Abstract
Background
Chronic immune activation in the blood and central nervous system is a consequence of human immunodeficiency virus (HIV) infection that contributes to disease morbidity and can occur despite virally suppressive antiretroviral therapy (ART). The trajectory of HIV-related inflammation may vary with the timing of ART initiation. We examined immune activation markers in cerebrospinal fluid (CSF) and blood specimens collected over 96 weeks from participants who initiated ART during acute HIV infection (AHI).
Methods
RV254/SEARCH010 study participants with AHI underwent CSF (n = 89) and plasma (n = 146) sampling before initiating ART and at weeks 24 and 96 of treatment. A majority participants (64.4%) received a standard ART regimen (hereafter, “standard ART”), with some (34.7%) also receiving maraviroc and raltegravir for the first 24 weeks (hereafter, “ART plus”). We compared neopterin, CXCL10, CCL2, and interleukin 6 (IL-6) levels in the AHI group to those in 18 healthy, uninfected controls.
Results
Following 24 and 96 weeks of treatment, levels of all CSF markers normalized while levels of several plasma markers remained elevated in the AHI group (P < .001). Participants receiving the ART-plus regimen had lower median plasma CCL2 levels at week 24 and lower plasma neopterin levels at week 96.
Conclusions
ART initiation during AHI differentially impacts the brain compartment, with markers of inflammation returning to normal levels in the CSF, where they were sustained at week 96, but not in plasma.
Keywords: CSF, immune activation, acute HIV, inflammation
(See the Editorial commentary by Gisslén and Hunt, on pages 1867–9.)
Current antiretroviral therapies (ARTs) are highly effective at suppressing replicating human immunodeficiency virus (HIV) but remain insufficient in ameliorating abnormal immune activation. Numerous studies have shown that, despite achieving an undetectable plasma HIV RNA load through sustained ART, people living with HIV often have elevated levels of immune activation markers, including soluble CD14, soluble CD163, and interleukin 6 (IL-6), a marker of early phase inflammation [1]. Other plasma markers with levels found to be abnormally elevated after ART initiation include neopterin, a pteridine biomarker of macrophage activation, and the chemokines CXCL10 (also known as IP-10) and CCL2 (also known as MCP-1), both of which regulate the trafficking of immunologic cells [2]. Similar to the peripheral blood system, abnormal immune activation has been observed in the central nervous system (CNS) in individuals receiving suppressive ART. Cerebrospinal fluid (CSF) evaluations in several cohorts indicate that neopterin levels in the CSF decline after successful viral suppression but do not invariably return to levels observed in HIV-negative controls [3, 4]. In one of these cohorts, after >10 years of ART, >50% of participants continue to have elevated CSF neopterin levels despite having undetectable plasma HIV RNA levels [5]. Additionally, HIV-infected participants with neurocognitive impairment have CSF neopterin levels 25% higher than those without neurocognitive impairment, suggesting that this persistent intrathecal immune activation may contribute to HIV-associated neurocognitive disorder [4]. Neuroimaging also supports persistent CNS immune activation in treated HIV, as brain positron emission tomography using a radioligand for microglial activation showed increased global uptake in individuals with virological suppression as compared to healthy controls [6], and brain magnetic resonance spectroscopy revealed elevated levels of cerebral metabolites in individuals undergoing ART, indicating brain inflammation [7].
It is unknown whether ART initiation during acute HIV infection (AHI) can alter this trajectory of persistent CNS immune activation. ART intervention during AHI is hypothesized to improve immunologic outcomes through a reduction in the latent HIV reservoir size. However, prior blood studies in the examined AHI cohort suggest that, after ART initiation, plasma levels of immune activation markers such as soluble CD14, neopterin, and CXCL10 do not return to levels observed in uninfected controls [8, 9]. In contrast, a study that evaluated intensification of standard ART regimens in 22 patients with AHI from this cohort found that CSF levels of neopterin, CCL2, and CXCL10 decreased from baseline to levels similar to those in controls after 24 weeks of therapy [9]. These data suggest a differential impact of very early ART initiation on the CNS as compared to plasma; however, the durability of the impact of ART in AHI has not been observed in a large sample over extended period. In this study, we analyzed levels of immune activation markers longitudinally in CSF as compared to plasma through 96 weeks of treatment in a large cohort of participants with AHI with very early ART intervention and compared them to levels in HIV-negative controls.
METHODS
Study Population
This study included individuals identified with AHI as part of the SEARCH010/RV254 cohort (clinical trials registration NCT00796146) in Bangkok, Thailand, as previously described. Participants with AHI were identified through real-time pooled nucleic acid testing and a sequential immunoassay at the Thai Red Cross Anonymous HIV clinic in Bangkok. Participants who agreed to enter the study rapidly underwent evaluation, provided before ART initiation (ie, week 0) samples, initiated ART, and were followed longitudinally. A majority of participants (64.4%) received efavirenz, tenofovir, and emtricitabine or lamivudine (hereafter, “standard ART”), with some (34.7%) also receiving maraviroc and raltegravir for the first 24 weeks (hereafter, “ART plus”) under a separate protocol (clinical trials registration NCT00796263). Eighteen HIV-negative participants were recruited as healthy controls (hereafter, “controls”) and enrolled and evaluated in an accompanying protocol (clinical trials registration NCT01397669).
All participants provided written, informed consent for participation in research. All involved institutional review boards approved the study. We adhered to the policies for protection of human subjects as prescribed in US Army Regulation 70.
Sample Collection and Analysis
Participants with AHI had a sample at one or more time point for blood (n = 146) and CSF collected by optional lumbar puncture (LP; n = 89) at baseline (just prior to ART initiation), at week 24 of ART (143 and 48, respectively), and at week 96 of ART (80 and 22, respectively). Controls provided CSF and blood samples at a single time point. CSF and plasma specimens were analyzed for HIV RNA, using the Roche COBAS AmpliPrep/COBAS TaqMan HIV test, in addition to standard assessments.
Levels of immune activation markers were determined as follows. Soluble CCL2, CXCL10, and IL-6 levels were assessed using multiplex Luminex Milliplex MAP immunology panels (MilliporeSigma, Burlington, MA), and neopterin levels were determined by a singleplex enzyme-linked immunoassay (Genway Biotech, San Diego, CA). All assays were performed in accordance with manufacturer protocols. Plates were read on a Bio-Plex 3D reader (Bio-Rad, Hercules, CA).
Analyses
Comparisons of demographic characteristics between groups were performed using the Fisher exact test for proportions, unpaired t tests for approximately normally distributed numeric data, and 2-tailed Mann-Whitney U tests for nonnormally distributed numeric data. Analyses were performed for participants with any available samples and, separately, for participants with samples collected at each of the 3 available time points (weeks 0, 24, and 96); analyses of the latter group are referred to as complete case analyses. Two-tailed Mann-Whitney U tests were used to compare differences in levels of markers analyzed for all available time points. Two-tailed Wilcoxon signed rank tests were used to analyze samples from the complete case analyses, except for comparisons to controls, which used 2-tailed Mann-Whitney U tests. Mann-Whitney U tests and logistic regression were used to assess the difference in levels of immune activation markers by ART regimen. To determine variables associated with having a level in the highest tertile, Fisher exact χ2 analyses were first performed to identify variables that may be associated with levels in this tertile. Any variables found to be significant or to trend toward significance (defined as a P value of < .20) were analyzed using logistic regression.
RESULTS
We examined baseline CSF samples from 89 participants with AHI (19 with complete case analysis) and plasma samples from 146 (49 with complete case analysis), as well as demographic and clinical information (Table 1). The median estimated infection duration at study entry was 17 days (range, 4–48 days). At weeks 24 and 96 of ART, 100% (34) and 95% (20), respectively, had a CSF HIV RNA load <80 copies/mL, and 91.1% and 98.6%, respectively, had a plasma HIV RNA <50 copies/mL. Values of demographic variables for individuals in the complete case analysis did not differ from those for all participants (P > .05), except for the median time to starting ART after the baseline visit (ie, 2 days and 1 day, respectively; P < .001). In all participants with AHI (n = 146), 76.7% experienced acute retroviral syndrome, defined as ≥3 qualifying signs or symptoms.
Table 1.
Demographic Characteristics for Participants With Acute Human Immunodeficiency Virus (HIV) Infection (AHI), Overall and for Those With Complete Case Analyses, and HIV-Uninfected Healthy Controls
| AHI Group | |||
|---|---|---|---|
| Characteristic | Control Group | Overall | Complete Casesa |
| Age, y | 26.5 (23–32)b | 28.0 (23–32)b | 32.5 (27.5–39) |
| Male sex, % | 95.9b | 89.8b | 50.0 |
| Estimated infection duration, d | 17 (13–23) | 15 (11–19) | … |
| Time from diagnosis to ART initiation, d | 1 (0–2)c | 2 (2–3) | … |
| Fiebig stage I/II, % | 43.0 | 45.8 | … |
| HIV subtype CRF01_AE, % | 82.2 | 83.7 | … |
| Plasma HIV RNA load, log10 copies/mLd | 5.75 (5.24–6.56) | 5.55 (4.89–6.34) | … |
| CSF HIV RNA load, log10 copies/mLd | 3.36 (2.34–4.32) | 3.37 (2.74–4.07) | … |
| CD4+ T cell count, cells/mm3,d | 387 (293–506) | 392 (311–555) | … |
| CD8+ T cell count, cells/mm3,d | 512 (306–928) | 472 (304–728) | … |
| CD4+ to CD8+ T-cell ratiod | 0.80 (0.45–1.17) | 0.86 (0.53–1.38) | … |
Data are median values (interquartile ranges), unless otherwise indicated.
aDefined as participants with AHI who had samples available from all time points.
bStatistically significantly different from the value for the control group.
cStatistically significantly different from the value for the complete case group.
dAt baseline.
Neurological symptoms of acute retroviral syndrome were found in 52.7% of participants. Headache was most common (with 52.1% reporting any headache and 13% reporting moderate or severe headache), and 1 participant reported a neurologic symptom (mild dizziness lasting 11 days) as part of acute retroviral syndrome.
CSF Immune Activation Markers
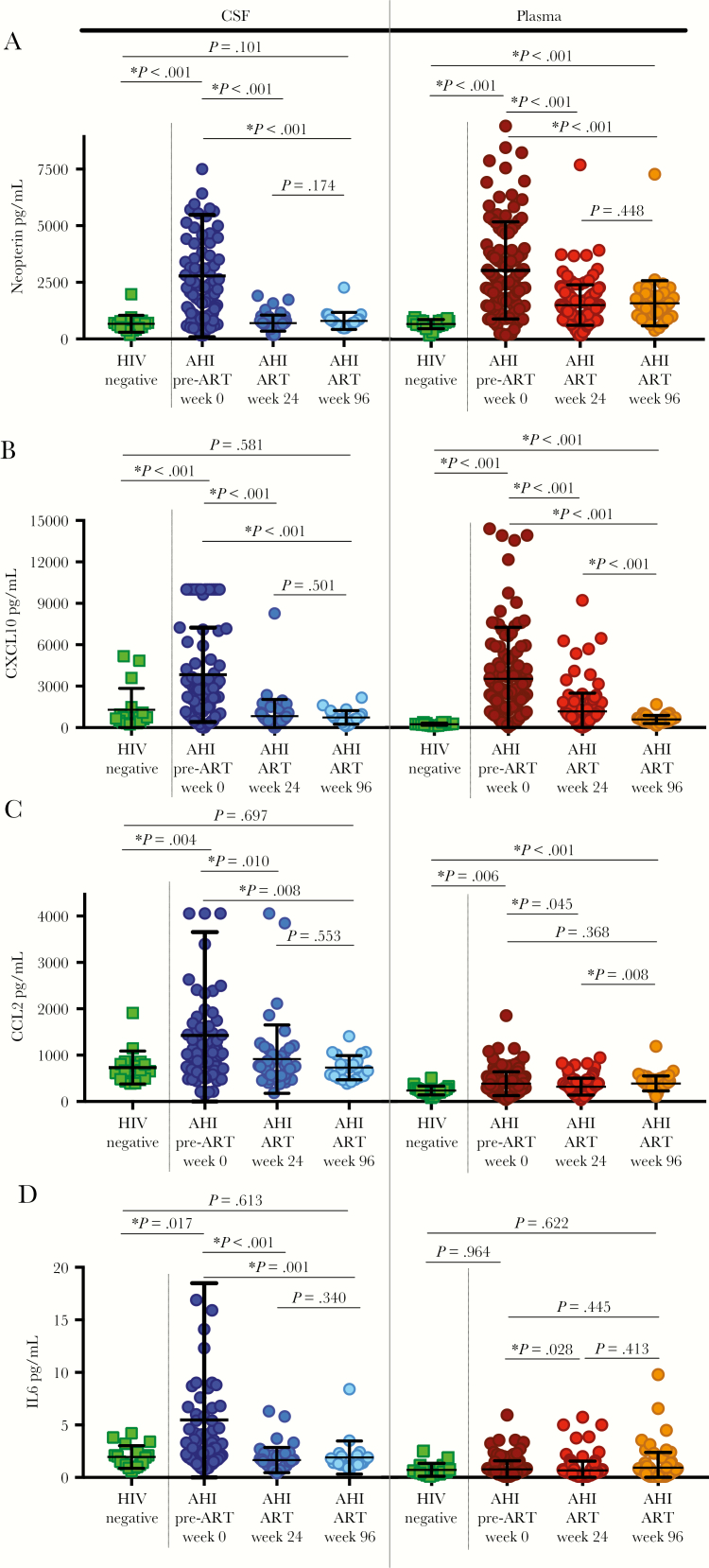
Among controls, CSF levels of immune activation markers were not associated with age or sex (Table 2). Analysis of all available samples from the AHI group revealed that median neopterin, CXCL10, CCL2, and IL-6 levels in CSF were elevated at week 0 (before ART initiation), compared with those in controls (Figure 1A–D). By week 24 of ART, median levels decreased, did not differ significantly from those of controls (all P < .05), and remained unchanged after 96 weeks of ART (all P > .05). At week 96, only 1 participant had an abnormal CSF neopterin level (>1341 pg/mL), similar to the proportion in the control group (χ2 = 0.021; P = .884). The complete case analyses of participants with CSF samples available (n = 19) mirrored the findings for CSF levels of neopterin and IL-6. The complete case analyses for median CSF CXCL10 levels revealed similar findings at week 0, compared with controls, although values decreased after 24 weeks of ART and were unchanged at week 96 (P = .096; Supplementary Figure 1). The median CSF CCL2 level among participants included in the complete case analyses did not differ from that among controls at any time point (Supplementary Figure 1).
Table 2.
Levels of Immune Activation Markers in Cerebrospinal Fluid (CSF) and Plasma Among Human Immunodeficiency Virus (HIV)–Uninfected Healthy Controls Before and 24 and 96 Weeks After Antiretroviral Therapy Initiation, and Participants With Acute HIV Infection (AHI)
| Control Group | AHI Group | |||||||
|---|---|---|---|---|---|---|---|---|
| Marker | Value | Participants, No. | Value, Wk 0a | Participants, No. | Value, Wk 24 | Participants, No. | Value, Wk 96 | Participants, No. |
| CSF | ||||||||
| Neopterin level, pg/mL | 651 (495–726) | 18 | 1986 (1025–3551) | 89 | 644 (542–756) | 48 | 722 (594–856) | 22 |
| CXCL10 level, pg/mL | 702 (388–1050) | 18 | 2739 (1276–5927) | 89 | 563 (306–891) | 48 | 645 (435–866) | 22 |
| CCL2 level, pg/mL | 673 (486–806) | 18 | 904 (680–1332) | 89 | 726 (559–990) | 48 | 704 (531–854) | 22 |
| IL-6 level, pg/mL | 1.7 (1.2–2.3) | 18 | 2.6 (1.7–4.7) | 83 | 1.4 (0.9–2.0) | 45 | 1.4 (1.2–2.0) | 22 |
| Plasma | ||||||||
| Neopterin level, pg/mL | 667 (568–758) | 18 | 2352 (1592–3503) | 136 | 1284 (937–1750) | 137 | 1371 (1107–1689) | 46 |
| CXCL10 level, pg/mL | 215 (179–249) | 18 | 2666 (1174–4058) | 140 | 798 (502–1396) | 141 | 494 (367–693) | 49 |
| CCL2 level, pg/mL | 209 (178–262) | 18 | 336 (196–467) | 140 | 318 (155–396) | 141 | 357 (292–417) | 49 |
| IL-6 level, pg/mL | 0.5 (0.3–0.8) | 18 | 0.5 (0.3–0.9) | 146 | 0.5 (0.2–0.7) | 143 | 0.6 (0.1–0.9) | 80 |
Data are median values (interquartile ranges).
aBefore ART initiation.
Figure 1.
Levels of the immune activation markers neopterin (A), CXCL10 (B), CCL2 (C), and interleukin 6 (IL-6; D) in cerebrospinal fluid and plasma in all available samples from participants with acute human immunodeficiency virus (HIV) infection (AHI) before (week 0) and 24 and 96 weeks after antiretroviral therapy initiation, compared with those in HIV-negative healthy controls. Mean values are denoted by central horizontal lines, and standard deviations are denoted by upper and lower horizontal lines.
Plasma Immune Activation Markers
In the control group, no measured immune activation levels were associated with age, and only the plasma level of CCL2 was related to sex, with the median value in men higher than that in women (103.8 pg/mL [IQR, 93.5–110.7 pg/mL] vs 76.7 pg/mL [IQR, 65–78.9 pg/mL]). As previously reported in this cohort [9], the median plasma neopterin level was elevated at week 0 (before ART initiation) in participants with AHI as compared to controls and declined after ART initiation but, at week 96, remained greater than that in controls (Figure 1A). Median plasma levels of CXCL10 (Figure 1B) and CCL2 (Figure 1C) followed a similar pattern. The median plasma level of IL-6 was not different from that in controls at any time point (Figure 1D). In the complete case analyses of participants with samples available at each time point (n = 49), the difference between the median plasma CCL2 level at week 0 and the level in controls (398 ± 325 pg/mL vs 228 ± 93 pg/mL; P = .061) approached significance. There were no other differences in median plasma levels of markers between controls and the complete case group.
Association Between ART Regimen and Immune Activation Levels Over Time
We compared samples collected from participants receiving the standard ART regimen to those from participants receiving the ART-plus regimen at weeks 24 and 96 after treatment initiation. Using Mann-Whitney U tests for comparison of all available samples, we found that the ART-plus arm had a lower median plasma CCL2 level at week 24 (200.9 pg/mL [n = 45] vs 338.5 pg/mL [n = 90]; P = .004), with trends toward a lower median plasma CXCL10 level (773.4 pg/mL [n = 49] vs 832.6 pg/mL [n = 90]; P = .126), median CSF CXCL10 level (496.0 pg/mL [n = 23] vs 620.2 pg/mL [n = 24]; P = .054), and median CSF IL-6 level (1.2 pg/mL [n = 20] vs 1.5 pg/mL [n = 24]; P = .088). At week 96, the only significant difference involved a lower median plasma neopterin level in the ART-plus group (1276 pg/mL [n = 32] vs 1692 pg/mL [n = 13]; P = .009). Using logistic regression accounting for baseline plasma HIV RNA load, the median plasma CCL2 level at week 24 in the ART-plus arm remained significantly lower (P = .015; odds ratio [OR], 1.00), along with trends toward lower median plasma CXCL10 (P = .075; OR, 1.00) and IL-6 (P = .109; OR, 1.41) levels at week 24. Median CSF levels at week 96 were not analyzed because there were only 4 participants in the standard ART arm and 16 in the ART-plus arm.
Factors Associated With Higher Immune Activation
In the complete case analysis, we examined baseline factors associated with having levels of plasma or CSF immune activation markers within the highest tertile of observed values at week 0 (before ART initiation) and week 24. Examined variables were age at baseline, sex, estimated infection duration, Fiebig stage, and HIV subtype, as well as plasma viral load, CSF viral load, CD4+ T-cell count, CD8+ T-cell count, and ratio of CD4+ to CD8+ T cells at weeks 0 and 24. Baseline plasma viral load was associated with having a highest tertile value for plasma levels of neopterin (P = .008; OR, 2.99), CXCL10 (P = .026; OR, 2.07), and CCL2 (P = .017; OR, 2.26) and CSF level of neopterin (P = .029; OR, 7.40; Supplementary Figure 2). Baseline CSF viral load was associated with the highest tertile for baseline plasma CXCL10 level (P = .044; OR: 4.21) and CSF CXCL10 level (P = .034; OR, 5.21). Baseline CD8+ T-cell count was associated with the highest tertile for plasma neopterin level (P = .026; OR, 1.00; Supplementary Figure 2). No baseline findings were associated with having a CSF CCL2 or CSF IL-6 level at week 0 in the highest tertile. Plasma IL-6 levels were not analyzed, owing to a similarity between values at week 0 and in the control group. No variables were associated with a highest tertile value at week 24.
DISCUSSION
This study identified that initiation of ART during AHI has a differential impact on the trajectory of immune activation in the CNS as compared to plasma. Very early ART initiation was associated with reversion of CSF levels of immune activation markers to levels in controls, with reversion sustained 96 weeks after treatment initiation, whereas plasma levels of neopterin, CXCL10, and CCL2 decreased during treatment but remained greater than those in controls at 96 weeks. The complete case analysis finding that CSF CCL2 levels among participants with AHI did not differ from those in controls may be related to a small sample size or to a delayed rise in the CCL2 level, which has been observed during primary HIV infection [10]. These overall findings are consistent with results of a study from the same cohort, which identified that initiating ART during AHI results in normalization of the YKL-40 level, a CSF marker of microglial activation, although some suggest it may be more generic to glial activation [11]. This work contrasts with the elevated CSF immunologic profiles when treatment is started during the chronic phase of HIV infection, when only a percentage of participants have a reduction and potential normalization of immune activation marker levels in CSF [3].
One possible explanation for the difference in the trajectory of levels between plasma and CSF immune activation markers in AHI is the impact of very early ART initiation on formation of the latent HIV reservoir [12]. Reservoir size may contribute to persistent immune activation, and the CNS is generally exposed to lower HIV loads during the early phases of infection. If early ART intervention differentially minimizes the CNS HIV reservoir as compared to reservoir sites in the peripheral system, it is possible that this reduced CNS reservoir removes the driving force behind persistent CNS immune activation. This hypothesis is supported by a recent study of participants treated during early HIV infection (estimated infection duration, 20–132 days) that examined anti-HIV antibody formation in the blood versus the CSF [13]. This work identified a “selective sparing” in acquisition of CNS HIV antibodies, suggesting that early treatment reduces establishment of the CNS reservoir [13].
We also found that very early ART initiation with an intensified regimen including maraviroc and raltegravir may further reduce levels of immune activation markers. However, it is notable that levels for the overall AHI group did not differ from those for the control group, and it is unknown whether patients would benefit clinically from reductions in immune activation marker levels. Previous work in this cohort with a smaller sample size did not identify an impact of ART regimen [9]. As maraviroc and raltegravir were only added to ART regimens for the first 24 weeks, it is unknown how sustained administration of an intensified regimen may influence immunologic profiles over time.
It is possible that trajectories of immune activation marker levels are multifactorial during HIV infection, resulting from plasma HIV RNA levels and host immunologic factors combined with the timing and type of ART initiation [12]. Recent work has shown that polymorphisms in the heme oxygenase-1 promoter region may influence HIV-related neuroinflammation [14]. Our work also identifies that higher baseline plasma and CSF viral loads and CD8+ T-cell count may be associated with worse baseline levels of immune activation markers.
The implications of these findings for long-term neuropathogenesis are unclear, but this work suggests that very early ART initiation may alleviate long-term neuroinflammation during HIV infection. After 24 weeks of ART, participants with AHI are known to have a normal level of the CSF marker of neuronal injury, neurofilament light chain, although elevated levels are observed in people with chronic HIV infection; it is not known whether this is mediated through suppressed CNS immune activation [15]. It is possible that the early initiation of ART may have a neuroprotective trajectory, although it is currently unknown whether participants with AHI will develop the common neurologic outcome of HIV-associated neurocognitive disorder, which affects up to 50% of people living with HIV [16].
This study is limited by demographic differences between controls and participants with AHI, which may impact the interpretability of our findings. As we did not include participants with AHI who were not treated with ART, the causality of our associations cannot be confirmed. Additionally, it is not known how accurately CSF measures reflect the immunologic state within the brain parenchyma or whether there are neuroanatomic regions with selective vulnerability to persistent CNS immune activation. In this work, we characterized the longitudinal trajectory of levels of 4 markers of immune activation in CSF after very early initiation of ART and found that CSF markers return to normal levels, which were sustained at week 96 of treatment. These findings lend further evidence for the protective effects of early ART initiation on the CNS and therefore provide strong support for prompt initiation of ART.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Disclaimer. The views expressed are those of the authors and should not be construed to represent the positions of the US Army, the Department of Defense, or the Department of Health and Human Services.
Financial support. This work was supported by the National Institute of Mental Health, National Institute of Health (grants K23MH114724, R01MH095613, R01NS061696, R21MH086341, R01NS084911, and K24MH098759, with additional support from the National Institutes of Mental Health); the Henry M. Jackson Foundation for the Advancement of Military Medicine and the Department of Defense (cooperative agreement W81XWH-11-2-0174); the Thai Government Pharmaceutical Organization (antiretroviral therapy [ART]); Gilead (ART); Merck (ART); and ViiV Healthcare (ART).
Potential conflicts of interest. V. V. has participated in advisory meetings for ViiV Healthcare and Merck. J. A. has participated in advisory meetings for ViiV Healthcare, Merck, AbbVie, Gilead, and Roche. S. S. cochairs a study through the AIDS Clinical Trials Group for which study antiretroviral medications are provided by ViiV Healthcare. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Lederman MM, Funderburg NT, Sekaly RP, Klatt NR, Hunt PW. Residual immune dysregulation syndrome in treated HIV infection. Adv Immunol 2013; 119:51–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Krebs SJ, Slike BM, Sithinamsuwan P, et al. ; SEARCH 011 study team Sex differences in soluble markers vary before and after the initiation of antiretroviral therapy in chronically HIV-infected individuals. AIDS 2016; 30: 1533–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Yilmaz A, Price RW, Spudich S, Fuchs D, Hagberg L, Gisslén M. Persistent intrathecal immune activation in HIV-1-infected individuals on antiretroviral therapy. J Acquir Immune Defic Syndr 2008; 47:168–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Edén A, Marcotte TD, Heaton RK, et al. . Increased intrathecal immune activation in virally suppressed HIV-1 infected patients with neurocognitive impairment. PLoS One 2016; 11:e0157160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ulfhammer G, Edén A, Mellgren Å, et al. . Persistent CNS immune activation following >10 years of effective HIV antiretroviral treatment. AIDS 2018 Sep 24; 32:2171–8. [DOI] [PubMed] [Google Scholar]
- 6. Vera JH, Guo Q, Cole JH, et al. . Neuroinflammation in treated HIV-positive individuals: A TSPO PET study. Neurology 2016; 86:1425–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Harezlak J, Buchthal S, Taylor M, et al. ; HIV Neuroimaging Consortium Persistence of HIV-associated cognitive impairment, inflammation, and neuronal injury in era of highly active antiretroviral treatment. AIDS 2011; 25:625–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sereti I, Krebs SJ, Phanuphak N, et al. ; RV254/SEARCH 010, RV304/SEARCH 013 and SEARCH 011 protocol teams Persistent, albeit reduced, chronic inflammation in persons starting antiretroviral therapy in acute HIV infection. Clin Infect Dis 2017; 64:124–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Valcour VG, Spudich SS, Sailasuta N, et al. ; SEARCH 010/RV 254 Study Group Neurological Response to cART vs. cART plus Integrase Inhibitor and CCR5 Antagonist Initiated during Acute HIV. PLoS One 2015; 10:e0142600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Spudich S, Gisslen M, Hagberg L, et al. . Central nervous system immune activation characterizes primary human immunodeficiency virus 1 infection even in participants with minimal cerebrospinal fluid viral burden. J Infect Dis 2011; 204:753–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Peluso MJ, Valcour V, Phanuphak N, et al. ; RV254SEARCH 010, RV304SEARCH 013, and SEARCH 011 Study Teams Immediate initiation of cART is associated with lower levels of cerebrospinal fluid YKL-40, a marker of microglial activation, in HIV-1 infection. AIDS 2017; 31:247–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gandhi RT, McMahon DK, Bosch RJ, et al. ; ACTG A5321 Team Levels of HIV-1 persistence on antiretroviral therapy are not associated with markers of inflammation or activation. PLoS Pathog 2017; 13:e1006285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Burbelo PD, Price RW, Hagberg L, et al. . Anti-human immunodeficiency virus antibodies in the cerebrospinal fluid: evidence of early treatment impact on central nervous system reservoir? J Infect Dis 2018; 217:1024–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gill AJ, Garza R, Ambegaokar SS, Gelman BB, Kolson DL. Heme oxygenase-1 promoter region (GT)n polymorphism associates with increased neuroimmune activation and risk for encephalitis in HIV infection. J Neuroinflammation 2018; 15:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Peluso MJ, Valcour V, Ananworanich J, et al. ; RV254/SEARCH 010 and SEARCH 011 Study Teams Absence of cerebrospinal fluid signs of neuronal injury before and after immediate antiretroviral therapy in acute HIV Infection. J Infect Dis 2015; 212:1759–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Heaton RK, Clifford DB, Franklin DR Jr, et al. ; CHARTER Group HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology 2010; 75:2087–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.