Abstract
Background
Causes of variations in outcomes from cancer care in developed countries are often unclear. Australia has developed health system pathways describing consensus standards of optimal cancer care across the phases of prevention through to follow-up or end-of-life. These Optimal Care Pathways (OCP) were introduced from 2013 to 14. We investigated whether care consistent with the OCP improved outcomes for colon cancer patients.
Methods
Colon patients diagnosed from 2008 to 2014 were identified from the Australian State of Victoria Cancer Registry (VCR) and cases linked with State and Federal health datasets. Surrogate variables describe OCP alignment in our cohort, across three phases of the pathway; prevention, diagnosis and initial treatment and end-of-life. We assessed the impact of alignment on (1) stage of disease at diagnosis and (2) overall survival.
Findings
Alignment with the prevention phase of the OCP occurred for 88% of 13,539 individuals and was associated with lower disease stage at diagnosis (OR = 0.33, 95% confidence interval 0.24 to 0.42), improved crude three-year survival (69.2% versus 62.2%; p < 0.001) and reduced likelihood of emergency surgery (17.7% versus 25.6%, p < 0.001). For patients treated first with surgery (n = 10,807), care aligned with the diagnostic and treatment phase indicators (44% of patients) was associated with a survival benefit (risk-adjusted HRnon-aligned vs aligned = 1.23, 95% confidence interval 1.13 to 1.35), better perioperative outcomes and higher alignment with follow-up and end-of-life care. The survival benefit persists adjusting for potential confounding factors, including age, sex, disease stage and comorbidity.
Interpretation.
This population-based study shows that care aligned to a pathway based on best principles of cancer care is associated with improved outcomes for patients with colon cancer.
Funding
None.
Keywords: Oncology, Optimal Cancer Pathways
Putting research into context
-
•
Factors accounting for cancer outcome disparities both between within and between countries in well-resourced health systems remain elusive.
-
•
In Australia, a nationally accepted evidenced based pathway of cancer care (the Optimal Care Pathway- OCP) has been developed through a multi-disciplinary clinician consensus based process, to establish the elements of quality care that should be offered to cancer patients. A project to assess the impact of the care that conforms with OCP standards on patient outcomes was undertaken.
-
•
A robust survival improvement was seen in the group of patients whose care was aligned to the OCP. This improvement in outcome did not depend on new diagnostic tests or treatments.
-
•
Alignment with pre-diagnosis pathway recommendations was associated with lower stage of disease at presentation, less emergency surgery and improved long term survival.
-
•
These findings have policy implications as it is demonstrated that subtle system level variation of care could impact favourably on outcomes.
1. Introduction
Despite improvements in treatment and the development of centers of excellence, variations in survival persist in many cancers when benchmarked both within and across countries [1], [2], [3]. The rising burden of cancer and persistent disparities in outcomes in developed countries has driven research into the delivery of more affordable, high quality and equitable cancer care [4], [5], [6]. Factors underlying outcome disparities between similar countries have remained elusive, with the contribution of health system dynamics to these disparities difficult to measure [7].
Research into context
Evidence Before This Study
Factors underlying outcome disparities in cancer patients between similar countries have remained elusive, with the contribution of health system dynamics to these disparities being unclear. As part of the Australian Government cancer reform agenda, the Optimal Care Pathways were developed to guide consistent cancer care. Prior to this process a literature review was undertaken to assess the evidence base for the use of pathways in cancer care. However no overall conclusion on the value of clinical pathways could be made.
Added Value of This Study
This population-based study, using high quality cancer registry data and linkage to government administrative data sets, is unique in its attempt evaluate patient outcomes in relation to alignment of care as designated in the seven-step Optimal Care Pathway, irrespective of the treatment provided. We conclude that a combination of often unrelated processes of clinical care can be strongly associated with positive outcomes from cancer care, including survival and patient support. Our data shows the significant impact of pre-diagnostic care in the primary health setting on outcomes and emphasizes the need for appropriate fusion of primary health care and cancer services.
Implications of All The Available Evidence
This study has important policy implications as system level organization of care may be an important explanatory variable of the differences in survival from colon cancer. Also important from a policy perspective is our finding of interdependency between good quality care in the early steps of the pathway and ongoing compliance in later steps. We highlight the need to address the organization and coordination of cancer care across the entire health system to embed practices consistent with agreed pathways representing optimal care. Although this study uses survival as the main endpoint, patient experience and costs of care delivery are also key determinants of appropriate and effective cancer care.
Alt-text: Unlabelled Box
Cancer care pathways define optimal trajectories through health systems and have been implemented in many countries [8], [9], [10], [11]. These pathways are generally designed for rapid referral, institutional conformity of practice and cost efficiencies [12], [7]. The American Society of Clinical Oncology published a policy statement on clinical pathways in oncology [13]. However, a meta-analysis of clinical pathway publications failed to show significant clinical benefits and the question of whether this approach to health systems improves overall outcomes remains open [10].
Over the last decade, the cancer reform agenda in Australia has concentrated on creating a uniform pathway of care for individual forms of cancers, the Optimal Care Pathway (OCP) [14]. The OCP are based on the best available evidence, with input from expert multidisciplinary clinical groups and broad consultation to define a consensus standard of optimal cancer care. The OCP describe seven phases of the pathway from prevention through to follow-up or end-of-life care. Critical elements of quality care, including time-frames for action, are described for each phase.
The concept of the OCP must be distinguished from the use of Clinical Practice Guidelines (CPG) which encourage standardised care at each step on the pathway. The OCP provides a guide for standards and performance of the health system and was designed to support the patient in finding the appropriate expert care, which includes CPG use.
The OCP standard of care applies for all populations (rural, regional or metropolitan), with wide acceptance of the principle that optimal care is both a goal and the right of all in society. Local area improvement networks across Victoria have used the OCPs to identify service gaps and to drive system and practice change toward alignment. The colorectal OCP was one of the first to be formally implemented in Victoria, following its release in November 2014, although a preliminary version was in circulation for several years prior to that date. Despite acceptance of the OCPs, evidence has been lacking as to the benefit to the patient from ‘following’ the OCP.
To test the hypothesis that improved patients' outcomes are associated with receiving care aligned to the OCP, a population based observational study was undertaken using Victorian Cancer Registry data linked to State and Federal government administrative datasets. To assess alignment, we first identified and assigned key surrogate variables available within the dataset as ‘indices’ describing steps within the OCP. Analyses identified the group which complied with these indices as aligned to the OCP and compared outcomes to the remainder of the cohort. The characteristics of both groups were compared to assess potential confounding factors.
2. Methods
The Australian Institute of Health and Welfare Ethics Committee approved (Approval EO2015/4/219) the use of linked, routinely collected State and Federal datasets for the study of Victorians diagnosed with colon cancer (ICD-10-AM diagnosis codes C18) between 2008 and 2014 through the Victorian Cancer Registry (VCR).
The VCR dataset was linked to public and private Victorian Government inpatient records (Victorian Admitted Episodes Dataset; VAED), and Australian Government data of ambulatory care (including all primary care), imaging and pathology payment data (Medicare Benefits Schedule; MBS) and prescription fill data (Pharmaceutical Benefits Scheme; PBS). This linked dataset provides information on patient demographics, tumour characteristics, comorbidities, cancer diagnosis and treatment including surgery and chemotherapy. Deaths, extracted from the Government Births, Deaths & Marriages Register by the VCR, were complete up to 31/12/2014. VCR provided stage at diagnosis data coded according to American Joint Committee on Cancer (AJCC). Patients with stage I, II, III or unknown stage disease who had an admission with a metastatic ICD-10-AM code (C78, C79) within four months of cancer diagnosis were reclassified as stage IV.
This linked dataset was held in a secure Electronic Data Warehouse at the Department of Health, Canberra (Australia) with access limited to three of the authors.
The Charlson Comorbidity Index [15] (CCI; excluding cancer) was extracted for each patient using admissions between one year prior and 30 days post cancer diagnosis. Comorbidity weights were applied according to Quan et al. [16] and grouped as zero vs. at least one. American Society of Anesthesiologists (ASA) Score, a global score that assesses the physical status of patients before surgery, was extracted from the first operative resection admission.
Phases of the OCP pathway were defined for this study as prevention (12 to 3 months prior to first treatment), diagnostic and initial surgical treatment (3 months prior up to and including the admission for surgery), chemotherapy after surgery (up to 4 months post-surgery), follow-up (6–18 months post initial surgery) and end-of-life (6 months prior to death).
Indices, chosen based on their inclusion in the OCP and data availability, were used to assess pathway compliance for each of these phases (Table 2). For the prevention phase, indices relate to the health prevention behaviours; opportunistic cancer screening (data from the National Bowel Cancer Screening Project were not available for this study) and cardiovascular disease prevention. In the diagnostic and initial treatment phase, indices were chosen to reflect alignment with key elements of the pathway rather than any specific impact from the index itself. For example, colonoscopy at diagnosis was an indicator selected but the result of the colonoscopy was not relevant to the assessment of alignment. Other indices are surrogates for attributes of quality; such as the number of lymph nodes removed (available from the VCR) reflecting clinical expertise and the annual operation load reflecting an experienced hospital.
Table 2.
List of Optimal Care Pathway (OCP) measures and data availability by OCP phase used to identify deviations from the OCP pathway.
| OCP description | Indices from datasets | Phase analysed |
|---|---|---|
Step 1. Prevention and early detection
|
GP visitsFOBTColonoscopyPSA testStatins useNo data available on family history or lifestyle factors | Prevention and early detection phase |
Step 2. Presentation, initial investigations and referrals:
|
No data available | Diagnosis and treatment phase |
Step 3. Diagnosis, staging and treatment planning:
|
CT scanColonoscopyNo data on MDM, clinical trials or communication | |
Step 4. Treatment:
|
12 plus nodes examinedHospital surgical volumeTime to adjuvant chemotherapy | |
Step 5. Care after initial treatment and recovery:
|
12 months follow-up ColonoscopyAbdominal CTCarcinoembryonic antigen (CEA) testNo data on survivorship | Care after initial treatment, recovery and survivorship and end of life care |
| Step 6. Managing recurrent, residual and metastatic disease | No data available | |
Step 7. End of life:
|
Palliative care referralChemotherapy in last 30 days of life | End-of-life phase |
MDM = Multidisciplinary meeting; CT = Computed Tomography; GP = General practitioner; FOBT = Faecal Occult Blood Test; PSA = Prostate Specific Antigen.
The main outcome of the study was risk-adjusted overall survival. Secondary outcomes include stage at diagnosis, the likelihood of emergency surgical admission and appropriate follow-up care (measured by timeliness to adjuvant therapy, and the performance of surveillance tests (colonoscopy, CT and CEA) in the post treatment period) and end-of-life care (measured by occurrence of a palliative care use of chemotherapy at end-of-life, both important elements in defining good quality end-of-life care [17], [18]).
2.1. Statistical Methods
Patients were assigned as aligned with the prevention phase (12 to 3 months prior to first treatment) when evidence of at least one of the prevention phase indices was present. The association compliance between the prevention phase pathway and stage at diagnosis was assessed using ordered logistic regression. For patients who had a surgical resection, logistic regression was used to assess the association between prevention pathway compliance and the likelihood of having an emergency surgical admission.
Patients were aligned with the diagnostic and treatment pathway (three months up to and including the date of surgery) when evidence of all of the diagnostic and treatment phase indices was present. Crude survival at one and three years following the date of surgery according to pathway compliance was estimated using the Kaplan–Meier method. Multivariable Cox proportional hazard models were used to assess if survival differences according to pathway compliance were independent from differences in age at diagnosis (cubic spline), CCI (0/1 +), ASA (1,2,3,4/5, unknown), hospital type (public/private), socioeconomic position (quintiles, categorical) and year of diagnosis (continuous). In order to satisfy the proportional hazards assumption, all survival models were stratified on AJCC summary stage at diagnosis (I, II, III, IV) and surgical admission type (emergency/non-emergency). Various sensitivity analyses were performed. The associations between pathway compliance and secondary outcomes were tested using Chi-squared tests for binary outcomes and Wilcoxon-rank-sum tests for continuous outcomes. P-values less than 0.05 were considered statistically significant. All analyses were performed using the statistical package R [19].
2.2. Role of the Funding Source
This project was funded entirely by Department of Health and Human Services (Victoria). The funders of the study had no role in study design, data collection, data analysis, data interpretation or writing of the manuscript. The corresponding author had full access to all the data in the study (in anonymized form) and the final responsibility for the decision to submit for publication.
3. Results
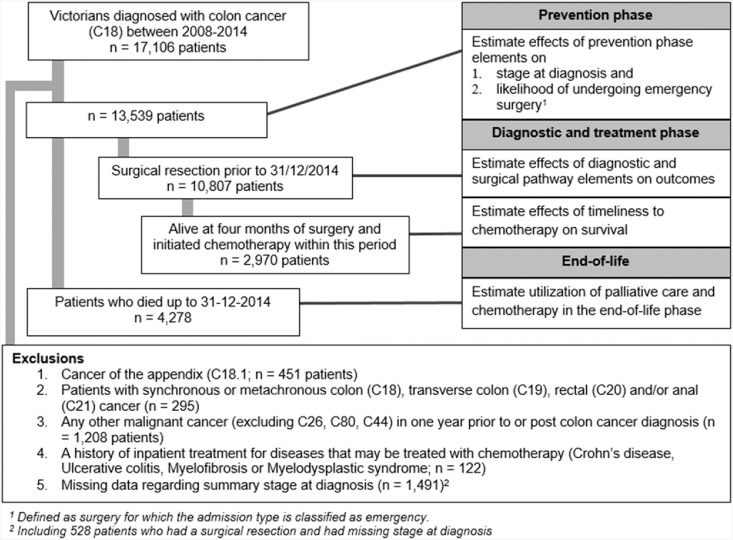
A total of 13,539 patients diagnosed with colon cancer between 2008 and 2014 were identified for analysis (Fig. 1 for exclusions; Table 1). Analysis of the whole group revealed the median age at diagnosis for these patients was 72 years, 51% were male and three-year survival was 68.1% [95% CI 67.2–69.0]. Stage-specific three-year survival from diagnosis was 92.8% [91.6–93.9], 85.3% [84.1–86.5], 73.0% [71.3–47.8] and 22.7% [21.1–24.3] for stage I, II, III and IV respectively.
Fig. 1.
Patient flowchart.
Table 1.
Patient demographics for each phase of the pathway.
| Characteristic | Prevention and early detection cohort (n = 13,539) |
Diagnostic & initial treatment cohort (n = 10,807) | End-of-life cohort (n = 4278) | |
|---|---|---|---|---|
| Sex | Female | 6691 (49%) | 5410 (50%) | 2056 (48%) |
| Male | 6848 (51%) | 5397 (50%) | 2222 (52%) | |
| Age at diagnosis | Under 50 | 911 (7%) | 719 (7%) | 207 (5%) |
| 50–59 | 1702 (13%) | 1376 (13%) | 382 (9%) | |
| 60–69 | 3176 (23%) | 2614 (24%) | 734 (17%) | |
| 70–74 | 1959 (14%) | 1584 (15%) | 546 (13%) | |
| 75–79 | 2159 (16%) | 1751 (16%) | 721 (17%) | |
| 80–84 | 2047 (15%) | 1630 (15%) | 847 (20%) | |
| 85 + | 1585 (12%) | 1133 (10%) | 841 (20%) | |
| Charlson Comorbidity Index | Zero | 11,218 (85%) | 9332 (86%) | 3128 (76%) |
| At least one | 1912 (15%) | 1475 (14%) | 985 (24%) | |
| Missinga | 409 | 0 | 165 | |
| Year of cancer diagnosis | 2008 | 1925 (14%) | 1515 (14%) | 894 (21%) |
| 2009 | 1887 (14%) | 1483 (14%) | 878 (21%) | |
| 2010 | 1967 (15%) | 1601 (15%) | 731 (17%) | |
| 2011 | 1928 (14%) | 1531 (14%) | 677 (16%) | |
| 2012 | 1932 (14%) | 1598 (15%) | 535 (13%) | |
| 2013 | 1947 (14%) | 1553 (14%) | 403 (9%) | |
| 2014 | 1953 (14%) | 1526 (14%) | 160 (4%) | |
| Socio-economic position (quintiles) | (Most disadvantaged) 1 | 2729 (24%) | 2141 (23%) | 1105 (27%) |
| 2 | 2544 (22%) | 2011 (22%) | 933 (23%) | |
| 3 | 2267 (20%) | 1868 (20%) | 797 (20%) | |
| 4 | 2035 (18%) | 1647 (18%) | 649 (16%) | |
| (Least disadvantaged) 5 | 1924 (17%) | 1549 (17%) | 603 (15%) | |
| Missingb | 2040 | 1591 | 191 | |
| Remoteness | Major cities | 7842 (68%) | 6302 (68%) | 2749 (67%) |
| Inner regional | 2875 (25%) | 2279 (25%) | 1056 (26%) | |
| Outer regional/remote | 835 (7%) | 678 (7%) | 299 (7%) | |
| Missingb | 1987 | 1548 | 174 | |
| Registry derived AJCC summary stage | I | 2646 (20%) | 2139 (20%) | 238 (6%) |
| II | 4208 (31%) | 3884 (36%) | 714 (17%) | |
| III | 3283 (24%) | 3000 (28%) | 892 (21%) | |
| IV | 3402 (25%) | 1784 (17%) | 2434 (57%) | |
Patients without hospitalisations.
Including all patients diagnosed in 2014.
3.1. Prevention and Early Detection Phase
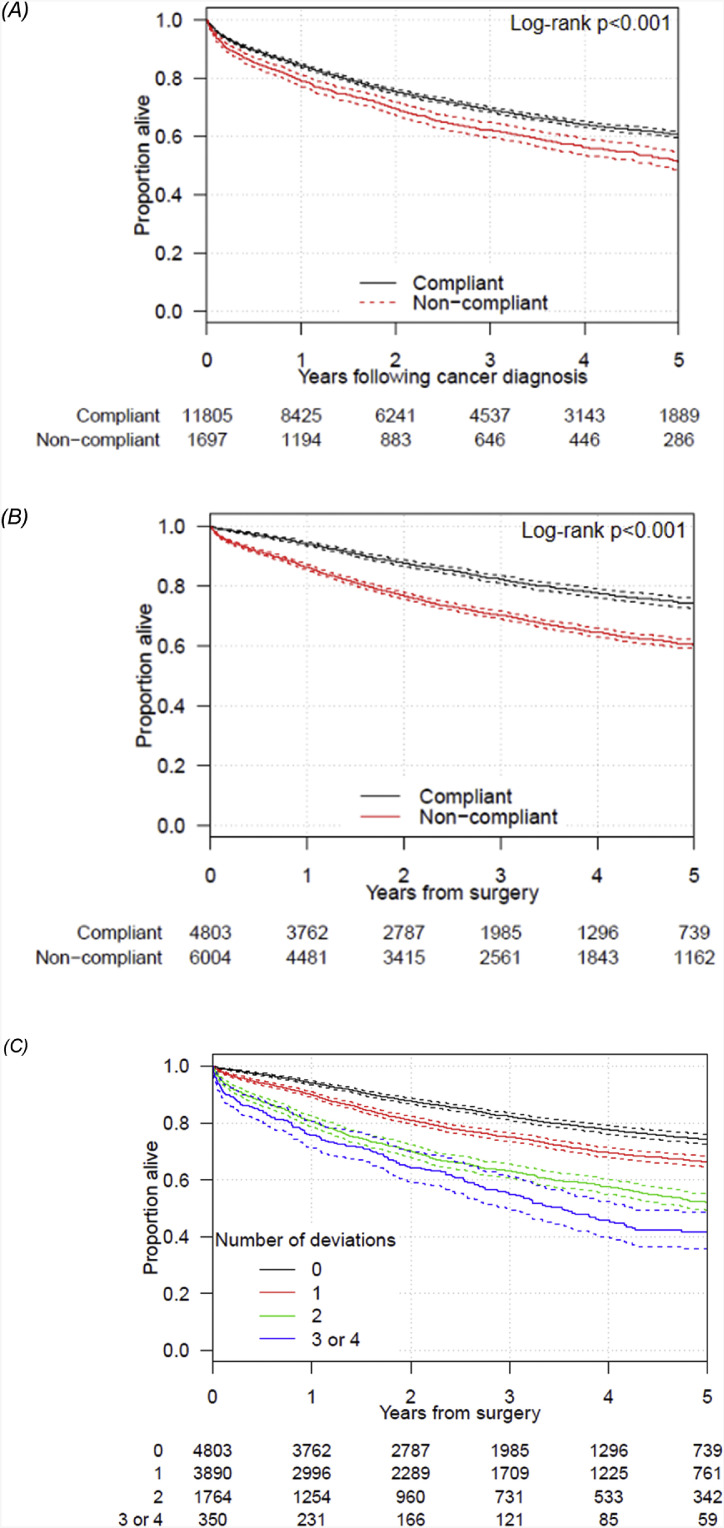
Index data were available for ad-hoc screening (FOBT and Colonoscopy) and a further three health-seeking behaviours (GP visit, Prostate Specific Antigen (PSA) test and prescription of Statins; Table 2). A record of one or more of the indices during the prevention and early detection phase was evident for 11,833/13,539 (87.4%) of patients. Those aligned with the prevention pathway were diagnosed with a lower stage of cancer (OR = 0.33 [95% CI = 0.24–0.42]; Table 3A). Restricted to the 10,807 patients who went to surgery, those aligned to the prevention pathway (88.6%) were less likely to have emergency surgery (OR = 0.64 [95% CI = 0.55–0.74]). Survival (with and without risk-adjustment) was higher for those aligned to the prevention phase compared to the non-aligned patient group (Fig. 2A; Table 3A). Each of the indices was independently associated with a reduction in the likelihood of emergency surgery (S1). Prevention and early detection pathway alignment was highest for 70–80 year old patients, females and those with comorbidities (Table 4).
Table 3.
Summary of outcomes according to evidence for (A) compliance with the prevention pathway and (B) compliance with the diagnostic and treatment pathway.
| (A) | |||
|---|---|---|---|
| Outcome variable | Alignment with prevention and early detection phasea |
P-value for difference | |
| Yes(n = 11,833; 87.4%) | No(n = 1706; 12.6%) | ||
| AJCC summary stage | |||
| I | 20.2% | 14.7% | < 0.001 |
| II | 31.2% | 30.1% | |
| III | 24.3% | 23.9% | |
| IV | 24.2% | 31.4% | |
| Emergency surgeryb | 17.7% | 25.6% | < 0.001 |
| Three-year crude survival [95% confidence interval]c | 69.2[68.3–70.2] | 62.2[59.7–64.8] | < 0.001 |
| (B) | |||
| Outcome variable |
Alignment with diagnostic and treatment phased |
P-value for difference |
|
| Yes(n = 4803; 44.4%) | No(n = 6004; 55.6%) | ||
| One-year crude survival [95% confidence interval] | 94.1%[93.4–94.8] | 86.8%[85.5–87.2] | < 0.001 |
| Three-year crude survival [95% confidence interval] | 82.4%[81.1–83.7] | 70.3%[69.0–71.6] | < 0.001 |
| Risk-adjusted hazard ratio restricted to one-year [95% confidence interval] | 1(Reference) | 1.35[1.16–1.57] | < 0.001 |
| Adjusted hazard ratio restricted to three-year survival [95% confidence interval] | 1(Reference) | 1.27[1.15–1.40] | < 0.001 |
| Length of stay initial surgery (days; median [IQR]) | 8[6–12] | 10[7–18] | < 0.001 |
| Prolonged (24 + hours) ICU stay initial surgery (%) | 22.4% | 26.4% | < 0.001 |
| Adjuvant chemotherapy utilisation (within four months of surgery) (%) | |||
| Full cohort | 1656/4803 (34.5%) | 1493/6004 (24.9%) | < 0.001 |
| Stage II | 314/1917 (16.4%) | 314/1967 (16.0%) | 0.76 |
| Stage III | 1196/1450 (82.5%) | 1012/1550 (65.3%) | < 0.001 |
| Stage III, under 80, no comorbidities | 1021/1114 (91.7%) | 857/986 (86.9%) | < 0.001 |
| Timely initiation of adjuvant chemotherapy (within 56 days of surgery) (%)e | 1177/1547 (75.8%) | 989/1423 (69.3%) | < 0.001 |
| Follow-up caref | |||
| – Colonoscopy utilisation (%) | 1984/2802 (70.8%) | 1991/3313 (60.0%) | < 0.001 |
| – Abdominal CT scan utilisation (%) | 1918/2802 (68.4%) | 1688/3313 (51.0%) | < 0.001 |
| – CEA testg | 1960/2802 (70.0%) | 1805/3313 (54.4%) | < 0.001 |
| Chemotherapy in last 30 days of life (%) | |||
| – Full cohort | 176/818 (21.5%) | 251/1855 (13.5%) | < 0.001 |
| – Subset of cohort aged < 80 at diagnosis and no recorded comorbidities | 135/484 (27.9%) | 201/823 (24.4%) | 0.19 |
| Palliative care in last 6 months of life (%) | 495/818 (60.5%) | 973/1855 (52.5%) | < 0.001 |
Patients with evidence of at least one of the five elements in the prevention phase were classified as compliant with the prevention pathway.
Restricted to 10,882 patients who had a resection.
Excluding patients whose cancer was only reported to the Victorian Cancer Registry by the death certificate; survival time measured from date of diagnosis.
Patients with evidence of all of the five elements in the diagnostic and surgical phase were classified as compliant with the diagnostic and surgical pathway.
Restricted to patients alive at four months and having commencing adjuvant chemotherapy within four months of surgery.
Alive and non-metastatic disease at 18 months following surgery.
Carcinoembryonic antigen test.
Fig. 2.
Kaplan–Meier curves with 95% confidence interval comparing survival of compliant and non-compliant patients for the (A) prevention phase, measured from the date of diagnosis to death or censor date1 (B) the diagnostic and initial treatment phase measured from the date of surgery to death or censor date and (C) showing survival by the number of deviations in the diagnostic and initial treatment phase.
1Excluding patients diagnosed based on the death certificate only.
Table 4.
Rate of pathway alignment for each of the OCP phases as grouped for analysis. P-values were extracted from multivariable logistic regression.
| Characteristic | Alignment with OCP (%) |
|||
|---|---|---|---|---|
| Prevention and early detection phase(n = 13,539) | Diagnosis and treatment phase(n = 10,807) | End of life phasea(n = 4278) | ||
| Age | Under 50 | 80% < 0.001b | 53% < 0.001b | 54% 0.50b |
| 50-59 | 84% | 50% | 52% | |
| 60–69 | 89% | 49% | 46% | |
| 70–74 | 94% | 45% | 47% | |
| 75–79 | 94% | 44% | 50% | |
| 80–84 | 89% | 40% | 48% | |
| 85 + | 75% | 29% | 45% | |
| Sex | Male | 86% 0.001 | 44% 0.15 | 46% 0.001 |
| Female | 88% | 45% | 51% | |
| Charlson Comorbidity Index | Zero At least one | 88% 0.005 89% | 47% < 0.001 31% | 49% 0.44 48% |
| Socio-economic status (SEIFA) | (Most disadvantaged) 1 | 87% 0.33c | 39% < 0.001c | 50% 0.033c |
| 2 | 87% | 42% | 47% | |
| 3 | 87% | 42% | 48% | |
| 4 | 88% | 46% | 48% | |
| (Least disadvantaged) 5 | 85% | 51% | 44% | |
| Remoteness | Major cities | 87% 0.08c | 47% < 0.001c | 50% 0.004c |
| Inner regional | 86% | 39% | 43% | |
| Outer regional | 87% | 26% | 46% | |
Abbreviations: SEIFA - Socio-Economic Indexes for Areas as described by the Index of Relative Socio-Economic Disadvantage (IRSD) based on the Statistical Area 1 of the address at the time of cancer diagnosis.
OCP aligned if no chemotherapy in last 30 days of life and palliative care in the last six months of life.
Test for quadratic trend.
Test for linear trend.
3.2. Diagnostic and Initial Treatment Phase
Data elements available for this OCP phase and relevant to the concept of expert care, including colonoscopy and CT scan within the three months period prior, up to and including the date of surgery. Hospital campus annual volume of all colon resections, (below median vs. median and above) and examination of 12 or more resected lymph nodes (Table 2) were also available. Patients with missing lymph node data (n = 1854) were assumed to have less than 12 lymph nodes examined (but see sensitivity analyses; Table S2).
Of the 13,539 colon cancer patients, 10,807 had a surgical resection prior to 31/12/2014 (Table 1). All stages of cancer (I-IV) are represented in this group with surgical resection. Overall, 9295 (86.0%) had a colonoscopy in the three months preceding their cancer surgery, 7456 (69.0%) patients had an abdominal CT scan, 10,031 (92.8%) had surgery in a campus with mean annual surgical volume of colorectal operations of 32 or more and 7955 (73.6%) of patients had 12 or more lymph nodes examined. The only index available for chemotherapy expertise, was time to commencement of adjuvant chemotherapy.
Overall, 44.4% of patients had care which was aligned with the diagnosis and treatment phase of the OCP (Table 3B). Diagnosis and treatment pathway alignment was non-linearly related to age, was higher for patients without comorbidities, higher socio-economic status and less remote Victorian residents (Table 4).
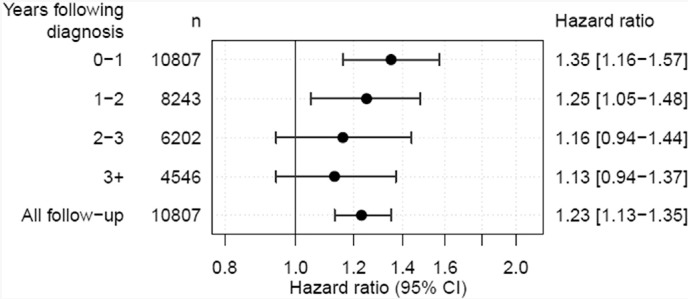
Survival was higher in patients whose care was aligned to the pathway than those who had one or more deviations from the pathway, both unadjusted (one-year survival crude survival = 94.1% vs. 86.3%; Fig. 2B; Table 3B) and after risk-adjusted for potential confounders (HRnon-aligned vs aligned = 1.23 [95% CI 1.13–1.35]). The survival benefit was greatest in the first year following surgery (HR = 1.35 [1.16–1.57]) and reduced in subsequent years (Fig. 3). There was an incremental survival effect with increasing number of deviations (Fig. 2C; P < 0.001; risk-adjusted HR = 1.17 95% CI 1.11–1.22 per deviation).
Fig. 3.
Risk-adjusted hazard ratios (with 95% confidence intervals) comparing patients whose care was not aligned with the pathway with pathway followers for various survival intervals (conditional survival). N is the number of patients alive at the start of the survival interval. Patients' follow up time was censored at the end of the interval.
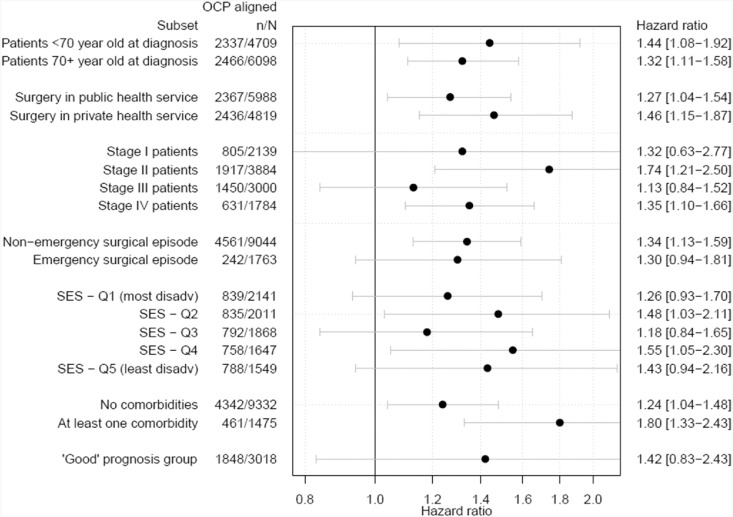
The survival benefit was persistent within each stage, age group, surgery admission type and hospital type of the surgery and patient comorbidities (Fig. 4) and adjusting for the socio-economic position had little impact (Table S2). There were a total of 15 unique diagnostic and initial treatment pathways in the group who were not fully aligned with the pathway. Outcomes for these are shown in Table S3.
Fig. 4.
Risk-adjusted hazard ratios (with 95% confidence interval) for surgical colon cancer patients with one or more deviations in the diagnostic and surgical pathway elements compared to patients whose care was aligned with the pathway, for various patient subsets. Subsets are not mutually exclusive.
The ‘Good’ prognosis group was defined as patients diagnosed under 80 years of age, stage I to III, ASA 1 to 3, non-emergency surgery a Charlson Comorbidity Index of zero.
3.3. Chemotherapy After Surgery
Of the 9784 patients who were alive four months following surgery, 2970 (30.4%) started chemotherapy (Stage I: 3%; Stage II: 17%; Stage III: 76%; Stage IV: 15%), of whom 2166 (72.9%) commenced chemotherapy within 56 days of surgery in accordance with the OCP (Table 3). Restricted to patients diagnosed with stage II and III cancers who commenced adjuvant chemotherapy, risk of death was higher for patients who started chemotherapy > 56 days after (but within four months of) surgery (one year adjusted hazard ratio (HR) = 1.51 [0.98–2.33]; three year adjusted HR = 1.39 [1.08–1.77]). Patients with deviations from any of the five elements in the surgical treatment path were more likely to commence chemotherapy outside the 56-day window (30.5%) compared to those without deviations in the surgical pathway elements (23.9%; p-diff < 0.001). Restricted to stage II and III colon patients aligned with the diagnostic and surgical pathway, no statistically significant effect of timeliness to adjuvant chemotherapy on survival could be detected although the best estimate is in favour of commencing chemotherapy within 56 days of surgery (n = 1418; one year adjusted HR = 1.10 [0.52–2.35]; three year adjusted HR = 1.42 [0.95–2.11]).
3.4. Follow-up and End-of-Life Care
Of the 10,807 patients who underwent surgical resection, 6115 were alive and free of metastases 1.5 years following surgery. Patients aligned with the diagnosis and initial treatment pathway were more likely to have had a follow-up colonoscopy (70.8% vs 60.1%; p-diff < 0.001), abdominal CT (68.4% vs 51.0%; p-diff < 0.001) and CEA test (70.0% vs 54.4%; p-diff < 0.001) between six and 18 months following surgery. Of the 13,539 colon cancer patients, 4278 died up to 31/12/2014 of which 57.3% had a palliative care contact in the last six months of life. In the subset of resected colon cancer patients, those who were aligned with the OCP were more likely to have had a palliative care contact in the last six months of life compared to those not aligned (60.5% vs. 52.5%; p < 0.001).
In total, 699 (16.0%) of the 4278 patients that died received chemotherapy in the last 30 days of life. The subset of resected colon cancer patients who followed the optimal surgical path were more likely to have had chemotherapy in the last 30 days of life compared to patients with any pathway deviation (21.5% vs. 13.5%; p < 0.001). Restricted to patients diagnosed before age 80 and those that have no recorded comorbidities, the difference in the use of chemotherapy in the last 30 days of life is not statistically significant (27.9% vs. 24.4%, p-diff = 0.19). End of life pathway alignment was highest for women, lower socio-economic position and less remote Victorian residents (Table 4).
4. Discussion
This population-based observational study of more than 13,500 consecutive colon cancer patients includes all patients diagnosed in Victoria with colon cancer over a seven-year period. It is to be noted that these patients were not managed according to any particular protocol. The median age of diagnosis was 72 years and the three-year survival of the whole group was 68.1% with appropriate disease stage specific survival. These results are in the upper range of survival compared with similar countries [4]. It is thus of extreme interest that given this background of excellent results, that there can be demonstrated a robust beneficial effect on survival in a large number (44%) of the surgical resection cohort was carried out when the individual patient's care is aligned to a series of pathway system indices derived from the OCP. Adjustment for potential confounding factors did not eliminate this effect and thus suggests that there is an impact of the health system on the outcomes of patients over and above the well-recognised patient characteristics of stage of disease, age sex and comorbidity.
Patients' care was classified as aligned with the OCP when evidence was found for four elements in the diagnostic and initial treatment phase. The four elements relate to diagnostic tests (CT, colonoscopy) and surrogates for clinical expertise and experience (surgical volume, lymph nodes examined). A possible mechanism by which a survival benefit could become manifest is, for example, through a direct effect of these tests on accuracy of disease staging, and therefore prescription of appropriate treatment. This is particularly relevant for stage III cancers (with positive nodes) where adjuvant chemotherapy is generally prescribed. Failure to detect positive nodes could lead to patients not receiving chemotherapy. Interestingly, although the effect of pathway alignment on survival is evident for all stages, the best estimate of the effect is largest in stage II disease, which may be due to the inclusion of incorrectly staged III patients. In this paper we have avoided this type of analysis preferring to aggregate all the indices examined into a health system effect as overall responsibility for the quality of service delivered, the competency of staff and the system of care rests at this level. Patients whose care is aligned with the pathway experience positive effects later in the treatment pathway. Increased alignment in later stages of the pathway is unlikely to be directly affected by the four measures of care alignment, and hence may reflect a health system effect. The IOM report describe seven features required for an effective health care system for the delivery of cancer care [7]. The health system has responsibility for professional factors (i.e. quality of care delivered) and structural factors, including time frames, facilities, multidisciplinary care and quality control. There is no current overall measure of such a complex system. OCP alignment may be such a surrogate measure of an effective health care system. We plan to apply this methodology across other cancer cohorts to confirm its utility.
Prior to surgical resection, the findings from the prevention phase indicate the importance of both screening actions and activities related to health seeking behaviour including visits to the general practitioner. We accept that the indices are interrelated and further investigation is required to clarify, for example, the impact of numbers of GP visits as compared with the prescription of statins (used as an indicator of health seeking behaviour). This study observes that these interventions in the prevention phase are associated with lower stage of the disease at diagnosis.
Following diagnosis, patients who did not have surgery were excluded from treatment group analysis in order to evaluate a consistent group of patients. However, it is noted that all stages of colon cancer were represented in this surgery group. The improved survival from following the pathway was present irrespective of the stage at diagnosis.
Patients of lower socioeconomic status, those domiciled in more remote areas of the State and those with comorbidities were less likely to be aligned with the OCP. However, these groups were still extensively represented in the group who complied with the OCP. Differences in OCP alignment between these groups might partly explain the disparities in survival within these groups. Further analyses are needed to estimate the magnitude of the direct effect of OCP alignment on the disparities between socio-economic or remoteness groups.
Deriving clinical information from administrative datasets is complex [20], [21]. However, a recent study demonstrated that administrative datasets similar to those used in this study did provide accurate clinical information [22]. Even though the administrative datasets are rich, data on many OCP elements was not available. For example, information on multidisciplinary treatment planning meetings as well as standards for communication, survivorship and clinical trials was contained within the OCP but indices with data collection capability were not available in the linked dataset. Furthermore, except for duplication of tests, not outcome data are available regarding patient experience of their cancer care (i.e. Patient Reported Outcome Measures).
Chemotherapy in last 30 days of life was higher in the cohort who followed the optimal surgical path (21.5% vs. 13.5%). Although this seem paradoxical at first, this difference is mostly driven by differences in age and comorbidity levels between the aligned and non-aligned cohort. For patients diagnosed at age under 80 and those that have no recorded comorbidities, the difference in the use of chemotherapy in the last 30 days of life is not statistically significant (27.9% vs. 24.4%, p-diff = 0.19).
5. Conclusions
The impact of a health care system on cancer care and thus patient outcomes may be an important contributor to the unexplained variation which occurs between countries and between different groups within any modern society. The reasons are still not clear and likely to be subtle and different in different contexts. We show that alignment with a set of measures based on the principles of the OCP is independently associated with improved colon cancer survival. The fact that alignment with the diagnostic and initial treatment phase was associated with better OCP alignment in later stages of the OCP (i.e. follow-up care and end-of-life care), suggests a possible ‘health service effect’.
Declaration of interests
All authors declare no competing interests.
Acknowledgments
Acknowledgements
Staff of the Victorian Cancer Registry, the Centre for Victorian Data Linkage from the Department of Health and Human Services, Victoria, and Data Integration Services Centre of the Australian Institute of Health and Welfare and Richard Hurley, Cathy Fussell, Loc Thai, Paul Lukong and Saravanan Satkumaran from the Health Analytics Branch, Health Economics and Research Division, Department of Health, Canberra.
Author Contribution
Study design: LtM, PMcN, KW & RJST.
Data analysis: LtM, PMcN, RJST.
Data interpretation & writing: LtM, PMcN, KW, PA, PB, RS, RJST.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.eclinm.2019.08.009.
Appendix A. Supplementary data
S1 Results of the multivariable logistic regression analysis assessing the effect of prevention phase variables on (A) stage at diagnosis and (B) the likelihood of having surgery in an emergency admission. PSA test is only assessed for men.
Table S2 Sensitivity analyses on overall survival.
Table S3. Pathways and associated one and three survival outcomes for colon cancer patients (2008–2014) undergoing surgery (n = 10,807). Outcomes for pathways with less than 100 patients were grouped.
Table S4: Patient demographics according to evidence of any health-seeking behaviour in the prevention phase (n = 13,359 patients).
Table S5: Evidence of pathway alignment in the diagnostic and initial treatment phase according to patient demographics (n = 10,807 patients).
References
- 1.Warwick J., Will O., Allgood P., Miller R., Duffy S., Greenberg D. Variation in colorectal cancer treatment and survival: a cohort study covering the East Anglia region. Colorectal Dis. 2013;15:1243–1252. doi: 10.1111/codi.12308. [DOI] [PubMed] [Google Scholar]
- 2.Keating N.L., Landrum M.B., Lamont E.B., Bozeman S.R., McNeil B.J. Area-level variations in cancer care and outcomes. Med Care. 2012;50:366–373. doi: 10.1097/MLR.0b013e31824d74c0. [DOI] [PubMed] [Google Scholar]
- 3.Downing A., Morris E.J., Corrigan N. High hospital research participation and improved colorectal cancer survival outcomes: a population-based study. Gut. 2017;66:89–96. doi: 10.1136/gutjnl-2015-311308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coleman M.P., Forman D., Bryant H. Cancer survival in Australia, Canada, Denmark, Norway, Sweden, and the UK, 1995-2007 (the International Cancer Benchmarking Partnership): an analysis of population-based cancer registry data. Lancet. 2011;377:127–138. doi: 10.1016/S0140-6736(10)62231-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maringe C., Walters S., Rachet B. Stage at diagnosis and colorectal cancer survival in six high-income countries: a population-based study of patients diagnosed during 2000-2007. Acta Oncol. 2013;52:919–932. doi: 10.3109/0284186X.2013.764008. [DOI] [PubMed] [Google Scholar]
- 6.Fang P., He W., Gomez D. Racial disparities in guideline-concordant cancer care and mortality in the United States. Adv Radiat Oncol. 2018;3:221–229. doi: 10.1016/j.adro.2018.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Committee on Improving the Quality of Cancer Care: Addressing the Challenges of an Aging Population, Board on Health Care Services, Institute of Medicine . National Academies Press; 2014. Delivering High-Quality Cancer Care: Charting a New Course for a System in Crisis. [PubMed] [Google Scholar]
- 8.Rachet B., Maringe C., Nur U., Quaresma M., Shah A., Woods L.M. Population-based cancer survival trends in England and Wales up to 2007: an assessment of the NHS cancer plan for England. Lancet Oncol. 2009;10(4):351–369. doi: 10.1016/S1470-2045(09)70028-2. [DOI] [PubMed] [Google Scholar]
- 9.Olsson L. Springer; 2017. Timely Diagnosis of Colorectal Cancer. [Google Scholar]
- 10.Rotter T., Kugler J., Koch R. A systematic review and meta-analysis of the effects of clinical pathways on length of stay, hospital costs and patient outcomes. BMC Health Serv Res. 2008;8:265. doi: 10.1186/1472-6963-8-265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Probst H.B., Hussain Z.B., Andersen O. Cancer patient pathways in Denmark as a joint effort between bureaucrats, health professionals and politicians—a national Danish project. Health Policy. 2012;105:65–70. doi: 10.1016/j.healthpol.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 12.Kreys E.D., Koeller J.M. Documenting the benefits and cost savings of a large multistate cancer pathway program from a payer's perspective. J Oncol Pract. 2013;9:e241–e247. doi: 10.1200/JOP.2012.000871. [DOI] [PubMed] [Google Scholar]
- 13.Zon R.T., Frame J.N., Neuss M.N. American society of clinical oncology policy statement on clinical pathways in oncology. J Oncol Pract. 2016;12:261–266. doi: 10.1200/JOP.2015.009134. [DOI] [PubMed] [Google Scholar]
- 14.Optimal cancer care pathways. Cancer Council. https://www.cancer.org.au/health-professionals/optimal-cancer-care-pathways.html (accessed Nov 26, 2018).
- 15.Charlson M.E., Pompei P., Ales K.L., MacKenzie C.R. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 16.Quan H., Li B., Couris C.M. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol. 2011;173:676–682. doi: 10.1093/aje/kwq433. [DOI] [PubMed] [Google Scholar]
- 17.Earle C.C., Park E.R., Lai B., Weeks J.C., Ayanian J.Z., Block S. Identifying potential indicators of the quality of end-of-life cancer care from administrative data. J Clin Oncol. 2003;21:1133–1138. doi: 10.1200/JCO.2003.03.059. [DOI] [PubMed] [Google Scholar]
- 18.Grunfeld E., Urquhart R., Mykhalovskiy E. Toward population-based indicators of quality end-of-life care: testing stakeholder agreement. Cancer. 2008;112:2301–2308. doi: 10.1002/cncr.23428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Core R. Computing; 2017. Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical. [Google Scholar]
- 20.Lee J.T., Huang N., Majeed A. The need for better linkage between administrative data and clinical datasets. BMJ. 2015 doi: 10.1136/bmj.h5816. [DOI] [PubMed] [Google Scholar]
- 21.Goldsbury D., Weber M., Yap S., Banks E., O'Connell D.L., Canfell K. Identifying incident colorectal and lung cancer cases in health service utilisation databases in Australia: a validation study. BMC Med Inform Decis Mak. 2017;17:23. doi: 10.1186/s12911-017-0417-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.MacCallum C., Da Silva N., Gibbs P., Thomson B.N.J., Skandarajah A., Hayes I. Accuracy of administrative coding data in colorectal cancer resections and short-term outcomes. ANZ J Surg. 2018;88:876–881. doi: 10.1111/ans.14714. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
S1 Results of the multivariable logistic regression analysis assessing the effect of prevention phase variables on (A) stage at diagnosis and (B) the likelihood of having surgery in an emergency admission. PSA test is only assessed for men.
Table S2 Sensitivity analyses on overall survival.
Table S3. Pathways and associated one and three survival outcomes for colon cancer patients (2008–2014) undergoing surgery (n = 10,807). Outcomes for pathways with less than 100 patients were grouped.
Table S4: Patient demographics according to evidence of any health-seeking behaviour in the prevention phase (n = 13,359 patients).
Table S5: Evidence of pathway alignment in the diagnostic and initial treatment phase according to patient demographics (n = 10,807 patients).