Short abstract
Objectives
To investigate the potential for pulpal regeneration via autologous transplantation of deciduous tooth pulp into immature necrotic permanent teeth using an experimental dog model.
Methods
Experimental apical periodontitis was induced in 60 teeth of six Beagle dogs. Following canal disinfection and pulpotomy, autologous deciduous pulp tissue was transplanted into the root canals (n = 30); as controls, contralateral teeth were treated in accordance with the recommendations of the American Association of Endodontists. Radiographic examinations were performed immediately before transplant, as well as 3 and 6 months after transplant. At the 6-month examination, root samples were collected and histological and immunohistochemical analyses were used to examine tissue regeneration.
Results
Radiographic analysis showed no significant differences in most histopathological parameters examined; however, apical diameter reduction was greater in the experimental group. Histological and immunohistochemical analyses showed that the canal walls of the experimental group had newly formed dentin-like tissue with dentinal tubules, while the control group had cementum-like deposits along the canal wall and apical foramina.
Conclusions
Autologous transplantation may be useful for regeneration of dental pulp in necrotic young permanent teeth.
Keywords: Young permanent teeth, deciduous teeth, dental pulp, implant, pulp revascularization, autologous transplantation, regeneration, dogs, tooth root, periapical periodontitis
Introduction
Regenerative endodontics utilizing the traditional revascularization technique was proposed more than 10 years ago1,2 and has since become an effective method to treat immature permanent teeth with pulpal necrosis.3–8 This technique relies on the introduction of stem cells from the apical papilla (SCAPs) and other stem cells (e.g., from Hertwig’s epithelial root sheath and surrounding periodontal tissues) into the root canal space by induced apical bleeding. When combined with the release of growth factors from dentinal walls, this procedure can initiate a series of biological events that support the growth of hard and connective tissues within the root canal space.9,10 The major goals of regenerative endodontic procedures are to eliminate symptoms, encourage periapical tissue healing, promote continued root development, strengthen dentinal walls to reduce the probability of tooth fracture, and achieve a positive pulp vitality response.11,12 These patient- and clinician-based outcomes are achieved by traditional revascularization procedures.4,13
Despite reports of clinical and radiographic success, current regenerative endodontic procedures are generally regarded as a reparative therapeutic strategy; in contrast, true regeneration is regarded as the development of de novo dental pulp tissue in the root canal space, accompanied by recovery of regular homeostatic function.3,14,15 True regeneration is also expected to include the establishment of biological mechanisms at the dentin–pulp complex that would control the activity of tertiary dentinogenesis, thus, preventing progressive pulp space obliteration.3,14,15 Finally, a fully regenerated pulp tissue is expected to exhibit immunocompetence and would thus be able to defend itself from bacterial infection in a manner similar to that of normal pulp tissue.3,14,15
Stem cells, signaling molecules (e.g., growth factors) and scaffolds are the three critical components for success in tissue engineering. In regenerative endodontics, deciduous tooth pulp meets this requirement for tissue engineering components because it contains undifferentiated stem cells and growth factors in a natural extracellular microenvironment for cell growth; thus, deciduous tooth pulp offers a unique and readily available biological tool for use in regenerative endodontic therapies.16,17 This study was designed to explore the feasibility of pulp tissue regeneration via autologous transplantation of deciduous tooth pulp into immature necrotic permanent teeth using an experimental dog model.
Methods
Animals and experimental protocols
All experimental protocols in this study that included the care and use of experimental animals were approved by the Animal Research Ethics Committee and the Institutional Biosafety Committee of the Affiliated Stomatological Hospital of Nanchang University (Jiangxi, PR China). All animals were handled in accordance with the guidelines established by the Institutional Animal Care Committee.18
Four-month-old male Beagle dogs (n = 6) were purchased from Shanghai Xingang Laboratory Animal Farm (Shanghai, China). In each animal, 10 teeth (four maxillary incisors and all six mandibular incisors) were used in a split mouth design, such that 30 teeth served as the experimental group; the contralateral teeth (n = 30) served as controls. The split mouth design also ensured randomized allocation into equal numbers of maxillary and mandibular teeth in each experimental group. Before the experiments, all teeth underwent radiographic examination to confirm that they exhibited incomplete root formation and open apices.8,19
Establishment of periapical periodontitis
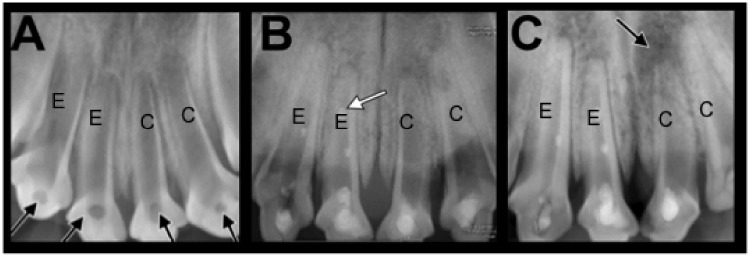
The dogs were anesthetized by intraperitoneal injection of 2% sodium pentobarbital; local anesthesia was administered using 2% lidocaine (Hubei Tianyao Pharmaceutical Co., Ltd. Xiangyang, China) without the application of a vasoconstrictor. Endodontic access cavities were prepared without isolation using water-cooled sterile spherical and cone diamond burs (K.G. Sorensen, São Paulo, Brazil), followed by tapered diamond burs to establish line access. The pulps were then extirpated, and the root canals were exposed to the oral cavity for 2 weeks to allow for the establishment of microbial biofilms and the development of inflammation. In immature teeth, the development of apical periodontitis typically occurs within 15 to 25 days.20 Accordingly, the onset of apical periodontitis, comprising radiolucent periapical lesions in open-apex teeth with thin and short roots, was verified by clinical and radiographic inspection at 2 weeks following induction of the lesions (Figures 1 and 2).
Figure 1.
Radiological analysis of maxillary incisors immediately prior to, and 3 and 6 months after treatment. Image A shows the apical periodontitis model established after 2 weeks (black arrows indicate unsealed access cavities). At this stage, maxillary incisors show thin root walls and open apices. Image B shows the model at 3 months postoperatively. At this stage, healing of periapical inflammation is indicated by reduction of radiolucency, narrowing of the apical foramen, thickening of root canal walls, and augmentation of root length. In some teeth, mineral trioxide aggregate fragmentation occurred, which is visible as small radiopaque spots within the canal (white arrow). Image C shows the model at 6 months postoperatively. At this stage, complete apical closure and advanced root growth are visible. Periapical radiolucency on the left maxillary central incisor indicates treatment failure (black arrow). Similar observations were made with mandible incisors (data not shown). E, experimental teeth; C, control teeth.
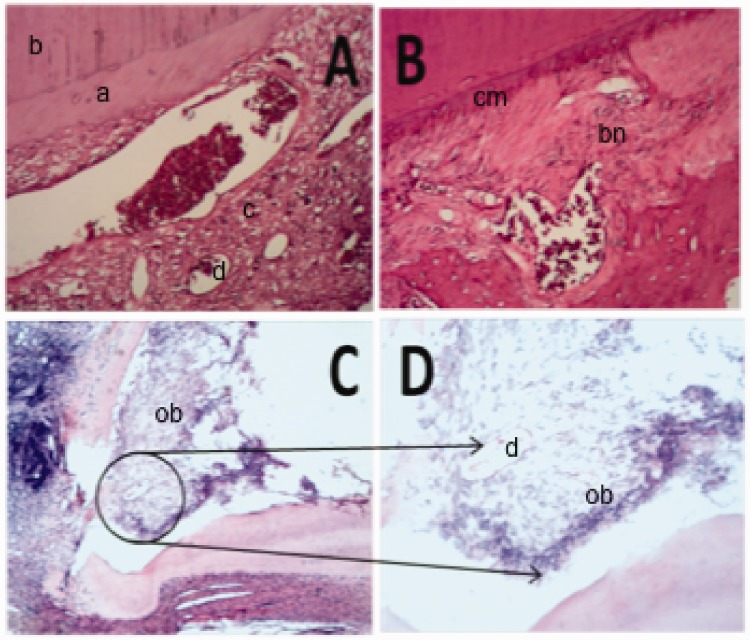
Figure 2.
Histological analysis via hematoxylin and eosin staining. Teeth that received transplanted deciduous pulp (panels A, C, and D) show generation of dentin-like structures, while teeth that underwent traditional revascularization (B) show mostly cementum-like repair tissue. Panel A is a representative image of central root tissues, showing dentin-like tissue formation with angular orientation of dentinal tubules (a) over existing dentin (b), as well as connective tissue (c) rich in blood vessels (d) and fibers (200× magnification). Panel B is a representative image of central root tissues, showing deposition of cementum-like (cm) repair tissue along the root canal wall and within the root space, incorporating bone lacunae (bn) (200×). Panel C is a representative image of the apical one-third region, showing odontoblast cells (ob) and connective tissue rich in blood vessels (d) and fibers (200×). Panel D is an enlargement of the region within the black circle in panel C, showing odontoblast cells (ob) and connective tissue (400×).
When periapical lesions were confirmed, the dogs were prepared for endodontic procedures: autologous pulp transplantation for experimental teeth and traditional revascularization for control teeth. All experimental procedures were performed under general and local anesthesia as described above, with rubber dam isolation. First, root canals were irrigated with 20 mL of 1.5% sodium hypochlorite, followed by 20 mL of sterile saline; they were then dried with sterile paper points. The canals were filled with a triple antibiotic paste that consisted of ciprofloxacin (Shanghai Zhongxi Sanwei Pharmaceutical Co., Ltd., Shanghai, China), minocycline (Wyeth Pharmaceutical Co., Ltd, Suzhou, China), and metronidazole (Chongqing Dikang Changjiang Pharmaceutical Co., Ltd., Chongqing, China) (at a ratio of 1:1:1) mixed with sterile water (approximately 1.0 g antibiotic powder per 1 mL of water); this mixture was applied below the cementoenamel junction. The access cavities were temporarily sealed with conventional glass ionomer cement (Suzhou Dental Co., Ltd., Suzhou, China). Four weeks later, the temporary cement was removed, and the canals were irrigated with 20 mL of sterile saline, followed by 20 mL of 17% EDTA and a brief final flush of sterile water.21 The root canals were dried with sterile paper points.
Autologous transplantation of deciduous tooth pulp
Teeth in the experimental group underwent pulp transplantation as follows: the deciduous cuspid was gently extracted under local anesthesia with 2% lidocaine (Hospira, Inc.) as described above. The crown was removed by creating a deep circumferential notch at the cementoenamel junction with a high-speed, water-cooled diamond disk, followed by separation of the crown from the root by the application of gentle digital pressure. The pulp tissue was then quickly removed using a small, sharp excavator with minimal trauma. Bleeding was then induced in permanent teeth by using a stainless steel file to gently agitate apical tissue 1 to 2 mm beyond the apical level; deciduous pulp tissues were immediately implanted into the root canal. The transplanted deciduous pulp was allowed to remain in contact with blood for 1 minute without interruption, and a thin layer (2–3-mm thickness) of ProRoot mineral trioxide aggregate (MTA, Dentsply Tulsa Dental, Tulsa, OK, USA) was placed over the blood clot.22 The remaining cavity space was filled with conventional glass ionomer cement temporarily as described above. One week later, the cavity space was filled with a resin composite (Z350, 3M/ESPE, St. Paul, MN, USA) by a total-etch adhesive protocol using Single Bond (3M/ESPE).
Traditional revascularization
Teeth in the control group were treated by following a traditional revascularization protocol.8,11,23 Briefly, apical bleeding was induced by using a stainless steel endodontic file to irritate the periapical tissues, such that the entire root canal was filled with blood to immediately below the cementoenamel junction. Following blot clot formation, MTA, glass ionomer, and Z350 resin were applied in a manner similar to that described for teeth in the experimental group.
Postoperative procedures, histological and immunohistochemical analysis
Radiographic examinations were carried out immediately, as well as at 3 and 6 months post-treatment, to evaluate continued periapical healing, apical closure, and thickening of the root canal wall. The resulting radiographs were evaluated three separate times by an oral radiologist, who was blinded to the different treatments. At the end of the experiments (6 months post-treatment), all animals were euthanized by an intravenous overdose of sodium pentobarbital (Merck, Darmstadt, Germany). Block sections of the jaws were dissected and fixed in 10% neutral-buffered formalin for 72 hours, then demineralized in 17% EDTA for 2 months; subsequently, sections were rinsed with buffered saline, dehydrated, and embedded in paraffin wax. Paraffin-embedded blocks were serially sectioned at 5-µm thickness using a Cryostat Microtome (Leica, Solms, Germany), and stained with hematoxylin and eosin (HE).19,24,25 Histological analysis under a light microscope (CX41, Olympus Optical Co., Tokyo, Japan) was conducted in triplicate by an oral pathologist who was also blinded to treatments. The following histopathological parameters were evaluated: the presence or absence of substantial new hard tissue; the presence or absence of healing of periapical inflammation that resulted in reduction of radiolucency; the presence or absence of continued thickening of the root canal wall; the presence or absence of apical foramen closure; and the presence or absence of an apparent reduction in apical diameter.
To evaluate angiogenesis, neurogenesis, and repair and/or regeneration of the pulp-dentin complex following autologous pulp transplant, immunohistochemical analyses were also performed. Briefly, the serial sections, prepared in a manner similar to that described above, were first subjected to antigen retrieval with proteinase K (30 µg/mL, Sigma-Aldrich, St. Louis, MO, USA), and then incubated with 0.3% hydrogen peroxide in methanol for 30 minutes. Subsequently, the sections were incubated with rabbit anti-CD31 (BA2966, Boster Bio, Wuhan, China), an immunomarker for vascularization,15,16 rabbit anti-Nestin (OM264981, Omin MAbs, Alhambra, CA, USA), a biomarker for nerve fibers, and rabbit anti-dentin sialoprotein (DSP) (BS-1748R, Bioss Antibodies, Biosynthesis Biotechnology Co., Ltd., Beijing, China), respectively; all antibodies were diluted in 1× phosphate-buffered saline.26 For negative controls, regular rabbit serum was used in place of the indicated antibodies. The sections were washed three times with phosphate-buffered saline, and then incubated with horseradish peroxidase-conjugated anti-rabbit IgG (SV0002, Boster Bio), diluted in 1× phosphate-buffered saline, for 30 minutes. After several additional washes with phosphate-buffered saline, the sections were incubated with 3,3’-diaminobenzidine substrate (DAB) (CWBIO, Beijing, China) to enable visualization of immunoreactivity. Sections were counterstained with hematoxylin and viewed with the CX41 microscope (Olympus Optical Co., Tokyo, Japan).
Statistical analysis
Results were analyzed by Fisher’s exact test using SPSS Statistics for Windows, Version 17.0 (SPSS Inc., Chicago, IL, USA). Differences with P < 0.05 were considered to be statistically significant.
Results
The animals were monitored closely throughout the experiments, and no unusual behaviors were observed, including changes to food intake. However, at the end of the experiment, 11 teeth were excluded from the study due to crown fractures, such that 25 teeth remained in the experimental group and 24 remained in the control group.
Radiographic analysis showed no major differences
Compared with radiographs taken prior to endodontic treatments, both the experimental and control groups displayed evidence of increased periapical healing at 3 months after the surgery, indicated by reduced radiolucency, reduced apical diameter, increased root canal width, and increased root length (Figure 1). At 6 months after the surgery, there was a marked increase in periapical healing (reduction in radiolucency), along with further reductions in apical diameters and closures of the apices of mandibular incisors, thickening of root canal walls, and further augmentation of root length in both groups (Figure 1). In most parameters evaluated, there were no significant differences, including in the amount of new hard tissue, healing of periapical inflammation, thickening of root canal walls, and closure of the apical foramen (Table 1); however, there was a significant reduction in apical diameter between the experimental group and the control group (P = 0.03, Table 1).
Table 1.
Radiographic and histologic analyses between groups*.
| Measurement | Experimental (n = 25) | Control (n = 24) | P value |
|---|---|---|---|
| Increase in root wall thickness | 0.64 ± 0.48 | 0.75 ± 0.43 | 0.41 |
| Apical closure | 0.64 ± 0.48 | 0.54 ± 0.50 | 0.49 |
| Periapical healing | 0.84 ± 0.37 | 0.67 ± 0.47 | 0.17 |
| New hard tissue deposition | 0.64 ± 0.48 | 0.75 ± 0.43 | 0.41 |
| Decrease in apical diameter | 0.92 ± 0.27 | 0.67 ± 0.47 | 0.03 |
*No significant differences were observed between groups in any parameters analyzed, except the apical diameter.
Considerable differences were detected in tissue regeneration
Both the experimental and control groups exhibited newly deposited mineralized tissue along the root walls and in the root canal space; however, the histological features of this new hard tissue considerably differed between the two groups (Figure 2). In the experimental group, HE staining revealed the presence of new dentin-like tissue along the canal walls, with apparent changes in dentinal tubule orientation (Figure 2). In contrast, the control group showed deposition of cementum-like tissue (Figure 2), which was present in a scattered fashion within bone fossae (data not shown).
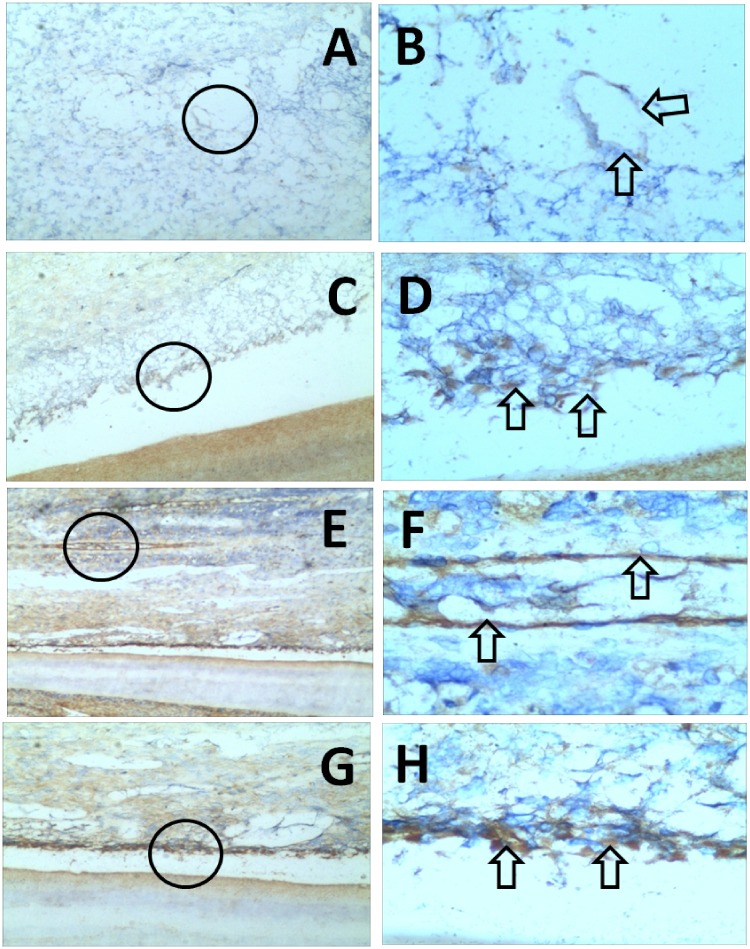
When further examination was performed by anti-DSP immunohistochemistry, odontoblast cells were found to be widely distributed in the regenerative tissue of the experimental group (Figure 3). In contrast, few odontoblasts were observed in the control group (data not shown), similar to the findings of HE staining analysis (Figure 2). In both the experimental and control groups, newly formed connective tissue within the root canals appeared to be rich in blood vessels (anti-CD31 reactive) and fibrous tissue (anti-Nestin reactive) (Figure 3), consistent with the findings of HE staining analysis (Figure 2). In the experimental group, the blood vessels also varied in size and distribution; the fibrous tissue contained large numbers of evenly distributed cells, and newly formed blood vessels were distributed in a cluster-like manner.
Figure 3.
Immunohistochemical analysis of the central one-third tissues of the experimental group. Panels A and B are representative images that show localization of CD31, an immunomarker for vascularization: A shows canal tissue rich in blood vessels (100×) and B is an enlargement of the region within the black circle in panel A (400×); arrows in panel B indicate a blood vessel. Panel C is a representative image that shows localization of dentin sialoprotein (DSP) in odontoblast cells (100×); D is an enlargement of the region within the black circle in panel C (400×), and arrows indicate odontoblast cells. Panels E–H are representative images that show localization of anti-Nestin antibody binding to nerve fibers and some transformed cells, such as pulp cells.49 Panel E shows odontoblast cells and connective tissue rich in blood vessels and fibers (40×); F is an enlargement of the region within the black circle in panel E (400×), and arrows indicate blood vessels and fibers. Panel G shows odontoblast cells (100×); H is an enlargement of the region within the black circle in panel G (400×), and arrows indicate odontoblast cells.
Discussion
To the best of our knowledge, this is the first report of a regenerative endodontic procedure that uses autologous deciduous tooth pulp transplant—a natural and vital three-dimensional scaffold, and a rich source of stem cells and growth factors—in the root canal of necrotic young permanent teeth. Since regenerative endodontics was introduced,2 most studies have used dental pulp stem cells, stem cells from exfoliated deciduous teeth, SCAPs, and bone marrow-derived stem cells.11,14,23,27–31 Deciduous tooth pulp contains a large number of deciduous pulp stem cells, which may serve as a good source of cells for pulp regeneration.32–38 The results from the present study indicate that the experimental autologous pulp transplantation approach can achieve clinical and radiographic outcomes similar to those reported in studies of the traditional revascularization technique,8,25 including healing of periapical inflammation, continued thickening of the root canal wall, augmentation of root length, and reduction of apical diameter.
Newly formed mineralized tissues were consistently observed in the root canal space, attached to the inner dentine wall or the inner lumen, at 3 months after treatment in both groups; this was similar to the findings of prior studies,8,25 although histological analysis showed some fundamental differences in mineralized tissues between the two groups. In the control group, tissue that formed over existing root dentin exhibited characteristics of cementum-like hard tissue; this was indicative of repair, rather than regeneration. Similarly, recent studies by da Silva et al.26,39,40 showed that hard tissues formed in the canals of revascularized immature teeth comprised heterogeneous mineralized tissues that resembled cementum or bone and soft tissues; new connective tissues in these canals were more similar to periodontal ligament than to normal pulp. Conversely, the mineralized tissue that developed in the experimental group closely resembled dentin, as it contained dentinal tubules; moreover, soft tissues within the root canals were rich in blood vessels and fibers. In addition, there were significant differences in apical diameter reduction between the experimental group and the control group. These differences may be partly attributed to the different types of stem cells used. Specifically, stem cells used in the control group were mainly derived from SCAPs, whereas those used in the experimental group were mostly from exfoliated deciduous teeth. In support of this theory, Arora et al. and Shi et al.33,37 have shown that stem cells from exfoliated deciduous teeth can differentiate into odontoblast-like cells and secrete dentin matrix, forming odontogenic dentin pulp-like tissue, odontoblast-like cells, and bone formation in vivo; eventually, these stem cells from exfoliated deciduous teeth can form mineralized dentin tissues (this process was recently reviewed by Xuan et al.32).
Of the steps involved in regenerative endodontics, the initial choice of an appropriate scaffold is crucial for success. An ideal scaffold should be biocompatible and facilitate the proliferation of the cell population required for structural and functional replacement of the target tissue.41 In terms of providing a biological scaffold, induced blood clots are often difficult to obtain in a clinical setting, and thus may require the use of autologous platelet concentrates (e.g., platelet plasma or platelet fibrin) as an alternative approach.41–45 Unlike plasma or fibrin, autologous deciduous dental pulp tissue does not require processing and can readily serve as a biological scaffold that contains multiple inherent growth factors, such as transforming growth factor-β, fibroblast growth factor-2, platelet-derived growth factor, and vascular endothelial growth factor. These growth factors attract stem cells and promote stem cell proliferation by promoting angiogenesis in the initial stages of healing.46 In addition, the ability of deciduous dental pulp stem cells to establish vascularization is more robust than that of dental pulp stem cells;47 in the presence of an open apical foramen, deciduous dental pulp stem cells may help establish vasculature to ensure sufficient nutrient supply for tissue regeneration. It should be noted that deciduous stem cells were not isolated and cultured separately in this experiment. Therefore, the microenvironment surrounding the stem cells was nearly identical to their native physiologic environment, which may have aided in their proliferation and differentiation.
Following root canal irrigation, multiple cytokines and dentin matrix proteins can be released from the root canal wall,48 and blood clots in the root canal can release a variety of growth factors, such as vascular endothelial growth factor and epidermal growth factor;10 together, these cytokines, matrix proteins, and growth factors promote the directional differentiation of stem cells. In the traditional revascularization approach, the blood clot formed by induced apical bleeding is believed to provide a scaffold that contains SCAPs and growth factors. Ideally, this promotes the growth and differentiation of stem cells and supports the growth of tissues into the root canal space, concomitant with continued root development.41–43 In deciduous pulp implantation, the implanted tissue is already vital and initiates a series of biological processes when it comes into contact with the induced blood; because of these processes, SCAPs and stem cells from exfoliated deciduous teeth are activated. Stem cells from exfoliated deciduous teeth are capable of extensive proliferation and multipotent differentiation, which might be have contributed to favorable biological outcomes in this study, including the dentin-like tissue morphology observed in the experimental group. Additionally, autologous deciduous pulp provides a matching donor with no risk of communicable disease, counteractive immune reactions, or tissue rejection, as well as no need for immunosuppression.
Conclusion
Dogs share many similarities with humans in terms of tooth structure and pathophysiological responses. Using a dog model, this study has provided evidence that implantation of deciduous tooth pulp tissue can yield favorable clinical, radiographic, and histological outcomes. These results suggest that autologous transplantation of deciduous tooth pulp can be used as a new and effective biologically based treatment approach to facilitate dental pulp regeneration in necrotic young permanent teeth. The procedure is simple and convenient, but further studies, including clinical trials, are needed before such an approach can be recommended for clinical application. Studies are underway to elucidate the structures and differentiation abilities of the implanted tissues and the underlying mechanisms of tissue formation.
Acknowledgments
The authors would like to thank Dr. Zezhang T. Wen and Dr. Janice A. Townsend at the LSUHSC School of Dentistry Department of Comprehensive Dentistry and Biomaterials, New Orleans, LA, USA for their insightful discussions during the preparation of the manuscript.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This research was funded in part by the Science and Technology Department of Jiangxi Province, PR China (grant #S2017YBYFE1007 to Yan Huang).
References
- 1.Thibodeau B, Trope M. Pulp revascularization of a necrotic infected immature permanent tooth: case report and review of the literature. Pediatr Dent 2007; 29: 47–50. [PubMed] [Google Scholar]
- 2.Banchs F, Trope M. Revascularization of immature permanent teeth with apical periodontitis: new treatment protocol? J Endod 2004; 30: 196–200. [DOI] [PubMed] [Google Scholar]
- 3.Diogenes A, Ruparel NB, Shiloah Y, et al. Regenerative endodontics: a way forward. J Am Dent Assoc 2016; 147: 372–380. [DOI] [PubMed] [Google Scholar]
- 4.Fouad AF, Verma P. Healing after regenerative procedures with and without pulpal infection. J Endod 2014; 40: S58–S64. [DOI] [PubMed] [Google Scholar]
- 5.El Ashiry EA, Farsi NM, Abuzeid ST, et al. Dental pulp revascularization of necrotic permanent teeth with immature apices. J Clin Pediatr Dent 2016; 40: 361–366. [DOI] [PubMed] [Google Scholar]
- 6.Nagata JY, Rocha-Lima TF, Gomes BP, et al. Pulp revascularization for immature replanted teeth: a case report. Aust Dent J 2015; 60: 416–420. [DOI] [PubMed] [Google Scholar]
- 7.Iwaya SI, Ikawa M, Kubota M. Revascularization of an immature permanent tooth with apical periodontitis and sinus tract. Dent Traumatol 2001; 17: 185–187. [DOI] [PubMed] [Google Scholar]
- 8.Rodriguez-Benitez S, Stambolsky C, Gutierrez-Perez JL, et al. Pulp revascularization of immature dog teeth with apical periodontitis using triantibiotic paste and platelet-rich plasma: a radiographic study. J Endod 2015; 41: 1299–1304. [DOI] [PubMed] [Google Scholar]
- 9.Huang GT, Sonoyama W, Liu Y, et al. The hidden treasure in apical papilla: the potential role in pulp/dentin regeneration and bioroot engineering. J Endod 2008; 34: 645–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wigler R, Kaufman AY, Lin S, et al. Revascularization: a treatment for permanent teeth with necrotic pulp and incomplete root development. J Endod 2013; 39: 319–326. [DOI] [PubMed] [Google Scholar]
- 11.American Association of Endodontists. Clinical considerations for a regenerative procedure. http://www.aae.og/specialty/wp-content/uploads/sites/2/2017/062018 [cited 2018].
- 12.Yang J, Yuan G, Chen Z. Pulp regeneration: current approaches and future challenges. Front Physiol 2016; 7: 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Trope M. Treatment of the immature tooth with a non-vital pulp and apical periodontitis. Dent Clin North Am 2010; 54: 313–324. [DOI] [PubMed] [Google Scholar]
- 14.Schmalz G, Smith AJ. Pulp development, repair, and regeneration: challenges of the transition from traditional dentistry to biologically based therapies. J Endod 2014; 40: S2–S5. [DOI] [PubMed] [Google Scholar]
- 15.Simon SR, Tomson PL, Berdal A. Regenerative endodontics: regeneration or repair? J Endod 2014; 40: S70–S75. [DOI] [PubMed] [Google Scholar]
- 16.Miura M, Gronthos S, Zhao M, et al. SHED: stem cells from human exfoliated deciduous teeth. Proc Natl Acad Sci U S A 2003; 100: 5807–5812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cehreli ZC, Isbitiren B, Sara S, et al. Regenerative endodontic treatment (revascularization) of immature necrotic molars medicated with calcium hydroxide: a case series. J Endod 2011; 37: 1327–1330. [DOI] [PubMed] [Google Scholar]
- 18.Couto M, Cates C. Laboratory guidelines for animal care. Methods Mol Biol 2019; 1920: 407–430. [DOI] [PubMed] [Google Scholar]
- 19.Thibodeau B, Teixeira F, Yamauchi M, et al. Pulp revascularization of immature dog teeth with apical periodontitis. J Endod 2007; 33: 680–689. [DOI] [PubMed] [Google Scholar]
- 20.Leonardo MR, da Silva LA, Leonardo Rde T, et al. Histological evaluation of therapy using a calcium hydroxide dressing for teeth with incompletely formed apices and periapical lesions. J Endod 1993; 19: 348–352. [DOI] [PubMed] [Google Scholar]
- 21.Berkhoff JA, Chen PB, Teixeira FB, et al. Evaluation of triple antibiotic paste removal by different irrigation procedures. J Endod 2014; 40: 1172–1177. [DOI] [PubMed] [Google Scholar]
- 22.Guimaraes BM, Tartari T, Marciano MA, et al. Color stability, radiopacity, and chemical characteristics of white mineral trioxide aggregate associated with 2 different vehicles in contact with blood. J Endod 2015; 41: 947–952. [DOI] [PubMed] [Google Scholar]
- 23.Chen MY, Chen KL, Chen CA, et al. Responses of immature permanent teeth with infected necrotic pulp tissue and apical periodontitis/abscess to revascularization procedures. Int Endod J 2012; 45: 294–305. [DOI] [PubMed] [Google Scholar]
- 24.Galler KM, D'Souza RN, Federlin M, et al. Dentin conditioning codetermines cell fate in regenerative endodontics. J Endod 2011; 37: 1536–1541. [DOI] [PubMed] [Google Scholar]
- 25.Yamauchi N, Yamauchi S, Nagaoka H, et al. Tissue engineering strategies for immature teeth with apical periodontitis. J Endod 2011; 37: 390–397. [DOI] [PubMed] [Google Scholar]
- 26.Yamauchi N, Nagaoka H, Yamauchi S, et al. Immunohistological characterization of newly formed tissues after regenerative procedure in immature dog teeth. J Endod 2011; 37: 1636–1641. [DOI] [PubMed] [Google Scholar]
- 27.Huang GT, Yamaza T, Shea LD, et al. Stem/progenitor cell-mediated de novo regeneration of dental pulp with newly deposited continuous layer of dentin in an in vivo model. Tissue Eng Part A 2010; 16: 605–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang J, Wang X, Sun Z, et al. Stem cells from human-exfoliated deciduous teeth can differentiate into dopaminergic neuron-like cells. Stem Cells Dev 2010; 19: 1375–1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang Y, Zeng Q. Regeneration of endodontics: a new direction of dental pulp regeneration. Int J Stomatol 2016; 43: 495–499. [Google Scholar]
- 30.Hargreaves KM, Diogenes A, Teixeira FB. Treatment options: biological basis of regenerative endodontic procedures. J Endod 2013; 39: S30–S43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Torabinejad M, Turman M. Revitalization of tooth with necrotic pulp and open apex by using platelet-rich plasma: a case report. J Endod 2011; 37: 265–268. [DOI] [PubMed] [Google Scholar]
- 32.Xuan K, Li B, Guo H, et al. Deciduous autologous tooth stem cells regenerate dental pulp after implantation into injured teeth. Sci Transl Med 2018; 10: pii: eaaf3227. [DOI] [PubMed] [Google Scholar]
- 33.Shi S, Bartold PM, Miura M, et al. The efficacy of mesenchymal stem cells to regenerate and repair dental structures. Orthod Craniofac Res 2005; 8: 191–199. [DOI] [PubMed] [Google Scholar]
- 34.Viale-Bouroncle S, Gosau M, Kupper K, et al. Rigid matrix supports osteogenic differentiation of stem cells from human exfoliated deciduous teeth (SHED). Differentiation 2012; 84: 366–370. [DOI] [PubMed] [Google Scholar]
- 35.Vakhrushev IV, Antonov EN, Popova AV, et al. Design of tissue engineering implants for bone tissue regeneration of the basis of new generation polylactoglycolide scaffolds and multipotent mesenchymal stem cells from human exfoliated deciduous teeth (SHED cells). Bull Exp Biol Med 2012; 153: 143–147. [DOI] [PubMed] [Google Scholar]
- 36.de Mendonca Costa A, Bueno DF, Martins MT, et al. Reconstruction of large cranial defects in nonimmunosuppressed experimental design with human dental pulp stem cells. J Craniofac Surg 2008; 19: 204–210. [DOI] [PubMed] [Google Scholar]
- 37.Arora V, Arora P, Munshi AK. Banking stem cells from human exfoliated deciduous teeth (SHED): saving for the future. J Clin Pediatr Dent 2009; 33: 289–294. [DOI] [PubMed] [Google Scholar]
- 38.Morsczeck C, Vollner F, Saugspier M, et al. Comparison of human dental follicle cells (DFCs) and stem cells from human exfoliated deciduous teeth (SHED) after neural differentiation in vitro. Clin Oral Investig 2010; 14: 433–440. [DOI] [PubMed] [Google Scholar]
- 39.da Silva LA, Nelson-Filho P, da Silva RA, et al. Revascularization and periapical repair after endodontic treatment using apical negative pressure irrigation versus conventional irrigation plus triantibiotic intracanal dressing in dogs' teeth with apical periodontitis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2010; 109: 779–787. [DOI] [PubMed] [Google Scholar]
- 40.Wang X, Thibodeau B, Trope M, et al. Histologic characterization of regenerated tissues in canal space after the revitalization/revascularization procedure of immature dog teeth with apical periodontitis. J Endod 2010; 36: 56–63. [DOI] [PubMed] [Google Scholar]
- 41.Lovelace TW, Henry MA, Hargreaves KM, et al. Evaluation of the delivery of mesenchymal stem cells into the root canal space of necrotic immature teeth after clinical regenerative endodontic procedure. J Endod 2011; 37: 133–138. [DOI] [PubMed] [Google Scholar]
- 42.Jadhav G, Shah N, Logani A. Revascularization with and without platelet-rich plasma in nonvital, immature, anterior teeth: a pilot clinical study. J Endod 2012; 38: 1581–1587. [DOI] [PubMed] [Google Scholar]
- 43.Narang I, Mittal N, Mishra N. A comparative evaluation of the blood clot, platelet-rich plasma, and platelet-rich fibrin in regeneration of necrotic immature permanent teeth: a clinical study. Contemp Clin Dent 2015; 6: 63–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bezgin T, Yilmaz AD, Celik BN, et al. Efficacy of platelet-rich plasma as a scaffold in regenerative endodontic treatment. J Endod 2015; 41: 36–44. [DOI] [PubMed] [Google Scholar]
- 45.Chmilewsky F, Jeanneau C, Dejou J, et al. Sources of dentin-pulp regeneration signals and their modulation by the local microenvironment. J Endod 2014; 40: S19–S25. [DOI] [PubMed] [Google Scholar]
- 46.Nishino Y, Yamada Y, Ebisawa K, et al. Stem cells from human exfoliated deciduous teeth (SHED) enhance wound healing and the possibility of novel cell therapy. Cytotherapy 2011; 13: 598–605. [DOI] [PubMed] [Google Scholar]
- 47.Mao JJ. Stem cells and the future of dental care. N Y State Dent J 2008; 74: 20–24. [PubMed] [Google Scholar]
- 48.Srisuwan T, Tilkorn DJ, Al-Benna S, et al. Revascularization and tissue regeneration of an empty root canal space is enhanced by a direct blood supply and stem cells. Dent Traumatol 2013; 29: 84–91. [DOI] [PubMed] [Google Scholar]
- 49.Neradil J, Veselska R. Nestin as a marker of cancer stem cells. Cancer Sci 2015; 106: 803–811. [DOI] [PMC free article] [PubMed] [Google Scholar]