Abstract
Background
Lung resection remains the gold standard treatment for early stage lung cancer; prediction of postoperative lung function is a key selection criterion for surgery with the aim of determining risk of postoperative dyspnoea. We aimed to identify the different prediction techniques used, and compare their accuracy.
Methods
A systematic review and meta-analysis sought to synthesise studies conducted that assess prediction of postoperative lung function up to 18/02/2018 (n = 135). PROBAST was used to assess risk of bias in studies, 17 studies were judged to be at low risk of bias.
Findings
Meta-analysis revealed CT volume and density measurement to be the most accurate (mean difference 71 ml) and precise (standard deviation 207 ml) of the reported techniques used for predicting FEV1; evidence for predicting gas transfer was lacking.
Interpretation
The evidence suggests using CT volume and density is the preferred technique in the prediction of postoperative FEV1. Further studies are required to ensure that the methods and thresholds we propose are linked to patient reported outcomes.
Funding
Salary support for NKO, RM, PN, BN, and AMT was provided by University Hospitals Birmingham NHS Foundation Trust.
Keywords: Thoracic surgery, Lung cancer, Lung function, Operability
Research in Context
Evidence Before This Review
Multiple methods of predicting postoperative lung function had been reported in patients undergoing surgery for lung cancer but the methods had not been objectively compared in a meta-analysis. Decisions about whether patients were suitable for curative lung cancer resection were being influenced by these predictions.
Added Value of This Review
A systematic review of available literature was performed and meta-analysis of predicting FEV1, but not gas transfer factor, was possible. This provides an objective comparison of different prediction methods.
Implications of all the Available Evidence
Using a more accurate and precise method to predict postoperative FEV1 may enable more accurate personalised decisions about lung cancer resection; CT volume and density measures appear outperform segment counting. Further evidence is required to compare the available methods to predict postoperative gas transfer factor.
Alt-text: Unlabelled Box
1. Introduction
Surgical resection with curative intent is the standard of care for stage I and II Non-small Cell Lung Cancer (NSCLC) but such invasive treatment requires careful preoperative assessment, including assessment of forced expiratory volume in 1 s (FEV1) and transfer factor (TLCO) [1]. The predicted postoperative values of FEV1 and TLCO are used to stratify risk of mortality and postoperative dyspnoea, a predicted value of between 30% and 60% expected being the cut off for requesting further investigation [1], [2], [3].
Different techniques to predict postoperative lung function have been reported but there has not been a quantitative review of their accuracy and precision. Predictions are used to inform important treatment decisions such as whether to proceed with surgical resection or recommend other treatment modalities (such as radiotherapy or chemotherapy). Predictions are a factor in counselling patients about their operative risk and a national audit reported that patient preference is a significant reason that patients with resectable tumours do not undergo resection [4]. In addition, prediction resulting in high-risk assessment may delay treatment to allow time for further investigations, such as cardiopulmonary exercise testing. Thus, there is a need for formal comparison of prediction methods.
2. Methods
This is a systematic review and meta-analysis of the techniques to predict postoperative lung function in patients undergoing lung cancer resection in line with the standards set out by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [5]. This review was registered on the International Prospective Register of Systematic Reviews (PROSPERO), record number [CRD42017058955].
2.1. Inclusion and Exclusion Criteria
The population for inclusion was adults with suspected or confirmed primary lung cancer. The intervention was lung resection with curative intent (pneumonectomy, lobectomy, segmentectomy, or wedge resection) with lung function measured before and after surgery; a technique of predicting postoperative lung function must have been applied and the results reported (outcome). Surgery for known benign conditions, palliation, diagnosis only, emergencies, and bronchoscopy only were excluded. Studies reporting combined results of surgery for both benign and malignant pathology were eligible provided the majority of the study population had primary lung cancer. Comparison between the predicted postoperative lung function and the actual measured postoperative lung function must have been performed; comparison of the accuracy and precision of the techniques was planned for meta-analysis. All retrospective and prospective studies were eligible, case reports and case series (n < 8) were excluded.
2.2. Search Strategy and Study Selection
The following electronic bibliographic databases were searched: EMBASE, MEDLINE, Cochrane Central Register of Controlled Trials (CENTRAL), Database of Abstracts of Reviews of Effects (DARE), NHS Economic Evaluation Database (NHS EED), Cochrane Database of Systematic Reviews (CDSR). The search strategy combined terms relating to lung surgery, terms relating measuring lung function, terms relating to the postoperative period and terms relating to prediction or correlation between measures (see Appendix 1 for details of search terms). No language, date or other restrictions were applied. The searches included all eligible studies from inception to 18/01/2018. We also considered additional sources of studies such as reference lists of included papers and contact with experts in the field.
Titles and abstracts of studies retrieved were screened independently by two reviewers to identify studies that potentially met the inclusion criteria. The full texts of potentially eligible studies were independently assessed for eligibility by two reviewers. Any disagreement about eligibility was resolved through consensus (with a third reviewer where necessary). Studies assessed as low risk of bias were included in the meta-analysis.
2.3. Data Management
A pre-piloted electronic form (Microsoft Excel 2010) was used to record extracted data from eligible studies and record risk of bias assessment. Extracted information included: study population and participant demographics; prediction technique; funding; study methodology; recruitment and study completion rates; type of surgery performed; lung function at baseline and postoperatively; postoperative time lung function was assessed. One reviewer extracted data independently (NO) and a second reviewer (JHS, RM, AT) checked the data, discrepancies were resolved through consensus (with a third author where necessary). Missing data from studies eligible for meta-analysis was requested from study authors via electronic mail.
2.4. Bias Assessment
There are few bias tools specific to prediction studies. A preliminary version of the Prediction study Risk Of Bias Assessment Tool (PROBAST) was sought from the authors of the tool and used to assess full papers that met eligibility criteria [6].
2.5. Evidence Synthesis
The principal summary measures were the mean difference between measured and predicted postoperative lung function (accuracy) and the standard deviation of the mean difference (precision). Meta-analysis of mean difference was performed using the generic inverse variance method in Revman Version 5.3 [7]. Multilevel meta-analysis of Variance Ratio, as described in detail by Senior et al., was used to analyse the Standard Deviation in RStudio Version 1.1.463 [8], [9]. Where necessary, patient level data was extracted from article scatter plots using online software WebPlotDigitizer Version 4.1 [10]. Meta-analysis was performed on absolute values; the corresponding authors of papers that provided percentage values only were contacted to provide additional data but no response was received to these enquiries. I2 or Cochran's Q was calculated as a measure of consistency across studies as provided by software packages. Analysis of funnel plots was planned to assess risk of publication bias.
2.6. Role of the Funding Source
Salary support for NKO, RM, PN, BN, and AMT is provided by University Hospitals Birmingham NHS Foundation Trust. The Trust had no role in the design or conduct of the study and no involvement with the writing of the manuscript or decision to submit for publication.
3. Results
3.1. Included Studies
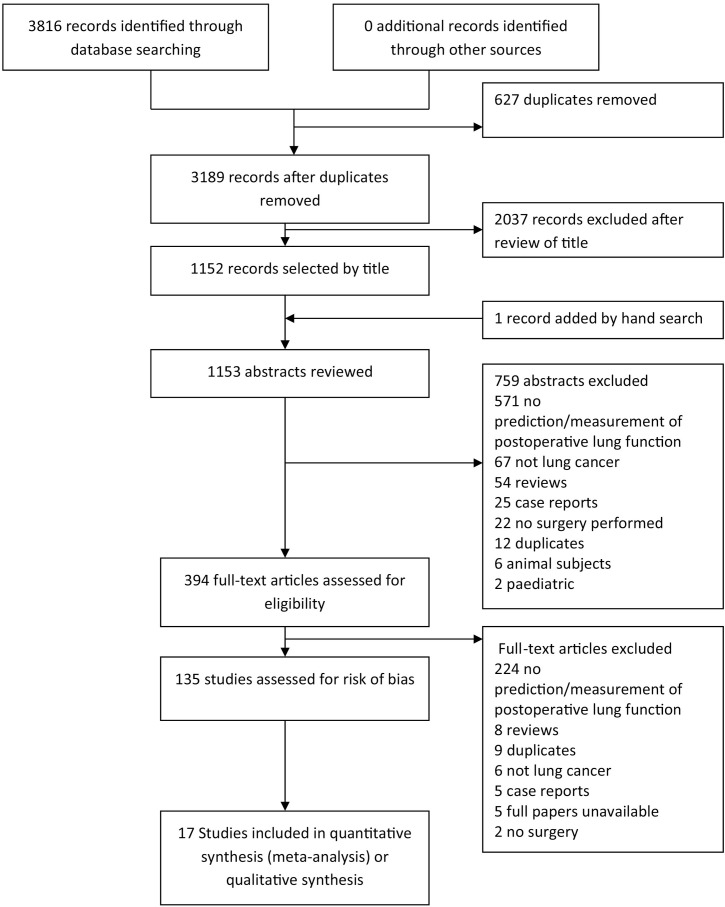
We found 135 full papers that met criteria for inclusion (Fig. 1). The most common reason for exclusion was that the article did not measure postoperative lung function or did not perform prediction of postoperative lung function.
Fig. 1.
Study screening and selection flow diagram.
Articles reported 16 different types of prediction tests, in order of frequency they reported:
-
1.
Perfusion scintigraphy
-
2.
Segment counting — 19 segments, Juhl and Frost formula [11]
-
3.
Ventilation scintigraphy
-
4.
Single Photon Emission Computed Tomography (SPECT)
-
5.
Computed Tomography volume and density (CT-VD)
-
6.
Vibration Response Imaging (VRI)
-
7.
Ventilation/perfusion scintigraphy
-
8.
Subsegment counting — 42 subsegments, Nakahara formula [12]
-
9.
Computed Tomography (CT) volume
-
10.
Co-registered SPECT–CT
-
11.
Perfusion Magnetic Resonance Imaging (MRI)
-
12.
Perfusion CT
-
13.
Newly derived regression equation
-
14.
Ventilation CT
-
15.
CT volumetry and partial densitometry
-
16.
Lateral position test
The tests broadly involve estimation of how much lung tissue is to be removed with or without estimation of the functionality of the lung. CT volume tests used the volume of the lobe to be resected as measured on CT relative to the total lung volumes as the proportion of function expected to be lost [13]. CT-VD tests take into account the density of lung tissue in Hounsfield units, a measure of emphysematous destruction, in addition to lung volumes to define functional lung tissue [13], [14], [15], [16], [17]. CT density masks to define functional lung tissue varied between − 1024 and − 910 for the lower limit − 650 and − 500 for the upper limit [13], [14], [15], [16]. CT volumetry and partial densitometry used the volume of lung tissue but only defined a lower limit of Hounsfield units below which the tissue was considered emphysematous; an upper limit was not defined [18].
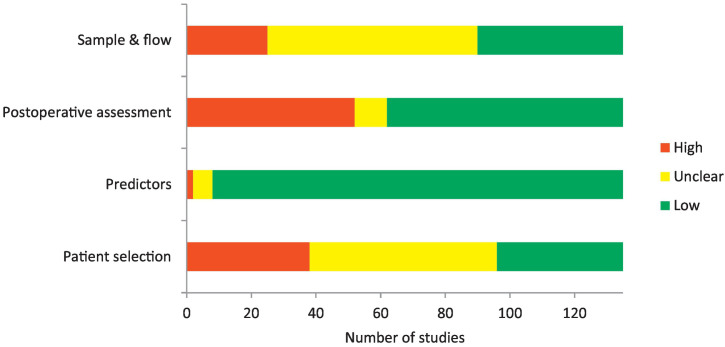
3.2. Risk of Bias and Applicability
The risk of bias assessments for all studies are presented in Appendix 2 and Fig. 2. The method of patient selection was often unclear or appeared to be based upon convenience; exclusion of patients based upon postoperative factors was also frequently described [19], [20], [21], [22], [23], [24], [25], [26], [27], [28]. The timings of postoperative lung function measurement were judged as a source of potential bias if they were performed within 3 months of surgery, before measurements plateau, or when measurements varied vastly in their timing such as a range between 24 days and 5 years [22], [28], [29], [30], [31], [32]. Follow up rates were low in many studies and few studies fully described patients who were lost to follow up [16], [21], [23], [33], [34], [35], [36], [37]. Study outcomes and predictors were generally applicable to the review question.
Fig. 2.
Risk of bias assessment for all eligible studies.
A total of 17 of the 135 full papers had sufficiently low risk of bias to warrant inclusion for meta-analysis. The characteristics of studies that were judged as low risk of bias are presented in Table 1. In some cases the analysis reported in the article was not adequate but sufficient patient level data or summary data was reported to allow the reviewers to perform agreement analysis independently and hence include the data in meta-analysis [38], [39], [40]. There were too few studies reporting each method (n < 10) to perform a valid funnel plot analysis of publication bias.
Table 1.
Study characteristics of the 17 with low risk of bias overall.
| Author, year Country, language | Population | Procedures | Prediction technique | Time to postoperative lung function | Sample analysed (total) | Outcomes | Analysis |
|---|---|---|---|---|---|---|---|
| Fourdrain et al., 2017 [11] France, English | N = 23 82.6% male Mean age 61 | Lobectomy & Pneumonectomy | Segment counting Subsegment counting Perfusion scintigraphy Ventilation scintigraphy CT partial density & volume | 3 months | 23 (37) | FEV1 | Mean difference Correlation |
| Yabuuchi, 2016[12] Japan, English | N = 49 53.1% male Mean age 67 | Lobectomy | Subsegment counting CT volumetry CT volume & density | 6–7 months | 49 (49) | FEV1 Change only | Mean difference Correlation |
| Ohno, 2015[13] Japan, English | N = 60 65.0% male Mean age 68 | Segmentectomy, lobectomy & bilobectomy | Segment counting Perfusion scintigraphy CT volume & density MRI perfusion | 6 months | 60 (60) | FEV1 % only | Mean difference Correlation |
| Chae, 2013[14] Korea, English | N = 51 74.5% male Mean age 64 | Lobectomy & pneumonectomy | Perfusion scintigraphy CT perfusion | 6 months | 51 (67) | FEV1 | Mean difference Correlation |
| Yanagita, 2013[15] Japan, English | N = 30 63.3% male Mean age 70 | Lobectomy & pneumonectomy | SPECT CT ventilation | 6 months | 30 (34) | FEV1 | Mean difference Correlation |
| Yamashita, 2010[16] Canada, English | N = 14 60.0% male Mean age 65 | Lobectomy & pneumonectomy | Perfusion scintigraphy CT perfusion | 3 months | 14 (25) | FEV1 | Mean difference Correlation |
| Yoshimoto, 2009[17] Japan, English | N = 37 59.4% male Mean age 65 | Lobectomy | Segment counting CT volume & density SPECT–CT | 3 months | 37 (37) | FEV1 | Mean difference Correlation |
| Ohno, 2007[18] Japan, English | N = 60 50.0% male Mean age 70 | Lobectomy & pneumonectomy | Perfusion scintigraphy Ventilation scintigraphy SPECT SPECT–CT | 6 months | 60 (60) | FEV1 % only | Mean difference Correlation |
| Sudoh, 2006[19] Japan, English | N = 22 86.4% male Mean age 71 | Lobectomy & segmentectomy | Subsegment counting SPECT–CT | 3–4 months | 22 (22) | FEV1 | Correlation |
| Wang, 2006[20] Canada, English | N = 28 61.0% male Mean age 65 | Segmentectomy, lobectomy & pneumonectomy | Segment counting | 12 months | 28 (57) | DLCO % only | Mean difference |
| Wu, 2002[21] Taiwan, English | N = 34 79.5% male Mean age 67 | Lobectomy & pneumonectomy | Perfusion scintigraphy CT volume & density | 3 months | 34(52) | FEV1 | Mean difference Correlation |
| Beccaria, 2001[22] Italy, English | N = 62 82.3% male Mean age 62 | Lobectomy & pneumonectomy | Segment counting | 6 months | 62 (93) | FEV1 | Correlation |
| Larsen, 1997[23] Denmark, English | N = 23 65.2% male Mean age 67 | Lobectomy & pneumonectomy | Perfusion scintigraphy | 6 months | 23 (41) | FEV1 | Mean difference |
| Bolliger, 1995[24] Switzerland, English | N = 22 68.0% male Mean age 63 | Wedge resection, segmentectomy, lobectomy & pneumonectomy | Perfusion scintigraphy | 6 months | 22 (25) | FEV1 & DLCO | Correlation |
| Wu, 1994[25] China, English | N = 38 86.8% male Mean age 68 | Lobectomy & pneumonectomy | CT volume & density | 3 months | 38 (38) | FEV1 | Mean difference Correlation |
| Egeblad, 1986[26] Denmark, English | N = 30 70.8% male Mean age 61 | Lobectomy & pneumonectomy | Segment counting | 6 months | 30 (30) | FEV1 | Correlation |
| Taube, 1980[27] Germany, German | N = 27 100% male Mean age 53 | Pneumonectomy | Perfusion scintigraphy | 6 months | 27 (29) | FEV1 | Correlation |
3.3. Quantitative Synthesis: Meta-analysis of Mean Difference
It was only possible to perform meta-analysis for studies predicting FEV1; their results are presented in Table 2, corresponding Forest plots are displayed in Appendix 3. CT-VD was shown to be the most accurate technique (mean difference 71 ml, 95% Confidence Interval 38–103). The minimum clinically important difference in FEV1 after surgery is not known but this has been established as 100 ml in the context of Chronic Obstructive Pulmonary Disease (COPD); as such a difference in FEV1 in the measured versus the predicted should not be noticeable clinically if it is less than 100 ml [41]. SPECT, CT ventilation and CT volumetry may fulfil these criteria but there was only one study that reported each of these techniques. Heterogeneity was low for CT-VD and CT perfusion, moderate for SPECT–CT, segment counting and subsegment counting but high for perfusion scintigraphy.
Table 2.
Meta-analysis results of prediction of postoperative FEV1: Mean difference.
| Prediction technique | Studies | Mean difference (measured − predicted FEV1) | 95% Confidence interval | I2 |
|---|---|---|---|---|
| CT — volume & density | 3 (n = 109) | 71 ml | 38 to 103 | 0% |
| Perfusion scintigraphy | 7 (n = 194) | 101 ml | − 11 to 214 | 88% |
| SPECT–CT | 2 (n = 59) | 107 ml | − 10 to 225 | 66% |
| CT — perfusion | 2 (n = 65) | 143 ml | 59 to 228 | 0% |
| Segment counting | 4 (n = 145) | 192 ml | 88 to 295 | 74% |
| Subsegment counting | 3 (n = 82) | 233 ml | 135 to 332 | 65% |
| SPECT | 1 (n = 30) | 10 ml | − 114 to 134 | Single study |
| CT — ventilation | 1 (n = 30) | 70 ml | − 24 to 164 | Single study |
| CT — volumetry | 1 (n = 30) | 90 ml | − 21 to 201 | Single study |
| CT — volume & partial density | 1 (n = 23) | 266 ml | 172 to 360 | Single study |
| Ventilation scintigraphy | 1 (n = 23) | 312 ml | 188 to 435 | Single study |
3.4. Quantitative Synthesis: Meta-analysis of Standard Deviation
Meta-analysis of precision was only possible for four prediction techniques because of missing data on variability of the mean difference in full papers. CT-VD was found to be the most precise FEV1 prediction method (SD 207 ml, Table 3), Cochran's Q did not provide evidence of heterogeneity in any of the techniques. A perfect test that predicted FEV1 within 100 ml of the measured postoperative value (as is accepted in spirometry repeatability standards) would have a standard deviation of 16·7 ml, as such all methods of prediction showed low precision.
Table 3.
Meta-analysis results of prediction of postoperative FEV1: Standard deviation.
| Prediction technique | Studies | Standard deviation | Cochran's Q test of heterogeneity (p value) |
|---|---|---|---|
| CT — volume & density | 2 (n = 75) | 207 ml | 1.569 (0.2103) |
| Subsegment counting | 2 (n = 60) | 274 ml | 0.047 (0.8279) |
| Perfusion scintigraphy | 5 (n = 109) | 285 ml | 3.690 (0.4495) |
| Segment counting | 4 (n = 145) | 331 ml | 3.361 (0.3392) |
| CT — volume & partial density | 1 (n = 23) | 229 ml | Single study |
| SPECT–CT | 1 (n = 37) | 249 ml | Single study |
| Ventilation scintigraphy | 1 (n = 23) | 303 ml | Single study |
| CT — perfusion | 1 (n = 14) | 329 ml | Single study |
This meta-analysis produces a combined standard deviation of the mean difference based on the standard deviations quoted in included studies. It should be noted this is mathematically distinct from the standard error of the mean for the reported meta-analysis mean difference. Null hypothesis for Cochran's Q test is homogeneity.
3.5. Qualitative Synthesis
Three studies of FEV1 prediction could not be meta-analysed but did describe comparisons of the accuracy different prediction techniques (Appendix 4). Yabuuchi et al. reported change in FEV1 rather than absolute values, they found CT-VD to outperform subsegment counting and CT volumetry [13]. Ohno et al. 2015 reported FEV1 percent of expected values rather than absolute values; they reported CT-VD and perfusion MRI (with and without contrast) to be comparable to each other but superior to both segment counting and perfusion scintigraphy [14]. Ohno et al. 2007 also reported FEV1 percent of expected values; they compared ventilation scintigraphy, perfusion scintigraphy, SPECT, and SPECT–CT. They found the latter two methods to be better than the former two methods [42].
Only two studies with low risk of bias and 23 of all eligible studies predicted TLCO. One of these papers reported segment counting and the other perfusion scintigraphy meaning quantitative synthesis was not possible [43], [44]. The mean difference was 2 percentage points (standard error 1·5) for segment counting and mean difference 11 percentage points (standard error 1·7) for perfusion scintigraphy.
4. Discussion
Our meta-analysis has shown that prediction of FEV1 after lung resection is most accurate and precise when using combined CT volume and density measures; however the precision of all methods to predict postoperative FEV1 is low. The common practise of using segment counting to guide treatment decisions and patient counselling may warrant changing in light of this finding. Prediction of TLCO has a limited evidence base but seems to be more accurate using segment counting than perfusion scintigraphy. These findings are of particular relevance to the future guidelines regarding assessment of fitness for lung cancer resection; the most recent British guidelines having been archived in 2017 [1].
4.1. Current Risk Assessment for Surgery
Patients undergo CT scanning at least twice prior to resection in the form of the initial diagnostic CT and subsequent staging Positron Emission Tomography (PET) CT; utilising existing imaging without further appointments and concomitant delays makes CT an appealing technique for predicting postoperative lung function. Additionally, CT densitometry has been shown to be superior to spirometry in predicting pulmonary complications, including prolonged air leak, after pulmonary resection and identification of patients at increased risk of these complications could facilitate targeted interventions or adjustments to surgical technique as preventative measures [45], [46]. A combined CT based risk assessment of predicting postoperative pulmonary function and the risk of postoperative pulmonary complications would be an attractive and relevant tool for clinicians.
4.2. Barriers to Clinical Implementation
Calculations of CT density and volume have been possible since 1994 but have not become routine clinical measures and the availability of analysis software to clinicians is an important problem [17]. Six different software programs were specified in papers eligible for this review including AZE VirtualPlace, Pulmo-CMS, Ziosoft M900 QUADRA, GE Healthcare Thoracic VCAR, Fujifilm Healthcare SYNAPSE VINCENT, and ‘standard software’ within a Somatom HiQ unit; this indicates a competitive market for image analysis. Developers of software may charge for novel types of image analysis and healthcare institutions are subsequently faced with additional expense that has no proof of clinical efficacy, additionally the number of patients requiring specific analysis may not justify subscription to software services even if benefit has been demonstrated. This can lead to new techniques not being utilised, these techniques then fail to be clinically validated, and patients cannot benefit from technological advancements.
4.3. Strengths and Limitations
This is the first systematic review and meta-analysis to consider how best to predict postoperative lung function as part of perioperative risk assessment. A key strength is that the results should be internationally applicable due to the wide range of countries articles originated from (34 from Japan, 28 from other countries outside Western Europe/North America). The clinical scenarios are broad meaning variation in operative procedure, time to follow up lung function, and precise details of prediction technique (including human error) could all contribute to heterogeneity. However, heterogeneity was only high for perfusion scintigraphy predicting FEV1. We were limited by insufficient information about study methodology being reported to enable risk of bias assessment resulting in bias being considered ‘unclear’ in many cases. Many papers did not report the primary outcome or sufficient data to enable independent calculation of this; as such the information from many additional patients could not be included. Five studies could not be retrieved in full but this is a small proportion of the total studies considered for full paper assessment (2·3%). Finally, reviews are generally subject to limitations of reporting bias, it was not possible to assess this using funnel plots due to the low number of full papers included.
4.4. Future research Recommendations
Future research in this field might focus on direct comparison of different prediction techniques to predict TLCO. Further validation of the utility of postoperative FEV1 and TLCO prediction might include assessments of how these relate to patient reported outcome measures (PROMs) such as quality of life, dyspnoea, ability to live independently and performance of preoperative activities. Postoperative quality of life has been shown to be equivalent in patients with impaired preoperative lung function compared to those without impairment, making the integration of PROMs an important complementary part of risk assessment when counselling patients about treatment options [47]. Prediction of postoperative lung function could potentially be combined with prediction of postoperative complications to give a global respiratory risk score. Finally the optimal CT protocol for prediction could be determined by direct comparison utilising different CT settings and different software to define functional lung tissue within the same patient group. The interaction between medical imaging companies and healthcare providers is likely to be critical in the implementation of research findings.
4.5. Conclusions
In conclusion, using CT volume and density is the most accurate and precise way to predict postoperative FEV1. There is limited evidence about predicting postoperative TLCO but segment counting appears to outperform perfusion scintigraphy.
Author Contributions
NKO, BN, and AMT conceived and designed the work. NKO, JH-S, RM, and AMT extracted and checked data. NKO and PN analysed data. NKO, BN, PN, and AMT interpreted data. NKO drafted the manuscript; JH-S, RM, PN, BN, and AMT revised it critically for content. All authors approved the final manuscript version for publication. All authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Declaration of Competing Interest
AMT reports grants from Grifols Biotherapeutics, grants from Alpha-1 Foundation, personal fees from CSL Behring, grants and non-financial support from Arrowhead Inc, outside the submitted work. NKO, JH-S, RM, PN, and BN have no conflict of interest to declare.
Acknowledgements
The authors would like to thank Mrs. Amanda Wood and Dr. Tae Kyung Park for their assistance in reviewing Chinese and Korean language articles.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.eclinm.2019.08.015.
Appendix A. Supplementary data
Appendices 1 to 4
References
- 1.Lim E., Baldwin D., Duffy J., Entwisle J., Faivre-Finn C., Kerr K. Guidelines on the radical management of patients with lung cancer. Thorax. 2010;65(Supplement III) doi: 10.1136/thx.2010.145938. Oct. [DOI] [PubMed] [Google Scholar]
- 2.Brunelli A., Charloux A., Bolliger C.T., Rocco G., Sculier J.-P., Varela G. ERS/ESTS clinical guidelines on fitness for radical therapy in lung cancer patients (surgery and chemo-radiotherapy) Eur Respir J. 2009;34(1):17–41. doi: 10.1183/09031936.00184308. Jul 1. [DOI] [PubMed] [Google Scholar]
- 3.Brunelli A., Kim W.K., Berger K.I., Addrizzo-Harris D.J. Physiologic evaluation of the patient with lung cancer being considered for resectional surgery. Chest. 2013;143(5) doi: 10.1378/chest.12-2395. e166S–e190S May. [DOI] [PubMed] [Google Scholar]
- 4.Royal College of Physicians . Royal College of Physicians; London: 2018. National Lung Cancer Audit annual report 2017 (for the period 2016) [Google Scholar]
- 5.Moher D., Shamseer L., Clarke M., Ghersi D., Liberati A., Petticrew M. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4:1. doi: 10.1186/2046-4053-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wolff R, Moons K, Riley R, Whiting P, Westwood M, Collins G, et al. PROBAST — a risk-of-bias tool for prediction-modelling studies | colloquium abstracts [internet]. 2017 [cited 2018 May 29]. Available from: /2017-global-evidence-summit/probast-%E2%80%93-risk-bias-tool-prediction-modelling-studies.
- 7.The Cochrane Collaboration . The Nordic Cochrane Centre; Copenhagen: 2014. Review manager (Revman) [Google Scholar]
- 8.Senior A.M., Gosby A.K., Lu J., Simpson S.J., Raubenheimer D. Meta-analysis of variance: an illustration comparing the effects of two dietary interventions on variability in weight. Evol Med Public Health. 2016;(1):244–255. doi: 10.1093/emph/eow020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.RStudio Integrated development environment for R [Internet]. Boston, MA. www.rstudio.org [cited 2018 Oct 25]. Available from.
- 10.Rohatgi A. WebPlotDigitizer [Internet] 2018. https://automeris.io/WebPlotDigitizer Austin, Texas, USA. Available from:
- 11.Fourdrain A., De F.D., Lafitte S., Iquille J., Prevot F., Lorne E. Quantitative computed tomography to predict postoperative FEV1 after lung cancer surgery. J Thorac Dis. 2017;9(8):2413–2418. doi: 10.21037/jtd.2017.06.118. Aug. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yabuuchi H., Kawanami S., Kamitani T., Yonezawa M., Yamasaki Y., Yamanouchi T. Prediction of post-operative pulmonary function after lobectomy for primary lung cancer: a comparison among counting method, effective lobar volume, and lobar collapsibility using inspiratory/expiratory CT. Eur J Radiol. 2016;85(11):1956–1962. doi: 10.1016/j.ejrad.2016.08.017. Nov. [DOI] [PubMed] [Google Scholar]
- 13.Ohno Y., Seki S., Koyama H., Yoshikawa T., Matsumoto S., Takenaka D. 3D ECG- and respiratory-gated non-contrast-enhanced (CE) perfusion MRI for postoperative lung function prediction in non-small-cell lung cancer patients: a comparison with thin-section quantitative computed tomography, dynamic CE-perfusion MRI, and perfusion scan. J Magn Reson Imaging JMRI. 2015;42(2):340–353. doi: 10.1002/jmri.24800. Aug. [DOI] [PubMed] [Google Scholar]
- 14.Chae E.J., Kim N., Seo J.B., Park J.-Y., Song J.-W., Lee H.J. Prediction of postoperative lung function in patients undergoing lung resection: dual-energy perfusion computed tomography versus perfusion scintigraphy. Invest Radiol. 2013;48(8):622–627. doi: 10.1097/RLI.0b013e318289fa55. Aug. [DOI] [PubMed] [Google Scholar]
- 15.Yanagita H., Honda N., Nakayama M., Watanabe W., Shimizu Y., Osada H. Prediction of postoperative pulmonary function: preliminary comparison of single-breath dual-energy xenon CT with three conventional methods. Jpn J Radiol. 2013;31(6):377–385. doi: 10.1007/s11604-013-0202-z. Jun. [DOI] [PubMed] [Google Scholar]
- 16.Yamashita C.M., Langridge J., Hergott C.A., Inculet R.I., Malthaner R.A., Lefcoe M.S. Predicting postoperative FEV1 using spiral computed tomography. Acad Radiol. 2010;17(5):607–613. doi: 10.1016/j.acra.2010.01.002. May. [DOI] [PubMed] [Google Scholar]
- 17.Yoshimoto K., Nomori H., Mori T., Kobayashi H., Ohba Y., Shibata H. Prediction of pulmonary function after lung lobectomy by subsegments counting, computed tomography, single photon emission computed tomography and computed tomography: a comparative study. Eur J Cardio-Thorac Surg Off J Eur Assoc Cardio-Thorac Surg. 2009;35(3):408–413. doi: 10.1016/j.ejcts.2008.10.057. Mar. [DOI] [PubMed] [Google Scholar]
- 18.Ohno Y., Koyama H., Takenaka D., Nogami M., Kotani Y., Nishimura Y. Coregistered ventilation and perfusion SPECT using krypton-81m and Tc-99m-labeled macroaggregated albumin with multislice CT utility for prediction of postoperative lung function in non-small cell lung cancer patients. Acad Radiol. 2007;14(7):830–838. doi: 10.1016/j.acra.2007.03.013. Jul. [DOI] [PubMed] [Google Scholar]
- 19.Sudoh M., Ueda K., Kaneda Y., Mitsutaka J., Li T.-S., Suga K. Breath-hold single-photon emission tomography and computed tomography for predicting residual pulmonary function in patients with lung cancer. J Thorac Cardiovasc Surg. 2006;131(5):994–1001. doi: 10.1016/j.jtcvs.2005.12.038. May. [DOI] [PubMed] [Google Scholar]
- 20.Wang J.-S., Abboud R.T., Wang L.-M. Effect of lung resection on exercise capacity and on carbon monoxide diffusing capacity during exercise. Chest. 2006;129(4):863–872. doi: 10.1378/chest.129.4.863. Apr. [DOI] [PubMed] [Google Scholar]
- 21.Wu M.-T., Pan H.-B., Chiang A.A., Hsu H.-K., Chang H.-C., Peng N.-J. Prediction of postoperative lung function in patients with lung cancer: comparison of quantitative CT with perfusion scintigraphy. AJR Am J Roentgenol. 2002;178(3):667–672. doi: 10.2214/ajr.178.3.1780667. Mar. [DOI] [PubMed] [Google Scholar]
- 22.Beccaria M., Corsico A., Fulgoni P., Zoia M.C., Casali L., Orlandoni G. Lung cancer resection: the prediction of postsurgical outcomes should include long-term functional results. Chest. 2001;120(1):37–42. doi: 10.1378/chest.120.1.37. Jul. [DOI] [PubMed] [Google Scholar]
- 23.Larsen K.R., Lund J.O., Svendsen U.G., Milman N., Petersen B.N. Prediction of post-operative cardiopulmonary function using perfusion scintigraphy in patients with bronchogenic carcinoma. Clin Physiol Oxf Engl. 1997;17(3):257–267. doi: 10.1111/j.1365-2281.1997.tb00005.x. May. [DOI] [PubMed] [Google Scholar]
- 24.Bolliger C.T., Wyser C., Roser H., Solèr M., Perruchoud A.P. Lung scanning and exercise testing for the prediction of postoperative performance in lung resection candidates at increased risk for complications. Chest. 1995;108(2):341–348. doi: 10.1378/chest.108.2.341. Aug. [DOI] [PubMed] [Google Scholar]
- 25.Wu M.T., Chang J.M., Chiang A.A., Lu J.Y., Hsu H.K., Hsu W.H. Use of quantitative CT to predict postoperative lung function in patients with lung cancer. Radiology. 1994;191(1):257–262. doi: 10.1148/radiology.191.1.8134584. Apr. [DOI] [PubMed] [Google Scholar]
- 26.Egeblad K., Aunsholt N.A., Funder V., Nielsen P.H. A simple method for predicting pulmonary function after lung resection. Scand J Thorac Cardiovasc Surg. 1986;20(2):103–107. doi: 10.3109/14017438609106484. Jan 1. [DOI] [PubMed] [Google Scholar]
- 27.Taube K., Konietzko N. Can the effect of pneumonectomy on cardiopulmonary function be anticipated? (Author's transl) Prax Klin Pneumol. 1980;34(9):548–554. Sep. [PubMed] [Google Scholar]
- 28.Juhl B., Frost N. A comparison between measured and calculated changes in the lung function after operation for pulmonary cancer. Acta Anaesthesiol Scand Suppl. 1975;57:39–45. doi: 10.1111/j.1399-6576.1975.tb05411.x. [DOI] [PubMed] [Google Scholar]
- 29.Nakahara K., Monden Y., Ohno K., Miyoshi S., Maeda H., Kawashima Y. A method for predicting postoperative lung function and its relation to postoperative complications in patients with lung cancer. Ann Thorac Surg. 1985;39(3):260–265. doi: 10.1016/s0003-4975(10)62591-x. Mar. [DOI] [PubMed] [Google Scholar]
- 30.Mizobuchi T., Wada H., Sakairi Y., Suzuki H., Nakajima T., Tagawa T. Spirometric and radiological evaluation of the remnant lung long after major pulmonary resection: can compensatory phenomena be recognized in clinical cases? Surg Today. 2014;44(9):1735–1743. doi: 10.1007/s00595-013-0702-6. Sep. [DOI] [PubMed] [Google Scholar]
- 31.Murakami J., Ueda K., Sano F., Hayashi M., Tanaka N., Hamano K. Prediction of postoperative dyspnea and chronic respiratory failure. J Surg Res. 2015;195(1):303–310. doi: 10.1016/j.jss.2015.01.018. [May 1] [DOI] [PubMed] [Google Scholar]
- 32.Smulders S.A., Smeenk F.W.J.M., Janssen-Heijnen M.L.G., Postmus P.E. Actual and predicted postoperative changes in lung function after pneumonectomy: a retrospective analysis. Chest. 2004;125(5):1735–1741. doi: 10.1378/chest.125.5.1735. May. [DOI] [PubMed] [Google Scholar]
- 33.Sekine Y., Iwata T., Chiyo M., Yasufuku K., Motohashi S., Yoshida S. Minimal alteration of pulmonary function after lobectomy in lung cancer patients with chronic obstructive pulmonary disease. Ann Thorac Surg. 2003;76(2):356–361. doi: 10.1016/s0003-4975(03)00489-2. Aug. [discussion 362] [DOI] [PubMed] [Google Scholar]
- 34.Imaeda T., Kanematsu M., Asada S., Seki M., Matsui E., Doi H. Prediction of pulmonary function after resection of primary lung cancer. Utility of inhalation-perfusion SPECT imaging. Clin Nucl Med. 1995;20(9):792–799. doi: 10.1097/00003072-199509000-00007. Sep. [DOI] [PubMed] [Google Scholar]
- 35.Ueda K., Murakami J., Sano F., Hayashi M., Kobayashi T., Kunihiro Y. Assessment of volume reduction effect after lung lobectomy for cancer. J Surg Res. 2015;197(1):176–182. doi: 10.1016/j.jss.2015.03.064. Jul. [DOI] [PubMed] [Google Scholar]
- 36.Khargi K., Duurkens V.A., Verzijlbergen F.F., Huysmans H.A., Knaepen P.J. Pulmonary function after sleeve lobectomy. Ann Thorac Surg. 1994;57(5):1302–1304. doi: 10.1016/0003-4975(94)91380-3. May. [DOI] [PubMed] [Google Scholar]
- 37.Sangalli M., Spiliopoulos A., Mégevand R. Predictability of FEV1 after pulmonary resection for bronchogenic carcinoma. Eur J Cardio-Thorac Surg Off J Eur Assoc Cardio-Thorac Surg. 1992;6(5):242–245. doi: 10.1016/1010-7940(92)90105-7. [DOI] [PubMed] [Google Scholar]
- 38.Yasukawa M., Nakagawa K., Sakaguchi M., Iwasaki T., Sasaki N. Prediction of postoperative pulmonary function after pneumonectomy for lung cancer. [Japanese] J Lung Cancer. 2004;6:683–687. [Oct] [Google Scholar]
- 39.Bria W.F., Kanarek D.J., Kazemi H. Prediction of postoperative pulmonary function following thoracic operations. Value of ventilation-perfusion scanning. J Thorac Cardiovasc Surg. 1983;86(2):186–192. Aug. [PubMed] [Google Scholar]
- 40.Comce F., Bingol Z., Kiyan E., Tanju S., Toker A., Cagatay P. Vibration-response imaging versus quantitative perfusion scintigraphy in the selection of patients for lung-resection surgery. Respir Care. 2011;56(12):1936–1941. doi: 10.4187/respcare.01059. Dec. [DOI] [PubMed] [Google Scholar]
- 41.Şimşek Veske N., Sökücü S.N., Günlüoğlu G., Seyhan E.C., Dalar L., Altın S. Place of vibration response imaging in preoperative lung cancer patients. Tuberk Ve Toraks. 2014;62(4):279–285. doi: 10.5578/tt.3138. [DOI] [PubMed] [Google Scholar]
- 42.Zeiher B.G., Gross T.J., Kern J.A., Lanza L.A., Peterson M.W. Predicting postoperative pulmonary function in patients undergoing lung resection. Chest. 1995;108(1):68–72. doi: 10.1378/chest.108.1.68. Jul. [DOI] [PubMed] [Google Scholar]
- 43.Kikuchi K., Ishii Y., Kitamura S. Prediction of postoperative lung function in patients with lung Cancer and chronic obstructive pulmonary disease. Jpn J Thorac Dis. 1996;34(10):1071–1076. Oct 25. [PubMed] [Google Scholar]
- 44.Detterbeck F., Gat M., Miller D., Force S., Chin C., Fernando H. A new method to predict postoperative lung function: quantitative breath sound measurements. Ann Thorac Surg. 2013;95(3):968–975. doi: 10.1016/j.athoracsur.2012.07.045. Mar. [DOI] [PubMed] [Google Scholar]
- 45.Ueda K., Kaneda Y., Sudo M., Mitsutaka J., Li T.-S., Suga K. Quantitative computed tomography versus spirometry in predicting air leak duration after major lung resection for cancer. Ann Thorac Surg. 2005;80(5):1853–1858. doi: 10.1016/j.athoracsur.2005.05.006. Nov 1. [DOI] [PubMed] [Google Scholar]
- 46.Kaplan T., Atac G.K., Gunal N., Kocer B., Alhan A., Cubuk S. Quantative computerized tomography assessment of lung density as a predictor of postoperative pulmonary morbidity in patients with lung cancer. J Thorac Dis. 2015;7(8):1391–1397. doi: 10.3978/j.issn.2072-1439.2015.07.26. Aug. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pompili C., Brunelli A., Refai M., Xiumè F., Sabbatini A. Does chronic obstructive pulmonary disease affect postoperative quality of life in patients undergoing lobectomy for lung cancer? A case-matched study. Eur J Cardio-Thorac Surg Off J Eur Assoc Cardio-Thorac Surg. 2010;37(3):525–530. doi: 10.1016/j.ejcts.2009.09.025. Mar. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendices 1 to 4