Short abstract
Objective
Breast cancer (BC) is a common malignancy among women worldwide. Fibroblast growth factor receptor 2 (FGFR2) rs2981582 is reported to play a vital role in BC development. However, the relationship between them remains unclear.
Methods
Ninety-five patients and 140 healthy controls were enrolled in the study. Plasma DNA was genotyped by the MassARRAY method. A meta-analysis was conducted to clarify the effect of FGFR2 polymorphism on BC risk.
Results
Our case-control study results revealed a significant difference in CC, TC, and TT genotypes between patients and controls. Logistic regression analysis showed that TT and TC genotype and the dominant mode were significantly correlated with BC development [odds ratio (OR) = 1.21, 95% confidence interval (CI): 1.050–2.27; OR = 1.81, 95% CI: 1.24–2.73; OR = 2.15, 95% CI: 1.25–5.31, respectively], even after adjusting for age, body weight, drinking, smoking, and estrogen receptor status. A meta-analysis of 15 studies showed significant differences among the dominant, recessive, heterozygote, and homozygote models between patients and controls.
Conclusions
Our results showed an association of FGFR2 rs2981582 polymorphism with BC in an Asian population. However, a more comprehensive study of the relationship between the polymorphism and BC is still needed.
Keywords: Breast cancer, FGFR2, rs2981582, meta-analysis, polymorphism, fibroblast growth factor receptor 2
Introduction
Breast cancer (BC) is one of the most common malignancies and a major cause of death among women. In China, BC accounts for 15% of new cases of cancer in women, with morbidity and mortality increasing year by year.1,2 Although the exact mechanism of BC is still not completely elucidated, a number of comprehensive analyses have shown that genomic and gene expression patterns contribute to the morbidity.3,4
Fibroblast growth factor receptor 2 (FGFR2, also known as CEK3), located at chromosome 10q26 and containing 22 exons, is a member of the FGFR family of tyrosine kinase receptors.5,6 It participates in the process of tumorigenesis by inducing mitogenic and survival signals and promoting invasiveness and angiogenesis.7,8 FGFR2 overexpression was observed in breast cancer cell lines and breast tumor tissues in estrogen receptor (ER)-positive BC as early as 1992.9 Since then, a large number of studies on the relationship between polymorphisms in the FGFR2 gene and BC have been implemented in different regions of the world.
Large genome-wide association studies (GWAS) have demonstrated that an intronic FGFR2 polymorphism, rs2981582, is significantly associated with BC.10–12 Meyer et al.13 reported that the T allele of rs2981582 has been linked to a higher level of FGFR2 transcription in both breast cancer cell lines and tumors. This link between rs2981582 and FGFR2 transcription is variable in patients of different ethnic origins and therefore sometimes contradictory. Murillo-Zamora et al.14 reported that the T allele of rs2981582 was significantly associated with an increased risk of BC in Mexican women [odds ratio (OR) = 1.24, 95% confidence interval (CI): 1.06–1.46). Ledwoń et al.15 reported a similar result in Polish women (OR = 1.31, 95% CI: 1.17–1.45). In contrast, Bayraktar et al. found no relationship of rs2981582 with BC in an American population.16 Chen et al.17 demonstrated that the CT and TT genotypes of rs2981582 reduced BC risk in a Han Chinese population (p = 0.006). Therefore, to clarify the association of FGFR2 rs2981582 polymorphism and sporadic BC, we performed a comprehensive association analysis in a case-control study in a Chinese Han population; we then conducted a meta-analysis on all case-control studies to make a more accurate assessment of the relationship.
Materials and methods
Patient recruitment
Patients were consecutively recruited from June to October 2016 among inpatients in Xi’an Hospital of Traditional Chinese Medicine. Ninety-five female patients who were histopathologically diagnosed with BC and did not have a family history of BC were included in the study. The control group comprised 140 unrelated healthy women. Patients with a history of other types of cancer, hormone replacement therapy, or other serious diseases were excluded from this study. This study was approved by the Ethical Committee of Xi’an Hospital of Traditional Chinese Medicine, and all patients and controls gave written informed consent for participation in this research. All of the studies were performed in accordance with the Declaration of Helsinki.
Genotyping
Venous blood samples were collected in tubes with EDTA anticoagulant and stored at −80°C until use. Genomic DNA was isolated from whole blood using the Wizard kit (Promega, Madison, WI, USA) according to the manufacturer’s protocol. Genotyping of rs2981582 was carried out following time-of-flight mass spectrometry on a MassARRAY iPLEX platform (Sequenom, San Diego, CA, USA). The average genotype call rate for the single nucleotide polymorphism (SNP) was >99%.
Meta-analysis
Studies published through 20 May 2019 were identified by a systematic literature search in PubMed, Embase, Web of Knowledge, China National Knowledge Infrastructure (CNKI), and WanFang Data. The medical subject headings (MeSH) and title/abstract were used to identify all eligible studies that mainly focused on the genotype of rs2981582 polymorphism. Eligible studies had to meet the following criteria: (1) the study evaluated the association between risk of BC and FGFR2 rs2981582 polymorphism in Asian populations; (2) the report focused on detailed genotype frequencies among patients with sporadic BC; and (3) the investigation was a case-control study. The exclusion criteria were as follows: (1) comment, review, or editorial articles; and (2) studies without detailed genotype data or with overlapping data. Publication bias was evaluated by using the Begg’s test.
Statistical analysis
All statistical analyses were performed using SPSS (version 17.0, SPSS Inc., Chicago, IL, USA). Pearson’s chi-squared test or the Mann-Whitney U test was performed to test the association between rs2981582 polymorphism in cases and controls. Logistic regression analysis was used to evaluate the contribution of genetic and nongenetic factors under the dominant, recessive, and additive models.
Stata software (version 12.0; Stata Corporation, College Station, TX, USA) was used for meta-analysis. The OR and 95% CI were calculated to assess the relationship between the polymorphism and BC. Pooled ORs were obtained from combinations of single study under the dominant model (TT + TC vs. CC), the recessive model (TT vs. TC + CC), the homozygous model, and the heterozygote model. Heterogeneity was tested by the Q test (Chi squared test; p < 0.10 was considered indicative of statistically significant heterogeneity) and the I2 statistic (values of 25%, 50%, and 75% were considered to represent low, medium, and high heterogeneity, respectively). If I2 > 50%, the random-effect model (DerSimonian–Laird method) was used to calculate the pooled ORs; otherwise, the fixed effect model (Mantel–Haenszel method) was used. Begg’s linear regression tests were applied to assess the potential publication bias.
Results
Characteristics of the study participants
Ninety-five patients and 140 healthy controls were recruited in our study. Seventy patients were at clinical stage I to II and 25 patients were at stage III. In the patient group, 24.3% had a drinking habit and 25.1% had a history of smoking. In the healthy control group, 18.5% had a drinking habit and 19.4% had a history of smoking. The demographic characteristics of the subjects in the patient and control groups are summarized in Table 1.
Table 1.
Demographic data of enrolled participants.
| Variable | Case (n = 95) | Controls (n = 140) | p-value |
|---|---|---|---|
| Age (years) | 47.22 ± 12.43 | 48.15 ± 10.61 | 0.192 |
| Body weight (kg) | 48.31 ± 11.64 | 45.20 ± 10.57 | 0.086 |
| Tobacco consumption | |||
| Yes | 25.1% | 19.4% | 0.091 |
| No | 74.9% | 80.6% | |
| Drinking | |||
| Yes | 24.3% | 18.5% | 0.065 |
| No | 75.7% | 76.5% | |
| Clinical stage | |||
| I-II | 70 | ||
| III | 25 | ||
| ER | |||
| Positives | 31 (32.6%) | ||
| Negative | 64 (67.4%) | ||
Genetics analysis
The minor allele frequency (MAF) was 0.419. A significant difference was observed between the patient group and the control group in the three genotypes, TT, TC, and CC (p = 0.021) (Table 2). A logistic regression analysis of rs2981582 SNP genotype between patients and controls was also performed. The results showed that the TT and TC alleles were significantly correlated with BC development (OR = 1.21, 95% CI: 1.050–2.27, p = 0.019; OR = 1.81, 95% CI: 1.24–2.73, p = 0.035), even after adjusting for age, drinking, smoking, and ER status (Table 3).
Table 2.
Genotypes of FGFR2 rs2981582 polymorphism between patient (case) and control group.
| rs2981582 | Control | Case | p-value |
|---|---|---|---|
| CC | 16 | 24 | 0.021 |
| TC | 59 | 33 | |
| TT | 65 | 38 | |
| C | 91 | 81 | 0.025 |
| T | 189 | 109 |
Table 3.
Logistic regression analysis of rs2981582 SNP genotype with breast cancer.
| rs2981582 | OR | 95% CI | p-value | OR* | 95% CI* | p-value* |
|---|---|---|---|---|---|---|
| CC | Reference | Reference | ||||
| TT | 1.21 | 1.050–2.27 | 0.019 | 1.19 | 1.172–2.957 | 0.017 |
| TC | 1.81 | 1.24–2.73 | 0.035 | 1.80 | 1.169–2.71 | 0.031 |
| Dominant model | 2.15 | 1.25–5.31 | 0.043 | 2.1 | 1.21–5.28 | 0.041 |
| Recessive model | 1.14 | 0.37–1.9 | 0.165 | 1.12 | 0.31–1.85 | 0.152 |
*Adjusting for age, body weight, smoking, drinking, and estrogen receptor status. Significant associations are marked in bold.
In addition, we assessed the influence of genetic models (dominant, recessive, and additive models) on BC risk; however, no significant associations in the recessive model were observed (OR = 1.14, 95% CI: 0.37–1.9). In contrast, there was a significant difference in the dominant model (OR = 2.15, 95% CI: 1.25–5.31, p = 0.043). These differences remained even after adjusting for age, drinking, smoking, and ER status (Table 3).
Meta-analysis
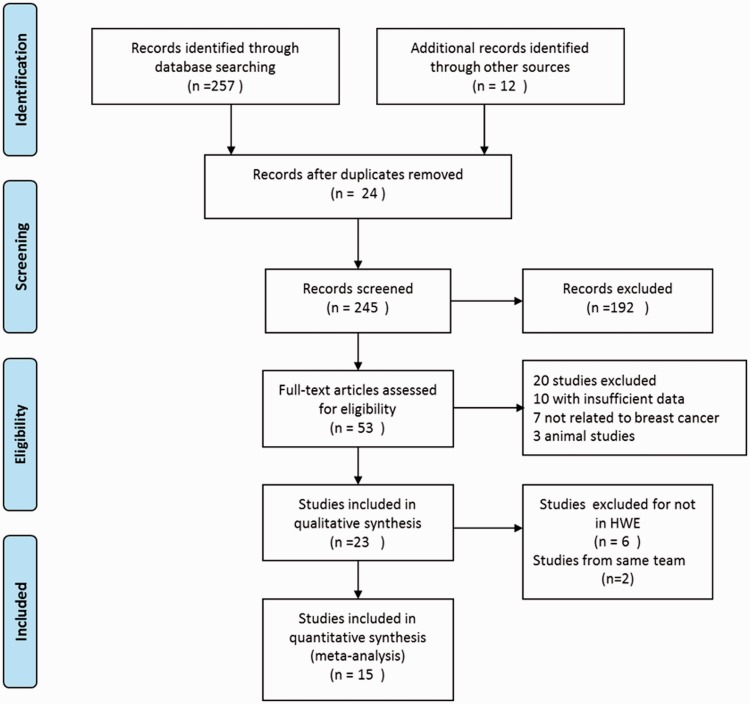
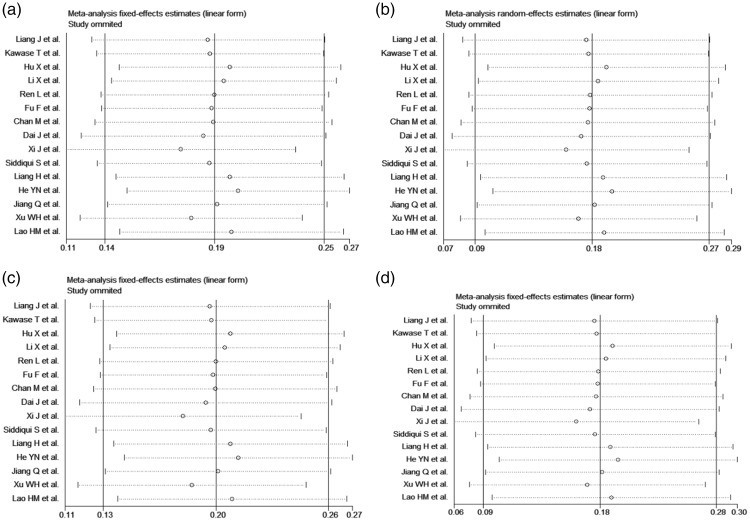
Fifteen relevant studies were identified from the literature search. The flow diagram of the search process is illustrated in Figure 1, and the characteristics of the enrolled studies are summarized in Table 4. Heterogeneity was identified by the Q test and I2 statistic, and no significant publication bias was found by the Begg’s test (p > 0.05) (Figure 2). We performed a sensitivity analysis to assess the influence of each individual study on the pooled OR by sequentially removing each eligible study. The results indicated that no single study influenced the quality of the pooled ORs in the sensitivity analysis (Figure 3).
Figure 1.
Flow sheet summarizing identification and selection of studies.
Table 4.
The basic characteristics of the 15 studies in the meta-analysis, showing FGFR2 rs2981582 single nucleotide polymorphism genotype and allele distribution in breast cancer patients (cases) and controls.
| Studies | Year | Total Number |
Case |
Control |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Case | Control | TT | TC | CC | T | C | TT | TC | CC | T | C | Method | HWE | ||
| Liang J et al.22 | 2008 | 1049 | 1073 | 119 | 460 | 447 | 698 | 1354 | 91 | 439 | 532 | 621 | 1503 | Taqman | 0.974 |
| Kawase T et al. 23 | 2009 | 456 | 912 | 42 | 192 | 221 | 276 | 634 | 63 | 347 | 502 | 473 | 1351 | TaqMan | 0.773 |
| Hu X et al.24 | 2011 | 203 | 200 | 47 | 78 | 78 | 172 | 234 | 26 | 95 | 79 | 147 | 253 | PCR-RFLP | 0.758 |
| Li X et al.25 | 2011 | 869 | 464 | 54 | 180 | 167 | 288 | 514 | 60 | 189 | 192 | 309 | 573 | MassArray | 0.219 |
| Ren L et al.26 | 2011 | 936 | 471 | 130 | 400 | 426 | 660 | 1252 | 56 | 181 | 234 | 293 | 649 | Taqman | 0.361 |
| Fu F et al.27 | 2012 | 118 | 104 | 21 | 55 | 42 | 97 | 139 | 8 | 47 | 49 | 63 | 145 | MassARRAY | 0.474 |
| Chan M et al.28 | 2012 | 1191 | 1534 | 155 | 527 | 486 | 837 | 1499 | 162 | 618 | 695 | 942 | 2008 | Taqman | 0.165 |
| Dai J et al.29 | 2012 | 914 | 967 | 216 | 820 | 732 | 1252 | 2284 | 164 | 796 | 884 | 1124 | 2564 | TaqMan | 0.424 |
| Xi J et al.30 | 2014 | 839 | 863 | 100 | 423 | 292 | 623 | 1007 | 94 | 376 | 379 | 564 | 1134 | MALDI-TOF | 0.959 |
| Siddiqui S et al.31 | 2014 | 368 | 484 | 56 | 168 | 144 | 280 | 456 | 53 | 205 | 226 | 311 | 657 | PCR-RFLP | 0.526 |
| Liang H et al.32 | 2015 | 609 | 882 | 103 | 266 | 238 | 472 | 742 | 111 | 375 | 370 | 597 | 1115 | MassARRAY | 0.298 |
| He YN et al.33 | 2015 | 253 | 343 | 41 | 103 | 109 | 185 | 321 | 49 | 157 | 137 | 255 | 431 | iMLDR | 0.710 |
| Jiang Q et al.34 | 2014 | 35 | 35 | 16 | 10 | 9 | 42 | 28 | 4 | 18 | 13 | 26 | 44 | DHPLC | 0.549 |
| Xu WH et al.35 | 2012 | 280 | 280 | 20 | 174 | 86 | 214 | 346 | 29 | 131 | 120 | 189 | 371 | PCR-RFLP | 0.439 |
| Lao HM et al.36 | 2012 | 623 | 620 | 46 | 150 | 155 | 242 | 460 | 48 | 151 | 151 | 247 | 453 | MassARRAY | 0.301 |
HWE, Hardy-Weinberg equilibrium; PCR-RFLP, polymerase chain reaction-restriction fragment length polymorphisms, MALDI-TOF, matrix-assisted laser desorption/ionization time-of-flight, iMLDR, improved multiplex ligase detection reaction; DHPLC, denaturing high performance liquid chromatography.
Figure 2.
The publication bias examined by Begg’s test in the dominant model (TC + TT vs. CC).
Figure 3.
Sensitivity analysis of the association between FGFR2 rs2981582 polymorphism and breast cancer susceptibility in the dominant model (TC + TT vs. CC).
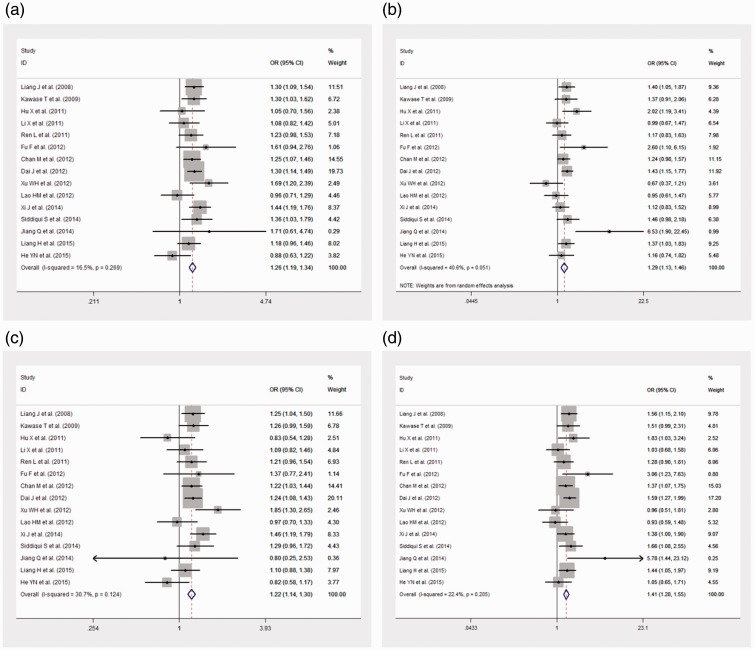
We used a pooled meta-analysis to determine BC risk and rs2981582 polymorphism in the Asian population under the recessive, dominant, homozygous, and heterozygote models (Figure 4). Our results showed that all four models could significantly increase the BC risk (dominant model: OR = 1.259, 95% CI: 1.187–1.336; recessive model: OR = 1.29, 95% CI: 1.13–1.46; heterozygote model: OR = 1.22, 95% CI: 1.14–1.30; homozygote model: OR = 1.41, 95% CI: 1.28–1.55).
Figure 4.
Forest plot of FGFR2 rs2981582 polymorphism and breast cancer susceptibility. (a) Dominant model (TC + TT vs. CC); (b) recessive model (TT vs. TC + CC); (c) heterogeneity model (TC vs. CC); and (d) homozygote model (TT vs. CC).
Discussion
Breast cancer is a disease that results from the interaction of environmental and genetic factors, and it is now a leading cause of cancer-related deaths in women worldwide.18,19 The incidence rate is increasing annually and patients are being diagnosed at an earlier age. However, the etiology, in terms of genetic risk factors associated with prognosis, is still not fully understood. FGFR2 has been reported to be associated with many diseases, especially with carcinogenesis. As a transmembrane protein, FGFR2 has an extracellular region, a transmembrane region, and an intracellular region. Once activated, it begins a cascade reaction through the RAS-MAPK and JAK-STAT signal pathways and then regulates the transcription of downstream genes involved in physiological and pathological activities, such as cell proliferation, differentiation, migration, and apoptosis.
In our study, the FGFR2 rs2981582 polymorphisms in the TT and TC genotypes were significantly correlated with BC development. These results were partly in accordance with reports by Liu et al.20 and Mazhar et al.21 However, those authors reported a significant association of genotype TT with the development of BC, whereas in our study both the TT and TC genotypes were significantly associated with BC. In addition, we found that either the dominant or additive model carried an increased risk of BC. To further elucidate the possible relationship of the polymorphism and BC, we performed a meta-analysis that included 15 studies in the Asian population. The meta-analysis results showed that the recessive model was also significantly associated with BC, which was different from our result in the case-control study.
Meyer et al.13 supplied the first experimental evidence of mechanisms between particular FGFR2 intronic SNPs and breast cancer risk. Those authors showed that 2 FGFR2 intronic SNPs (rs7895676 and rs2981578) altered the binding affinity of FGFR2 for transcription factors and increased FGFR2 expression.13 However, based on published studies, the associations of rs2981582 polymorphism with BC are still inconsistent. These different conclusions may result from the diversity of genetic backgrounds and carcinogenic factors. As a member of the FGFR family, FGFR2 has been found to be overexpressed in breast cancer cell lines. Aberrant expression of alternatively spliced isoforms of FGFR2 has been shown to activate cell mitogenesis and differentiation and lead to transformation in breast cancer cells. Nevertheless, this could be influenced by age, family history, reproductive and gynecologic factors, as well as lifestyle factors, such as alcohol consumption and lack of physical activity. Thus, the association of FGFR2 rs2981582 polymorphism with BC is still worth exploring.
Although our results might be useful in helping to identify patients with BC risk, the results should be considered preliminary and further research is necessary. However, we cannot ignore some of the limitations. First, the mechanism of BC is complex and we observed only one SNP; therefore, our results cannot completely explain the association of genetic polymorphism and BC. Second, our sample size for BC cases was relatively small and all patients were from ShaanXi Province in northwest China, which may bias the final results. Third, the meta-analysis results were not representative because all enrolled studies were from an Asian population. Furthermore, the existence of confounding factors, such as family history, hormone metabolism, and menopausal status, might have distorted the meta-analysis, explaining the inconsistent results between the case-control study and the meta-analysis.
In summary, our results showed an association of the FGFR2 rs2981582 polymorphism with BC in an Asian population. However, these data need to be replicated in a larger cohort of patients, and functional studies are necessary to investigate whether and how the polymorphism might influence key pathways involved in the pathogenesis of BC.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was funded by China Postdoctoral Science Foundation (No. 2017M613179) and The National Natural Science Foundation of China (No. 81691877).
References
- 1.Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin 2016; 66: 115–132. [DOI] [PubMed] [Google Scholar]
- 2.Zhang ZL, Zhang CZ, Li Y, et al. Association between ERalpha gene Pvu II polymorphism and breast cancer susceptibility: a meta-analysis. Medicine (Baltimore) 2018; 97: e317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Patel N, Weekes D, Drosopoulos K, et al. Integrated genomics and functional validation identifies malignant cell specific dependencies in triple negative breast cancer. Nat Commun 2018; 9: 1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gusterson B, Eaves CJ. Basal-like breast cancers: from pathology to biology and back again. Stem Cell Reports 2018; 10: 1676–1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xia P, Li B, Geng T, et al. FGFR2 gene polymorphisms are associated with breast cancer risk in the Han Chinese population. Am J Cancer Res 2015; 5: 1854–1861. [PMC free article] [PubMed] [Google Scholar]
- 6.Haworth KE, Farrell WE, Emes RD, et al. Methylation of the FGFR2 gene is associated with high birth weight centile in humans. Epigenomics 2014; 6: 477–491. [DOI] [PubMed] [Google Scholar]
- 7.Salehi Z, Afzali S, Shabanipour S, et al. Evaluation of FGFR2 gene polymorphism in women with breast cancer. Cell Mol Biol (Noisy-le-grand) 2015; 61: 94–97. [PubMed] [Google Scholar]
- 8.Dedes KJ, Wetterskog D, Ashworth A, et al. Emerging therapeutic targets in endometrial cancer. Nat Rev Clin Oncol 2011; 8: 261–271. [DOI] [PubMed] [Google Scholar]
- 9.Luqmani YA, Graham M, Coombes RC. Expression of basic fibroblast growth factor, FGFR1 and FGFR2 in normal and malignant human breast, and comparison with other normal tissues. Br J Cancer 1992; 66: 273–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Easton DF, Pooley KA, Dunning AM, et al. Genome-wide association study identifies novel breast cancer susceptibility loci. Nature 2007; 447: 1087–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hunter DJ, Kraft P, Jacobs KB, et al. A genome-wide association study identifies alleles in FGFR2 associated with risk of sporadic postmenopausal breast cancer. Nat Genet 2007; 39: 870–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Katoh M. Cancer genomics and genetics of FGFR2 (Review). Int J Oncol 2008; 33: 233–237. [PubMed] [Google Scholar]
- 13.Meyer KB, Maia AT, O'Reilly M, et al. Allele-specific up-regulation of FGFR2 increases susceptibility to breast cancer. PLoS Biol 2008; 6: e108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murillo-Zamora E, Moreno-Macias H, Ziv E, et al. Association between rs2981582 polymorphism in the FGFR2 gene and the risk of breast cancer in Mexican women. Arch Med Res 2013; 44: 459–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ledwoń JK, Hennig EE, Maryan N, et al. Common low-penetrance risk variants associated with breast cancer in Polish women. BMC Cancer 2013; 13: 510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bayraktar S, Thompson PA, Yoo SY, et al. The relationship between eight GWAS-identified single-nucleotide polymorphisms and primary breast cancer outcomes. Oncologist 2013; 18: 493–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen Y, Shi C, Guo Q. TNRC9 rs12443621 and FGFR2 rs2981582 polymorphisms and breast cancer risk. World J Surg Oncol 2016; 14: 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lichtenstein P, Holm NV, Verkasalo PK, et al. Environmental and heritable factors in the causation of cancer–analyses of cohorts of twins from Sweden, Denmark, and Finland. N Engl J Med 2000; 343: 78–85. [DOI] [PubMed] [Google Scholar]
- 19.Qi XL, Yao J, Zhang Y. No association between the progesterone receptor gene polymorphism (+331G/a) and the risk of breast cancer: an updated meta-analysis. BMC Med Genet 2017; 18: 123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu CL, Hu XP, Guo WD, et al. Case-control study on the fibroblast growth factor receptor 2 gene polymorphisms associated with breast cancer in Chinese Han women. J Breast Cancer 2013; 16: 366–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mazhar A, Jamil F, Bashir Q, et al. Genetic variants in FGFR2 and TNRC9 genes are associated with breast cancer risk in Pakistani women. Mol Med Rep 2016; 14: 3443–3451. [DOI] [PubMed] [Google Scholar]
- 22.Liang J, Chen P, Hu Z, et al. Genetic variants in fibroblast growth factor receptor 2 (FGFR2) contribute to susceptibility of breast cancer in Chinese women. Carcinogenesis 2008; 29: 2341–2346. [DOI] [PubMed] [Google Scholar]
- 23.Kawase T, Matsuo K, Suzuki T, et al. FGFR2 intronic polymorphisms interact with reproductive risk factors of breast cancer: results of a case control study in Japan. Int J Cancer 2009; 125: 1946–1952. [DOI] [PubMed] [Google Scholar]
- 24.Hu X. Association of FGFR2 polymorphisms with the risk of breast cancer in Chinese women of Ningxia Han population. Ningxia Medical University, 2011. [Google Scholar]
- 25.Li X. The breast cancer susceptive locus screening in Han Chinese women and meta-analysis on common breast cancer risk factors. Southern Medical University, 2011. [Google Scholar]
- 26.Ren L, Zhang B, Cao XC, et al. Association of FGFR2 gene polymorphism with estrogen receptor positive breast cancer detected by fluorescent quantitative PCR. Zhonghua Yi Xue Yi Chuan Xue Za Zhi 2010; 27: 445–448. [DOI] [PubMed] [Google Scholar]
- 27.Fu F, Wang C, Huang M, et al. Polymorphisms in second intron of the FGFR2 gene are associated with the risk of early-onset breast cancer in Chinese Han women. Tohoku J Exp Med 2012; 226: 221–229. [DOI] [PubMed] [Google Scholar]
- 28.Chan M, Ji SM, Liaw CS, et al. Association of common genetic variants with breast cancer risk and clinicopathological characteristics in a Chinese population. Breast Cancer Res Treat 2012; 136: 209–220. [DOI] [PubMed] [Google Scholar]
- 29.Dai J, Hu Z, Jiang Y, et al. Breast cancer risk assessment with five independent genetic variants and two risk factors in Chinese women. Breast Cancer Res 2012; 14: R17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xi J, Su Y, Beeghly FA, et al. Association of physical activity and polymorphisms in FGFR2 and DNA methylation related genes with breast cancer risk. Cancer Epidemiol 2014; 38: 708–714. [DOI] [PubMed] [Google Scholar]
- 31.Siddiqui S, Chattopadhyay S, Akhtar MS, et al. A study on genetic variants of Fibroblast growth factor receptor 2 (FGFR2) and the risk of breast cancer from North India. PLoS One 2014; 9: e110426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liang H, Yang X, Chen L, et al. Heterogeneity of breast cancer associations with common genetic variants in FGFR2 according to the intrinsic subtypes in southern Han Chinese women. Biomed Res Int 2015; 2015: 626948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.He YN, Chen Q, Liu H, et al. The relationship between four GWAS-identified single nucleotide polymorphisms and female breast cancer in Henan population. Chin J Endocr Surg 2015; 5: 367–371. [Google Scholar]
- 34.Jiang Q. Relative study between the FGFR2 rs298l582 polymorphism and breast cancer in the Qing Hai Tibetan population. Journal of Qing Hai Medical College, pp. 22–25.
- 35.Xu WH, Wang SB. Relative research of FGFR2 gene SNPs and breast neoplasm. Chin Modern Med 2012; 19: 17–18. [Google Scholar]
- 36.Lao HM. Study on the screening and identification of sporadic breast cancer susceptible gene polymorphism in women from Guangdong, Chongqing, Shangdong and Nanchang. Southern Medical University, 2008. [Google Scholar]