Abstract
Actinidia chinensis Planch. (A. chinensis), commonly known as Chinese kiwifruit, is a China native fruit, which becomes increasingly popular due to attractive economic, nutritional, and health benefits properties. The whole plant including fruits, leaves, vines, and roots of A. chinensis are used mainly as food or additive in food products and as folk medicine in China. It is a good source of triterpenoids, polyphenols, vitamin C, carbohydrate, amino acid, and minerals. These constituents render the A. chinensis with a wide range of pharmacological properties including antitumor, antioxidant, anti-inflammatory, immunoregulatory, hypolipemic, antidiabetic, and cardiovascular protective activities, suggesting that it may possibly be value in the prevention and treatment of pathologies associated to cancer, oxidative stress, and aging. This minireview provides a brief knowledge about the recent advances in chemistry, biological activities, utilization, and storage of Chinese kiwifruit. Future research directions on how to better use of this crop are suggested.
Keywords: Actinidia chinensis, nutritional composition, chemistry, pharmacological properties, antitumor, antioxidant
Introduction
Actinidia chinensis Planch. (A. chinensis), commonly known as “Chinese kiwifruit” (English), “中华猕猴桃” (Chinese), and characterized by excessive vegetative vigor, is a woody perennial, deciduous, and functionally dioecious medicinal plant in the family Actinidiaceae (Flora of China, 2007; The Plant List, 2013). It is native to China and has been cultivated in New Zealand, United States, Greece, Italy, Chile, France, Japan, and Korea (Li and Zhu, 2017; Ma et al., 2017). In China, they are mainly distributed in temperate to warm-temperate zones such as Shaanxi, Gansu, Henan, Guangdong, Guangxi, Fujian, Guizhou, Yunnan, Sichuan, as well as the middle and lower reaches of the Yangtze River basin, especially in Yiling district in Yichang city, Hubei province ( Figure 1 ) (Flora of China, 2007). There are 13 A. chinensis cultivars, especially “Hongyang,” “Jintao,” and “Huayou,” are developed for commercial production in China (Sharon, 2016), and more than three ones such as “Sungold,” “Charm,” and “Hort16A” developed in New Zealand (Henare, 2016) ( Table 1 ).
Figure 1.
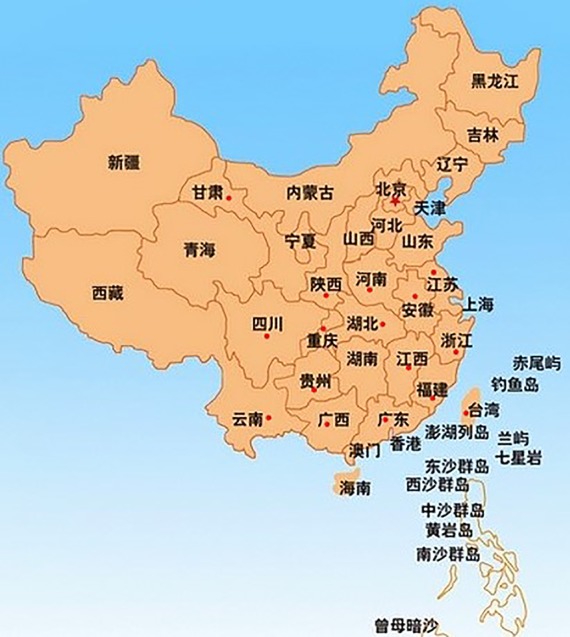
The red spots in the map depicted the main region of A. chinensis distribution in China (Flora of China, 2007; https://www.newasp.net/soft/105257.html).
Table 1.
A. chinensis cultivars developed for commercial production (Henare, 2016).
| Origin country | Cultivar | Fruit shape | Avg. weight | Fruit skin | Fruit flesh |
|---|---|---|---|---|---|
| China | Cuiyu (Liangmei No. 1) | Ovoid | 90 g | Greenish brown with short hairs | Green |
| Wuzhi No. 3 (Wuzhi 81-36) | Ellipsoid | 85 g | Dark green with soft hairs | Bright green | |
| Chuhong (Panda™ Forest Red Kiwi) |
Long ellipsoid | 80 g | Dark green and hairless | Green with red flesh around white core | |
| Qihong | Cylindric | 100 g | Green with sparse or absent hairs | Light green to yellow | |
| Hongyang (Red Sun, RS1) | Obovoid | 60-70 g | Dark green or greenish brown with fine hairs | Green-yellow to yellow, circle of red around white core | |
| Jintao (C6, WIB-C6, Jingold™) | Long cylindric | 90 g | Yellow with brown hairs | Green-yellow to orange-yellow | |
| Huayou (Panda™ Golden Kiwi) | Ellipsoid | 90 g | Ellipsoid | Light green to yellow | |
| Ganmi No. 1 (Zaoxian No. 1, FT-79-5) |
Cylindric | 85 g | Green-brown to pale brown with soft hairs | Greenish-yellow to yellow | |
| Ganmi No. 3 (Jinfeng, FT 79-3) |
Ellipsoid | 80-90 g | Yellow-brown or dark brown with short, fine hairs | Yellow | |
| Jinyan | Cylindrical | 100-110 g | Yellow brown with short, fine hairs | Yellow | |
| Ganmi No. 2 (Kuimi, FY 79-1) | Apple shaped | 100 g | Green-brown to dark brown with fine hairs | Yellow-green to yellow | |
| Hort16A | Ovoid | 95-100g | Green-brown to brown with soft hairs | Yellow-green to bright yellow | |
| Wanhong | Cylindrical | 110-140g | Green-brown with rare hairs | Yellow-green to bright yellow | |
| New Zealand | Charm (Zespri® Charm) | Ovoid | Brown with soft hairs | Yellow | |
| Sungold (Zespri® Sungold) | Brown with smooth skin | Yellow | |||
| Hort16A (Zespri®
Gold, Earligold) |
Ovoid | 95-100g | Green-brown to brown with soft hairs | Yellow-green to bright yellow | |
| Italy | Soreli (Ac 171.76) | Oblong | > 100g | Brown with sparse hairs | Yellow |
| Japan | Sanuki Gold | Squat | 160-180g | Brown with soft hairs | Bright yellow |
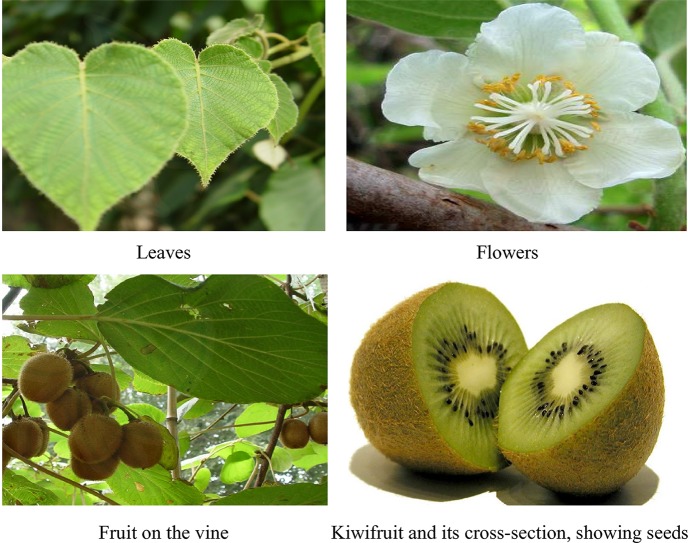
There are two varieties accepted by The Plant List that include A. chinensis and A. chinensis var. setosa H.L.Li (The Plant List, 2013). The fruit of A. chinensis is the largest one in Actinidia genus, and it has the greatest economic, medicinal, and edible significance in terms of production and utilization. Its relevant pictures are showed in Figure 2 . Generally, Chinese kiwifruit with a cross-sectional radius of about 3 cm is oval-shaped densely covered with yellowish-brown hairs. The flesh color of fruit skin is green to yellow, and the average fruit weight is 20–120 g. The fruit is a tasty, nutritious food that can be eaten fresh directly. Today, a range of kiwifruit processed products with the attractive eating quality and nutritional benefits has been developed including juice, preserved fruit, yogurt, wine, canned fruit, dried kiwi slices, fruit vegetable juice drinks, milk beverage, and vinegar. Apart from being a food and natural health product, the whole plant (fruits, branches and leaves, vines and roots) of A. chinensis has been used as traditional folk medicine in China (He et al., 2017; Wei et al., 2018; Fang et al., 2019). The ripe kiwifruit, tastes sweet and sour, acts on the spleen, stomach, and kidney meridians, has improving properties on dyspepsia, loss of appetite, and vomiting. The branches and leaves have been used to treat arthronalgia, bleeding, empyrosis, and ulcer. The vine has appetizing, heat clearing, and wind-dampness dispelling effects and is used to treat indigestion, aundice, and urolithiasis. The root and bark of A. chinensis taste bitter and astringent, and they have various medical effects such as wind and heat dispelling, blood circulation improving, and detumescence properties, and are used for the treatment of rheumatoid arthritis, bruises, furuncle, swelling, filariasis, hepatitis, and dysentery (Xie, 1975). However, people with weak spleen and stomach should be cautious in taking A. chinensis (Xie, 1975). To date, only very few modern studies have been done on potential toxic and side effects of A. chinensis, which should be highlighted in future research.
Figure 2.
The leaves, flowers, vines, and fruits of A. chinensis.
The principal chemical composition of the whole plant of A. chinensis include polyphenol, triterpenoids and derivatives, carotenoids, polysaccharides, amino acids, vitamins, essential oils, and microelements. (Papunidze et al., 2001; Chang and Case, 2005; Ma et al., 2017; Wang et al., 2017; Twidle et al., 2018). Among these ingredients, the main bioactive constituents are phenolic compounds, triterpenes, and the major nutritional composition are vitamin C, vitamin E, dietary fiber, and microelements, which make up a relatively significant share of the daily value ( Table 2 ). Pharmacological results have revealed various promising bioactivities to A. chinensis including antitumor, antioxidant, anti-inflammatory, antimicrobial, immunoregulatory, hypolipemic and antidiabetic, cardiovascular protective, hypnotic effects, and ACE inhibitory activities (Deng et al., 2013; Niu et al., 2016; Sun et al., 2017; Xia et al., 2017; Deng et al., 2018; Hou et al., 2018; Fang et al., 2019). Much of these bioactivities of A. chinensis are consistent with those observed in traditional folk medicine. More importantly, A. chinensis showed significantly antitumor and antioxidant properties, and these effects could be depended on the presence of a range of triterpenoids, polysaccharide, and phenolic compounds (Chang and Case, 2005; Wei et al., 2018; Fang et al., 2019). However, the information on the chemical and biological activities of A. chinensis is scattered. In this review, we intend to systematically summarize the recent advances in nutritional composition, chemistry, and biological activities of A. chinensis and also provide future research directions for better utilize and develop it as a sustainable crop.
Table 2.
Nutritional composition of Zespri® sun-gold kiwifruit.
| Nutrient | Unit | Kiwifruit 81g | Value per 100 g |
|---|---|---|---|
| Proximates | |||
| Water | g | 66.78 | 82.44 |
| Energy | kcal | 51 | 63 |
| Protein | g | 0.83 | 1.02 |
| Total lipid (fat) | g | 0.23 | 0.28 |
| Carbohydrate, by difference | g | 12.79 | 15.79 |
| Fiber, total dietary | g | 1.1 | 1.4 |
| Sugars, total | g | 9.96 | 12.3 |
| Minerals | |||
| Calcium, Ca | mg | 14 | 17 |
| Iron, Fe | mg | 0.17 | 0.21 |
| Magnesium, Mg | mg | 10 | 12 |
| Phosphorus, P | mg | 20 | 25 |
| Potassium, K | mg | 255 | 315 |
| Sodium, Na | mg | 2 | 3 |
| Zinc, Zn | mg | 0.06 | 0.08 |
| Vitamins | |||
| Vitamin C, total ascorbic acid | mg | 130.7 | 161.3 |
| Thiamin | mg | 0.000 | 0.000 |
| Riboflavin | mg | 0.060 | 0.074 |
| Niacin | mg | 0.187 | 0.231 |
| Vitamin B-6 | mg | 0.064 | 0.079 |
| Folate, DFE | µg | 25 | 31 |
| Vitamin B-12 | µg | 0.06 | 0.08 |
| Vitamin A, RAE | µg | 1 | 1 |
| Vitamin A, IU | IU | 19 | 23 |
| Vitamin E (alpha-tocopherol) | mg | 1.13 | 1.40 |
| Vitamin D (D2 + D3) | µg | 0.0 | 0.0 |
| Vitamin D | IU | 0 | 0 |
| Vitamin K (phylloquinone) | µg | 4.9 | 6.1 |
| Lipids | |||
| Fatty acids, total saturated | g | 0.053 | 0.065 |
| Fatty acids, total monounsaturated | g | 0.019 | 0.023 |
| Fatty acids, total polyunsaturated | g | 0.090 | 0.111 |
| Fatty acids, total trans | g | 0 | 0 |
| Cholesterol | mg | 0 | 0 |
Source: USDA Food Composition Databases, https://ndb.nal.usda.gov/ndb/ Accessed on April, 2018.
Chemical Composition
Nutritional Composition
Chinese kiwifruit, known as the “king of fruits,” is a fruit with high-pulp juices, thick flesh, delicious taste, and rich nutrition and has a higher commercial and economic value. It is a rich source of various nutrients including vitamins, carbohydrate, sugar, minerals, amino acids, protein, fatty acids (e.g., linoleic acid), and carotenoids. Table 2 lists the nutritional composition of sun-gold kiwifruit reported from the USDA Food Composition Database (United States Department of Agriculture, USDA Food Composition Databases, 2018). Table 3 shows the chemical content of A. chinensis fruit (Chang and Case, 2005; Cui et al., 2007; Zhou et al., 2009; Xu et al., 2010; He et al., 2014; He et al., 2015a; He et al., 2015b; Xu et al., 2016; Twidle et al., 2018; Sivakumaran et al., 2018; Wei et al., 2018; Zhang et al., 2018). Of particular note, nutritional composition in kiwifruit is vitamin C (1.61 mg/g) and minerals K (3.15 mg/g). The average vitamin C content of Huayou, Jintao, Ganmi-1, Ganmi-2, Ganmi-3, Wuzhi-3, and Cuiyu cultivated in China are 1.59, 1.49, 0.86, 1.34, 0.97, 2.88, and 1.18 mg/g, respectively. Meanwhile, the vitamin C in SunGold was 1.61 mg/g edible flesh, followed by other varieties Sweet Green (1.5 mg/g) and green “Hayward” (0.85 mg/g) (Sivakumaran et al., 2018). Especially, the vitamin C content in kiwifruit is higher than that determined in lemon, orange, strawberry, and grapefruits (Ma et al., 2017).
Table 3.
The nutritional composition or phytochemicals content of A. chinensis fruit.
| Composition | Cultivar location | Genotype | Method | Plant part | Content | Ref. |
|---|---|---|---|---|---|---|
| Vitamin C (ascorbic acid) | Colomicta | A. chinensis | HPLC | Ripe fruits | 0.82 mg/g FW; 4.34 mg/g DW | Kvesitadze et al., 2001 |
| Vitamin C | Cardinal | A. chinensis | HPLC | Ripe fruits | 0.74 mg/g FW; 4.30 mg/g DW | Kvesitadze et al., 2001 |
| Vitamin C | Bruno | A. chinensis | HPLC | Ripe fruits | 0.76 mg/g FW; 4.28 mg/g DW | Kvesitadze et al., 2001 |
| Vitamin C | Monti | A. chinensis | HPLC | Ripe fruits | 0.76 mg/g FW; 4.33 mg/g DW | Kvesitadze et al., 2001 |
| Vitamin C | Purpuria | A. chinensis | HPLC | Ripe fruits | 0.78 mg/g FW; 4.27 mg/g DW | Kvesitadze et al., 2001 |
| Vitamin C | Gaivard | A. chinensis | HPLC | Ripe fruits | 0.72 mg/g FW; 4.14 mg/g DW | Kvesitadze et al., 2001 |
| Vitamin C | Gaivard | A. chinensis | HPLC | Skin | 0.21 mg/g FW; 0.63 mg/g DW | Kvesitadze et al., 2001 |
| Vitamin C | Gaivard | A. chinensis | HPLC | Pulp | 0.85 mg/g FW; 4.75 mg/g DW | Kvesitadze et al., 2001 |
| Vitamin C | Gaivard | A. chinensis | HPLC | Core | 0.48 mg/g FW; 2.67 mg/g DW | Kvesitadze et al., 2001 |
| Vitamin C | Gaivard | A. chinensis | HPLC | Fresh juice | 0.55 mg/g FW; 3.44 mg/g DW | Kvesitadze et al., 2001 |
| Vitamin C | Gaivard | A. chinensis | HPLC | Juice after 24 h | 0.55 mg/g FW; 3.44 mg/g DW | Kvesitadze et al., 2001 |
| Vitamin C | Shaanxi, China | Huayou | 2,6-dichloroindophenol titration method | Ripe fruits | 1.59 mg/g FW | Ma et al., 2017 |
| Total starch content in tissue | Pukekohe | Zespri® SunGold Kiwifruit | Total starch assay kit | Outer pericarp | 38.6% DW | Li and Zhu, 2017 |
| Total starch content in tissue | Auckland | Gold9 | Total starch assay kit | Outer pericarp | 51.8% DW | Li and Zhu, 2017 |
| Total starch content in tissue | New Zealand | Hort16A | Total starch assay kit | Outer pericarp | 44.8% DW | Li and Zhu, 2017 |
| Apparent amylose content | Pukekohe | Zespri® SunGold Kiwifruit | Total starch assay kit | Outer pericarp | 27.5% DW | Li and Zhu, 2017 |
| Apparent amylose content | Auckland | Gold9 | Total starch assay kit | Outer pericarp | 24.5% DW | Li and Zhu, 2017 |
| Apparent amylose content | New Zealand | Hort16A | Total starch assay kit | Outer pericarp | 25.3% DW | Li and Zhu, 2017 |
| True amylose content | Pukekohe | Zespri® SunGold Kiwifruit | Total starch assay kit | Outer pericarp | 17.8% DW | Li and Zhu, 2017 |
| True amylose content | Auckland | Gold9 | Total starch assay kit | Outer pericarp | 15.7% DW | Li and Zhu, 2017 |
| True amylose content | New Zealand | Hort16A | Total starch assay kit | Outer pericarp | 15.5% DW | Li and Zhu, 2017 |
| Total dietary fibre | New Zealand | Hort 16A | Megazyme method | puree | 34.1 mg/g FW | Yuliarti et al., 2008 |
| Total dietary fibre | New Zealand | Hort 16A | Megazyme method | Skin and cores | 13.84% DW | Yuliarti et al., 2008 |
| Insoluble dietary fibre | New Zealand | Hort 16A | Megazyme method | Puree | 26.1 mg/g FW | Yuliarti et al., 2008 |
| Insoluble dietary fibre | New Zealand | Hort 16A | Megazyme method | Skin and cores | 11.39% DW | Yuliarti et al., 2008 |
| Soluble dietary fibre | New Zealand | Hort 16A | Megazyme method | Puree | 8 mg/g FW | Yuliarti et al., 2008 |
| Soluble dietary fibre | New Zealand | Hort 16A | Megazyme method | Skin and cores | 2.45% DW | Yuliarti et al., 2008 |
| Nonstarch polysaccharide | New Zealand | gold kiwifruit | Acid extraction | Pomace | 77.59% DW | Yuliarti et al., 2015a |
| Nonstarch polysaccharide | New Zealand | gold kiwifruit | Acid extraction | Early-harvested Fruits |
69.14% DW | Yuliarti et al., 2015b |
| Nonstarch polysaccharide | New Zealand | gold kiwifruit | Acid extraction | Main-harvested fruits |
64.49% DW | Yuliarti et al., 2015b |
| Nonstarch polysaccharide | New Zealand | gold kiwifruit | Water extraction | Pomace | 79.16% DW | Yuliarti et al., 2015a |
| Nonstarch polysaccharide | New Zealand | gold kiwifruit | Water extraction | Early-harvested fruits |
60.74% DW | Yuliarti et al., 2015b |
| Nonstarch polysaccharide | New Zealand | gold kiwifruit | Water extraction | Main-harvested fruits |
63.77% DW | Yuliarti et al., 2015b |
| Nonstarch polysaccharide | New Zealand | gold kiwifruit | Enzymatic extraction | Pomace | 80.12% DW | Yuliarti et al., 2015a |
| Nonstarch polysaccharide | New Zealand | gold kiwifruit | Enzymatic extraction | Early-harvested fruits |
39.21% DW | Yuliarti et al., 2015b |
| Nonstarch polysaccharide | New Zealand | gold kiwifruit | Enzymatic extraction | Main-harvested fruits |
64.02% DW | Yuliarti et al., 2015b |
| Total free amino acids | Shaanxi, China | Hort16A | Hitachi L-8900 amino acid analyzer | Ripe fruits | 8.31 mg/g FW | Ma et al., 2017 |
| Total free amino acids | New Zealand | Hort16A | Hitachi L-8900 amino acid analyzer | Ripe fruits | 8.01 mg/g FW | Ma et al., 2017 |
| Total free amino acids | Shaanxi, China | Huayou | Hitachi L-8900 amino acid analyzer | Ripe fruits | 7.15 mg/g FW | Ma et al., 2017 |
| Total essential amino acids | Shaanxi, China | Huayou | Hitachi L-8900 amino acid analyzer | Ripe fruits | 1.55 mg/g FW | Ma et al., 2017 |
| Total essential amino acids | Shaanxi, China | Hort16A | Hitachi L-8900 amino acid analyzer | Ripe fruits | 2.09 mg/g FW | Ma et al., 2017 |
| Total essential amino acids | New Zealand | Hort16A | Hitachi L-8900 amino acid analyzer | Ripe fruits | 2.06 mg/g FW | Ma et al., 2017 |
| Nonessential amino acids | New Zealand | Hort16A | Hitachi L-8900 amino acid analyzer | Ripe fruits | 5.95 mg/g FW | Ma et al., 2017 |
| Nonessential amino acids | Shaanxi, China | Hort16A | Hitachi L-8900 amino acid analyzer | Ripe fruits | 6.22mg/g FW | Ma et al., 2017 |
| Nonessential amino acids | Shaanxi, China | Huayou | Hitachi L-8900 amino acid analyzer | Ripe fruits | 5.60 mg/g FW | Ma et al., 2017 |
| Total phenolic | New Zealand | Zespri® SunGold Kiwifruit | Folin-Ciocalteu method | Thinned young fruits (20 days) | ∼80 mg GAE/g FDW | Jiao et al., 2019 |
| Total phenolic | Shanxi Province | Red sun | Folin-Ciocalteu method | Ripe fruits | 0.87 mg GAE/g FW | Wang et al., 2018 |
| Total phenolic | Shanxi Province | Cuiyu | Folin-Ciocalteu method | Ripe fruits | 0.83 mg GAE/g FW | Wang et al., 2018 |
| Total flavonoid | New Zealand | Zespri® SunGold Kiwifruit | UV/Vis | Thinned young fruits (20days) | ∼30 mg CE/g FDW | Jiao et al., 2019 |
| Total flavanol | New Zealand | Zespri® SunGold Kiwifruit | UV/Vis | Thinned young fruits (20days) | ∼20 mg CE/g FDW | Jiao et al., 2019 |
| Total flavonoid | Shanxi Province | Red sun | UV/Vis | Ripe fruits | 0.68 mg CE/g FW | Wang et al., 2018 |
| Total flavonoid | Shanxi Province | Cuiyu | UV/Vis | Ripe fruits | 0.68 mg CE/g FW | Wang et al., 2018 |
| Total carotenoid | New Zealand | Hort16A | HPLC | Main-harvested fruits |
0.62 mg/100 g FW | McGhie and Ainge, 2002 |
| Total chlorophylls | New Zealand | gold kiwifruit | HPLC | Outer Pericarp | 0.07 mg/100 g FW | Montefiori et al., 2005 |
| Total anthocyanins | New Zealand | Hongyang | HPLC | Pericarp | 2.99 mg/100 g FW | Montefiori et al., 2005 |
| Total organic acids | China | Hongyang | HPLC | Ripe Fruits | 39.86 mg/g FW | Montefiori et al., 2005 |
| Total organic acids | China | Cuiyu | HPLC | Ripe Fruits | 29.65 mg/g FW | Montefiori et al., 2005 |
The data show evidence that the sun-gold kiwifruit is high in carbohydrate (15.79%) and sugars (12.3%). The total starches contents were found for outer pericarp and core tissues ranged from 38.6% to 51.8% and 34.6% to 40.7% DW in three harvesting A. chinensis varieties, and the starches in core have higher amylose content (20.7%–23.3%) and enzyme susceptibility. However, the crystallinity degree, granule size, and gelatinization parameters of starches in core are somewhat lower (Li and Zhu, 2017). The kiwifruit peel contains a higher total pectin content (3.7%–4.2%) than that of pulp (1.6%–2.1%) (Meng et al., 2017). Xia et al. (2017) analyzed the polysaccharide from Hongyang using water extraction, followed by column chromatography, high performance gel permeation chromatography, HPLC, and Fourier transform infrared spectroscopy. The results indicated that the polysaccharide of Hogyang fruit consisted of the following monosaccharides: D-galactose (25.45%), D-galacturonic acid (25.25%), L-arabinose (20.51%), L-rhamnose (17.78%), D-glucose (6.14%), D-mannose (2.13%), D-xylose (1.03%), D-glucuronic acid (0.97%), and D-fucose (0.74%). These studies confirmed the utilization potential of Chinese kiwifruit as an incredibly healthy food and loaded with important nutrients and health benefits for human consumption.
Kiwifruit contained 18 free amino acids. Briefly, the total essential amino acid contents in Jintao, Hongyang, Huayou, and Hort16A cultivated in China are 2.59, 1.55, 2.0, and 2.09 mg/g FW, whereas the total essential amino acid in Hort16A cultivated in New Zealand was 2.06 mg/g FW. Meanwhile, Jintao, Hort16A and Hongyang also had a high amount of nonessential amino acid and total free amino acid. The most abundant amino acids detected in the kiwifruit were arginine, glutamic acid, lysine, phenylalanine, aspartic acid, and tyrosine (Ma et al., 2017). There are a number of total saturated lipids including C8:0, C10:0, C12:0, C14:0, C16:0, and C18:0 with the content of 1.49, 0.05, 0.14, 0.14, 0.09, 0.9, and 0.14 mg/g in edible flesh portion of A. chinensis. Meanwhile, the content of monounsaturated fatty acids C16:1 and C18:1 are 0.09 and 0.27 mg/g, and the polyunsaturated fatty acids C18:2 and C18:3 are 1.13 and 0.77 mg/g (Drummond, 2013). In fact, the kiwifruit seed oil is rich in unsaturated fatty acids (89.92%), notably linolenic acid, which accounts for 60.59% of total seed oil (Luan et al., 2017). Υ-tocopherol, Υ-tocotrienol, and ƍ-tocotrienol are identified in kiwifruit seed oil (Fiorentino et al., 2009). Besides, minerals like calcium, iron, potassium, magnesium, sodium, phosphorus, copper, manganese, zinc, iodine, selenium, and vitamins including vitamin A, β-carotene, lutein, zeaxanthin, riboflavin, niacin, pantothenic acid, vitamin B6, folate, tocopherol, vitamin E, vitamin K, and choline are identified in kiwifruit (Sivakumaran et al., 2018). Thus, these data suggest that kiwifruit is an interesting fruit for daily nutrition and energy suppliers.
Phytochemicals
A range of phytochemicals, including triterpenoids, saponins, and phenolic compounds (flavonoids, polyphenols, anthraquinones, and coumarins) varying in structures, were found and identified in A. chinensis. The major constituents isolated and identified in leaves and roots of A. chinensis are listed in Table 4 .
Table 4.
Chemical constituents isolated from A. chinensis.
| NO | Name | Cas | Formula | Source | Ref. |
|---|---|---|---|---|---|
| Triterpenoids | |||||
| 1. | (2α,3β,4α)-2,3,23-Trihydroxyursa-12,20(30)-dien-28-oic acid; Actinidic acid | 341971-45-7 | C30 H46 O5 | roots, unripe fruit | Ji and Liang, 1985; Lahlou et al., 2001 |
| 2. | Maslinic acid | 4373-41-5 | C30 H48 O4 | roots | Cui et al., 2007 |
| 3. | Ursolic acid acetate | 7372-30-7 | C32 H50 O4 | roots | Cui et al., 2007 |
| 4. | 23-Hydroxyursolic acid | 94414-19-4 | C30 H48 O4 | roots | Cui et al., 2007 |
| 5. | Ergosta-4,6,8(14),22-tetraen-3-one | 19254-69-4 | C28 H40 O | roots | Cui et al., 2007 |
| 6. | 2α,3β,24-Trihydroxyurs-12-en-28-oic acid | 143839-02-5 | C30 H48 O5 | roots | Ji and Liang, 1985 |
| 7. | 2α,3α,24-Trihydroxyurs-12,20(30)-dien-28-oic acid | 341503-22-8 | C30 H46 O5 | roots | Ji and Liang, 1985 |
| 8. | Pygenic acid A (3- epi-corosolic acid) | 52213-27-1 | C30 H48 O4 | roots | Chen et al., 2011 |
| 9. | 2α,3β-Dihydroxyurs-12-en-28,30-olide | 1198363-27-7 | C30 H46 O4 | roots | Zhou et al., 2009 |
| 10. | 2α,3β,24-Trihydroxyurs-12-en-28,30-olide | 1198363-28-8 | C30 H46 O5 | roots | Zhou et al., 2009 |
| 11. | 3β-Hydroxyurs-12,18-dien-28-oic acid | 14021-14-8 | C30 H46 O3 | roots | Zhou et al., 2009 |
| 12. | 2α,3α,23-Trihydroxyursa-12, 20(30)-dien-28-oic acid | 1187824-97-0 | C30 H46 O5 | roots | Zhou et al., 2009 |
| 13. | 2α,3α,19α,23, 24-Pentahydroxyurs-12-en-28-oic acid | 1309360-33-5 | C30 H48 O7 | roots | Xu et al., 2010 |
| 14. | Ursolic acid | 74984-66-0 | C30 H48 O3 | roots | Xu et al., 2010 |
| 15. | Pseudotaraxasterol | 464-98-2 | C30 H50 O | roots | Xu et al., 2010 |
| 16. | 2α,3α,23-Trihydroxyurs-12-en-28-oic acid | 103974-74-9 | C30 H48 O5 | roots | Xu et al., 2010 |
| 17. | 2α,3β,24-Trihydroxyurs-12-en-28-oic acid | 475631-15-3 | C30 H48 O5 | roots | Xu et al., 2010 |
| 18. | 2α,3β,19α, 23-Tetrahydroxyurs-12-en-28-oic acid | 70868-78-9 | C30 H48 O6 | roots | Xu et al., 2010 |
| 19. | 2α,3α,19α, 24- Tetrahydroxyurs-12-en-28-oic acid 28-O-β-D-glucopyranoside | 153753-66-3 | C36 H58 O11 | roots | Xu et al., 2010 |
| 20. | Oleanolic acid acetate | 4339-72-4 | C32 H50 O4 | roots | Zhu et al., 2013 |
| 21. | Corosolic acid | 4547-24-4 | C30 H48 O4 | roots | Zhu et al., 2013 |
| 22. | Arjunic acid | 31298-06-3 | C30 H48 O5 | roots | Zhu et al., 2013 |
| 23. | Euscaphic acid | 53155-25-2 | C30 H48 O5 | roots | Zhu et al., 2013 |
| 24. | Oleanolic acid | 508-02-1 | C30 H48 O3 | roots | He et al., 2014 |
| 25. | 2α,3α,24-Trihydroxyolean-12-en-28-oic acid | 150821-16-2 | C30 H48 O5 | roots | He et al., 2015a |
| 26. | 2α,3α,19α,24-Tetrahydroxyurs-12-en-28-oic acid | 153753-65-2 | C30 H48 O6 | roots | He et al., 2015a |
| 27. | Jacoumaric acid | 63303-42-4 | C39 H54 O6 | roots | Cui, 2016 |
| 28. | 3β-Hydroxystigmast-5-en-7-one | 2034-74-4 | C29 H48 O2 | roots | Xu et al., 2016 |
| 29. | (2α,3α)-2,3,23,24-Tetrahydroxyurs-12-en-28-oic acid; 2α,3α,23,24-Tetrahydroxy ursan-12-en-28-acid | 143773-49-3 | C30 H48 O6 | roots | Xu et al., 2016 |
| 30. | Oleanan-28-oic acid, 12-chloro-2,3,13,23-tetrahydroxy-, γ-lactone, (2α,3β,4α,12α)- | 1309360-32-4 | C30 H47 Cl O5 | roots | Xu et al., 2016 |
| 31. | Urs-13(18)-en-28-oic acid, 2,3,23-trihydroxy-, (2α,3β,4α)- | 1980812-62-1 | C30 H48 O5 | roots | Xu et al., 2016 |
| 32. | Urs-13(18)-en-28-oic acid, 2,3,19,23-tetrahydroxy-, β-D-glucopyranosyl ester, (2α,3β,4α)- | 1980812-63-2 | C36 H58 O11 | roots | Xu et al., 2016 |
| 33. | Pygenic acid B (2α,3α,24-trihydroxyurs-12-en-28-oic acid) | 89786-83-4 | C30 H48 O5 | roots | Xu et al., 2016 |
| 34. | 2α,3α,23,24-Tetrahydroxyursa-12, 20(30)-dien-28-oic acid | 2220160-45-0 | C30 H46 O6 | roots | Wei et al., 2018 |
| 35. | 2α,3β,23,24-Tetrahydroxyurs-12-en-28-oic acid | 116787-94-1 | C30 H48 O6 | roots | Wei et al., 2018 |
| 36. | 2α,3β,23-Trihydroxyurs-12-en-28-oic acid | 114580-55-1 | C30 H48 O5 | roots | Wei et al., 2018 |
| 37. | 3β-Hydroxyurs-12-en-28-oic acid | 77-52-1 | C30 H48 O3 | roots | Wei et al., 2018 |
| 38. | 3β-Hydroxyolean-12-en-28-oic acid | 28283-45-6 | C35 H56 O7 | roots | Wei et al., 2018 |
| 39. | 2β,3α,23-Trihydroxyurs-12-en-28-oic acid | 175132-32-8 | C30 H48 O5 | roots | Wei et al., 2018 |
| 40. | 2β,3β,23-Trihydroxyurs-12-en-28-oic acid | 116348-15-3 | C3o H48 O5 | roots | Wei et al., 2018 |
| 41. | Spathodic acid 28-O-β-glucopyranoside | 870559-41-4 | C36 H58 O10 | root barks | Zhang et al., 2018 |
| 42. | Fupenzic acid | 119725-20-1 | C3o H44 O5 | root barks | Zhang et al., 2018 |
| Phenols | |||||
| 43. | Planchol A | 883238-17-3 | C14 H14 O6 | roots | Chang and Case, 2005 |
| 44. | Planchol B | 883238-19-5 | C15 H16 O6 | roots | Chang and Case, 2005 |
| 45. | Planchol C | 883238-20-8 | C16 H18 O6 | roots | Chang and Case, 2005 |
| 46. | Planchol D | 883238-21-9 | C16 H16 O7 | roots | Chang and Case, 2005 |
| 47. | Benzeneacetic acid, 2-[(3,4-dihydroxybenzoyl)oxy]-4,6-dihydroxy-, methyl ester | 911315-93-0 | C16 H14 O8 | leaves | Wurms and Cooney, 2006 |
| 48. | Tachioside (methoxyhydroquinone-3-O-β-D-glucopyranoside) | 109194-60-7 | C13 H18 O8 | roots | Zhou et al., 2010 |
| 49. | Isotachioside (methoxyhydroquinone-1-O-β-D-glucopyranoside) | 31427-08-4 | C13 H18 O8 | roots | Zhou et al., 2010 |
| 50. | Vanillic acid | 121-34-6 | C8 H8 O4 | roots | Zhou et al., 2010 |
| 51. | 1-O-(β-D-glucosyl)-2-[2-methoxy-4-(ω-hydroxypropyl)-phenoxy]-propan-3-ol | 68340-35-2 | C19 H30 O10 | roots | Zhou et al., 2010 |
| 52. | Protocatechualdehyde | 139-85-5 | C7 H6 O3 | roots | He et al., 2014 |
| 53. | rel-(1R,2R)-1,2-Bis(4-hydroxy-3-methoxyphenyl)-1,3-propanediol | 69887-40-7 | C17 H20 O6 | roots | He et al., 2014 |
| 54. | rel-(1R,2S)-1,2-Bis(4-hydroxy-3-methoxyphenyl)-1,3-propanediol | 69887-41-8 | C17 H20 O6 | roots | He et al., 2014 |
| 55. | p-Hydroxyl benzoic acid | 99-96-7 | C7 H6 O3 | roots | He et al., 2014 |
| 56. | Chlorogenic acid | 327-97-9 | C16 H18 O9 | roots | He et al., 2015a |
| 57. | Caffeic acid | 331-39-5 | C9 H8 O4 | roots | He et al., 2015a |
| 58. | Cryptochlorogenic acid | 905-99-7 | C16 H18 O9 | roots | He et al., 2015a |
| 59. | Neochlorogenic acid | 906-33-2 | C16 H18 O9 | roots | He et al., 2015a |
| 60. | 5-O-Coumaroylquinic acid | 87099-71-6 | C16 H18 O8 | roots | He et al., 2015a |
| 61. | Dihydroxy-dihydrochalcone-2’-O-β-D-glucopyranoside | 23140-78-5 | C21 H24 O9 | roots | Xu et al., 2016 |
| Flavonoids | |||||
| 62. | Epicatechin | 490-46-0 | C15 H14 O6 | unknown | Michaud and Ane-Margail, 1977 |
| 63. | epi-Afzelechin | 24808-04-6 | C15 H14 O5 | unknown | Michaud and Ane-Margail, 1977 |
| 64. | Procyanidin C1 | 37064-30-5 | C45 H38 O18 | unknown | Michaud and Ane-Margail, 1977 |
| 65. | 2-(3,4-Dihydroxyphenyl)-3,4-dihydro-4-[(phenylmethyl)thio]-2H-1-benzopyran-3,5,7-triol | 66052-27-5 | C22 H20 O6 S | unknown | Michaud and Ane-Margail, 1977 |
| 66. | 2,2’-Bis(3,4-dihydroxyphenyl)-3,3’,4,4’-tetrahydro-4’-[(phenylmethyl)thio][4,8’-bi-2H-1-benzopyran]-3,3’,5,5’,7,7’-hexol | 66293-44-5 | C37 H32 O12 S | unknown | Michaud and Ane-Margail, 1977 |
| 67. | Afzelechin | 2545-00-8 | C15 H14 O5 | roots | Chang and Case, 2005 |
| 68. | Procyanidin B3 | 23567-23-9 | C30 H26 O12 | roots | Chang and Case, 2005 |
| 69. | Procyanidol B2 | 29106-49-8 | C30 H26 O12 | roots | Chang and Case, 2005 |
| 70. | Afzelechin-(4α→8)-afzelchin | 101339-37-1 | C30 H26 O10 | roots | Chang and Case, 2005 |
| 71. | (2R,2’R,3R,3’R,4R)-3,3’,4,4’-Tetrahydro-2,2’-bis(4-hydroxyphenyl)[4,8’-bi-2H-1-benzopyran]-3,3’,5,5’,7,7’-hexol | 114715-48-9 | C30 H26 O10 | roots | Chang and Case, 2005 |
| 72. | Quercetin | 117-39-5 | C15 H10 O7 | fruits | Lee et al., 2010 |
| 73. | (+)-Catechin | 154-23-4 | C15H14O6 | roots | Zhou et al., 2010 |
| 74. | (-)-Epicatechin-5-O-β-D-glucopyranoside | 131831-20-4 | C21 H24 O11 | roots | He et al., 2014 |
| Anthraquinones | |||||
| 75. | Emodic acid | 478-45-5 | C15 H8 O7 | roots | Ji and Liang, 1985 |
| 76. | Hydroxyemodin | 481-73-2 | C15 H10 O6 | roots | Ji and Liang, 1985 |
| 77. | Emodin | 518-82-1 | C15 H10 O5 | roots | Ji and Liang, 1985 |
| 78. | Emodin 3-methyl ether | 521-61-9 | C16 H12 O5 | roots | Ji and Liang, 1985 |
| 79. | Questin | 3774-64-9 | C16 H12 O5 | roots | Ji and Liang, 1985 |
| Coumarins | |||||
| 80. | 5-Hydroxy-6-methoxy-7-O-β-D-glucosyl coumarin | 141238-32-6 | C16 H18 O10 | roots | Zhou et al., 2010 |
| 81. | Fraxin | 524-30-1 | C16 H18 O10 | roots | Zhou et al., 2010 |
| 82. | Esculin | 531-75-9 | C15 H16 O9 | roots | He et al., 2015b |
| 83. | Isofraxoside | 24778-11-8 | C16 H18 O10 | roots | He et al., 2015b |
| Other compouds | |||||
| 84. | β-Sitosterol | 83-46-5 | C29 H50 O | roots | Ji and Liang, 1985 |
| 85. | Butyl β-D-fructopyranoside | 67884-27-9 | C10 H20 O6 | roots | Zhou et al., 2010 |
| 86. | Lignoceric acid | 557-59-5 | C24 H48 O2 | roots | Chen et al., 2011 |
| 87. | (-)-Quinic acid γ-lactone | 665-27-0 | C7 H10 O5 | roots | Chen et al., 2011 |
| 88. | Stearyl-β-D-glucopyranoside | 76739-16-7 | C24 H48 O6 | roots | Chen et al., 2011 |
| 89. | Daucosterol | 474-58-8 | C35 H60 O6 | roots | Chen et al., 2011 |
| 90. | Indole-3-carboxylic acid | 771-50-6 | C9 H7 N O2 | roots | He et al., 2014 |
| 91. | Stigmastane-3,6-diol | 112244-29-8 | C29 H52 O2 | roots | Cui, 2016 |
| 92. | Sitoindoside Ⅰ | 18749-71-8 | C51 H90 O7 | roots | Cui, 2016 |
Triterpenoids
Currently, triterpenoids have been the major research focus of A. chinensis components due to their promising antitumor properties. To date, 42 triterpenoids have been isolated and identified mainly from roots of A. chinensis. The commonly triterpenoids found in roots of A. chinensis are 12-en-28-oic acids of oleanane and ursane type. It is noteworthy that some of these triterpenoids (1-2, 7, 15-18, 21, 25-26, 29-30, and 34-40) have significant antitumor activity and deserve further research and development.
Phenolic Compounds
The phenolic compounds abundantly presented in different botanical parts of A. chinensis, and they have drawn increasing attention. These compounds include phenols, flavonoids, and flavanols are characterized by antitumor, antioxidant, and free radicals scavenging properties. HPLC-PAD and UPLC-QqQ-MS/MS-based methods have been used generally for the identification and quantification of these phenolic compounds (Ma et al., 2017; Jiao et al., 2019). The total phenolic, flavonoid, and flavanol contents from young A. chinensis kiwifruits “Zespri® SunGold Kiwifruit” growing in 20 days are 82.84 mg GAE/g FDW, 30.08 catechin/g equivalents FDW, and 20.20 catechin/g equivalents FDW. Meanwhile, the total phenolic, flavonoid, and flavanol contents presented in young A. chinensis kiwifruits growing in 60 days and mature kiwifruits are gradually decreasing, indicating polyphenol content possesses a decreasing pattern during fruit ripening (Jiao et al., 2019). The major chemical composition of phenolics detected in young “Zespri® SunGold Kiwifruit” are epicatechin, quercitrin, rutin, catechin, chlorogenic acid, ferulic acid, and vanillic acid. Based on UPLC-TOF/MS and UPLC-QqQ/MS method, Zhao et al., 2014 showed that the radix A. chinensis contained catechin derivatives, quinic acid derivatives, coumarin derivatives, caffeic acid, and p-coumaric acid (Zhao et al., 2014), showing that A. chinensis appears to be a good source of phenolics.
Volatile Compound and Essential Oil
The volatile components of A. chinensis var. chinensis fruit and flowers have been profiled by GC-MS. The dominant volatile components of eating-ripe firmness fruit are straight-chain aldehydes, alcohols, and esters, such as hexanal, decanal, octanal, nonanal, benzaldehyde, acetaldehyde, hex-E2-enal, 1,8-cineole, ethanol, hexanol, methyl butanoate, and ethyl octanoate (Wang et al., 2011). The volatile components of flowers included (3E,6E)-α-farnesene (38.8%), pentadecane (12.49%), (+)-germacrene D (8.55%), heptadecane (8.01%), (8Z)-heptadecene (7.72%), 2-phenylethano (4.69%), (3Z,6Z,9Z)-heptadecatriene (2.54%), and nonadecane (1.98%) (Twidle et al., 2018). It can be found that terpenes and straight chain alkenes were dominant in flowers of A. chinensis var. chinensis, which contained nearly >92% of the total ion counts. Importantly, many of these compounds possess strong and interesting aroma. However, the volatile components gradually changed during maturation. The essential oil of roots of A. chinensis have been profiled by GC-MS, and the major essential oil in roots are dodecane (29.39%), octane (5.16%), decane (2.94%), paeonal (2.81%), camphor (2.77%), n-decanoic acid (2.64%), 4-Methyldodecane (2.45%), undecane (2.16%), and linalool oxide (2.1%) (Yu et al., 2009).
Carotenoid and Chlorophyll
Carotenoids and chlorophyll are responsible for the color and attractiveness of kiwifruit fruits, as well as provide nutritional values. The carotenoids detected in the red-fleshed genotypes of A. chinensis fruit (Hort16A) are 9′-cis-neoxanthin, violaxanthin, antheraxanthin, lutein, zeaxanthin, β-cryptoxanthin, and β-carotene (McGhie and Ainge, 2002; Montefiori et al., 2005). Chlorophylls a and b are the dominant chlorophylls in Hort16A (McGhie and Ainge, 2002).
Quality Determination
Ripe kiwifruit is susceptible to environmental and itself. Usually, human sensory evaluation method can directly identify the fruit shape, color, surface, pulp, and flavor, but there is little information about swelling, ripening, and other agents present in fruit. Physical and chemical method including firmness and microbial are used effectively to determine the quality condition of kiwifruit. Some new instrument detection methods with accurate analysis ability such as GC, GC-MS, HPLC, UPLC-QqQ-MS/MS, and electronic nose combined with surface acoustic wave resonator are developed for the fruit and its products quality rapid analysis (Kvesitadze et al., 2001; Montefiori et al., 2005; Liu and Hui, 2015; Jiao et al., 2019). As to radix A. chinensis, the systematical method like UPLC-TOF/MS and UPLC-QqQ/MS is commonly applied to quality evaluation and active components analysis for A. chinensis (Zhao et al., 2014). Therefore, there are many high accurate analysis methods for rapid quality evaluation, but there is a lack of effective and standardized quality and safety standard for kiwifruit in China. Thus, there is an urgent demand for developing specific functional components and quality evaluation indicators for standardization and quality control of the fruit and its products.
Biological Activities
A. chinensis contains a range of bioactive compounds accounting for natural pharmacological properties including antitumor, antioxidant, anti-inflammatory, immunoregulatory, hypolipemic, antidiabetic, and cardiovascular protective activities, and most of these biological activities support its traditional use. Table 5 shows the major biological activities of compound or extract from A. chinensis.
Table 5.
Biological activities of compounds or extracts of A. chinensis.
| Effect | Compound/Extract | Class of compounds | In vitro | In vivo | Ref. |
|---|---|---|---|---|---|
| a | 1 | A | Showed cytotoxicities against HepG2, A549, MCF-7, and SK-OV-3 with IC50 (48 h) values of 36.4, 40.37, 44. 3, and 16.33 μM. | Wei et al., 2018 | |
| 2 | A | Showed cytotoxicities against A549, LoVo, and HepG2 with IC50 (48 h) values of 23.2, 6, and 34.9 μg/ml. | Wei et al., 2018; Xu et al., 2010 | ||
| 7 | A | Showed cytotoxicities against A549, MCF-7, SK-OV-3, and HeLa with IC50 (48 h) values of 16.63, 47.93, 22.91, and 15.27 μM. | Wei et al., 2018 | ||
| 15 | A | Showed cytotoxicities against LoVo, and HepG2 with IC50 (48 h) values of 31.1, and 33.9 μg/ml. | Xu et al., 2010 | ||
| 16 | A | Showed cytotoxicities against HepG2, MCF-7, SK-OV-3, and HeLa with IC50 (48 h) values of 12.22, 36.29, 45.13, and 49.71 μM. | Wei et al., 2018 | ||
| 17 | A | Showed cytotoxicities against A549, MCF-7, SK-OV-3, and HeLa with IC50 (48 h) values of 39.3, 11.01, 40.9 and 41.6 μM. | Wei et al., 2018 | ||
| 18 | A | Showed cytotoxicities against HepG2, A549, MCF-7, and HeLa with IC50 (48 h) values of 19.08, 32.08, 35.74, and 15.05 μM. | Wei et al., 2018 | ||
| 21 | A | Inhibited HCC cells migration by targeting the VEGFR2/Src/FAK pathway. | Ku et al., 2015 | ||
| 21 | A | Showed cytotoxicities against A549, LoVo, and HepG2 with IC50 (48 h) values of 34.6, 2.9, and 9.2 μg/ml. | Xu et al., 2010 | ||
| 25 | A | Showed cytotoxicities against A549 and SK-OV-3 with IC50 (48 h) values of 42.74 and 25.83 μM. | Wei et al., 2018 | ||
| 26 | A | Showed cytotoxicities against A549 and HeLa with IC50 (48 h) values of 22.6 and 29.35 μM. | Wei et al., 2018 | ||
| 29 | A | Showed cytotoxicities against A549 and SK-OV-3 with IC50 (48 h) values of 31.3 and 37.9 μM. | Wei et al., 2018 | ||
| 30 | A | Showed cytotoxicities against A549, LoVo, and HepG2 with IC50 (48 h) values of 30.4, 31.1, and 25.5 μg/ml. | Xu et al., 2010 | ||
| 34 | A | Showed cytotoxicities against HepG2, A549, MCF-7, and HeLa with IC50 (48 h) values of 19.62, 18.86, 45.94 and 28.74 μM. | Wei et al., 2018 | ||
| 35 | A | Showed cytotoxicities against HepG2, MCF-7, and SK-OV-3 with IC50 (48 h) values of 11.76, 12, and 10.3 μM. | Wei et al., 2018 | ||
| 36 | A | Showed cytotoxicities against HepG2, MCF-7, and SK-OV-3 with IC50 (48 h) values of 14.22, 16.99, 28.9 μM. | Wei et al., 2018 | ||
| 37 | A | Showed cytotoxicities against HepG2, A549, MCF-7, and SK-OV-3 with IC50 (48 h) values of 48.4, 12.7, 11.2, and 31.7 μM. | Wei et al., 2018 | ||
| 38 | A | Showed cytotoxicities against A549, MCF-7, and SK-OV-3 with IC50 (48 h) values of 34.45, 42.2 and 49.55 μM. | Wei et al., 2018 | ||
| 39 | A | Showed cytotoxicities against HepG2 with IC50 (48 h) values of 32.5 μM. | Wei et al., 2018 | ||
| 40 | A | Inhibited NCI-H460 cell proliferation by decreasing NF-κB expression. Showed cytotoxicities against SK-OV-3 with IC50 of 37.21 μM. | Cheng et al., 2015; Wei et al., 2018 | ||
| 43 | B | Showed cytotoxic activity against P-388 and A-549 cell lines with IC50 of 2.5 and 1.42 μM. | Chang and Case, 2005 | ||
| 44 | B | Showed cytotoxic activity against P-388 and A-549 cell lines with IC50 of 3.85 and 2.88 μM. | Chang and Case, 2005 | ||
| 45 | B | Showed cytotoxic activity against P-388 and A-549 cell lines with IC50 of 5.02 and 4.5 μM. | Chang and Case, 2005 | ||
| 46 | B | Showed cytotoxic activity against P-388 and A-549 cell lines with IC50 of 3.52 and 2.6 μM. | Chang and Case, 2005 | ||
| b | vitamin E (ƍ-Tocomonoenol) | C | Radical-scavenging capacities on DPPH and O2 were 23.96 and 29.20%; hydroperoxide conjugate dienes formation and TBARS were 26.88 and 46.70%. | Fiorentino et al., 2009 | |
| vitamin E (α-tocopherol) | C | Radical-scavenging capacities on DPPH and O2 were 25.21 and 27.07%. hydroperoxide conjugate dienes formation and TBARS were 33.08 and 53.01%. | Fiorentino et al., 2009 | ||
| vitamin E (ƍ-tocopherol) | C | Radical-scavenging capacities on DPPH and O2 were 23.4 and 29.273%; hydroperoxide conjugate dienes formation and TBARS were 25.48 and 43.2%. | Fiorentino et al., 2009 | ||
| polymeric proanthocyanidins fractionated by methanol- water (80:20, v/v) | D |
IC50 for DPPH, ABTS were 105.3 and 74.7μg/ml; FRAP values is 7.4 mM VCE/g. | Chai et al., 2014 | ||
| polymeric proanthocyanidins fractionated by acetone-methanol-water (40:40:20, v/v/v) | D | IC50 for DPPH, ABTS were 67.7 and 60.1 μg/ml; FRAP values is 9.6 mM VCE/g. | Chai et al., 2014 | ||
| polymeric proanthocyanidins fractionated by acetone-water (70:30, v/v) | D | IC50 for DPPH, ABTS were 69.3 and 39.5 μg/ml; FRAP values is 9.6 mmol VCE/g. | Chai et al., 2014 | ||
| polyphenols compounds (55.10 mg GAE/g DW), contain p-hydroxybenzoic acid, protocatechuic acid, and p-coumaric acid. | B | 10-50 µg/ml showed DPPH free radical scavenging. | Deng et al., 2016 | ||
| seed oil rich in unsaturated fatty acid from Hongyang | E | IC50 for DPPH, HO·scavenging capacity were 31.4 and 1.09; FRAP and ORAC values were 107.3 mg and 1.09 Trolox/kg. | Deng et al., 2018 | ||
| seed oil rich in unsaturated fatty acid from Huayou | E | IC50 for DPPH, HO·scavenging capacity were 33.7 and 1.12; FRAP and ORAC values were 72.0 mg and 1.72 Trolox/kg. | Deng et al., 2018 | ||
| seed oil rich in unsaturated fatty acid from Hort 16A | E | IC50 for DPPH, HO·scavenging capacity were 32.4 and 1.04; FRAP and ORAC values were 3.3 mg and 1.69 Trolox/kg. | Deng et al., 2018 | ||
| water-soluble polysaccharides | F | 0.5-3 mg/ml showed DPPH radical scavenging activity, protection of the HEK 293 cells from H2O2 damage. | Zhang et al., 2015 | ||
| c | polymeric proanthocyanidins fractionated by methanol- water (80:20, v/v) | D | Inhibited monophenolase and diphenolase activity with IC50 of 180.2 and 390.2 μg/ml. | Chai et al., 2014 | |
| polymeric proanthocyanidins fractionated by acetone-methanol- water (40:40:20, v/v/v) | D | Inhibited monophenolase activity with IC50 of 80.1 and 192.6 μg/ml. | Chai et al., 2014 | ||
| polymeric proanthocyanidins fractionated by acetone-water (70:30, v/v) | D | Inhibited monophenolase activity with IC50 of 48.9 and 64.9 μg/ml. | Chai et al., 2014 | ||
| d | polyphenols compounds (55.10 mg GAE/g DW), contain p-hydroxybenzoic acid, protocatechuic acid, p-coumaric acid, etc. | B | 20, 40, 60 µg/ml for 12 h inhibit IL-1β and TNF-α secretion in LPS-induced RAW 264.7 cells. | Deng et al., 2016 | |
| seed oil rich in fatty acids | E | 1.0 and 3.0 ml/kg/day for 84 days down-regulated TNF-α, IL-6, IL-1β, COX-2 and iNOS in high-fat diet induced mice. | Qu et al., 2019 | ||
| water-soluble polysaccharides | F | 50, 100, 200, 300 μg/ml reduce NO production of RAW 264.7 cells, and 100, 200 and 300 μg/ml enhanced phagocytic activity of RAW 264.7 cells. | Zhang et al., 2015 | ||
| e | seed oil rich in fatty acids | E | 1.0 and 3.0 mL/kg/day for 84 days decreased bodyweight and ameliorated serum TC, TG, HDL-C, and LDL-C levels in high-fat diet treated mice. | Qu et al., 2019 | |
| f | flavonoid-rich extract | G | IC50 of ACE inhibitory activity was 12.81 mg/ml. | Hettihewa et al., 2018 | |
| g | actinidin | H | Enhanced gastric protein α-, β-, and κ-caseins digestion under simulated gastric conditions. | Kaur et al., 2010 | |
| h | thaumatin-like protein | H | Inhibited Botrytis cinereal, Mycosphaerella arachidicola and Coprinus comatus, inhibit HIV-1 reverse transcriptase. | Wang and Ng, 2002 | |
| i | 41 | A | 100 μg/ml inhibited tobacco mosaic virus with inhibition rate of 45.70%. | Zhang et al., 2018 | |
| j | 21 | A | 50 μg/ml showed inhibitory effects on CYP2C19, CYP2D6, and CYP3A4 with 69.3,71.0 and 39.3 of remaining activity. | Xu et al., 2016 | |
| 25 | A | 10 μg/ml showed inhibitory effects on CYP2C9, CYP2C19, CYP2D6, and CYP3A4 with 28.3, 59.9, 31.8, and 37.1% of remaining activity. | Xu et al., 2016 | ||
| 30 | A | 10 μg/ml showed inhibitory effects on CYP2C9 and CYP3A4 with 67.1 and 9.8% of remaining activity. | Xu et al., 2016 | ||
| 33 | A | 50 μg/ml showed inhibitory effects on CYP2C19 and CYP3A4 with 75.0 and 35.0 of remaining activity | Xu et al., 2016 | ||
| 61 | B | 10 μg/ml showed inhibitory effects on CYP2C9 with 69.0% of remaining activity. | Xu et al., 2016 |
a, Antitumor effects; b, Antioxidant activity; c. Antityrosinase activity; d, Anti-inflammatory activity; e, Hypolipidemic activity; f, ACE inhibitory activity; g, Digestive activity; h, Antifungal activity; i, Antiviral activity; j, Cytochrome P450 enzyme inhibitory activity. A, Triterpenoid; B, Phenols; C, Vitamin; D, Proanthocyanidins; E, Oil; F, Polysaccharides; G, Flavonoids; H, Protein.
Antitumor Activity
Crude extracts, fractions, and isolated compounds from A. chinensis exhibited strong inhibition against tumor growth in various forms of human cancer cells. These cancer cells were hepatocellular carcinoma cells HepG2 (Xu et al., 2010; Zuo et al., 2012), Hep3B, SMMC7721, MHCC97L, MHCC97H, HCCLM3 (Fang et al., 2019), HL-7702 (He et al., 2017), Huh7 (Hou et al., 2018), lung cancer cells NCI-H460 and NCI-H1299 (Lv et al., 2018), colon cancer cells HT-29, LoVo, and SW480, pharyngeal carcinoma cell lines Fadu and HEP-2, gastric cancer cells SGC-7901, BGC-823, MKN-49P, and MFC, as well as other cancer cells like A549, P-388, MCF-7, SK-OV-3, and HeLa (Chang and Case, 2005; Xu et al., 2010; Xu et al., 2010; Zuo et al., 2012; Shen et al., 2014; Xia et al., 2017; Gu et al., 2017; Wang et al., 2017; Wei et al., 2018). These reported antitumor activities are consistent with the traditional usage such as liver cancer, lung cancer, colon cancer, esophagus cancer, and gastric cancer.
A large number of triterpenoids in roots of A. chinensis especially those with carboxyl group showed marked cytotoxicity against various types of cancer cells in vitro. Especially, compounds 1-2, 7, 15-18, 21, 25-26, 29-30, 34-40, and 43-46 exhibited remarkable antitumor activity against on A549, HepG2, LVOV, MCF-7, HeLa, and/or HepG2 in vitro ( Table 5 ). Additionally, the polysaccharide of Hogyang fruit showed notable inhibitory against tumor cells lines SGC7901, MCF-7, HT29, HepG2, and NCI-H460 with IC50 of were 0.28, 0.31, 0.58, 0.64, and 0.65 µM, respectively (Xia et al., 2017). In vivo, a polysaccharide isolated from the roots of A. chinensis showed antitumor activity by prolonging the life of EAC or P388 cells-induced tumor mice and inhibiting the DNA synthesis in EAC cells (Lin, 1988). Early treatment and long-term treatment with water extracts of roots from A. chinensis with 2 g/kg/day strongly attenuated the malignant behavior of HCC in mice by decreasing DLX2 expression (Fang et al., 2019).
The molecular mechanism of the inhibition against tumor growth and the apoptosis promoting of the fractions and isolated compounds were due to downregulate DLX2 gene expression and VEGFR2/Src/FAK pathway, inhibit cholesterol metabolism by upregulating PCSK9 signaling pathway, regulate gene encoding laminin subunit beta-3 pathways, and decreased NF-κB and EP3 expression. Meanwhile, the antioxidation and anti-inflammation are also important and possible mechanisms. The triterpenoids, polysaccharides, and phenolic compounds were identified as the major bioactive compounds in the extract from A. chinensis roots with antitumor properties (Chang and Case, 2005; Wei et al., 2018), which provides new way to search for treating cancers with natural therapeutic compounds. Overall, A. chinensis has prominent antitumor potential and has a good health benefit for people, however, the further in vivo and clinical studies on antitumor properties of A. chinensis are needed for confirmation.
Antioxidant Activity
Antioxidant activity of bioactive compounds of A. chinensis have been the mostly studied by various in vitro and in vivo assays. These in vitro assays consisted of both chemical and biological assays like DPPH, ABTS, FRAP, HO·, ORAC, oxidative stress by H2O2, and lipid oxidation (Chai et al., 2014; Lee et al., 2015; Hwang et al., 2017; Deng et al., 2018). The in vivo assays were based on SOD, GSH, ALT, AST, oxidative DNA damage, and lipid oxidation (Iwasawa et al., 2011; Sun et al., 2017; Deng et al., 2018; Wang et al., 2018). The above results showed that A. chinensis is a good source of bioactive compounds with antioxidant properties to various extents. The antioxidant capacities of kiwifruit are greatly attributed to polyphenols, flavonoid, unsaturated fatty acid, and vitamin C. In addition, the different extraction methods, different plant parts, and genetic diversity of kiwifruit demonstrated different antioxidant activities. The peel showed the strongest antioxidant activity, followed by the pulp and the core. The antioxidant activity of kiwifruit peel was mainly depended on plenty of phenolic substances, and the antioxidant activity of the pulp was mainly attributed to the existence of a large amount of vitamin C (Zhang et al., 2016). The seed oil of Hort 16A and Hongyang are attractive materials rich in unsaturated fatty acid demonstrated radical scavenging capacities for FRAP, DPPH, HO·, and ORAC with IC50 of 3.3 mgTrolox/kg, 32.4 mg/ml, 1.04 mg/ml, 1.69 mgTrolox/kg, and 107.3 mgTrolox/kg, 31.4 mg/ml, 1.09 mg/ml, 1.99 mgTrolox/kg, respectively (Deng et al., 2018). The radical scavenging capacities of fresh and freeze-dried Hort 16A rich in phenolics and flavonoids for ABTS, DPPH, and ORAC were 8.8, 8.8, 98.3, and 6.0, 5.0, and 40.3 mg VCE/g, respectively (Hwang et al., 2017). The radical scavenging capacities of Red sun and Cuiyu rich in phenolics and flavonoids for ABTS, DPPH, ORAC, and FRAP were 1.35, 1.01, 10.78, 1.50 and 1.32, 0.9, 8.87, 1.28 mg VCE/g, respectively (Wang et al., 2018). Oral administration of kiwifruit protected lymphocytes against oxidative DNA damage, inhibit lipid oxidation in mice, increased SOD and GSH, and lowered ALT and AST levels in the patients (Sun et al., 2017). Therefore, A. chinensis possess confirmed antioxidant capacity and it seems that appropriate extraction methods, appropriate genotypes, and plant parts can be screened to maximize the antioxidant properties of A. chinensis.
Anti-Inflammatory Activity
Anti-inflammatory activity of A. chinensis has been proved in vivo and in vitro models. On high-fat diet-induced obese C57BL/6 mice models, consecutive consumption the seeds oil of A. chinensis with 1.0 and 3.0 ml/kg·bw ameliorated obesity-induced inflammation by down-regulating the mRNA expression of related to inflammation adipokines, such as TNF-α, IL-6, IL-1β, COX-2, and iNOS (Qu et al., 2019). The aqueous and ethyl acetate extracts demonstrated anti-inflammatory activity in inflammatory bowel disease models of the IL-10 gene-deficient mice (Edmunds et al., 2012). In patients with type-2 diabetes mellitus, the fruit juice of A. chinensis showed preventative activity on inflammation by activating Keap1 and Nrf2 via upregulating miR-424 (Sun et al., 2017). On the cellular level, polyphenols mainly composed of protocatechuic acid, p-hydroxybenzoic acid, p-coumaric acid, caffeic acid, and ferulic acid from seeds of A. chinensis at concentration of 40 and 60 μg/ml for 12 h decreased the secretion of pro-inflammatory cytokines IL-1β and TNF-α in LPS-induced RAW 264.7 cells (Deng et al., 2016). Therefore, the anti-inflammatory potential A. chinensis seeds mainly depend on the synergetic effect of these polyphenols, and it may be used to prevent a variety of inflammation related diseases.
Antibacterial Activity
All the extracts including skin, pulp, seeds, and stems showed bactericidal against Staphylococcus aureus, Streptococcus pyogenes, S. faecalis, Salmonella typhi, Proteus mirabilis, Pseudomonas aeruginosa, Escherichia coli, and Klebsiella pneumonia. The skin and pulp extracts showed inhibition activity against S. aureus and S. pyogenes with MIC values of 8 and 4 μg/ml, but they showed moderate inhibition activity against S. faecalis, S. typhi, P. mirabilis, P. aeruginosa, E. coli, and K. pneumonia with MIC values ranging from 16 to 128 μg/ml. The leaves and stems extract just inhibited S. pyogenes and P. aeruginosa with MIC values of both 64 and 32 μg/ml. The seeds extracts showed an exclusively bacteriostatic activity against these selected strains of bacteria with MIC values of between 1 and 8 μg/ml (Basile et al., 1997). Polyphenol from seeds of A. chinensis showed significant bactericidal against Bacillus cereus, B. subtilis, Shigella flexneri, and Salmonella Typhi, and bacteriostatic against B. thuringiensis. We can find that the antimicrobial activity of the polyphenol extract on gram-positive bacteria is higher than that of gram-negative bacteria (Deng et al., 2013). Therefore, kiwifruit seeds are potential food processing material for their antimicrobial activity.
Immunoregulatory Activity
Consumption of the aqueous extracts of whole fresh fruit of Hort16Aat 375 mg/kg for 12 days enhanced both innate and acquired immunity in cholera vaccine and tetanus/diphtheria vaccine models in Balb/c mice, showing a beneficial effect on healthy (Shu et al., 2008). The homopolysaccharide derivatived by O-sulfation from the roots of A. chinensis at concentration of 10 and 50 μg/ml activated phagocytic activity and increased NO production of RAW 264.7 macrophages, and the activity of sulfated polysaccharides is strongly related to the degree of the sulfation (Niu et al., 2016), and treatment with 50-300 μg/ml water-soluble polysaccharides dose-dependently stimulated NO production and phagocytic activity of RAW 264.7macrophages (Zhang et al., 2015). It remains to clarify the detailed mechanism of immunoregulatory activity and the responsible compositions for this valid action.
Hypolipemic and Antidiabetic Activities
Administered the seed oil of A. chinensis rich in fatty acids at 1.0 and 3.0 ml/kg·bw daily over 12 consecutive weeks significantly lowered bodyweight gain, inguinal fat tissue weight, and the accumulation of TC, TG, HDL-C, and LDL-C in liver of the high-fat diet-induced obese C57BL/6 mice. Meanwhile, long-term consumption of the seed oil of A. chinensis up-regulated the expression of thermogenesis-related genes like PPAR-γ, UCP1, PGC1-α, and PRDM16, down-regulated FAS expression, and altered the gut microbiota by decreasing the Firmicutes-to-Bacteroidetes ratio (Qu et al., 2019). In addition, the seed oil from A. chinensis supplementation improved insulin resistance and alleviated hyperglycemia by reducing HOMA-IR index and blood glucose in high fat diet-induced obese mice (Qu et al., 2019). Thus, the lipid lowering potential of A. chinensis seed provide a basis theory for food industries.
Cardiovascular Protective Effects
In H9c2 rat cardiac myocytes cells induced by hypoxia in cardiomyocytes treated with angiotensin II, treatment with 1.25 and 2.5 mg/ml polysaccharide of A. chinensis alleviated cardiac hypertrophy, decreased mitochondrial dysfunction and reduced cardiomyocytes apoptosis by decreasing the apoptosis-associated genes expression like mitochondria associated-1 and caspases3/8/9, and cleaving caspases-3/8/9. Additionally, the protective effects of polysaccharide against hypoxia-induced apoptosis may be attributable to inactivate the ERK1/2 and PI3K/AKT signaling pathways (Wang et al., 2018). The polysaccharide of A. chinensis can be potentially used in the treatment of heart disease. However, it is noteworthy that polysaccharide at high dose (10 mg/ml) suppressed the cardiomyocytes viability.
Hypnotic Effects
Oral administration of ethanol extracts from A. chinensis peel at dose of 250, 500, and 1,000 mg/kg dose-dependently decreased sleep latency and increased sleep duration in pentobarbital-treated mice. Especially, the sequentially partitioned with ethyl acetate fraction rich in flavonoids (1.63 mg QE/g) at 250 mg/kg exert significantly hypnotic effects and this sedative-hypnotic activity could be inhibited by GABAA-BZD receptor antagonist flumazenil. The flavonoids may be attributable to hypnotic activity via allosteric GABAA-BZD receptor modulation, but the precise mechanisms and the existing individual flavonoids are needed to be evaluated in the future (Yang et al., 2013).
Ace Inhibitory Activity
The 70% aqueous acetone extracts partitioning with hexane rich in flavonoid from Hort 16A dose-dependently inhibited ACE activity with IC50 of 12.81 mg/ml using a fluorescence-based biochemical assay. LC-MS/MS showed that the higher total phenolic and total flavonoid contents are identified in this extract. UPLC-MS/MS showed that polyphenols (231.32 µg/g DW) in the extract are mainly flavonols, flavanols, and phenolic acids. Specifically, quercetin-3-O-galactoside (205.19 µg/g DW), quercetin-3-O-glucoside (0.45 µg/g DW), quercetin-3-O-rhamnoside (0.61 µg/g DW), quercetin-3-O-rutinoside (0.29 µg/g DW), epicatechin (5.15 µg/g DW), catechin (0.75 µg/g DW), epigallocatechin (0.61 µg/g DW), phloridzin (2.03 µg/g DW), and isoferulic acid (15.12 µg/g DW) are major compounds in the extract (Hettihewa et al., 2018). These compounds could be responsible for the observed in vitro ACE inhibitory activity of Hort 16A fruit, though the active compounds identifying and in vivo animal studies remain to be investigated and conducted.
Dermatological Activity
The raw polysaccharides with >90% carbohydrate and 5.2% residual protein from the fresh fruit of A. chinensis at 10 μg/ml showed a significantly proliferation-promoting on cell proliferation rates of HaCaT cell line and primary keratinocytes (NHK), and it also significantly promoted proliferation of human dermal fibroblasts at 132 and 198 μg/ml. Meanwhile, treatment of the polysaccharides at 200 μg/ml significantly stimulated ATP-synthesis, promoted mitochondrial activity and energy metabolism of HaCaT keratinocytes, and significantly increased collagen synthesis in dermal skin equivalents (Deters et al., 2005). Kiwifruit pericarp proanthocyanidins mainly contained B-type propelargonidins, procyanidins, procyanidins gallate, and prodelphinidins showed strongly inhibition activity on tyrosinase, indicating that it can be used as whitening agents (Chai et al., 2014).
Cytochrome P450 Enzyme Inhibitory Activities
Cytochrome P450 system in liver plays an important role in drug metabolism. It transforms drug from hydrophobic to hydrophilic, which is easier to excrete. The 90% EtOH extract of A. chinensis root at 50 μg/ml exhibited inhibition activities on CYP2C9, CYP2D6, and CYP3A4 in human liver tissue with the 69.0, 76.3, and 53.3% of remaining activity, respectively. The inhibitory effect of the crude extract could be largely attributed to the presence of triterpenoids (Xu et al., 2016). It is worth noting that the combination of crude extracts or these triterpenoids with other medical herbs or drugs may lead to drug interaction with cytochrome CYPs at pharmacokinetic and pharmacodynamic levels, which indicates that people should cautiously consume A. chinensis fruit when taken medicine.
Processing and Utilization
Chinese kiwifruit is a very high nutritional value of nourishing and consumers’ favorite fruit, which has shown application potential in food, medicine, and health products industry. China is the largest kiwifruit producer in the world. In 2016, kiwifruit production in China reached 2.41 million tons per year, accounting for 56.0% of the world’s total kiwifruit production (United Nations Food and Agriculture Organization, 2016). To date, a series of commercially available products has been processed due to abundant nutrient substance and claimed health benefits. These Chinese kiwifruit related products include sliced fruit, juice, preserved fruit, yogurt, wine, canned fruit, dried kiwi slices, fruit vegetable juice drinks, biscuits, milk beverage, whipped cream, baked goods, vinegar, and oil capsule. Furthermore, various different parts of A. chinensis showed different uses. Briefly, the leaves contain protein, starch, and polyphenols, which may be developed as an excellent source of natural products. The beautiful and fragrant of Chinese kiwifruit flowers rich in honey juice and volatiles can be used as high-quality honey source. Kiwifruit peel residue as sources of high-quality pectin can be used as functional ingredient for food products. Chinese kiwifruit seeds rich in essential fatty acids, protein, and dietary fiber can be used in food and health products industry (Xie, 1975; Garcia et al., 2012). The roots and barks contain ursolic acid, oleanolic acid, and quercetin, which have antitumor effect against liver cancer, lung cancer, gastric cancer, esophageal cancer, colorectal cancer, and cervical cancer (Chang and Case, 2005; Xu et al., 2010; Wei et al., 2018). The different parts of A. chinensis are widely used as pharmaceutical raw materials in medicine for prevention and treatment of tumors. In addition, the various claimed nutritional and pharmacological properties including strong antitumor, antioxidation, and anti-inflammatory potential of various extracts or active compounds of A. chinensis indicated that they could be further developed for functional food with added-commercial value or effective and safe drug formulations.
Storage Methods
Chinese kiwifruit has a short postharvest life because of fast softening and serious decay. Preservation of Chinese kiwifruit for prolonged periods is particularly important. Freezing and frozen storage is currently the most common method, which can effectively inhibit the softening of kiwifruit and prolong its postharvest life. However, kiwifruit is cold-sensitive and very susceptible to chilling injury when storage at the temperature between −2°C and 2.5°C for a long time (Gerasopoulos et al., 2006; Ma et al., 2014). Interestingly, dipped by water for 10 min at 45°C to low temperature storage can prevent chilling injury development to kiwifruit. Meanwhile, the kiwifruit pretreated at 45°C and then stored at 0°C for 90 days showed higher firmness and soluble solids content, and MDA content and lipoxygenase activity in kiwifruit are reduced. However, pretreated at 20 and 55°C were ineffective at alleviating chilling tolerance (Ma et al., 2014). Various other treatments including preharvest calcium chloride sprays (Gerasopoulos and Drogoudi, 2005), putrescine (Yang et al., 2016), preharvest chilling (Sfakiotakis et al., 2005), and gradual cooling (Yang et al., 2013) have also been used to alleviate chilling injury in kiwifruit.
After harvest, kiwifruit is highly perishable, and its nutritional ingredients and quality decline rapidly due to the influence of internal biochemical reactions and external environment. The modified atmosphere packaging, chitosan, 1-methylcyclopropene, ClO2, ozone, tea polyphenols, protein, lipid composite film, oxalate, salicylic acid, and citric acid have been used individually or combined to alleviate physicochemical quality changes for postharvest of kiwifruit (Huang et al., 2017). The ozone treatment induced the ripening process, delayed the microbial growth, and influenced the content of vitamin C, polyphenols, flavonoids, and carotenoids (Goffi et al., 2019). The chitosan combined with salicylic acid treatment during storage at room temperature for 14 days provides a significantly effective preservative effect by delayed vitamin C and soluble solids decomposition, inhibiting moisture loss and acidity change, and maintaining texture and surface color of Chinese kiwifruit in 14 days of storage at room temperature (Huang et al., 2017).
Conclusions
Chinese kiwifruit and related products are increasingly popular throughout the world due to the remarkably economic, nutritional, and health benefits values. It is a good source of phenolic compounds, vitamin C, carbohydrates, sugars, amino acids, and minerals. Of particular note in kiwifruit is vitamin C and minerals K. The phenolic compounds present in Chinese kiwifruit are organic acids and flavonoids, and fruit peel and flesh, leaf, vine, and roots also contain a variety of these phenolic components. The major components of the roots are triterpenoids characterized by 12-en-28-oic acids of oleanane and ursane type. Terpenes, straight chain alkenes, alcohols, and esters were dominant volatile components in flowers and roots of A. chinensis. These chemical compounds render the A. chinensis with a range of sensory quality, nutritional, and pharmacological properties as proved by in vitro and in vivo studies. The claimed biological activity of isolated compounds, fractions, or crude extracts include antitumor, antioxidant, anti-inflammatory, antibacterial, immunoregulatory, hypolipemic, antidiabetic, and cardiovascular protective effects. Of particular note is that these claimed biological activities such as antitumor, antioxidant, and immunoregulatory may be greatly attributed to the existence of triterpenoids, polyphenols, flavonoid, polysaccharide, unsaturated fatty acid, and vitamin C. These findings suggest that Chinese kiwifruit can be useful in the prevention and treatment of pathologies associated to cancer, oxidative stress, and aging.
There are also research opportunities to better development, utilization, and protection kiwifruit for human consumption. Cytochrome P450 inhibitory activities, toxicity analysis, qualitative and quantitative metabolite research, effective and standardized quality standard building, and clinical studies should be encouraged to conducted for safe daily consumption. Meanwhile, the synergism and attenuation effects, metabolic behavior of various ingredients, as well as the in vivo and molecular mechanisms studies responsible for the observed biological properties should be conducted. It is also found that some of the A. chinensis cultivars were only supported by a few studies, and confirmative studies should be conducted to verify their health effects. Apart from the fruit, other plant parts of kiwifruit including leaves and roots should also be explored for effective utilization. The effective method and technology for the storage and preservation of kiwifruit during preharvest and postharvest remain to be explored to avoid the frequent chilling damage, soft rot, and mildew, and also decrease and improve the change of the chemical profile and bioactivity properties during storage.
Author Contributions
XC and YM obtained the literatures. JF, ZZ, and XH wrote the manuscript. XH, LH, and YL gave ideas and edited the manuscript. All authors approved the paper for publication.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
A549, Human alveolar basal epithelial cells; ABTS, 2′-azino-bis (3-ethylbenzthiazoline-6-sulfonic acid; ACE, Angiotensin converting enzyme; ALT, Alanine aminotransferase; AST, Aspartate aminotransferase; CE, Catechin equivalents; COX-2, Cyclooxygenase-2; CYP2C9, Cytochrome P450 2C9; CYP2D6, Cytochrome P450 2D6; CYP3A4, Cytochrome P450 3A4; CYPs, Cytochrome P450; DLX2, Distal-less-like homeobox protein; DPPH, 2,2-diphenyl-1-picrylhydrazyl; DW, Dry weight; EP3, Prostaglandin E2 receptor subtype 3; ERK1/2, Extracellular regulated protein kinases; FAS, Fatty acid synthase; FDW, Freeze-dried weight; FRAP, Ferric ion reducing antioxidant power; FW, flesh weight; GABA-BZD, γ-aminobutyric acid-benzodiazepine; GAE, Gallic acid equivalents; GC-MS, Gas chromatography-mass spectrometer; GSH, Glutathione; HaCaT, Human immortalized keratinocytes; HCC, Hepatocellular carcinoma; HDL-C, High density lipoprotein cholesterol; HEK 293, Human embryonic kidney 293 cells; HepG2, Liver hepatocellular cells; HO·, Hydroxyl radical; HOMA-IR, Homeostasis model assessment for insulin resistance; HPLC, High-performance liquid chromatography; IC50, Half maximal inhibitory concentration; IL-1β, Interleukin-1β; IL-6, Interleukin-6; iNOS, Inducible nitric oxide synthase; Keap1, Kelch-like ECH-associated protein 1; LC-MS/MS, Liquid chromatography-tandem mass spectrometry; LDL-C, Low density lipoprotein cholesterol; LoVo, Colorectal cancer cell line; MCF-7, Human breast adenocarcinoma cell line; MDA, Malondialdehyde; MIC, Minimum inhibitory concentration; NCI-H460, Large cell lung cancer cells; NF-κB, Nuclear factor-kappa B; NO, Nitric oxide; Nrf2, Nuclear factor (erythroid-derived 2)-like 2; ORAC, Oxygen radical absorbance capacity; P-388, Mouse leukemia cells; PGC1-α, Peroxisome proliferator-activated receptor gamma coactivator 1-alpha; PI3K/AKT, Phosphatidylinositol 3-kinase/protein kinase B; PPAR-γ, Peroxisome proliferators-activated receptors; PRDM16, PR domain containing 16; QE, Quercetin equivalents; RAW 264.7, Mouse leukaemic monocyte macrophage cell line; SK-OV-3, Human ovarian epithelial cancer cells; SOD, Superoxide dismutase; TC, Total cholesterol; TG, Triglyceride; TNF-α, Tumor necrosis factor alpha; UCP1, Uncoupling protein 1; UPLC-MS/MS, Ultra-high-performance liquid chromatography-tandem mass spectrometry; UPLC-QqQ-MS/MS, Ultra-high-performance hydrophilic interaction liquid chromatography-triple quadrupole tandem mass; UPLC-TOF/MS, Ultra-performance liquid chromatography quadrupole-time of flight mass spectrometry; USDA, United States Department of Agriculture; UV/Vis, Ultraviolet-visible spectroscopy; VCE, Vitamin C equivalents; VEGFR2/Src/FAK, Vascular endothelial growth factor receptor 2/Src/focal adhesion kinase.
References
- Basile A., Vuotto M. L., Violante U., Sorbo S., Martone G., Castaldo-Cobianchi R. (1997). Antibacterial activity in Actinidia chinensis, Feijoa sellowiana and Aberia caffra . Inter. J. Antimicrob. Ag. 8, 199–203. 10.1016/S0924-8579(97)00376-2 [DOI] [PubMed] [Google Scholar]
- Chai W. M., Shi Y., Feng H. L., Xu L., Xiang Z. H., Gao Y. S., et al. (2014). Structure characterization and antityrosinase mechanism of polymeric proanthocyanidins fractionated from kiwifruit pericarp. J. Agric. Food Chem. 62, 6382–6389. 10.1021/jf501009v [DOI] [PubMed] [Google Scholar]
- Chang J., Case R. (2005). Cytotoxic phenolic constituents from the root of Actinidia chinensis . Planta Med. 71, 955–959. 10.1055/s-2005-871225 [DOI] [PubMed] [Google Scholar]
- Chen X., Yang S., Bai S. (2011). Chemical constituents in root of Actinidia chinensis . Zhongcaoyao. 42, 841–843. 10.7666/d.y1883489 [DOI] [Google Scholar]
- Cheng Q. L., Li H. L., Huang Z. Q., Chen Y. J., Liu T. S. (2015). 2β, 3β, 23-trihydroxy-urs-12-ene-28-olic acid (TUA) isolated from Actinidia chinensis radix inhibits NCI-H460 cell proliferation by decreasing NF-κB expression. Chem.Biol. Interact. 240, 1–11. 10.1016/j.cbi.2015.06.038 [DOI] [PubMed] [Google Scholar]
- Cui Y. (2016). Study on chemical constituents in ethyl acetate extracts from the root of Actinidia chinensis. Hubei Agri. Sci. 55, 5359–5360. 10.14088/j.cnki.issn0439-8114.2016.20.048. [DOI] [Google Scholar]
- Cui Y., Zhang X., Chen J., Zhang Y., Lin X., Zhou L. (2007). Chemical constituents from root of Actinidia chinensis . Zhongguo Zhongyao Zazhi. 32, 1663–1665. 10.3321/j.issn:1001-5302.2007.16.016 [DOI] [PubMed] [Google Scholar]
- Deng J., Liu Q., Zhang C., Cao W., Fan D., Yang H. (2016). Extraction optimization of polyphenols from waste kiwi fruit seeds (Actinidia chinensis Planch.) and evaluation of its antioxidant and anti-inflammatory properties. Molecules 21, pii: . 10.3390/molecules21070832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng J. J., Liu Q. Q., Zhang Q., Zhang C., Liu D., Fan D. D., et al. (2018). Comparative study on composition, physicochemical and antioxidant characteristics of different varieties of kiwifruit seed oil in China. Food Chem. 264, 411–418. 10.1016/j.foodchem.2018.05.063 [DOI] [PubMed] [Google Scholar]
- Deng J. J., Yang H. X., Fan D. D., Cao W., Luo Y. E. (2013). Antibacterial activities of polyphenolic extract from kiwi fruit (Actinidia chinensis Planch.) seeds. J. Pure Appl. Microbio. 7, 491–494. [Google Scholar]
- Deters A. M., Schröder K. R., Hensel A. (2005). Kiwi fruit (Actinidia chinensis) polysaccharides exert stimulating effects on cell proliferation via enhanced growth factor receptors, energy production, and collagen synthesis of human keratinocytes, fibroblasts, and skin equivalents. J. Cell. Physiol. 202, 717–722. 10.1002/jcp.20161 [DOI] [PubMed] [Google Scholar]
- Drummond L. (2013). The composition and nutritional value of kiwifruit. Adv. Food Nutr. Res. Chapter 3 . 68, 33–54. 10.1016/B978-0-12-394294-4.00003-1 [DOI] [PubMed] [Google Scholar]
- Edmunds S. J., Roy N. C., Davy M., Cooney J. M., Barnett M. P., Zhu S., et al. (2012). Effects of kiwifruit extracts on colonic gene and protein expression levels in IL-10 gene-deficient mice. Br. J. Nutr. 108, 113–129. 10.1017/S0007114511005241 [DOI] [PubMed] [Google Scholar]
- Fang T. T., Fang Y., Xu X. J., He M. Y., Zhao Z. Y., Huang P. X., et al. (2019). Actinidia chinensis Planch. root extract attenuates proliferation and metastasis of hepatocellular carcinoma by inhibiting epithelial-mesenchymal transition. J. Ethnopharm. 231, 474–485. 10.1016/j.jep.2018.11.014 [DOI] [PubMed] [Google Scholar]
- Fiorentino A., Mastellone C., D’Abrosca B., Pacifico S., Scognamiglio M., Cefarelli G., et al. (2009). ƍ-Tocomonoenol: a new vitamin E from kiwi (Actinidia chinensis) fruits. Food Chem. 115, 187–192. 10.1016/j.foodchem.2008.11.094 [DOI] [Google Scholar]
- Flora of China (2007). Actinidia chinensis Planchon Vol. 12 Beijing: Science Press. [Google Scholar]
- Garcia C. V., Quek S. Y., Stevenson R. J., Winz R. A. (2012). Kiwifruit flavour: a review. Trends Food Sci. & Tech. 24, 82–91. 10.1016/j.tifs.2011.08.012 [DOI] [Google Scholar]
- Gerasopoulos D., Drogoudi P. D. (2005). Summer-pruning and preharvest calcium chloride sprays affect storability and low temperature breakdown incidence in kiwifruit. Postharvest Biol. Technol. 36, 303–308. 10.1016/j.postharvbio.2005.01.005 [DOI] [Google Scholar]
- Gerasopoulos D., Chlioumis G., Sfakiotakis E. (2006). Non-freezing points below zero induce low-temperature breakdown of kiwifruit at harvest. J. Sci. Food Agric. 86, 886–890. 10.1002/jsfa.2429 [DOI] [Google Scholar]
- Goffi V., Zampella L., Forniti R., Petriccione M., Botondi R. (2019). Effects of ozone postharvest treatment on physicochemical and qualitative traits of Actinidia chinensis‘Soreli’ during cold storage. J. Sci. Food Agric. 99, 5654–5661. 10.1002/jsfa.9823 [DOI] [PubMed] [Google Scholar]
- Gu J., Li J., Yang X. D., Shang Z. W., Wei H. Q., Jiang X. J., et al. (2017). Study on inhibition effect of Actinidia chinensis Planch. extracted by chloroform proliferation of HEP-2 Cells. Chin. Arch. Tradi. Chin. Med. 35, 2835–2838. 10.13193/j.issn.1673-7717.2017.11.028 [DOI] [Google Scholar]
- He G. N., Hu X. M., Wang H., Fan L., Wang B. C. (2015. a). Chemical constituents in root of Actinidia chinensis Planch. Zhonghua Zhongyiyao Zazhi. 30, 498–500. [Google Scholar]
- He J., Ma B. Z., Wang X. X., Liu F., Qin W. J., Zhang X. L., et al. (2015. b). Chemical constituents from the root of Actinidia chinensis (II). Zhongguo Yaoxue Zazhi. 50, 1960–1963. 10.11669/cpj.2014.03.005 [DOI] [Google Scholar]
- He J., Ma B. Z., Zhao T., Wang W., Wei F. L., Lu J., et al. (2014). Chemical constituents from the roots of Actinidia chinensis. Zhongguo Yaoxue Zazhi. 49, 184–186. 10.11669/cpj.2014.03.005 [DOI] [Google Scholar]
- He M. Y., Hou J. J., Wang L. Y., Zheng M. H., Fang T. T., Wang X. D., et al. (2017). Actinidia chinensis Planch. root extract inhibits cholesterol metabolism in hepatocellular carcinoma through upregulation of PCSK9. Oncotarget 8, 42136–42148. 10.18632/oncotarget.15010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hettihewa S. K., Hemar Y., Rupasinghe H. P. V. (2018). Flavonoid-rich extract of Actinidia macrosperma (a wild kiwifruit) inhibits angiotensin-converting enzyme in vitro . Food 7, 146. 10.3390/foods7090146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henare S. J. (2016). The nutritional composition of kiwifruit (Actinidia spp.). Nutr. Compos. Fruit Cultivars, Chapter 15, 337–368. 10.1016/B978-0-12-408117-8.00015-5 [DOI] [Google Scholar]
- Hou J. J., Wang L. Y., Wu D. J. (2018). The root of Actinidia chinensis inhibits hepatocellular carcinomas cells through LAMB3. Cell Biol. Toxicol. 34, 321–332. 10.1007/s10565-017-9416-7 [DOI] [PubMed] [Google Scholar]
- Huang Z. Y., Li J., Zhang J. F., Gao Y. Y., Hui G. H. (2017). Physicochemical properties enhancement of Chinese kiwi fruit (Actinidia chinensis Planch.) via chitosan coating enriched with salicylic acid treatment. J. Food Meas. Charact. 11, 184–191. 10.1007/s11694-016-9385-1 [DOI] [Google Scholar]
- Hwang J. S., Cho C. H., Baik M. Y., Park S. K., Heo H. J., Cho Y. S., et al. (2017). Effects of freeze-drying on antioxidant and anticholinesterase activities in various cultivars of kiwifruit (Actinidia spp.). Food Sci. Biotechnol. 26, 221–228. 10.1007/s10068-017-0030-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasawa H., Morita E., Yui S., Yamazaki M. (2011). Anti-oxidant effects of kiwi fruit in vitro and in vivo. Biol. Pharm. Bull. 34, 128–134. 10.1248/bpb.34.128 [DOI] [PubMed] [Google Scholar]
- Ji Z., Liang X. (1985). Chemical constituents of the root of Actinidia chinensis Planch. Yaoxue Xuebao 20, 778–781. [PubMed] [Google Scholar]
- Jiao Y., Chen D. L., Fan M. T., Young, Qu S. (2019). UPLC-QqQ-MS/MS-based phenolic quantification and antioxidant activity assessment for thinned young kiwifruits. Food Chem. 281, 97–105. 10.1016/j.foodchem.2018.12.062 [DOI] [PubMed] [Google Scholar]
- Kaur L., Rutherfurd S. M., Moughan P. J., Drummond L., Boland M. J. (2010). Actinidin enhances gastric protein digestion as assessed using an in vitro gastric digestion model. J. Agri. Food Chem. 58, 5068–5073. 10.1021/jf903332a [DOI] [PubMed] [Google Scholar]
- Ku C. Y., Wang Y. R., Lin H. Y., Lu S. C., Lin J. Y. (2015). Corosolic acid inhibits hepatocellular carcinoma cell migration by targeting the VEGFR2/Src/FAK pathway. PLoS One. 10, e0126725. 10.1371/journal.pone.0126725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kvesitadze G. I., Kalandiya A. G., Papunidze S. G., Vanidze M. R. (2001). Identification and quantification of ascorbic acid in kiwi fruit by high-performance liquid chromatography. Appl. Biochem. Micro. 37, 215–218. 10.1023/A:1002848302873 [DOI] [Google Scholar]
- Lahlou E. H., Hirai N., Kamo T., Tsuda M., Ohigashi H. (2001). Actinidic acid, a new triterpene phytoalexin from unripe kiwi fruit. Biosci. Biotech. Bioch. 65, 480–483. 10.1271/bbb.65.480 [DOI] [PubMed] [Google Scholar]
- Lee D. E., Shin B. J., Hur H. J., Kim J. H., Kim J., Kang N. J., et al. (2010). Quercetin, the active phenolic component in kiwifruit, prevents hydrogen peroxide-induced inhibition of gap-junction intercellular communication. Brit. J. Nutr. 104, 164–170. 10.1017/S0007114510000346 [DOI] [PubMed] [Google Scholar]
- Lee I., Lee B. H., Eom S. H., Oh C. S., Kang H., Cho Y. S., et al. (2015). Antioxidant capacity and protective effects on neuronal PC-12 cells of domestic bred kiwifruit. Korean J. Hortic. Sci. Technol. 33, 259–267. 10.7235/hort.2015.14123 [DOI] [Google Scholar]
- Li D. X., Zhu F. (2017). Physicochemical properties of kiwifruit starch. Food Chem. 220, 129–136. 10.1016/j.foodchem.2016.09.192 [DOI] [PubMed] [Google Scholar]
- Lin P. F. (1988). Antitumor effect of Actinidia chinensis polysaccharide on murine tumor. Zhonghua Zhong Liu Za Zhi. 10, 441–444. [PubMed] [Google Scholar]
- Liu W., Hui G. H. (2015). Kiwi fruit (Actinidia chinensis) quality determination based on surface acoustic wave resonator combined with electronic nose. Bioengineered 6, 1–9. 10.1080/21655979.2014.996430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luan X., Li X. J., Guo M. M. (2017). Xiangxi kiwifruit seed component and characteristics of kiwifruit seed oil. Chin. Oils Fats. 42, 136–139. 10.3969/j.issn.1003-7969.2017.08.030 [DOI] [Google Scholar]
- Lv J. P., Wang L. Y., Shen H., Wang X. D. (2018). Regulatory roles of OASL in lung cancer cell sensitivity to Actinidia chinensis Planch. root extract (acRoots). Cell Biol. Toxicol. 34, 207–218. 10.1007/s10565-018-9422-4 [DOI] [PubMed] [Google Scholar]
- Ma Q. S., Suo J. T., Huber D. J., Dong X. Q., Han Y., Zhang Z. K., et al. (2014). Effect of hot water treatments on chilling injury and expression of a new C-repeat binding factor (CBF) in ‘Hongyang’ kiwifruit during low temperature storage. Postharvest Biol. Technol. 97, 102–110. 10.1016/j.postharvbio.2014.05.018 [DOI] [Google Scholar]
- Ma T. T., Sun X. Y., Zhao J. M., You Y. L., Lei Y. S., Gao G. T., et al. (2017). Nutrient compositions and antioxidant capacity of kiwifruit (Actinidia) and their relationship with flesh color and commercial value. Food Chem. 218, 294–304. 10.1016/j.foodchem.2016.09.081 [DOI] [PubMed] [Google Scholar]
- McGhie T. K., Ainge G. D. (2002). Color in ruit of the genus Actinidia: carotenoid and chlorophyll compositions. J. Agri. Food Chem. 50, 117–121. 10.1021/jf010677l [DOI] [PubMed] [Google Scholar]
- Meng F. C., Cao J. F., Zhang Y. T., Chang M. Y. (2017). Optimization of extraction technology of pectin from kiwifruit peel and pulp by ultrasound-assisted extraction. J. Green Sci. Technol. 14, 283–285. 10.16663/j.cnki.lskj.2017.14.101 [DOI] [Google Scholar]
- Michaud J., Ane-Margail M. (1977). Analytical study of catechuic tannins. I. Flavanolic oligomers of Actinidia chinensis Planch. Babin, Bull. Soc. Parma Bord. 116, 52–64. [Google Scholar]
- Montefiori M., McGhie T. K., Costa G., Ferguson A. R. (2005). Pigments in the fruit of red-fleshed kiwifruit (Actinidia chinensis and Actinidia deliciosa). J. Agric. Food Chem. 53, 9526–9530. 10.1021/jf051629u [DOI] [PubMed] [Google Scholar]
- Niu H., Song D., Sun Y. L., Zhang W. X., Mu H. B., Duan J. Y. (2016). Preparation and sulfation of an α-glucan from Actinidia chinensis roots and their potential activities. Int. J. Biol. Macromol. 92, 981–987. 10.1016/j.ijbiomac.2016.07.091 [DOI] [PubMed] [Google Scholar]
- Papunidze G., Kalandiya A., Papunidze S., Vanidze M. (2001). Kiwifruit chemical composition. Bull. Ge. Acad. Sci. 164, 544–546. [Google Scholar]
- Qu L., Liu Q., Zhang Q., Tuo X., Fan D., Deng J., et al. (2019). Kiwifruit seed oil prevents obesity by regulating inflammation, thermogenesis, and gut microbiota in high-fat diet-induced obese C57BL/6 mice. Food Chem. Toxicol. 125, 85–94. 10.1016/j.fct.2018.12.046 [DOI] [PubMed] [Google Scholar]
- Sfakiotakis E., Chlioumis G., Gerasopoulos D. (2005). Preharvest chilling reduces low temperature breakdown incidence of kiwifruit. Postharvest Biol. Technol. 38, 169–174. 10.1016/j.postharvbio.2005.06.010 [DOI] [Google Scholar]
- Sharon J. H. (2016). The nutritional composition of kiwifruit (Actinidia spp.). Nutr. Compos. Fruit Cultivars. 15, 343–345. 10.1016/B978-0-12-408117-8.00015-5 [DOI] [Google Scholar]
- Shen L., Zhang G. J., Zhang G. S., Song W. Y., Xu G. H. (2014). Effect of Actinidia chinensis polysaccharide on apoptosis of MFC and their orthotopic transplanted tumor of gastric cancer. Chin. Trad. Herb. Drugs. 45, 673–678. 10.7501/j.issn.0253-2670.2014.05.015 [DOI] [Google Scholar]
- Shu Q., De Silva U. M., Chen S., Peng W. D., Ahmed M., Lu G. J., et al. (2008). Kiwifruit extract enhances markers of innate and acquired immunity in a murine model. Food Agri. Immunol. 19, 149–161. 10.1080/09540100802117198 [DOI] [Google Scholar]
- Sivakumaran S., Huffman L., Sivakumaran S., Drummond L. (2018). The nutritional composition of Zespri SunGold kiwifruit and ZespriSweet green kiwifruit. Food Chem. 238, 195–202. 10.1016/j.foodchem.2016.08.118 [DOI] [PubMed] [Google Scholar]
- Sun L. F., Li X. F., Li G., Dai B., Tan W. (2017). Actinidia chinensis Planch. improves the indices of antioxidant and anti-inflammation status of type 2 diabetes mellitus by activating keap1 and nrf2 via the upregulation of microRNA-424. Oxid. Med. Cell. Longev. 2017, 7038789. 10.1155/2017/7038789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Plant List (2013). Version 1.1. Published on the internet; http://www.theplantlist.org/ (accessed 1st January).
- Twidle A. M., Barker D., Seal A. G., Fedrizzi B., Suckling D. M. (2018). Identification of floral volatiles and pollinator responses in kiwifruit cultivars, Actinidia chinensis var. chinensis . J. Chem. Ecol. 44, 406–415. 10.1007/s10886-018-0936-2 [DOI] [PubMed] [Google Scholar]
- United Nations Food and Agriculture Organization (2016). Kiwifruit production in 2016. “Crops/World Regions/Production Quantity from pick lists”. Retrieved 2018-02-20.
- United States Department of Agriculture USDA Food Composition Databases (2018). https://ndb.nal.usda.gov/ndb/ Accessed on April, 2018.
- Wang H. X., Ng T. B. (2002). Isolation of an antifungal thaumatin-like protein from kiwi fruits. Phytochem. 61, 1–6. 10.1016/S0031-9422(02)00144-9 [DOI] [PubMed] [Google Scholar]
- Wang M. Y., MacRae E., Wohlers M., Marsh K. (2011). Changes in volatile production and sensory quality of kiwifruit during fruit maturation in Actinidia deliciosa‘Hayward’ and A. chinensis‘Hort16A’. Postharvest Biol. Technol. 59, 16–24. 10.1016/j.postharvbio.2010.08.010 [DOI] [Google Scholar]
- Wang Q., Lei X. X., Yang X. D., Xin T., Li Y. Q. (2017). Purification of total triterpenes from the root of Actinidia chinensis Planch. and its antitumor activity. Guangdong Med. J. 38, 514–518. 10.3969/j.issn.1001-9448.2017.04.005 [DOI] [Google Scholar]
- Wang Q., Xu Y. F., Gao Y., Wang Q. (2018). Actinidia chinensis Planch. polysaccharide protects against hypoxia-induced apoptosis of cardiomyocytes in vitro . Mol. Med. Rep. 18, 193–201. 10.3892/mmr.2018.8953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Zhao C. L., Li J. Y., Liang Y. J., Yang R. Q., Liu J. Y., et al. (2018). Evaluation of biochemical components and antioxidant capacity of different kiwifruit (Actinidia spp.) genotypes grown in China. Biotec. Biotechnol. Equip. 32, 1–8. 10.1080/13102818.2018.1443400 [DOI] [Google Scholar]
- Wei L. B., Ma S. Y., Liu H. X., Huang C. S., Liao N. (2018). Cytotoxic triterpenoids from roots of Actinidia chinensis . Chem. Biodivers. 15, e1700454. 10.1002/cbdv.201700454 [DOI] [PubMed] [Google Scholar]
- Wurms K. V., Cooney J. M. (2006). Isolation of a new phenolic compound, 3, 5-dihydroxy-2-(methoxycarbonylmethyl) phenyl 3, 4-dihydroxybenzoate, from leaves of Actinidia chinensis (kiwifruit). Asian J. Biochem. 1, 325–332. 10.3923/ajb.2006.325.332 [DOI] [Google Scholar]
- Xia C., Chen J., Zhang Y. J., Zhu Y. Q., Li H. J., Deng J. L. (2017). Isolation, purification, monosaccharide composition and anticancer proliferation activity of polysaccharide fraction from Hongyang kiwifruit (Actinidia chinensis). Food Sci. 38, 126–131. 10.7506/spkx1002-6630-201721020 [DOI] [Google Scholar]
- Xie Z. W. (1975). National compilation of Chinese herbal medicine Vol. I. Beijing: People’s Health Publishing House, 820–821.
- Xu Y. X., Xiang Z. B., Jin Y. S., Shen Y., Chen H. S. (2010). Two new triterpenoids from the roots of Actinidia chinensis. Fitoterapia. 81, 920–924. 10.1016/j.fitote.2010.06.007 [DOI] [PubMed] [Google Scholar]
- Xu Y. X., Xiang Z. B., Jin Y. S., Xu W., Sun L. N., Chen W. S., et al. (2016). Constituents from the roots of Actinidia chinensis and their cytochrome P450 enzyme inhibitory activities. Chem. Biodivers. 13, 1454–1459. 10.1002/cbdv.201500518 [DOI] [PubMed] [Google Scholar]
- Yang H. L., Lee Y. C., Han K. S., Singh H. S., Yoon M., Park J. H., et al. (2013). Green and gold kiwifruit peel ethanol extracts potentiate pentobarbital-induced sleep in mice via a GABAergic mechanism. Food Chem. 136, 160–163. 10.1016/j.foodchem.2012.07.111 [DOI] [PubMed] [Google Scholar]
- Yang Q., Rao J., Yi S., Meng K., Wu J., Hou Y. (2013). Antioxidant enzyme activity and chilling injury during low-temperature storage of kiwifruit cv Hongyang exposed to gradual postharvest cooling. Horticul. Environ. Biotechnol. 53, 505–512. 10.1007/s13580-012-0101-8 [DOI] [Google Scholar]
- Yang Q., Wang F., Rao J. (2016). Effect of putrescine treatment on chilling injury, fatty acid composition and antioxidant system in kiwifruit. PLoS One. 11, e0162159. 10.1371/journal.pone.0162159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu A. N., Tian D. T., Qu W. Y., Tan Z. W., Song X. J. (2009). Chemical composition of the essential oil of the roots of Actinidia chinensis from China. Chem. Nat. Com. 45, 108–109. 10.1007/s10600-009-9235-z [DOI] [Google Scholar]
- Yuliarti O., Goh K. K., Matia-Merino L., Mawson J., Brennan C. (2015. a). Extraction and characterisation of pomace pectin from gold kiwifruit (Actinidia chinensis). Food Chem. 187, 290–296. 10.1016/j.foodchem.2015.03.148 [DOI] [PubMed] [Google Scholar]
- Yuliarti O., Goh K. K., Matia-Merino L., Mawson J., Drummond L., Brennan C. S. (2008). Effect of extraction techniques and conditions on the physicochemical properties of the water soluble polysaccharides from gold kiwifruit (Actinidia chinensis). Inter. J. Food Sci. Tec. 43, 2268–2277. 10.1111/j.1365-2621.2008.01866.x [DOI] [Google Scholar]
- Yuliarti O., Matia-Merino L., Goh K. K., Mawson J., Williams M. A., Brennan C. (2015. b). Characterization of gold kiwifruit pectin from fruit of different maturities and extraction methods. Food Chem. 166, 479–485. 10.1016/j.foodchem.2014.06.055 [DOI] [PubMed] [Google Scholar]
- Zhang L., Zhang W. X., Wang Q. J., Wang D. D., Dong D. Q., Mu H. B., et al. (2015). Purification, antioxidant and immunological activities of polysaccharides from Actinidia Chinensis roots. Int. J. Biol. Macromol. 72, 975–983. 10.1016/j.ijbiomac.2014.09.056 [DOI] [PubMed] [Google Scholar]
- Zhang T., Li C., Luo A. W., Tang M. L., Chen H., Li X. L., et al. (2016). Antioxidant activities in vitro of different fruit parts of eight kiwifruit varieties. Food Sci. 37, 88–93. 10.7506/spkx1002-6630-201619015 [DOI] [Google Scholar]
- Zhang X. Y., Zhou Y., Wei Z. P., Shen J., Wang L. K., Ma Z. Q., et al. (2018). Antiphytoviral toxins of Actinidia chinensis root bark (ACRB) extract: laboratory and semi-field trials. Pest Manag. Sci. 74, 1630–1636. 10.1002/ps.4854 [DOI] [PubMed] [Google Scholar]
- Zhao T., He J., Wang X., Ma B., Wang X., Zhang L., et al. (2014). Rapid detection and characterization of major phenolic compounds in radix Actinidia chinensis Planch. by ultra-performance liquid chromatography tandem mass spectrometry. J. Pharm. Biomed. Anal. 98, 311–320. 10.1016/j.jpba.2014.05.019 [DOI] [PubMed] [Google Scholar]
- Zhou X. F., Liu Y. H., Tang L., Zhang P., Wu J. Z. (2010). Chemical constituents from the roots of Actinidia chinensis . Chem. Nat. Compd. 46, 308–309. 10.1007/s10600-010-9599-0 [DOI] [Google Scholar]
- Zhou X. F., Zhang P., Pi H. F., Zhang Y. H., Ruan H. L., Wang H., et al. (2009). Triterpenoids from the roots of Actinidia chinensis . Chem. Biodivers. 6, 1202–1207. 10.1002/cbdv.200800214 [DOI] [PubMed] [Google Scholar]
- Zhu W. J., Yu D. H., Zhao M., Lin M. G., Lu Q., Wang Q. W., et al. (2013). Antiangiogenic triterpenes isolated from Chinese herbal medicine Actinidia chinensis Planch. Anticancer Ag. Med. Chem. 13, 195–198. 10.2174/1871520611313020002 [DOI] [PubMed] [Google Scholar]
- Zuo L. L., Wang Z. Y., Fan Z. L., Tian S. Q., Liu J. R. (2012). Evaluation of antioxidant and antiproliferative properties of three Actinidia (Actinidia kolomikta, Actinidia arguta, Actinidia chinensis) extracts in vitro . Inter. J. Mol. Sci. 13, 5506–5518. 10.3390/ijms13055506 [DOI] [PMC free article] [PubMed] [Google Scholar]