Abstract
Background
This study aimed to investigate the effects of three-dimensional (3D) printed titanium (3DTi) scaffolds on osteogenic differentiation and new bone formation by 3D cultured adipose tissue-derived stem cells (ADSCs) in vitro, and the effects of bone regeneration in vivo using a full-thickness mandibular defect rat model, and the mechanisms involved.
Material/Methods
Alpha-beta titanium alloy (Ti6Al4V) 3DTi scaffolds were prepared with Cellmatrix hydrogel and 3D culture medium. ADSCs were impregnated into the 3DTi scaffolds. ADSC viability and proliferation were assessed using the cell counting kit-8 (CCK-8) assay, and alkaline phosphatase (ALP) levels were measured. Real-time polymerase chain reaction (RT-PCR) and Western blot were performed to assess the expression of osteogenesis-related mRNA for RUNX2, OPN, OCN, and IGF-1 genes and proteins. A rat model of full-thickness mandibular defect was evaluated with micro-computed tomography (microCT) scanning, and histochemistry with Alizarin red and von Giesen’s stain were used to evaluate osteogenesis.
Results
ADSC viability and proliferation were not affected by culture with 3DTi scaffolds. Expression of osteogenesis-related mRNA and proteins for RUNX2, OPN, OCN, and IGF-1, expression of ALP, and histochemical findings showed that the use of 3DTi scaffolds enhanced osteogenic differentiation and new bone formation by ADSCs, with upregulation of components of the IGF-1R/AKT/mTORC1 pathway.
Conclusions
The 3D culture of ADSCs with 3DTi scaffolds enhanced osteogenic differentiation and new bone formation through the IGF-1R/AKT/mTORC1 pathway. This improved method of osteointegration may have clinical application in the preparation of bone grafts before implantation for improved repair of mandibular bone defects.
MeSH Keywords: Bone Regeneration, Insulin-Like Growth Factor I, Mesenchymal Stromal Cells, Osteogenesis
Background
Worldwide, mandibular bone defects are an important public health issue. The ability of bone grafts to repair bone defects is enhanced using mesenchymal stem cells. The reconstruction of facial bone defects that result from infection, trauma, tumor, or surgery remains a significant challenge for maxillofacial surgeons, mainly when repairing the mandibular ramus. The mandibular nerve branches are required not only for daily eating but also for facial aesthetics. Traditional methods of repair, including autogenous and allogeneic bone grafts, are limited by complications at donor sites, risk of disease transmission, limited sources, and can lack an accurate fit with the defects [1]. Through the development of tissue engineering, 3D-printed titanium (3DTi) scaffolds have attracted recent attention because of their excellent biocompatibility and increased control of the macro-structure and micro-structure. 3DTi scaffolds have been widely used in plastic surgery and reconstructive surgery, for example, in the repair of osteonecrosis of the femoral head [2]. However, the poor bioactivity of titanium limits the application of 3DTi scaffolds, bone tissue may not grow into the scaffold. Therefore, the use of mesenchymal stem cell implantation in bone graft repair has recently been evaluated as an alternative approach [3].
Adipose tissue-derived stem cells (ADSCs) have several advantages for clinical use in bone graft repair, including their availability, low trauma associated with their use, and high their high content in tissues. Studies have shown that the proliferation and osteogenic effects of bone mesenchymal stem cells gradually decrease with age, while the osteogenic capacity of ADSCs does not [4]. Therefore, ADSCs show potential use in bone graft repair and may replace bone mesenchymal stem cells in bone regenerative medicine. However, during the culture of ADSCs in vitro, their differentiation occurs in a complex 3D environment, which is completely different from the traditional two-dimensional (2D) laboratory culture methods previously used [5]. The 3D culture conditions contribute to the functional differentiation of stem cells [5], and is more suitable for applications in tissue engineering and regenerative medicine. However, the osteogenic effects of ADSCs in the 3D culture environments and their potential mechanisms remain unknown.
Therefore, this study aimed to investigate the effects of three-dimensional (3D) printed titanium (3DTi) scaffolds on osteogenic differentiation and new bone formation by 3D cultured adipose-derived stem cells (ADSCs) in vitro, and the effects of bone regeneration in vivo using a full-thickness mandibular defect rat model, and the mechanisms involved.
Material and Methods
Three-dimensional printed titanium (3DTi) scaffolds
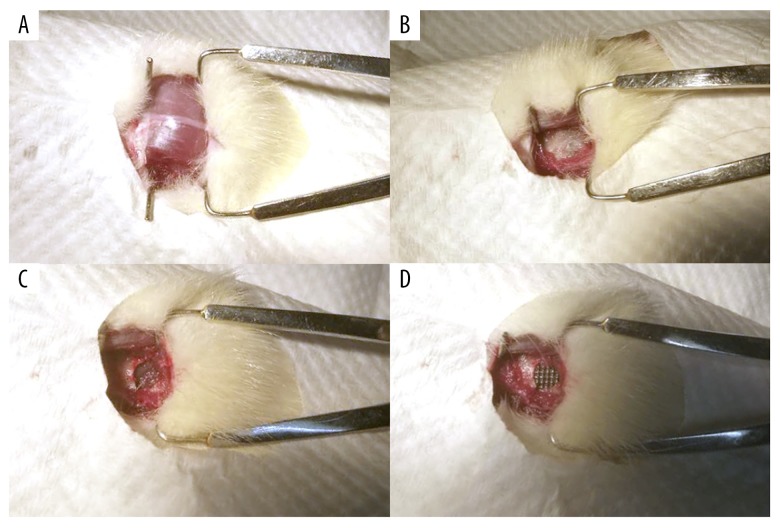
The 3DTi scaffolds were generated from the powdered form of the alpha-beta titanium alloy, Ti6Al4V, as previously described by He et al. [6] (Beijing ShapeDream Information Technology Co. Ltd, Beijing, China). The 3DTi scaffolds were designed with a diameter of 5 mm, a height of 1 mm, a strut width of 200 μm, and a 600 μm pore size, with >80% porosity, for use in animal experiments (Figure 1A). All scaffolds were prepared by selective laser melting and post-production heat treatment. The morphological characteristics of the scaffolds were evaluated by scanning electron microscopy (SEM) (Supplementary Figure 1).
Figure 1.
The three-dimensional printed titanium (3DTi) scaffold and the surgical procedure used for the rat mandibular defect model. The macroscopic appearance of the 3DTi scaffold (A). The incision in the skin of the rat model (B). The exposure of the rat mandibular ramus (C). The 5 mm full-thickness mandibular bone defect created in the rat model (D). The porous titanium scaffold implanted into the bone defect.
Cell culture of adipose tissue-derived stem cells (ADSCs)
Human adipose tissue-derived stem cells (ADSCs) were purchased from ScienCell Company (Carlsbad, CA, USA) and cultured in ADSC complete growth media (HUXMD-90011) (Cyagen, Santa Clara, CA, USA) supplemented with 10% fetal bovine serum, 1% penicillin-streptomycin, and glutamine. ADSCs were cultured at 37°C in saturated humidity containing 5% CO2. When the cells had grown to 70–80% confluence, they were digested with 0.25% trypsin without EDTA (Solarbio, Beijing, China).
3D cell culture in Cellmatrix collagen gel
ADSCs were cultured in a Cellmatrix Type I-A (631-00651) 3D collagen gel (Nitta Gelatin, Inc., Kerala, India), according to the manufacturer’s instructions. Cell pellets were collected by centrifugation and mixed with the Cellmatrix mixture solution (1×106 cells/mL). The Cellmatrix mixture was dispensed into culture plates (96-well or 6-well) and incubated at 37°C for 30 min to form a hydrogel. The hydrogel was overlaid with an appropriate volume of serum-free culture solution, and standard cell culture was performed. Images of the 3D culture of ADSCs are shown in Supplementary Figure 2.
Cell viability and proliferation
After 7 days of culture in the 2D plates or 3D hydrogel, the cells were evaluated with the LIVE/DEAD® Viability/Cytotoxicity assay kit (L3224) (Invitrogen, Carlsbad, CA, USA). After incubation at 37°C for 30 min in the dark, the cells were imaged by fluorescence microscopy with and 80i Eclipse fluorescence microscope (Nikon, Tokyo, Japan).
The proliferation rates of cells cultured in the 2D or 3D culture medium were detected with a cell counting kit-8 (CCK-8) assay kit (Dojindo, Kumamoto, Japan) after the ADSCs were cultured in the hydrogel for 1, 3, 5, or 7 days. ADSCs cultured on 2D plates served as controls. The absorbance of the supernatant was detected at 450 nm with a microplate reader (Thermo Fisher Scientific, Waltham, MA, USA).
Histochemical staining with Alizarin red
ADSCs were cultured in Cellmatrix (3D group) or in the multi-well plates (2D group) to investigate the osteogenic effect of 3D culture. In the 2D group, ADSCs were cultured with either normal culture medium (2D-N group) or osteogenic medium (2D-O group). After culture for 14 days, all ADSCs were fixed in 70% ice-cold ethanol for 30 min and rinsed with double-distilled H2O2 and then stained with 0.1% Alizarin Red S (Sigma-Aldrich, St. Louis, MO, USA) to identify calcium deposits. The bound stain was eluted using 10% acetic acid (Sigma-Aldrich, St. Louis MO, USA) to quantify the orange-red staining of Alizarin red, followed by neutralization with 10% ammonium hydroxide (Sigma-Aldrich, St. Louis MO, USA). The optical density at 405 nm was analyzed with a microplate reader (Thermo Fisher Scientific, Waltham, MA, USA).
Measurement of alkaline phosphatase (ALP) levels
Alkaline phosphatase (ALP) was measured using a commercial kit (Beyotime, Jiangsu, China). After 7 days of culture, all ADSCs were lysed by 0.1% Triton X-100 and Tris-HCl (10 mM, pH 7.4) for 2 h at 4°C. Then, p-nitrophenyl phosphate and lysate were mixed and incubated at 37°C for 15 min. The optical densities at 405 nm were quantified using a microplate reader (Thermo Fisher Scientific, Waltham, MA, USA).
Real-time polymerase chain reaction (RT-PCR)
RT-PCR was used to quantify the mRNA expression of the osteogenic genes, RUNX2, OPN, and OCN in ADSCs after 7 days and 14 days of incubation. Total mRNA was extracted from ADSCs with TRIzol reagent (Invitrogen, Carlsbad, CA, USA), and reversed-transcribed into cDNA using a cDNA synthesis kit (Thermo Fisher Scientific, Waltham, MA, USA). The primer sequences of selected genes are listed in Table 1. The expression levels of osteoblastic markers were calculated based on the 2−ΔΔCt method by normalizing the values to those of the housekeeping gene, GAPDH. RT-PCR was performed to evaluate the mRNA expression levels of the insulin-like growth factor-1 (IGF-1) gene. IGF-1 was evaluated because it plays an essential role in bone regeneration under stress, and the hydrogel could provide some stress to ADSCs in the 3D cultured group, and so the 2D and 3D cultures were evaluated with osteogenic media (the 2D-O group and 3D group).
Table 1.
Primer pair sequences.
| Gene | Sequences, (5′-3′) | Annealing temperature (°C) | |
|---|---|---|---|
| Forward | Reverse | ||
| GAPDH | GACTTCAACAGCAACTCCCAC | TCCACCACCCTGTTGCTGTA | 58 |
| RUNX2 | TTCTCCAACCCACGAATGCAC | CAGGTACGTGTGGTAGTGAGT | 58 |
| OPN | ATCTCCTAGCCCCACAGACCC | CACACTATCACCTCGGCCATC | 58 |
| OCN | CTCACACTCCTCGCCCTATTG | CGCCTGGGTCTCTTCACTAC | 58 |
| IGF-1 | GTGTTGCTTCCGGAGCTGTG | CAAATGTACTTCCTTCTGAGTC | 58 |
RUNX2 – runt-related transcription factor 2; OPN – osteopontin; OCN – osteocalcin; IGF-1 – insulin-like growth factor-1.
Western blot
ADSCs were harvested and lysed in lysis buffer (Solarbio, Beijing, China) after culture for 14 days. Total protein levels were quantified with a bicinchoninic acid (BCA) kit (Solarbio, Beijing, China). Equal amounts of protein were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to polyvinylidene fluoride (PVDF) membranes. The membranes were incubated overnight with primary antibodies to runt-related transcription factor 2 (RUNX2) (1: 1000) (Abcam, Cambridge, UK), osteopontin (OPN) (1: 1000) (Abcam, Cambridge, UK), and osteocalcin (OCN) (1: 500) (Abcam, Cambridge, UK). GAPDH was used as the control. The blots were detected using an enhanced chemiluminescent (ECL) kit (Invitrogen, Carlsbad, CA, USA) and quantified with Quantity One software (Bio-Rad, Hercules, CA, USA).
Based on the RT-PCR results for IGF-1, Western blot was performed to evaluate the levels of proteins downstream of IGF-1, including, IGF-1R (1: 1000) Abcam, Cambridge, UK), phosphorylated (p) IGF-1R (1: 1000) (Abcam, Cambridge, UK), AKT (1: 500) (Abcam Cambridge, UK), pAKT (1: 500) (Abcam, Cambridge, UK), S6 (1: 10000) (Abcam, Cambridge, UK), and pS6 (1: 10000) (Abcam, Cambridge, UK).
Enzyme-linked immunosorbent assay (ELISA)
An enzyme-linked immunosorbent assay (ELISA) was performed to measure the insulin-like growth factor (IGF-1) protein content in the medium, using a commercially available ultrasensitive ELISA kit (ab100545; Abcam, Cambridge, UK), according to the manufacturer’s instructions.
The rat model of full-thickness mandibular defect
Sprague-Dawley (SD) rats (n=9) at 8 weeks of age were obtained from the Peking Union Medical College Hospital (PUMCH). All animal experiments were approved by the Institutional Animal Care and Use Committee of PUMCH. In the model, 5 mm full-thickness standardized mandibular ramus defects were made surgically in each SD rat to examine the influence of 3D cultured ADSCs on bone repair (Figure 1). All rats were randomly divided into three groups: the Control group, in which only 3D printed titanium alloy scaffolds were applied to the defect; the SC group, in which 250 μL of phosphate-buffered saline (PBS) containing 1×106 of ADSCs were injected into the scaffold after implantation; and the SCH group, in which Cellmatrix hydrogel containing 1×106 ADSCs was impregnated into the 3D printed titanium alloy scaffolds before implantation.
Micro-computed tomography (microCT) scanning
The mandible from each SD rat was harvested at 12 weeks postoperatively to track new bone formation. All samples were scanned using a microCT system (Bruker, Billerica, MA, USA). The bone volume/total volume (BV/TV) ratio was calculated using Materialise Mimics software (Materialise, Leuven, Belgium) to determine the degree of bone regeneration.
Histological analysis
Tissues from the bone defect area of the rat mandible were sectioned at a thickness of 50 μm and underwent van Gieson’s histochemical staining that included 1.2% trinitrophenol solution and 1% acid fuchsin solution. The stained sections were imaged using light microscopy. The newly formed scaffolds and bone were identified as black and red areas within the tissue sections. The proportion of bone tissue was calculated with ImageJ software (NIH, Bethesda, MD, USA).
Statistical analysis
All experiments were performed in triplicate. Data were expressed as the mean±standard deviation (SD). Student’s t-test and one-way analysis of variance (ANOVA) were used to compare the differences between groups. A p-value <0.05 was considered to be statistically significant.
Results
Culture in three-dimensional (3D) printed titanium (3DTi) scaffolds did not affect the viability or proliferation rate of adipose tissue-derived stem cells (ADSCs)
Adipose tissue-derived stem cells (ADSCs) were studied in the three groups, including the 2D-N group (ADSCs cultured in standard culture medium), the 2D-O group (ADSCs cultured in osteogenic medium), and the 3D group (ADSCs cultured in Cellmatrix). The cell viability of ADSCs in 2D and 3D culture was assessed by the LIVE/DEAD® Viability/Cytotoxicity assay. Live and dead cells showed green and red fluorescence, respectively. As shown in Figure 2A and 2B, almost no dead cells were observed in either group, and no significant differences were found between the 2D and 3D culture groups (Figure 2C). A cell counting kit-8 (CCK-8) assay was used to evaluate the proliferation rate of ADSCs on days 1, 3, 5, and 7 (Figure 2D). The cell number steadily increased until 7 days in the two groups, and similar cell proliferation rates were observed at each time point. These results indicated that the cell viability and proliferation of ADSCs were not affected by 3D culture.
Figure 2.
The results of the LIVE/DEAD® Viability/Cytotoxicity assay of adipose tissue-derived stem cells (ADSCs). The ADSCs cultured in the two-dimensional (2D) plate (A), and in three-dimensional (3D) hydrogel (B) (Scale bars, 100 μm). The quantitative results of the LIVE/DEAD® Viability/Cytotoxicity assay (C). Quantitative analysis of the ADSC proliferation rate using the cell counting kit-8 (CCK-8) assay during 7 days (D).
Culture with 3DTi scaffolds enhanced osteogenic differentiation of ADSCs, including alkaline phosphatase (ALP) activity and Alizarin red staining
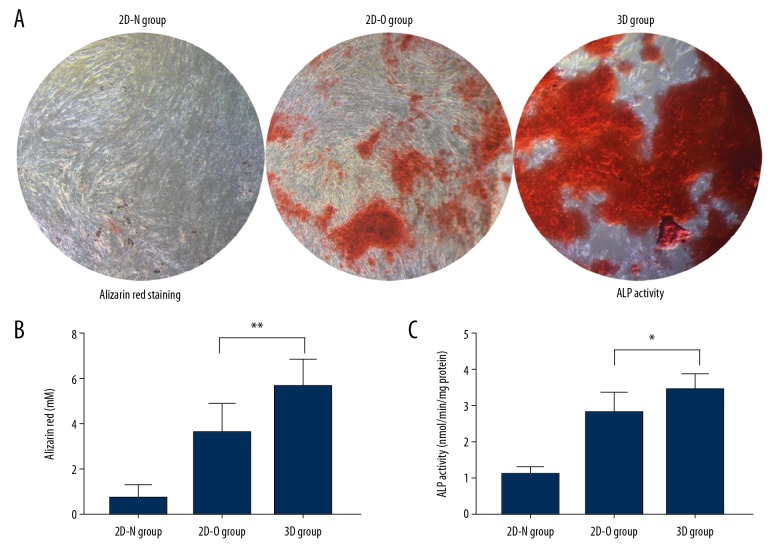
After incubation in 2D culture plates or 3D hydrogel for 14 days, Alizarin red was used to detect the calcium deposition in ADSCs. As shown in Figure 3A, the deposited mineralized matrix stained red. Quantitative analysis of the newly formed calcium deposits was performed by colorimetric detection (Figure 3B). There were substantial increases in calcium deposits in the 3D group, indicating that 3D culture increased the osteogenic potential of ADSCs.
Figure 3.
Adipose tissue-derived stem cell (ADSC) mineralization was assessed by Alizarin red staining for calcium and measurement of alkaline phosphatase (ALP) levels. ADSCs cultured in different environments were stained with Alizarin red after 14 days (A) and quantified (B) in the 2D-N group (ADSCs cultured in standard culture medium), the 2D-O group (ADSCs cultured with osteogenic medium), and the 3D group (ADSCs cultured in Cellmatrix). ALP levels in the ADSCs in the three groups after 7 days of incubation are shown (C). * p<0.05, ** p<0.01.
Because ALP activity plays an important role in the matrix mineralization process, the bone differentiation capability of ADSCs was evaluated by measuring ALP activity at day 7. The ALP activity in the 3D group was significantly increased compared with 2D-N and 2D-O groups on days 7 (p <0.05) (Figure 3C). This result indicated that 3D culture promoted osteogenic differentiation of ADSCs.
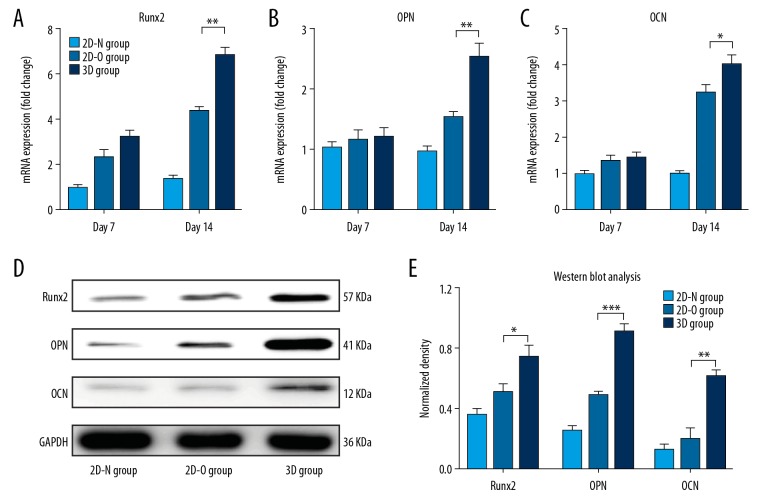
Real-time polymerase chain reaction (RT-PCR) and Western blot
Real-time polymerase chain reaction (RT-PCR) and Western blot were performed to assess the expression of osteogenesis-related mRNA and proteins, respectively. The mRNA expression levels gradually increased over time from day 7 to day 14. At day 7, osteocalcin (OCN) and osteopontin (OPN) were not significantly increased in the 3D group. However, all mRNAs, including runt-related transcription factor 2 (RUNX2), OCN, and OPN, showed significantly increased in the 3D group compared with the 2D groups after 14 days of culture (p<0.05) (Figure 4A–4C). On day 14, the levels of osteogenesis-related proteins, Runx2, OPN, and OCN were also significantly increased in the 3D group compared with the 2D group (p<0.05) (Figure 4D, 4E). These results confirmed the osteogenic ability of ADSCs in the 3D hydrogel. Also, the osteogenic potential of ADSCs in 3DTi scaffolds in vitro was also evaluated and the results corresponded with osteogenesis-related mRNA expression of ADSCs without 3DTi scaffolds (Supplementary Figure 3).
Figure 4.
Real-time polymerase chain reaction (RT-PCR) and Western blot to evaluate the expression levels of osteogenic mRNAs and proteins. The expression of mRNA for the runt-related transcription factor 2 (RUNX2) gene (A), the osteopontin (OPN) gene (B), and the osteocalcin (OCN) gene (C) in adipose tissue-derived stem cells (ADSCs) in the three groups, the 2D-N group (ADSCs cultured in standard culture medium), the 2D-O group (ADSCs cultured with osteogenic medium), and the 3D group (ADSCs cultured in Cellmatrix), was quantified by RT-PCR. The osteogenic protein expression for Runx2, OPN, and OCN was detected by Western blot (D). The density of proteins in each group is shown (E). * p<0.05, ** p<0.01, *** p<0.001.
3D culture enhanced osteogenic differentiation through the IGF-1R/AKT/mTORC1 pathway
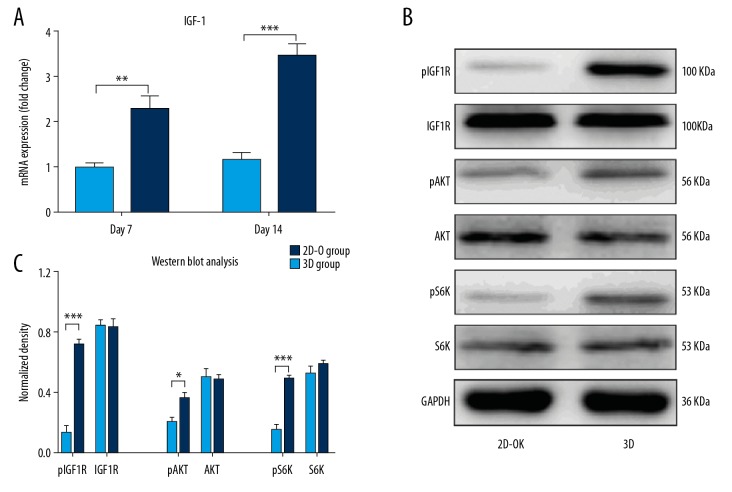
Insulin-like growth factor-I (IGF-1) plays an important role in the development of the skeleton and the healing of fractures associated with stress [7,8]. It has previously been shown that skeletal unloading significantly reduced IGF-1 expression levels [9]. Therefore, the Cellmatrix hydrogel was used to provide loading onto the ADSCs during 3D culture. The results showed that the IGF-1 protein levels in the supernatant were highly expressed in the 3D culture environment (Supplementary Figure 4). It has been previously shown that after IGF-1 is released, it combines with its receptor, IGF-1R, on the cell membrane and then activates the downstream mTORC1 signaling pathway to promote osteogenesis [10]. In this study, the expression levels of IGF-1 mRNA were assessed by real-time polymerase chain reaction (RT-PCR), which showed that the concentration of IGF-1 in the 3D group gradually increased with the incubation time. There were no changes between different time points (Figure 5A).
Figure 5.
Real-time polymerase chain reaction (RT-PCR) and Western blot analysis of insulin-like growth factor-1 (IGF-1) mRNA in adipose tissue-derived stem cells (ADSCs) and the expression of the downstream proteins involved in the IGF-1R/AKT/mTORC1 signaling pathway in the 2D-O group (ADSCs cultured with osteogenic medium) and 3D group (ADSCs cultured in Cellmatrix). The IGF-1 mRNA expression at days 7 and 14 was quantified by RT-PCR (A). Expression of pIGF-1R, IGF-1R, pAKT, AKT, pS6K, and S6K in adipose tissue-derived stem cells (ADSCs) was determined by Western blot after 14 days of culture (B). Quantitative analysis of the Western blot results and comparison between the two groups (C). * p<0.05, ** p<0.01, and *** p<0.001.
The protein expression levels of the IGF-1R/AKT/mTORC1 signaling pathway molecules were measured by Western blot to explore the mechanisms of osteogenesis in this study further. The results showed that the protein levels of pIGF-1R, pAKT, and pS6 were significantly increased in the 3D group but not in the 2D group (Figure 5B, 5C). Therefore, the 3D culture of ADSCs enhanced osteogenesis by activating the IGF-1R/AKT/mTORC1 signaling pathway.
3D culture enhanced bone growth in the 3D-printed alpha-beta titanium alloy, Ti6Al4V, scaffolds in vivo
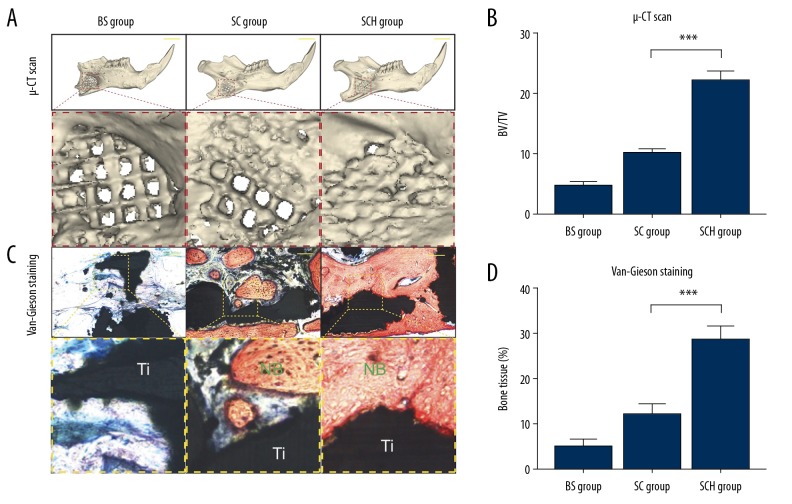
At 12 weeks following surgery, micro-computed tomography (microCT) and histology were performed to detect the newly formed bone within the scaffolds. As shown in Figure 6A, the Control group showed almost no bone formation, and the SC group showed only minor new bone formation. However, microCT scanning showed that newly formed bone and scaffold were well-integrated in the SCH group. Quantitative analysis showed that the bone volume/total volume (BV/TV) ratio was highest in the SCH group (p <0.001) (Figure 6B). Histology supported the findings from microCT imaging. Only fibrous tissue was observed in the Control group. However, in the SCH group, the 3DTi scaffold was filled with newly formed bone, and the amount of new bone formation was greatest in the SCH group, as shown by Van-Gieson staining (p <0.001) (Figure 6C, 6D). No complications were observed in any of the animals used in the in vivo experiments.
Figure 6.
A rat model of full-thickness mandibular defect evaluated by micro-computed tomography (microCT) scanning and van Gieson’s histochemical staining. The volume of regenerated bone evaluated by ex vivo microCT scans at 12 weeks (A). Scale bars, 5 mm. The bone volume/total volume (BV/TV) ratio was calculated using Materialise Mimics software (B). Histological analysis of the osteogenesis and osteointegration in the three groups by van Gieson’s histochemical staining at 12 weeks (C). Scale bars, 100 mm. Adipose tissue-derived stem cells (ADSCs) were studied in the three groups, including the 2D-N group (ADSCs cultured in standard culture medium), the 2D-O group (ADSCs cultured in osteogenic medium), and the 3D group (ADSCs cultured in Cellmatrix). Quantitative analysis of newly formed bone among three groups (D). NB – new bone formation; Ti – 3D-printing scaffold. *** p<0.001.
Discussion
A three-dimensional (3D) culture environment can promote the osteogenic differentiation of adipose tissue-derived stem cells (ADSCs) induced by dexamethasone in vitro [11]. However, traditional two-dimensional (2D) culture involves seeding cells into plastic plates, which greatly reduces their differentiation potential [12]. In the present study, Cellmatrix hydrogel was used to load ADSCs into the 3D culture medium. The 3D culture technique for ADSCs did not affect stem cell viability and proliferation, as the culture conditions in Cellmatrix mimicked physiological conditions found in vivo, which may enhance adhesion and communication between cells [13].
Interactions between the extracellular matrix (ACM) and neighboring cells define cellular behaviors, including survival, differentiation, and proliferation [14]. In the present study, ADSCs were investigated in three groups, including the 2D-N group (ADSCs cultured in standard culture medium), the 2D-O group (ADSCs cultured in osteogenic medium), and the 3D group (ADSCs cultured in Cellmatrix). After culturing the cells for 14 days, Alizarin red staining showed more mineralized deposits from ADSCs in the 3D cell culture group than in the 2D culture group. The levels of alkaline phosphatase (ALP), an osteogenic marker [15], were also promoted by 3D culture. Real-time polymerase chain reaction (RT-PCR) and Western blot were performed to assess the expression of osteogenesis-related mRNA for RUNX2, OPN, OCN, and IGF-1 genes and proteins, and the findings supported osteogenesis associated with the ADSCs, with increased expression in the 3D culture environment. These findings are supported by the results from previously published studies that showed increased expression of osteogenic factors and ALP in osteoblast-like MC3T3-E1 and MG63 cells in the 3D culture associated with increased gone mineral deposits [16]. Similar findings were previously reported using SaOS2, a human osteosarcoma cell line, where the expression levels of markers of early osteoblastic differentiation markers such as Runx2 and ALP were increased [17].
A 3D cell culture environment for ADSCs may promote osteogenesis through the IGF-1R/AKT/mTROC1 signal pathway. IGF-1 is an important growth factor involved in the process of bone healing, which acts synergistically. Also, IGF-1 modulates skeletal growth through paracrine and autocrine mechanisms [18]. Several cell types release IGF-1 under conditions of mechanical loading [19]. The Cellmatrix hydrogel was used in the present study because it provided mechanical loading during 3D culture. Therefore, this study investigated the expression levels of IGF-1 and activation of IGF-1R/AKT/mTORC1 signaling pathways (Figure 7). In the 3D culture environment, IGF-1 was released into the matrix by ADSCs, where it bound to IGF-1R, which may further activate mTORC1 signaling and initiate its downstream physiological functions [20]. Also, mTORC1 has previously been reported to play a critical role in bone homeostasis [21]. The expression levels of pIGF-1R, pAKT, and pS6K, a specific downstream protein of mTORC1, were found to be increased under the culture conditions used in this study. Previous studies showed cross-talk between the integrin and IGF-I signaling pathways to enhance bone formation in response to mechanical loading [22]. These data suggest that high expression levels of IGF-1 upregulate the phosphorylation of IGF-1R and activate its downstream mTORC1 pathway, thereby confirming the involvement of the IGF-1R/AKT/mTORC1 signaling pathway in the 3D culture environment of ADSCs.
Figure 7.
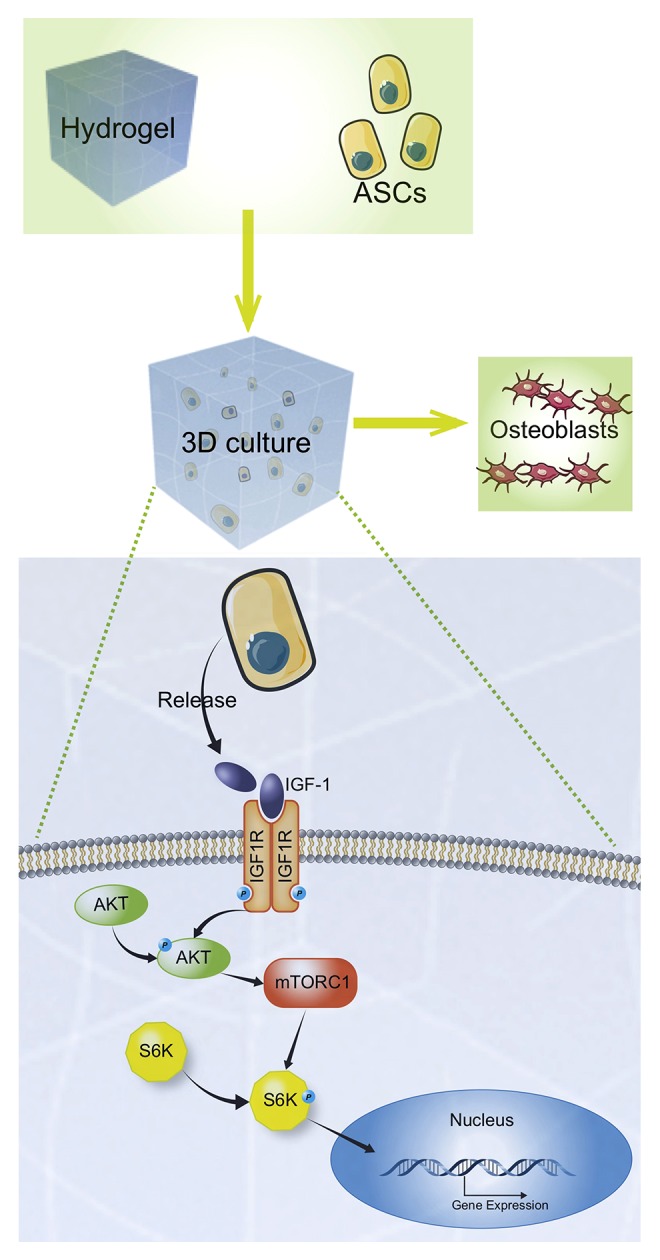
Potential mechanism of osteogenesis associated with the culture of adipose tissue-derived stem cells (ADSCs) using the three-dimensional (3D) printed titanium (3DTi) scaffolds. Cells produced insulin-like growth factor-1 (IGF-1) that was released into the matrix and bound to IGF-1R on the cell membrane, which further activated the downstream AKT/mTORC1 signaling pathway to enhance gene expression.
The alpha-beta titanium alloy, Ti6Al4V, is one of the most promising candidates for use in the scaffold among the metal alloys due to the low cytotoxicity and mechanical properties of Ti6Al4V. The 3D titanium alloy (3DTi) scaffold has an interconnected porous architecture, which provides the necessary space for bone ingrowth and angiogenesis and can provide sufficient mechanical support [23]. The 3DTi scaffold cannot be degraded, and its excellent biocompatibility has been previously described [24]. However, the bio-inert properties of Ti6Al4V significantly limit its applications in orthopedic procedures, and 3DTi scaffolds used alone have little osteogenic capability [6]. Previous studies have used hydrogel/titanium micro hybrids to improve bone integration [25].
Therefore, the present study aimed to investigate whether 3D cultured ADSCs promoted bone grown into the 3DTi scaffold in vivo. Also, a 5 mm critical size mandibular bone defect rat model was established, which could not spontaneously heal with bone grafting [26]. The ADSCs were cultured in the Cellmatrix hydrogel before implantation. The findings from the in vivo study in the rat model showed that in the SCH group, in which Cellmatrix hydrogel containing ADSCs were impregnated into the 3DTi scaffolds before implantation, there was improved new bone formation when compared with other study groups. Previous studies showed that stem cell injected grafting method significantly reduced the survival rate of cells due to acute inflammation or oxidative stress [27], and the incorporation of the surrounding muscle into the scaffolds also restricted bone regeneration. Chen et al. [28] reported that hypoxic conditions could improve the survival of mesenchymal stem cells and improved osteogenesis in vivo, and the hydrogel microenvironment is moderately hypoxic. Cellmatrix contains Type I collagen, which has previously been reported to induce osteogenic differentiation of mesenchymal stem cells [29]. Hydrogel can retain the viability of mesenchymal stem cells and increase the osteogenic potential of ADSCs. In the present study, a rat model with a full-thickness mandibular defect was used, which was evaluated with micro-computed tomography (microCT) scanning and histological analysis. In this in vivo model, mandibular bone defects implanted with 3D cultured ADSCs combined with the 3DTi scaffold showed more mineralized bone tissue formation in the scaffolds when compared with the acellular scaffolds (in the Control group) and the scaffold impregnated with ADSCs alone (the SC group).
Conclusions
This study aimed to investigate the effects of three-dimensional (3D) printed titanium (3DTi) scaffolds on osteogenic differentiation and new bone formation by 3D cultured adipose tissue-derived stem cells (ADSCs) in vitro, and the effects of bone regeneration in vivo using a full-thickness mandibular defect rat model, and the mechanisms involved. The use of 3DTi scaffolds enhanced osteogenic differentiation and new bone formation by ADSCs through the IGF-1R/AKT/mTORC1 pathway. The use of 3D culture conditions of ADSCs combined with the 3DTi scaffold was shown to improve osteointegration and may have a clinical role in improving bone grafts before implantation for the repair of mandibular bone defects.
Supplementary Data
The morphological characteristics of scaffolds were evaluated by scanning electron microscope (SEM).
Representation of passage 4 of the adipose tissue-derived stem cells (ADSCs). The hydrogel three-dimensional (3D) culture (A), with the three-dimensional (3D) printed titanium (3DTi) scaffold (B). (Scale bar: 100 μm).
The osteogenic potential of adipose tissue-derived stem cells (ADSCs) in three-dimensional (3D) printed titanium (3DTi) scaffolds in vitro was evaluated at 14 days. The mRNA expression levels of the RUNX2, OPN, and OCN genes in ADSCs in the three groups were quantified by real-time polymerase chain reaction (RT-PCR). Adipose tissue-derived stem cells (ADSCs) were studied in the three groups, including the 2D-N group (ADSCs cultured in standard culture medium), the 2D-O group (ADSCs cultured in osteogenic medium), and the 3D group (ADSCs cultured in Cellmatrix).
Enzyme-linked immunosorbent assay (ELISA) detection of secreted IGF-1 in the three study groups at 3 days and 7 days of culture. Adipose tissue-derived stem cells (ADSCs) were studied in the three groups, including the 2D-N group (ADSCs cultured in standard culture medium), the 2D-O group (ADSCs cultured in osteogenic medium), and the 3D group (ADSCs cultured in Cellmatrix).
Footnotes
Conflict of interest
None.
Source of support: This study was supported by the funds from the Guangzhou Institute of Pediatrics/Guangzhou Women and Children’s Medical Center (No. IP-2019-023) and the Zhongwen Wang Academic Experience Heritage Studio Special Fund of Guangdong Provincial Hospital of Traditional Chinese Medicine (The Second Affiliated Hospital of Guangzhou University of Chinese Medicine) (2018. No.7)
References
- 1.Larsson L, Decker AM, Nibali L, et al. Regenerative medicine for periodontal and peri-implant diseases. J Dent Res. 2016;95:255–66. doi: 10.1177/0022034515618887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhu W, Zhao Y, Ma Q, et al. 3D-printed porous titanium changed femoral head repair growth patterns: Osteogenesis and vascularisation in porous titanium. J Mater Sci Mater Med. 2017;28:62. doi: 10.1007/s10856-017-5862-2. [DOI] [PubMed] [Google Scholar]
- 3.Shou K, Huang Y, Qi B, et al. Induction of mesenchymal stem cell differentiation in the absence of soluble inducer for cutaneous wound regeneration by a chitin nanofiber-based hydrogel. J Tissue Eng Regen Med. 2018;12:e867–80. doi: 10.1002/term.2400. [DOI] [PubMed] [Google Scholar]
- 4.Chen HT, Lee MJ, Chen CH, et al. Proliferation and differentiation potential of human adipose-derived mesenchymal stem cells isolated from elderly patients with osteoporotic fractures. J Cell Mol Med. 2012;16:582–93. doi: 10.1111/j.1582-4934.2011.01335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McKee C, Chaudhry GR. Advances and challenges in stem cell culture. Colloids Surf B Biointerfaces. 2017;159:62–77. doi: 10.1016/j.colsurfb.2017.07.051. [DOI] [PubMed] [Google Scholar]
- 6.He Y, Yu L, Liu J, et al. Enhanced osteogenic differentiation of human bone-derived mesenchymal stem cells in 3-dimensional printed porous titanium scaffolds by static magnetic field through up-regulating Smad4. FASEB J. 2019;33(5):6069–81. doi: 10.1096/fj.201802195R. [DOI] [PubMed] [Google Scholar]
- 7.Tian F, Wang Y, Bikle DB. IGF-1 signaling mediated cell-specific skeletal mechano-transduction. J Orthop Res. 2018;36:576–83. doi: 10.1002/jor.23767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yan B, Huang M, Zeng C, et al. Locally produced IGF-1 promotes hypertrophy of the ligamentum flavum via the mTORC1 signaling pathway. Cell Physiol Biochem. 2018;48:293–303. doi: 10.1159/000491729. [DOI] [PubMed] [Google Scholar]
- 9.Drissi H, Lomri A, Lasmoles F, et al. Skeletal unloading induces biphasic changes in insulin-like growth factor-I mRNA levels and osteoblast activity. Exp Cell Res. 1999;251:275–84. doi: 10.1006/excr.1999.4539. [DOI] [PubMed] [Google Scholar]
- 10.Reijnders CM, Bravenboer N, Tromp AM, et al. Effect of mechanical loading on insulin-like growth factor-I gene expression in rat tibia. J Endocrinol. 2007;192:131–40. doi: 10.1677/joe.1.06880. [DOI] [PubMed] [Google Scholar]
- 11.Zhang Q, Lin S, Liao J, Cai X. Physical cues drive chondrogenic differentiation. Curr Stem Cell Res Ther. 2018;13(7):576–82. doi: 10.2174/1574888X13666180102121455. [DOI] [PubMed] [Google Scholar]
- 12.Gupta D, Grant DM, Zakir Hossain KM, et al. Role of geometrical cues in bone marrow-derived mesenchymal stem cell survival, growth and osteogenic differentiation. J Biomater Appl. 2018;32:906–19. doi: 10.1177/0885328217745699. [DOI] [PubMed] [Google Scholar]
- 13.Baker BM, Chen CS. Deconstructing the third dimension: How 3D culture microenvironments alter cellular cues. J Cell Sci. 2012;125:3015–24. doi: 10.1242/jcs.079509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weber GF, Bjerke MA, DeSimone DW. Integrins and cadherins join forces to form adhesive networks. J Cell Sci. 2011;124:1183–93. doi: 10.1242/jcs.064618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arvidson K, Abdallah BM, Applegate LA, et al. Bone regeneration and stem cells. J Cell Mol Med. 2011;15:718–46. doi: 10.1111/j.1582-4934.2010.01224.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Honda M, Hariya R, Matsumoto M, Aizawa M. Acceleration of osteogenesis via stimulation of angiogenesis by combination with scaffold and connective tissue growth factor. Materials (Basel) 2019;12(13) doi: 10.3390/ma12132068. pii: E2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bedouin Y, Pellen Mussi P, Tricot-Doleux S, et al. 3D cell culture to determine in vitro biocompatibility of bioactive glass in association with chitosan. Biomed Mater Eng. 2015;26:169–81. doi: 10.3233/BME-151555. [DOI] [PubMed] [Google Scholar]
- 18.Yakar S, Isaksson O. Regulation of skeletal growth and mineral acquisition by the GH/IGF-1 axis: Lessons from mouse models. Growth Horm IGF Res. 2016;28:26–42. doi: 10.1016/j.ghir.2015.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li P, Liang ML, Zhu Y, et al. Resveratrol inhibits collagen I synthesis by suppressing IGF-1R activation in intestinal fibroblasts. World J Gastroenterol. 2014;20:4648–61. doi: 10.3748/wjg.v20.i16.4648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bakker AD, Gakes T, Hogervorst JM, et al. Mechanical stimulation and IGF-1 enhance mRNA translation rate in osteoblasts via activation of the AKT-mTOR pathway. J Cell Physiol. 2016;231:1283–90. doi: 10.1002/jcp.25228. [DOI] [PubMed] [Google Scholar]
- 21.Shen G, Ren H, Qiu T, et al. Mammalian target of rapamycin as a therapeutic target in osteoporosis. J Cell Physiol. 2018;233:3929–44. doi: 10.1002/jcp.26161. [DOI] [PubMed] [Google Scholar]
- 22.Kapur S, Mohan S, Baylink DJ, Lau KH. Fluid shear stress synergizes with insulin-like growth factor-I (IGF-I) on osteoblast proliferation through integrin-dependent activation of IGF-I mitogenic signaling pathway. J Biol Chem. 2005;280:20163–70. doi: 10.1074/jbc.M501460200. [DOI] [PubMed] [Google Scholar]
- 23.Tsai CH, Hung CH, Kuo CN, et al. Improved bioactivity of 3D printed porous titanium alloy scaffold with chitosan/magnesium-calcium silicate composite for orthopaedic applications. Materials (Basel) 2019;12(2) doi: 10.3390/ma12020203. pii: E203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ran Q, Yang W, Hu Y, et al. Osteogenesis of 3D printed porous Ti6Al4V implants with different pore sizes. J Mech Behav Biomed Mater. 2018;84:1–11. doi: 10.1016/j.jmbbm.2018.04.010. [DOI] [PubMed] [Google Scholar]
- 25.Koenig G, Ozcelik H, Haesler L, et al. Cell-laden hydrogel/titanium microhybrids: Site-specific cell delivery to metallic implants for improved integration. Acta Biomater. 2016;33:301–10. doi: 10.1016/j.actbio.2016.01.023. [DOI] [PubMed] [Google Scholar]
- 26.Zhang W, Zhang Z, Chen S, et al. Mandibular jaw bone regeneration using human dental cell-seeded tyrosine-derived polycarbonate scaffolds. Tissue Eng Part A. 2016;22:985–93. doi: 10.1089/ten.tea.2016.0166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Uemura M, Refaat MM, Shinoyama M, et al. Matrigel supports survival and neuronal differentiation of grafted embryonic stem cell-derived neural precursor cells. J Neurosci Res. 2010;88:542–51. doi: 10.1002/jnr.22223. [DOI] [PubMed] [Google Scholar]
- 28.Chen J, Yang Y, Shen L, et al. Hypoxic preconditioning augments the therapeutic efficacy of bone marrow stromal cells in a rat ischemic stroke model. Cell Mol Neurobiol. 2017;37:1115–29. doi: 10.1007/s10571-016-0445-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kihara T, Hirose M, Oshima A, Ohgushi H. Exogenous type I collagen facilitates osteogenic differentiation and acts as a substrate for mineralization of rat marrow mesenchymal stem cells in vitro. Biochem Biophys Res Commun. 2006;341:1029–35. doi: 10.1016/j.bbrc.2006.01.059. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The morphological characteristics of scaffolds were evaluated by scanning electron microscope (SEM).
Representation of passage 4 of the adipose tissue-derived stem cells (ADSCs). The hydrogel three-dimensional (3D) culture (A), with the three-dimensional (3D) printed titanium (3DTi) scaffold (B). (Scale bar: 100 μm).
The osteogenic potential of adipose tissue-derived stem cells (ADSCs) in three-dimensional (3D) printed titanium (3DTi) scaffolds in vitro was evaluated at 14 days. The mRNA expression levels of the RUNX2, OPN, and OCN genes in ADSCs in the three groups were quantified by real-time polymerase chain reaction (RT-PCR). Adipose tissue-derived stem cells (ADSCs) were studied in the three groups, including the 2D-N group (ADSCs cultured in standard culture medium), the 2D-O group (ADSCs cultured in osteogenic medium), and the 3D group (ADSCs cultured in Cellmatrix).
Enzyme-linked immunosorbent assay (ELISA) detection of secreted IGF-1 in the three study groups at 3 days and 7 days of culture. Adipose tissue-derived stem cells (ADSCs) were studied in the three groups, including the 2D-N group (ADSCs cultured in standard culture medium), the 2D-O group (ADSCs cultured in osteogenic medium), and the 3D group (ADSCs cultured in Cellmatrix).