Abstract
Introduction
The World Health Organization recommends initiation of lifelong antiretroviral therapy (ART) in all HIV-infected pregnant women (“Option B+"); however, disengagement from care has been documented postnatally and thereafter. The community-based adherence club (AC) system has been widely implemented in Cape Town, South Africa, and provides HIV care to stable adults on ART, but women who initiated ART in antenatal care services are currently referred to local ART clinics postnatally.
Methods
The Postpartum Adherence Clubs for Antiretroviral Therapy (PACART) study is a pragmatic randomised controlled trial evaluating ACs to deliver long-term HIV care to women who initiated ART antenatally. Consecutive eligible women seeking care postnatally at a large primary health care facility in Cape Town were randomised to either the local ART clinic (standard of care), or the AC service. The primary objective is to compare maternal HIV viral suppression up to 24 months postpartum. Six study visits are scheduled through 24 months; measurements at each visit include phlebotomy for viral load and questionnaires assessing maternal health, infant health, and ART adherence. Qualitative interviews examining issues of ART adherence and retention, and assessments of costs and cost-effectiveness will also be done.
Results
Enrolment is complete, with 412 women enrolled. Follow-up visits are ongoing.
Discussion
There is an urgent need to improve ART delivery for maternal and child health. With a pragmatic trial design, we aim to assess use of the community-based AC system to improve maternal engagement in HIV care in the postpartum period and beyond.
Keywords: Adherence clubs, Antiretroviral therapy, HIV, Option B+, Postpartum, Pregnancy
1. Introduction
In 2013, the World Health Organization (WHO) recommended initiation of lifelong antiretroviral therapy (ART) in all HIV-infected pregnant women regardless of clinical status (“Option B+") [1]. All 21 Global Plan priority countries in Africa have adopted this policy, and the number of HIV-infected pregnant and postpartum women on ART has increased substantially [2,3]. In addition to reducing mother-to-child transmission of HIV, foreseen benefits of this approach include a reduction in horizontal transmission of HIV, a reduction in maternal mortality, and operational simplification [3].
The full benefit of Option B+ is dependent on adherence to ART and retention in care during pregnancy, delivery, the postpartum period and beyond [[4], [5], [6]]. However, several studies have documented suboptimal levels of adherence to ART and/or retention in care in women started on ART during pregnancy, and the postpartum period has been identified as particularly high-risk [[7], [8], [9], [10]]. In Malawi, for example, 29% (7543/25849) and 30% (7748/25535) of women starting ART under Option B+ between 2011 and 2012 were lost to follow-up (LTFU) after 24 and 36 months, respectively [11]. While many prevention of mother-to-child transmission (PMTCT) programmes focus on providing ART during pregnancy and delivery [2,10], there is thus an urgent need for evidence-based approaches to support mothers on ART beyond these periods [5,12]. Further, the increasing number of HIV-infected women started on lifelong ART during pregnancy has placed strain on health systems [6], and service delivery models that accommodate the rising number of women on ART are needed.
Few interventions to increase long-term engagement in ART care in women who started ART in pregnancy have been evaluated. In the general population, several differentiated ART care models have been implemented to accommodate ART expansion and minimise LTFU. These include the adherence club (AC) system, in which services are decentralised into communities and run by community health workers (CHWs), supported by nurses [[13], [14], [15]]. There is observational evidence that stable general adult patients managed via ACs have a reduced risk of LTFU compared to those managed in routine clinic services [14,16], and a study among postpartum women showed similar virological outcomes at six months post-delivery in those managed in ACs vs routine clinic services [17]. There are, however, to our knowledge, no individually randomised studies examining outcomes in patients managed in ACs, and no studies of longer-term outcomes in women referred postnatally.
South Africa adopted Option B+ in 2013; >95% of eligible pregnant women in South Africa in 2015 received ART [18]. The AC model has been widely implemented in the Cape Metro health district in Cape Town, South Africa since 2011; by 2015 there were more than 30,000 patients in 1,308 ACs at 55 health facilities [19]. Currently, women in the postpartum period are referred to general adult ART clinics. We designed the Postpartum Adherence Clubs for Antiretroviral Therapy (PACART) study to evaluate the AC model as a means of delivering ART to postpartum women who started ART in pregnancy; follow-up is through 24 months to assess long-term outcomes. This paper describes the study design and methods, and reports on progress to date.
2. Materials and methods
2.1. Study design
PACART is a pragmatic randomised controlled trial comparing two strategies to provide ART to HIV-infected postpartum women who started ART in pregnancy. Enrolled women were randomised to either (a) referral to ART services at the local adult ART clinic, following the current standard of care (control); or (b) referral directly to the community-based ART AC service (intervention). Randomisation was a 1:1 allocation using a computer-generated dynamic permuted block design. Women in both arms are scheduled to attend study measurement visits conducted separately from routine ART service appointments through 24 months postpartum. The primary objective is to compare maternal HIV viral suppression in the two arms; we hypothesise that women randomised to attend ACs will be more likely to maintain viral suppression (defined as viral load [VL] < 1000 copies/ml) up to 24 months postpartum. Secondary objectives include comparison of maternal retention in care, maternal health, routine health care service use in the HIV-exposed children of participating mothers, cost and cost-effectiveness, and acceptability of each ART service.
2.2. Setting and population
The study is taking place in Gugulethu, Cape Town, at the Gugulethu Community Health Centre (CHC) and the associated Midwife Obstetric Unit (MOU). This large public sector primary care facility serves a population of approximately 350,000 which is predominantly of low socioeconomic status [20]. The CHC includes an ART clinic which provides HIV care to the general adult population. The MOU is operated by nurse-midwives, and provides antenatal care (ANC), PMTCT services, obstetric services and postnatal care to >4000 women annually [21]. ANC uptake in the community is high (>95%), as is antenatal HIV prevalence, estimated at 30% in 2013 [20].
HIV-infected women were eligible for enrolment if they were 18 years or older and within 28 days postpartum. Additional eligibility criteria were based on AC admission criteria and included a suppressedVL (VL < 400 copies/ml) in the preceding three months and the absence of any comorbidities requiring regular clinical follow-up. Women were excluded if they intended to relocate out of Cape Town during the study period or had lost their foetus or infant. Once enrolment began, we noted a larger than expected number of women attending their first postpartum clinic visit at the MOU beyond 28 days postpartum; eligibility criteria were thus expanded in April 2016 to include women up to 70 days postpartum.
2.3. Routine postnatal and ART services
2.3.1. Postnatal and MOU ART services
At the Gugulethu MOU, PMTCT services are provided at a specific integrated ART clinic. The first-line ART regimen is tenofovir 300 mg, emtricitabine 200 mg, and efavirenz 600 mg, taken once daily as a fixed-dose combination. Clinical follow-up occurs one-to-two monthly during pregnancy, with VL measured after three months on ART.
Postnatally, mother-infant pairs are scheduled to attend the postnatal clinic within seven days of delivery for basic neonatal support and infant feeding monitoring. In addition, mothers on ART are scheduled to attend the MOU ART clinic with their infants, usually within one month of delivery, for counselling, review of maternal and infant health status and dispensing of ART. Mothers are referred from the MOU ART clinic to the general adult ART service at the CHC for further HIV care, and infants are referred separately to clinics that provide routine “well-baby” services, including vaccinations and HIV testing. Referrals are typically made at the first postnatal visit to the MOU ART clinic.
2.3.2. General adult ART clinic services
At the first general adult ART clinic visit, usually within one month of referral, women undergo a clinical assessment by either a doctor or clinical nurse practitioner, with further visits scheduled at least monthly for the first four months (Table 1). Subsequent visits are two monthly for medication refill, with three months of medication given over the December holiday period. Clinician review is six monthly. Patients with clinical or psychosocial concerns may be reviewed more frequently or referred to higher levels of care. Routine laboratory investigations include VL testing at months four and twelve on ART and annually thereafter.
Table 1.
Comparison of control and intervention arms.
| General Adult ART Clinic (Control) | Adherence Club (Intervention) | |
|---|---|---|
| Venue | ART clinic at the Gugulethu CHC | Offsite community hall, ~450 m from the CHC |
| Modality of care | Individualised | Group-based |
| Usual eligibility criteria | All patients eligible | On ART > 6 months, latest VL < 400 copies/ml, no comorbidities requiring regular clinical follow-up |
| First appointment date | Patients with a referral letter can attend on any day; appointment not necessary | Patients must attend the club office at the Gugulethu CHC with clinic folder and referral letter - CHW will provide first AC visit date |
| Attending staff | Clinician (CNP or doctor) | CHWs attend all club visits, CNP attends 2 club visits per year (blood visit and clinical visit) |
| Prescription frequency | 1-2 monthly, may be more frequent based on clinician discretion | 2 monthly |
| End of year holiday arrangements | 3 months of ART for stable patients from mid-October | 4 months of ART for all patients from beginning of October |
| Treatment buddy collections | Buddies may collect medication on behalf of the patient unless clinician requests to see the patient | Buddies may collect medication on behalf of the patient on alternate visits, but blood and clinical visits must be attended by the patient |
| Counselling | Daily waiting room CHW talks, patients at high risk for non-adherence have pill counts and further counselling | Group counselling session at every visit |
| Laboratory testing | Months 4 and 12 on ART and then annually, may be more frequent based on clinician discretion | Annually |
| Visit duration | From 3 to 4 h to the whole day | ~1 h for standard visit, up to 3 h for clinical visit |
| Follow-up of non-attendance | Every 4 months, patients who have not had an ART refill in this period have a home visit by a CHW | If medication not collected within 5 days of scheduled AC visit, participant referred to the general adult ART clinic |
| Management of clinical complications or VL > 400 copies/ml | Managed by CHC clinicians, with up-referral if necessary | Referred to general adult ART clinic for further management |
| Provision of routine infant care | Local “well-baby” clinic | Local “well-baby” clinic |
AC: Adherence club; ART: Antiretroviral therapy; CHC: Community health centre, CHW: Community health worker; CNP: Clinical nurse practitioner; h: Hour; VL: Viral load.
2.3.3. Adherence clubs
Adults attending the general ART clinic who fulfil the following eligibility criteria may be referred from the ART clinic to the ACs: on ART for at least six months, suppressed VL (VL < 400 copies/ml) and no comorbidities requiring regular clinical follow-up.
Clubs meet for approximately 60 minutes, every two months, with four months of medication given over the December holiday period [15]. At routine visits, there is a short CHW-led group discussion and patients are weighed, screened for symptoms and dispensed pre-packed ART. A nurse attends an annual visit to perform VL assessment and attends the subsequent visit to perform a clinical assessment and check VL results. Patients who have lost weight, are symptomatic, have an elevated VL, or who have not collected their medication within five days of any scheduled visit are referred to the general ART clinic immediately. Patients have the option of sending a “buddy” (i.e., a partner, relative, or friend) to attend non-clinical club visits, but must attend every second session.
2.4. Description of control and intervention
2.4.1. Control
Based on the local standard of care, mothers randomised to the control arm were referred out from the MOU ART service to the general adult ART clinic at the CHC, and their infants to the “well-baby” child health services. All maternal and infant clinical care and follow-up are provided by government health services and are based on public sector policies.
2.4.2. Intervention
Women randomised to the intervention arm were referred out from the MOU ART service to the ACs postnatally. Per current guidelines, only patients on ART for at least six months are eligible for referral to ACs, meaning that many postpartum women who initiated ART in pregnancy would be ineligible. For this study, women were referred to ACs provided they were on ART for at least three months and met the other AC eligibility criteria. Infants were referred to “well-baby” child health services, per the local standard of care. All maternal and infant clinical care and follow-up are provided by government health services and are based on public sector policies.
2.5. Study procedures
2.5.1. Recruitment, enrolment and randomisation
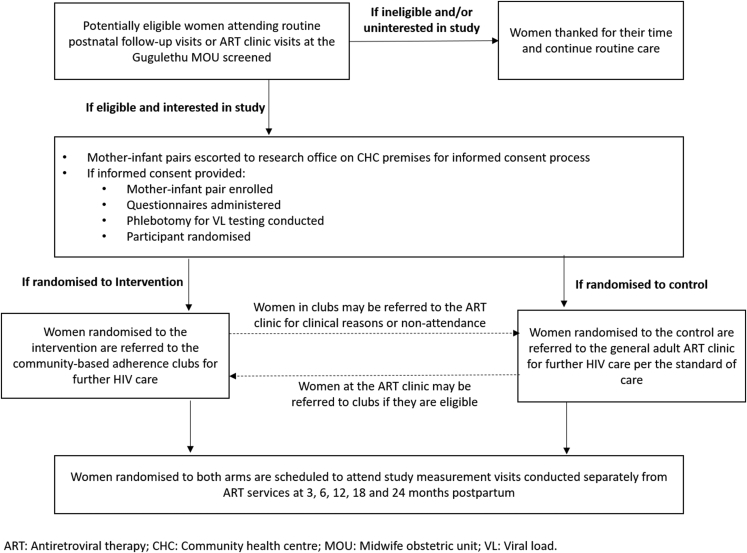
Recruitment took place at either postpartum visits to the MOU ART clinic or postnatal clinic visits (Fig. 1). Consecutive potentially eligible women were provided with basic information about the study and, if interested, were screened. Those interested and eligible were referred to study counsellors for the informed consent process. Women who provided consent underwent an enrolment interview and VL testing and were then randomised to either the general adult ART clinic management strategy or the AC strategy. Randomisation allocations were via opaque sealed envelopes kept securely and accessed by the study coordinator, who communicated the allocations to study staff.
Fig. 1.
PACART screening, enrolment and randomisation process.
After enrolment, women in both arms were seen at the MOU ART clinic where, following standard procedures, they were counselled, prescribed ART and provided with a referral letter; those referred to the general adult ART clinic were advised to visit the referral clinic within a month, while those referred to the AC were escorted by a counsellor to the AC club office at the CHC for a club appointment. In both arms, infants were referred for routine infant care to local primary care “well baby” clinics.
2.5.2. Measurements
Study measurement visits are scheduled at approximately three, six, twelve, eighteen and twenty-four months postpartum. All study visits are conducted separately from ART services, to allow participants to engage in study procedures independent of their site of care. In addition to study visits, we call participants at nine, fifteen and twenty-one months postpartum to ask about general maternal and infant health status and remind them of their next study visit date.
Quantitative assessments include questionnaires administered at every visit to collect data on both mothers and infants, including demographics, medical history, a range of measures of ART adherence, and infant feeding (Table 2). Questionnaires examining behavioural, psychosocial and mental health status, which may affect adherence, are also administered, including measures of depression, anxiety, alcohol abuse, availability of social support, the patient-provider relationship and HIV-related stigma. VL measurement is conducted at each study measurement visit; this is done separate from routine clinical monitoring, with batch testing by the South African National Health Laboratory Services using the Abbott Realtime HIV-1 assay (Abbott Laboratories, Waltham, MA). Additional study data come from the review of routine medical records. Specifically, we abstract data from local health care services on maternal follow-up in routine care, and infant health and health care.
Table 2.
Schedule of measurements.
| Item for completion | <70 days pp (study visit 1) | 3 months pp (study visit 2) | 6 months pp (study visit 3) | 12 months pp (study visit 4) | 18 months pp (study visit 5) | 24 months pp (study visit 6) |
| Questionnaires | ||||||
| Maternal | ||||||
| Demographics | X | X | X | X | X | X |
| Medical history | X | X | X | X | X | X |
| Family planning use/pregnancy intentions | X | X | X | X | X | X |
| Maternal adherence1 | X | X | X | X | X | X |
| K-102 | X | X | X | X | X | X |
| EPDS2 | X | X | X | X | X | X |
| Patient-provider relationship scale | X | X | X | X | X | X |
| Alcohol screen4 | X | X | X | X | X | |
| Social impact scale | X | X | X | |||
| Availability of social support scale | X | X | X | X | ||
| Resource Interview | X | X | ||||
| Infant | ||||||
| Infant demographics | X | X | X | X | X | X |
| Medical history | X | X | X | X | X | X |
| Infant adherence1 | X | X | X | X | X | X |
| Feeding practices | X | X | X | X | X | X |
| Resource Interview | X | X | ||||
| Study laboratory measures | ||||||
| Phlebotomy for maternal HIV viral load testing and dried blood spot testing for tenofovir diphosphate levels | X | X | X | X | X | X |
| Qualitative acceptability interviews | X | |||||
| Clinical data abstraction | X | |||||
pp: Postpartum.
1 Adherence assessments will include maternal ART adherence and questions regarding infant adherence to antiretroviral and cotrimoxazole prophylaxis.
2 Kessler-10 (screening questionnaire for non-specific psychological distress), Edinburgh Postnatal Depression Survey.
3 Alcohol use disorders identification test (AUDIT).
Qualitative in-depth interviews will be conducted, with the aim of assessing acceptability of the AC service vs the general adult ART clinic. Interviews will be conducted by an isiXhosa-speaking research assistant in a subset of 30 participants (15 in each trial arm) interviewed at approximately 24 months postpartum. Participants will be selected purposively using maximum variation sampling with respect to age and will sign separate informed consent forms for this part of the study.
For the cost-effectiveness analysis, data on health services costs will be abstracted from routine expenditure reports, facility accounts and staffing plans, and pharmacy accounts maintained by the province. Questionnaires will be administered to collect data on health service use by participants. Programme costs will be based on local standards for training and materials development, and overhead costs will be based on provincial accounts.
2.6. Sample size and analysis plan
2.6.1. Sample size estimation
The projected minimum sample size for the trial is 388 participants. This is based on the following assumptions: a superiority comparison of the AC model and standard of care; 90% power (β = 0.1) and 2-sided α = 0.05 using a log-rank test of survival proportions; 1:1 randomisation; 10% withdrawal during follow-up (due to death, relocation, or refusal); an expected proportion of women remaining with VL <1000 copies/ml of 70% in the SOC arm; an expected proportion of women remaining with VL <1000 copies/ml in the intervention arm of 85%; and thus the expected minimum difference in the primary outcome at 24 months postpartum in the two arms of 15%. A contamination rate of 10% is included to account for the possibility that some participants in the SOC control may be more likely to adhere to ART and/or be retained in care if they are aware of the AC intervention. The proposed sample size will also provide >80% power to detect appreciable differences for most secondary outcomes of interest.
2.6.2. Outcomes and analysis plan
The primary study outcome is time to detectable viraemia, defined as time to VL > 1000 copies/ml; this VL threshold has been chosen to account for viral blips and local definitions of clinically meaningful viraemia (Table 3). Secondary outcomes will consider other VL cutpoints, including time to VL > 400 copies/ml and time to VL > 50 copies/ml. Additional secondary outcomes include time to virological failure, defined as time to two consecutive VLs >1000 copies/ml; retention, defined as time to LTFU (no visit within three months of scheduled clinic visit); and a combined retention/VL outcome.
Table 3.
Primary and secondary outcomes.
| Indicator | Source | |
|---|---|---|
| Primary Outcome | ||
| Time to detectable VL | Time to VL > 1000 copies/ml | Maternal blood at 3, 6, 12, 18 and 24 months pp |
| Secondary Outcomes | ||
| Time to detectable VL | Time to VL > 400 copies/ml | Maternal blood at 3, 6, 12, 18 and 24 months pp |
| Time to detectable VL | Time to VL > 50 copies/ml | Maternal blood at 3, 6, 12, 18 and 24 months pp |
| Time to virological failure | Time to two consecutive VLs >1000 copies/ml | Maternal blood at 3, 6, 12, 18 and 24 months pp |
| Time to LTFU | Time to missed clinic visit and no visit within 3 months of scheduled clinic visit | Data abstraction from routine facility records |
| Time to combined LTFU/detectable VL | Time to VL > 1000 copies/ml, or missed clinic visit and no visit within 3 months of scheduled clinic visit | Maternal blood at 3, 6, 12, 18 and 24 months pp and data abstraction from routine facility records |
| Maternal Health | Includes maternal health care service use (including hospitalisation), mental health and maternal deaths | Participant questionnaires, data abstraction from routine facility records |
| Infant Health | Includes infant health care service use (including hospitalisation, HIV PCR testing uptake, vaccination uptake), feeding practices, infant HIV infection and infant deaths | Participant questionnaires, data abstraction from routine facility records |
| Cost and Cost-Effectiveness | Mean cost per woman from the start of postnatal care | Facility and provincial expenditure reports and accounts, participant questionnaires |
| Acceptability | Assessed using patient provider relationship scale and qualitative interviews | Questionnaires, qualitative interviews |
LTFU: Loss to follow-up; PCR: Polymerase chain reaction; pp: Postpartum; VL: Viral load.
Primary analyses will be by intention-to-treat, with secondary modified intention-to-treat analyses conducted in participants with VL < 1000 copies/ml at the time of randomisation, and per protocol analyses in participants attending at least one visit at the intended service. Demographic, clinical, and relevant behavioural characteristics in the two arms at baseline will be compared. For the primary analysis, product-limit methods stratified by study arm will be used to describe time to detectable viraemia; study arms will be compared using log-rank tests. The primary analysis will be presented by estimated hazard ratio (95% confidence interval), with the threshold for statistical significance adjusted for the interim analyses required by the Data Monitoring Committee. All other statistical tests will be two-sided at α = 0.05. Proportional hazards models will be used to examine the influence of participant demographic, behavioural and/or clinical characteristics on the primary outcome, independent of study arm.
A priori subgroup analyses include: (1) timing of maternal ART initiation during pregnancy, with subgroups of (i) women who initiated ART early in gestation, defined as the first two trimesters, versus (ii) women who initiated ART late in gestation, defined as the third trimester; and (2) prior antiretroviral exposure, distinguishing women who initiated lifelong ART before pregnancy but defaulted from care and are restarting treatment in pregnancy, from women who received antiretrovirals previously for PMTCT only, from women who are antiretroviral naïve. Both of these covariates are strongly associated with maternal adherence to ART, and may modify the impact of the AC intervention on patient outcomes; previous antiretroviral exposure also allows for stratification by potential for antiretroviral resistance. Additional subgroup analyses will be done based on timing of randomisation, age categories, parity, education, employment, timing of HIV diagnosis, timing of first antenatal visit, CD4 nadir, gestation at ART initiation, weeks on ART at randomisation, viral suppression at randomisation, breastfeeding status and whether or not doses were missed in the 30 days prior to randomisation.
In cost-effectiveness analyses, capital costs will be annuitised using the recommended rate of 3%. Costs will be defined as the mean cost per woman from the start of postnatal care; calculations will follow standard methods with costs as quantities of resources utilised multiplied by unit costs. Resources include health care visits and programme-level costs for each study arm. Human resource costs will be calculated separately to enable estimation of the number of different cadres of staff needed to provide services in each trial arm at different levels of scale. We will use trial outcome data to calculate the incremental cost required to improve maternal outcomes; costs and outcomes will be discounted appropriately. Multi-way probabilistic (Bayesian) sensitivity analyses will be used to assess uncertainty.
Qualitative data will be analysed using the NVivo software package (QSR International, Australia). Analysis will focus on commonalities and divergences in participants’ responses to key domains and constructs, including perceptions of postnatal services, barriers and facilitators of adherence and retention during the postpartum period, as well as the possibility of contamination between trial arms.
2.7. Ethical considerations
Ethical approval for the study has been obtained from the Human Subjects Research Ethics Committee of the Faculty of Health Sciences at the University of Cape Town (REF 194/2015); approval has also been obtained from the local government and the facility manager.
3. Results
3.1. Study progress
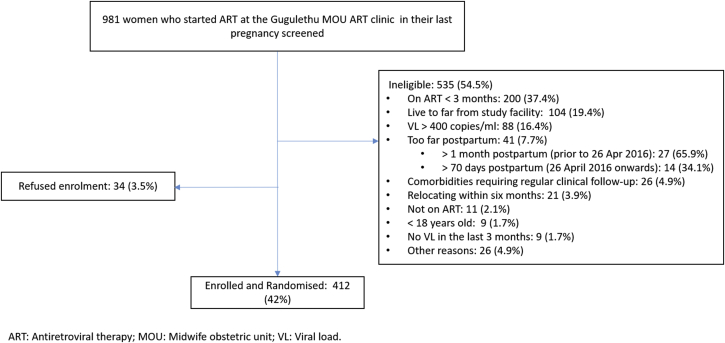
Screening began on 6 January 2016 and the first participant was enrolled on 11 January 2016. In total, 981 women who started ART in their last pregnancy at the MOU ART clinic were screened, of whom 412 were enrolled (Fig. 2). The most common reason for ineligibility was duration on ART of <3 months. Enrolment was completed on 10 November 2017. Four-hundred and twelve participants were enrolled; this is more than the minimum sample size and was done for logistical reasons. Three-, six- and twelve-month follow-up visits have been completed; eighteen- and twenty-four-month visits are currently ongoing. Follow-up visits are expected to conclude in November 2019.
Fig. 2.
Flow chart of recruitment, screening and enrolment.
4. Discussion
4.1. Pragmatic trial design
The PACART study has been designed to answer urgent questions regarding the implementation of ART services in the postpartum period and beyond for women who started ART in pregnancy. We have taken a pragmatic approach, the aim being to assess whether this intervention can work under usual conditions. This is partly borne of consultations with local health service representatives, who advocated for an assessment of currently well-established systems, to maximise feasibility in a real-world setting. Pragmatic design features include the eligibility criteria for club referral in the study, which, other than those related to the intervention, mimic the criteria for club referral in usual care (Table 4). Participants are recruited from routine clinic visits and are incorporated into well-established routine AC club and clinic systems; no additional resources or expertise are required in either study arm. Per standard guidelines, participants randomised to adherence clubs may be referred to the general ART clinic for clinical reasons or due to visit non-attendance, and those randomised to the ART clinic may be referred to ACs should they become eligible. While this complicates the assessment of outcomes for the study, it reflects current practices, and we have accounted for possible contamination in the calculation of sample size. The primary outcome is based on VL quantification, which is the primary mode of assessment of ART effectiveness in routine services. Follow-up study visits have been kept to a minimum, with visits between six and twenty-four months scheduled at six monthly intervals, to minimise any effect on retention or adherence outcomes. Thus, with this trial design, we hope to assess whether this intervention will work under real-world conditions in this setting.
Table 4.
PRECIS-2[23] scores for trial domains.
| Domain | Score | Rationale |
|---|---|---|
| Eligibility Criteria | 4/5 | Eligibility criteria for club referral in the study, other than those related to the intervention, are based on criteria used in usual care including VL < 400 copies/ml and the absence of comorbidities requiring regular clinical follow-up. In routine services, patients on ART for <6 months are ineligible. As part of the intervention, we referred women in the immediate postpartum period, and reduced the required duration on ART to ≥3 months to accommodate women started on ART in pregnancy. |
| Recruitment path | 4/5 | Potential participants were approached at routinely scheduled service visits at the facility. While we had specific study recruiters who engaged with patients, no additional effort was made to engage patients. |
| Setting | 5/5 | Patients were referred to obtain HIV care at the routine service ACs or routine ART clinics, with no changes made to these systems. |
| Organisation intervention |
5/5 |
Patients are incorporated into routine AC club and clinic systems upon referral; no additional resources or organisation of care delivery are required. |
| Flexibility of experimental intervention – Delivery | 5/5 | The intervention is administered as part of routine services and flexibility for study and other patients is identical. |
| Flexibility of experimental intervention - Adherence | 4/5 | Flexibility for participants and routine patients attending the ACs is identical and follows standard guidelines: participants randomised to ACs may be referred to the general ART clinic for clinical reasons or due to visit non-attendance. In addition, those randomised to the ART clinic may be referred to ACs should they become eligible. For ethical reasons, study participants who attend study visits and are found to have defaulted ART are counselled to return to care and are given a referral letter. |
| Follow-up | 4/5 | Follow-up visits are 1–2 monthly at clinics and 2 monthly at ACs. Study follow-up is less frequent (at 3, 6, 12, 18 and 24 months), in order to minimise effects on adherence and retention. |
| Outcome | 5/5 | The primary outcome is based on VL measurement and is relevant in practice: it indicates effectiveness of ART and risk of HIV transmission and is used to make decisions regarding treatment failure and possible virologic resistance in routine care. |
| Analysis | 5/5 | The primary analysis will be intention to treat. |
AC: Adherence club; ART: Antiretroviral therapy; PRECIS-2: PRagmatic-Explanatory Continuum Indicator Summary 2; VL: Viral load.
4.2. Use of viral suppression up to 24 months postpartum as the outcome measure
The primary outcome is viral suppression through 24 months postpartum. While previous studies of adherence have used LTFU as the primary outcome measure, this has been difficult to assess in our setting due to poor documentation of routine service visits and high levels of mobility between facilities. VL provides an objective and direct indication of ART adherence and effectiveness, and the level is directly proportional to the risk of HIV transmission [22]; this will allow us to closely assess risk over the study period. In addition, VL levels are the predominant means of assessment of ART effectiveness in routine care; management decisions regarding treatment failure and possible virologic resistance are largely based on VL results. While VL testing is done as part of routine care, we conduct separate VL testing as part of the study. This ensures more frequent measures; study VL testing is conducted at least six monthly, while stable patients in routine care are tested 12 monthly. In addition, this allows assessment of adherence separate to retention in usual care. The duration of follow-up is up to 24 months, and we will thus have data on longer-term outcomes. WHO infant feeding guidelines recommend that HIV-infected mothers breastfeed for at least 12 months and up to 24 months or beyond [22]. The risk of HIV transmission is ongoing throughout breastfeeding, and this study will provide data on ART adherence and viral suppression in this crucial period. Considering the increasing numbers of pregnant women initiated on lifelong ART, future studies examining adherence beyond 24 months might be required.
4.3. Interdisciplinary approach
Barriers to adherence to ART in sub-Saharan Africa include individual-level factors (including depression, shock and denial), community-level factors (including the extent of partner/community support, fear of disclosure and stigma) and health system-level factors (including lack of resources, service accessibility and negative staff interactions) [7]. The AC model has been designed to address some of these elements: patients on long-term ART are supported by peers within communities [16], with the aim of removing some of the structural barriers associated with attending clinical facilities [14]. To fully understand the range of factors which may directly affect or mediate outcomes in this study, we have included a range of individual, interpersonal and psychosocial measures including assessments of depression, anxiety, social support, stigma, disclosure of HIV status and partner involvement.
Patient acceptability is critical to the success of any intervention aiming to maintain or improve adherence to chronic treatment and retention in long-term health care services; we have included both quantitative measures, using a locally adapted version of the patient provider relationship scale, and qualitative methods, in the form of qualitative in-depth interviews, to assess acceptability. Lastly, cost-effectiveness is an important consideration. Costing data will be used alongside findings on clinical outcomes to understand the cost-effectiveness of the two strategies for ART services during the postnatal period and will be analysed from both patient and health systems perspectives.
5. Conclusions
Increasing numbers of HIV-infected pregnant women are being initiated on lifelong ART; however, high levels of non-retention in care, and/or inadequate adherence to treatment have been documented postpartum and thereafter. The PACART study is being conducted to assess the use of the community-based AC system to improve adherence and retention in care in the postpartum period and beyond. The questions posed by this trial are urgent considering that most high-burden HIV countries have already implemented Option B+ and, while it is being conducted in South Africa, the results may have implications for other low- and middle-income countries with high HIV burdens, where poor access to care and resource scarcity require innovative approaches to care.
Conflicts of interest
None.
Disclosures
None.
Funding
This work was supported by the Medical Research Council [grant number MR/M007464/1]. Jasantha Odayar received training in research that was supported by the Fogarty International Centre of the National Institutes of Health under Award Number D43 TW010559. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- 1.World Health Organization . 2nd. World Health Organization; Geneva, Switzerland: 2016. Consolidated Guidelines on the Use of Antiretroviral Drugs for Treating and Preventing HIV Infection: Recommendations for a Public Health Approach.http://apps.who.int/iris/bitstream/10665/208825/1/9789241549684_eng.pdf Available at: [PubMed] [Google Scholar]
- 2.UNAIDS . UNAIDS; Geneva, Switzerland: 2016. Get on the Fast-Track.http://www.unaids.org/en/resources/documents/2016/get-on-the-fast-track [Google Scholar]
- 3.Kalua T., Tippett Barr B.A., van Oosterhout J.J., Mbori-Ngacha D., Schouten E.J., Gupta S., Sande A., Zombe G., Tweya H., Lungu E., Kajoka D., Tih P., Jahn A. Lessons learned from option B+ in the evolution toward “test and start” from Malawi, Cameroon, and the United Republic of Tanzania. J. Acquir. Immune Defic. Syndr. 2017;75(1):S43–S50. doi: 10.1097/QAI.0000000000001326. PMID: 28398996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization . World Health Organization; Geneva, Switzerland: 2015. Guideline on when to Start Antiretroviral Therapy and on Pre-exposure Prophylaxis for HIV.http://www.who.int/hiv/pub/guidelines/earlyrelease-arv/en/ [PubMed] [Google Scholar]
- 5.Shaffer N., Abrams E.J., Becquet R. Option B+ for prevention of mother-to-child transmission of HIV in resource-constrained settings: great promise but some early caution. AIDS. 2014;28:599–601. doi: 10.1097/QAD.0000000000000144. [DOI] [PubMed] [Google Scholar]
- 6.Chi B.H., Bolton-Moore C., Holmes C.B. Prevention of mother-to-child HIV transmission within the continuum of maternal, newborn, and child health services. Curr. Opin. HIV AIDS. 2013;8(5):498–503. doi: 10.1097/COH.0b013e3283637f7a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nachega J.B., Uthman O.A., Anderson J., Peltzer K., Wampold S., Cotton M.F., Mills E.J., Ho Y.-S., Stringer J.S.A., McIntyre J.A., Mofenson L.M. Adherence to antiretroviral therapy during and after pregnancy in low-, middle, and high-income countries: a systematic review and meta-analysis. AIDS. 2012;26(16):2039–2052. doi: 10.1097/QAD.0b013e328359590f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim M.H., Ahmed S., Hosseinipour M.C., Giordano T.P., Chiao E.Y., Yu X., Nguyen C., Chimbwandira F., Kazembe P.N., Abrams E., J. The impact of option B + on the antenatal PMTCT cascade in Lilongwe , Malawi. J. Acquir. Immune Defic. Syndr. 2015;68(5):e77–83. doi: 10.1097/FQAI.0000000000000692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Phillips T., Thebus E., Bekker L.-G., McIntyre J., Abrams E.J., Myer L. Disengagement of HIV-positive pregnant and postpartum women from antiretroviral therapy services: a cohort study. J. Int. AIDS Soc. 2014;17(1) doi: 10.7448/IAS.17.1.19242. PMID: 25301494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Myer L., Phillips T.K. Beyond “option B+”: understanding antiretroviral therapy (ART) adherence, retention in care and engagement in ART services among pregnant and postpartum women initiating therapy in sub-Saharan Africa. J. Acquir. Immune Defic. Syndr. 2017;75(2):S115–S122. doi: 10.1097/QAI.0000000000001343. PMID: 28498180. [DOI] [PubMed] [Google Scholar]
- 11.Haas A.D., Tenthani L., Msukwa M.T., Tal K., Jahn A., Gadabu O.J., Spoerri A., Chimbwandira F., van Oosterhout J.J., Keiser O. Retention in care during the first 3 years of antiretroviral therapy for women in Malawi’s option B+ programme: an observational cohort study. Lancet HIV. 2016;3:e175–e182. doi: 10.1016/S2352-3018(16)00008-4. PMID: 27036993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Glass R.I., Birx D.L. Advancing PMTCT implementation through Scientific Research : a vital agenda for combating the global AIDS epidemic in low- and middle-income countries. J. Acquir. Immune Defic. Syndr. 2016;72 doi: 10.1097/QAI.0000000000001076. [DOI] [PubMed] [Google Scholar]
- 13.Wilkinson L., Harley B., Sharp J., Solomon S., Jacobs S., Cragg C., Kriel E., Peton N., Jennings K., Grimsrud A. Expansion of the Adherence Club model for stable antiretroviral therapy patients in the Cape Metro, South Africa 2011-2015. Trop. Med. Int. Health. 2016;21(6):743–749. doi: 10.1111/tmi.12699. PMID: 27097834. [DOI] [PubMed] [Google Scholar]
- 14.Luque-Fernandez M.A., Van Cutsem G., Goemaere E., Hilderbrand K., Schomaker M., Mantangana N., Mathee S., Dubula V., Ford N., Hernan M.A., Boulle A. Effectiveness of patient adherence groups as a model of care for stable patients on antiretroviral therapy in Khayelitsha, Cape Town, South Africa. PLoS One. 2013;8(2) doi: 10.1371/journal.pone.0056088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grimsrud A., Sharp J., Kalombo C., Bekker L.-G., Myer L. Implementation of community-based adherence clubs for stable antiretroviral therapy patients in Cape Town, South Africa. J. Int. AIDS Soc. 2015;18 doi: 10.7448/IAS.18.1.19984. PMID: 26022654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grimsrud A., Lesosky M., Kalombo C., Bekker L.-G., Myer L. Community-based adherence clubs for the management of stable antiretroviral therapy patients in Cape Town , South Africa: a cohort study. J. Acquir. Immune Defic. Syndr. 2016;71(1):e16–23. doi: 10.1097/QAI.0000000000000863. [DOI] [PubMed] [Google Scholar]
- 17.Myer L., Iyun V., Zerbe A., Phillips T.K., Brittain K., Mukonda E., Allerton J., Kalombo C.D., Nofemela A., Abrams E.J. Differentiated models of care for postpartum women on antiretroviral therapy in Cape Town, South Africa: a cohort study. J. Int. AIDS Soc. 2017;20(4):32–40. doi: 10.7448/IAS.20.5.21636. PMID: 28770593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.UNAIDS . UNAIDS; Geneva, Switzerland: 2017. UNAIDS Data 2017.https://www.unaids.org/sites/default/files/media_asset/20170720_Data_book_2017_en.pdf [Google Scholar]
- 19.Tsondai P.R., Wilkinson L.S., Grimsrud A., Mdlalo P.T., Ullauri A., Boulle A. High rates of retention and viral suppression in the scale-up of antiretroviral therapy adherence clubs in Cape Town, South Africa. J. Int. AIDS Soc. 2017;20(4):51–57. doi: 10.7448/IAS.20.5.21649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Myer L., Phillips T., Manuelli V., McIntyre J., Bekker L.-G., Abrams E.J. Evolution of antiretroviral therapy services for HIV-infected pregnant women in Cape Town, South Africa. J. Acquir. Immune Defic. Syndr. 2015;69(2):e57–e65. doi: 10.1097/QAI.0000000000000584. PMID: 24655651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Myer L., Phillips T.K., Zerbe A., Ronan A., Hsiao N.-Y., Mellins C.A., Remien R.H., Le Roux S.M., Brittain K., Ciaranello A., Petro G., McIntyre J.A., Abrams E.J. Optimizing antiretroviral therapy (ART) for maternal and child health (MCH): rationale and design of the MCH-ART study. J. Acquir. Immune Defic. Syndr. 2016;72(2):S189–S196. doi: 10.1097/QAI.0000000000001056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Myer L., Essajee S., Broyles L.N., Watts D.H., Lesosky M., El-Sadr W.M., Abrams E.J. Pregnant and breastfeeding women: a priority population for HIV viral load monitoring. PLoS Med. 2017;14(8) doi: 10.1371/journal.pmed.1002375. PMID: 28809929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Loudon K., Treweek S., Sullivan F., Donnan P., Thorpe K.E., Zwarenstein M. The PRECIS-2 tool: designing trials that are fit for purpose. BMJ. 2015;350 doi: 10.1136/bmj.h2147. [DOI] [PubMed] [Google Scholar]