Abstract
The efficacy of immunotherapy varies widely among different gastrointestinal cancers. Response to immune checkpoint inhibitors is shown to correlate with tumor mutation load (TML), mismatch repair deficiency (dMMR) status, and programmed cell death-ligand 1 (PD-L1) expression. Herein, we quantify TML, dMMR, and PD-L1 expression and determine their interrelationship in gastrointestinal cancers. Here, a total of 4,125 tumors from 14 different gastrointestinal cancer sites were studied using validated assays. Next-generation sequencing was performed on genomic DNA isolated from formalin-fixed paraffin-embedded tumor specimens using the NextSeq platform. TML was calculated using only somatic nonsynonymous missense mutations sequenced with a 592-gene panel. Microsatellite instability (MSI) was assessed using direct analysis of altered known MSI loci in the target regions of the sequenced genes. PD-L1 expression was analyzed by IHC. Interestingly, right-sided colon and small-bowel adenocarcinomas had the highest prevalence of TML-high tumors (14.6% and 10.2%, respectively). Pancreatic neuroendocrine tumors and gastrointestinal stromal tumors had the lowest rates of TML-high (1.3% and 0%, respectively). TML-high was strongly associated with MSI-H (P < 0.0001). However, all TML-high anal cancers (8.3%) were microsatellite stable (MSS). Higher PD-L1 expression was more likely to be seen in MSI compared with MSS tumors (20.6% vs. 7.8%, P < 0.0001).
Introduction
Gastrointestinal cancers are generally considered to be less responsive to immunotherapy. However, the newer generation of immunotherapies using immune checkpoint inhibitors (ICI) has demonstrated significant clinical benefits and prolonged duration of response in smaller subsets of patients with colorectal cancers (1), gastroesophageal cancers (2, 3), hepatocellular carcinomas (HCC; ref. 4), and anal cancers (5). Recently, FDA approvals were granted for use of the checkpoint inhibitors pembrolizumab (Keytruda, Merck & Co., Inc.) and nivolumab (Opdivo, Bristol-Myers Squibb) in selected gastrointestinal cancers. Pembrolizumab was approved for the treatment of metastatic nonhematologic cancers that are characterized by microsatellite instability–high (MSI-H) status or mismatch repair deficiency (dMMR) and for programmed cell death-ligand 1 (PD-L1)–positive advanced gastric or gastroesophageal junction (GEJ) adenocarcinomas (6). Nivolumab was approved for the treatment of patients with MSI-H/dMMR mCRC (1), and for the treatment of HCC in patients who have been previously treated with sorafenib (4). ICIs are currently approved for only a small biomarker-defined subset of gastrointestinal cancers; thus, there is a need for the refinement of predictive biomarkers to identify more, yet undefined, potential responders to ICIs.
MMR is an important DNA repair mechanism that ensures genomic integrity and is mediated by key proteins that form heterodimers to recognize and remove DNA errors. The loss of MMR proteins leads to an accumulation of DNA replication errors, a phenomenon known as microsatellite instability (MSI), and eventually to somatic mutations. Proteins resulting from some of these mutated genes are immunogenic and provoke an antitumor immune response by increasing immune cell infiltration and thereby improving sensitivity to ICIs (7, 8). As tumor mutation load (TML) measures the total number of nonsynonymous, somatic mutations identified per megabase (MB) of the genome coding area, tumors with MSI-H are characterized by a higher TML. Furthermore, current evidence suggests that tumors with a TML-high status are associated with improved sensitivity to ICIs (9-11). Tumor cells also express several immunoregulatory molecules, such as PD-L1. PD-L1 binds to the immunoinhibitory receptor PD-1 on T cells, downregulates T-cell activation, and disrupts the local antitumor immune response. Increased PD-L1 expression by IHC has been shown to predict response to ICI in NSCLC (12), melanoma (13), gastric cancer and GEJ adenocarcinoma (6), and bladder cancer (14). For these reasons, TML, MSI, and PD-L1 seem to be biologically intertwined; however, the extent of this relationship and the mechanisms that regulate it are unclear.
dMMR/MSI-H phenotype can be detected by directly enumerating known MSI loci using targeted deep sequencing [MSI next-generation sequencing (MSI-NGS); ref. 15]. The Cancer Genome Atlas (TCGA) showed that three quarters of hypermutated colorectal cancers (defined as tumors harboring a mutation rate of >12 × 106) were MSI-H, whereas the rest had somatic MMR gene and polymerase ε (POLE) mutations, indicating the strong association between MSI-H and TML-high (16). Stadler and colleagues (17) recently used next-generation sequencing (NGS) analysis and suggest that, in colorectal cancer, TML is a highly accurate means of screening for dMMR. Defects in DNA polymerase δ and ε, which usually have proofreading capabilities (18, 19), and the DNA MMR system have been shown to cause hypermutation in tumors (20). Nonetheless, our understanding of the processes that cause hypermutational status in most cancer types is limited (21).
In the current study, we characterize the prevalence of MSI, TML, and PD-L1 in 4,125 gastrointestinal tumors and attempt to understand the relationship between these three biomarkers. To our knowledge, this is the largest study to examine the prevalence and correlation of MSI, TML, and PD-L1 in gastrointestinal cancers.
Materials and Methods
Patients
Tumors from patients with various gastrointestinal cancers were molecularly profiled using a single platform between 2009 and July of 2017, deidentified, and retrospectively analyzed. Gastrointestinal tumors tested were consecutive samples submitted for molecular profiling. Histologic and clinical diagnoses were obtained from submitted pathology reports that were confirmed by board-certified pathologists. For colorectal cancers, only tumors that were documented to arise from a specified location in the colon or rectum were included as primary tumors. Samples taken from locations other than that of the primary cancer were classified as metastases.
NGS
NGS was performed on genomic DNA isolated from formalin-fixed paraffin-embedded (FFPE) tumor samples using the NextSeq platform (Illumina, Inc.). Matched normal tissue was not sequenced. A custom-designed SureSelect XT assay was used to enrich 592 whole-gene targets (Agilent Technologies). All variants were detected with >99% confidence based on allele frequency and amplicon coverage, with an average sequencing depth of coverage of >500 and an analytic sensitivity of 5%. Prior to molecular testing, tumor enrichment was achieved by harvesting targeted tissue using manual microdissection techniques.
TML
TML was measured by counting all nonsynonymous missense mutations found per tumor that had not been previously described as germline alterations (592 genes and 1.4 MB sequenced/tumor). The threshold to define TML-high was greater than or equal to 17 mutations/MB and was established by comparing TML with MSI by fragment analysis in colorectal cancer cases, based on reports of TML having high concordance with MSI-H in colorectal cancer (22).
MSI
MSI was examined using over 7,000 target microsatellite loci and compared with the reference genome hg19 from the University of California, Santa Cruz (Santa Cruz, CA) Genome Browser database. The number of microsatellite loci that were altered by somatic insertion or deletion was counted for each sample. Only insertions or deletions that increased or decreased the number of repeats were considered. Genomic variants in the microsatellite loci were detected using the same depth and frequency criteria as used for mutation detection. MSI-NGS results were compared with results from over 2,000 matching clinical cases analyzed with traditional PCR-based methods. The threshold to determine MSI by NGS was determined to be 46 or more loci with insertions or deletions to generate a sensitivity of >95% and specificity of >99%.
PD-L1 expression
PD-L1 IHC analysis was performed on full slides of FFPE tumor samples using automated staining techniques. The primary antibody used was SP142 (Spring Biosciences). The staining was regarded as positive if its intensity on the membrane of the tumor cells was ≥2+ (on a semiquantitative scale of 0–3: 0 for no staining, 1+ for weak staining, 2+ for moderate staining, and 3+ for strong staining) and the percentage of positively stained cells was 5%.
Ethics statement
Human subjects had been deidentified prior to analysis, with this research being exempt per the Western Institutional Review Board.
Results
Tumor characteristics
In total, 4,125 tumor specimens obtained from patients with 14 different gastrointestinal cancer types were analyzed (Table 1). Overall, 56% of patients were male and 44% were female. The mean age was 61 years (range, 12–90). Most specimens were from primary tumor sites (59%) compared with the metastatic sites (37%). The origin of the remaining 4% of tumors was unclear.
Table 1.
Tumor characteristics of 4,125 patients divided by tumor location
| Gender |
Specimen site |
Age |
||||||
|---|---|---|---|---|---|---|---|---|
| Cancer types | Total (N) |
Female n (%) |
Male n (%) |
Local/primary n (%) |
Mets/distant n (%) |
Unclear n (%) |
Average age |
Age range |
| Anal cancer | 72 | 55 (76) | 17 (23) | 20 (27) | 35 (48) | 17 (23) | 62.1 | 38-82 |
| Biliary tract cancer | 402 | 214 (53) | 189 (46) | 319 (79) | 84 (20) | 0 (0) | 62.7 | 28-88 |
| Esophageal adenocarcinoma | 211 | 24 (11) | 187 (88) | 134 (63) | 76 (36) | 1 (0) | 63.8 | 24-90 |
| Esophageal squamous cancer | 57 | 21 (36) | 36 (63) | 37 (64) | 20 (35) | 0 (0) | 63.1 | 31-86 |
| Gastric cancer | 373 | 148 (39) | 225 (60) | 238 (63) | 125 (33) | 10 (2) | 60.7 | 14-90 |
| GEJ adenocarcinoma | 97 | 26 (26) | 71 (73) | 53 (54) | 23 (23) | 21 (21) | 61.7 | 26-84 |
| GIST | 130 | 57 (43) | 73 (56) | 72 (55) | 39 (30) | 19 (14) | 58.8 | 14-90 |
| Hepatocellular cancer | 135 | 38 (28) | 97 (71) | 86 (63) | 46 (34) | 3 (2) | 62.4 | 12-89 |
| Left-sided colon cancer | 515 | 251 (48) | 264 (51) | 372 (72) | 141 (27) | 2 (0) | 58.2 | 22-90 |
| Pancreatic adenocarcinoma | 870 | 400 (45) | 470 (54) | 371 (42) | 499 (57) | 0 (0) | 63.5 | 24-90 |
| Pancreatic NET | 75 | 31 (41) | 44 (58) | 24 (32) | 51 (68) | 0 (0) | 55.7 | 29-82 |
| Rectal cancer | 541 | 212 (39) | 329 (60) | 259 (47) | 232 (42) | 50 (9) | 58.7 | 26-90 |
| Right-sided colon cancer | 500 | 249 (49) | 251 (50) | 373 (74) | 116 (23) | 11 (2) | 62.9 | 17-90 |
| Small bowel adenocarcinoma | 147 | 79 (53) | 68 (46) | 91 (61) | 54 (36) | 2 (1) | 61.7 | 25-84 |
| Total | 4,125 | 1,805 (44) | 2,320 (56) | 2,449 (59) | 1,540 (37) | 136 (4) | 61.4 | 12-90 |
TML distribution
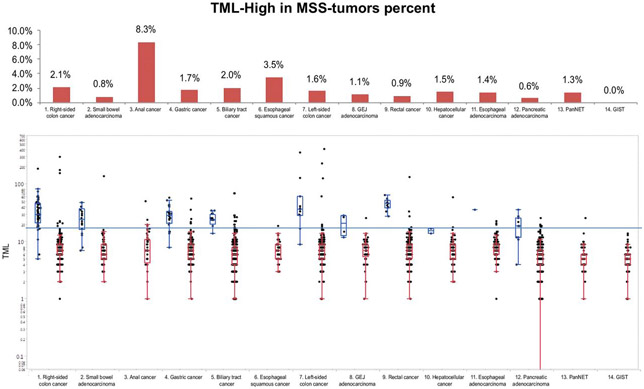
Right-sided colon cancers and small-bowel adenocarcinomas exhibited the highest average TML (13 and 10.2 mutations/MB, respectively) and had the greatest prevalence of TML-high tumors (14.6% and 10.2%, respectively; Fig. 1). Both anal and gastric cancers had a rate of TML-high of 8.3%, whereas pancreatic adenocarcinomas and pancreatic neuroendocrine tumors (pancreatic NET) had a TML-high rate of ≤1.5%. None of the gastrointestinal stromal tumors (GIST) demonstrated TML-high (0%). In addition, a considerable difference in the prevalence of TML-high between gastric cancers and GEJ adenocarcinomas was observed (8.3% vs. 3.1%, respectively). We then analyzed the relative TML distribution between primary and metastatic sites (Table 2). Overall, primary tumors had a greater TML-high rate than metastatic sites (5.7% vs. 3.0%, P < 0.001). However, significant differences were seen in right-sided colon cancers (16.9% vs. 6.9%, P = 0.0064) and small-bowel adenocarcinomas (14.4% vs. 3.7%, P = 0.0495) only. The lower frequency of TML-high in metastatic tumors may be related to the finding that primary tumors with TML-high are less likely to develop metastatic disease than TML-low primary tumors.
Figure 1.
TML distribution across 14 gastrointestinal cancer types. Top, percentage of tumors with TML-high (≥17 mutations/MB) in descending order; bottom, boxplots of TML distributions. The green lines indicate the average TML in each cancer type, and the dotted line indicates the cutoff of TML = 17 mut/MB.
Table 2.
TML distribution in 14 gastrointestinal cancer types highlighting the difference between primary and metastatic sites.
| Cancer types | All tumors | Primary tumors |
Distant/mets tumors |
Unclear origin |
P (mets. vs. local) |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Average TML |
TML-high % |
Average TML |
SE | TML-high % |
Average TML |
SE | TML-high % |
Average TML |
SE | ||
| Right-sided colon cancer | 13.0 | 16.9 | 14.0 | 1.3 | 6.9 | 9.8 | 0.8 | 18.2 | 14.7 | 7.0 | 0.0064 |
| Small-bowel adenocarcinoma | 10.2 | 14.4 | 10.5 | 1.0 | 3.7 | 9.8 | 2.5 | 0.0 | 9.3 | 2.9 | 0.0495 |
| Gastric cancer | 9.3 | 9.7 | 9.7 | 0.5 | 4.8 | 8.2 | 0.4 | 20.0 | 13.4 | 5.3 | 0.2524 |
| GEJ adenocarcinoma | 7.8 | 5.8 | 8.1 | 0.8 | 0.0 | 7.7 | 0.4 | 0.0 | 7.3 | 0.5 | 0.5473 |
| Anal cancer | 8.8 | 4.8 | 8.8 | 1.2 | 8.6 | 9.1 | 1.4 | 12.5 | 8.2 | 1.3 | 1 |
| Pancreatic NET | 5.8 | 4.2 | 6.4 | 0.9 | 0.0 | 5.5 | 0.4 | 0.32 | |||
| Left-sided colon cancer | 10.1 | 4.0 | 10.9 | 1.6 | 2.1 | 7.9 | 0.3 | 0.0 | 9.0 | 0.0 | 0.4224 |
| Hepatocellular cancer | 7.6 | 3.5 | 8.1 | 0.7 | 0.0 | 6.8 | 0.4 | 0.0 | 6.0 | 0.6 | 0.5512 |
| Biliary tract cancer | 6.9 | 3.1 | 6.8 | 0.2 | 6.0 | 7.1 | 0.4 | 0.1052 | |||
| Rectal cancer | 8.4 | 3.1 | 8.2 | 0.6 | 2.6 | 8.3 | 0.4 | 4.0 | 9.7 | 1.0 | 0.792 |
| Esophageal squamous cancer | 7.5 | 2.7 | 7.4 | 0.5 | 5.0 | 7.7 | 0.8 | 1 | |||
| Esophageal adenocarcinoma | 8.1 | 1.5 | 7.7 | 0.3 | 2.6 | 8.7 | 0.4 | 0.0 | 11.0 | 0.6216 | |
| Pancreatic adenocarcinoma | 6.1 | 1.3 | 5.7 | 0.1 | 1.4 | 6.4 | 0.2 | 1 | |||
| GIST | 5.1 | 0.0 | 5.0 | 0.2 | 0.0 | 5.1 | 0.4 | 0.0 | 5.6 | 0.7 | 1 |
| Grand total | 8.5 | 5.7 | 8.8 | 0.3 | 3.0 | 7.5 | 0.1 | 5.9 | 9.2 | 0.8 | <0.001 |
NOTE: Tumors are placed in descending order according to the TML-high% in primary tumors. In bold, % of TML-high tumors that were compared between primary and metastatic sites.
Association between TML and MSI
We examined the association between TML and MSI status. In most gastrointestinal cancers, TML-high was strongly associated with MSI-H (P < 0.0001), suggesting that MSI is the main cause of TML-high (Fig. 2). However, it is noteworthy that all TML-high anal cancers were MSS. Interestingly, among MSS tumors, the highest TML-high rate was seen in squamous-cell cancers, namely anal and esophageal cancers (8.3% and 3.5%, respectively). In addition, MSI-H was not seen in anal cancers, esophageal squamous cancers, pancreatic NETs, and GISTs; however, in these tumors, TML-high frequencies of 8.3%, 3.5%, 1.3%, and 0%, respectively, were observed, indicating that additional causes exist for TML-high that could result in increased neoantigen production. As expected, in all cancer types when MSI-H was seen, a significant correlation between MSI and TML was observed.
Figure 2.
TML and MSI status in 14 gastrointestinal cancer types. Top, percentage of TML-high in MSS tumors; bottom, TML in MSS (red) and MSI-H (blue) tumors. The blue line indicates the threshold of TML-high (≥17 mutations/MB).
Interrelationship between TML, MSI, and PD-L1
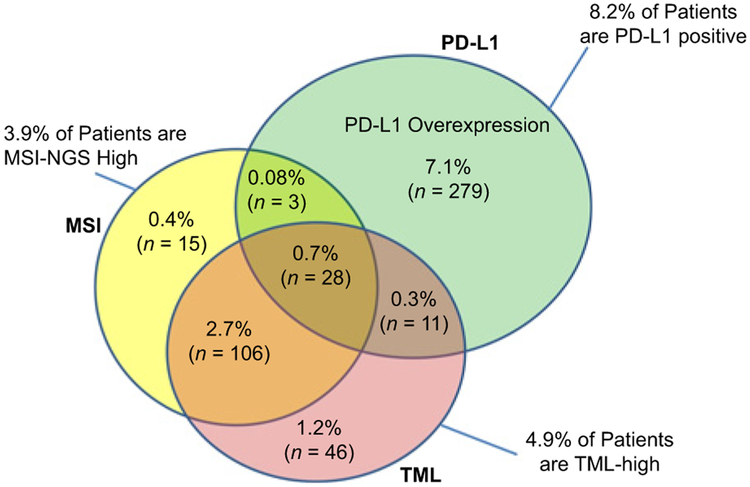
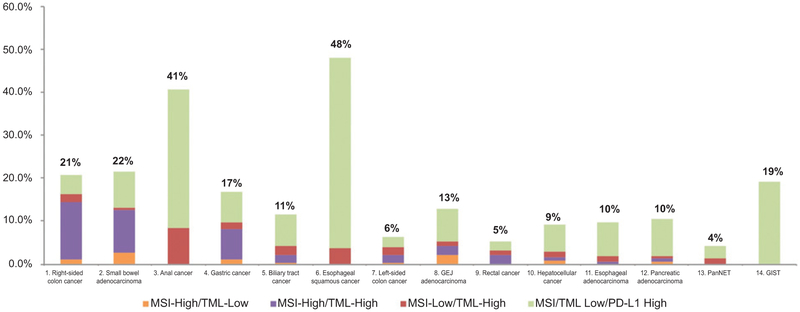
The distribution of PD-L1 tumor expression was variable. GISTs, anal cancers, and esophageal squamous cancer types showed low MSI/TML but high PD-L1 expression, whereas others such as right-sided colon cancers and small-bowel adenocarcinomas showed the reverse profile. We further assessed the interrelationship between TML, MSI, and PD-L1, which is highlighted in the Venn diagram (Fig. 3). MSI-H and TML-high together identified ≤17% of tumors; however, the integration of PD-L1 overexpression has shown to potentially broaden the identification of predictive biomarkers in gastrointestinal tumors. For example, 48% of esophageal squamous cancers may potentially be candidates for therapy with an ICI (Fig. 4).
Figure 3.
Venn diagram showing the overlap of PD-L1 overexpression, MSI-NGS-high, and TML-high in all gastrointestinal tumors, with all three markers tested (N = 3,896). N indicates the number of cases within each category. A total of 3,408 tumors were negative for all three markers.
Figure 4.
Prevalence of MSI, TML, and PD-L1 expression in 14 types of gastrointestinal cancers.
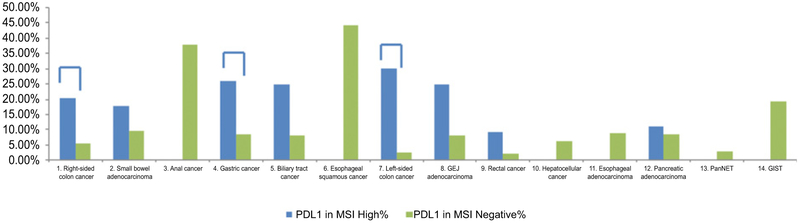
We further analyzed the impact of MSI-H on PD-L1 expression (Fig. 5; Supplementary Table S1): overall, MSI-H tumors showed higher PD-L1 expression in comparison with MSS tumors (20.6% vs. 7.8%, P < 0.0001). Analyzing each tumor type separately, this relationship remained significant only for right-sided colon cancers (20.3% vs. 5.4%, P < 0.0001), left-sided colon cancers (30.0% vs. 2.5%, P < 0.0001), and gastric cancers (25.9% vs. 8.4%, P = 0.003). However, a similar trend was seen in small-bowel adenocarcinomas, biliary tract cancers, GEJ adenocarcinomas, rectal cancers, and pancreatic adenocarcinomas. Several tumor sites that did not demonstrate MSI-H status (anal cancer, esophageal squamous, pancreatic NET, and GIST) showed PD-L1-high expression.
Figure 5.
PD-L1 expression frequency in the MSI-negative cohort (green) and MSI-high cohort (blue). A connective line indicates that PD-L1 expression is significantly higher in the MSI-H cohort.
Finally, we compared PD-L1 expression frequency between MSI/TML-negative tumors and in MSI or TML-positive tumors. PD-L1 expression was significantly higher in MSI-H/TML-high primary tumor samples from right-sided colon cancers (18% vs. 5%, P = 0.0002), left-sided colon cancers (17% vs. 3%, P = 0.003), gastric cancers (27% vs. 8%, P = 0.0004), biliary tract cancers (31% vs. 8%, P = 0.0009), and esophageal adenocarcinomas (67% vs. 8%, P = 0.0003).
Discussion
The introduction of immune checkpoint (PD-1/PD-L1/CTLA4) inhibitors represents a major step forward in cancer therapy, resulting in a dramatic paradigm shift for the standard-of-care in many solid tumors. However, currently ICIs have shown activity in only a select subset of patients with gastrointestinal neoplasms, identified using MSI, TML, and PD-L1 expression separately in individual cancers. Whether the integration of these predictive biomarkers could better select patients who would benefit from these drugs is still unclear. The three biomarkers that were studied have been associated with the prediction of patient response to immune checkpoint inhibitors in gastrointestinal and nongastrointestinal malignances. However, the prevalence of these biomarkers and their interrelationship is still poorly understood. Describing the relative prevalence of these biomarkers at different disease sites and their association with each other can shed light on the biology of these diseases and provide a better understanding of the subsets of patients who may benefit from immunotherapy.
Higher levels of mutations are believed to induce the expression of immunogenic and cancer-specific neoantigens, leading to a robust antitumor immune response. Even though TML is tightly associated with MSI status, it is possible to observe TML-high in MSS tumors (23). In the current study, a cutoff of ≥17 mutations per MB was used to define TML-high tumors based on an established concordance (>99%) with MSI-H in colorectal cancers. However, different thresholds to define TML-high were used in other studies, making it harder to compare data.
In the current study, right-sided colon cancers and small-bowel adenocarcinomas had the highest TML-high rate. Recent genomic profiling of small-bowel adenocarcinomas demonstrated that 9.5% were TML-high (24). In addition, 7.6% of small-bowel adenocarcinomas were MSI-H, and all MSI-H tumors had an intermediate to high TML, which is consistent with other reports (25). A multicenter phase II study in patients with advanced small-bowel adenocarcinomas is ongoing to examine the efficacy of pembrolizumab within this patient population, even though patient selection or stratification according to the MSI or TML status is not planned (NCT02949219; ref. 26).
Here, we showed that TML-high is strongly associated with MSI-H status in most gastrointestinal cancers, except for anal cancers, esophageal squamous cell cancers, and pancreatic NETs. These tumors did not show MSI-H, even though they exhibited TML-high rates of 8.3%, 3.5%, and 1.3%, respectively. Therefore, it is possible that additional factors are responsible for increased neoantigen production leading to TML-high (Fig. 2). Anal cancers and esophageal squamous cancers have two risk factors in common: human papillomavirus (HPV; refs. 27,28) and smoking. HPV+ and smoking-related tumors have been shown to harbor higher mutation rates in comparison with their HPV-negative and nonsmoking counterparts (29, 30); thus, it is possible that HPV infection and smoking have a role in causing a high TML in squamous gastrointestinal cancers. In addition, HPV infection is present in >80% of anal cancers and is recognized as the main cause of anal squamous-cell carcinoma (28). Moreover, HPV-negative and HPV-positive cancers have been shown to have different mutation profiles (31, 32). It has been demonstrated that HPV-induced master regulators play crucial roles with regard to mutation and neoantigen load in cervical cancer (30). However, as we found that only 8.3% of anal cancers are TML-high, other factors may be involved in driving mutations. This knowledge will help us to better select patients and identify the subgroup that will benefit best from ICIs. This is especially important considering the recent demonstration of nivolumab efficacy in patients with anal cancers (5).
We also observed a considerable difference between the percentage of TML-high tumors in gastric cancers and GEJ adenocarcinomas (8.3% vs. 3.1%). Generally, gastric cancers and GEJ adenocarcinomas are grouped together, and many trials examining PD-1/PD-L1 blockade in gastroesophageal cancers enroll patients with gastric cancers and GEJ cancers without distinction between the two sites (33). However, given these results, patient stratification into two groups is probably needed in future clinical trials of anti-PD-1/PD-L1 agents.
Another important observation was the difference between primary and metastatic sites. Overall, primary tumors carried a greater TML-high frequency than metastatic tumors. Nevertheless, considering each type of tumor separately, only right-sided colon cancers and small-bowel adenocarcinomas reached a significant difference (Table 2). Primary MSI-H colorectal cancers are less likely to metastasize to a distant organ, a finding that likely explains the differences in TML observed here. In addition, TML-high has been related to better prognosis (34). As mentioned above, an increased number of mutations increases the probability of having more immunogenic proteins that can be recognized as neoantigens by our immune system. This phenomenon may prevent cancer cells from metastasizing and could be the reason why we saw a greater TML-high rate in primary than in metastatic sites.
Recently, PD-L1 expression has been extensively studied as a prognostic and predictive biomarker in different tumor types. However, many clinical studies have reported controversial results (35), likely due to lack of consistency. Various PD-L1 antibody clones have been used, as well as different thresholds to define positive and negative staining. For a meaningful comparison among all gastrointestinal cancer types investigated here, a single clone and a uniform threshold is needed. We adopted SP142, a widely used clone that has generated highly concordant results in various cancer types with other commonly used antibodies (36). The threshold adopted in this study for PD-L1 positive is 5%, higher than the 1% required for entry into many clinical studies in gastrointestinal cancers. In various gastrointestinal cancers, including gastroesophageal cancer (3, 37) and HCC (4), further analysis of the clinical results showed that higher expression of PD-L1 is associated with improved survival in patients treated with immune checkpoint inhibitors. The most recent approval of pembrolizumab in gastroesophageal cancer adopts a threshold of 1% of combined positive score and takes into account staining on tumor cells, lymphocytes, and macrophages (6). For completeness of results, when we set the threshold for PD-L1 positivity at 1%, the overall prevalence of tumors with PD-L1 overexpression increased in all tumor types, as expected, maintaining the same distribution (Supplementary Table S2).
Although further analysis among patients with different PD-L1 expression levels is not yet available, it is not unreasonable to speculate that high PD-L1 expressers may have an increased likelihood to respond to the agent based on previously published studies. Here we found that PD-L1 expression on tumor cells varies widely throughout gastrointestinal cancers, with esophageal squamous and anal cancers showing the highest rate of PD-L1 expression (Fig. 4). More interestingly, PD-L1 expression does not always co-occur with MSI-H/TML-high. In fact, as highlighted in Fig. 4, in anal cancers, esophageal squamous cancers, pancreatic NETs, and GISTs, PD-L1 expression is high, even though MSI and TML are low or negative. Indeed, a wide range of response rates (RR) to ICI in gastrointestinal cancers has been demonstrated in several clinical trials (Supplementary Table S3), suggesting that ICI responses are likely driven by different biomarkers. For instance, squamous esophageal cancers showed promising RR to nivolumab without harboring MSI or TML-high but having more than 40% of PD-L1+ tumors. In contrast, colorectal cancers harbor low PD-L1 expression but higher rates of MSI-H/TML-high.
GISTs are rare tumors, not strictly considered typical gastrointestinal tract tumors, characterized by a gain-of-function mutation of c-KIT in approximately 95% of cases. However, we recently demonstrated that mutations in oncogenic driver genes are associated with lower TML-high, which may explain why in our cohort, no GISTs were TML-high (38). Although the response of GISTs to imatinib is impressive, almost every patient relapses. Recently, Seifert and colleagues (39) demonstrated that the inhibitory receptors (i.e., PD-1, LAG3, and TIM3) were upregulated on the tumor-infiltrating T-cells in GIST samples, while intratumoral PD-L1 expression was variable and heterogeneous. More importantly, PD-1 and PD-L1 blockade in vivo enhanced the antitumor effects of imatinib by increasing T-cell effector function in the presence of KIT and IDO inhibition. The fact that 19% of GISTs showed PD-L1 expression provides us with further rationale for attempting treatment with PD-L1 inhibitors, alone or in combination with tyrosine kinase inhibitors. Some clinical trials are now enrolling GIST patients to confirm this hypothesis (40).
We certainly acknowledge that our study has several limitations, such as the retrospective nature of the analysis, heterogeneous population of patients and tumor samples but, more importantly, the lack of clinical data and outcomes for these patients that did not allow us to correlate biomarkers with outcomes. Therefore, future validations of these findings in prospective cohorts are needed.
Finally, our findings suggest that the integration of the three biomarkers may be a better way to characterize the prevalence of immune-related biomarkers in gastrointestinal cancers, which might result in identifying more potential responders to ICI treatment. For instance, based on our data, small-bowel adenocarcinomas and GISTs may benefit from immunotherapy: the former due to the high rate of TML-high that is correlated to MSI-H status, and the latter due to the high rate of PD-L1 expression. Future clinical trials in these populations are warranted to validate this hypothesis.
Conclusions
This is the first comprehensive analysis of TML, MSI, and PD-L1 expression in gastrointestinal cancers. TML-high rate varied widely among gastrointestinal cancers. MSI-H is conceivably the main driver for TML, even though other factors may be involved, such as HPV infection, and deserve future study. PD-L1 expression does not always associate with either MSI or TML-high, yet could potentially identify additional responders to ICI treatment, regardless of MSI status or mutation load. Our findings provide baseline data for the prevalence of molecular and histologic parameters potentially associated with responsiveness to ICI in gastrointestinal malignancies. Our results also suggest that testing for all three markers (TML, MSI, and PD-L1) may be necessary to broaden the identification of responders to ICI treatment and better stratify patients. Future investigations in prospective trials are needed to evaluate the integration of these biomarkers with other potential factors (e.g., CD8+ tumor-infiltrating lymphocytes and IFNg gene signature). Finally, standardized methodologies and thresholds are needed, as well as the exploration and standardization of new methods to measure TML, such as liquid biopsy-based assays (41).
Supplementary Material
Implications: TML-high rate varied widely among gastrointestinal cancers. Although MSI is conceivably the main driver for TML-high, other factors may be involved. Future clinical trials are needed to evaluate whether the integration of TML, MSI, and PD-L1 could better identify potential responders to immunotherapy.
Footnotes
Disclosure of Potential Conflicts of Interest
D. Raghavan is a consultant/advisory board member for Caris Life Sciences Medical Advisory Board. R.M. Goldberg is a consultant/advisory board member for Caris Life Sciences. W.M. Korn has ownership interest (including patents) in OncoCyte and is a consultant/advisory board member for Eli Lilly and Merck. B.A. Weinberg reports receiving a commercial research grant from Novartis, has received speakers bureau honoraria from Eli Lilly, and is a consulting/advisory board member for Caris Life Sciences. A.F. Shields reports receiving commercial research support from Caris Life Sciences. J.L. Marshall is the director (POA) at Caris Life Sciences. No potential conflicts of interest were disclosed by the other authors.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked advertisement in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Note: Supplementary data for this article are available at Molecular Cancer Research Online (http://mcr.aacrjournals.org/).
References
- 1.Overman MJ, McDermott R, Leach JL, Lonardi S, Lenz HJ, Morse MA, et al. Nivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer (CheckMate 142): an open-label, multicentre, phase 2 study. Lancet Oncol 2017;18:1182–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kudo T, Hamamoto Y, Kato K, Ura T, Kojima T, Tsushima T, et al. Nivolumab treatment for oesophageal squamous-cell carcinoma: an open-label, multicentre, phase 2 trial. Lancet Oncol 2017;18:631–9. [DOI] [PubMed] [Google Scholar]
- 3.Muro K, Chung HC, Shankaran V, Geva R, Catenacci D, Gupta S, et al. Pembrolizumab for patients with PD-L1-positive advanced gastric cancer (KEYNOTE-012): a multicentre, open-label, phase 1b trial. Lancet Oncol 2016;17:717–26. [DOI] [PubMed] [Google Scholar]
- 4.El-Khoueiry AB, Sangro B, Yau T, Crocenzi TS, Kudo M, Hsu C, et al. Nivolumab in patients with advanced hepatocellular carcinoma (Check-Mate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet 2017;389:2492–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morris VK, Salem ME, Nimeiri H, Iqbal S, Singh P, Ciombor K, et al. Nivolumab for previously treated unresectable metastatic anal cancer (NCI9673): a multicentre, single-arm, phase 2 study. Lancet Oncol 2017;18:446–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fuchs CS, Doi T, Jang RW, Muro K, Satoh T, Machado M, et al. KEYNOTE-059 cohort 1: Efficacy and safety of pembrolizumab (pembro) monotherapy in patients with previously treated advanced gastric cancer. J Clin Oncol 2017;35:15s (suppl; abstr 4003). [Google Scholar]
- 7.Le DT, Durham JN, Smith KN, Wang H, Bartlett BR, Aulakh LK, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science 2017;357:409–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med 2015;372:2509–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Champiat S, Ferté C, Lebel-Binay S, Eggermont A, Soria JC. Exomics and immunogenics: Bridging mutational load and immune checkpoints efficacy. Oncoimmunology 2014;3:e27817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zibelman M, Ramamurthy C, Plimack ER. Emerging role of immunotherapy in urothelial carcinoma-advanced disease. Urol Oncol 2016;34:538–47. [DOI] [PubMed] [Google Scholar]
- 11.Bellmunt J, Powles T, Vogelzang NJ. A review on the evolution of PD-1/PD-L1 immunotherapy for bladder cancer: the future is now. Cancer Treat Rev 2017;54:58–67. [DOI] [PubMed] [Google Scholar]
- 12.Reck M, Rodriguez-Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med 2016;375:1823–33. [DOI] [PubMed] [Google Scholar]
- 13.Gandini S, Massi D, Mandala M. PD-L1 expression in cancer patients receiving anti PD-1/PD-L1 antibodies: a systematic review and meta-analysis. Crit Rev Oncol Hematol 2016;100:88–98. [DOI] [PubMed] [Google Scholar]
- 14.Powles T, Eder JP, Fine GD, Braiteh FS, Loriot Y, Cruz C, et al. MPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature 2014;515:558–62. [DOI] [PubMed] [Google Scholar]
- 15.Chalmers ZR, Connelly CF, Fabrizio D, Gay L, Ali SM, Ennis R, et al. Analysis of 100,000 human cancer genomes reveals the landscape of tumor mutational burden. Genome Med 2017;9:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cancer Genome Atlas Network. Comprehensive molecular characterization of human colon and rectal cancer. Nature 2012;487:330–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stadler ZK, Battaglin F, Middha S, Hechtman JF, Tran C, Cercek A, et al. Reliable detection of mismatch repair deficiency in colorectal cancers using mutational load in next-generation sequencing panels. J Clin Oncol 2016;34:2141–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Briggs S, Tomlinson I. Germline and somatic polymerase epsilon and delta mutations define a new class of hypermutated colorectal and endometrial cancers. J Pathol 2013;230:148–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kane DP, Shcherbakova PV. A common cancer-associated DNA polymerase epsilon mutation causes an exceptionally strong mutator phenotype, indicating fidelity defects distinct from loss of proofreading. Cancer Res 2014;74:1895–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roberts SA, Gordenin DA. Hypermutation in human cancer genomes: footprints and mechanisms. Nat Rev Cancer 2014;14:786–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alexandrov LB, Nik-Zainal S, Wedge DC, Aparicio SA, Behjati S, Biankin AV, et al. Signatures of mutational processes in human cancer. Nature 2013;500:415–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Salem ME, Weinberg BA, Xiu J, El-Deiry WS, Hwang JJ, Gatalica Z, et al. Comparative molecular analyses of left-sided colon, right-sided colon, and rectal cancers. Oncotarget 2017;8:86356–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gong J, Sy M, Fakih M. Tumor mutational burden of microsatellite stable metastatic colorectal tumors by patient factors and KRAS, BRAF, and PIK3CA mutation status. J Clin Oncol 2017;35:4s (suppl; abstr 627). [Google Scholar]
- 24.Schrock AB, Devoe CE, McWilliams R, Sun J, Aparicio T, Stephens PJ, et al. Genomic profiling of small-bowel adenocarcinoma. JAMA Oncol 2017;3:1546–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Salem ME, Lenz HJ, Shields A, Xiu J, Tabari Baker T, Hwang JJ, et al. Molecular variations between small bowel adenocarcinomas (SBAs), right-sided colon cancers (RT-Colon), and gastroesophageal cancers (GEC).Ann Oncol 2017;28:mdx262.017. [Google Scholar]
- 26.Lech G, Korcz W, Kowalczyk E, Søotwiński R, Søodkowski M. Primary small bowel adenocarcinoma: current view on clinical features, risk and prognostic factors, treatment and outcome. Scand J Gastroenterol 2017;52:1194–202. [DOI] [PubMed] [Google Scholar]
- 27.Ludmir EB, Stephens SJ, Palta M, Willett CG, Czito BG. Human papillomavirus tumor infection in esophageal squamous cell carcinoma. J Gastrointest Oncol 2015;6:287–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bernardi MP, Ngan SY, Michael M, Lynch AC, Heriot AG, Ramsay RG, et al. Molecular biology of anal squamous cell carcinoma: implications for future research and clinical intervention. Lancet Oncol 2015;16:e611–21. [DOI] [PubMed] [Google Scholar]
- 29.Govindan R, Ding L, Griffith M, Subramanian J, Dees ND, Kanchi KL, et al. Genomic landscape of non-small cell lung cancer in smokers and never-smokers. Cell 2012;150:1121–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Qin Y, Ekmekcioglu S, Forget MA, Szekvolgyi L, Hwu P, Grimm EA, et al. Cervical cancer neoantigen landscape and immune activity is associated with human papillomavirus master regulators. Front Immunol 2017;8:689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chung JH, Sanford E, Johnson A, Klempner SJ, Schrock AB, Palma NA, et al. Comprehensive genomic profiling of anal squamous cell carcinoma reveals distinct genomically defined classes. Ann Oncol 2016;27:1336–41. [DOI] [PubMed] [Google Scholar]
- 32.Weinberg BA, Lenz HJ, Arguello D, El-Deiry WS, Xiu J, Gatalica Z, et al. Molecular characterization of squamous cell carcinoma of the anal canal (SCCA). J Clin Oncol 2017;35:4s (suppl; abstr 538). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jin Z, Yoon HH. The promise of PD-1 inhibitors in gastro-esophageal cancers: microsatellite instability vs. PD-L1. J Gastrointest Oncol 2016;7:771–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Danilova L, Wang H, Sunshine J, Kaunitz GJ, Cottrell TR, Xu H, et al. Association of PD-1/PD-L axis expression with cytolytic activity, mutational load, and prognosis in melanoma and other solid tumors. Proc Natl Acad Sci U S A 2016;113:E7769–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mino-Kenudson M Programmed cell death ligand-1 (PD-L1) expression by immunohistochemistry: could it be predictive and/or prognostic in non-small cell lung cancer? Cancer Biol Med 2016;13:157–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gatalica Z, Vanderwalde AM, Rose I, Chen S, Reddy SK, Hamid O, et al. Distribution of PD-L1 expression in diverse cancer types: Experience with over 10,000 cases. J Clin Oncol 2016;34:15s (suppl; abstr 11548). [Google Scholar]
- 37.Chung HC, Arkenau HT, Wyrwicz L, Oh DY, Lee KW, Infante JR, et al. Avelumab (MSB0010718C; anti-PD-L1) in patients with advanced gastric or gastroesophageal junction cancer from JAVELIN solid tumor phase Ib trial: Analysis of safety and clinical activity. J Clin Oncol 2016;34:15s (suppl; abstr 4009). [Google Scholar]
- 38.Salem ME, Xiu J, Lenz HJ, Atkins MB, Philip PA, Hwang JJ, et al. Characterization of tumor mutation load (TML) in solid tumors. J Clin Oncol 2017;35:15s (suppl; abstract 11517). [Google Scholar]
- 39.Seifert AM, Zeng S, Zhang JQ, Kim TS, Cohen NA, Beckman MJ, et al. PD-1/PD-L1 blockade enhances T-cell activity and antitumor efficacy of imatinib in gastrointestinal stromal tumors. Clin Cancer Res 2017;23:454–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tan Y, Trent JC, Wilky BA, Kerr DA, Rosenberg AE. Current status of immunotherapy for gastrointestinal stromal tumor. Cancer Gene Ther 2017;24:130–3. [DOI] [PubMed] [Google Scholar]
- 41.Gandara DR, Kowanetz M, Mok TSK, Rittmeyer A, Fehrenbacher L, Fabrizio D, et al. Blood-based biomarkers for cancer immunotherapy: tumor mutational burden in blood (bTMB) is associated with improved atezolizumab (atezo) efficacy in 2L+ NSCLC (POPLAR and OAK). Ann Oncol 2017;28:(suppl 5): v460–9. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.