Short abstract
Objective
This study aimed to determine the most appropriate cognitive and cerebrospinal fluid (CSF) biomarker setting to distinguish frontotemporal dementia (FTD) from Alzheimer’s disease (AD).
Method
Patients with FTD, those with AD, and those without dementia were enrolled in this study. CSF amyloid-ß 42 (Aß42), total (t)-tau, and phosphorylated (p)-tau concentrations were determined by enzyme-linked immunosorbent assays. Cognition was evaluated by the Mini-Mental State Examination (MMSE) and its domain scores. The associations of CSF biomarkers with cognitive measures were examined using regression models and the diagnostic value of CSF biomarkers was determined by receiver operating characteristics curves.
Results
CSF Aß42 levels were lower, whereas t-tau/Aß42 and p-tau/Aß42 ratios were higher in patients with AD compared with those with FTD. Some MMSE domain scores were different in FTD and AD, but they did not improve the ability to distinguish between the two pathologies. Poor temporal orientation scores were associated with low Aß42 levels only in patients with FTD. The p-tau/Aß42 ratio reached sufficient levels of sensitivity and specificity to discriminate FTD with primary progressive aphasia from AD.
Conclusions
The ratio of CSF p-tau/Aß42 is a sensitive and specific biomarker for discriminating patients with primary progressive aphasia from those with AD.
Keywords: Alzheimer’s disease, frontotemporal dementia, biomarker, cerebrospinal fluid, Mini-Mental State Examination, p-tau/Aß42 ratio
Introduction
Frontotemporal dementia (FTD) is a neurodegenerative disorder that is characterized by neuronal loss in the frontal and temporal lobes.1 Clinically, patients affected by FTD can be broadly divided into two main subtypes. One subtype is found in those with predominant behavioral and social comportment disorders (behavioral variant frontotemporal dementia [bvFTD]). The other subtype is found in those with primary language disturbances (primary progressive aphasia [PPA]), which includes semantic PPA (svPPA) and non-fluent/agrammatic PPA (naPPA).2–6
FTD is neuropathologically characterized by aggregates of three different proteins in the frontal and temporal lobes, including tau, TAR DNA-binding protein 43, and fused-in-sarcoma protein.7 FTD is inherited in approximately one third of cases and the most common pathogenic mutations have been found in the genes for progranulin (GRN), tau (MAPT), and C9orf72.8–12
A major challenge for early and correct treatment and for development of novel therapies in FTD is accurate diagnosis because its symptoms overlap with those of Alzheimer’s disease (AD). FTD is often associated with changes in personality, motivation, social behavior, and organizational abilities. “AD is characterized by a progressive amnestic disorder with episodic and semantic memory deficit”, followed by a decline in other attentional, perceptual, and visuospatial abilities. In bvFTD, neuropsychiatric changes are the most prominent symptoms and usually precede or hide cognitive deficits. AD appears initially with cognitive deficits in the episodic memory domain and only later clinically manifests with alterations in social conduct, personality changes, and aphasia. However, non-cognitive disorders may sometimes characterize the onset of AD, making differential diagnosis a challenging task.13
Neuropathological diagnosis of AD, which is defined by the presence at autopsy of both amyloid-ß plaques and tau neurofibrillary tangles, has been found in up to 30% of clinically diagnosed FTD cases.14–17 A high proportion of FTD syndromes are associated with AD pathology,18 including 7% with bvFTD, 44% with naPPA, and 10% with svPPA. A recent study showed that 39.5% of a group of patients diagnosed with PPA were characterized by an AD-underlying pathology (22.2%, 35.7%, and 75% with naPPA, svPPA, and logopenic progressive aphasia, respectively).19 Therefore, differentiation of AD from FTD spectrum disorders poses a serious diagnostic problem for clinicians.
Cerebrospinal fluid (CSF) biomarkers have the potential to optimize diagnostic accuracy and detect AD in the early phases when it could be possible to administer a potentially effective treatment. CSF values of major constituents of AD pathology, amyloid-ß 42 (Aß42), and total and phosphorylated tau (t-tau and p-tau), have been widely studied. These are associated with key features of AD, such as Aß42 for amyloid plaque load, t-tau for neuronal degeneration, and p-tau for neurofibrillary tangles.20,21 A CSF profile characterized by low Aß42 levels and high concentrations of t-tau and p-tau accurately discriminates patients with AD from healthy older individuals, but, it is not equally useful in differential diagnosis of other dementias.22–25 Many studies have analyzed CSF biomarkers to verify possible diagnostic applications to distinguish FTD from AD and control subjects, but values of specificity and sensitivity are insufficient for diagnostic application.26–31 Interestingly, the most promising approach appears to be use of a biomarker ratio, rather than single measurements.32–37
This study aimed to compare cognitive and biomarker characteristics of patients with FTD, those with AD, and non-demented (ND) patients, and to determine the best CSF biomarker setting in distinguishing FTD (bvFTD and PPA) from AD cases.
Materials and methods
Study population
The study population consisted of 96 patients who were recruited at the INRCA Hospital, Neurology Unit, Ancona, Italy. Participants underwent a comprehensive clinical investigation that included medical history, neuropsychological and functional evaluation, neuroimaging, and laboratory tests. All subjects were administered the Mini-Mental State Examination (MMSE) according to standard procedures.38,39 MMSE scores were calculated by grouping various items of the MMSE by domain: temporal orientation (0–5 points), spatial orientation (0–5 points), short-term memory (0–3 points), attention (0–5 points), verbal episodic memory (0–3 points), language (0–8 points), and visuospatial function (0 or 1 point). Brain atrophy was estimated on magnetic resonance imaging (MRI) scans using a modified version of the Pasquier scale,40 where the final score was the mean of the values collected throughout the cerebrum. The score ranged from 0 to 3 as follows: 0 = no cortical atrophy, 1 = mild atrophy (opening of sulci), 2 = moderate atrophy (volume loss of gyri), and 3 = severe atrophy (“knife blade” atrophy); a value of 2 was considered pathological. Only patients with a score ≤2 were included in the study.
The work-up findings allowed participants to be divided into three groups as follows: patients with probable AD (n = 55), patients with FTD (n = 21), and ND patients (n = 20). The diagnosis had to be confirmed after at least a 2-year follow-up. Probable AD was diagnosed according to joint Alzheimer’s Association Workgroup and National Institute of Aging guidelines.41,42 FTD was diagnosed by current European Federation of Neurological Sciences-European Neurological Society guidelines of disorders associated with dementia, which included a revision on dementia syndromes outside of AD.43,44 bvFTD and various forms of PPA were diagnosed according to specific established criteria.2,45,46 Twelve patients in the FTD group predominantly had bvFTD, whereas nine patients predominantly had the PPA phenotype. The ND group consisted of individuals with peripheral nervous system disorders caused by trauma, degeneration, or other conditions. Exclusion criteria were age <60 years, family history of disease, cerebrovascular accidents, anamnesis of delirium, cognitive decline induced by head injury, recently diagnosed or untreated thyroid disease, vitamin B12 or folic acid deficiency, intoxication with drugs or medications, severe depression (pseudodementia), chromosome 21 trisomy (Down syndrome), neurosyphilis, and human immunodeficiency virus dementia. The study was conducted according to the principles of the Declaration of Helsinki and was approved by the local ethics committee (Comitato Etico Regionale Marche [CERM] 2016 0259; 15 December 2016). Patients who participated in this study did not provide written or verbal informed consent because the study was a non-genetic, retrospective, observational study, and as such, did not require informed consent.
CSF sampling and assays
CSF was obtained by lumbar puncture in the L3/L4 or L4/L5 intervertebral space. CSF samples were collected in polypropylene vials and delivered to the laboratory within 3 hours. Part of the sample was used for routine clinical laboratory analyses, including total cell count and total protein determination. A volume of 2 to 3 mL was centrifuged at 2000 × g for 10 minutes to pellet residual cells and other insoluble material. The supernatant was then aliquoted into polypropylene tubes and stored at −80°C until use for biomarker determination. CSF levels of Aß42, t-tau, and p-tau were determined using commercially available ELISA kits (Fujirebio Inc., Tokyo, Japan) according to the manufacturer’s instructions. Assay performance was monitored using internal and external quality control samples. All analyses were performed by the same investigators who were blinded to patients’ demographic, clinical, and cognitive data. The t-tau/Aß42 and p-tau/Aß42 ratios, which were derived from biomarker measurements, were also calculated. All of the subjects signed informed consent for lumbar puncture and analysis of CSF.
Statistical analysis
All variables were checked for normality by the Kolmogorov–Smirnov test. The characteristics of participants included in each group (AD, FTD, and ND) were compared using ANOVA for normally distributed variables, followed by Bonferroni’s post-hoc analysis. The Kruskal–Wallis test and Mann–Whitney test were used for non-normally distributed variables. χ2 analysis was applied to compare dichotomous variables.
Linear regression was performed separately for each patient group to analyze relations between CSF biomarkers and MMSE scores. CSF biomarker concentrations and ratios were independent variables, MMSE and domain scores were dependent variables, and age and sex were covariates. Bonferroni correction for multiple comparisons was applied.
Receiver operating characteristics (ROC) curve analysis was performed to evaluate the discriminating power of CSF biomarkers in clinical diagnosis of AD and FTD. The area under the curve (AUC) was used as a measure of the overall performance of each ROC curve (with a 95% confidence interval), and optimal cutoff points of biomarkers were calculated by selecting the point on the ROC curve that maximized both sensitivity and specificity. Statistical significance was set at p < 0.05. All analyses were performed using IBM SPSS Statistics for Windows, Version 19.0 (IBM Corp., Armonk, NY, USA).
Results
Comparative assessment of the patient groups
The demographic, clinical, and cognitive characteristics of the patient population are shown in Table 1. The mean age in the FTD group was significantly lower than that in the AD group (p < 0.05), whereas the sex distribution was almost identical among the groups. Assessment of MRI scans showed a significantly higher percentage of patients with brain atrophy in patients with AD and FTD compared with ND patients (both p < 0.05). The MMSE total score, as well as verbal episodic memory, were significantly different among the three groups (all p < 0.05). The AD group showed the lowest scores and the ND group showed the highest scores. However, visuospatial function and short-term memory scores were essentially the same among the three groups. Attention domain scores were significantly lower in the AD and FTD groups compared with the ND group (both p < 0.05). Language and spatial orientation scores were significantly lower in the AD group compared with the ND group (both p < 0.05). Finally, the temporal orientation domain was significantly impaired in patients with AD compared with those in the other two groups (both p < 0.05).
Table 1.
Demographic, clinical, and cognitive characteristics of the patient groups.
| AD (n = 55) | FTD (n = 21) | ND (n = 20) | |
|---|---|---|---|
| Age (years) | 77.3 (7.1)a | 72.0 (5.8) | 72.8 (8.2) |
| Sex (female) | 32 (58.2) | 12 (57.1) | 8 (40.0) |
| Cerebral atrophy (pathological score) | 42 (76.4)b | 11 (52.4)c | 3 (15.0) |
| MMSE | 14.5 (6.1)a,b | 19.0 (6.2)c | 28.1 (1.4) |
| Temporal orientation | 1.9 (1.4)a,b | 3.4 (1.7) | 5.0 (0.0) |
| Spatial orientation | 2.8 (1.6)b | 3.9 (1.4) | 5.0 (0.0) |
| Short-term memory | 2.7 (0.8) | 2.6 (0.9) | 3.0 (0.0) |
| Attention | 2.0 (1.6)b | 2.5 (1.9)c | 5.0 (0.0) |
| Verbal episodic memory | 0.2 (0.5)a,b | 1.1 (0.9)c | 2.4 (0.5) |
| Language | 5.4 (1.7)b | 5.7 (2.0) | 7.6 (0.5) |
| Visuospatial function | 0.2 (0.4) | 0.4 (0.5) | 0.6 (0.5) |
Data are presented as mean (standard deviation) of continuous variables and as number (%) of dichotomous variables (sex and cerebral atrophy). ANOVA, followed by Bonferroni’s post-hoc analysis, was used to compare continuous variables. The χ2 test was applied to evaluate differences in percentages. ap < 0.05, AD versus FTD; bp < 0.05, AD versus ND; cp < 0.05, FTD versus ND. AD: Alzheimer’s disease; FTD: frontotemporal dementia; ND: non-demented; MMSE: Mini-Mental State Examination.
CSF clinical laboratory data and biomarker values are reported in Table 2. These data were not normally distributed. Therefore, we applied the Kruskal–Wallis test to compare the groups. None of the clinical laboratory parameters were significantly different among the three groups. However, concentrations and ratios of biomarkers showed differences among the groups. Aß42 concentrations were significantly lower, and t-tau/Aß42 and p-tau/Aß42 ratios were higher in the AD group compared with the other two groups (all p < 0.05). Two other indices, t-tau and p-tau concentrations, were significantly higher in the AD and FTD groups compared with the ND group (all p < 0.05). Biomarker values of patients with bvFTD and PPA are reported in Table 3. There were no significant differences in CSF biomarker values between the two groups of patients.
Table 2.
CSF clinical laboratory data and biomarker values.
| AD (n = 55) | FTD (n = 21) | ND (n = 20) | |
|---|---|---|---|
| CSF total protein (mg/dL) | 39.6 (18.8) | 37.9 (16.7) | 40.4 (21.9) |
| CSF cell count (cells/mm3) | 0.0 (3.5) | 2.0 (5.0) | 0.0 (2.8) |
| CSF chloride (mEq/L) | 124.5 (7.0) | 124.0 (8.5) | 127.0 (6.8) |
| CSF albumin (mg/dL) | 23.8 (18.5) | 24.4 (12.3) | 24.7 (17.2) |
| CSF immunoglobulin G (mg/dL) | 2.8 (2.1) | 2.7 (1.7) | 3.5 (3.1) |
| Aß42 (pg/mL) | 381.1 (198.6)a,b | 713.3 (378.5) | 689.5 (410.7) |
| t-tau (pg/mL) | 449.8 (483.5)b | 308.0 (263.8)c | 167.5 (141.7) |
| p-tau (pg/mL) | 65.0 (45.0)b | 51.8 (30.6)c | 43.4 (25.5) |
| t-tau/Aß42 | 1.2 (1.4)a,b | 0.5 (0.6)c | 0.2 (0.1) |
| p-tau/Aß42 | 0.2 (0.1)a,b | 0.1 (0.1) | 0.1 (0.0) |
Data are presented as median (interquartile range). The Kruskal–Wallis test was used to compare the groups. ap < 0.05, AD versus FTD; bp < 0.05, AD versus ND; cp < 0.05, FTD versus ND. AD: Alzheimer’s disease; FTD: frontotemporal dementia; ND: non-demented; CSF: cerebrospinal fluid; Aß42: amyloid-ß 42; t-tau: total tau; p-tau; phosphorylated tau.
Table 3.
CSF biomarker values of patients with bvFTD and PPA.
| bvFTD (n = 12) | PPA (n = 9) | p value | |
|---|---|---|---|
| Aß42 (pg/mL) | 522.0 (327.7) | 811.7 (156.9) | 0.075 |
| t-tau (pg/mL) | 351.6 (183.8) | 268.1 (257.2) | 0.829 |
| p-tau (pg/ml) | 58.8 (25.3) | 44.9 (27.9) | 0.122 |
| t-tau/Aß42 | 0.48 (0.52) | 0.36 (0.29) | 0.515 |
| p-tau/Aß42 | 0.10 (0.09) | 0.06 (0.04) | 0.055 |
Data are presented as median (interquartile range). The Mann–Whitney test was used to compare the groups. CSF: cerebrospinal fluid; bvFTD: behavioral variant frontotemporal dementia; PPA: primary progressive aphasia; Aß42: amyloid-ß 42; t-tau: total tau; p-tau; phosphorylated tau.
Relations between the cognitive profile and CSF biomarkers
Linear regression analyses were performed to evaluate if variations in specific cognitive domains, as measured by MMSE scores, were correlated with values of CSF biomarkers. The AD and ND groups failed to show significant associations for any of the CSF biomarkers, whereas a significant relationship was obtained in patients with FTD. In patients with FTD, poor temporal orientation scores were associated with low Aß42 levels after correction for age and sex (R2 = 0.520; p = 0.028).
ROC curve analyses
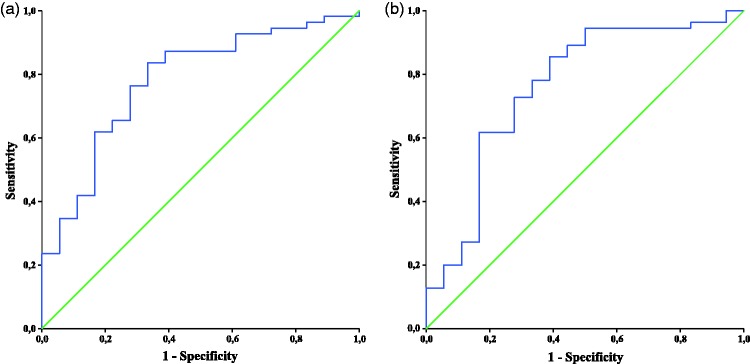
When ROC analyses were performed for each biomarker and ratio, only p-tau/Aß42 and t-tau/Aß42 ratios were able to significantly differentiate AD from FTD (Figure 1). The AUC was 0.761 for p-tau/Aß42 (p = 0.001). Using a cutoff value of 0.0098, the CSF p-tau/Aß42 ratio had a sensitivity of 85.5% and a specificity of 61.1% for distinguishing FTD from AD. The AUC was 0.780 for t-tau/Aß42 (p < 0.001). Using a cutoff value of 0.565, the CSF t-tau/Aß42 ratio had a sensitivity of 83.6% and specificity of 66.7% for distinguishing FTD from AD. These two ratios were also applied in distinguishing AD from bvFTD and AD from PPA separately (Table 4). Both of these ratios were better at differentiating AD from PPA than for differentiating AD from bvFTD. The p-tau/Aß42 ratio showed acceptable values of sensitivity (92.7%) and specificity (75.0%) in distinguishing AD from PPA. According to a consensus report, a useful diagnostic biomarker should have sensitivity and specificity approaching or exceeding 80%.47 Therefore, the p-tau/Aß42 ratio appears to fulfill these requirements in differentiating AD from PPA.
Figure 1.
ROC curve analysis of p-tau/Aß42 and t-tau/Aß42 for discriminating AD from FTD. (a) ROC curve of p-tau/Aß42. The ratio distinguished patients with AD from those with FTD with a sensitivity of 85.5% and a specificity of 61.1% using a cutoff value of 0.0098. (b) ROC curve of t-tau/Aß42. The ratio distinguished patients with AD from those with FTD with a sensitivity of 83.6% and a specificity of 66.7% using a cutoff value of 0.565. ROC: receiver operating characteristics; Aß42: amyloid-ß 42; p-tau; phosphorylated tau; t-tau: total tau; AD: Alzheimer’s disease; FTD: frontotemporal dementia.
Table 4.
ROC analyses for discriminating AD from bvFTD and PPA.
| Cutoff | Sensitivity (%) | Specificity (%) | Area under the curve (95% CI) | p | |
|---|---|---|---|---|---|
| p-tau/Aß42 | |||||
| AD versus bvFTD | 0.162 | 61.8 | 80.0 | 0.695 | 0.052 |
| AD versus PPA | 0.073 | 92.7 | 75.0 | 0.843 | 0.002 |
| t-tau/Aß42 | |||||
| AD versus bvFTD | 0.504 | 87.3 | 60.0 | 0.749 | 0.013 |
| AD versus PPA | 0.738 | 70.9 | 87.5 | 0.818 | 0.004 |
ROC: receiver operating characteristics; AD: Alzheimer’s disease; bvFTD: behavioral variant frontotemporal dementia; PPA: primary progressive aphasia; CI: confidence interval; Aß42: amyloid-ß 42; t-tau: total tau; p-tau; phosphorylated tau.
Discussion
This study examined CSF biomarkers and cognitive status in a population of patients with AD, FTD, or no dementia. We found that these three patient groups showed numerous differences regarding cognitive domain scores, and Aß42, t-tau, and p-tau levels. Additionally, we found that low Aß42 levels were associated with a worse performance in the temporal orientation domain only in the FTD group. Analysis of CSF biomarkers for differential diagnosis of FTD and AD showed that only the p-tau/Aß42 ratio reached sufficient levels of sensitivity and specificity to discriminate AD from PPA. None of the CSF biomarkers were adequate for distinguishing AD from bvFTD, or for distinguishing AD from FTD considered as a whole group.
Cognitive evaluation results indicated that distinct domain scores were different among the groups. Patients with AD showed greater deficits on tests of verbal episodic memory and temporal orientation than did those with FTD and ND. These findings are consistent with clinical observations that AD predominantly affects memory and orientation functioning, while language, executive skills, and attention are more likely to be impaired in FTD. Nevertheless, we did not find lower scores in these domains in the FTD group. Additionally, MMSE score analysis showed significant differences between AD and ND in five domains, while differences between FTD and ND were found only in three domains. Our results confirm previous observations on the limited usefulness of the MMSE in evaluating patients with FTD.48 People with AD are expected to perform worse than people with FTD with a comparable stage of dementia. This is because the cognitive domains that are assessed by the MMSE are those most prominently affected in AD. Even the Montreal Cognitive Assessment, with its added executive function items, is significantly worse in those with AD compared with those with FTD.49 Other global cognitive tests, such as the Addenbrooke’s Cognitive Examination test and its subsequent versions, or specific tests for frontal executive function (e.g., Frontal Assessment Battery, Trail Making Test, and Weigl Color Form Sorting Test) could be more informative.
CSF analysis in the present study showed that Aß42 was a biomarker for AD, while t-tau and p-tau were effective for distinguishing AD and FTD from ND, which is in line with the role of tau proteins in neurodegeneration. Although lower CSF Aß42 levels in AD compared with FTD and controls have been reported by many investigators, there is more variability with regard to t-tau and p-tau among groups.26,27,35,50 The main reasons for these inconsistencies are the potential presence of mixed pathologies in patients with dementia, a significant degree of underlying AD pathology in healthy older people, and variability in CSF measurements.30
When analyzing the relations between MMSE domain scores and CSF biomarkers among the three groups, we found only one significant association in the FTD group of temporal orientation domain scores and Aß42 levels. The lower the temporal orientation domain score was, the lower Aß42 levels were. Impaired orientation as measured by the MMSE is a specific deficit, it is not due to diffuse cognitive dysfunction, and it is likely to be associated with changes in specific neural substrates, such as the hippocampus and parietal cortex.51,52 Our findings suggest a possible association between a higher degree of AD pathology and a profile of more temporal orientation disabilities on cognitive tests in FTD. Animal models and in vitro studies have shown that Aß, tau, and α-synuclein mutually promote their accumulation, thus providing a partial explanation for the simultaneous presence of different neuropathological features in the same subject.53,54 This also suggests the possibility of onset, during aging, of different pathogenic processes in multiple brain regions that eventually enhance their respective development in a synergic way. Therefore, the phenomenon of AD neuropathology could be a comorbidity in several dementia syndromes, including FTD, indicating that “pure” etiologies are relatively rare.
The lack of a relationship between CSF biomarker levels and MMSE scores in patients with AD supports the view that different degrees of cognitive impairment in AD are not reflected in Aß42, t-tau, and p-tau levels. According to the accepted temporal trend of CSF biomarkers in AD, they reach a peak in the preclinical stage of the disease and plateau after diagnosis, and show no further changes with worsening of the clinical condition.55 The lack of a relationship between CSF biomarker levels and MMSE scores in the ND group deserves some discussion. Associations between amyloid burden and specific cognitive domains, and between tau values and visuospatial episodic memory have been described in cognitively healthy individuals.56,57 However, a number of methodological differences, such as group composition, sensitivity of the cognition measures used, and sample size, prevent comparison of the results of such studies with our data. Additionally, our sample was unlikely to have included a significant number of individuals with preclinical AD. Therefore, a relationship between biomarkers and cognitive abilities was unlikely to emerge.
We tested CSF biomarkers levels and ratios to evaluate the usefulness of CSF biomarkers in differential diagnosis of FTD and AD in our clinically defined cases. We found that the p-tau/Aß42 ratio was a sensitive and specific biomarker for discriminating PPA, which is the FTD form with primary language disturbance, from AD. Other reports showed that the combination of p-tau and Aß42 could be considered useful in distinguishing FTD from AD. Schoonenboom and colleagues showed that the combined Aß42 and p-tau dosage allowed differentiation of patients with AD from those with FTD, with a sensitivity of 72% and a specificity of 93%.58 More recently, De Souza et al.35 reported high sensitivity and specificity of the p-tau/Aß42 ratio in differentiation of AD from bvFTD (sensitivity = 95% and specificity = 85.2) and from svPPA (sensitivity = 91.7% and specificity = 84.2). However, some reports have shown that t-tau/Aß42 is the best ratio for distinguishing AD and FTD.28,34 Our study showed, by a rigorous statistical approach, the validity of the p-tau/Aß42 ratio in discriminating PPA, as a specific group, from AD. To the best of our knowledge, previous reports analyzed other diagnostic cohorts included in the FTD spectrum or they did not calculate the p value associated with ROC curve analysis.
Our study has some limitations. We did not have pathological confirmation of our diagnoses. To reduce this bias, we attempted to achieve the highest clinical accuracy by means of adopting strict clinical criteria and a clinical follow-up of 2 years. Use of the MMSE as the sole measure of cognition may have determined low sensitivity and accuracy in evaluating cognitive changes. However, temporal orientation and verbal episodic memory MMSE scores have been reported to correlate well with the Free and Cued Selective Reminding Test.59 Moreover, the MMSE score is the only independent predictor for postoperative cognitive dysfunction in patients with severe systemic diseases.60 Another limitation is that the small sample size could have limited the validity of our results. However, the sample size calculated for the present study was aimed at identifying marked differences among groups, whereas investigation of small differences would have required a larger number of subjects.
Regardless of these limitations, our findings suggest that FTD and AD have comparatively distinct CSF biomarker profiles. Patients with FTD are characterized by higher levels of CSF Aß42, and lower t-tau/Aß42 and p-tau/Aß42 values compared with patients with AD. The ratio of p-tau/Aß42 appears to be the most sensitive and specific biomarker for discriminating FTD with primary language disturbances from AD.
Conclusion
A major challenge for early and correct treatment of neurodegenerative disorders is an accurate diagnosis. In particular, differential diagnosis of AD and FTD may be a difficult task because of overlapping symptoms and a lack of specific biomarkers for FTD. Our study suggests that the p-tau/Aß42 ratio could be useful in differential diagnosis of AD and FTD with primary language disturbances. However, the p-tau/Aß42 ratio does not function well in distinguishing AD from behavioral variant FTD, or from FTD considered as a whole group. Exploratory analyses for novel biomarkers that have diagnostic utility in FTD are ongoing. Promising data are available concerning a possible diagnostic role of TAR DNA-binding protein 43, progranulin, and tau isoforms, but their definitive validation as diagnostic tools for the clinical setting is still lacking.
Acknowledgements
The authors wish to thank Ms. Belinda Giorgetti and Mr. Moreno Solazzi for their technical contribution to CSF storage and biomarker determination, and Dr. Annarita Bonfigli for her support in ethics committee procedures.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This work was funded by the Italian Ministry of Health.
References
- 1.Olney NT, Spina S, Miller BL. Frontotemporal dementia. Neurol Clin 2017; 35: 339–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ghosh S, Lippa CF. Clinical subtypes of frontotemporal dementia. Am J Alzheimers Dis Other Demen 2015; 30: 653–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rascovsky K, Hodges JR, Knopman D, et al. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain 2011; 134: 2456–2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mesulam MM. Primary progressive aphasia. Ann Neurol 2001; 49: 425–432. [PubMed] [Google Scholar]
- 5.Hodges JR, Patterson K. Semantic dementia: a unique clinicopathological syndrome. Lancet Neurol 2007; 6: 100–114. [DOI] [PubMed] [Google Scholar]
- 6.Turner RS, Kenyon LC, Trojanowski JQ, et al. Clinical, neuroimaging, and pathologic features of progressive nonfluent aphasia. Ann Neurol 1996; 39: 166–173. [DOI] [PubMed] [Google Scholar]
- 7.Wang X, Shen Y, Chen W. Progress in frontotemporal dementia research. Am J Alzheimers Dis Other Demen 2013; 28: 15–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baker M, Mackenzie IR, Pickering-Brown SM, et al. Mutations in progranulin cause tau-negative frontotemporal dementia linked to chromosome 17. Nature 2006; 442: 916–919. [DOI] [PubMed] [Google Scholar]
- 9.See TM, LaMarre AK, Lee SE, et al. Genetic causes of frontotemporal degeneration. J Geriatr Psychiatry Neurol 2010; 23: 260–268. [DOI] [PubMed] [Google Scholar]
- 10.Hutton M, Lendon CL, Rizzu P, et al. Association of missense and 5’-splice-site mutations in tau with the inherited dementia FTDP-17. Nature 1998; 393: 702–705. [DOI] [PubMed] [Google Scholar]
- 11.Dejesus-Hernandez M, Mackenzie IR, Boeve BF, et al. Expanded GGGGCC hex-anucleotide repeat in noncoding region of C9ORF72 causes chromosome 9p-linked FTD and ALS. Neuron 2011; 72: 245–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Renton AE, Majounie E, Waite A, et al. A hexanucleotide repeat expansion in C9ORF72 is the cause of chromosome 9p21-linked ALS-FTD. Neuron 2011; 72: 257–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Silveri MC. Frontotemporal dementia to Alzheimer's disease. Dialogues Clin Neurosci 2007; 9: 153–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kertesz A, McMonagle P, Blair M, et al. The evolution and pathology of frontotemporal dementia. Brain 2005; 128: 1996–2005. [DOI] [PubMed] [Google Scholar]
- 15.Knopman DS, Boeve BF, Parisi JE, et al. Antemortem diagnosis of frontotemporal lobar degeneration. Ann Neurol 2005; 57: 480–488. [DOI] [PubMed] [Google Scholar]
- 16.Forman MS, Farmer J, Johnson JK, et al. Frontotemporal dementia: clinicopathological correlations. Ann Neurol 2006; 59: 952–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Knibb JA, Xuereb JH, Patterson K, et al. Clinical and pathological characterization of progressive aphasia. Ann Neurol 2006; 59: 156–165. [DOI] [PubMed] [Google Scholar]
- 18.Alladi S, Xuereb J, Bak T, et al. Focal cortical presentations of Alzheimer’s disease. Brain 2007; 130: 2636–2645. [DOI] [PubMed] [Google Scholar]
- 19.Paraskevas GP, Kasselimis D, Kourtidou E, et al. Cerebrospinal fluid biomarkers as a diagnostic tool of the underlying pathology of primary progressive aphasia. J Alzheimers Dis 2017; 55: 1453–1461. [DOI] [PubMed] [Google Scholar]
- 20.Shea YF, Chu LW, Zhou L, et al. Cerebrospinal fluid biomarkers of Alzheimer's disease in Chinese patients: a pilot study. Am J Alzheimers Dis Other Demen 2013; 28: 769–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blennow K, Hampel H, Weiner M, et al. Cerebrospinal fluid and plasma biomarkers in Alzheimer disease. Nat Rev Neurol 2010; 6: 131–144. [DOI] [PubMed] [Google Scholar]
- 22.Shaw LM, Vanderstichele H, Knapik-Czajka M, et al. Cerebrospinal fluid biomarker signature in Alzheimer’s disease neuroimaging initiative subjects. Ann Neurol 2009; 65: 403–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paraskevas GP, Kapaki E, Liappas I, et al. The diagnostic value of cerebrospinal fluid tau protein in dementing and nondementing neuropsychiatric disorders. J Geriatr Psychiatry Neurol 2005; 18: 163–173. [DOI] [PubMed] [Google Scholar]
- 24.Trojanowski JQ, Vandeerstichele H, Korecka M, et al. Update on the biomarker core of the Alzheimer’s Disease Neuroimaging Initiative subjects. Alzheimers Dement 2010; 6: 230–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weiner MW, Aisen PS, Jack CR, Jr, et al. The Alzheimer’s disease neuroimaging initiative: progress report and future plans. Alzheimers Dement 2010; 6: 202–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Riemenschneider M, Wagenpfeil S, Diehl J, et al. Tau and Abeta42 protein in CSF of patients with frontotemporal degeneration. Neurology 2002; 58: 1622–1628. [DOI] [PubMed] [Google Scholar]
- 27.Grossman M, Farmer J, Leight S, et al. Cerebrospinal fluid profile in frontotemporal dementia and Alzheimer's disease. Ann Neurol 2005; 57: 721–729. [DOI] [PubMed] [Google Scholar]
- 28.Bian H, Van Swieten JC, Leight S, et al. CSF biomarkers in frontotemporal lobar degeneration with known pathology. Neurology 2008; 70: 1827–1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Körtvélyessy P, Gukasjan A, Sweeney-Reed CM, et al. Progranulin and amyloid-β levels: relationship to neuropsychology in frontotemporal and Alzheimer's disease. J Alzheimers Dis 2015; 46: 375–380. [DOI] [PubMed] [Google Scholar]
- 30.Irwin DJ, Trojanowski JQ, Grossman M. Cerebrospinal fluid biomarkers for differentiation of frontotemporal lobar degeneration from Alzheimer's disease. Front Aging Neurosci 2013; 2: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ewers M, Mattsson N, Minthon L, et al. CSF biomarkers for the differential diagnosis of Alzheimer's disease: a large-scale international multicenter study. Alzheimers Dement 2015; 11: 1306–1315. [DOI] [PubMed] [Google Scholar]
- 32.Vergallo A, Carlesi C, Pagni C, et al. A single center study: Aβ42/p-Tau181 CSF ratio to discriminate AD from FTD in clinical setting. Neurol Sci 2017; 38: 1791–1797. [DOI] [PubMed] [Google Scholar]
- 33.Blasko I, Lederer W, Oberbauer H, et al. Measurement of thirteen biological markers in CSF of patients with Alzheimer's disease and other dementias. Dement Geriatr Cogn Disord 2006; 21: 9–15. [DOI] [PubMed] [Google Scholar]
- 34.Kapaki E, Paraskevas GP, Papageorgiou SG, et al. Diagnostic value of CSF biomarker profile in frontotemporal lobar degeneration. Alzheimer Dis Assoc Disord 2008; 22: 47–53. [DOI] [PubMed] [Google Scholar]
- 35.De Souza LC, Lamari F, Belliard S, et al. Cerebrospinal fluid biomarkers in the differential diagnosis of Alzheimer's disease from other cortical dementias. J Neurol Neurosurg Psychiatry 2011; 82: 240–246. [DOI] [PubMed] [Google Scholar]
- 36.De Rino F, Martinelli-Boneschi F, Caso F, et al. CSF metabolites in the differential diagnosis of Alzheimer's disease from frontal variant of frontotemporal dementia. Neurol Sci 2012; 33: 973–977. [DOI] [PubMed] [Google Scholar]
- 37.Struyfs H, Niemantsverdriet E, Goossens J, et al. Cerebrospinal fluid P-Tau181P: biomarker for improved differential dementia diagnosis. Front Neurol 2015; 17: 138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975; 12: 189–198. [DOI] [PubMed] [Google Scholar]
- 39.Magni E., Binetti G, Bianchetti A, et al. Mini-Mental State Examination: a normative study in Italian elderly population. Eur J Neurol 1996; 3: 198–202. [DOI] [PubMed] [Google Scholar]
- 40.Pasquier F, Leys D, Weerts JG, et al. Inter- and intraobserver reproducibility of cerebral atrophy assessment on MRI scans with hemispheric infarcts. Eur Neurol 1997; 36: 268–272. [DOI] [PubMed] [Google Scholar]
- 41.McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement 2011; 7: 263–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dubois B, Feldman HH, Jacova C, et al. Advancing research diagnostic criteria for Alzheimer's disease: the IWG-2 criteria. Lancet Neurol 2014; 13: 614–629. [DOI] [PubMed] [Google Scholar]
- 43.Sorbi S, Hort J, Erkinjuntti T, et al. EFNS-ENS Guidelines on the diagnosis and management of disorders associated with dementia. Eur J Neurol 2012; 19: 1159–1179. [DOI] [PubMed] [Google Scholar]
- 44.Neary D, Snowden JS, Gustafson L, et al. Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology 1998; 51: 1546–1554. [DOI] [PubMed] [Google Scholar]
- 45.Rascovsky K, Hodges JR, Kipps CM, et al. Diagnostic criteria for the behavioral variant of frontotemporal dementia (bvFTD): current limitations and future directions. Alzheimer Dis Assoc Disord 2007; 21: S14–S18. [DOI] [PubMed] [Google Scholar]
- 46.Gorno-Tempini ML, Hillis AE, Weintraub S, et al. Classification of primary progressive aphasia and its variants. Neurology 2011; 76: 1006–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Consensus Report of the Working Group on: “Molecular and Biochemical Markers of Alzheimer’s Disease”. The Ronald and Nancy Reagan Research Institute of the Alzheimer’s Association and, National Institute on Aging Working Group. Neurobiol Aging 1998; 19: 109–116. [PubMed] [Google Scholar]
- 48.Beber BC, Chaves MLF. Evaluation of patients with behavioral and cognitive complaints: misdiagnosis in frontotemporal dementia and Alzheimer's disease. Dement Neuropsychol 2013; 7: 60–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Deutsch MB, Liang LJ, Jimenez EE, et al. Are we comparing frontotemporal dementia and Alzheimer disease patients with the right measures? Int Psychogeriatr 2016; 28: 1481–1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bibl M, Mollenhauer B, Wolf S, et al. Reduced CSF carboxyterminally truncated Abeta peptides in frontotemporal lobe degenerations. J Neural Transm 2007; 114: 621–628. [DOI] [PubMed] [Google Scholar]
- 51.Eichenbaum H, Dudchenko P, Wood E, et al. The hippocampus, memory, and place cells: is it spatial memory or a memory space? Neuron 1999; 23: 209–226. [DOI] [PubMed] [Google Scholar]
- 52.Delpolyi AR, Rankin KP, Mucke L, et al. Spatial cognition and the human navigation network in AD and MCI. Neurology 2007; 69: 986–997. [DOI] [PubMed] [Google Scholar]
- 53.Clinton LK, Blurton-Jones M, Myczek K, et al. Synergistic interactions between Abeta, tau, and alpha-synuclein: acceleration of neuropathology and cognitive decline. J Neurosci 2010; 30: 7281–7289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Giasson BI, Forman MS, Higuchi M, et al. Initiation and synergistic fibrillization of tau and alpha-synuclein. Science 2003; 300: 636–640. [DOI] [PubMed] [Google Scholar]
- 55.Jack CR, Jr, Knopman DS, Jagust WJ, et al. Hypothetical model of dynamic biomarkers of the Alzheimer's pathological cascade. Lancet Neurol 2010; 9: 119–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hedden T, Oh H, Younger AP, et al. Meta-analysis of amyloid-cognition relations in cognitively normal older adults. Neurology 2013; 80: 1341–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pettigrew C, Soldan A, Moghekar A, et al. Relationship between cerebrospinal fluid biomarkers of Alzheimer's disease and cognition in cognitively normal older adults. Neuropsychologia 2015; 78: 63–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schoonenboom NS, Pijnenburg YA, Mulder C, et al. Amyloid ß 1–42 and phosphorylated tau in CSF as markers for early onset Alzheimer disease. Neurology 2004; 62: 1580–1584. [DOI] [PubMed] [Google Scholar]
- 59.Carcaillon L, Amieva H, Auriacombe S, et al. A subtest of the MMSE as a valid test of episodic memory? Comparison with the free and cued reminding test. Dement Geriatr Cogn Disord 2009; 27: 429–438. [DOI] [PubMed] [Google Scholar]
- 60.Radtke FM, Franck M, Herbig TS, et al. Incidence and risk factors for cognitive dysfunction in patients with severe systemic disease. J Int Med Res 2012; 40: 612–620. [DOI] [PubMed] [Google Scholar]