Short abstract
Objectives
Alzheimer’s disease (AD) and behavioral variant frontotemporal dementia (bvFTD) are among the leading causes of early-onset dementia. This study aimed to assess the rate of whole brain atrophy by comparing bvFTD and AD.
Methods
Two patients (one man with AD, and one woman with bvFTD) had neuropsychological and neuroimaging assessment by using automated techniques for cross-sectional and longitudinal atrophy measurements.
Results
In the patient with AD, magnetic resonance imaging (MRI) showed decreased bilateral hippocampal and mesial-temporal volume. However, conventional images showed no difference between baseline (T0) and after 1 year (T1). In the patient with bvFTD, MRI showed bilateral frontotemporal lobe atrophy and a moderate increase in atrophy between T0 and T1, particularly in the temporal lobes. A cross-sectional cerebral volume examination showed a considerable reduction in brain volume in the patient with bvFDT and a moderate reduction in the patient with AD. A longitudinal cerebral volume examination showed a lower percentage brain volume change in the patient with bvFTS compared with the patient with AD.
Conclusions
Our results suggest that bvFTD has more neurodegenerative progression. MRI findings should be considered as a reliable marker of disease progression in the brain. Our findings offer potential for monitoring treatment outcomes.
Keywords: Alzheimer’s disease, brain atrophy, cognitive impairment, frontotemporal dementia, cerebral volume, magnetic resonance imaging
Introduction
Alzheimer’s disease (AD) and behavioral variant frontotemporal dementia (bvFTD) are among the leading causes of early-onset dementia.1–5 The revised diagnostic criteria for AD include disproportionate atrophy on structural magnetic resonance imaging (MRI) in the medial, basal, and lateral temporal lobes, and medial parietal cortex.3 Diagnostic criteria for bvFTD include disproportionate atrophy in the frontal, insular, or anterior temporal lobe.5 Assessment of disease progression in dementia is a pressing modern challenge to understanding its natural history and for evaluating the therapeutic response, especially in AD and bvFTD where signs and symptoms are overlapping. Changes in regional and global brain atrophy over time, which are detectable in vivo by MRI, are extensively used as a sensitive and objective marker of disease progression. These changes may also provide outcome measures in clinical trials to evaluate the efficacy of potentially disease-modifying treatments.
The condition of bvFTD is characterized by early and progressive changes in personality, emotional blunting, and/or loss of empathy. Patients with bvFTD experience difficulty in modulating behavior, and this often results in socially inappropriate responses or activities.6 These patients show a deficit in executive functioning, including attentional control, inhibitory control, working memory, cognitive flexibility, reasoning, problem solving, and planning. Therefore, cognitive deficit in patients with bvFTD is characterized by alteration in different areas of the prefrontal cortex. Memory, constructive praxis, and spatial orientation skills are generally preserved in the early stages of this disease.
In contrast, the earliest and most common clinical manifestation of AD is impairment in episodic memory, which is the incapacity to consciously retrieve a previously experienced item or specific episode in life.7 Indeed, the earliest neurofibrillary changes in AD usually occur in medial temporal lobe structures (hippocampus, entorhinal cortex), with interruption of the neural network, which is critical for episodic memory function.8
Recently, whole brain atrophy rates in AD were reported to range from 1% to 4% per year, while atrophy rates in similarly aged people without AD ranged from 0.3% to 0.7% per year.9 The majority of regional cerebral atrophy studies in AD versus bvFTD are cross-sectional,10–14 while there is a paucity of longitudinal studies. A previous study showed a greater reduction in orbitofrontal grey matter and temporal white matter (WM) structures in bvFTD than in AD over 12 months.15 A longitudinal study16 of cortical and subcortical atrophy showed that patients with AD had greater cortical atrophy in the bilateral inferior parietal cortex and in the right posterior cingulate cortex/percuneus than did those with bvFTD. Furthermore, patients with bvFTD had greater atrophy in the striatum than did those with AD over time.
To the best of our knowledge, no studies have evaluated the rate of whole brain atrophy by comparing bvFTD and AD directly. We report here one patient who was affected by AD and one who was affected by bvFTD, and they were followed up to 12 months. Whole brain atrophy rates over time were evaluated by using Structural Image Evaluation, Using Normalization, of Atrophy (SIENA)- and Structural Image Evaluation, Using Normalization, of Atrophy Cross-sectional (SIENAX)-normalized measurements. Additionally, the annual change in the whole brain profile that was calculated for each subject from two MRI scans was correlated with results of clinical and neuropsychological testing.
Materials and methods
Two patients (one man with AD, 55 years old and one woman with bvFTD, 57 years old, disease duration for 4 years for both patients) had baseline clinical and neuropsychological assessment. The patients were originally requested to attend two imaging visits at 0 and 12 months from baseline. These time points were selected to provide a range of different intervals over which cognitive impairment and brain atrophy could be determined.
Diagnosis was reached by consensus according to current clinical diagnostic criteria for typical AD (i.e., amnestic presentation) or probable bvFTD3,5 following a comprehensive clinical assessment. This assessment included neuropsychological assessment and a structural magnetic resonance imaging (MRI) brain scan. The two patients were matched for age, sex, education and disease duration. Five healthy controls were selected and matched to the patients according to age, sex, and education for baseline comparisons. Exclusion criteria included degenerative neurological disease (other than AD or bvFTD for the patients) and contraindication to MRI. The study was approved by the local research ethics committee and written consent was obtained.
Neuropsychological assessment was performed at baseline (T0) and after 1 year (T1) by using the Mini Mental State examination (MMSE) and Brief Neuropsychological Examination (ENB2).17 The MMSE was used to screen cognitive decline. The ENB2 battery was used to investigate the following different cognitive domains: attention, memory, executive functions, perceptive and praxis abilities. The ENB2 includes the following 16 subtests for five cognitive domains: digit span, immediate and delayed recall prose memory, interference memory (10 and 30 seconds), trail making test parts A and B, token test, word phonemic fluency, abstraction, cognitive estimation, overlapping figures, spontaneous drawing, copy drawing, clock drawing, and ideational and ideomotor praxis tests. An ENB2 total score was calculated to obtain a general measure of the cognitive status.
Two patients and the five controls underwent an MRI examination by using a 3.0 T magnetic resonance scanner (Achieva; Philips Healthcare, Best, the Netherlands) at T0 and T1. Identical conventional T1-weighted three-dimensional fast field echo images used for brain volume analysis (repetition time/echo time = 8.2/3.7 ms, 256 × 256 matrix, 1 × 1 × 1-mm acquisition voxel MPS, 1 signal average, 250-mm field of view, 180 slices of 1-mm thickness, sagittal orientation) were acquired in each subject.
We used the automated techniques SIENAX and SIENA18 for cross-sectional and longitudinal atrophy measurements, respectively. These methods are part of the FMRIB software library, which is freely available for academic use (http: //www.fmrib.ox-.ac.uk/fsl).
SIENAX uses the cross-sectional version of structural image evaluation using the normalization of atrophy method18 to estimate global and regional brain tissue volume, which is normalized for the subject’s head size. Brain volume was calculated by measuring the difference in volumes inside a mesh over the exterior surface of the brain and inside the ventricles. A partial volume correction was used to limit the effect of cerebrospinal fluid within the cerebral sulci. The algorithm extracted the skull and brain masks from an image at one time point. The brain image was then affine-registered to a canonical image (MNI152 image) in a standardized space (using the skull image to provide the scaling cue). This procedure provided a spatial normalization (scaling) factor for each subject. Tissue-type segmentation with partial volume estimation was then performed to calculate the total volume of brain tissue, including separate estimates of volumes of grey matter, WM, and ventricular cerebrospinal fluid.18 Finally, standard space masks were used to provide estimates of ventricular cerebrospinal fluid and peripheral grey matter volumes.20 All volumetric outputs are provided as normalized for head size and un-normalized. The primary output is referred to as total normalized brain volume.
SIENA was used to assess changes in brain volume by directly estimating the local shifts in brain edges across the entire brain and then converting the edge displacement into a global estimate of percentage brain volume change (PBVC) between the two time points. SIENA was started by extracting brain and skull images from two time point whole head input data.18 For each individual subject, the baseline and follow-up brain images were aligned to each other20 using the skull images to constrain the registration scaling. Both brain images were then resampled halfway into the standard space. Tissue-type segmentation was then carried out21 to identify brain/non-brain edge points, and then perpendicular edge displacement (between the two time points) was estimated at these edge points. Finally, the mean edge displacement across the whole brain was converted into a global estimate of PBVC between the two time points.
Interaction effect analysis (improved time) was performed by calculating the T1–T0 differences to report the frequency and variance of cognitive clinical scores. Analyses were performed using an open source R3.0 software package (www.r-project.org).
Results
We found mild cognitive impairment in both patients at T0 (Table 1). In particular, the patient with AD showed impairment of memory (immediate and delayed recall), and impaired attention and visuo-spatial orientation. The patient with bvFDT presented with greater impairment in executive functions (trail making test B) and in language performance (word phonemic fluency) compared with the patient with AD (Table 1). At follow-up (T1), we observed greater global cognitive decline in the patient with bvFDT compared with the patient with AD, with severe impairment in memory, attention, and in executive function. In particular, we observed a worse performance (100%) in abstraction and cognitive estimation. The patient with bvFDT showed greater impairment compared with the patient with AD (Table 1). These data were partially confirmed by structural MRI. In the patient with AD, MRI showed bilateral hippocampal and mesial-temporal decreased volume, which was associated with high-signal intensity of the hippocampi and ex vacuo bilateral enlargement of the temporal horn. However, conventional images showed no significant differences between T0 and T1 (Figure 1). In the patient with bvFTD, conventional MRI showed bilateral frontotemporal lobe atrophy and a moderate increase in atrophy between T0 and T1, particularly in the temporal lobes (Figure 2).
Table 1.
Representation of variations in percentage and variance of cognitive clinical scores.
|
FDT |
AD |
|||
|---|---|---|---|---|
| Test | Frequency (%) | Variance | Frequency (%) | Variance |
| MMSE | −41.67 | −10.00 | −31.82 | −7.00 |
| Digit span | −75.00 | −3.00 | −33.33 | −1.00 |
| Immediate recall prose memory | −60.00 | −3.00 | −50.00 | −2.00 |
| Delayed recall prose memory | −77.78 | −7.00 | −75.00 | −6.00 |
| Brown Peterson technique 10 s | −66.67 | −2.00 | −33.33 | −1.00 |
| Brown Peterson technique 30 s | 0.00 | 0.00 | −66.67 | −2.00 |
| Trail making test A | 260.00 | 390.00 | −10.00 | −5.00 |
| Trail making test B | 128.57 | 450.00 | 140.00 | 350.00 |
| Token test | 0.00 | 0.00 | 0.00 | 0.00 |
| Word phonemic fluency | −80.00 | −4.00 | −37.50 | −3.00 |
| Abstraction tests | −100.00 | −2.00 | 0.00 | 0.00 |
| Cognitive estimation test | −100.00 | −2.00 | −50.00 | −2.00 |
| Intricate figures test | −33.33 | −9.00 | 0.00 | 0.00 |
| House figure copy | 0.00 | 0.00 | −50.00 | −1.00 |
| Daisy drawing test | 0.00 | 0.00 | −50.00 | −1.00 |
| Clock drawing test | −100.00 | −6.00 | −28.57 | −2.00 |
| Ideomotor apraxia test | 0.00 | 0.00 | 0.00 | 0.00 |
| Global score | −32.00 | −16.00 | −26.42 | −14.00 |
FDT, frontotemporal dementia; AD, Alzheimer’s disease; MMSE, Mini Mental State examination.
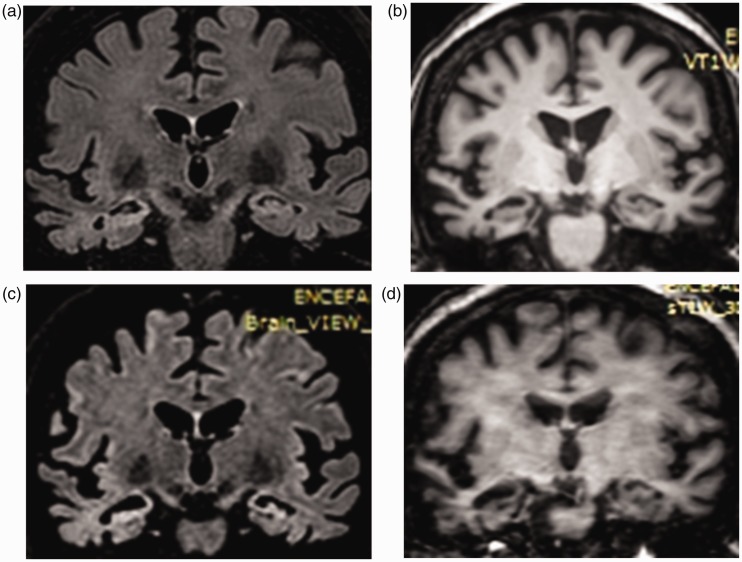
Figure 1.
Alzheimer’s disease. (a) fluid-attenuated inversion recovery coronal image at baseline. (b) Fast spin echo T2-weighted coronal image at baseline. (c) Fluid-attenuated inversion recovery coronal image after 1 year. (d) Fast spin echo T2-weighted coronal image after 1 year. Conventional magnetic resonance imaging shows bilateral hippocampal and mesial-temporal atrophy, which is associated with high-signal intensity of the hippocampi. Ex vacuo bilateral enlargement of the temporal horn can be seen. Conventional magnetic resonance imaging shows no difference between baseline and after 1 year.
Figure 2.
Frontotemporal dementia. (a) Fluid-attenuated inversion recovery coronal image at baseline. (b) Multiplanar reconstruction gradient echo T1-weighted coronal image at baseline. (c) Fluid-attenuated inversion recovery coronal image after 1 year. (d) Multiplanar reconstruction gradient echo T1-weighted coronal image after 1 year. Conventional magnetic resonance imaging shows bilateral frontotemporal lobe atrophy. Conventional images show a slight increase in atrophy between baseline and after 1 year, particularly in the temporal lobes.
A cross-sectional cerebral volume examination, which was performed by automatic SIENAX software, showed a considerable reduction in brain volume in the patient with bvFDT compared with controls as follows: grey matter, −14.33%; WM, −1.09%; and normalized brain volume, −7.94%. The patient with AD showed a moderate reduction in brain volume compared with controls as follows: grey matter, −0.24%; WM, −3.16%; and normalized brain volume, −1.44% (Figure 3). A longitudinal cerebral volume examination, which was performed by SIENA software, showed a lower PVBC (−5.06%) in the patient with bvFTS compared with the patient with AD (−1.83%) (Figure 3).
Figure 3.
Graphical representation of variations in the percentage of brain structure in the patients with AD and bvFTD between baseline and after 1 year. AD, Alzheimer’s disease; bvFTD, behavioral variant frontotemporal dementia.
Discussion
This study examined patterns of whole and cortical atrophy in the two most common younger-onset dementia syndromes, AD and bvFTD. We found disease-specific decreased cortical cerebral volume and expression of neurodegeneration was related to disease progression. Specifically, our structural MRI findings showed that the patient with AD had decreased bilateral hippocampal and mesial-temporal volume, with no difference between T0 and T1. In the patient with bvFTD, conventional MRI showed bilateral frontotemporal lobe atrophy and showed a moderate increase in atrophy between T0 and T1.
An automatic longitudinal cerebral volume examination showed a considerable reduction in brain volume in the patient with bvFDT and a moderate reduction in the patient with AD. Additionally, the patient with AD showed decreased grey matter volume, and even greater reduction in WM, as previously reported.22 The pathophysiological association between WM damage and cortical changes in AD is currently unclear. Changes in WM might be secondary to cortical degeneration. Alternatively, other causes could generate WM and cortical damage in AD, such as co-existing vascular injury or neurodegenerative pathogenesis of AD. Therapeutic interventions specifically targeting processes related to deterioration of WM might slow progression of the disease and should thus be considered.23 These findings were associated with neuropsychological data. The patient with AD showed impairment in memory (immediate and delayed recall), and impairment in attention and visuo-spatial orientation. The considerable decrease in grey matter volume in the patient with bvFTD was associated with worse cognitive scores and expression of more progressive degeneration. In fact, a longitudinal cerebral volume examination showed a lower PVBC in the patient with bvFTS compared with the patient with AD. The different profiles of progression of atrophy suggest that each syndrome is associated with a distinct pattern of disease propagation, and is most likely driven by its specific underlying pathology.
Prospective, longitudinal imaging studies of dementia are difficult to conduct. Our findings, even if limited to two single subjects, indicate that deficits in grey matter and WM may represent a diagnostic biomarker for differential progression of AD and bvFTD. Additionally, over 1 year, the patient with bvFTD showed a greater decrease in brain volume compared with the patient with AD, as shown by automatic evaluation and only partially confirmed by conventional structural MRI. Information on the location and severity of brain atrophy in these patient populations and their evolution over time is likely to be informative for diagnosis of these progressive neurodegenerative disorders. Our results suggest that bvFTD has a more neurodegenerative progression than AD, as shown by neuropsychological assessment. These findings should be considered as reliable markers of disease progression in the brain, and could provide potential for monitoring treatment outcome in future drug trials.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1.Vieira RT, Caixeta L, Machado S, et al. Epidemiology of early-onset dementia: a review of the literature. Clin Pract Epidemiol Ment Health 2013; 9: 88–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jahn H. Memory loss in Alzheimer's disease. Dialogues Clin Neurosci 2013; 4: 445–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging and the Alzheimer’s Association workgroup. Alzheimers Dement 2011; 3: 263–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rosness TA, Engedal K, Chemali Z. Frontotemporal dementia: an updated clinician’s guide. J Geriatr Psychiatry Neurol 2016; 29: 271–280. [DOI] [PubMed] [Google Scholar]
- 5.Rascovsky K, Hodges JR, Knopman D, et al. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain 2011; 134: 2456–2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Levenson RW, Sturm VE, Haase CM. Emotional and behavioral symptoms in neurodegenerative disease: a model for studying the neural bases of psychopathology. Annu Rev Clin Psychol 2014; 10: 581–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cover KS, Van Schijndel RA, Van Dijk BW, et al. Alzheimer’s Disease Neuroimaging Initiative. Assessing the reproducibility of the SienaX and Siena brain atrophy measures using the ADNI back-to-back MP-RAGE MRI scans. Psychiatry Res 2011; 193: 182–190. [DOI] [PubMed] [Google Scholar]
- 8.Kitagaki H, Mori E, Yamaji S, et al. Frontotemporal dementia and Alzheimer disease: evaluation of cortical atrophy with automated hemispheric surface display generated with MR images. Radiology 1998; 208: 431–439. [DOI] [PubMed] [Google Scholar]
- 9.Frisoni GB, Laakso MP, Beltramello A, et al. Hippocampal and entorhinal cortex atrophy infrontotemporal dementia and Alzheimer's disease. Neurology 1999; 52: 91–100. [DOI] [PubMed] [Google Scholar]
- 10.Du AT, Schuff N, Kramer JH, et al. Different regional patterns of cortical thinning in Alzheimer’s disease and frontotemporal dementia. Brain 2007; 130: 1159–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rabinovici GD, Seeley WW, Kim EJ, et al. Distinct MRI atrophy patterns in autopsy-proven Alzheimer's disease and frontotemporal lobar degeneration. Am J Alzheimers Dis Other Demen 2007; 22: 474–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Möller C, Hafkemeijer A, Pijnenburg YAL, et al. Joint assessment of white matter integrity, cortical and subcortical atrophy to distinguish AD from behavioral variant FTD: A two-center study. Neuroimage Clin 2015; 9: 418–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frings L, Yew B, Flanagan E, et al. Longitudinal grey and white matter changes in frontotemporal dementia and Alzheimer’s disease. Plos One 2014; 9: e90814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Landin-Romero R, Kumfor F, Leyton CE, et al. Disease-specific patterns of cortical and subcortical degeneration in a longitudinal of Alzheimer’s disease and behavioural-variant frontotemporal dementia. Neuroimage 2017; 151: 72–80. doi: 10.1016/j.neuroimage.2016.03.032. [DOI] [PubMed] [Google Scholar]
- 15.Mondini S, Mapelli D, Vestri A, et al. Esame neuropsicologico breve 2. Milano: Raffaello Cortina, 2011. [Google Scholar]
- 16.Smith SM, Zhang Y, Jenkinson M, et al. Accurate, robust, and automated longitudinal and cross-sectional brain change analysis. Neuroimage 2002; 17: 479–489. [DOI] [PubMed] [Google Scholar]
- 17.De Stefano N, Matthews PM, Filippi M, et al. Evidence of early cortical atrophy in MS. Neurology 2003; 60: 1157–1162. [DOI] [PubMed] [Google Scholar]
- 18.Jenkinson M, andSmith S.. A global optimisation method for robust affine registration of brain images. Med Image Anal 2001; 5: 143–156. [DOI] [PubMed] [Google Scholar]
- 19.Zhang Y, Brady M, Smith S. Segmentation of brain MR images through a hidden Markov random field model and the expectation–maximization algorithm. IEEE Trans Med Imaging 2001; 20: 45–57. [DOI] [PubMed] [Google Scholar]
- 20.Yin RH, Tan L, Liu Y, et al. Multimodal voxel-based meta-analysis of white matter abnormalities in Alzheimer's disease. J Alzheimers Dis 2015; 47: 495–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee SH, Coutu JP, Wilkens P, et al. Alzheimer’s Disease Neuroimaging Initiative (ADNI). Tract-based analysis of white matter degeneration in Alzheimer’s disease. Neuroscience 2015; 301: 79–89. [DOI] [PMC free article] [PubMed] [Google Scholar]