Abstract
Objective:
The aim of this study was to assess the efficacy of maintenance pembrolizumab in patients with extensive-stage SCLC after treatment with platinum and etoposide.
Methods:
Patients with extensive-stage SCLC with a response or stable disease after induction chemotherapy were eligible. Pembrolizumab at a dose of 200 mg administered intravenously every 3 weeks was initiated within 8 weeks of the last cycle of chemotherapy. The primary end point of the study was progression-free survival (PFS) from study registration, with overall survival (OS) as a key secondary end point. Available tumor tissue was assessed for expression of programmed death ligand 1 (PD-L1) both in the tumor cells and in the surrounding stroma. Blood for circulating tumor cells was collected before the first, second, and third cycles of pembrolizumab.
Results:
Of the 45 patients enrolled, 56% were male and 22% had treated brain metastases. The median PFS was 1.4 months (95% confidence interval [CI]: 1.3–2.8), with a 1-year PFS of 13%. The median OS was 9.6 months (95% CI: 7.0–12), with a 1-year OS of 37%. Of the 30 tumors that could be assessed, three had PD-L1 expression (≥1%) in the tumor cells. A total of 20 tumors could be assessed for PD-L1 expression in the stroma. The median PFS in the eight patients with tumors positive for expression of PD-L1 at the stromal interface was6.5 months (95% CI: 1.1–12.8) compared with 1.3 months (95% CI: 0.6–2.5) in 12 patients with tumors negative for this marker. No unexpected toxicities were observed.
Conclusion:
Maintenance pembrolizumab did not appear to improve median PFS compared with the historical data. However, the 1-year PFS rate of 13% and OS rate of 37% suggest that a subset of patients did benefit from pembrolizumab.
Keywords: SCLC, Maintenance, Pembrolizumab, Metastatic
Introduction
More than 30,000 new cases of SCLC are diagnosed each year in the United States, and most of these patients have extensive-stage disease at diagnosis.1 Despite a high response rate with platinum-etoposide combination therapy, the median progression-free survival (PFS) after completion of initial chemotherapy is only 2 months and the median overall survival (OS) is about 10 months.2 Therefore, there remains a need to evaluate novel agents for the management of these patients.
Recently, immune checkpoint inhibitors, specifically, drugs targeting the programmed cell death protein 1 (PD-1) pathway in T cells, have shown clinical benefit in several tumor types.3 Pembrolizumab, which is an antibody targeting programmed cell death protein 1 (PD-1) is approved for several tumor types, including advanced NSCLC, in both the frontline and recurrent settings.4 Available data suggest that immune checkpoint inhibitors are more likely to benefit patients with NSCLC who are smokers and whose tumors have a high mutational burden.
SCLC occurs almost exclusively in patients who are smokers, and generally these tumors have a high mutational burden. Therefore, there is an expectation that immune checkpoint inhibitors will be beneficial in these patients. We speculated that immunotherapy may be better tolerated and more effective in patients after completion of chemotherapy because these patients are likely to have better performance status and fewer symptoms than at the time of disease progression. In addition, clinical and preclinical data suggest that chemotherapy may enhance the susceptibility of the tumor to immunotherapy.5,6
Therefore, we conducted a single-arm phase II study to evaluate the ability of maintenance pembrolizumab to improve PFS and OS in patients with extensive-stage SCLC.
Methods
Study Population
Patients with extensive-stage SCLC were eligible if they were at least 18 years of age and had a response or stable disease after four to six cycles of platinum-etoposide chemotherapy. Patients were required to have an Eastern Cooperative Oncology Group performance status of 0 or 1 and adequate hematologic, hepatic, and renal function. Patients with treated brain metastases were eligible. Patients were excluded if they had autoimmune disease, including a paraneoplastic disorder of autoimmune nature that required systemic treatment (disease-modifying agents, corticosteroids, or other immunosuppressive drugs) within the previous 3 months or active interstitial lung disease or pneumonitis. Prior therapy with immune checkpoint inhibitors was not allowed. Prophylactic cranial radiation and thoracic radiation were permitted.
The study procedures were approved by the institutional review boards at each participating institution. Good Clinical Practice guidelines, Declaration of Helsinki ethical standards, and all local and national regulations were followed. All patients provided written informed consent before participation.
Study Design and Assessments
Imaging studies, including brain scans confirming response or disease stability, had to be done no more than 3 weeks before the patient started treatment with pembrolizumab. Patients treated with brain radiation, either prophylactic or therapeutic, or radiation to any other site, were required to have completed radiation therapy at least 7 days before starting treatment with pembrolizumab.
All eligible patients were treated with pembrolizumab at a dose of 200 mg intravenously every 3 weeks. Pembrolizumab had to be started within 8 weeks of the start of the last cycle of chemotherapy. Therapy was continued for a total of 2 years unless disease progression or unacceptable toxicity developed or the patient withdrew consent. A patient could continue to receive pembrolizumab despite progression defined on the basis of the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 if it was determined that the patient was deriving clinical benefit. However, if there was further RECIST-defined progression after two more cycles, pembrolizumab was discontinued. Adverse events were assessed according to National Cancer Institute Common Toxicity Criteria version 4.0. Treatment delays or discontinuations were defined for drug-related toxicities, including immune-related adverse events. No modification of the pembrolizumab dose was permitted.
Patients underwent imaging studies to assess disease status after every two cycles for the first six cycles and then at the discretion of the treating physician, but no less than every four cycles. Retrieval of pretreatment biopsy specimens for assessment of PD-L1 expression in tumor cells and stromal tissue was conducted by Qualtek Clinical Laboratories (Newton, PA). Assessment of tumor PD-L1 level was conducted by using the DAKO 22C3 antibody (Dako, Carpinteria, CA). A sample was considered adequate for PD-L1 assessment only if there were at least 50 viable tumor cells or five viable tumor cells with PD-L1 staining. A modified proportion score was used to assess PD-L1 expression in tumor cells. The modification in modified proportion score is that mononuclear cells within the tumor cell nests staining for PD-L1 were counted in combination with tumor cells positive for PD-L1. In addition to PD-L1 expression in the tumor, PD-L1 expression in the surrounding stroma was also assessed. The stromal interface was considered positive for PD-L1 if a lichenoid pattern of PD-L1 membrane-stained cells surrounding the tumor nests was identified at low power (a 4× objective). At higher power (a 20× objective), most such cells appeared to represent macrophages, though other mononuclear cells may have been present.
In addition, blood was collected before the first, second, and third cycles of pembrolizumab to assess circulating tumor cells (CTCs). CTC assessment and analysis was performed by using the CELLSEARCH system from Menarini Silicon Biosystems, Inc (Athyn, PA). Blood samples were processed within 72 hours of the draw. These assessments were conducted by the translational core laboratories of the Karmanos Cancer Institute (Detroit, MI).
Statistical Analysis
The primary analysis was designed to estimate the PFS of patients with SCLC who received maintenance pembrolizumab. PFS was defined as duration of time from registration to time of progression by RECIST 1.1 criteria. Patients who died without reported prior progression were considered to have progressed on the day of their death. The median PFS in patients with SCLC who have stable or responding disease after initial chemotherapy is approximately 2 months. We expected that maintenance pembrolizumab would improve the median PFS by 50% (to 3 months). Assuming an exponential and one-sided α value of 0.05, 38 patients accrued in 18 months with a minimum follow-up time of 6 months would achieve 80% power. An enrollment of 43 patients was planned, assuming a dropout rate of approximately 10%. In addition to the primary end point, secondary end points were OS and response rate. Descriptive statistics were performed for baseline characteristics. Response rate was summarized with the Wilson confidence interval (CI). Fisher’s exact test was used for subgroup analysis of the categorical data. PFS and OS were estimated by using Kaplan-Meier methods, from which the median and 95% CI were calculated. The exact binary method was used to calculate CI estimates for toxicity and response rates. As for the exploratory analyses, Cox regression was used to evaluate the association between time-to-event outcomes and covariates such as PD-L1 status and CTCs. Landmark analysis was performed for the association between nonbaseline CTC levels and time-to-event end points. All statistical tests were two sided, and p values less than 0.05 were considered statistically significant. All analyses were performed with the use of SAS software (version 9.4, SAS Institute Inc., Cary, NC) and R version 3.4 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Patients
Between February 25, 2015, and November 7, 2016, a total of 45 patients were enrolled at five sites in the United States. The baseline demographics are shown in Table 1. The median patient age was 66 years (range 50–87); 56% of the patients were male and 22% had brain metastases at baseline. All patients with brain metastases received whole brain radiation, and one patient received prophylactic cranial radiation.
Table 1.
Baseline Demographics
| Characteristic | n (%) (N = 45) |
|---|---|
| Median age, y (range) | 66 (50–86) |
| Sex | |
| Male | 20 (44) |
| Female | 25 (56) |
| Race | |
| White | 37 (82) |
| Black | 6 (13) |
| Other | 2 (5) |
| Current or former smoker | 44 (98) |
| Brain metastases | 10 (22) |
| Chemotherapy cycles | |
| 4 | 23 (51) |
| 5–6 | 22 (49) |
| Median time to first dose of pembrolizumab, wk (range) | 5 (3–9) |
| Measurable disease | 34 (76) |
The median time from the last cycle of chemotherapy to the first dose of pembrolizumab was 5 weeks (range 3–9 weeks). The median number of cycles administered was 4 (range, one–26). Four patients continue to receive therapy and have received 18 to 26 cycles of therapy. The median duration of follow-up is 14.6 months (95% CI: 12.3–16.6).
Outcomes
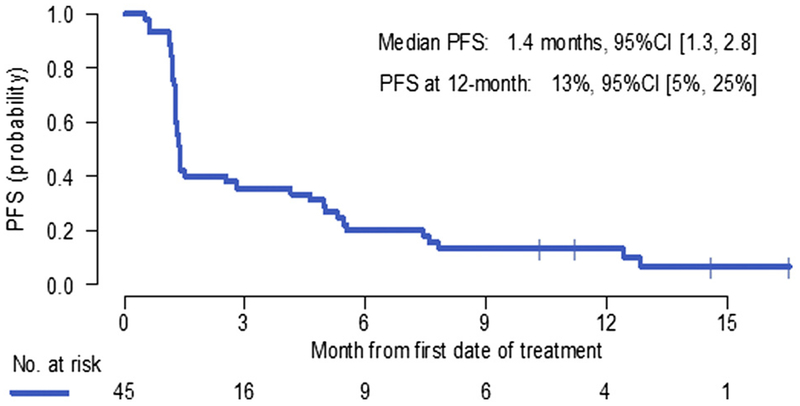
The median PFS from study registration was 1.4 months (95% CI: 1.3–2.8) (Fig. 1). The 6-month and 12-month PFS rates were 20% and 13%, respectively. The response rate in all enrolled patients was 11.1% (one complete response and four partial responses [PRs]) (95% CI: 4.8–23.5). Among 34 patients with measurable disease before they started taking pembrolizumab, the response rate was 14.7% (95% CI:6.4–30.1). The median duration of response was 10.8 months (95% CI: 5.8–not reached).
Figure 1.
Progression-free survival (PFS) from study registration (N = 45). CI, confidence interval.
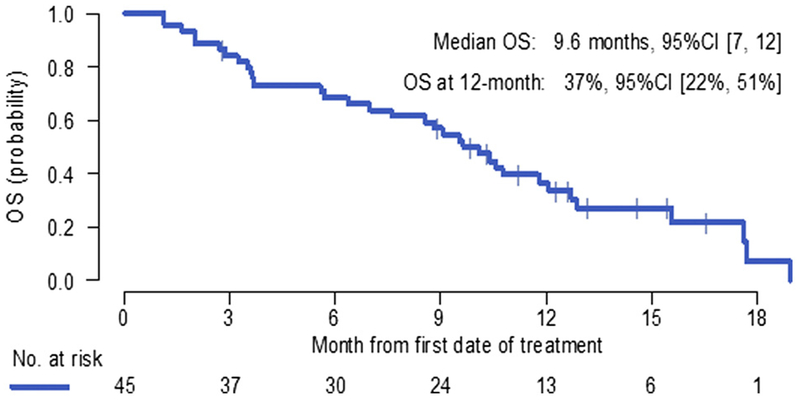
Of the 41 patients who had disease progression, 14 did not receive any further therapy, 21 received chemotherapy, and two received immunotherapy; in four patients subsequent therapy was not known. The median OS was 9.6 months (95% CI: 7.0–12.0), with 6-month and 12-month OS rates of 68% and 37%, respectively (Fig. 2).
Figure 2.
Overall survival (OS) from study registration (N = 45). CI, confidence interval.
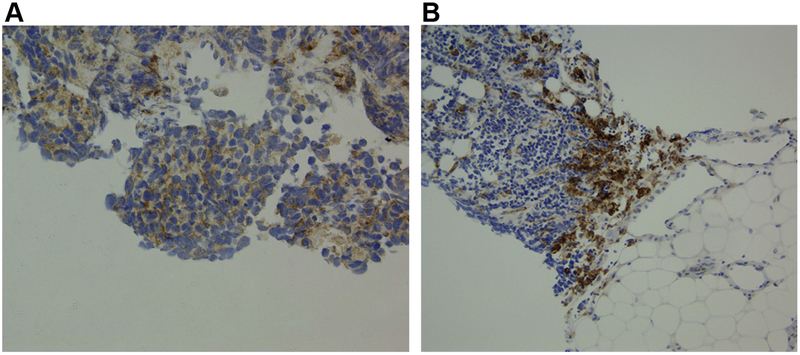
The tumors of 30 patients were deemed adequate for assessment of PD-L1 expression (Fig. 3). Only three patients had PD-L1 expression detected in their tumor cells. The PFS times in these three patients were 10, 11, and 13 months, and two of these three patients are continuing to receive therapy without progression. Two of the three patients whose tumors were PD-L1- positive had measurable disease at baseline, and both had a PR. PD-L1 expression at the stromal interface could be assessed in 20 tumors, of which eight were positive. Of the three tumors that were positive for PD-L1 expression in tumor cells, two were also positive for PD-L1 expression at the stromal interface. In the eight patients with PD-L1 expression at the stromal interface, the median PFS and OS times were6.5 months (95% CI: 1.1–12.8) and 12.8 months (95% CI: 1.1–17.6), respectively (Table 2). In the 12 patients whose tumors were negative for stromal PD-L1, the median PFS and OS times were 1.3 months (95% CI: 0.6–2.5) and 7.6 months (95% CI: 2.0–12.7), respectively. Of the eight patients with tumors that were positive for stromal PD-L1, three had a PR (37.5% [95% CI: 13.7–69.4]) compared with only one of the 12 patients with tumors negative for stromal PD-L1(8.3% [95% CI: 1.5–35.4]).
Figure 3.
Programmed death ligand 1 (PD-L1) expression in the tumor cells (A) and at the stromal interface (B).
Table 2.
Outcomes Based on PD-L1 Expression at the Stromal Interface (n = 20)
| PD-L1 Status | n | PFS, mo (95% CI) | OS, mo (95% CI) |
|---|---|---|---|
| Positive | 8 | 6.5 (1.1–12.8) | 12.8 (1.1–17.6) |
| Negative | 12 | 1.3 (0.6–2.5) | 7.6 (2.0–12.7) |
PD-L1, programmed death ligand 1; PFS, progression-free survival; CI, confidence interval; OS, overall survival.
CTCs at baseline were assessed in 37 patients and were detected in 19 (51%). The median number of CTCs was 1 (range 0–256). PFS and OS did not correlate with the baseline CTC count or with changes in the CTC count during therapy.
Safety
The most common treatment-emergent adverse events observed were fatigue, nausea, pruritus, constipation, and cough (Table 3). Three adverse event categories were considered immune related: rash developed in eight patients, hypothyroidism developed in four patients (all ≤grade 2), and type I diabetes mellitus with diabetic ketoacidosis developed in one patient. Serious adverse events included two patients with acute coronary syndrome, one of whom died from the adverse event and one who developed complete heart block without evidence of myocarditis but died from complications of the event. The only grade 3 toxicity that occurred in at least 5% of the patients was hyponatremia, which occurred in four patients.
Table 3.
Incidence of Treatment-Emergent Adverse Events of All Grades with a Frequency of 10% or Higher
| Toxicity | Frequency, n (%) |
|---|---|
| Fatigue | 18 (40) |
| Nausea | 12 (27) |
| Pruritus | 10 (22) |
| Cough | 10 (22) |
| Constipation | 10 (22) |
| Dyspnea | 9 (20) |
| Dizziness | 9 (20) |
| Rash | 8 (18) |
| Diarrhea | 7 (16) |
| Increased aspartate transaminase level | 6 (13) |
| Back pain | 6 (13) |
| Headache | 6 (13) |
| Noncardiac chest pain | 6 (13) |
| Neuropathy | 5 (11) |
| Abdominal pain | 5 (11) |
| Generalized weakness | 5 (11) |
| Decreased lymphocyte count | 5 (11) |
Note: All the toxicities listed were grade 2 or lower.
Discussion
Despite a high response rate with initial chemo-therapy, the OS in patients with extensive-stage SCLC is poor.7 We conducted this single-arm phase II study to test the hypothesis that drugs targeting the PD-1 pathway would improve the outcomes of patients with SCLC, particularly after the cancer had been controlled with induction chemotherapy. In our study, maintenance pembrolizumab did not improve the median PFS compared with that of historical controls. However, the respective PFS and OS rates of 13% and 37% at 1 year after patients began receiving pembrolizumab suggest that the drug did benefit a subset of patients. The benefit appears to be sustained, as is suggested by the four patients who continued to receive therapy beyond 18 cycles. The median number of cycles administered was 4, and there were no new toxicities observed with pembrolizumab in this study.
Many trials have evaluated maintenance therapy in SCLC, and most of these have failed to show significant improvement in clinical outcomes.8 CALGB 30504, which was a randomized phase II trial, evaluated sunitinib (a vascular endothelial growth factor receptor inhibitor) as maintenance therapy.2 The median PFS was 3.7 months with sunitinib versus 2.1 months with placebo (p = 0.02), whereas the median OS times were 9.0 months and 6.9 months, respectively (p = 0.16). The median PFS with pembrolizumab in the current study is similar to that reported with placebo in CALGB 30504, but the median OS with pembrolizumab in the current study was similar to that reported with sunitinib. In recurrent NSCLC trials, PD-1–directed agents have not consistently improved PFS but have improved OS, possibly because these drugs result in sustained benefit in only a minority of patients.9,10
Drugs targeting the PD-1 pathway have been evaluated in patients with recurrent SCLC. Ott et al. published the results from the SCLC cohort of the KEYNOTE 028 trial, which evaluated the efficacy of pembrolizumab in several different tumor types, including recurrent PD-L1–positive (≥1% of tumor cells) SCLC.11 Of 163 patients screened, only 24 were treated during the trial. Despite an impressive response rate of 33%, the median PFS was only 1.9 months and the median OS was 9.7 months. The CheckMate 032 trial evaluated nivolumab with or without ipilimumab in patients with recurrent SCLC, regardless of tumor PD-L1 status.12 All efficacy parameters were better with the combination than with single-agent nivolumab; these parameters included included response rate (21% versus 12%), 3-month PFS (30% versus 18%), and 1-year OS (40% versus 27%). The results with maintenance pembrolizumab in our study are very similar to these outcomes, which suggests that the efficacy of immune checkpoint inhibitors when they are used as maintenance therapy may be no better than when they are used at the time of disease recurrence. The potential advantage of maintenance therapy is that a greater proportion of patients would receive immunotherapy because at relapse, some patients may not be considered for any further therapy owing to declining performance status.
The efficacy of immune checkpoint inhibitors in SCLC suggests that, as with other tumor types, these agents only benefit a small subset of patients. Therefore, there is a need to define biomarkers that can prospectively identify those patients who will benefit from this therapy. Recently, the CheckMate 032 investigators reported on the utility of tumor mutational burden as a predictive biomarker.13 Their retrospective analysis showed that high tumor mutational burden could predict for clinical benefit with checkpoint inhibitors, particularly with the combination of nivolumab and ipilimumab. Tumor mutational burden was not assessed in our study.
Tumor cell PD-L1 level has been assessed as a predictive biomarker in several tumor types.14 In our study, we were able to analyze tumor cell PD-L1 expression in tumors of 30 patients, and only three tumors were PD-L1–positive (≥1%). This low rate of PD-L1 positivity in SCLC is consistent with the findings from CheckMate 032 study, in which only 18% of tumors were PD-L1–positive.10 In addition, tumor PD-L1 expression did not predict for efficacy with either nivolumab or the combination of nivolumab and ipilimumab. In KEYNOTE 028, tumor cell PD-L1 expression was identified in 32% of SCLCs assessed. It is possible that the higher response rate of 33% that was observed in this study is due to restriction of enrollment to patients SCLC who have PD-L1–positive tumors.11 Both PFS and OS in KEYNOTE 028 were similar to those reported with nivolumab in CHECKMATE 32 and with pembrolizumab in our current study. Recently Yasuda, et al. assessed PD-L1 expression in 39 SCLCs by utilizing the 22c3 antibody (the same antibody used in our study); they found only one tumor to have PD-L1 expression (≥1%) in tumor cells.15
It is well recognized that PD-L1 expression may be observed not only in tumor cells but also in the tumor microenvironment. Recent data have shown that PD-L1 expression in the host cells may be a better indicator of efficacy of PD-1–directed agents than tumor cell PD-L1 expression is.16,17 In an exploratory analysis, we found that eight of the 20 tumors that could be analyzed for PD-L1 expression at the stromal interface were positive for PD-L1. Schultheis et al. analyzed 94 SCLCs and found that none of the tumors demonstrated PD-L1 expression in tumor cells but 18.5% had PD-L1 expression in the stroma.18 Kim et al. assessed PD-L1 expression in tumor cells and in infiltrating immune cells in high-grade neuroendocrine lung carcinomas.19 PD-L1 expression was observed in tumor cells in 14% of the 120 SCLCs and in infiltrating immune cells in 22% of them. There was no correlation between PD-L1 expression in tumor cells and in infiltrating immune cells, though there was a correlation between PD-L1 expression in the infiltrating immune cells and high tumor mutational burden. They also found that patients with PD-L1 expression in tumor-infiltrating immune cells had a longer PFS. These data suggest that in SCLC, PD-L1 expression is infrequent in tumor cells and more frequent in stromal cells, and that stromal PD-L1 expression may be a predictive biomarker.
In our study, the eight patients with stromal PD-L1 expression in their tumors had a higher median PFS (6.8 versus 1.3 months) and a higher median OS (12.8 versus 7.6 months). However, it is not possible in a single-arm study to determine whether a biomarker has prognostic or predictive utility. It is important to consider that none of the observed differences were statistically significant and that they could be merely a function of the small number of evaluated patients. The reasons for improved benefit in these patients treated with pembrolizumab are unclear. PD-L1 expression at the stromal interface may represent presence of effector T cells in the tumor microenvironment that are limited by PD-L1 expression at the stromal interface.20 Treatment with pembrolizumab may release these effector T cells from the inhibitory effects of PD-1 signaling.
In conclusion, maintenance pembrolizumab did not appear to improve median PFS in patients with extensive-stage SCLC compared with that in the historical data. However, the 1-year PFS rate of 13% and 1-year OS rate of 37% after patients started taking pembrolizumab suggest that a subset of patients did derive clinical benefit. The role of pembrolizumab and other PD-1/PD-L1–directed agents in the management of patients with SCLC will be defined by identifying biomarkers that can predict for clinical benefit and by ongoing clinical trials such as KEYNOTE-604. which is evaluating the addition of pembrolizumab to frontline chemotherapy in patients with extensive-stage SCLC.
Acknowledgments
Funding support and pembrolizumab were provided by Merck & Co. (Kenilworth, NJ).
Disclosure: Dr. Gadgeel reports grants from Merck during the conduct of the study and personal fees from Genentech/Roche, Astra-Zeneca, Ariad/Takeda, and Abbvie outside the submitted work. Dr. Pennell reports personal fees from Eli Lilly, AstraZeneca, and Regeneron outside the submitted work. Dr. Fidler reports personal fees from Genentech/Roche, Abbvie, Boehringer Ingelheim, Takeda, AstraZeneca, Celegene, and Merck outside the submitted work. Dr. Halmos reports grants from Merck, AstraZeneca, and Mirati; personal fees from Genentech, Foundation Medicine, Guardant Health 360, and Ignyta; and grants and personal fees from Novartis, Boehringer Ingelheim, Pfizer, and Takeda outside the submitted work. Dr. Bonomi reports personal fees from Astra Zeneca, Biodesix, Genentech, Imedex, Merck, Pfized, Spectrum, and Trovagene outside the submitted work. Dr. Stevenson reports grants from Merck during the conduct of the study, as well as grants from Merck, Bristol-Myers Squibb, Bayer, and Aduro Biotech outside the submitted work. Dr. Sukari reports grants from Eisai and personal fees from Merck outside the submitted work. Dr. Wozniak reports personal fees from Boehringer Ingelheim, AstraZeneca, and Takeda, as well as grants from Boehringer Ingelheim outside the submitted work. Dr. Kalemkerian reports grants from Merck outside the submitted work. The remaining authors declare no conflict of interest.
Footnotes
Presented in part at the 2017 Annual Meeting of the American Society of Clinical Oncology. June 2–6, 2017; Chicago, IL.
References
- 1.American Cancer Society. Key statistics for small cell lung cancer. https://www.cancer.org/cancer/small-cell-lung-cancer/about/key-statistics.html. Accessed April 2, 2018.
- 2.Ready NE, Pang HH, Gu L, et al. Chemotherapy with or without maintenance sunitinib for untreated extensive-stage small-cell lung cancer: a randomized, double-blind, placebo-controlled phase II study- CALGB 30504 (Alliance). J Clin Oncol. 2015;33:1660–1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shin DS, Ribas A. The evolution of checkpoint blockade as a cancer therapy: what’s here, what’s next? Curr Opin Immunol. 2015;33:23–35. [DOI] [PubMed] [Google Scholar]
- 4.Meng X, Liu Y, Zhang J, Teng F, Xing L, Yu J. PD-1 blockade in advanced NSCLC: a focus on pembrolizumab. Cancer Treat Rev. 2018;62:39–49. [DOI] [PubMed] [Google Scholar]
- 5.Bracci L, Schiavoni G, Sistigu A, Belardelli F. Immune-based mechanisms of cytotoxic chemotherapy: implications for the design of novel and rationale-based combined treatments against cancer. Cell Death Differ. 2014;21:15–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lesterhuis WJ, Punt CJ, Hato SV, et al. Platinum-based drugs disrupt STAT6-mediated suppression of immune responses against cancer in humans and mice. J Clin Invest. 2011;121:3100–3108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Farago AF, Keane FK. Current standards for clinical management of small cell lung cancer. Transl Lung Cancer Res. 2018;7:69–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rossi A, Garassino MC, Cinquini M, et al. Maintenance or consolidation therapy in small-cell lung cancer: a systematic review and meta-analysis. Lung Cancer. 2010;70:119–128. [DOI] [PubMed] [Google Scholar]
- 9.Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced non-squamous non-small-cell lung cancer. N Engl J Med. 2015;373:1627–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rittmeyer A, Barlesi F, Waterkamp D, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicenter randomised controlled trial. Lancet. 2017;389:255–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ott PA, Elez E, Hiret S, et al. Pembrolizumab in patients with extensive-stage small-cell lung cancer: results from the phase Ib KEYNOTE-028 study. J Clin Oncol. 2017;35:3823–3829. [DOI] [PubMed] [Google Scholar]
- 12.Hellmann MD, Ott PA, Zugazagoitia J, et al. Nivolumab+/−ipilimumab in advanced small-cell lung cancer (SCLC): first report of a randomized expansion cohort from CheckMate 032 [abstract]. J Clin Oncol. 2017;35:8503. [Google Scholar]
- 13.Antonia S, Callahan MK, Awad MM, et al. Impact of tumor mutation burden on the efficacy of nivolumab or nivolumab+ipilimumab in small cell lung cancer: an exploratory analysis of CheckMate 032 [abstract]. J Thorac Oncol. 2017;12:OA07.03a. [Google Scholar]
- 14.Patel SP, Kurzrock R. PD-L1 expression as a predictive biomarker in cancer immunotherapy. Mol Cancer Ther. 2015;14:847–856. [DOI] [PubMed] [Google Scholar]
- 15.Yasuda Y, Ozasa H, Kim YH. PD-L1 expression in small cell lung cancer. J Thorac Oncol. 2018;13:e40–e41. [DOI] [PubMed] [Google Scholar]
- 16.Lin H, Wei S, Hurt EM, et al. Host expression of PD-L1 determines efficacy of PD-L1 pathway blockade-mediated tumor regression. J Clin Invest. 2018;128: 805–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tang H, Liang Y, Anders RA, et al. PD-L1 on host cells is essential PD-L1 blockade mediated tumor regression. J Clin Invest. 2018:580–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schultheis AM, Scheel AH, Ozretic L, et al. PD-L1 expression in small cell neuroendocrine carcinomas. Eur J Cancer. 2015;51:421–426. [DOI] [PubMed] [Google Scholar]
- 19.Kim HS, Lee JH, Nam SJ, et al. Association of PD-L1 expression with tumor-infiltrating immune cells and mutation burden in high-grade neuroendocrine carcinoma of the lung. J Thorac Oncol. 2018;13:636–648. [DOI] [PubMed] [Google Scholar]
- 20.Gajewski TF, Schreiber H, Fu YX. Innate and adaptive immune cells in the tumor microenvironment. Nat Immunol. 2013;14:1014–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]