Abstract
Background
Robotic-assisted percutaneous coronary intervention (R-PCI) has been successfully employed in the United States since 2011. Performing R-PCI from a remote location has never been reported but if feasible would extend availability of treatment to many patients with coronary artery disease (CAD) who would otherwise go without.
Objective
To assess the feasibility of remote tele-R-PCI with the operator 20 miles away from the patients.
Methods
Five patients with single, type A coronary artery lesions treatable by PCI consented to participate. The primary endpoint was procedural success with no major adverse cardiac events (MACE) before discharge. Procedural success was defined as achieving < 10% diametric stenosis of the occluded target vessel utilizing tele-R-PCI balloon angioplasty and stent deployment (CorPath GRX®, Corindus Vascular Robotics, USA) without converting to in-lab manual PCI by an on-site standby team. Procedural, angiographic, and safety data were collected as were questionnaire scores from the remote operator evaluating the robot-network composite, image clarity, and overall confidence in the procedure.
Results
The primary endpoint was achieved in 100% of patients. No procedural complications or adverse events occurred, and all patients were discharged the following day without MACE. The operator scores were favorable with the operators rating the procedure as equivalent to an in-lab procedure.
Conclusions
Performing long distance tele-R-PCI in patients with CAD is feasible with predictably successful outcomes if reliable network connectivity and local cardiac catheterization facilities are available.
Keywords: Percutaneous coronary intervention, Robotic, Remote, Coronary artery disease, Long distance PCI
Research in context
Prior to this study being planned and carried out, a thorough search and review of all the papers published from 2000 to the present was conducted on robotic-assisted PCI (R-PCI) as well as robotic–assisted surgery in general. Moreover, the authors keep abreast of all new publications in the field. Robotic-assisted PCI has only been clinically utilized since 2011 and the only FDA- and CE Mark-approved robotic platform for PCI, is the CorPath GRX® manufactured by Corindus Vascular Robotics, Waltham, MA. Thus, the number of publications in the field is comparatively small but is continually increasing as interest in the field grows. The safety, effectiveness, and advantages of R-PCI with balloon angioplasty and stent placement for treatment of simple and complex coronary artery lesions, compared to manual PCI, have been established in several trials on hundreds of patients. Remote R- PCI “telestenting” has recently been successfully performed on anesthetized pigs. The present study was the first-in-human experience of performing tele-R-PCI from a truly remote location, with the operator 20 miles away from the patients. Although only five patients with simple type A coronary lesions were treated, the study was a significant achievement in the development of R-PCI because the results demonstrated that long distance tele-R-PCI is feasible if good internet connectivity and cardiac catheterization facilities are locally available. If utilized effectively, remote R-PCI could potentially be employed to provide standard of care treatment to many patients with CAD who live in remote areas or underdeveloped countries who would otherwise not receive treatment.
Alt-text: Unlabelled Box
1. Introduction
Although mortality rates from coronary artery disease (CAD) have stabilized over the past several decades in developed countries, the global burden of cardiovascular disease is steadily increasing in developing and underdeveloped countries [1], [2]. Percutaneous coronary intervention (PCI) with balloon angioplasty and stent placement is the standard of care for treating atherosclerotic coronary artery lesions. However, the availability of hospitals with cardiac catheterization facilities and trained interventionalists is extremely limited in remote and underdeveloped areas. As a result, very few patients receive therapy. Thus, a real need exists to expand patient accessibility to revascularization procedures such as PCI. This need is underscored by the steadily decreasing number of trained interventional cardiologists [3].
Robot-assisted PCI (R-PCI) has been successfully utilized in the US since 2011, and its popularity continues to increase as technological improvements in robotic platforms are introduced and the advantages of R-PCI compared to manual PCI become better known [4]. The only endovascular robotic device with FDA and CE Mark approval for PCI is the CorPath GRX robotic platform (and its predecessor, the CorPath 200) manufactured by Corindus Vascular Robotics (Waltham, MA USA). When utilizing the CorPath system, the interventionalist operator performs the procedure from a lead-shielded robotic workstation usually placed several feet from the patient in the cardiac catheterization laboratory. The workstation is connected via cables to the robotic arm and cassette deck located at the patient's bedside. The primary advantage of R-PCI with the CorPath GRX system is a marked reduction in radiation exposure to the interventional cardiologist of up to 97% [4]. Since R-PCI eliminates the need for the interventionalist to wear heavy, lead-lined protective garments, it presumably ameliorates the risk of orthopedic injuries known to occur from repeated wearing of such garments [5]. In addition, recent software advances in the CorPath GRX robotic system have increased the accuracy and precision of measuring lesion size, stent length, and stent placement, as well as enhanced close-up viewing of angiographic images [4], [6], [7]. Remote robotic-assisted surgical procedures are also being increasingly employed in urology as well as in general surgical patients [8], [9].
Madder et al., recently conducted a successful in vivo R-PCI “telestenting” feasibility study in anesthetized pigs with the operator located 166 km from the animal housing facility [10]. The same authors then performed hard-wired R-PCI in humans from a room adjacent to the cardiac catheterization laboratory [11]. Their findings intrigued both operators and administrators regarding the potential for utilizing R-PCI in humans from a long distance remote location using secure mainstream internet at generically available connection parameters. Such an achievement would be a major step forward in making interventional revascularization treatment available to patients with CAD in remote or underdeveloped areas. To this end, the purpose of this study was to assess the feasibility of performing remote tele-R-PCI in patients with CAD with the interventionalist cardiologist operator located 20 miles away from the cardiac catheterization laboratory.
2. Method and Materials
2.1. Study Design and Patient Population
This was a single center, open label, one-arm study conducted at the Apex Heart Institute in Ahmedabad, India, in 5 patients with confirmed CAD, treatable by PCI. The study protocol and Informed Consent Form were approved by the Apex Heart Institute Ethics Committee and the study was conducted in compliance with Good Clinical Practice standards. All patients provided informed, written voluntary consent prior to attending a screening visit within seven days of the PCI procedure to determine eligibility. Study inclusion criteria included: presence of a de novo, previously untreated, native coronary artery stenosis (> 80% occlusion by visual estimate), lesion length ≤ 20 mm by visual estimate, and vessel diameter between 2.5 and 4.0 mm. Patients were excluded from participating in the study if any of the following criteria were met: left ventricular ejection fraction < 40%, acute coronary syndrome in the past 48 h, prior stent placement within 5 mm proximal or distal to the target vessel lesion, a lesion requiring planned treatment with atherectomy, or any device except for balloon dilatation prior to stent placement, evidence of intraluminal thrombus or moderate to severe tortuosity (> 90°) proximal to the target lesion, total vessel occlusion, severe target vessel calcification proximal to target lesion, lesion located in a native vessel distal to an anastomosis with a saphenous vein graft or a left/right internal mammary artery bypass that must be approached through the by-pass graft, or presence of > 50% diameter stenosis in the left main coronary artery.
2.2. Description of Robotic System Device
The CorPath GRX® robotic system (Corindus Vascular Robotics, Waltham, MA, USA) consists of the CorPath GRX bedside Unit and a lead-shielded, Robotic Control Workstation. The Bedside Unit has three components: (1) Extended Reach Arm, (2) Robotic Drive and (3) single-use sterile Cassette (“Cassette”). The Extended Reach Arm supports the Robotic Drive, which houses the Cassette. The Bedside Unit is connected via cables to the local Robotic Control Workstation which is located in the cardiac catheterization laboratory several feet away from the patient. For this study, a second identical remote Robotic Control Workstation was placed 20 miles away in Akshardham, Gandhinagar, India, a cultural and religious complex with high-quality modern information technological and networking infrastructure. The primary interventionalist operator performed the PCI procedures from the Remote Robotic Control Workstation. The Robotic Control Workstations contain a (1) robot control subsystem, and (2) telepresence communication subsystem.
The robot control substation contains a control computing system, monitors, networking equipment (LAN/MAN/WAN connectivity) and a robotic console with three joysticks. The monitors display (1) real-time hemodynamic variables and (2) fluoroscopic video, providing the operator enhanced visualization of the PCI procedure. One joystick is for balloon/stent manipulation, one for guidewire manipulation, and the third joystick is for guide catheter manipulation. The guidewire joystick allows for both linear and rotational movement (clockwise and counterclockwise) of the guidewire. The balloon/stent joystick allows for precise control of linear motion (advancement and retrieval) of its respective devices. The guide catheter joystick allows for precise control of linear motion (advancement and retrieval) and for rotational movement (clockwise and counterclockwise) of the guide catheter. The devices are controlled independently (which allows operations to be performed individually by using one joystick at a time) or simultaneously by activating multiple joysticks at once. For more precise positioning and discrete manipulation, the balloon/stent, guidewire and guide catheter can be advanced in1-mm increments via the touch-screen buttons on the user interface.
The telepresence subsystem in the Robotic Control Workstations contains local and remote cameras, monitors, and conferencing hubs which enable real-time human-to-human bidirectional audio and video communication between the remote interventional cardiologist and the Cath Lab team. Fig. 1 shows photographs of (A) the cardiac catheterization laboratory, (B) bedside robotic arm and cassette, and (C) the remote robotic control workstation with monitors for displaying real-time hemodynamic variables and angiographic images.
Fig. 1.
Photographs of the catheterization laboratory (A), CorPath GRX bedside Robotic Arm (B), Cassette and Remote Robotic Control Work Station (C).
2.3. Interventional Procedure
Vascular access was obtained via the radial or the femoral artery by the in-lab interventional cardiologist and a 6 French introducer sheath was introduced. The robotic Cassette was prepared by the in-lab team and a sterile guide catheter, guidewire, and balloon catheter were loaded in the system. The scrub technician helped position the imaging unit, performed contrast injections, and inflated/deflated the angioplasty balloon upon direction of the remote operator. These functions could also be performed by the remote operator. The guide catheter was advanced through the introducer sheath into the coronary ostium by the in-lab cardiologist. At this point, the remote operator, working from the Remote Robotic Workstation advanced the guidewire, advanced the balloon catheter over the guidewire, and after successful pre-dilation of the stenosis, deployed a stent. The guide wire was then withdrawn, final angiograms were recorded, and the guide catheter was withdrawn by the in-lab cardiologist. In view of the unprecedented nature of this procedure, the in-lab interventional cardiologist and a cardio-thoracic surgical team were available throughout the procedure on-standby to provide emergency support if needed. Following completion of the procedure, a 5-item questionnaire was answered by the primary operator (Appendix 1). The procedures were performed on December 1 and 2, 2018.
2.4. Study Endpoints
The primary endpoint was Procedural Success with no MACE. Procedural success was defined as remote achievement of < 10% diametric stenosis of target vessel and establishment of normal flow, with no operative or direct cognitive or interpretive input from the onsite staff or standby team. MACE is defined as major adverse cardiac events, including cardiac death, clinically relevant Q-wave or non-Q-wave myocardial infarction after coronary revascularization, or clinically driven target vessel revascularization (TVR by PCI or CABG). The secondary endpoint was Technical Device Success (defined as successful completion of the remote R-PCI without unplanned conversion to manual for guidewire or balloon/stent catheter inability to navigate vessel anatomy or poor guide catheter support).
Descriptive statistics were employed to describe demographic and procedural parameters. Categorical variables were expressed as proportions and continuous variables were expressed as mean and standard deviation or median and interquartile range as deemed appropriate. SPSS 24 (IBM Corporation, Normonk, NY) was used to perform these analyses.
3. Results
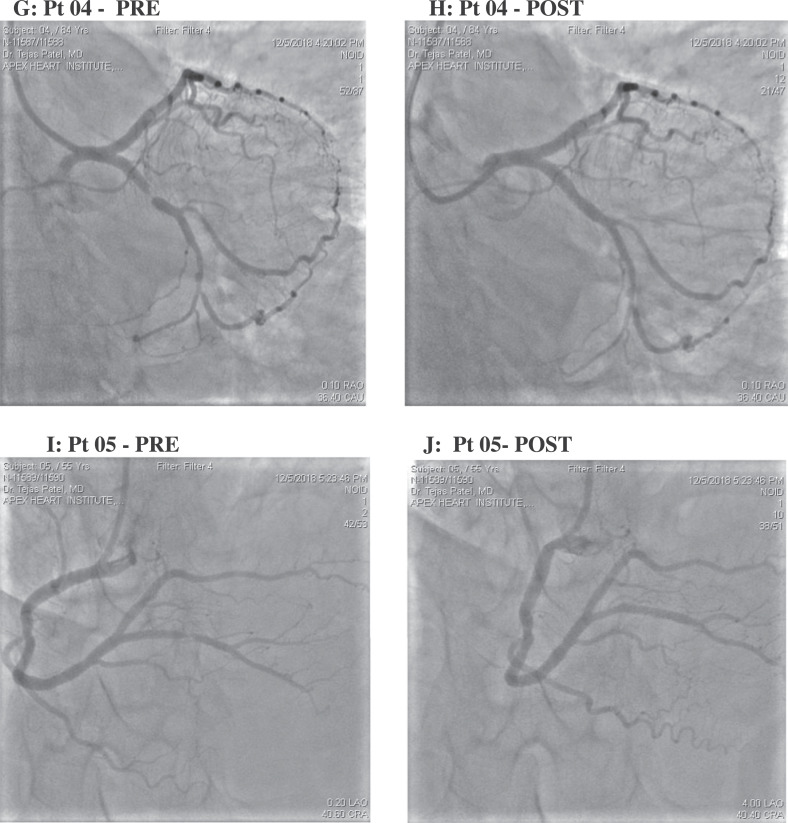
Patient demographics and procedural characteristics are shown in Table 1. The patients (2 female, 3 male) ranged in age from 52 to 84 years; 4 had undergone a previous PCI procedure. Mean (range) duration of the PCI procedure was 23.6 (19–29) minutes. The primary and secondary endpoints were achieved in 100% of patients. Procedural success was evidenced by pre- and post-R-PCI angiograms from each patient as shown in Fig. 2. Individual patient radiation exposure from fluoroscopic imaging during R-PCI ranged from 989 to 1713 mGy (Table 2). All five patients at 24 h after the procedure were free of MACE. Twelve-lead electrocardiograms performed immediately after the procedure and on the subsequent morning showed no evidence of significant ischemia. Continuous electrocardiographic monitoring of cardiac rhythm from after the procedure to discharge showed no evidence of arrhythmia in any patient. All patients remained hemodynamically stable and asymptomatic throughout the hospital course and at the time of discharge. No access site complications occurred. The CorPath GRX robotic system, controlled remotely using an internet-based connection, showed excellent performance. The operator's rating of the response time, device control, and ability to communicate with the local in-lab team were all rated as Satisfied or Extremely Satisfied. The mean (SD) time delay between the remote console and the in-lab robotic system measured by the robot software was 53 (11) ms.
Table 1.
Patient demographics and procedural characteristics.
| Patient | Gender | Age (y) |
Height (cm) |
Weight (kg) |
Access | Lesion Length (mm) |
Pre Stenosis (%) |
Post Stenosis (%) |
Procedure Timea (min) |
Indication for PCI |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 54 | 174 | 80 | R Femoral | 12 | 95 | 0 | 28 | Stable Angina |
| 2 | M | 53 | 176 | 60 | L Radial | 15 | 90 | 0 | 21 | Stable Angina |
| 3 | F | 52 | 165 | 85 | R Radial | 18 | 90 | 0 | 21 | Stable Angina |
| 4 | F | 84 | 152 | 50 | R Radial | 10 | 99 | 0 | 29 | Stress Test ECG |
| 5 | M | 55 | 169 | 62 | L Radial | 09 | 90 | 0 | 19 | Stable Angina |
y = years, cm = centimeters, Kg = kilograms, R = right, L = left.
Time from insertion of sheathed catheter to withdrawal of guide catheter.
Fig. 2.
Pre and post R-PCI angiograms from five patients in the cardiac catheterization laboratory located 20 miles away from the interventionalist-operator.
Table 2.
Procedural fluoroscopy time and total radiation exposure by patient.
| Fluoroscopy Time |
Radiation Dose |
||
|---|---|---|---|
| (min) | DAP (cGy/cm2) | Ka (mGy) | |
| Patient 1 | 4·39 | 5614 | 989 |
| Patient 2 | 9·58 | 5763 | 1103 |
| Patient 3 | 7·26 | 7928 | 1429 |
| Patient 4 | 5·18 | 7609 | 1414 |
| Patient 5 | 6·51 | 8672 | 1713 |
DAP = dose area product; Ka = Air kerma values.
4. Discussion
This telerobotic long distance intervention study represents the first-in-human experience of R-PCI with balloon angioplasty and stent deployment interventions conducted from a remote location 20 miles from the patient and the cardiac catheterization laboratory. This was made possible by interfacing the advanced robotic vascular technology of the CorPath GRX platform with reliable networking equipment (LAN/MAN/WAN connectivity) and an advanced audio-visual telecommunications system between the remote and local Robotic Control Workstations. Feasibility of R-PCI has been demonstrated in anesthetized pig model in the recent past [10], although our report is the first demonstration of feasibility in humans.
The remote R-PCI procedure was successful in all aspects. The remote interventionalist operator rated the functionality of the robotic platform and the network connection system as equivalent to an in-lab manual PCI procedure with no significant procedural delays or technical difficulties. This was corroborated by the 53 ms mean delay recorded by the network which is most likely imperceptible to the operator. Procedure times in 2 patients (28 and 29 min) were slightly longer than those previously reported for simple lesion R-PCI, but still within the range of normal variability. Patient radiation exposure from fluoroscopic imaging was similar to or below that observed for manual PCI for type A lesions [12], [13], [14]. The patients did not report any symptoms during the procedure nor did they express any subjective lack of confidence in the operator or procedure.
Because this was an exploratory proof-of-principle study, we decided to enroll only five patients with single, type A coronary lesions. Robotic-assisted PCI however, has been shown to be safe and effective for the treatment of complex coronary lesions. Mahmud et al., compared R-PCI with manual PCI in 315 patients with complex CAD in a real-life hospital setting [6]. The results showed that (1) the clinical success of manual PCI versus R-PCI was comparable (100% versus 98.8%, respectively), and (2) robotic-assisted PCI was feasible, safe, and highly technically successful in treating more complex types of coronary lesions. Robotic Technical Success was 91.7% (defined as clinical success and completion of the PCI procedure entirely robotically or with partial manual assistance), the rate of manual assistance was 11.1%, and the rate of manual conversion was 7.4%. Other studies have reported similar findings on the utility of R-PCI for treating complex CAD [7].
The results of this study represent a milestone in the development of remote robotic PCI in that they open myriad possibilities for remote interventional treatment of CAD in underdeveloped countries or in any rural area that does not have ready access to an interventional cardiologist or a local hospital that specializes in emergency cardiovascular care. Presently, the vast majority of patients with CAD or acute coronary syndrome in developing countries have little or no access to immediate interventional therapy [15]. Current robotic technology combined with improvements in network connectivity and operator expertise in R-PCI procedures can be employed as a front-line service in regions where such expertise is not available. The CorPath GRX robotic system could also be used remotely as a supplemental or expert back-up service for local operators to provide advanced interventional revascularization expertise to a larger number of patients.
In addition to treatment of CAD, long distance robotic vascular technology has therapeutic neurovascular applications, particularly for treatment of acute cerebrovascular ischemic events (stroke), where delay of treatment can critically impact successful patient outcomes. Reports from underdeveloped countries indicate wide gaps in availability of acute neurointerventional therapeutic facilities [16], [17]. Effective utilization of remote telerobotic surgery could hypothetically provide a major part of the solution to these deficits after feasibility and safety are established.
Several limitations of this study need to be mentioned. This was a non-randomized, open-label, single-arm study in which tele-R-PCI was performed on a small sample size of five patients with simple coronary artery lesions. No comparisons were made with manual PCI and the same operator performed all the procedures. Since this was a “first-in-human” study, a team was available in the laboratory to ensure patient safety in the event of problems such as network failure or equipment malfunction. These limitations should be viewed in the context that this was an exploratory, proof of concept study. Further multicenter evaluation of feasibility and safety of tele-R-PCI, without an in-lab interventional cardiology operator, and in a larger population of patients with both simple and complex coronary lesions, will need to be conducted.
In conclusion, this was the first time tele-R-PCI with balloon angioplasty and stent deployment has been performed with the interventional cardiologist located several miles away from the patient in the cardiac catheterization laboratory. Remote tele-R-PCI utilizing the CorPath GRX telerobotic platform is feasible with predictably successful outcomes, if reliable network connectivity and local cardiac catheterization facilities are available.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.eclinm.2019.07.017.
Acknowledgments
Acknowledgements
The authors would like to thank Edward V. Avakian, MA, PhD for medical writing and editorial assistance in preparing the manuscript and Tina Ridgeway, RN, BS for clinical data management. They are both employees of Corindus Vascular Robotics, Inc.
Disclosures
Funding for this study was provided by Corindus Vascular Robotics, Waltham, MA, USA. Dr. Patel was the Principal Investigator and is a paid consultant for Corindus Vascular Robotics.
Appendix A. Supplementary Data
The following are the supplementary data related to this article.
Primary Interventionalist Physician Questionnaire.
References
- 1.Gaziano T.A., Bitton A., Anand S., Abrahams-Gessel S., Murphy A. Growing epidemic of coronary heart disease in low- and middle-income countries. Curr Probl Cardiol. 2010;35(2):72–115. doi: 10.1016/j.cpcardiol.2009.10.002. Feb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kulkarni V.T., Ross J.S., Wang Y., Nallamothu B.K., Spertus J.A., Normand S.L. Regional density of cardiologists and rates of mortality for acute myocardial infarction and heart failure. Circ Cardiovasc Qual Outcomes. 2013;6:352–359. doi: 10.1161/CIRCOUTCOMES.113.000214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Granada J.F., Delgado J.A., Uribe M.P., Fernandez A., Blanco G., Leon M.B. First-in-human evaluation of a novel robotic-assisted coronary angioplasty system. JACC Cardiovasc Interv. 2011;4(4):460–465. doi: 10.1016/j.jcin.2010.12.007. Apr. [DOI] [PubMed] [Google Scholar]
- 4.Smilowitz N.R., Moses J.W., Sosa F.A., Lerman B., Qureshi Y., Dalton K.E. Robotic-enhanced PCI compared to the traditional manual approach. J Invasive Cardiol. 2014;26(7):318–321. Jul. [PubMed] [Google Scholar]
- 5.Goldstein J.A., Balter S., Crowley M., Hodgson J., Klein L.W. Occupational hazards of interventional cardiologists: prevalence of orthopedic health problems in contemporary practice. Catheter Cardiovasc Interv. 2004;63:407–411. doi: 10.1002/ccd.20201. [DOI] [PubMed] [Google Scholar]
- 6.Mahmud E., Naghi J., Ang L., Harrison J., Behnamfar O., Pourdjabbar A. Demonstration of the safety and feasibility of robotically assisted percutaneous coronary intervention in complex coronary lesions: results of the CORA-PCI study (complex robotically assisted percutaneous coronary intervention) JACC Cardiovasc Interv. 2017;10(13):1320–1327. doi: 10.1016/j.jcin.2017.03.050. [DOI] [PubMed] [Google Scholar]
- 7.Smitson C.C., Ang L., Pourdjabbar A., Reeves R., Patel M., Mahmud E. Safety and feasibility of a novel, second-generation robotic-assisted system for percutaneous coronary intervention: first-in-human report. J Invasive Cardiol. 2018;30(4):152–156. [PubMed] [Google Scholar]
- 8.Abbou C.C., Hoznek A., Salomon L., Olsson L.E., Lobontiu A., Saint F. Laparoscopic radical prostatectomy with a remote controlled robot. J Urol. 2017;197(2S):S210–S212. doi: 10.1016/j.juro.2016.10.107. [DOI] [PubMed] [Google Scholar]
- 9.Marescaux J., Leroy J., Gagner M., Rubino F., Mutter D., Vix M. Transatlantic robot-assisted telesurgery. Nature. 2001;413(6854):379–380. doi: 10.1038/35096636. [DOI] [PubMed] [Google Scholar]
- 10.Madder R.D., Van Oosterhout S.M., Jacoby M.E., Collins J.S., Borgman A.S., Mulder A.N. Percutaneous coronary intervention using a combination of robotics and telecommunications by an operator in a separate physical location from the patient: an early exploration into the feasibility of telestenting (the REMOTE-PCI study) EuroIntervention. 2017;12(13):1569–1576. doi: 10.4244/EIJ-D-16-00363. [DOI] [PubMed] [Google Scholar]
- 11.Madder R.D., VanOosterhout S., Mulder A., Bush J., Martin S., Rash A. Feasibility of robotic Telestenting over long geographic distances a pre-clinical ex vivo and in vivo study. EuroIntervention. 2019 doi: 10.4244/EIJ-D-19-00106. Apr 16. pii: EIJ-D-19-00106. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 12.Fetterly A.F., Lennon R.J., Bell M.R., Holmes D.R., Rihal C.S. Clinical determinants of radiation dose in percutaneous coronary interventional procedures. JACC. Cardiovascular Interventions. 2011;4(3):336–343. doi: 10.1016/j.jcin.2010.10.014. [DOI] [PubMed] [Google Scholar]
- 13.Chambers C.E. Radiation dose in percutaneous coronary intervention. JACC Cardiovasc Interv. 2011;4(3):344–346. doi: 10.1016/j.jcin.2010.11.009. [DOI] [PubMed] [Google Scholar]
- 14.Sciabasi A., Ferrante G., Fishetti D. Radiation dose among different cardiac and vascular invasive procedures: the RODEO study. Int J Cardiol. 2017;240:92–96. doi: 10.1016/j.ijcard.2017.03.031. [DOI] [PubMed] [Google Scholar]
- 15.Mohanan P.P., Mathew R., Harikrishnan S. Presentation, management, and outcomes of 25 748 acute coronary syndrome admissions in Kerala, India: results from the Kerala ACS registry. Eur Heart J. 2013;34(2):121–129. doi: 10.1093/eurheartj/ehs219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fujiwara K., Osanai T., Kobayashi E., Tanikawa T., Kazumata K., Tokairin K. Accessibility to tertiary stroke centers in Hokkaido, Japan: use of novel metrics to assess acute stroke care quality. J Stroke Cerebrovasc Dis. 2018;27(1):177–184. doi: 10.1016/j.jstrokecerebrovasdis.2017.08.013. [DOI] [PubMed] [Google Scholar]
- 17.Alberts M.J., Range J., Spencer W., Cantwell V., Hampel M.J. Availability of endovascular therapies for cerebrovascular disease at primary stroke centers. Interv Neuroradiol. 2017;23(1):64–68. doi: 10.1177/1591019916678199. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Primary Interventionalist Physician Questionnaire.