Abstract
Recent studies demonstrate that peripheral amylin treatment reduces pathology in mouse models of Alzheimer’s disease (AD). However, soluble and aggregated amylin are distinct species; while amylin is a physiological neuropeptide, amylin aggregation is a pathological factor for diabetes. We thus hypothesized that because of their similarity in secondary structures, amylin antagonizes amyloid-β peptide (Aβ) induced AD pathology in neurons with a dose dependent pattern. To test the hypothesis, we conducted both in vitro and in vivo experiments with different doses of amylin and with its analog, pramlintide. Here we report that a high concentration of either Aβ or amylin alone induced tau phosphorylation (pTau) in primary neurons. Interestingly, with a low concentration, amylin had direct effects to reverse the Aβ induced pTau, as well as damaged neuronal synapses and neurite disorganization. However, when the concentration was high (10.24 μM), amylin lost the effects against the Aβ induced cellular AD pathology and, together with Aβ, worsened tauopathy in neurons. In the 5XFAD AD mouse model, daily peripheral amylin treatment with a low dose (200 μg/kg) more effectively reduced amyloid burden, and increased synapse, but with a high dose (800 μg/kg) it more effectively reduced tauopathy. Correspondingly, amylin treatment improved learning and memory in these mice. It demonstrates that amylin has a dose-dependent U-shape effect against AD pathogenesis. Within a physiological range, amylin is a neuroprotective hormone against AD in neurons; but when both Aβ and amylin concentrations are elevated, imbalance of Aβ and amylin may contribute to brain AD pathogenesis.
Keywords: Alzheimer’s disease, Amylin, pramlintide, amyloid-β peptide (Aβ), tauopathy, synapse, U-shape
Background
Amylin (also known as islet amyloid polypeptide or IAPP) is a highly conserved 37–amino acid peptide produced and secreted by the β-cells in the pancreas, and functions as a gut-brain axis hormone [1–3]. While AD patients are found to have a lower concentration of plasma amylin than controls [4, 5], a genome-wide interaction study showed that an amylin gene polymorphism is associated with brain amyloid burden and cognitive impairment in AD [6].
It is found that amylin and amyloid-β peptide (Aβ), a major component of AD pathology [7], share several features, including exhibiting similar β-sheet secondary structures [8] and binding to the same amylin receptor (AMY receptor) [9]. Because that the primary amino acid sequences of amylin and Aβ are largely different (Figure 1)[10], it is possible that a large amount of Aβ in the AD brain blocks or interferes with AMY receptor to bind amylin and its signal transduction. Thus, providing monomeric amylin type peptides could rebalance these two peptides and be therapeutic for AD [5, 11, 12]. On the other hand, while amylin can aggregate and become neurotoxic when the concentration is high in cell cultures [13, 14], amylin aggregates are found co-localized with amyloids in the AD brain [15].
Figure 1. Primary amino acid sequences of Aβ and amylin-type peptides.
The amino acid sequences of Aβ1–42, amylin and pramlintide are presented. Amino acids of amylin are in red, and the amino acids of other peptides are in red if they are identical to that in amylin. The different amino acids of pramlintide from amylin are in blue, while the different amino acids of Aβ1–42 from amylin are in black.
Amylin’s direct effects in the presence of Aβ for AD pathology on neurons are unclear. It is possible that when amylin level is low relative to the amount of Aβ, AMY receptor is occupied by Aβ and cannot bind amylin effectively to mediate physiological function; when amylin concentration is high or under some pathological environment like diabetes, amylin aggregates and loses its ability to bind AMY receptor in aging brain [4]. To understand the mechanism of amylin for AD, we think it is important to address the two aspects, angel and devil, of this peptide, so it can help to determine whether and what type of amylin peptides should be translated into a therapeutic for AD in humans [4]. We hypothesized that imbalance of Aβ and amylin may contribute to AD pathologenesis. Here, using both primary neuron cultures and AD mouse models, we report dose-dependent and U-shape effects of amylin derived from their direct actions against Aβ induced AD pathology on the target neurons.
Materials & methods
Reagent preparation
HFIP treated human Aβ 1–42 was purchased from AnaSpec (Cat# AS-64129–05). Aβ 1–42 was suspended in sterile filtered DMSO to a concentration of 2.2 mM. This was sonicated in a water bath on high for 1 minute and then was diluted to a concentration of 1 mM Aβ with Neurobasal Medium (final concentrations of 90% Neurobasal Mediumand 10% DMSO) and rotated for 24 hours at 4 ° Celsius (°C) [16]. After 24 hours, different concentrations of this amyloid peptide solution diluted in control medium were incubated with primary neurons for different durations.
Human amylin was purchased from AnaSpec (Fremont, CA) and pramlintide was purchased from Amylin Pharmaceutical (San Diego, CA). They were resolved in water to a concentration of 256μM and diluted in control solution to working concentration before being used [14].
Mice and experimental treatments
All animal procedures were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Boston University Animal Care and Use Committee.
CD1 pregnant mice were purchased from Charles River laboratories International, Inc. All mice were maintained in a microisolator of the animal facility at Boston University School of Medicine, MA, USA. An AD mouse model, 5XFAD mice, which are amyloid precursor protein (APP)/presenilin 1 (PS1) double transgenic mice with five familial AD mutations [17] were purchased from the Jackson Laboratory (Bar Harbor, ME, USA). Female 5XFAD mice aged 4 months were used for the experiments. The mice were treated with intraperitoneal (i.p.) injections of amylin 200 μg/kg vs. 800 μg/kg vs. phosphate-buffered saline (PBS) once daily for 6 weeks. Experimental groups (n = 14–16 per cohort) were matched for age and weight.
Primary neuronal cultures
For primary mouse neuronal cultures, primary cultures were prepared from cortical tissue obtained from the brains of postnatal 0–1 day (P0-P1) CD1 mouse pups as described by Beaudoin III et al. [nature protocols 7(9): 1741–1754 (2012)] with some modifications. Briefly, early postnatal mouse cortex was dissected, incubated in 0.25% trypsin (Gibco, 15090–046) with DNase I Solution (1:100; Stemcell, 7900) for 15 min, and dispersed by trituration in Neurobasal medium (Invitrogen, 21103–049) with 2% B-27 supplement (Gibco, 17504–044), 2 mM L-Glutamine (Invitrogen, 25030–081), 0.3% Glucose (Sigma, G6152) and 5% FBS (fetal bovine serum). Dissociated cells were plated onto 24-well plates precoated with 0.1mg/mL poly-D-Lysine (Sigma, P-9155) at a density of 1×104 cells/cm2 for immunofluorescence assay or 6-well plates precoated with 0.1mg/mL poly-D-Lysine at a density of 2×105 cells/cm2 for western blot assay. Cells were maintained in Neurobasal medium with 2% B-27 supplement, 2 mM L-glutamine, and 0.3% Glucose and incubated in 5% CO2 at 37 °C. The medium was changed twice a week. Experiments were carried out at 14 days in vitro (DIV). On Day 14 of culture, cells were pre-incubated with different concentrations of amylin peptides for 1 hour, followed by the addition of different concentrations of aggregated Aβ1–42.
Rat cortical neurons were cultured, and the experiments were conducted as described [18, 19]. Pregnant female rats at gestational day 15 were sacrificed by cervical dislocation (Rats Wistar; Janvier), and the fetuses were removed from the uterus. The cells from the cortex were seeded at a density of 30 000 cells/well in 96 well-plates pre-coated with poly-L-lysine (10 μg/ml, Greiner, 655930) and cultured at 37°C in a humidified air (95%)/CO2 (5%) atmosphere (6 wells/condition, 1 culture). On Day 12 of culture, cells were pre-incubated with different concentrations of different amylin peptides for 1 hour, followed by the addition of aggregated Aβ1–42 (10 μM).
Rat hippocampal neurons were cultured, and the experiments were conducted as described by Callizot et al. [20]. Briefly, pregnant female rats at gestational day 17 were killed by cervical dislocation (Rats Wistar; Janvier). The fetuses were removed from the uterus, and the hippocampi were dissociated by trypsinization for 20 min at 37°C (Trypsin EDTA 1X; PanBiotech, P10–023100). After the reaction was stopped, cells were then mechanically dissociated by 3 passages through a 10-ml pipette and then centrifuged at 515 × g for 10 min at 4°C. The supernatant was discarded, and the cells of the pellet were re-suspended in a defined culture medium consisting of neurobasal medium (Nb, Invitrogen, 21103) supplemented with 2% B27 (Invitrogen, 17504), 2 mM L-glutamine (Panbiotech, P04–80100), 2% PS solution and 10 ng/ml brain-derived neurotrophic factor (BDNF, PanBiotech, CB-1115002). Viable cells were counted in a Neubauer cytometer using the trypan blue exclusion test. The cells were seeded at a density of 20 000 cells/well in 96 well-plates pre-coated with poly-D-lysine (10 μg/ml, Greiner, 655930) and cultured at 37°C in a humidified air (95%)/CO2 (5%) atmosphere (6 wells/condition, 1 culture). After 17 days of culture, the cells were pre-incubated with test peptides for 1 hour before the application of Aβ1–42 (0.6 μM).
Immunocytochemistry
Primary mouse neurons cultured in coverslips were washed using 1XPBS and fixed with 4% PFA for 15 min. Then neurons were permeabilized with 0.05% Triton X-100 and non-specific sites were blocked with 10% donkey serum for 1 h at room temperature. Later neurons were incubated with primary antibody. After washing, anti-mouse antibody conjugated with Alexa Fluor® 488 (Life Technology, A21202; 1:1000) and anti-rabbit antibody conjugated with Alexa Fluor® 594 (Life Technology, A21207; 1:1000) staining was performed for 1 h at room temperature. Neurons were counterstained with DAPI (4’, 6-Diamidino-2-Phenylindole, Dihydrochloride) for nuclear staining. The stained neurons were observed under the fluorescence microscopy (Carl Zeiss, Germany).
For primary rat neurons, cells were fixed with a cold solution of ethanol (95%, Sigma, 32221) /acetic acid (5%, Sigma, 33209) for 5 min. Cells were then permeabilized, and non-specific sites were blocked with a solution of PBS (PanBiotech, P04–36500) containing 0.1% saponin (Sigma Aldrich, S7900) and 1% FCS for 15 min. Cells were then incubated with primary antibody overnight at 4°C. The antibody stainings for different proteins were revealed with an Alexa Fluor 488 goat anti-mouse antibody (Molecular probe, A11001; 1:400) for 1 hour. The nuclei of cells were labeled by a fluorescent marker (Hoechst solution, SIGMA, B1155). Twenty pictures per well were taken using InCell AnalyzerTM 2000 (GE Healthcare) with 20x magnification.
To study the effects of amylin on tauopathy formation in primary neurons, mouse neurons were incubated with mouse monoclonal antibody anti-PHF-1 (1:200) [21, 22] or mouse monoclonal antibody anti-CP13 (1:200) [23] overnight at 4°C. PHF-1 antibody recognizes Tau phosphorylated at Ser396 and Ser404 and CP13 antibody recognizes Tau phosphorylated at Ser202. A co-staining was done using either chicken polyclonal microtubule-associated protein 2 (MAP-2) (Abcam, ab5392; 1:500) or rabbit monoclonal antibody anti-NeuN (Abcam, ab177487; 1:300) primary antibody. Rat neurons were exposed to 2.5 μM of Aβ1–42 in a defined medium (Callizot et al. 2013) for 16 hours. BDNF (50 ng/ml) was used as positive control. Cells were incubated with a mouse monoclonal primary against human hyperphosphorylated tau paired helical filament (Fisher scientific, MN1060; 1:400) overnight in the same solution. This antibody recognizes PHF-tau phosphorylated at Ser212 and Thr214. A co-staining was done using a chicken polyclonal microtubule-associated protein 2 (MAP-2) primary antibody (Abcam, ab5392; 1:400). The area and intensity of phosphorylated tau was analyzed by ImageJ software (http://rsbweb.nih.gov/ij/) as described (Jensen et al. 2013) (Hoboken 2013, tau phos) and the ratio with the number of cell bodies was calculated.
To investigate the effects of amylin on synapse formation, rat hippocampal cells were pretreated with amylin or pramlintide for one hour, and then treated with 0.6 μM of Aβ1–42 in the presence of test peptide(s). One culture was used per condition. After 48 hours of the treatment, cells were fixed, permeabilized and blocked. Cells were then incubated with a mouse monoclonal primary antibody against post-synaptic density 95 kDa (PSD95) (Abcam, ab2723, 1:100) and a rabbit polyclonal primary antibody against Synaptophysin (Sigma, ab7837, 1:100) overnight at 4°C. The nuclei of cells were labeled by a fluorescent marker (Hoechst solution, SIGMA, B1155). The results are expressed in μm2 per field [20].
To study the effects of amylin on neurite outgrowth and neuronal survival, cells were treated by adding 10 μM of Aβ1–42 in a defined medium in the presence of test peptides for 24 hours. Cells were incubated with a mouse monoclonal primary microtubule-associated protein 2 antibody (MAP-2, Sigma M4403; 1:400) for 2 hours in the same solution. This antibody specifically stains the cell bodies and neurites of neurons [20].
All of the images were taken in the same conditions and there were 3 replications.
Tissue Processing
Animals were anesthetized deeply and perfused with ice-cold PBS, then followed by brain dissection. The brains were separated into left and right hemispheres. From one of the hemispheres, the cerebral cortex tissues were separately dissected, flash-frozen in dry ice and stored at −80°C until biochemical analysis were performed as described below. The other hemisphere was fixed in 4% paraformaldehyde/PBS solution for 24 hr and transferred to 30% sucrose/PBS solution until tissue dropped to the bottom of the container. And then the brain was freezed in OCT media for cryosectioning, cut into 30-μm coronal sections and stored at −20 °C in 30% glycerol/30% ethylene glycol/PBS solution until used.
Western blots
Western blot assays were performed as described before [12]. Sarkosyl-insoluble fractionation was used to separate the soluble and insoluble fractions of brain extracts from 5XFAD mice. Briefly, primary neurons or brain tissues were lysed in the radioimmunoprecipitation assay buffer (Thermo Scientific, PI89900) mixed with protease inhibitor cocktail (Thermo Scientific, 78440; 1:400) and homogenized with a sonicator. Protein concentration of each sample was determined by the BCA protein assay kit (Thermo Scientific, 23227). Protein (50 μg) was electrophoresed on 4%−20% Tris-Glycine Mini Gels (Thermo Scientific, XP04200BOX) and transferred to the PVDF transfer membrane (Thermo Scientific, 88520). The membrane was blocked by 5% non-fat dry milk in Tris Buffered Saline with Tween-20 (TBST) (American Bioanalytical, Inc., AB14330–04000) and then incubated with the primary antibody diluted in the blocking buffer overnight at 4°C. Anti-PSD95 (cell signaling, 3450T; 1:1000), anti-Synaptophysin (Santa Cruz Biotech, sc-17750; 1:1000), anti-actin (Santa Cruz Biotech, sc-81178, 1:500), PHF-1 (1:300), CP13 (1:500) and total Tau (Santa Cruz, sc-32274; 1:1000) were used. After washing, the blots were incubated with m-IgGκ BP-HRP (anti-mouse IgG-HRP; Santa Cruz Biotech, sc-516102; 1:2000) or mouse anti-rabbit IgG-HRP (Santa Cruz Biotech, sc-2357; 1:2000) for 1 hour at room temperature. Immunoblots were visualized with the ECL™ Blotting Reagents (GE Healthcare, RPN2109) according to the manufacturer’s instruction. The molecular weight was identified by Precision Plus Protein™ Dual Color Standards (Bio-Rad, #1610374). The control protein bands, β-actin for mouse proteins and neurofilament for rat proteins, were used in Western Blots.
Immunohistochemistry of mouse brains
Immunohistochemistry was used to characterize the mouse brain pathology (at least 10 animals were used in each treatment group). 30-μm brain sections were prepared as described above. After air drying and washing with PBS, quenching of endogenous peroxidase activity was performed by incubating the sections for 30 min in 0.3% H2O2 in methanol followed by PBS washes.
Slides were preincubated in blocking solution (5% [vol/vol] goat serum [Sigma, St Louis, MO, USA] and 1% bovine serum albumin in TBST for 1 hr at room temperature, followed by incubation in Mouse on Mouse Blocking Reagent (Vector Labs, Inc., MKB-2213) for 1 h. Primary antibodies against different components were incubated individually with the slides overnight. For Aβ, mouse mAb 6E10 (Biolegend, SIG-39320; 1:2000) was used. The secondary antibodies used were biotinylated mouse antibodies (Vector Labs, Inc.; 1:4000) for immunohistochemistry. Immunobinding of primary antibodies was detected by biotin-conjugated secondary antibodies and a Vectastain ABC kit (Vector Labs, Inc.) using DAB (3,3′-diaminobenzidine; Vector Labs, Inc.) as a substrate for peroxidase and counter-staining with hematoxylin. The end products were visualized as eight-bit RBG images using Image J software at a total magnification of × 10.
End products were visualized as 8-bit RBG images using NIS Elements BR 3.2 software (http://www.nikoninstruments.com/Products/Software/NIS-Elements-Br-Microscope-Imaging-Software) at a total magnification of 40x. For analysis of the amyloid burden, ImageJ software (http://rsbweb.nih.gov/ij/) was used. After adjusting for threshold, ImageJ was used to count all plaques and to measure the area taken up by plaques, average size of the plaques, and the percent of total brain area occupied by plaques. The intensity × size values of the plaques were measured by analyzing average raw gray levels of the plaques in the image, normalizing them to the background of the slide not taken up by brain, and multiplying by the average size of the plaques in the image. The brain area (cortex or hippocampus) was outlined using the edit plane function. The number of plaques in the outlined structure was also recorded. Data were pooled from 3 independent readers who were blind to the treatment for all three sections and averaged. The outcomes of amyloid burden in the brain between treatment and controls were compared by using one-way ANOVA.
Morris water maze test
Morris water maze test with spatial learning and memory performance [24] was used with 5XFAD mice (at least 10 animals were used in each treatment group) after the completion of treatment. All mice underwent reference memory training with a hidden platform in one quadrant of the pool for 10 days with four trials per day. After the last trial of day 10, the platform was removed, and each mouse received one 60 s swim probe trial. Escape latency (seconds) was recorded and analyzed using an HVS image video tracking system.
Statistical Analysis
Statistical analyses were performed using the GraphPad Prism 8 (version 8.1.0, GraphPad data analysis software, USA). For Morris water maze test, two-way between-subjects ANOVA with Tukey’s post hoc testing was performed. For other experiments, one-way ANOVA with Tukey’s post hoc testing was used for multiple comparisons. For western blots (Fig. 5E), there are four replications. For all other cell experiments, there are three replications. All values are expressed as the mean ± SE. In all cases, p < 0.05 was considered significant.
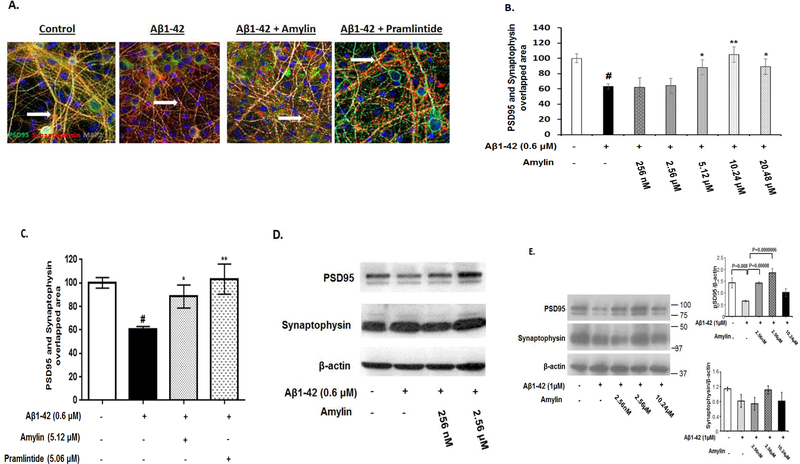
Figure 5. Amylin reversed Aβ induced synapse loss in primary neurons.
Rat fetal hippocampal neurons were grown and treated with aggregated Aβ1–42 (0.6 μM), causing loss of synapses, defined by co-localization of PSD95 and Synaptophysin, in these neurons. The cultures were treated with control medium or medium containing different concentrations of amylin or pramlintide before adding Aβ1–42. The neurons were then fixed, incubated with different antibodies to reveal PSD95 and Synaptophysin, and quantified. The experiment was repeated three times and the representative images are shown. The photos of controls, Aβ, Aβ+amylin (10.24 μM) and Aβ+pramlintide (5.06 μM) are shown (A) and quantified (C); Aβ+different concentrations of amylin were quantified (B). Western blots were conducted to reveal the expressions of PSD95 and Synaptophysin in primary rat hippocampal neurons. The experiment was repeated three times and the representative WB gels are shown (D). In addition, Western blots of mouse cortical neurons in the conditions of control, Aβ alone, Aβ plus amylin were conducted and quantitated to detect the expressions of PSD95 and Synaptophysin (E). The experiment was repeated four times and the representative WB gels are shown. # represents the condition of synapse loss after adding aggregated Aβ and compared with that in the control condition (p<0.001). Compared to the incubation condition with aggregated Aβ alone, differences with statistical significance are shown with * p<0.05; ** p<0.01.
Results
Aβ or amylin alone vs. mixed for cellular tauopathy
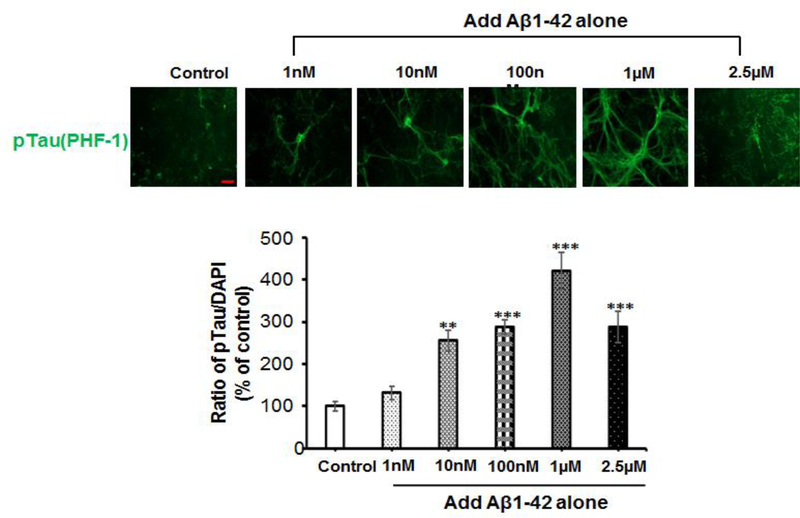
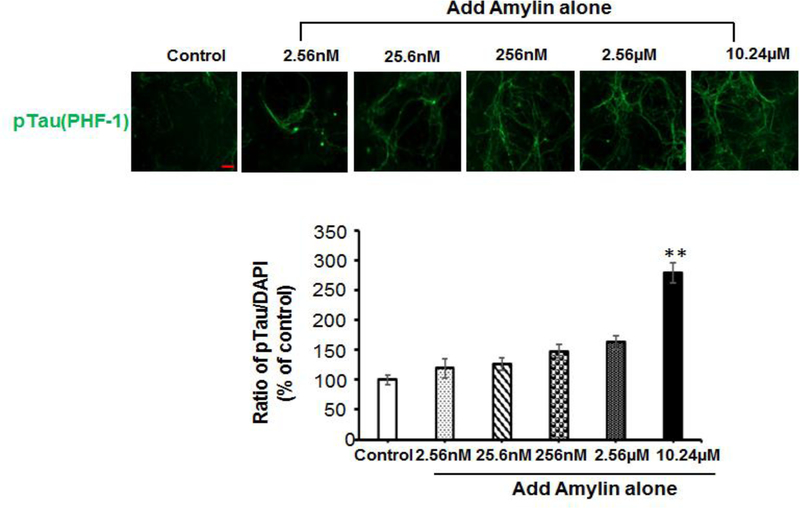
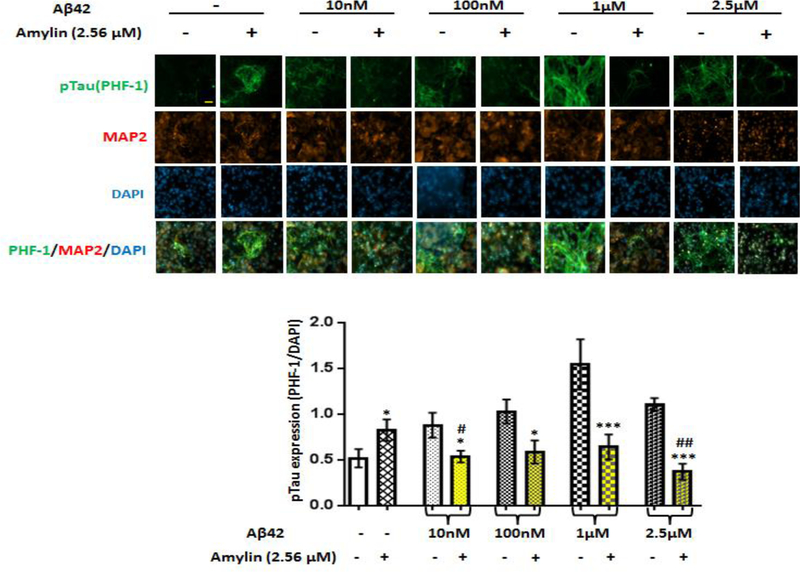
To test the effect of either Aβ or amylin alone on the cellular tauopathy, we first added different concentrations of Aβ1–42 (Figure 2A) or amylin (Figure 2B) alone to the primary mouse cortical neurons. Either peptide alone with elevating concentrations increased celluar tau phosphorylation (pTau) detected by PHF-1 antibody (Ser396/Ser404) immunostaining. Aβ1–42 (from 10 nM to 1 μM) significantly increased the expression of pTau in a dose-dependent manner and the maximum amount of Aβ1–42 (2.5 μM) induced less pTau than 1 μM Aβ1–42 due to some neuronal death. Increasing concentration of human amylin alone also induced the pTau levels in primary neurons. The data demonstrated that both Aβ and amylin alone could induce the cellular tauopathy when the concentration is high.
Figure 2. Either Aβ or amylin alone could induce cellular tau phosphorylation in primary neurons.
Mouse cortical neurons were grown and treated with Aβ1–42 alone (A), or human amylin alone (B), or Aβ1–42 plus amylin (C). The neurons were fixed, incubated with the pTau antibody, PHF-1. When the concentration of either peptide was increased, both Aβ1–42 (A) and human amylin (B) alone induced cellular tau phosphorylation (pTau) (Scale bars: 50 μm). The maximum amount of 2.5 μM Aβ1–42 induced less pTau than 1 μM Aβ1–42 due to some neuronal death. The level of tau phosphorylation was normalized by DAPI to allow comparisons among different experimental groups. Values are expressed relative to the controls (untreated mouse primary neurons), which were set as 100%. Values are the mean ± SE from all experiments. For A and B, compared to the control condition, differences with statistical significance are shown with * p<0.05; ** p<0.01; *** p<0.001. For C, compared to the condition of Aβ1–42 alone, the differences of condition of amylin plus Aβ1–42 are shown with statistical significance # p<0.05; ## p<0.01; the differences between the conditions with amylin alone and amylin plus Aβ1–42 are shown with statistical significance ^ p<0.05; ^^ p<0.01. Each experiment was repeated three times and the representative imagings are shown.
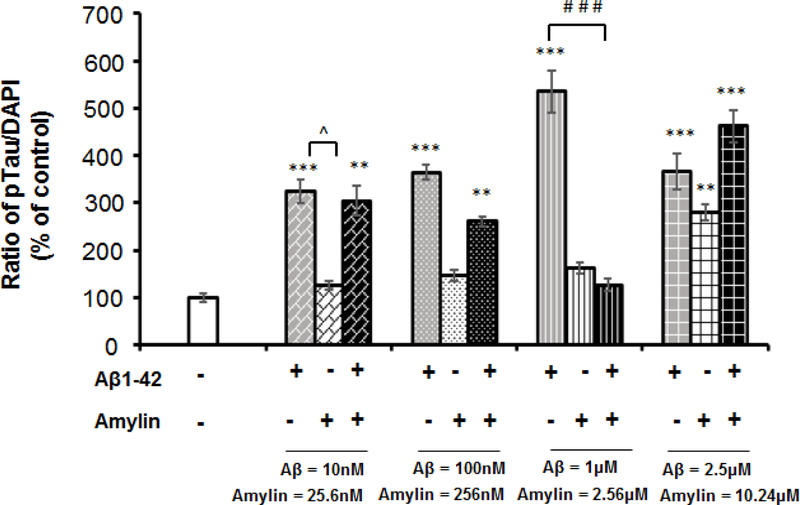
We next examined if amylin had synergistic or antagonizing effects for Aβ inducing cellular pTau expression by the parallel addition of increasing amounts of amylin and Aβ1–42 to primary neurons (Figure 2C and supplement Figure 1). Amylin had a dose-dependent antagonizing effect against Aβ toxicity, e.g. reduced the levels of tau phosphorylation, when it was up to the concentration of 2.56 μM or 1:2.56 ratio with Aβ, but only the ratio of 1 μM Aβ: 2.56 μM amylin reached statistical significance. However, when the higher amylin concentration, 10.2 μM, was added with 4:1 ratio with Aβ (2.5 μM), amylin lost the antagonizing effect and tended to have a synergistic effect with Aβ to increase pTau expression.
Dose-dependent and U-shape effects of amylin-type peptides for reducing Aβ induced tauopathy
The Figure 2 data suggested that that imbalance of Aβ and amylin could be critical for cellular tauopathy formation. To address this, we first preincubated the primary neurons with a fixed level of amylin (2.56μM), and then added the increasing concentrations of Aβ1–42. Interestingly, in the presence of the fixed amount of amylin, increasing amounts of Aβ1–42, unlike Aβ1–42 alone, did not increase, but decrease, the cellular pTau levels (Figure 3). The best ratio of Aβ1–42 to amylin against pTau expression was 1:2.56 in mouse cortical neurons.
Figure 3. Preincubation with amylin dramatically reduced Aβ induced cellular tau phosphorylation in primary neurons regardless of increasing amounts of Aβ.
Mouse cortical neurons were grown and pretreated without and with amylin (2.56 μM) for 1 hr before adding increasing amounts of aggregated Aβ1–42 for 16 hrs. The neurons were fixed, and incubated with the pTau antibody, PHF-1, to detect cellular tauopathy. The neurons were also immunostained by MAP2 antibody and DAPI (Scale bars: 50 μm). The differences between the absence and the presence of amylin for each concentration of Aβ1–42 are shown with statistical significance * p<0.05; ** p<0.01; *** p<0.001. The differences between the condition with amylin alone and amylin plus Aβ1–42 are shown with statistical significance #p<0.05; ##p<0.01. The experiment was repeated three times and the representative imagings are shown.
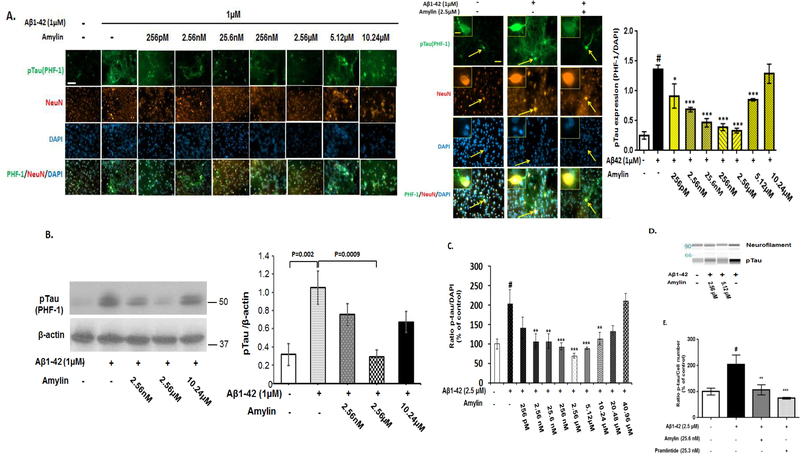
Next, we tested the effects of increasing concentrations of amylin but with a fixed amount of Aβ1–42 (1 μM) on the cellular tauopathy detected by two different antibodies, PHF-1 antibody (Figure 4A) and CP13 (Supplement Figure 2). In the presence of the fixed amount of Aβ1–42, preincubating with increasing concentrations of amylin to the primary neurons reversed Aβ induced tau phosphorylation with a dose-dependent U shape pattern. A lower concentration of amylin, from 2.56 nM (10 ng/ml) to 2.56 μM (10 μg/ml), was beneficial against Aβ induced pTau in primary neurons (Figures 2–4). The lowest effective concentration of amylin was 2.56 nM (10 ng/ml) was also the EC50 of amylin to inhibit Aβ1–42 induced tauopathy and the maximum effective concentration of amylin to reduce pTau was 2.56 μM. However, when the concentrations was higher than 2.56 μM, in the presence of Aβ amylin became less effective or not effective as well as caused neurotoxicity in a U-shape pattern. Western blot results confirmed that 2.56 μM amylin, but not a higher concentration than this, significantly decreased the cellular pTau levels induced by 1 μM Aβ1–42 in primary neurons (Figure 4B; Supplement Figure 3).
Figure 4. Amylin’s protective effects against the pathological process of tauopathy in cortical neurons are a dose-dependent, U shaped pattern.
Mouse cortical neurons were grown and pretreated without and with increasing concentrations of amylin for 1 hr before adding the fixed amount of aggregated Aβ1–42 (1 μM) for 16 hrs. The neurons were fixed, incubated with the pTau antibody, PHF-1, to detect cellular tauopathy and quantitated. The neurons were immunostained by NeuN antibody and DAPI as well (Scale bars: 50 μm). An indicated (arrow) PHF-1 positive-neuron is showed in a higher-magnification inset in the right panel (Scale bars: 10 μm) (A). Western blots were conducted to reveal the expressions of pTau (PHF-1 antibody) in neurons in the conditions of control, Aβ alone, Aβ plus amylin (B). To confirm the result, we also used rat fetal cortical neurons and conducted the immunohistochemistry (C) as described for mouse cortical neurons except adding aggregated Aβ1–42 (2.5 μM-best concentration to induce tauopathy in rat neurons). Western blot (D) was conducted to confirm the immunohistochemistry in (C). The same concentrations of amylin and pramlintide were added to the rat neurons for 1 hr before adding aggregated Aβ1–42 in the immunostainning experiment for pTau expression (E). The neurons were fixed, incubated with different antibodies to reveal the pathological component of pTau, and quantified, and the representative photographs are shown. # represents the comparisons between the control condition of treatment (PBS) and the condition with adding aggregated Aβ (p<0.001). Compared to the condition with aggregated Aβ, differences in the preincuation of either amylin or pramlintide are shown with statistical significance * p<0.05; ** p<0.01; *** p<0.001. Each experiment was repeated three times and the representative images and the WB gels are shown.
To confirm that the balance of Aβ and amylin is critical for amylin’s beneficial effects in neurons and imbalance of these two peptides causes cellular toxicity, we additionally used rat cortical neuron cultures to repeat the same experiment. Although the concentrations of Aβ to induce the maximum tauopathy and the concentrations of amylin to antagonize the pathology formation were different in the mouse and rat cultures, adding amylin had dose-dependent effects antagonizing the cellular tauopathy induced by Aβ1–42 with a similar U-shape in both cultures (Figure 4C). The U-shape effects of amylin suggest that at more than 10.24 μM, amylin lost its ability to antagonize Aβ induced pTau in neurons. Similar to amylin, adding pramlintide, an amylin analog, also reversed the tau phosphorylation caused by Aβ1–42 in these neurons (Figure 4E). Taken together, there were two factors determining the amylin’s effects against Aβ induced tauopathy: 1) the amount of amylin and 2) the ratio between Aβ and amylin (Figures 2, 3 and 4A). The best ratio of Aβ1–42 to amylin against pTau expression in neurons was 1:2.56 in the mouse cortical cultures, while the best ratio for the rat neurons was 1:1 (Figure 4C).
Dose-dependent effects of amylin peptides for reversing synapse loss and neurite network damage caused by Aβ
Since synaptic damage is the key step in AD pathological process to cause cognitive decline, we predicted that adding amylin would enhance synapses formation in the presence of Aβ. Neuronal synapses were evaluated by measuring the number of cellular sites showing colocalization of PSD95 and Synaptophysin (yellow color in Figure 5) in primary rat hippocampal neurons. While significant synapse loss (40% decrease in the number of synapses) was observed after 48 hours of incubation with Aβ1–42 (0.6 μM) [20], preincubation with amylin resulted in an effect to reverse the Synapse loss caused by Aβ1–42 (Figure 5A). Interestingly, these effects tended to be presented or were presented as dose-dependent, inverted U-shape to enhance synapse formation in both rat and mouse neurons (Figure 5B and 5E; Supplement Figure 4). Pramlintide also effectively reversed the synaptic damage induced by Aβ (Figure 5A and 5C). Western blots showed that amylin significantly increased the expression level of PSD95 in a dose dependent way with an inverted U-shape, but did not affect Synaptophysin expression, in both rat and mouse neurons (Figure 5E).
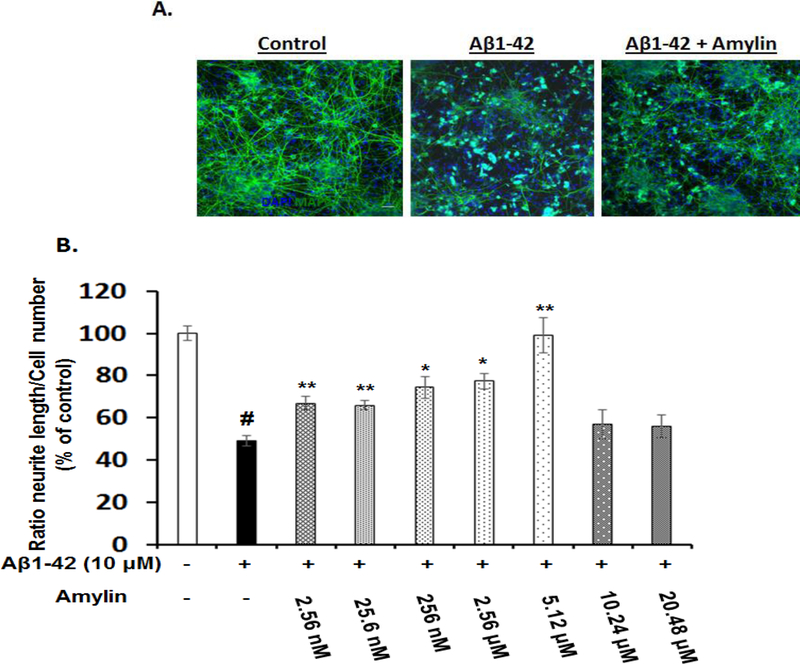
As expected, Aβ1–42 elicited a 50% loss of neurite length in primary cortical cultures (Figure 6). Preincubation with amylin resulted in a dose-dependent effect to rescue the deficit in neurite length outgrowth caused by Aβ1–42, and the concentration of amylin that completely reversed the toxicity of Aβ1–42 was 5.12 μM (Figure 6B). Again, this effect of amylin presented as an inverted U-shape. Adding pramlintide to the primary cortical culture also showed a trend in similar effect on neurite outgrowth (data not shown).
Figure 6. Amylin type peptides’ effects on Aβ induced neurite damage in primary cortical neurons.
Rat fetal cortical neurons were grown and treated with aggregated Aβ1–42, causing reduced neurite outgrowth in these neurons. The neurons were fixed, incubated with the antibodies to reveal MAP2 and nuclei, and quantified for the neurite length. The representative photos of controls, Aβ, and Aβ plus amylin (2.56 μM) are shown (A). The average length of neurites in rat neurons which pretreated without and with increasing concentrations of amylin for 1 hour before adding the fixed amount of aggregated Aβ1–42 (10 μM) for 24 hours were quantified (B). # represents the condition of incubation after adding aggregated Aβ and compared with that in the control condition (p<0.001). Compared to the incubation condition with aggregated Aβ alone, differences with statistical significance are shown with * p<0.05; ** p<0.01. The experiment was repeated three times and the representative images are shown.
Peripheral amylin treatment with a low vs. a high dose reduces AD pathology in the brain of an AD mouse model
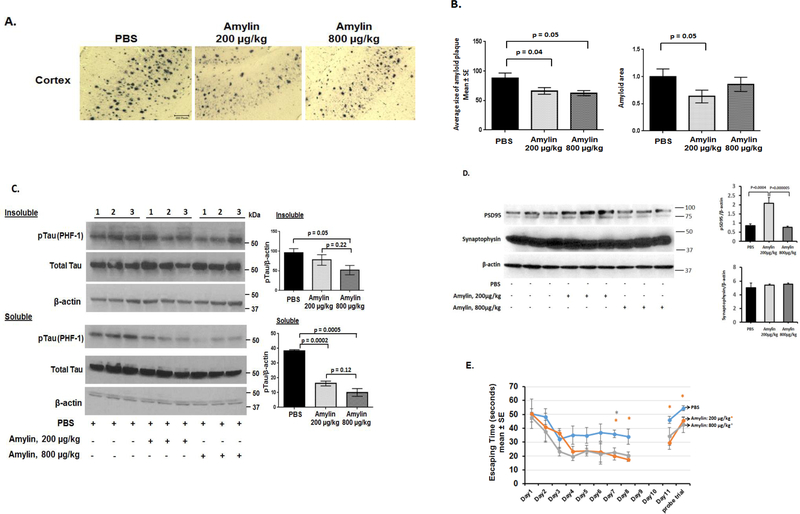
We conducted an in vivo experiment to investigate whether amylin treatment with different doses would show different results in reducing AD pathology in the brain. 5XFAD mice aged 4 months, which is an age when there are prominent Aβ amyloid deposits in the brain, were used. The mice were given a 6 week course of daily i.p. injections of PBS saline vs. different doses of human amylin (200 μg/kg vs. 800 μg/kg) (n = 14–16 per group) (Figure 7). Figure 7A and Supplement Figure 5 shows that immunostaining of amyloid plaques in the cortex regions in the treatment of PBS vs. low dose vs. high dose of amylin. Quantitatively, compared to PBS treatment, amylin treatment with either 200 μg/kg or 800 μg/kg significantly reduced the amyloid pathology in the cortex, e.g. the average size of amyloid plaques, the total amyloid area and the amounts of Aβ (p <0.05) (Figure 7B). While amylin treatment with 200 μg/kg dose reduced the percentage area of amyloid burden and the amount of Aβ1–42 determined by ELISA in the brain, the higher dose of amylin (800 μg/kg) only showed the trend and did not reach the statistical significance (Supplement Figure 6). A dose of amylin 400 μg/kg had the effects between 200 μg/kg and 800 μg/kg on amyloid burden in the brain (data not shown).
Figure 7. Peripheral amylin treatment with a low vs. a high dose reduces AD pathology and improves learning and memory in an AD mouse model.
Amylin treatment of 5XFAD mice reduces the AD pathology and improves their learning and memory. At 4 months of age, 5XFAD mice were treated by i.p. injection of PBS or amylin (200 μg/kg vs. 800 μg/kg) daily for 6 weeks (n = 14–16 per group). (A) Dense-cored Aβ plaque burden is significantly reduced by the amylin 200 μg/kg treatment in the cortex and less so by the amylin 800 μg/kg treatment. The photos of cerebral cortex are shown (Scale bars: 200 μm). (B) Amylin treated (200 μg/kg vs. 800 μg/kg) mice had a significant reduction in Aβ amyloid burden in cortex quantitated by average plaque size, total amyloid area. Again, the dose of 800 μg/kg was less effective than 200 μg/kg. (C) Protein extracts from the brains were fractionated into soluble fraction by TBS extraction and insoluble fraction by the sarkosyl-extraction followed by Western blots with the PFH-1 antibody against pTau and quantitated. Total tau and actin were detected by Western blots and are shown. The amylin treatment with 800 μg/kg was more effective to reduce tauopathy in the brain than the dose of 200 μg/kg. (D) Total protein from the brains was extracted followed by Western blots with the antibody against PSD95 and the one against Synaptophisin. While the amylin treatment with the dose of 200 μg/kg, but not the dose of 800 μg/kg, effectively increased the expression of PSD95, both amylin doses did not influence the expression of Synaptophysin. (E) The amylin treated 5XFAD mice illustrated improved cognition compared to the PBS treatment by showing shortened times in Morris water maze test in finding the hidden platform at day 7 and 8 (D7–8), in memory at day 11 (D11) after the completion of training and skipping two days, and in the probe trial. Mean ± SE was used with *p < 0.05.
We next investigated whether amylin treatment could influence the other key component, tauopathy, in the brain of 5XFAD mice. Western blots with PHF-1 antibody demonstrated that amylin treatments with both doses reduced the levels of pTau in the soluble and insoluble brain fractions, while the higher the dose, the better the effects (Figure 7C, Supplement Figure 7). We examined the amylin’s effects on synaptic proteins. Consistent with the in vitro data in neurons (Figure 5), the in vivo data show that compared to PBS treatment, amylin treatment with the dose of 200 μg/kg, but not 800 μg/kg, increased the level of PSD95 expression (p = 0.00005) (Figure 7D). Again, the treatment did not influence the expression of Synaptophysin in the brain. The results indicated that amylin treatment with a high dose tended to be less effective to reduce AD pathology.
Peripheral amylin treatment with different doses improved learning and memory
The Morris water maze test was conducted to investigate the effects of amylin with different doses on learning and memory. While both amylin treatments with either 200 μg/kg or 800 μg/kg equally improved learning and memory in these 5XFAD mice, the dose of 200 μg/kg was more statistically significant than the dose of 800 μg/kg to improve cognition in the AD mouse models (Figure 7E). When compared to PBS treatment, the 200 μg/kg amylin treatment reduced the time for acquisition during maze training at day 7 (p = 0.02) and day 8 (p = 0.04), and shortened the time for memory retention (p = 0.02) and for probe trial (p = 0.01) two days after maze training in these 5XFAD mice. Similarly but less significant in p values, the 800 μg/kg amylin treatment only showed statistical significance for acquisition at day 8. The amylin treatments regardless of the doses used had no such effect in wild type mice (data not shown).
Discussion
The significant finding in this study was the observation of a U-shape or inverted U-shape of amylin effects on AD pathogenesis. A high concentration of an individual peptide, Aβ or amylin alone, induced cellular AD pathology; interestingly, when mixed together, amylin presented with reduction for the Aβ induced cellular tauopathy in neurons. However, when a high concentration (μM) of amylin was mixed with a high concentration (μM) of Aβ to a level over four times (Figure 2C), amylin’s antagonizing effects against Aβ induced tauopathy disappeared.. The data imply that while Aβ accumulation is an initial step in AD pathogenesis, imbalanced level of Aβ to the amylin level may also play an important role in AD pathogenesis and can serve as an alternative drug target for the disease. Indeed, AD patients are found to have a low level of amylin in plasma [4, 5]. On the other hand, accumulated and aggregated amylin was found in AD brains [15].
Aβ induced tau phosphorylation and aggregation, which is the key element in the pathogenesis of AD [25–27]. Amylin treatment antagonized this Aβ1–42 induced cytopathic process in primary mouse and rat neurons with a dose-dependent U shape (Figures 2–4). Although we did not observe the U-shape effects of amylin against tauopathy in vivo with two doses we used (Figure 7C), while a low dose of amylin (200 μg/kg) effectively attenuates AD pathology, e.g. increasing the expression of PSD95, in the brain, a high dose of amylin (800 μg/kg) was less effective (Figure 7D). Our data suggest that when the concentration of amylin was low, it could not bind its receptor to antagonize the Aβ induced neurodegenerative process effectively; however, when the concentration of amylin was too high, it could cause amylin aggregation, lost its beneficial effects against tauopathy formation and probably became synergistically neurotoxic. It is possible that a large amount of Aβ can block or interfere with amylin’s effects as a neuroprotective peptide in the AD brain [11]. However, while some studies show that Aβ binds AMY receptors, others do not agree so that the binding of Aβ to an amylin receptor is controversial [9, 28, 29], [10]. Importantly, amylin induces intracellular cAMP, but Aβ does not [10], as activation of cAMP signaling can be neuroprotective [30, 31]. A recent paper demonstrates that low vs. high concentrations of amylin bind different receptors [29], suggesting that amylin has beneficial effects against neurodegeneration through its cognate receptor, AMY receptor, to increase intracellular cAMP, which is a different receptor from the one binding to aggregated amylin [4] [32]. In addition, one study suggested that aggregated human amylin induces β cell apoptosis through FAS-associated death receptor [33]. Further experiments are needed to explain how low and high concentrations of amylin affect neurodegeneration.
Results from cortical and hippocampal neurons showed that both amylin and pramlintide antagonized Aβ induced synaptic damage [20] by increasing the expression and colocalization of post-synaptic marker PSD95 and pre-synaptic marker Synaptophysin, which define synaptic contact and density, and enhanced the neurite network damaged by Aβ (Figures 5 and 6). Again, an inverted U-shape of amylin effects on PSD95 was observed (Figure 5E). Consistently, the higher dose was less effective to reduce the AD pathology, although amylin treatment with both low and high doses improved learning and memory, in AD mice (Figure 7E). Two studies show that PSD95 levels are lower in the brains of AD [34] and MCI [35] subjects than in control brains, and one study shows that both PSD95 and Synaptophysin levels are low in AD brains [36]. PSD95 levels and synapses negatively correlate with Aβ42 accumulation and with cognitive function in sporadic AD brains [37]. In addition, the PSD95-NMDA receptor complex has been shown to regulate tau phosphorylation at the post-synaptic region [38]. Currently there is no drug yet targeting synapse loss in AD. Amylin-type peptides may serve to restore synapses via AMY receptor while independently reducing the pathological cascade in AD brain.
Despite that amylin effects against the cellular pathology induced by Aβ presented with a similar dose-dependent U-shape, our experiments showed that the concentrations of amylin and the amylin ratios to Aβ for the maximum benefits were different in two culture systems and were different between in vitro and in vivo systems. In addition, the concentrations of amylin and the amylin ratios to Aβ for the maximum benefits for different AD pathology components were also different in vitro and in vivo. AMY receptor is a complex receptor, which is one type of G-protein coupled receptor and is composed of the calcitonin receptor (CTR) complexed with three different receptor-activity-modifying proteins (RAMPs) [39, 40] [32]. Heterodimers between the CTR and RAMP1 or RAMP2 or RAMP3 or CTR alone have been shown to preferentially bind amylin to mediate physiological functions in the brain [41] [42]. The possible explanation is that the different AMY subtypes probably mediate different activities against different AD components, and they are expressed differently in different tissues and in different species so that the best concentrations of amylin to mediate each beneficial effect were different. Thus developing different amylin analogs to specific AMY subtype in humans is probably necessary for the AD drug development.
Pramlintide is an amylin analog, and our experiments showed that as with amylin, pramlintide had similar beneficial effects on neurons (Figures 4C, 5C). Pramlintide is also an FDA approved diabetes drug, which was optimized by substituting prolines at positions 25, 28 and 29 of human amylin to prevent oligomerization and aggregation [43]. Pramlintide shows its safety profile for diabetes [28, 44, 45]. While the average fasting plasma amylin concentrations are in the range of 4–25 pM in healthy humans [46], amylin aggregates only when the concentration reaches to the μM level [13, 47]. The peak of the amylin concentration in the amylin treatment we conducted for AD mouse models was ~1–4 nM, and the half-life of exogenous amylin or pramlintide is approximately 20–45 min in vivo [48, 49] (our own data not shown). Given that pramlintide does not aggregate, even the peak concentration of the amylin treatment in vivo was far from the concentration leading to amylin aggregation. In addition, several in vitro studies demonstrate that amylin can form cross-interactions [50, 51] and inhibit the formation of Aβ aggregates [52–56].
There could be two strategies in drug discovery for AD: 1) to stop or reduce the neurotoxic components of AD pathology such as Aβ and pTau and 2) to restore or rescue the lost neuronal activity blocked or interfered by AD pathology such as synapse loss. Our study supports that amylin type peptide treatment can accomplish both. As amylin-type peptides antagonize the AD degenerative cascade, instead of a single pathological component, in AD mouse models [12], and our recent clinical trial in humans demonstrates that pramlintide use is safe in non-diabetic patients under fasting conditions [12], pramlintide and other non-aggregating amylin analogs might become therapeutic for AD.
Supplementary Material
ACKNOWLEDGEMENTS
This work was supported by grants from Alzheimer’s Disease Association, IIRG-13–284238; NIA, R21AG045757 and RO1AG-022476; and Ignition Award (W.Q.Q), and from BU ADC pilot grant (H.Z). Support was provided through P30 AG13864 (N.K.). Hongbo Yao was supported by Chinese National Foundation for Abroad Scholarship.
Footnotes
The remaining authors have nothing to disclose.
REFERENCES
- [1].Nishi M, Sanke T, Seino S, Eddy RL, Fan YS, Byers MG, Shows TB, Bell GI, Steiner DF (1989) Human islet amyloid polypeptide gene: complete nucleotide sequence, chromosomal localization, and evolutionary history. Molecular endocrinology (Baltimore, Md.) 3, 1775–1781. [DOI] [PubMed] [Google Scholar]
- [2].Lutz TA (2013) The interaction of amylin with other hormones in the control of eating. Diabetes, obesity & metabolism 15, 99–111. [DOI] [PubMed] [Google Scholar]
- [3].Mietlicki-Baase EG (2016) Amylin-mediated control of glycemia, energy balance, and cognition. Physiol Behav. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Qiu WQ, Zhu H (2014) Amylin and its analogs: a friend or foe for the treatment of Alzheimer’s disease? Frontiers in aging neuroscience 6, 186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Adler BL, Yarchoan M, Hwang HM, Louneva N, Blair JA, Palm R, Smith MA, Lee HG, Arnold SE, Casadesus G (2014) Neuroprotective effects of the amylin analogue pramlintide on Alzheimer’s disease pathogenesis and cognition. Neurobiol Aging 35, 793–801. [DOI] [PubMed] [Google Scholar]
- [6].Roostaei T, Nazeri A, Felsky D, De Jager PL, Schneider JA, Pollock BG, Bennett DA, Voineskos AN (2016) Genome-wide interaction study of brain beta-amyloid burden and cognitive impairment in Alzheimer’s disease. Mol Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Hardy J, Selkoe DJ (2002) The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science (New York, N.Y.) 297, 353–356. [DOI] [PubMed] [Google Scholar]
- [8].Lim YA, Ittner LM, Lim YL, Gotz J (2008) Human but not rat amylin shares neurotoxic properties with Abeta42 in long-term hippocampal and cortical cultures. FEBS Lett 582, 2188–2194. [DOI] [PubMed] [Google Scholar]
- [9].Fu W, Ruangkittisakul A, MacTavish D, Shi JY, Ballanyi K, Jhamandas JH (2012) Amyloid beta (Abeta) peptide directly activates amylin-3 receptor subtype by triggering multiple intracellular signaling pathways. The Journal of biological chemistry 287, 18820–18830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Gingell JJ, Burns ER, Hay DL (2014) Activity of pramlintide, rat and human amylin but not Abeta1–42 at human amylin receptors. Endocrinology 155, 21–26. [DOI] [PubMed] [Google Scholar]
- [11].Zhu H, Wang X, Wallack M, Li H, Carreras I, Dedeoglu A, Hur JY, Zheng H, Li H, Fine R, Mwamburi M, Sun X, Kowall N, Stern RA, Qiu WQ (2015) Intraperitoneal injection of the pancreatic peptide amylin potently reduces behavioral impairment and brain amyloid pathology in murine models of Alzheimer’s disease. Mol Psychiatry 20, 252–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Zhu H, Xue X, Wang E, Wallack M, Na H, Hooker JM, Kowall N, Tao Q, Stein TD, Wolozin B, Qiu WQ (2017) Amylin receptor ligands reduce the pathological cascade of Alzheimer’s disease. Neuropharmacology 119, 170–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].May PC, Boggs LN, Fuson KS (1993) Neurotoxicity of human amylin in rat primary hippocampal cultures: similarity to Alzheimer’s disease amyloid-beta neurotoxicity. J Neurochem 61, 2330–2333. [DOI] [PubMed] [Google Scholar]
- [14].Lorenzo A, Razzaboni B, Weir GC, Yankner BA (1994) Pancreatic islet cell toxicity of amylin associated with type-2 diabetes mellitus. Nature 368, 756–760. [DOI] [PubMed] [Google Scholar]
- [15].Jackson K, Barisone GA, Diaz E, Jin LW, Decarli C, Despa F (2013) Amylin deposition in the brain: A second amyloid in Alzheimer disease? Ann Neurol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Dahlgren KN, Manelli AM, Stine WB Jr., Baker LK, Krafft GA, LaDu MJ (2002) Oligomeric and fibrillar species of amyloid-beta peptides differentially affect neuronal viability. J Biol Chem 277, 32046–32053. [DOI] [PubMed] [Google Scholar]
- [17].Oakley H, Cole SL, Logan S, Maus E, Shao P, Craft J, Guillozet-Bongaarts A, Ohno M, Disterhoft J, Van Eldik L, Berry R, Vassar R (2006) Intraneuronal beta-amyloid aggregates, neurodegeneration, and neuron loss in transgenic mice with five familial Alzheimer’s disease mutations: potential factors in amyloid plaque formation. The Journal of neuroscience : the official journal of the Society for Neuroscience 26, 10129–10140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Singer CA, Figueroa-Masot XA, Batchelor RH, Dorsa DM (1999) The mitogen-activated protein kinase pathway mediates estrogen neuroprotection after glutamate toxicity in primary cortical neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience 19, 2455–2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Chumakov I, Nabirotchkin S, Cholet N, Milet A, Boucard A, Toulorge D, Pereira Y, Graudens E, Traore S, Foucquier J, Guedj M, Vial E, Callizot N, Steinschneider R, Maurice T, Bertrand V, Scart-Gres C, Hajj R, Cohen D (2015) Combining two repurposed drugs as a promising approach for Alzheimer’s disease therapy. Scientific reports 5, 7608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Callizot N, Combes M, Steinschneider R, Poindron P (2013) Operational dissection of beta-amyloid cytopathic effects on cultured neurons. J Neurosci Res 91, 706–716. [DOI] [PubMed] [Google Scholar]
- [21].Greenberg SG, Davies P (1990) A preparation of Alzheimer paired helical filaments that displays distinct tau proteins by polyacrylamide gel electrophoresis. Proc Natl Acad Sci U S A 87, 5827–5831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Hasegawa M, Morishima-Kawashima M, Takio K, Suzuki M, Titani K, Ihara Y, Wille H, Drewes G, Biernat J, Mandelkow EM, Mandelkow E (1992) Protein sequence and mass spectrometric analyses of tau in the Alzheimer’s disease brain Alzheimer-like paired helical filaments and antiparallel dimers formed from microtubule-associated protein tau in vitro. The Journal of biological chemistry 267, 17047–17054. [Google Scholar]
- [23].Lewis J, McGowan E, Rockwood J, Melrose H, Nacharaju P, Van Slegtenhorst M, Gwinn-Hardy K, Paul Murphy M, Baker M, Yu X, Duff K, Hardy J, Corral A, Lin WL, Yen SH, Dickson DW, Davies P, Hutton M (2000) Neurofibrillary tangles, amyotrophy and progressive motor disturbance in mice expressing mutant (P301L) tau protein. Nat Genet 25, 402–405. [DOI] [PubMed] [Google Scholar]
- [24].Janus C, Pearson J, McLaurin J, Mathews PM, Jiang Y, Schmidt SD, Chishti MA, Horne P, Heslin D, French J, Mount HT, Nixon RA, Mercken M, Bergeron C, Fraser PE, St George-Hyslop P, Westaway D, Janus C, Chishti MA, Westaway D (2000) A beta peptide immunization reduces behavioural impairment and plaques in a model of Alzheimer’s disease Transgenic mouse models of Alzheimer’s disease. Nature 408, 979–982. [DOI] [PubMed] [Google Scholar]
- [25].Tsai LH, Takahashi T, Caviness VS Jr., Harlow E (1993) Activity and expression pattern of cyclin-dependent kinase 5 in the embryonic mouse nervous system. Development (Cambridge, England) 119, 1029–1040. [DOI] [PubMed] [Google Scholar]
- [26].Baumann K, Mandelkow EM, Biernat J, Piwnica-Worms H, Mandelkow E (1993) Abnormal Alzheimer-like phosphorylation of tau-protein by cyclin-dependent kinases cdk2 and cdk5. FEBS Lett 336, 417–424. [DOI] [PubMed] [Google Scholar]
- [27].Tsai LH, Delalle I, Caviness VS Jr., Chae T, Harlow E (1994) p35 is a neural-specific regulatory subunit of cyclin-dependent kinase 5. Nature 371, 419–423. [DOI] [PubMed] [Google Scholar]
- [28].Gebre-Medhin S, Olofsson C, Mulder H (2000) Islet amyloid polypeptide in the islets of Langerhans: friend or foe? Diabetologia 43, 687–695. [DOI] [PubMed] [Google Scholar]
- [29].Zhang N, Yang S, Wang C, Zhang J, Huo L, Cheng Y, Wang C, Jia Z, Ren L, Kang L, Zhang W (2017) Multiple target of hAmylin on rat primary hippocampal neurons. Neuropharmacology 113, 241–251. [DOI] [PubMed] [Google Scholar]
- [30].Tanaka Y, Tanaka R, Liu M, Hattori N, Urabe T (2010) Cilostazol attenuates ischemic brain injury and enhances neurogenesis in the subventricular zone of adult mice after transient focal cerebral ischemia. Neuroscience 171, 1367–1376. [DOI] [PubMed] [Google Scholar]
- [31].Bao Y, Jiang L, Chen H, Zou J, Liu Z, Shi Y (2015) The Neuroprotective Effect of Liraglutide is Mediated by Glucagon-Like Peptide 1 Receptor-Mediated Activation of cAMP/PKA/CREB Pathway. Cellular physiology and biochemistry : international journal of experimental cellular physiology, biochemistry, and pharmacology 36, 2366–2378. [DOI] [PubMed] [Google Scholar]
- [32].Qiu WQ (2017) Amylin and its G-protein-coupled receptor: A probable pathological process and drug target for Alzheimer’s disease. Neuroscience 356, 44–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Zhang S, Liu H, Yu H, Cooper GJ (2008) Fas-associated death receptor signaling evoked by human amylin in islet beta-cells. Diabetes 57, 348–356. [DOI] [PubMed] [Google Scholar]
- [34].Whitfield DR, Vallortigara J, Alghamdi A, Howlett D, Hortobagyi T, Johnson M, Attems J, Newhouse S, Ballard C, Thomas AJ, O’Brien JT, Aarsland D, Francis PT (2014) Assessment of ZnT3 and PSD95 protein levels in Lewy body dementias and Alzheimer’s disease: association with cognitive impairment. Neurobiol Aging 35, 2836–2844. [DOI] [PubMed] [Google Scholar]
- [35].Sultana R, Banks WA, Butterfield DA (2010) Decreased levels of PSD95 and two associated proteins and increased levels of BCl2 and caspase 3 in hippocampus from subjects with amnestic mild cognitive impairment: Insights into their potential roles for loss of synapses and memory, accumulation of Abeta, and neurodegeneration in a prodromal stage of Alzheimer’s disease. J Neurosci Res 88, 469–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Love S, Siew LK, Dawbarn D, Wilcock GK, Ben-Shlomo Y, Allen SJ (2006) Premorbid effects of APOE on synaptic proteins in human temporal neocortex. Neurobiol Aging 27, 797–803. [DOI] [PubMed] [Google Scholar]
- [37].Shinohara M, Fujioka S, Murray ME, Wojtas A, Baker M, Rovelet-Lecrux A, Rademakers R, Das P, Parisi JE, Graff-Radford NR, Petersen RC, Dickson DW, Bu G (2014) Regional distribution of synaptic markers and APP correlate with distinct clinicopathological features in sporadic and familial Alzheimer’s disease. Brain : a journal of neurology 137, 1533–1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Mondragon-Rodriguez S, Trillaud-Doppia E, Dudilot A, Bourgeois C, Lauzon M, Leclerc N, Boehm J (2012) Interaction of endogenous tau protein with synaptic proteins is regulated by N-methyl-D-aspartate receptor-dependent tau phosphorylation. The Journal of biological chemistry 287, 32040–32053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Gebre-Medhin S, Mulder H, Pekny M, Westermark G, Tornell J, Westermark P, Sundler F, Ahren B, Betsholtz C (1998) Increased insulin secretion and glucose tolerance in mice lacking islet amyloid polypeptide (amylin). Biochem Biophys Res Commun 250, 271–277. [DOI] [PubMed] [Google Scholar]
- [40].Hay DL, Chen S, Lutz TA, Parkes DG, Roth JD (2015) Amylin: Pharmacology, Physiology, and Clinical Potential. Pharmacol Rev 67, 564–600. [DOI] [PubMed] [Google Scholar]
- [41].Christopoulos G, Perry KJ, Morfis M, Tilakaratne N, Gao Y, Fraser NJ, Main MJ, Foord SM, Sexton PM (1999) Multiple amylin receptors arise from receptor activity-modifying protein interaction with the calcitonin receptor gene product. Mol Pharmacol 56, 235–242. [DOI] [PubMed] [Google Scholar]
- [42].Hay DL (2017) Amylin. Headache 57 Suppl 2, 89–96. [DOI] [PubMed] [Google Scholar]
- [43].Roth JD, Erickson MR, Chen S, Parkes DG (2012) GLP-1R and amylin agonism in metabolic disease: complementary mechanisms and future opportunities. Br J Pharmacol 166, 121–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Moriarty DF, Raleigh DP (1999) Effects of sequential proline substitutions on amyloid formation by human amylin20–29. Biochemistry (Mosc) 38, 1811–1818. [DOI] [PubMed] [Google Scholar]
- [45].Pencek R, Roddy T, Peters Y, De Young MB, Herrmann K, Meller L, Nguyen H, Chen S, Lutz K (2010) Safety of pramlintide added to mealtime insulin in patients with type 1 or type 2 diabetes: a large observational study. Diabetes, obesity & metabolism 12, 548–551. [DOI] [PubMed] [Google Scholar]
- [46].Nyholm B, Fineman MS, Koda JE, Schmitz O (1998) Plasma amylin immunoreactivity and insulin resistance in insulin resistant relatives of patients with non-insulin-dependent diabetes mellitus. Hormone and metabolic research = Hormon- und Stoffwechselforschung = Hormones et metabolisme 30, 206–212. [DOI] [PubMed] [Google Scholar]
- [47].Jhamandas JH, Mactavish D (2012) beta-Amyloid protein (Abeta) and human amylin regulation of apoptotic genes occurs through the amylin receptor. Apoptosis : an international journal on programmed cell death 17, 37–47. [DOI] [PubMed] [Google Scholar]
- [48].Colburn WA, Gottlieb AB, Koda J, Kolterman OG (1996) Pharmacokinetics and pharmacodynamics of AC137 (25,28,29 tripro-amylin, human) after intravenous bolus and infusion doses in patients with insulin-dependent diabetes. J Clin Pharmacol 36, 13–24. [DOI] [PubMed] [Google Scholar]
- [49].Young A (2005) Central nervous system and other effects. Advances in pharmacology (San Diego, Calif.) 52, 281–288. [DOI] [PubMed] [Google Scholar]
- [50].Andreetto E, Yan LM, Tatarek-Nossol M, Velkova A, Frank R, Kapurniotu A (2010) Identification of hot regions of the Abeta-IAPP interaction interface as high-affinity binding sites in both cross- and self-association. Angewandte Chemie (International ed. in English) 49, 3081–3085. [DOI] [PubMed] [Google Scholar]
- [51].Seeliger J, Evers F, Jeworrek C, Kapoor S, Weise K, Andreetto E, Tolan M, Kapurniotu A, Winter R (2012) Cross-amyloid interaction of Abeta and IAPP at lipid membranes. Angewandte Chemie (International ed. in English) 51, 679–683. [DOI] [PubMed] [Google Scholar]
- [52].Yan LM, Velkova A, Tatarek-Nossol M, Andreetto E, Kapurniotu A (2007) IAPP mimic blocks Abeta cytotoxic self-assembly: cross-suppression of amyloid toxicity of Abeta and IAPP suggests a molecular link between Alzheimer’s disease and type II diabetes. Angewandte Chemie (International ed. in English) 46, 1246–1252. [DOI] [PubMed] [Google Scholar]
- [53].Sellin D, Yan LM, Kapurniotu A, Winter R (2010) Suppression of IAPP fibrillation at anionic lipid membranes via IAPP-derived amyloid inhibitors and insulin. Biophys Chem 150, 73–79. [DOI] [PubMed] [Google Scholar]
- [54].Andreetto E, Yan LM, Caporale A, Kapurniotu A (2011) Dissecting the role of single regions of an IAPP mimic and IAPP in inhibition of Abeta40 amyloid formation and cytotoxicity. Chembiochem : a European journal of chemical biology 12, 1313–1322. [DOI] [PubMed] [Google Scholar]
- [55].Yan LM, Velkova A, Kapurniotu A (2013) Molecular Characterization of the Hetero-Assembly of beta-Amyloid Peptide with Islet Amyloid Polypeptide. Curr Pharm Des. [DOI] [PubMed] [Google Scholar]
- [56].Yan LM, Velkova A, Tatarek-Nossol M, Rammes G, Sibaev A, Andreetto E, Kracklauer M, Bakou M, Malideli E, Goke B, Schirra J, Storr M, Kapurniotu A (2013) Selectively N-Methylated Soluble IAPP Mimics as Potent IAPP Receptor Agonists and Nanomolar Inhibitors of Cytotoxic Self-Assembly of Both IAPP and Abeta40. Angewandte Chemie (International ed. in English) 52, 10378–10383. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.