Abstract
Environmental temperature plays a role in the variation of blood pressure. Maternal cold stress could affect the physiological phenotype of the offspring, including blood pressure elevation. In the present study, we found that adult offspring of dams exposed to cold have increased systolic and diastolic blood pressure, and decreased urine volume and sodium excretion, accompanied by increased heart rate and heart rate variability, secondary to increased activity of the sympathetic nervous system. Renal denervation or adrenergic receptor blockade decreased blood pressure and increased sodium excretion. The increase in peripheral sympathetic nerve activity can be ascribed to the central nervous system because administration of clonidine, a centrally acting α2 adrenergic receptor agonist, lowered blood pressure to a greater degree in the prenatal cold-exposed than control offspring. Moreover, these prenatal cold-exposed offspring had hypothalamic paraventricular nucleus (PVN) disorder because magnetic resonance spectroscopy showed decreased N-acetylaspartate and increased choline and creatine ratios in the PVN. Additional studies found that prenatal cold exposure impaired the balance between inhibitory and excitatory neurons. This led to PVN overactivation that was related to enhanced PVN-angiotensin II type 1 (AT1) receptor expression and function. Microinjection of the AT1 receptor antagonist losartan in the PVN lowered blood pressure to a greater extent in prenatal cold-exposed that control offspring. The present study provides evidence for overactive peripheral and central sympathetic nervous systems in the pathogenesis of prenatal cold-induced hypertension. Central AT1 receptor blockade in the PVN may be a key step for treatment of this type hypertension.
Introduction
Hypertension is the leading cause of death and other cardiovascular diseases globally [1,2]. Several pieces of evidence have shown the association between the environment and blood pressure [3,4]; the environmental temperature has been recognized to be one of the regulators of blood pressure. A retrospective study of 95,277 subjects found that the systolic blood pressure (SBP) increased by 3.3 ± 0.1 mm Hg and the prevalence of hypertension increased by 8.2% in the cold months [5].
The impact of parental disease and environment in prenatal programing can be transmitted to future generations [6]. Our previous studies have shown that prenatal maternal stresses, such as infection and inflammation, lead to hypertension in the offspring [7,8]. Cold stress may also cause physiological alterations in dams and affect the physiological traits of their offspring [9–11]. Whether or not cold stress during pregnancy results in blood pressure elevation in the offspring remains unclear.
The risk of preeclampsia is increased by exposure to cold temperature at late in pregnancy [12]. Cooling of acral skin increases blood pressure to a greater extent in preeclampsia than normal pregnancy. Pregnant rats exposed to cold develop human preeclampsia-like abnormalities, including an increase in sympathetic nervous system (SNS) activity, renal, adrenal, and liver pathology [13,14], and increased blood pressure in the last week of gestation [13,14]. Cold-induced hypertension and increased activity of central and peripheral SNS also occur in male rats [15]. The increase in SNS activity stimulates the renin-angiotensin-aldosterone system (RAAS), both of which regulate sodium homeostasis, peripheral resistance, and central arterial stiffness [16,17]. Chronically enhanced sympathetic nervous and RAAS activities impair the development of the fetus [18]. Therefore, we hypothesized that overactivation of the SNS during gestation is involved in the prenatal cold-induced hypertension in the adult offspring. In order to test this hypothesis, we studied the effect of maternal cold stress on blood pressure and sodium excretion in the offspring, and investigated the role of the central and renal SNS and RAAS in these phenotypes.
Methods
Prenatal cold stress exposed offspring rats
Virgin female Sprague–Dawley (SD) rats (250–280 g) were purchased from the Animal Centre of The Third Military Medical University, Chongqing, China. And all the in vivo studies were performed at the Animal Centre of The Third Military Medical University. The rats were fed a standard chow (with 0.18% sodium and 0.68% potassium) and drank water ad libitum. After breeding with male SD rats, the pregnant rats were randomly divided into cold stress and control groups. The cold-exposed dams were housed individually in cold (4°C) rooms from 14 to 21 days of gestation [9], whereas the room temperature was 25 ± 2°C for the control group. After weaning, the offspring of both control and cold-exposed dams were fed regular rat chow at 4 weeks of age. Only male offspring were included in the present study, since female offspring are protected against maternal hypertension due to the protective effect of female sex hormones [19].
At 11 weeks of age, the offspring from six litters in prenatal cold exposure group and control were randomly assigned into four groups: (1) propranolol (non-selective β antagonist, 30 mg/kg, Sigma–Aldrich, St Louis, MO) group; (2) prazosin (selective α1 adrenergic receptor antagonist, 1 mg/kg, Sigma–Aldrich) group; (3) clonidine (centrally acting α2 adrenergic receptor agonist, 0.2 mg/kg, Sigma–Aldrich) group; and (4) renal denervation group. All the antihypertensive agents were administrated into the stomach via a gastric tube twice daily for 1 week.
The present study was approved by the Third Military Medical University Animal Use and Care Committee. All experiments conformed to the guidelines of the ethical use of animals, and all efforts were made to minimize animal suffering and to reduce the number of animals used.
Rat renal denervation model
Renal sympathetic denervation of 11-week-old rats was performed by chemical ablation as described previously [20,21]. Briefly, after anesthetizing the rats with pentobarbital (50 mg/kg, i.p.), both renal arteries were exposed through a ventral abdominal median incision. The renal artery was stripped of its adventitia and painted with a solution of phenol in ethanol (10% v/v) for 3 min, using cotton swabs. The surgical incisions were closed and the rats were allowed to recover with free access to food and water for 1 week.
Basic characteristics measurement and urine collection in the offspring
Several vital signs and basic characteristics, including adult weight, body temperature, heart rate, blood glucose, blood lipids, and blood pressure, were measured at 12 weeks of age and the birth weight was measured at 1 day of age. Body temperature was measured by a thermometer (MC-347, OMRON, Kyoto, Japan), about 4 cm past the anal sphincter. Blood glucose was measured by the blood glucose meter and test strips (CareSens N, Seoul, Korea). Blood lipids, including total cholesterol and triglycerides, were measured by the ELISA kit (Jiancheng, Nanjing, China). The blood pressure and heart rate were also measured at 4, 8, 12, 16, 20, and 24 weeks of age in conscious rats using a computerized noninvasive tail-cuff manometry (BP-98A; Softron, Tokyo, Japan) as described in previous studies [22,23]. Blood pressure and heart rate were measured five times between 3:00–5:00 PM; the average of the five measurements was used in the calculations. The offspring was kept in metabolic cages for 24 h urine collection. Urine sodium was measured by an electrolyte analyzer (Ciba Corning Diagnostics, Norwood, MA, U.S.A.).
Heart rate variability assessment
Heart rate variability (HRV) indices of rat at 12 weeks of age, including low-frequency and high-frequency ratio (LF/HF), standard deviation of the N-N intervals (SDNN) and square root of the mean squared differences of successive N-N intervals (RMSSD), were obtained using the Powerlab ECG system (AD Instruments, Australia) and Chart 7.0 software. The time-domain parameters, including SDNN and RMSSD, were calculated by the R–R intervals, while the time-domain parameter of HRV was determined by LF/HF, according to the Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology [24].
Magnetic resonance spectroscopic examination
Magnetic Resonance Spectroscopy (MRS) was performed on a 7.0T MRI scanner (Bruker PharmaScan, Germany) with a rat head coil. During the scan, the rats at 12 weeks of age were anesthetized with pentobarbital (50 mg/kg, i.p.) and the heads were fixed to remain stationary. The scanning range was 1.5 mm × 1.5 mm × 1.5 mm in the specified hypothalamic paraventricular nucleus (PVN) region. The scanning parameters were T2 weight imaging (T2WI): repetition time/echo time (TR/TE) = 3500/45 ms, matrix 256 × 256 and field of view = 40 mm × 40 mm, slice thickness = 1 mm, gap = 0 mm). The proton spectra were acquired and analyzed by Topspin software (Bruker PharmaScan, Germany) to calculate the N-acetyl aspartate/Creatine (NAA/Cr) and Choline/Creatine (Cho/Cr).
Intracerebroventricular injection
The control and prenatal cold-exposed offspring at 12 weeks of age were anesthetized with pentobarbital (50 mg/kg, i.p.) and placed on a heated board to maintain body temperature at 37°C. The rat head was mounted on a stereotaxic apparatus (Kopf, Tujunga, CA, U.S.A.) and a 4 mm-diameter hole was drilled in the skull using a high-speed drill (Muromachi Kikai, Tokyo, Japan). Intracerebral injections were given bilaterally by a microliter syringe (diameter, 50 μm) into the lateral ventricle (1.0 mm posterior, 1.5 mm lateral from the midline, and 4.5 mm ventral). Losartan (11.45 nmol/kg) was injected intracerebroventricularly to block the AT1 receptor in the paraventricular nucleus (PVN). Control rats were injected with the same volume of saline.
Immunohistochemistry
The brains were isolated, washed several times with PBS, fixed with 4% paraformaldehyde for 24 h, embedded in paraffin, sectioned (4 μm), and mounted on glass slides. The slides were blocked sequentially with 5% goat serum in PBS for 1 h at 37°C for immunohistochemistry. The tissues were incubated with rabbit anti-AT1 receptor (1:100, Proteintech, Wuhan, China), rabbit anti-GRIN2B antibody, (excitatory glutamatergic neuron marker [25], 1:100, Proteintech, Wuhan, China) and rabbit anti-GAD65 antibody (inhibitory GABAergic neurons marker [26], 1:100, Proteintech, Wuhan, China) overnight at 4°C. The optical density (OD) of the stained slides was quantitated by ImageJ (NIH, Bethesda, MD, U.S.A.).
Immunoblotting
SDS-PAGE loaded with the designated amount of lysed brain protein were transferred onto PVDF membranes. After blocking with 1% BSA in TBST buffer for 1 h, the transblots were probed with the rabbit anti-AT1 receptor antibody (1:500, Proteintech, Wuhan, China). The amount of protein transferred onto the membranes was verified by immunoblotting for β-actin (1:500, Santa Cruz, CA, U.S.A.). The membranes were then washed three times with TBST. The bound complex was detected using the Odyssey Infrared Imaging System (Li-Cor Bioscience). The images were analyzed using the Odyssey Application Software to obtain the integrated intensities.
The measurement of pro-inflammation cytokines levels, NADPH oxidase activity, and renin/Angiotensin II levels in PVN
The PVN region of the brain was homogenized in buffer (10 mM Tris HCl, 250 mM sucrose, 2 mM PMSF, protease inhibitor cocktail; pH 7.4), and centrifuged at 24,000 g for 25 min at 4°C. The pro-inflammation cytokines, including interleukin (IL)-1β, IL-6, and tumor necrosis factor (TNF)-α in the samples were quantitated by ELISA (Beyotime Biotechnology, Shanghai, China). The NADPH oxidase activity was checked by the commercial kit (Beyotime Biotechnology, Shanghai, China). And the levels of renin/Ang II were measured by chemiluminescence immunoassay (CLIA) kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China).
Dihydroethidium staining
PVN reactive oxygen species (ROS) was evaluated by dihydroethidium (DHE, 10−5 mol/l, Beyotime, Shanghai, China) staining on frozen sections of PVN tissues from prenatal cold-exposed offspring or control rats. After being washed for 30 min, images were taken with a fluorescence microscope at excitation wavelengths of 490 nm and emission of 590 nm. All sections were processed under the same conditions. Settings for image acquisition were identical for all sections (exposure time, 20 ms). The DHE fluorescence intensity was quantitated by ImageJ (NIH, Bethesda, MD, U.S.A.).
Echocardiography
Transthoracic echocardiography of rats at 12 weeks of age was performed with an GE vivid 9D ultrasound. After anesthetizing with 2% isoflurane, heart rate was kept at 270–300 beats/min and hearts were viewed in the short-axis between the two papillary muscles and each measurement was obtained with M-mode by averaging results from three consecutive heart beats. Left ventricle fraction shortening (FS) was calculated by FS (%) = (LVIDd-LVIDs)/LVIDd × 100%, and Left ventricle ejection fraction (%) = (LVIDd3 – LVIDs3)/LVIDd3 × 100% (LVIDd: LV diastolic LV internal diameter, LVIDs: LV internal diameter).
Statistical analysis
The data are expressed as mean ± S.E.M. Comparison amongst and within groups (n ≥3) was made by one-way or repeated measures ANOVA and significant differences were determined by the Holm–Sidak test. Student’s t test was used when only two groups were being compared. A value of P<0.05 was considered significant.
Results
Blood pressure is increased while sodium excretion is decreased in adult rat offspring of dams exposed to cold
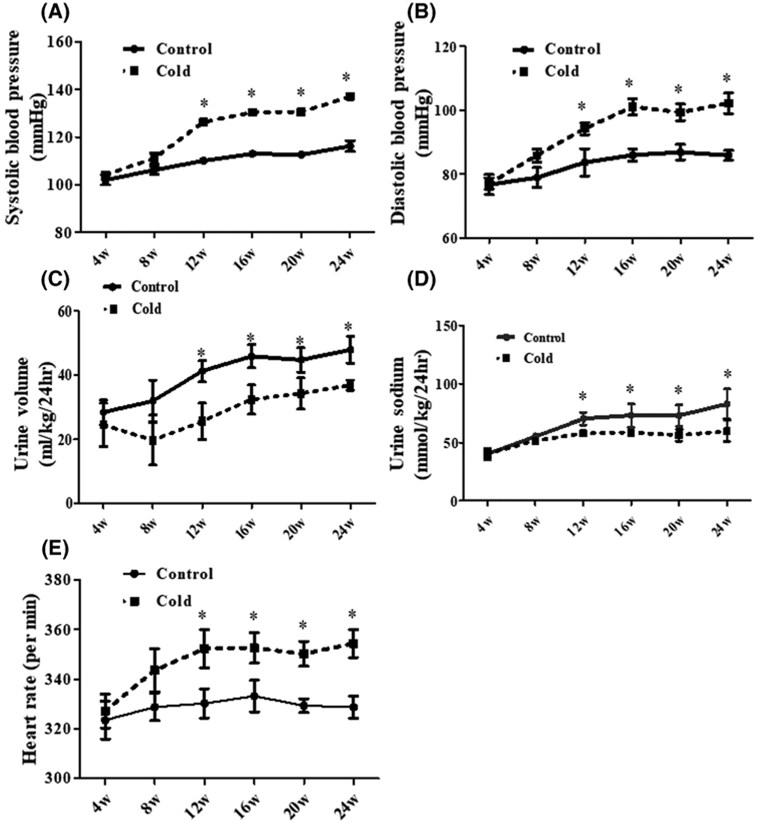
SBP and diastolic blood pressure (DBP) were increased in an age-dependent manner in both the control and prenatal cold-exposed rats. However, at 12 weeks of age the SBP and DBP were higher in the prenatal cold-exposed than the control offspring (Figure 1A,B). Additional studies showed decreased urine volume and sodium excretion in prenatal cold-exposed offspring as compared with the controls (Figure 1C,D). Moreover, the heart rate was higher in prenatal cold-exposed than control offspring (Figure 1E), suggesting an over activity of the SNS. Prenatal cold stress decreased the birth weight of rats, whereas other parameters, including body weight, body temperature, plasma glucose, lipids, cardiac hypertrophy, and function were not different between the groups (Supplemental Figure S1 and 2).
Figure 1. Effect of prenatal cold stress on blood pressure, urine volume, sodium excretion, and heart rate in adult rat offspring.
(A,B) Effect of prenatal cold stress on systolic (A) and diastolic (B) blood pressure in adult offspring. SBP and DBP were recorded by the tail-cuff method in conscious prenatal cold-exposed and control offspring at 4–24 weeks of age (*P<0.05 vs control, n=10/group). (C,D) Effect of prenatal cold stress on urine volume (C) and urine sodium (D) in adult offspring. Urine was collected in prenatal cold-exposed and control offspring at 4–24 weeks of age (*P<0.05 vs cold group, n=10/group). (E) Effect of prenatal cold stress on heart rate in adult offspring. The heart rate was measured at 4, 8, 12, 16, 20, and 24 weeks of age in conscious rats (*P<0.05 vs control, n=10/group).
It should be noted that 1 week of cold exposure also increased the SBP of the dams at the 21 day of gestation, but no significant changes were observed with other parameters, such as heart rate, body weight, and body temperature (Supplemental Figure S3).
Increased SNS activity is involved in the increased blood pressure of adult offspring of dams exposed to cold
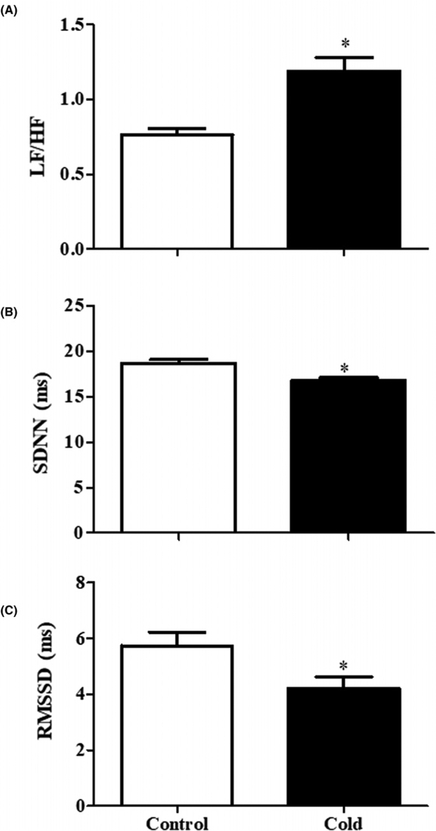
As indicated above, in the basal state, the adult rat offspring of dams exposed to cold stress had increased heart rate, suggestive of increased activity of the SNS. Additional evidence for increased sympathetic activity in adult rat offspring of dams exposed to cold came from measurement of HRV. LF/HF was increased while SDNN and RMSSD were decreased in adult offspring of dams exposed to cold (Figure 2). We confirmed that the increased activity of the SNS is responsible for the prenatal cold-induced hypertension in offspring from the effects of two different adrenergic receptor blockers. The non-selective β adrenergic receptor antagonist propranolol (30 mg/kg) and the α1 adrenergic receptor antagonist prazosin (1 mg/kg) caused a greater decrease in SBP and DBP in adult prenatal cold-exposed than control offspring (Figure 3A,B). The decreased urine volume and sodium excretion observed in adult prenatal cold-exposed offspring were normalized by treatment with propranolol or prazosin (Figure 3C,D).
Figure 2. Effect of prenatal cold stress on HRV.
Three HRV parameters, including LF/HF (A), SDNN (B), and RMSSD (C), were analyzed in prenatal cold-exposed and control offspring at 12 weeks of age to determine the SNS activity (*P<0.05 vs control, n=5/group).
Figure 3. Effect of adrenergic receptor blockers, propranol and prazosin, on blood pressure and natriuresis.
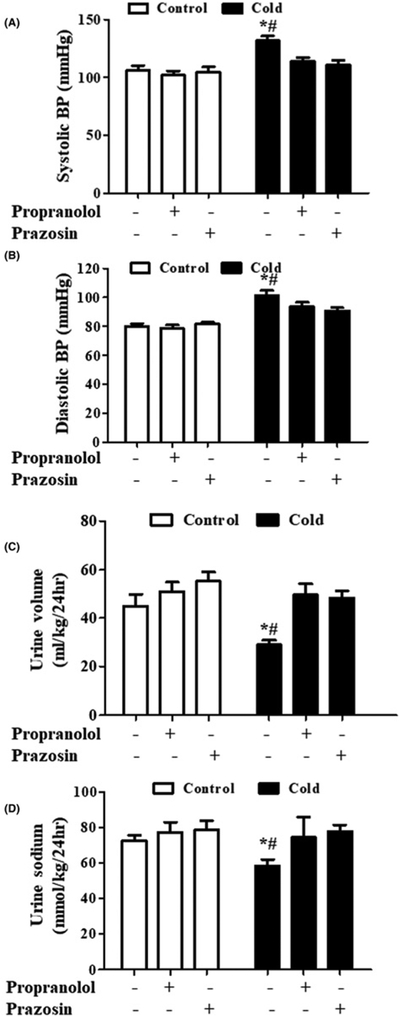
Effect of adrenergic receptor blockers, propranolol and prazosin, on SBP (A), DBP (B), urine volume (C), and urine sodium (D) in adult rat prenatal cold-exposed and control offspring. Propranolol (non-selective β antagonist, 30 mg/kg) and prazosin (specific α1 adrenergic receptor antagonist, 1 mg/kg) were administered into the stomach with a gastric tube twice daily for 1 week from 11 to 12 weeks of age. SBP and DBP were recorded by the tail-cuff method in conscious prenatal cold-exposed and control offspring. (*P<0.05 vs control, #P<0.05 vs others, n=5/group).
Renal denervation lowers the blood pressure of adult rat offspring of dams exposed to cold
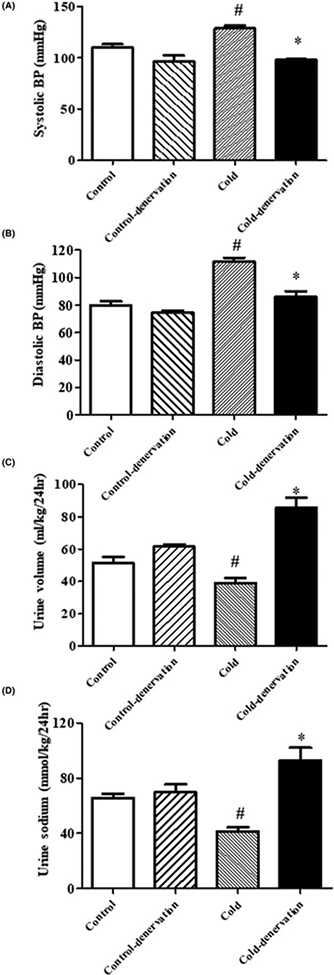
To determine if the increased SNS activity and high blood pressure of adult rat offspring of dams exposed to cold are transmitted directly to the kidney, we performed renal denervation studies. Renal denervation decreased blood pressure and increased urine volume and sodium excretion in both rats. However, the changes were greater in adult offspring of dams exposed to cold than those not exposed to cold, i.e., control rats (Figure 4A–D). Moreover, the blood pressure elevation and renal functional impairment were not caused by the destruction of renal tissue (Supplemental Figure S4). These results support a role for renal sympathetic nervous over-activation in the blood pressure elevation caused by prenatal cold exposure.
Figure 4. Effect of renal denervation on blood pressure, urine volume, and urine sodium in adult rat prenatal cold-exposed and control offspring at 12 weeks of age.
(A,B) Effect of renal denervation on blood pressure in adult rat prenatal cold-exposed offspring. SBP (A) and DBP (B) were recorded by the tail-cuff method in conscious prenatal cold-exposed and control offspring. (*P<0.05 vs control, #P<0.05 vs others, n=4/group). (C) Effect of renal denervation on urine volume in prenatal cold-exposed and control offspring (*P<0.05 vs control, #P<0.05 vs others, n=4/group). (D) Effect of renal denervation on urine sodium in adult prenatal cold-exposed and control offspring (*P<0.05 vs control, #P<0.05 vs others, n=4/group).
The central SNS is also involved in the increased blood pressure of adult rat offspring of dams exposed to cold
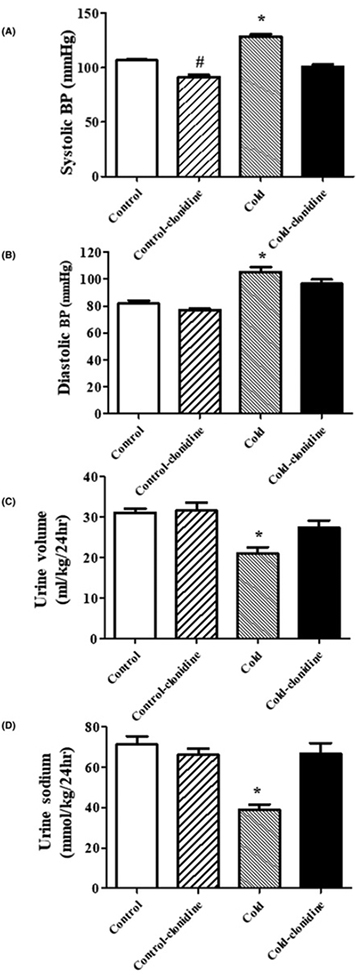
To determine if the central SNS is also involved in the increased blood pressure of adult rat offspring of dams exposed to cold, we studied the effect of clonidine, a centrally acting α2 adrenergic receptor agonist (0.2 mg/kg, oral administration). We found that clonidine also reduced the blood pressure (Figure 5A,B) and increased the urine volume and sodium excretion to a greater degree (Figure 5C,D) in prenatal cold-exposed than control offspring.
Figure 5. Effect of centrally acting α2 adrenergic receptor agonist, clonidine, on blood pressure and natriuresis.
Effect of centrally acting α2 adrenergic receptor agonist, clonidine, on SBP (A), diastolic blood pressure (B), urine volume (C), and urine sodium (D) in adult rat prenatal cold-exposed and control offspring at 12 weeks of age. Clonidine (0.2 mg/kg) was administrated into the stomach with a gastric tube twice daily for 1 week from 11 to 12 weeks of age. The blood pressure, urine volume, and urine sodium were measured after the last gavage. (*P<0.05 vs others, #P<0.05 vs control group without treatment, n=5/group).
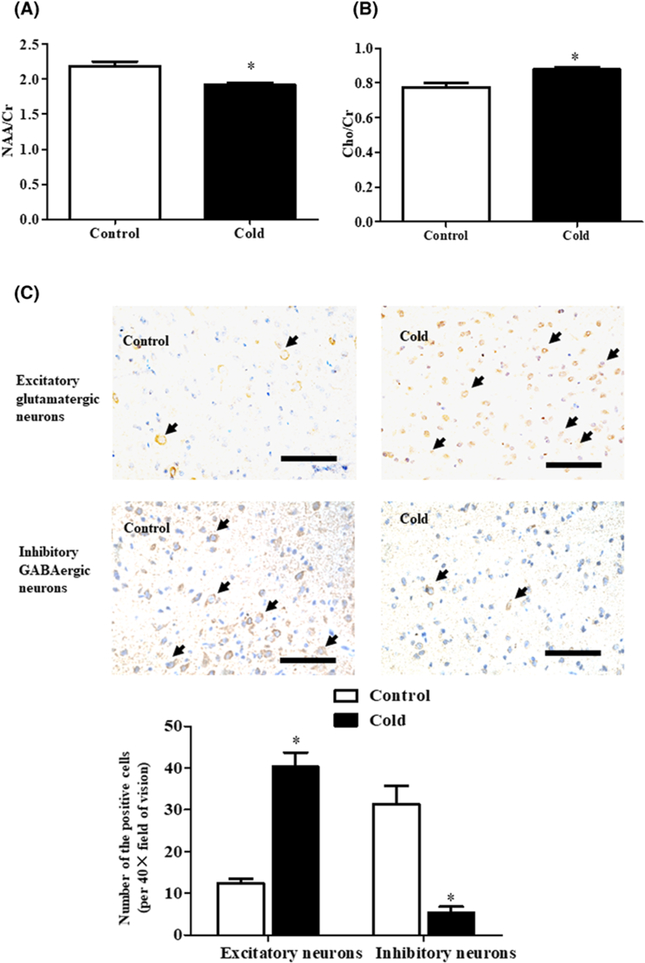
The hypothalamic PVN is the most important site for central integration of sympathetic outflow [27,28]. Therefore, we checked whether there is PVN disorder in rat adult offspring with prenatal cold exposure by measuring NAA/Cr and Cho/Cr in the PVN region; increased ratios are indicative of neuronal cell loss [29]. MRS showed that NAA/Cr was lower in the prenatal cold-exposed than control group, whereas Cho/Cr was higher in the prenatal cold-exposed than control group (Figure 6A,B, Supplemental Figure S5). Since there are two types of pre-sympathetic neurons, i.e., inhibitory and excitatory neurons, we then checked the intensity of the staining of specific markers of inhibitory and excitatory neurons. We found that prenatal cold-exposed adult rats had increased number of glutamergic excitatory neurons and decreased number of inhibitory GABAergic neurons (Figure 6C). Thus, we concluded that prenatal cold-exposure altered the balance between inhibitory and excitatory neurons that led to PVN overactivation.
Figure 6. Effect of prenatal cold stress on the metabolic and structural changes of PVN neurons in adult rat offspring at 12 weeks of age.
(A,B) MRS values of adult prenatal cold-exposed and control offspring. The ratios of NAA/Cr (A) and Cho/Cr (B) of the PVN region were acquired by the MRS to detect the central SNS disorder (*P<0.05 vs control, n=6). (C) The intensity of inhibitory and excitatory neurons changes in adult prenatal cold-exposed and control offspring. The excitatory glutamatergic neurons were identified by GRIN2B staining and the inhibitory GABAergic neurons were identified by GAD65 staining. The number of positive cells (black arrow) was counted in ten random fields of the PVN zone from each rat. (*P<0.05 vs control, scale bar = 100 μm, n=4/group).
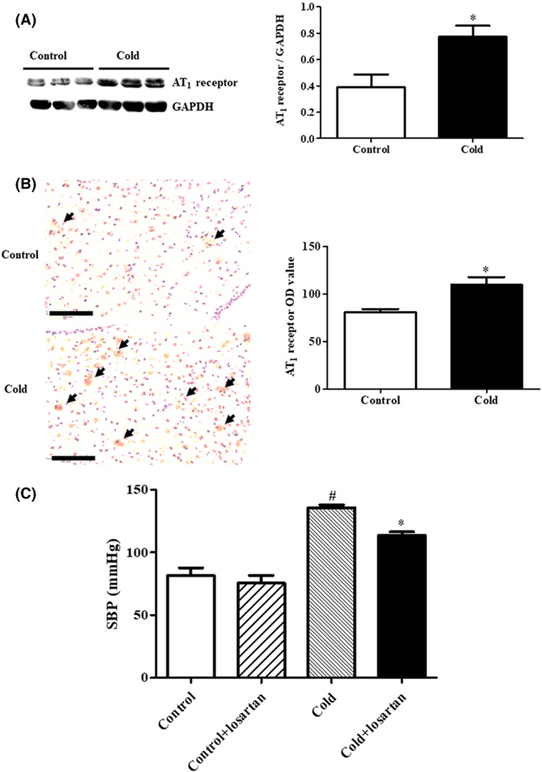
Higher AT1 receptor expression in the PVN leads to higher central sympathetic system in the rat adult offspring of dams exposed to cold
PVN function is regulated by the AT1 receptor [27,28,30]; our additional study showed that PVN AT1 receptor expression was higher in the prenatal cold-exposed than control offspring (Figure 7A,B). And prenatal cold stress significantly increased the levels of renin and Angiotensin II (Ang II) in PVN, as compared with the control offspring, whereas the prenatal cold exposure did not affect PVN AT2 receptor expression (Supplemental Figure S6). Moreover, the increased AT1 receptor expression is of pathological significance because the microinjection of losartan, an AT1 receptor blocker (11.45 nmol/kg body weight) in the hypothalamic PVN lowered the blood pressure to a greater degree in prenatal cold-exposed than control offspring (Figure 7C).
Figure 7. Effect of prenatal cold stress on AT1 receptor expression and function in PVN of adult rat offspring at 12 weeks of age.
(A,B) AT1 receptor expression was detected by immunoblotting (A) and immunohistochemistry (B). (A) Results of immunoblotting are expressed as the ratio of AT1 receptor and GAPDH (*P<0.05 vs control, n=6). (B) The staining in each group was repeated at least five times, and ten fields of vision in each slide were chosen for OD assessment (*P<0.05 vs control, scale bar = 100 μm, shown as black arrow). (C) Effect of the AT1 receptor antagonist, losartan, on SBP in adult prenatal cold-exposed and control offspring. Losartan (AT1 receptor antagonist) was administrated into the hypothalamic PVN by microinjection (11.45 nmol/kg). The rats were anesthetized with pentobarbital and blood pressure was measured from the femoral artery. Blood pressures were obtained 1 h after the losartan PVN injection (*P<0.05 vs others, #P<0.05 vs control group without treatment, n=8/group).
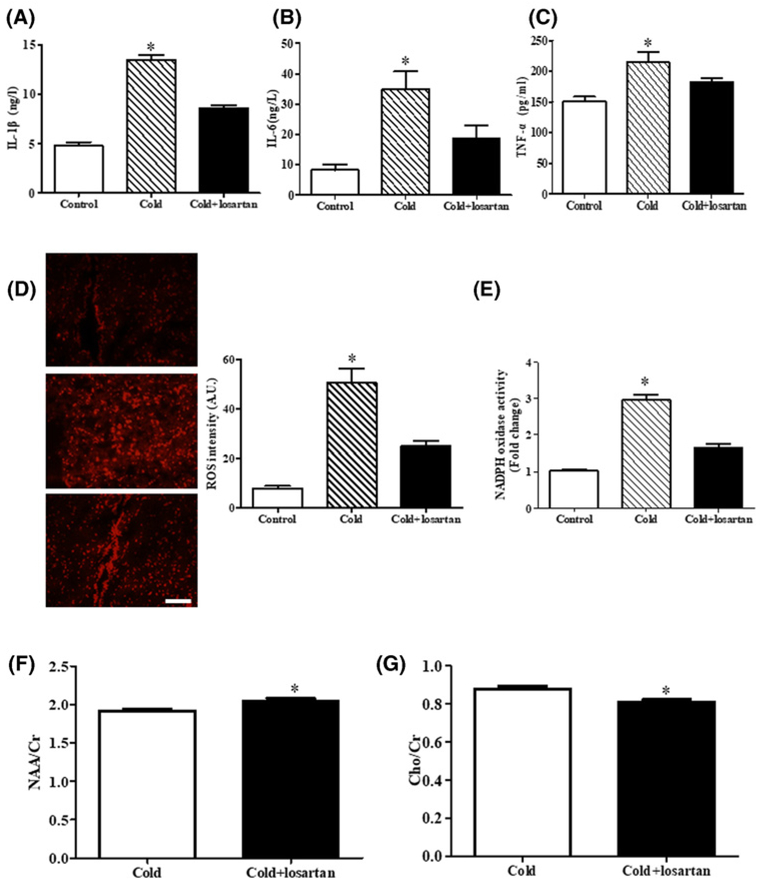
Inflammation and oxidase stress are important mediators of increased AT1 receptor and PVN dysfunction [27,28,31,32,58–60]. Therefore, we also checked the inflammatory state and level of ROS in the PVN region. We found that the pro-inflammatory cytokine levels, such as IL-1β, IL-6, and TNF-α were higher in PVN region in prenatal cold-exposed than control offspring (Figure 8A–C), moreover, the PVN tissue subjected to prenatal cold treatment showed enhanced DHE fluorescent intensity and increased NADPH oxidase activity (Figure 8D,E), the above mentioned increased inflammatory state and ROS level were reduced by losartan treatment in PVN tissue from prenatal cold-exposed offspring (Figure 8A–E). It was also noticed that the effect of losartan in recovery of PVN dysfunction was evident in MRS analysis (Figure 8F,G, Supplemental Figure S5). These results imply that increased AT1 receptor, via increased inflammation and oxidative stress, leads to overactive PVN function in rat adult offspring with prenatal cold exposure.
Figure 8. Effect of losartan on the levels of inflammation and oxidase stress in PVN of prenatal cold exposed offspring at 12 weeks of age.
(A–C) The levels of the pro-inflammatory cytokines IL-1β (A), IL-6 (B) and TNF-α (C) were measured by ELISA kits (*P<0.05 vs others, n=8). (D,E) The ROS level was measured by DHE staining and NADPH oxidase activity kit (*P<0.05 vs others, n=5). (F,G) MRS values of cold-exposed offspring with or without losartan. The ratios of NAA/Cr (F) and Cho/Cr (G) of the PVN region were acquired by the MRS to detect the central SNS disorder (*P<0.05 vs prenatal cold treated group, n=6).
Discussion
Hypertension may be caused by changes in environment temperature. Blood pressure is increased with decreased outdoor temperature [33]. Epidemiological studies have found that cold season increases the risk of developing hypertension [33,34]. To date, no studies have examined the association between prenatal cold exposure and hypertension in the offspring. Our current study showed that cold exposure during gestation increases the blood pressure and decreases urine volume and sodium excretion in the adult offspring.
Essential hypertension is associated with increased activity of the SNS [35,36]. Increased sympathetic activity and elevated basal blood pressure have been observed in children and young adults with family history of hypertension [37,38]. Our present study found that the heart rate was higher in cold exposed offspring, indicating the higher sympathetic activity may play a major role in the pathogenesis of hypertension associated with prenatal cold exposure. This phenomenon was confirmed by the measurement of HRV; increased LF/HF and decreased SDNN and RMSSD were found in cold-exposed offspring. Moreover, the adrenergic receptor antagonists, propranolol (non-selective β antagonist) and prazosin (α1 adrenergic receptor antagonist), decreased SBP and increased the urine volume and sodium excretion to a greater extent in prenatal cold-exposed than control offspring.
The renal nerves participate in the long-term control of blood pressure by regulating renal blood flow, sodium and water handling, and release of hormones, including renin and prostaglandins [35,39]. In our current study, we found that renal denervation decreased the blood pressure and increased sodium excretion in greater degree in prenatal-cold exposed offspring, suggested increased renal nerve activity, similar to those observed in maternal obesity [40,41], diabetes [42], and drug abuse [43]. Central SNS activity is regulated by the hypothalamic PVN through projections to the rostral ventrolateral medulla and the intermediolateral region of the spinal cord [44]. The PVN is a critical brain region [27,28] involved in the development and maintenance of hypertension [45–47]. Prenatal cold stress has been associated with behavioral and neurological disorders, with alteration of the structure of the hippocampus [10,11,48]. Our present study showed that the prenatal cold stress led to neuronal cell loss in the PVN region, identified by MRS. Since PVN is tuned by both excitatory glutamatergic and inhibitory GABAergic inputs [29,49], we further determined which type of neurons were impaired by prenatal cold stress. We found the loss of inhibitory neurons and gain of excitatory neurons may have led to hyperactivity of PVN and elevation of blood pressure. The phenomenon is similar to that found in the genetically hypertensive animals. Some studies in spontaneously hypertensive rats have shown that impaired inhibitory and enhanced excitatory inputs into PVN neurons participate in the pathogenesis of hypertension [50,51].
Anatomical and functional studies suggest that Ang-II and the AT1 receptor participate in the regulation of PVN function [52]. The AT1 receptor antagonist, losartan, significantly decreased the sympathoexcitatory response and the firing rate of labeled neurons in PVN [53,54]. Our present study showed that AT1 receptor expression in the PVN is increased and microinjection of losartan into PVN decreased blood pressure to a greater extent in prenatal cold-exposed than control offspring. Ang-II, via the AT1 receptor, can act as a proinflammatory hormone [55]. Neuroinflammation is a trigger and sustainer of excitotoxicity in the central nervous system [56]. AT1 receptor associated inflammation within the subfornical organ, PVN, and rostral ventrolateral medulla has emerged as an important contributor to sympathoexcitation in several cardiovascular diseases, especially in hypertension [57,58]. Besides, the sympathetic responses to central Ang-II in hypertension, via AT1 receptor, are mediated by superoxide within the PVN [59,60]. Since both inflammation and ROS are critical AT1 receptor-mediated events within the PVN with major pathophysiological consequences in hypertension, our data showed increased AT1 receptor could lead to inflammation and oxidative stress, which causes in PVN overactivation and blood pressure elevation, and losartan significantly reduced PVN inflammation and oxidative stress, and lowered blood pressure.
In conclusion, we provided evidence that renal sympathetic nervous overactivation increased blood pressure and decreased urine volume and sodium excretion in prenatal cold-exposed offspring. Increased AT1 receptor expression led to PVN disorder and increased central SNS activity, consequently, higher blood pressure. Blockade of the AT1 receptor or renal sympathetic nerve activity could prevent the fetal programming of hypertension specifically that related to the hypertension caused by prenatal cold exposure.
Supplementary Material
Clinical perspectives.
The impact of parental disease and environment in prenatal programing can be transmitted to future generations, and maternal cold stress could affect the physiological phenotype of the offspring. Whether or not cold stress during pregnancy results in blood pressure elevation in the offspring remains unclear.
In the present study, we found that renal sympathetic nervous overactivation increased blood pressure and decreased urine volume and sodium excretion in prenatal cold-exposed offspring. Increased AT1 receptor expression led to PVN disorder and increased central SNS activity, consequently, higher blood pressure.
This finding provides novel insight into the prenatal cold stress-induced elevation of blood pressure and imply that avoiding low-temperature environment during pregnancy may reduce the risk of hypertension in offspring. Central AT1 receptor blockade in the PVN may be a key step for treatment of this type hypertension.
Funding
These studies were supported in part by grants from the National Natural Science Foundation of China [grant numbers 31430043, 81500536, and 81700371], National Key R&D Program of China [grant number 2018YFC1312700], Program of Innovative Research Team by National Natural Science Foundation [grant number 81721001], Program for Changjiang Scholars and Innovative Research Team in University [grant number IRT1216], and the National Institutes of Health [grant numbers 5R01DK039308-31, 5P01HL074940-13, and 5R01HL092196-10].
Abbreviations
- DBP
diastolic blood pressure
- FS
fraction shortening
- HRV
Heart rate variability
- IL
interleukin
- LF/HF
low-frequency and high-frequency ratio
- MRS
magnetic resonance spectroscopy
- OD
optical density
- PVN
paraventricular nucleus
- RAAS
renin-angiotensin-aldosterone system
- RMSSD
square root of the mean squared differences of successive N-N interval
- ROS
reactive oxygen species
- SD
Sprague-Dawley
- SDNN
standard deviation of the N-N interval
- SNS
sympathetic nervous system
- SBP
systolic blood pressure
- TNF
tumor necrosis factor
Footnotes
Competing Interests
The authors declare that there are no competing interests associated with the manuscript.
References
- 1.Basu S and Millett C (2013) Social epidemiology of hypertension in middle-income countries: determinants of prevalence, diagnosis, treatment, and control in the WHO SAGE study. Hypertension 62, 18–26, 10.1161/HYPERTENSIONAHA.113.01374 [DOI] [PubMed] [Google Scholar]
- 2.Cooper RS, Kaufman JS and Bovet P (2017) Global burden of disease attributable to hypertension. JAMA 317, 2017–2018, 10.1001/jama.2017.4213 [DOI] [PubMed] [Google Scholar]
- 3.Arnett DK and Claas SA (2012) Preventing and controlling hypertension in the era of genomic innovation and environmental transformation. JAMA 308, 1745–1746, 10.1001/jama.2012.28747 [DOI] [PubMed] [Google Scholar]
- 4.Lopez-Jaramillo P, Camacho PA and Forero-Naranjo L (2013) The role of environment and epigenetics in hypertension. Expert Rev. Cardiovasc. Ther 11, 1455–1457, 10.1586/14779072.2013.846217 [DOI] [PubMed] [Google Scholar]
- 5.Ponjoan A, Garcia-Gil MM, Marti R, Comas-Cufi M, Alves-i-Cabratosa L, Sala J et al. (2014) Derivation and validation of BOREAS, a risk score identifying candidates to develop cold-induced hypertension. Environ. Res 132, 190–196, 10.1016/j.envres.2014.03.039 [DOI] [PubMed] [Google Scholar]
- 6.Herrera-Garcia G and Contag S (2014) Maternal preeclampsia and risk for cardiovascular disease in offspring. Curr. Hypertens. Rep 16, 475, 10.1007/s11906-014-0475-3 [DOI] [PubMed] [Google Scholar]
- 7.Wang X, Luo H, Chen C, Chen K, Wang J, Cai Y et al. (2014) Prenatal lipopolysaccharide exposure results in dysfunction of the renal dopamine D1 receptor in offspring. Free Radic. Biol. Med 76, 242–250, 10.1016/j.freeradbiomed.2014.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang X, Wang J, Luo H, Chen C, Pei F, Cai Y et al. (2015) Prenatal lipopolysaccharide exposure causes mesenteric vascular dysfunction through the nitric oxide and cyclic guanosine monophosphate pathway in offspring. Free Radic. Biol. Med 86, 322–330, 10.1016/j.freeradbiomed.2015.05.040 [DOI] [PubMed] [Google Scholar]
- 9.Lian S, Wang D, Xu B, Guo W, Wang L, Li W et al. (2018) Prenatal cold stress: effect on maternal hippocampus and offspring behavior in rats. Behav. Brain Res 346, 1–10, 10.1016/j.bbr.2018.02.002 [DOI] [PubMed] [Google Scholar]
- 10.Saetta M, Noworaj A and Mortola JP (1988) Cold exposure of the pregnant rat and neonatal respiration. Exp. Biol 47, 177–181 [PubMed] [Google Scholar]
- 11.Tazumi T, Hori E, Uwano T, Umeno K, Tanebe K, Tabuchi E et al. (2005) Effects of prenatal maternal stress by repeated cold environment on behavioral and emotional development in the rat offspring. Behav. Brain Res 162, 153–160, 10.1016/j.bbr.2005.03.006 [DOI] [PubMed] [Google Scholar]
- 12.Auger N, Siemiatycki J, Bilodeau-Bertrand M, Healy-Profitos J and Kosatsky T (2017) Ambient temperature and risk of preeclampsia: biased association? Paediatr. Perinat. Epidemiol 31, 267–271, 10.1111/ppe.12362 [DOI] [PubMed] [Google Scholar]
- 13.Kanayama N, Tsujimura R, She L, Maehara K and Terao T (1997) Cold-induced stress stimulates the sympathetic nervous system, causing hypertension and proteinuria in rats. J. Hypertens 15, 383–389, 10.1097/00004872-199715040-00009 [DOI] [PubMed] [Google Scholar]
- 14.Khatun S, Kanayama N, Belayet HM, Masui M, Sugimura M, Kobayashi T et al. (1999) Induction of preeclampsia like phenomena by stimulation of sympathetic nerve with cold and fasting stress. Eur. J. Obstet. Gynecol. Reprod. Biol 86, 89–97, 10.1016/S0301-2115(99)00050-0 [DOI] [PubMed] [Google Scholar]
- 15.Papanek PE, Wood CE and Fregly MJ (1991) Role of the sympathetic nervous system in cold-induced hypertension in rats. J. Appl. Physiol. (1985) 71, 300–306, 10.1152/jappl.1991.71.1.300 [DOI] [PubMed] [Google Scholar]
- 16.Nizar JM and Bhalla V (2017) Molecular mechanisms of sodium-sensitive hypertension in the metabolic syndrome. Curr. Hypertens. Rep 19, 60, 10.1007/s11906-017-0759-5 [DOI] [PubMed] [Google Scholar]
- 17.Schutten MT, Houben AJ, de Leeuw PW and Stehouwer CD (2017) The link between adipose tissue renin-angiotensin-aldosterone system signaling and obesity-associated hypertension. Physiology 32, 197–209, 10.1152/physiol.00037.2016 [DOI] [PubMed] [Google Scholar]
- 18.Alexander BT, Dasinger JH and Intapad S (2015) Fetal programming and cardiovascular pathology. Compr. Physiol 5, 997–1025, 10.1002/cphy.c140036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xue B, Beltz TG, Guo F and Johnson AK (2018) Sex differences in maternal gestational hypertension-induced sensitization of angiotensin II hypertension in rat offspring: the protective effect of estrogen. Am. J. Physiol. Regul. Integr. Comp. Physiol 314, R274–R81, 10.1152/ajpregu.00216.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eriguchi M and Tsuruya K (2016) Renal sympathetic denervation in rats. Methods Mol. Biol 1397, 45–52, 10.1007/978-1-4939-3353-2_5 [DOI] [PubMed] [Google Scholar]
- 21.Tudorancea I, Lohmeier TE, Alexander BT, Pieptu D, Serban DN and Iliescu R (2018) Reduced renal mass, salt-sensitive hypertension is resistant to renal denervation. Front. Physiol 9, 455, 10.3389/fphys.2018.00455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lu X, Ye Z, Zheng S, Ren H, Zeng J, Wang X et al. (2018) Long-term exposure of fine particulate matter causes hypertension by impaired renal D1 receptor-mediated sodium excretion via upregulation of G-protein-coupled receptor kinase type 4 expression in Sprague-Dawley rats. J. Am. Heart Assoc 7, 10.1161/JAHA.117.007185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ye Z, Lu X, Deng Y, Wang X, Zheng S, Ren H et al. (2018) In utero exposure to fine particulate matter causes hypertension due to impaired renal dopamine D1 receptor in offspring. Cell. Physiol. Biochem 46, 148–159, 10.1159/000488418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.(1996) Heart rate variability: standards of measurement, physiological interpretation and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Circulation 93, 1043–1065, 10.1161/01.CIR.93.5.1043 [DOI] [PubMed] [Google Scholar]
- 25.Glendining KA, Fisher LC and Jasoni CL (2018) Maternal high fat diet alters offspring epigenetic regulators, amygdala glutamatergic profile and anxiety. Psychoneuroendocrinology 96, 132–141, 10.1016/j.psyneuen.2018.06.015 [DOI] [PubMed] [Google Scholar]
- 26.Song M, Kim H, Park S, Kwon H, Joung I and Kim Kwon Y (2018) Aucubin promotes differentiation of neural precursor cells into GABAergic neurons. Exp. Neurobiol 27, 112–119, 10.5607/en.2018.27.2.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carmichael CY and Wainford RD (2015) Hypothalamic signaling mechanisms in hypertension. Curr. Hypertens. Rep 17, 39, 10.1007/s11906-015-0550-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hurr C and Young CN (2016) Neural control of non-vasomotor organs in hypertension. Curr. Hypertens. Rep 18, 30, 10.1007/s11906-016-0635-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cao Z, Ye BD, Shen ZW, Cheng XF, Yang ZX, Liu YY et al. (2015) 2D-1H proton magnetic resonance spectroscopic imaging study on brain metabolite alterations in patients with diabetic hypertension. Mol. Med. Rep 11, 4232–4238, 10.3892/mmr.2015.3305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kleiber AC, Zheng H, Sharma NM and Patel KP (2010) Chronic AT1 receptor blockade normalizes NMDA-mediated changes in renal sympathetic nerve activity and NR1 expression within the PVN in rats with heart failure. Am. J. Physiol. Heart Circ. Physiol 298, H1546–55, 10.1152/ajpheart.01006.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bardgett ME, Holbein WW, Herrera-Rosales M and Toney GM (2014) Ang II-salt hypertension depends on neuronal activity in the hypothalamic paraventricular nucleus but not on local actions of tumor necrosis factor-alpha. Hypertension 63, 527–534, 10.1161/HYPERTENSIONAHA.113.02429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li T, Chen Y, Gua C and Wu B (2018) Elevated Oxidative Stress and Inflammation in Hypothalamic Paraventricular Nucleus Are Associated With Sympathetic Excitation and Hypertension in Rats Exposed to Chronic Intermittent Hypoxia. Front. Physiol 9, 840, 10.3389/fphys.2018.00840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Su D, Du H, Zhang X, Qian Y, Chen L, Chen Y et al. (2014) Season and outdoor temperature in relation to detection and control of hypertension in a large rural Chinese population. Int. J. Epidemiol 43, 1835–1845, 10.1093/ije/dyu158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kawazoe N, Zhang X, Chiang C, Liu H, Li J, Hirakawa Y et al. (2018) Prevalence of hypertension and hypertension control rates amongst elderly adults during the cold season in rural Northeast China: a cross-sectional study. J. Rural Med 13, 64–71, 10.2185/jrm.2959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.DiBona GF (2013) Sympathetic nervous system and hypertension. Hypertension 61, 556–560, 10.1161/HYPERTENSIONAHA.111.00633 [DOI] [PubMed] [Google Scholar]
- 36.Rajashekar RK, Niveditha Y and Ghosh S (2003) Blood pressure response to cold pressor test in siblings of hypertensives. Indian J. Physiol. Pharmacol 47, 453–458 [PubMed] [Google Scholar]
- 37.Rathi P, Agarwal V and Kumar A (2013) Sympathetic hyperactivity in children of hypertensive parents. Ann. Neurosci 20, 4–6, 10.5214/ans.0972.7531.200103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Whelton PK, Carey RM, Aronow WS, Casey DE Jr., Collins KJ, Dennison Himmelfarb C et al. (2018) 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J. Am. Coll. Cardiol 71, e127–e248, 10.1016/j.jacc.2017.11.006 [DOI] [PubMed] [Google Scholar]
- 39.Sata Y, Head GA, Denton K, May CN and Schlaich MP (2018) Role of the sympathetic nervous system and its modulation in renal hypertension. Front. Med. (Lausanne) 5, 82, 10.3389/fmed.2018.00082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Samuelsson AM, Morris A, Igosheva N, Kirk SL, Pombo JM, Coen CW et al. (2010) Evidence for sympathetic origins of hypertension in juvenile offspring of obese rats. Hypertension 55, 76–82, 10.1161/HYPERTENSIONAHA.109.139402 [DOI] [PubMed] [Google Scholar]
- 41.Samuelsson AS, Mullier A, Maicas N, Oosterhuis NR, Eun Bae S, Novoselova TV et al. (2016) Central role for melanocortin-4 receptors in offspring hypertension arising from maternal obesity. Proc. Natl Acad. Sci. U.S.A 113, 12298–12303, 10.1073/pnas.1607464113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.de Almeida Chaves Rodrigues AF, de Lima IL, Bergamaschi CT, Campos RR, Hirata AE, Schoorlemmer GH et al. (2013) Increased renal sympathetic nerve activity leads to hypertension and renal dysfunction in offspring from diabetic mothers. Am. J. Physiol. Renal. Physiol 304, F189–F197, 10.1152/ajprenal.00241.2012 [DOI] [PubMed] [Google Scholar]
- 43.Riezzo I, Fiore C, De Carlo D, Pascale N, Neri M, Turillazzi E et al. (2012) Side effects of cocaine abuse: multiorgan toxicity and pathological consequences. C. Curr. Med. Chem 19, 5624–5646, 10.2174/092986712803988893 [DOI] [PubMed] [Google Scholar]
- 44.Ranson RN, Motawei K, Pyner S and Coote JH (1998) The paraventricular nucleus of the hypothalamus sends efferents to the spinal cord of the rat that closely appose sympathetic preganglionic neurones projecting to the stellate ganglion. Exp. Brain Res 120, 164–172, 10.1007/s002210050390 [DOI] [PubMed] [Google Scholar]
- 45.Chen QH, Andrade MA, Calderon AS and Toney GM (2010) Hypertension induced by angiotensin II and a high salt diet involves reduced SK current and increased excitability of RVLM projecting PVN neurons. J. Neurophysiol 104, 2329–2337, 10.1152/jn.01013.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li DP and Pan HL (2007) Role of gamma-aminobutyric acid (GABA)A and GABAB receptors in paraventricular nucleus in control of sympathetic vasomotor tone in hypertension. J. Pharmacol. Exp. Ther 320, 615–626, 10.1124/jpet.106.109538 [DOI] [PubMed] [Google Scholar]
- 47.Zhou JJ, Yuan F, Zhang Y and Li DP (2015) Upregulation of orexin receptor in paraventricular nucleus promotes sympathetic outflow in obese Zucker rats. Neuropharmacology 99, 481–490, 10.1016/j.neuropharm.2015.08.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shi XY, Ju J, Zou LP, Wang J, Shang NX, Zhao JB et al. (2016) Increased precipitation of spasms in an animal model of infantile spasms by prenatal stress exposure. Life Sci 152, 171–177, 10.1016/j.lfs.2016.03.047 [DOI] [PubMed] [Google Scholar]
- 49.Li DP, Yang Q, Pan HM and Pan HL (2008) Pre- and postsynaptic plasticity underlying augmented glutamatergic inputs to hypothalamic presympathetic neurons in spontaneously hypertensive rats. J. Physiol 586, 1637–1647, 10.1113/jphysiol.2007.149732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Allen AM (2002) Inhibition of the hypothalamic paraventricular nucleus in spontaneously hypertensive rats dramatically reduces sympathetic vasomotor tone. Hypertension 39, 275–280, 10.1161/hy0202.104272 [DOI] [PubMed] [Google Scholar]
- 51.Li DP and Pan HL (2007) Glutamatergic inputs in the hypothalamic paraventricular nucleus maintain sympathetic vasomotor tone in hypertension. Hypertension 49, 916–925, 10.1161/01.HYP.0000259666.99449.74 [DOI] [PubMed] [Google Scholar]
- 52.Pan HL (2004) Brain angiotensin II and synaptic transmission. Neuroscientist 10, 422–431, 10.1177/1073858404264678 [DOI] [PubMed] [Google Scholar]
- 53.Chen QH and Toney GM (2001) AT(1)-receptor blockade in the hypothalamic PVN reduces central hyperosmolality-induced renal sympathoexcitation. Am. J. Physiol. Regul. Integr. Comp. Physiol 281, R1844–53, 10.1152/ajpregu.2001.281.6.R1844 [DOI] [PubMed] [Google Scholar]
- 54.Li DP and Pan HL (2005) Angiotensin II attenuates synaptic GABA release and excites paraventricular-rostral ventrolateral medulla output neurons. J. Pharmacol. Exp. Ther 313, 1035–1045, 10.1124/jpet.104.082495 [DOI] [PubMed] [Google Scholar]
- 55.Forrester SJ, Booz GW, Sigmund CD, Coffman TM, Kawai T, Rizzo V et al. (2018) Angiotensin II signal transduction: an update on mechanisms of physiology and pathophysiology. Physiol. Rev 98, 1627–1738, 10.1152/physrev.00038.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Olmos G and Llado J (2014) Tumor necrosis factor alpha: a link between neuroinflammation and excitotoxicity. Mediators Inflamm 2014, 861231, 10.1155/2014/861231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Capone C, Faraco G, Peterson JR, Coleman C, Anrather J, Milner TA et al. (2012) Central cardiovascular circuits contribute to the neurovascular dysfunction in angiotensin II hypertension. J. Neurosci 32, 4878–4886, 10.1523/JNEUROSCI.6262-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Biancardi VC, Stranahan AM, Krause EG, de Kloet AD and Stern JE (2016) Cross talk between AT1 receptors and Toll-like receptor 4 in microglia contributes to angiotensin II-derived ROS production in the hypothalamic paraventricular nucleus. Am. J. Physiol. Heart Circ. Physiol 310, H404–15, 10.1152/ajpheart.00247.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Campese VM, Shaohua Y and Huiquin Z (2005) Oxidative stress mediates angiotensin II-dependent stimulation of sympathetic nerve activity. Hypertension 46, 533–539, 10.1161/01.HYP.0000179088.57586.26 [DOI] [PubMed] [Google Scholar]
- 60.Erdos B, Broxson CS, King MA, Scarpace PJ and Tumer N (2006) Acute pressor effect of central angiotensin II is mediated by NAD(P)H-oxidase-dependent production of superoxide in the hypothalamic cardiovascular regulatory nuclei. J. Hypertens 24, 109–116, 10.1097/01.hjh.0000198026.99600.59 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.