bronchus in addition to surgery to correct the heart deformity and thus relieve the pressure on the trachea.
In theory, this novel approach ensures the structural integrity of the trachea with long-term growth potential and no restenosis. It is also simple, low-cost, and time-saving and yields a favorable clinical prognosis. Therefore, we believe that it deserves widespread application in clinical practice. Patients can recover quickly, restore a high quality of life, and achieve a high survival rate. No long-term tracheal restenosis occurred in this study. Early- and mid-term clinical follow-up confirmed that the tracheal compression did not recur. Consequently, the novel surgical procedures employed in this investigation are effective and safe approaches in the treatment of bronchial or tracheal compression.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This study was supported by the National Nature Science Foundation of China (grant No. 81470026), Gansu Provincial Health Department Research Plan (grant No. GSWST2012-02) and Longyuan Youth Innovative Talents Support Programs (2109901).
References
- 1.Kussman BD, Geva T, McGowan FX. Cardiovascular causes of airway compression. Paediatr Anaesth 2004; 14: 60–74. [DOI] [PubMed] [Google Scholar]
- 2.Kim ES, Yoon JY, Kim TKet al. Severe airway obstruction in an infant with congenital tracheal stenosis and congenital heart disease -a case report. Korean J Anesthesiol 2012; 62: 285–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sebening C, Jakob H, Tochtermann Uet al. Vascular tracheobronchial compression syndromes-experience in surgical treatment and literature review. Thorac Cardiovasc Surg 2000; 48: 164–174. [DOI] [PubMed] [Google Scholar]
- 4.Kaneko Y, Achiwa I. Congenital heart surgery in patients with coexisting congenital anomalies of respiratory or digestive system. Kyobu Geka 2012; 65: 665–668. [PubMed] [Google Scholar]
- 5.Xu ZW, Li WH. One stage surgical correction of congenital cardiac disease and congenital tracheal stenosis in infants and children. J Card Surg 2009; 24: 558–560. [DOI] [PubMed] [Google Scholar]
- 6.Berlinger NT, Long C, Foker Jet al. Tracheobronchial compression in acyanotic congenital heart disease. Ann Otol Rhinol Laryngol 1983; 92: 387–390. [DOI] [PubMed] [Google Scholar]
- 7.Stanger P, Lucas RV, Jr, Edwards JE. Anatomic factors causing respiratory distress in acyanotic congenital cardiac disease. Special reference to bronchial obstruction. Pediatrics 1969; 43: 760–769. [PubMed] [Google Scholar]
- 8.Grillo HC, Wright CD, Vlahakes GJet al. Management of congenital tracheal stenosis by means of slide tracheoplasty or resection and reconstruction, with long-term follow-up of growth after slide tracheoplasty. J Thorac Cardiovasc Surg 2002; 123: 145–152. [PubMed] [Google Scholar]
- 9.Lai SH, Liao SL, Wong KS. Cardiovascular-associated tracheobronchial obstruction in children. Cardiol Young 2013; 23: 233–238. [DOI] [PubMed] [Google Scholar]
- 10.Nomura N, Asano M, Mizuno Aet al. Translocation of dilated pulmonary artery for relief of bronchial compression associated with ventricular septal defect. Eur J Cardiothorac Surg 2007; 32: 937–939. [DOI] [PubMed] [Google Scholar]