Abstract
In synaesthesia, stimulation of one sensory modality evokes additional experiences in another modality (e.g. sounds evoking colours). Along with these cross-sensory experiences, there are several cognitive and perceptual differences between synaesthetes and non-synaesthetes. For example, synaesthetes demonstrate enhanced imagery, increased cortical excitability and greater perceptual sensitivity in the concurrent modality. Previous models suggest that synaesthesia results from increased connectivity between corresponding sensory regions or disinhibited feedback from higher cortical areas. While these models explain how one sense can evoke qualitative experiences in another, they fail to predict the broader phenotype of differences observed in synaesthetes. Here, we propose a novel model of synaesthesia based on the principles of stochastic resonance. Specifically, we hypothesize that synaesthetes have greater neural noise in sensory regions, which allows pre-existing multisensory pathways to elicit supra-threshold activation (i.e. synaesthetic experiences). The strengths of this model are (a) it predicts the broader cognitive and perceptual differences in synaesthetes, (b) it provides a unified framework linking developmental and induced synaesthesias, and (c) it explains why synaesthetic associations are inconsistent at onset but stabilize over time. We review research consistent with this model and propose future studies to test its limits.
This article is part of a discussion meeting issue ‘Bridging senses: novel insights from synaesthesia’.
Keywords: synaesthesia, noise, neural model, multisensory, hallucinations, hyperexcitability
1. Introduction
Synaesthesia is a brain-based phenomenon in which a stimulus presented to one sensory modality (the inducing sensation) triggers a simultaneous and involuntary qualitative experience in another cognitive or perceptual modality (the concurrent sensation) [1–3]. For instance, in grapheme–colour synaesthesia, present in approximately 4% of the population [4], letters and numbers evoke the experience of (or are strongly associated with) distinct colours. However, the inducer is not perceptually supplanted by the concurrent sensation, as a grapheme–colour synaesthete viewing a black number 5 would see the physical colour of the text plus additional colour qualia (e.g. a specific red hue). These experiences not only are subjectively reported by individuals but also can be characterized and verified using neuroimaging techniques. Specifically, research shows that viewing numbers or letters associated with a synaesthetic experience causes increased activation in perceptual colour areas in grapheme–colour synaesthetes, supporting the perceptual nature of the synaesthetic associations [5–7]. Notably, however, these results have not been universally replicated (see [8–10] for a critical review).
In the most fundamental sense, synaesthesia is simply one sense evoking a conscious experience in a second cognitive or perceptual modality [1,11]. As synaesthesia remains a behaviourally defined condition, researchers have argued for the expansion of this definition to include other common characteristics of synaesthesia to serve as further diagnostic criteria [2]. The most often reported aspects of these are the following: automaticity (synaesthetes have very little conscious control over the onset or content of the concurrent once an inducer is encountered), consistency of inducer and concurrent associations (a specific inducer is always associated with specific concurrent (e.g. 2 is always blue)), generic nature of synaesthetic experiences (associations are relatively basic as opposed to elaborate and complex images), synaesthetic experiences are laden with affect, memorable and useful [2]. However, these aspects are only strongly associated with the experience of synaesthesia and their requirement as diagnostic criteria remains open to debate [12–14]. The ambiguity in what constitutes a ‘genuine’ form of synaesthesia has contributed to the question of whether the genetic form (developmental synaesthesia) is a phenomenon distinct from acquired or drug-induced types, with each arising through unique neural mechanisms [12,15,16].
The two most established models of synaesthesia, the disinhibited feedback model and the cross-activation model (described in greater detail below), suggest competing mechanisms to explain the most important aspect of synaesthesia: that stimulation of one modality evokes activity in a second modality, leading to altered qualitative experiences. However, data in support of these models are controversial (reviewed below in detail, e.g. [6,8]), they fail to account for the broader phenotype of differences observed in synaesthetes [17–19], and they have not substantially evolved from their original proposal despite the wealth of new findings from the broader field of multisensory research. First, in §§2–5, we recount evidence for perceptual and neuroanatomical differences between synaesthetes and non-synaesthetes, as well as evidence for the broader phenotype of synaesthesia. Secondly, in §§6–9, we briefly discuss these two models, review evidence supporting them, and highlight their limitations. Finally, we propose a novel ‘stochastic resonance’ model of synaesthesia based on recent research that addresses the limitations of the previous models and provides a unified framework for developmental and induced synaesthesias.
Reviewed in detail in §10 of this manuscript, stochastic resonance is the process by which the addition of noise to a neural system can counterintuitively increase the quality of signal transmission or detection [20,21]. According to the stochastic resonance model of synaesthesia (SRS), the primary neurobiological difference in synaesthetes is the presence of higher levels of neural noise in the concurrent modality (usually a broad distributed network of visual areas involved in colour processing) that allows cross-modal signals to be experienced as supra-threshold conscious percepts. These increased levels of noise are also predicted to lead to the numerous altered perceptual and cognitive differences observed in the broader phenotype of synaesthesia. Furthermore, this model can explain several forms of synaesthesia—drug-induced, developmental and acquired synaesthesia—all within the same framework. We review current literature that supports this model and suggest studies to challenge its validity.
2. What makes a synaesthete different from non-synaesthete?
The defining behavioural feature of synaesthesia is that stimulation of an inducing modality (e.g. sounds) evokes qualitative experiences in an unrelated modality (e.g. colours). However, recent research has shown that there are neurobiological, perceptual and cognitive differences between synaesthetes and non-synaesthetes that are seemingly unrelated to these altered qualitative experiences [2]. In the §§3–5, we review these differences to examine whether they can be integrated with previous neurobiological models of synaesthesia.
3. Neurobiological differences (functional)
Neuroimaging studies demonstrate that synaesthetes differ from non-synaesthetes in several aspects. Most notably, inducing stimuli evoke stronger co-activation of concurrent sensory areas in synaesthetes compared with non-synaesthetes. For example, grapheme–colour synaesthetes show stronger co-activation of the visual word form area (VWFA) with perceptual colour areas (e.g. V4) [5,7]. Notably, however, these findings have not been universally replicated [8]. Furthermore, magnetoencephalography (MEG) studies show that the activation of colour regions follows activation of the VWFA by only a few milliseconds [6]. Similarly, functional connectivity is increased between grapheme and colour areas during the synaesthetic experience [22]. These co-activation findings have been expanded to other forms of synaesthesia as well. In visual–olfactory synaesthesia (images induce olfactory sensations), synaesthetic experiences were associated with primary olfactory cortex activation [23] and lexical–gustatory synaesthetes (who experience tastes on hearing specific words) showed increased activation in taste areas [24].
Neuroimaging research additionally shows that functional differences between synaesthetes and non-synaesthetes are not restricted to the inducing and concurrent modalities, nor do they require the conscious experience of synaesthesia. For example, functional connectivity between grapheme and colour areas is present even when graphemes do not induce a synaesthetic experience [22] and even during rest [25]. Furthermore, functional activation differences in grapheme–colour synaesthetes and non-synaesthetes are found not only in the corresponding sensory modalities but also in parietal and frontal regions as well (see [9,26] for review), along with increased functional connectivity between parietal and visual areas [27] and increased connectivity between the frontoparietal and visual intrinsic networks (ICNs) during resting state [25].
More generally, several studies have demonstrated that these differences are present even at hierarchically earlier levels of sensory processing. For example, grapheme–colour synaesthetes show significant differences in visual evoked potentials as measured by electroencephalography (EEG), even to stimuli that do not evoke colours. These differences occur in the primary visual areas within 70 ms, indicating differences in early processing of stimuli [19]. Similarly, studies report that synaesthesia-related activity occurs as early as V1 [28]. Thus, even basic visual cortical processes are altered in grapheme–colour synaesthesia. However, previous models of synaesthesia do not easily explain these early processing differences.
4. Neurobiological differences (anatomical)
In tandem with the functional differences, synaesthetes and non-synaesthetes demonstrate anatomical differences in these similar neural structures. Grapheme–colour synaesthetes have increased grey matter volume in the posterior fusiform gyrus (FG) [29] and increased white matter in the bilateral retrosplenial cortex [8]. Similarly, other researchers have reported increased cortical thickness, volume and surface area in the FG and adjacent regions, such as the lingual gyrus and the calcarine cortex in grapheme–colour synaesthetes [30]. Diffusion tensor imaging (DTI) shows that grapheme–colour synaesthetes also have increased connectivity in the inferior temporal cortex, putatively in a region that could link VWFA and colour regions [31]. Similarly, sound–colour synaesthetes have greater white matter integrity in the inferior fronto-occipital fasciculus, the major white matter tract connecting visual and auditory regions [32].
However, the anatomical differences observed in synaesthetes extend beyond the inducer and concurrent modalities to numerous other neural structures. Several studies have demonstrated that synaesthetes show connectivity (as measured using DTI) and cortical thickness differences in parietal and frontal regions [31,33–36]. Synaesthetes have also been reported to possess anatomical differences in subcortical and cortical emotion processing regions of the brain [37]. More generally, synaesthetes have a globally altered structural network topology reflected by reduced small-worldness (i.e. the neural networks are more locally clustered and have a reduced long-range connectivity) [38], indicating large-scale changes exist in the brain of a synaesthete.
5. Perceptual and cognitive differences
In addition to functional and structural differences, research over the past decade indicates that synaesthetes possess a myriad of cognitive and perceptual alterations compared with non-synaesthetes. Best replicated among these are benefits on memory. For example, several studies have shown that grapheme–colour synaesthetes have an enhanced memory [39–41]. Time–space synaesthetes demonstrate similar benefits in terms of improved spatial working memory and spatial processing abilities including mental rotation [42–44]. Subjective reports suggest that these cognitive improvements are due to the recruitment of synaesthetic associations as an extra dimension to encode information, or facilitated memory processes in general [39–41,45,46].
In terms of perceptual differences between synaesthetes and non-synaesthetes, synaesthetes demonstrate enhanced perception in the concurrent modality of synaesthesia [47,48] and enhanced imagery in the concurrent [18,49–51], and have altered cortical excitability in early processing areas [17,52]. Before reviewing these perceptual differences in detail, it is important to consider how these sensory differences are causally related to the cross-sensory experiences of synaesthesia. One possibility, as argued by previous models, is that these perceptual differences are a consequence of experiencing synaesthetic associations. However, given the breadth of these perceptual changes and their presence in early sensory regions (e.g. primary visual cortex), it is also possible that these differences are not causally related to synaesthetic experiences themselves, but to the broader phenotype of synaesthesia or factors contributing to its development.
In general, the perceptual differences present in synaesthetes occur in modalities related to their synaesthetic experiences. For example, time–space synaesthetes have enhanced spatial processing abilities [42] and those with mirror-touch synaesthesia have enhanced tactile sensitivity [48]. Interestingly, grapheme–colour synaesthetes show improved colour sensitivity [47], a larger McCollough effect [53] and enhanced visual imagery [18,49,50,54]. Most importantly, these perceptual benefits are trait-like differences in synaesthetes, such that they are observed even with stimuli that do not trigger synaesthetic experiences reflecting alterations of low-level colour processing. Critically, however, studies have demonstrated reduced motion sensitivity [47] and normal non-visual imagery in synaesthetes [54], indicating that perceptual enhancements are specific to the synaesthetic concurrent modality (i.e. synaesthetes are not simply better on every task) and might have associated trade-offs. Indeed, these perceptual alterations have been associated with substantially increased cortical excitability in the primary visual cortices of synaesthetes [17,52]. Importantly, research indicates that synaesthesia can be induced in non-synaesthetes by modulating cortical excitability in primary sensory areas [55].
6. Previous models of synaesthesia
A complete neural model of synaesthesia should account for not just the localized anatomical differences observed, but also the diffuse brain-wide alterations and perceptual enhancements that are present in those with synaesthesia. Previous models of synaesthesia, the disinhibited feedback and cross-activation models, sought to explain the experience of synaesthesia in neuroanatomical terms, and provided an excellent characterization of how one sensory system could trigger activation in another. However, these models are generally unable to account for the broader phenotype of cognitive, perceptual and neuroanatomical differences that is present in synaesthesia. The following sections (§§7 and 8) review these two established models, summarizes evidence in support of them, and highlights their limitations.
7. The disinhibited feedback theory
According to the disinhibited feedback theory [11], synaesthetic experiences arise because of disinhibited feedback to the concurrent modality from higher-level brain areas where information from the inducer and the concurrent pathways converge. For example, in grapheme–colour synaesthesia, information about grapheme stimuli first propagates through the cortical hierarchy to arrive at the temporo-parietal junction. From this multisensory nexus, the signal travels back down to V4 through disinhibition of a normally inhibited feedback pathway and causes activation of a representation in V4, leading to the synaesthetic experience.
This theory makes two predictions: (a) synaesthetes and non-synaesthetes have similar brain connectivity, and (b) there is a delay between when the inducer is processed and the concurrent is activated, since it takes time for information to traverse this distributed network. The first prediction of the theory suggests that synaesthesia uses the pre-existing neural pathways in normal adults, rather than being dependent on abnormal pathways or the formation of new connections in synaesthetes. This prediction is supported by the ability of hallucinogenic drugs to induce synaesthetic experiences in non-synaesthetes [16] as well as training studies that show synaesthesia can be induced over time [56].
The second prediction suggests that in grapheme–colour synaesthesia, following presentation of a grapheme, activity will propagate from the grapheme processing areas (VWFA), to higher processing areas (possibly in parietal areas) and then backpropagate to the colour processing area of V4. This is partially supported by the neuroanatomical differences between grapheme–colour synaesthetes and non-synaesthetes, seen in the parietal regions [27,31]. However, this model requires a substantial processing delay between activation of the VWFA and colour area V4. MEG and EEG studies, conversely, show that activity within V4 is significant within a few milliseconds after the grapheme-responsive area, and there is an absence of activation in higher processing regions before onset of activity in V4 [6]. This slight delay in the propagation of activity from grapheme to colour in synaesthesia is insufficient for signals to travel to higher processing regions and back to V4 [57,58]. However, these studies do not investigate the differences between associator and projector synaesthetes. In the light of recent connectivity research showing that the associators (and not projectors) use top-down pathways as suggested by the disinhibited feedback model [59], further research using MEG should be conducted in associator synaesthetes alone to provide more convincing evidence against the disinhibited feedback model of synaesthesia. Nonetheless, it is unclear how differences in the disinhibition of later feedback pathways would explain early visual processing differences seen in grapheme–colour synaesthetes [19]. Together, these results provide evidence against the disinhibited feedback model of synaesthesia.
8. The cross-activation theory
The cross-activation theory was proposed to explain grapheme–colour synaesthesia and was inspired by its congenital nature and the adjacency of the VWFA (the grapheme processing area) and V4 (a colour processing area) [45,60]. This theory suggests that genetic factors lead to decreased pruning of neural connections, resulting in the abnormal connection of two sensory regions. Thus, while V4 and the VWFA are initially connected in all individuals (suggesting that all individuals are born synaesthetes; [61,62]), this connection is pruned away in non-synaesthetes during development. These abnormal connections then lead to a ‘cross-activation’ of concurrent experiences in the presence of an inducer (i.e. graphemes evoking colours). The cross-activation model, when extended to other forms of synaesthesia, suggests hyper-connectivity between brain regions specific to the form of synaesthesia. For example, grapheme–colour synaesthetes have a hyperconnected V4 and VFWA, and tone–colour synaesthetes a hyperconnected V4 and auditory cortex [31,32].
The cross-activation model makes three main predictions: (1) inducer and concurrent modalities should be in densely interconnected regions, (2) genetic factors alter neural pruning and should predict presence of synaesthesia, and (3) synaesthetes should show anatomical pathways that are not present in adult non-synaesthetes.
Evidence for the cross-activation model is mixed. For example, this model first predicts that the synaesthetic experience is largely accounted for by anatomical changes only in the network connecting the inducer and concurrent modalities. DTI-based evidence in grapheme–colour synaesthetes supports this hypothesis to an extent [29,31]. However, critical review of neuroimaging differences in synaesthesia reveals that anatomical and functional alterations in synaesthetes are not regionally specific as hypothesized by this model, but instead are broadly distributed throughout the brain (also see §4) [8,38].
Second, this model predicts that genetic factors lead to decreased pruning between concurrent and inducer modalities in synaesthetes. Thus, synaesthesia should have a strong genetic component and the genetic factors implicated in synaesthesia should play a critical role in neural pruning. Developmental synaesthesia has indeed been documented to be largely heritable and studies show that there is 73.9% concordance of synaesthesia in monozygotic twins [63]. Tilot et al. [64] provided preliminary evidence that genetic factors common to three synaesthetic families may play a role in axonogenesis, in support of the view that genetic differences affect connectivity differences in synaesthetes. However, genetic differences and altered axonogenesis only explain the predisposition of an individual to acquire synaesthesia and not the exact form of synaesthesia that will be acquired. Furthermore, since most of the neural pruning occurs early on in development, differences between synaesthetes and non-synaesthetes should be seen early in life. Indeed, synaesthetic associations are observed at least by age 6; however, they do not become consistent until later adolescence [65]. The cross-activation theory does not directly explain the inconsistency of associations throughout childhood or how novel associations can be acquired through training [56,65] or following foreign language exposure [66] during adulthood.
The cross-activation model also emphasizes that the connections in synaesthetes are unique, critical and sufficient for synaesthetic experiences to arise since they are hypothesized to be pruned away during typical development of non-synaesthetes. However, research over the past 10 years has shown that connections linking sensory regions are present not only during development, but also through adulthood in all individuals (for reviews, see [67,68]). For example, the cross-activation model suggests that auditory and visual areas should be anatomically connected by pathways that are unique to tone–colour synaesthetes, and that the presence of these connections is what gives rise to these synaesthetic experiences [27,69,70]. However, substantial research from human and non-human primates indicates that monosynaptic axons directly link auditory and visual areas in all individuals [71,72]. Moreover, these connections serve vital roles in everyday sensory communication. For example, simple sounds can elicit changes in visual cortex even when no visual stimuli are present [73–79]. Indeed, auditory activity modulates the excitability of the visual cortex [73,80–82], which leads to an improved detection and discrimination of visual stimuli [74,75,83,84]. These results underscore that differences between synaesthetes and non-synaesthetes are not completely explained by the existence of anatomical pathways between inducer and concurrent modalities.
Critically, one could argue that the cross-activation theory could be modified to allow for the existence of the pathways mentioned above in all individuals by suggesting that these pathways are simply stronger in synaesthetes. However, as described above, the literature identifying these structural differences is mixed. No longitudinal study has systematically shown that synaesthesia is a consequence of selective sparing of these connections. Furthermore, the cross-activation theory predicts that synaesthesia cannot be induced or acquired unless there is enough time and reason that causes reorganization of anatomical networks in non-synaesthetes. However, research shows that synaesthesia can be acquired quickly as a result of sensory deprivation or alterations in cortical excitability [55]. Recent evidence from our laboratory indicates that auditory–visual synaesthesia can be induced in 50% of undergraduate non-synaesthetes with as little as 5 min of visual deprivation [85]. Indeed, patients with neural trauma leading to sensory deprivation who acquire synaesthesia tend to do so within 1–3 days (though onset can occur after several months or not at all) [86,87]. Drugs like lysergic acid diethylamide (LSD), mescaline and psilocybin among others can also induce transient synaesthetic experiences in minutes that last for only a few hours [16]. The cross-activation model fails to explain how synaesthesia can be acquired in a short span of time.
Why do acquired synaesthesias (due to drugs or sensory deprivation) fail to fit into the cross-activation framework? One justification often given is that acquired synaesthesias are completely different from developmental synaesthesia in their neural origin and phenomenological properties. The strongest argument to this effect is the lack of consistency in associations during induced synaesthesia. However, several researchers have discussed [12,13] that consistency is not a required trait of synaesthetic associations, especially during onset [65]. The cross-activation framework thus requires acquired and induced synaesthesias to be due to separate neurobiological mechanisms, with very limited research suggesting existence of such a discrepancy. Alternatively, it remains possible that a single model can account for developmental, acquired and induced synaesthesias.
9. Need for a new model
According to the disinhibited feedback model of synaesthesia, the cross-sensory experiences in synaesthesia arise due to the disinhibition of feedback from higher-level brain areas to the concurrent modality. The major evidence against this long-range disinhibited feedback model is that the delay between activity in the inducer and concurrent is too quick, such that there is no activation in higher processing areas before onset of activity in concurrent. On the other hand, the cross-activation model suggests that synaesthetes have decreased pruning and strengthened connections between inducer and concurrent. The major limitations of this model are that it assumes acquired synaesthesias are inherently different from the developmental form, and that the connectivity differences between synaesthetes and non-synaesthetes are often seen in areas other than those expected according to the model. Along with the problems discussed above with each model, the previous models of synaesthesia (a) fail to predict the broader phenotype of synaesthesia, (b) do not predict the formation of new associations in synaesthesia, and (c) cannot explain cortical excitability differences in synaesthetes nor (d) how synaesthesia can be induced by modulating the cortical excitability of primary sensory areas.
More generally, the cross-activation model emphasizes a genetic basis for the differences between synaesthetes and non-synaesthetes. However, it cannot account for why individuals with a similar genetic composition sometimes have different forms of synaesthesia [63] and why there are individual differences in onset age for synaesthesia [65]. Furthermore, if the connections between regions are pruned during development, synaesthetic associations cannot be acquired without substantial reorganization. However, individuals with synaesthesia can quickly develop (in as little as 10 min) new inducer–concurrent associations in adulthood [66,88]. Furthermore, research indicates that mood, even within the normal range, affects synaesthetic colours experienced by grapheme–colour synaesthetes [89]. Modulations of the synaesthetic experiences such as changes in the concurrent colour in grapheme–colour synaesthesia or reduction of the number of inducers over time have been self-reported by synaesthetes [2,90–92]. The cross-activation theory and the disinhibited feedback model cannot explain why only some stimuli in the inducer modality produce a concurrent sensation but others do not. They fail to explain how new associations develop or are altered during adulthood. These models do not predict the developmental trajectory of associations—that they are inconsistent when first acquired (during development or adulthood) and become consistent over time [65,66,93].
Lastly, these models cannot account for induced synaesthesia in healthy adults by increased cortical excitability. For example, increasing sensorimotor excitability of non-synaesthetes using transcranial direct current stimulation (tDCS) results in behavioural patterns that mimic the characteristics of mirror-touch synaesthesia [55]. Indeed, both the cross-activation model and disinhibited feedback model cannot explain how synaesthesia can be caused by changes in cortical excitability (if connections are already pruned or if hyperexcitability is induced in earlier processing areas than the multisensory nexus).
10. The stochastic resonance model of synaesthesia
A neural model of synaesthesia should be able to explain how synaesthetic experiences arise (developmentally and through acquisition or induction). Additionally, as synaesthetes demonstrate numerous other trait-like differences, a holistic model of this phenomenon should account for these broader phenotypical differences. Considering the evidence reviewed, we propose a new model of synaesthesia, suggesting that a simple change in levels of neural noise in the sensory systems can lead to the experience of synaesthesia (both acquired and developmental forms), as well as produce a broader phenotype of anatomical, functional and perceptual changes seen in synaesthetes.
While engineers often focus on improving mechanical systems by noise-reduction and filtration, biologists, especially neuroscientists, have discovered the presence and importance of noise in the neural system [94–96]. One such critical role of neural noise, counterintuitively, is to increase the quality of signal transmission or detection [20]. This improvement in signal detection is achieved through the principles of stochastic resonance. Stochastic resonance is a phenomenon popularly studied in physics, in which an increase in the input noise can result in an improvement in the output signal-to-noise ratio [21]. Thus, noise makes a system more excitable through stochastic resonance [97] and stochastic resonance can be broadly defined as ‘noise benefits’ in the context of neural systems (see [98] for review and figure 1 for a simple graphical representation). In one specific application of stochastic resonance, adding noise can be beneficial to attain synchronization among coupled excitable systems; this is also known as stochastic synchronization [97,99–103].
Figure 1.
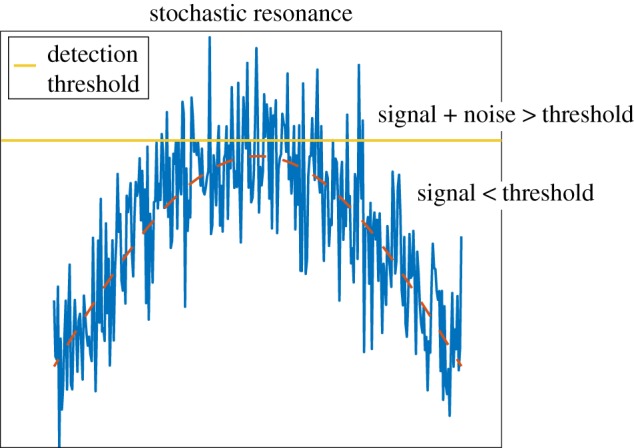
Graphical representation of stochastic resonance. The red dashed line shows a signal in the absence of noise, the blue line this signal in the presence of noise and the horizontal orange line the detection threshold. In isolation, the signal remains below detection threshold, but with the addition of noise it surpasses this threshold. (Online version in colour.)
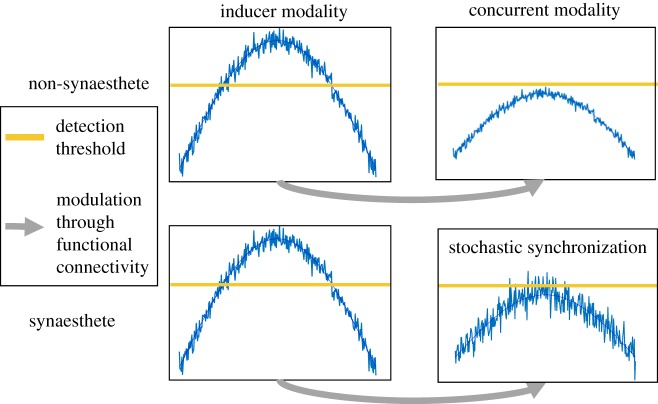
Here, we propose a neural model of synaesthesia based on the principles of stochastic resonance and synchronization (figure 2). Previous models of synaesthesia suggest altered connectivity between brain regions as an explanation for synaesthetic experiences, while largely ignoring the broader perceptual changes. By contrast, the stochastic resonance model suggests that the phenotype of synaesthesia primarily arises due to higher levels of noise in the concurrent modality (figures 3 and 4). Normal activity sent along pre-existing multisensory connections by the inducer hyperactivates the concurrent modality through stochastic resonance, leading to the additional sensory experience in the concurrent sensory system.
Figure 2.
Graphical representation of how stochastic resonance leads to synaesthesia. The blue line shows a signal in the presence of noise, the horizontal orange line the detection threshold, and the grey arrow modulatory functional connections present in synaesthetes and non-synaesthetes alike. Increased noise in the concurrent modality of synaesthetes leads to stochastic synchronization between the inducer and the concurrent. Thus, the sub-threshold modulatory effects breach detection thresholds, leading to synaesthetic experiences. (Online version in colour.)
Figure 3.
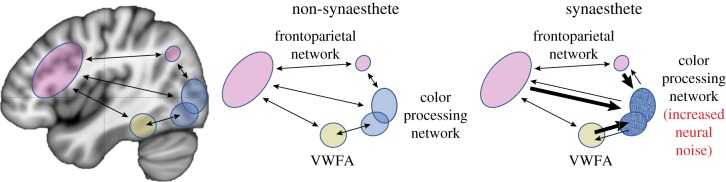
A comparison between non-synaesthetes and grapheme–colour synaesthetes based on the stochastic resonance model. Synaesthetes have higher levels of noise in the colour processing network, which leads to stochastic synchronization between the VWFA and colour processing network. The colour processing areas also have greater cortical excitability and increased connectivity with other frontoparietal areas in non-synaesthetes. (Online version in colour.)
Figure 4.
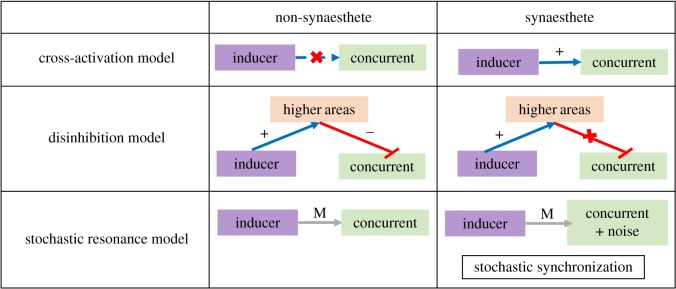
A comparison between non-synaesthetes and synaesthetes according to the cross-activation model, disinhibited feedback model and the stochastic resonance model. ‘M’ indicates modulatory effects, symbol ‘+’ indicates excitatory input, and symbol ‘−’ represents inhibitory input. According to the cross-activation model, synaesthetes have additional connections between the inducer and concurrent, while the disinhibited feedback model suggests the absence of inhibitory signal from higher areas to the concurrent in synaesthetes. The stochastic resonance model suggests that synaesthetes have higher levels of noise in the concurrent, which leads to stochastic synchronization between the inducer and the concurrent. (Online version in colour.)
This model argues that the major difference between synaesthetes and non-synaesthetes is noise levels in the concurrent modality and not the neuroanatomical connections between the inducer and the concurrent. Specifically, as anatomical connections linking the sensory systems are present and functionally important in all individuals [71,72] (see §8 for detailed discussion), one sense can modulate activity in a second [74,75,83,84]. However, these cross-sensory signals are typically only modulatory, such that a sound will change the excitability of visual neurons but will not cause visual neurons to generate action potentials [74,81,104], explaining why these cross-sensory modulations do not tend to reach perceptual awareness in non-synaesthetes. However, in the presence of higher sensory noise in synaesthetes (e.g. increased noise in visual areas), these cross-sensory signals could be sufficient to produce supra-threshold activity in the concurrent modality owing to stochastic resonance, leading to the experience of synaesthesia. Thus, this model predicts that the associations one experiences in synaesthesia initially change each time synaesthesia is evoked, based on the current state of the concurrent modality. However, with repeated and consistent evocation of these supra-threshold experiences, the synaesthetic associations would be predicted to stabilize over time. For example, a grapheme–colour synaesthete early in development will have a synaesthetic experience of different colours on every encounter with the number 2 and only after a significant time would the association between 2 and a specific hue become consistent. Indeed, this account is consistent with the wealth of evidence indicating that synaesthetic associations are based on experiences in the environment and are refined over several years [65].
The SRS is based on four main assumptions: (1) synaesthesia occurs through common pathways that are present and functionally important in all individuals, (2) synaesthetic associations are unpredictable at onset and stabilize over time, (3) neural noise is higher in the concurrent modality in synaesthetes than non-synaesthetes, and (4) the broader phenotype of cognitive, perceptual, anatomical and functional differences in synaesthetes occurs in response to the combined presence of increased neural noise and the resulting years of experiencing synaesthesia. Here, we review evidence based on the current literature supporting this model.
11. Evidence supporting the stochastic resonance model
The first assumption of this model is that synaesthesia occurs through pathways that are present and functionally relevant in all individuals. This assumption is consistent with the observation made by several other researchers [105–110] that synaesthesia may be an extension of typical multisensory processes. In addition to physiology research supporting the presence of these connections (also reviewed in §8), additional support comes from the ability of certain drugs like LSD to induce synaesthesia in non-synaesthetic adults in a transient fashion [14]. Human neuroimaging research shows increased resting state activity in the visual cortex following LSD induction compared with placebo control [111,112], indicating that psychedelic drugs may give rise to synaesthetic experiences by increasing the basal neural activity in the visual cortex, which in turn could lead to stochastic resonance between the inducer and the visual cortex.
If cross-sensory anatomical connections are present and functionally relevant in the general population, then why are not all individuals synaesthetes? According to the stochastic resonance model of synaesthesia, modulations of the concurrent modality by the inducer are not strong enough to breach thresholds and reach awareness in normal settings. Functionally, this might be because bottom-up sensory input into the concurrent modality is several times stronger than the modulatory effects of the inducer. Thus, the proclivity to override any signal from the inducer makes detection and stabilization of synaesthesia impossible in non-synaesthetes in most natural contexts. However, non-synaesthetes can detect these modulations when this sensory input is removed, such as under sensory deprivation [113] and in special laboratory settings. Several studies have shown that sounds modulate visual cortex [73,74,81,84] in non-synaesthetes. For example, light flashes that are coupled with two tones are perceived as two flashes instead of one [114]. Similarly, auditory stimulation can lead to the perception of a second phosphene during stimulation of the occipital lobe [115]. Furthermore, sensory deprivation caused by either short-term eye-closure or traumatic injury can lead to even the induction of auditory–visual synaesthesias [86,87]. Therefore, if the synaesthetic experiences are simply a consequence of the modulations reaching perceptual awareness (through stochastic resonance), then amplifying the signal in the concurrent modality should increase the perception of synaesthetic experiences. Animal research shows that drugs like LSD do exactly this by altering noise levels in the visual cortex [116]. Drug-induced synaesthetic experiences thus might be a consequence of inducer modulations reaching awareness owing to altered noise in the concurrent modality.
The strength of the SRS is that it can explain developmental and acquired/induced synaesthesias in the same framework. The main argument against acquired synaesthesia being genuine is its inconsistent nature [15]. The SRS rather highlights this aspect and emphasizes that synaesthetic associations are not fixed at onset and stabilize over the course of development in all forms of synaesthesia. These assumptions are supported by Simner & Bain [65], showing that children aged 6–7 are limited to 34% consistent associations on average (e.g. around 12 letters and numbers) and the longitudinal tracking demonstrates that the number of consistent associations increases with age to 48% by age 7–8 and 71% by age 10–11. Interestingly, some children lose their synaesthetic experiences during this period, further highlighting the idiosyncratic nature of synaesthetic associations during development. The associations not only are inconsistent during childhood but also are inconsistent in adult synaesthetes during the formation of new associations. For example, when adults learn a new language, synaesthetic associations that emerge may initially be inconsistent within the first session, but typically become consistent over time [66].
The SRS does not explicitly predict which associations a synaesthete acquires or how they stabilize over time. However, we hypothesize that repeated exposure and usefulness of the associations will be key factors in consolidating these associations. Specifically, repeated supra-threshold activation of the concurrent modality through stochastic resonance may increase the strength of connections between inducer and concurrent through Hebbian learning. Partial support comes from research demonstrating that synaesthesia can be trained [56]. During training, the repeated exposure not only allowed the associations to stabilize, but also resulted in individuals experiencing synaesthetic qualia. Similarly, studies suggest that many associations in synaesthesia are based on learned statistical regularities in the environment [117]. This is supported by studies showing that at a population level, non-synaesthetes have similar associations to synaesthetes [118]. That is, individuals implicitly identify and use statistical regularities in the environment. Finally, research additionally indicates that synaesthetic associations may be useful during learning for synaesthetes [119,120], adding ecological validity to why the associations stabilize.
The final and most important assumption of the SRS is the presence of increased neural noise in the concurrent modality of synaesthetes. This requirement for the model is indirectly supported by research showing that (a) synaesthetes have altered processing and excitability in the concurrent modality, (b) the concurrent shows altered neural connectivity, (c) the inducing modality appears anatomically and functionally normal, and (d) altering noise levels in sensory modalities can induce synaesthesia. In terms of giving rise to synaesthetic associations, increased noise levels in the concurrent modality of synaesthetes should lead to altered processing specifically within the concurrent even in the absence of the inducer. As discussed in §5, grapheme–colour synaesthetes have enhanced visual imagery and normal non-visual imagery [54]. Similarly, grapheme–colour synaesthetes have enhanced colour processing but normal tactile sensitivity, while mirror-touch synaesthetes have higher tactile sensitivity but normal colour perception [48]. Furthermore, research shows that grapheme–colour synaesthetes have early perceptual differences compared with non-synaesthetes in visual processing [19,57,58].
Older animal research in primates and achromatopsia patients suggested that area V4 is the primary colour processing region in the cortex [121–130]. However, a growing body of the literature in animals and humans suggests that colour processing is a broadly distributed process, not restricted to V4, and that colour-selective neurons are present in as early as primary visual cortex [131–142]. Similarly, V4 is involved in more than just colour processing, including the processing of three-dimensional shapes, target comparison, object orientation and motion [143–147]. Thus, we hypothesize that differences between grapheme–colour synaesthetes and non-synaesthetes are present in the broad network of visual areas that process colour information. This hypothesis is supported by research showing that grapheme–colour synaesthetes have greater cortical excitability compared with non-synaesthetes in three different sites in the visual cortex, but do not differ in their motor cortex excitability [52]. Additionally, perceptual differences between grapheme–colour synaesthetes and non-synaesthetes are present in early visual processing [19]. Furthermore, neuroimaging studies suggest that activation differences in synaesthetes and non-synaesthetes are not limited to V4 but are also seen in adjacent regions [8,148]. However, perceptual differences in synaesthetes are not a general feature of the synaesthetic brain but are specific to the concurrent modality (for example, visual, but not auditory, differences in the case of grapheme–colour synaesthesia). The SRS explains the broader phenotype of perceptual differences in synaesthetes through higher noise levels in the concurrent modality. Specifically, perceptual sensitivity is strongly associated with cortical excitability as measured using transcranial magnetic stimulation (TMS)/tDCS [149–151], which indicates a link between cortical excitability and perceptual sensitivity as seen in synaesthetes.
Recent research shows that individuals with autism spectrum disorder (ASD) and synaesthesia share an atypical profile of increased sensory sensitivity [152,153]. Thus, the sensory processing deficits seen in individuals with ASD or Asperger syndrome may explain the observation of a higher incidence of synaesthesia in this population [154–157]. Autism has been associated with an imbalance in excitatory/inhibitory levels in the sensory systems and the social–emotional system [158–162], including altered levels and action of inhibitory gamma-aminobutyric acid (GABA) (see [163] for review). Similarly, autism research also shows that neural variability/neural noise is higher in adults with ASD than in healthy adults [158,162,164]. One could speculate that in the SRS framework, both conditions are due, in part, to the increased neural noise, but differ in terms of where these differences are observed: in autism, there may be a more broadly distributed increase in noise levels, including emotional, limbic and occasionally sensory areas (that in turn cause synaesthesia to arise in such cases), while they are focal and only in sensory regions in synaesthesia. Since the balance of excitatory/inhibitory neurotransmitters controls noise levels in the brain [165,166], a greater understanding of how this balance is altered in both autism and synaesthesia may help clarify the observed link between the two conditions.
The maintenance of neural noise levels in a neural network is modulated by the balance of excitatory and inhibitory neurotransmitter levels [165,166]. Magnetic resonance spectroscopy-based estimation of major inhibitory neurotransmitter GABA and excitatory neurotransmitter glutamate suggests that there is reduced GABAergic action but conserved glutamatergic action in the visual cortex of individuals with ASD compared with healthy controls. This reduction of GABAergic levels affects perceptual suppression in the visual cortex in autism [167]. Similarly, the GABRB3 gene, which codes for an important GABA receptor, has been associated with autism and increased tactile sensitivity [168]. GABA levels have also been associated with increased tactile sensitivity as well as visual discrimination ability in a region-specific manner [169,170]. Furthermore, GABA levels are region-specific even in healthy human adults [171,172]. Thus, altered neurotransmitter levels in the concurrent modality can explain the region-specificity (i.e. changes restricted to the concurrent modality) of increased neural noise in synaesthesia.
Altered noise levels in the concurrent modality may also explain observed patterns of altered connectivity in synaesthetes, particularly between the concurrent and connected brain regions. Higher noise levels may lead to repetitive co-activation of the concurrent in the presence of an inducer, leading to increased connectivity between them. Indeed, in epilepsy, which most often occurs owing to hyperexcitable neurons in the medial temporal lobe, even though the seizure begins in a small cluster of neurons, the increased responsivity of this area dramatically alters patterns of connectivity throughout the brain [173–175]. Additional research will be needed to confirm the extent to which this model explains and predicts elements of the synaesthesia phenotype. In synaesthetes, this might be observed as hyper-connectivity between the inducer and concurrent, which is the premise of the cross-activation model. However, according to this model, while increased pathway strength between two modalities can enhance synaesthetic associations, they are not necessary for the development of synaesthesia. This is supported by longitudinal researching showing that neural connectivity between the auditory cortex and somatosensory cortex increased in a synaesthete who acquired sound–touch synaesthesia following a stroke [176]. Similarly, decreased pruning between inducer and concurrent will preserve the connectivity between the two, allowing easier modulation of the concurrent through the inducer via stochastic resonance. In this regard, the SRS is not completely incompatible with the cross-activation model, as it is possible that synaesthesia could emerge from either cause (increased connectivity or increased neural noise). However, we predict that synaesthetes who develop synaesthesia through increased connectivity and not neural noise will have a different phenotype (e.g. no perceptual or excitability changes). Further research should investigate the neural changes in development of both acquired and developmental synaesthesia to explore these possibilities.
If the synaesthetic experiences are guided mainly by the inducer–concurrent stochastic resonance, then how can semantic information or top-down influences lead to differences in synaesthetic experiences [45,177]? Sensory processing at both cortical and thalamic levels is strongly modulated by top-down influences [178–180] (for example, semantic priming can alter visual perception [181]). Interestingly, colour information has also been shown to invoke semantic networks in the brain [182] and semantic networks have been shown to invoke early colour perception [183,184]. Thus, in the SRS model framework, semantic effects observed in synaesthesia are a byproduct of modulatory effects on sensory perception and are not unique to synaesthesia. However, there might be differences in the strength of these modulations between non-synaesthetes and synaesthetes owing to altered connectivity between the concurrent and frontoparietal regions [31,38].
Along with altered connectivity with the inducer, the noisier concurrent also has altered connectivity with other brain regions. For example, in grapheme–colour synaesthetes, the colour processing regions (including primary visual (V1) and V4) might be hyperconnected to other connected regions. This is consistent with research showing activity in neighbouring brain regions other than V4, including texture areas in grapheme–colour synaesthesia [8,148]. Neural noise levels in the early stages of synaesthesia might determine the strength of these connections as well as where they project. Visual cortex will thus have differences in its connectivity to other brain regions that it is normally connected with. This may explain the frontal and parietal connectivity differences observed in synaesthetes [31,38]. Thus, we suggest that the neural connectivity differences in synaesthetes will be more global than merely being restricted to the inducer–concurrent pair (figure 3).
The SRS suggests that the difference between synaesthetes is in the concurrent modality and, therefore, there is nothing unique about the inducer. This is consistent with population-based clustering studies showing that synaesthetes often have more than one form of synaesthesia with a common concurrent (but not always) [185]. Indeed, monozygotic twins may have distinct forms of synaesthesia with different inducers, but will generally share the same concurrent modality [63]. These results demonstrate that during development there is nothing unique about the inducer modality. In this framework, increased neural noise in the inducer modality is not required for development of synaesthesia but might aid stochastic synchronization between inducer and concurrent.
Most of the evidence presented supporting a model of altered neural noise in synaesthetes is indirect. Future research should explicitly test this prediction directly, especially during the onset of synaesthesia. In the next section, we suggest studies that can be used to examine the model's limits as well as future directions for expanding this framework.
12. Future directions
The stochastic resonance model of synaesthesia makes several predictions that can readily be tested. Here, we mention a few:
-
1.
It predicts differences between synaesthetes and non-synaesthetes in terms of their neural noise. Noise measures, such as EEG entropy, can be used to measure variability in neural activity to compare synaesthetes and non-synaesthetes.
-
2.
It suggests that the type of synaesthesia that develops should be based on (a) experiences during development and (b) the sensory modality with an increased level of noise. For example, if one has excessive noise in perceptual colour areas and extensive exposure to musical training early in life then one should be more likely to develop auditory–visual synaesthesia. Similarly, populations more immersed in the use of tonal languages like Chinese should have a greater prevalence of tone–colour synaesthesia. This seems likely since absolute pitch is more prevalent in tonal languages [186,187] and synaesthesia is more prevalent in individuals with absolute pitch [188].
-
3.
There is no critical period to acquire synaesthesia in the SRS framework. However, as neural noise levels are higher during development [189,190], it should be easier to acquire synaesthesia during development. As neural noise levels decrease and stabilize during adolescence, synaesthesia may be lost for some individuals. Indeed, beneficial noise declines in older adults, consistent with reports that synaesthesia weakens or is lost late in life. Conversely, the induction of synaesthesia in adulthood is expected to require external pressure in the form of drugs that increase neural noise in sensory areas, sensory deprivation or potentially extensive training. Longitudinal tracking of synaesthetes in terms of their synaesthetic experiences and associations along with neural measures of noise can verify each of these predictions.
-
4.
Enhanced plasticity present during development can enable neural noise differences in the concurrent modality to produce broader phenotypical differences in synaesthetes. Thus, synaesthetes who acquire synaesthesia earlier in life (when there is greater plasticity) will exhibit more brain-wide differences (global connectivity changes), while those who acquire synaesthesia later will have more restricted differences (largely in the concurrent modality).
-
5.
One way to measure neural noise in the concurrent is to quantify its excitability. Our model suggests that synaesthetes should have increased excitability in the specific concurrent modality and not necessarily other brain regions. It is possible that the expression of synaesthesia (projector versus associator) or the number of synaesthesias one experiences is associated positively with the degree of excitability in a region-specific manner.
-
6.
In developmental synaesthetes, any area that has high input to the concurrent modality should have increased connectivity with the concurrent (owing to stochastic resonance; figure 3). Thus, in synaesthetes, altered connectivity should be seen not only between concurrent and inducer, but also with other brain regions.
-
7.
Cortical excitability, neural noise and excitation/inhibition balance differences should be measurable in synaesthetes even before the onset of synaesthetic experiences. Thus, we should be able to use cortical excitability or neurotransmitter levels as a predictor for whether children of synaesthetes will themselves develop synaesthesia.
-
8.
Similarly, certain drugs induce specific cortical changes (e.g. LSD alters levels of neural noise and connectivity in visual cortex [111,116]). Thus, one should be able to track drug-induced cortical excitability changes in a modality-specific manner to predict the onset and type of synaesthesia induced.
Although synaesthesia is genetic, it only affects the propensity to develop synaesthesia. For example, sometimes only one of a monozygotic pair of twins develops synaesthesia [63]. According to the SRS, the form of synaesthesia and the associations acquired will be determined by environmental factors. However, further research should help develop the exact genetic and molecular mechanisms that alter neural noise and in turn predispose individuals to synaesthesia. Although the cross-activation model can determine the predisposition of an individual to acquire synaesthesia, it cannot predict when (the onset age) an individual will develop synaesthesia or how new associations will develop; future studies, like those mentioned above, will further refine the SRS to address critical issues such as these. Importantly, as recent studies have failed to replicate the findings of concurrent activation and connectivity differences in synaesthetes [8–10], future research should incorporate recent advances in our understanding of normal colour processing to further promote the development of better models of synaesthesia.
Computational models of synaesthesia may advance our understanding of how associations develop between the inducer and the concurrent with stochastic resonance. For example, a recent computational model proposed by Shriki and colleagues demonstrated that synaesthesia can emerge in a neuronal network through changes in neuronal plasticity and sensitivity [191]. This model places developmental and acquired synaesthesias within the same framework, and accounts for increased cortical excitability in synaesthetes compared with non-synaesthetes as well as a monotonic mapping between inducer and concurrent. The SRS partly overlaps with this computational model, and the latter could be extended to test whether synaesthesia is predicted to emerge owing to greater noise in the concurrent modality (as our model predicts). In their current form, the two models make different predictions that can be addressed in future studies: (a) the computational model suggests that the connectivity (cross-talk in their language) between inducer and concurrent modalities is zero in non-synaesthetes, whereas the SRS model predicts that there is a continuum linking synaesthetes and non-synaesthetes, wherein the inducer and concurrent are connected and exhibit sub-threshold cross-talk in non-synaesthetes; (b) the simple computational model, unlike the SRS, is limited to the inducer and concurrent modalities and does not predict brain-wide differences, early perceptual differences between synaesthetes and non-synaesthetes or explain top-down influences on synaesthetic experiences; (c) the SRS, unlike the computational model, does not implicitly require plasticity differences (in duration or amount) during childhood in developmental synaesthesia.
Most of the evidence presented in this article is based on grapheme–colour synaesthesia and does not distinguish between projectors and associators. It would be imprudent to assume the validity of the SRS for different expressions and forms of synaesthesia without further testing. Indeed, it is possible that more than one mechanism underlies different forms of synaesthesia. Similarly, most previous models of synaesthesia, including the SRS, are limited in their ability to explain only the percept in the concurrent but not its non-veridicality (knowledge or awareness that the concurrent percept is not real). The predictive processing theory of sensorimotor contingencies provides one framework that can account for the presence of a percept and yet the absence of veridicality in synaesthesia [192]. Finally, the SRS can be combined with other models and theories to explain different aspects of synaesthesia, such as how associations become consistent through the role of the environment, the non-veridicality of the synaesthetic percepts, and why individuals differ in their experiences (projector versus associator).
13. Conclusion
The stochastic resonance model of synaesthesia can be thoroughly validated with future studies as suggested in the previous section. The model suggests that the various perceptual differences in synaesthetes are not a simple consequence of cross-sensory experiences but rather a part of the broader phenotype of synaesthesia. We hypothesize that these perceptual differences might provide a better marker of synaesthesia than the conventional correlates such as consistency (which we argue is not a requirement for synaesthetic experiences). The SRS also does not discriminate between types of synaesthesia—induced versus developmental synaesthesia—in terms of providing a neurological basis. The stochastic resonance model of synaesthesia incorporates several of the recent findings from research on the perceptual systems and neuroanatomical connectivity, and addresses limitations in older models to provide a better theoretical framework to understanding the neurological basis of synaesthesia and neural noise in general.
Acknowledgements
The authors thank Dr John Plass for valuable comments on the previous draft.
Data accessibility
This article has no additional data.
Authors' contributions
P.L. and D.B. jointly designed the model, and drafted the manuscript. Both authors gave final approval for publication and agree to be held accountable for the work performed herein.
Competing interests
The authors declare no competing financial interests.
Funding
This study was supported by NIH grant no. R00 DC013828.
References
- 1.Baron-Cohen S, Burt L, Smith-Laittan F, Harrison J, Bolton P. 1996. Synaesthesia: prevalence and familiality. Perception 25, 1073–1079. ( 10.1068/p251073) [DOI] [PubMed] [Google Scholar]
- 2.Cytowic RE. 2002. Synesthesia: a union of the senses. New York, NY: MIT press. [Google Scholar]
- 3.Hubbard EM, Ramachandran VS. 2005. Neurocognitive mechanisms of synesthesia. Neuron 48, 509–520. ( 10.1016/j.neuron.2005.10.012) [DOI] [PubMed] [Google Scholar]
- 4.Simner J, Mulvenna C, Sagiv N, Tsakanikos E, Witherby SA, Fraser C, Scott K, Ward J. 2006. Synaesthesia: the prevalence of atypical cross-modal experiences. Perception 35, 1024–1033. ( 10.1068/p5469) [DOI] [PubMed] [Google Scholar]
- 5.Sperling JM, Prvulovic D, Linden DE, Singer W, Stirn A. 2006. Neuronal correlates of colour-graphemic synaesthesia: AfMRI study. Cortex 42, 295–303. ( 10.1016/S0010-9452(08)70355-1) [DOI] [PubMed] [Google Scholar]
- 6.Brang D, Hubbard EM, Coulson S, Huang M, Ramachandran VS. 2010. Magnetoencephalography reveals early activation of V4 in grapheme-color synesthesia. Neuroimage 53, 268–274. ( 10.1016/j.neuroimage.2010.06.008) [DOI] [PubMed] [Google Scholar]
- 7.Nunn JA, et al. 2002. Functional magnetic resonance imaging of synesthesia: activation of V4/V8 by spoken words. Nat. Neurosci. 5, 371–375. ( 10.1038/nn818) [DOI] [PubMed] [Google Scholar]
- 8.Hupé J-M, Bordier C, Dojat M. 2012. The neural bases of grapheme–color synesthesia are not localized in real color-sensitive areas. Cereb. Cortex. 22, 1622–1633. ( 10.1093/cercor/bhr236) [DOI] [PubMed] [Google Scholar]
- 9.Hupé J-M, Dojat M. 2015. A critical review of the neuroimaging literature on synesthesia. Front. Hum. Neurosci. 9, 103 ( 10.3389/fnhum.2015.00103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dojat M, Pizzagalli F, Hupé J-M. 2018. Magnetic resonance imaging does not reveal structural alterations in the brain of grapheme-color synesthetes. PLoS ONE 13, e0194422 ( 10.1371/journal.pone.0194422) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grossenbacher PG, Lovelace CT. 2001. Mechanisms of synesthesia: cognitive and physiological constraints. Trends Cogn. Sci. 5, 36–41. ( 10.1016/S1364-6613(00)01571-0) [DOI] [PubMed] [Google Scholar]
- 12.Simner J. 2012. Defining synaesthesia: a response to two excellent commentaries. Br. J. Psychol. 103, 24–27. ( 10.1111/j.2044-8295.2011.02059.x) [DOI] [PubMed] [Google Scholar]
- 13.Cohen Kadosh R, Terhune DB. 2012. Redefining synaesthesia? Br. J. Psychol. 103, 20–23. ( 10.1111/j.2044-8295.2010.02003.x) [DOI] [PubMed] [Google Scholar]
- 14.Simner J. 2012. Defining synaesthesia. Br. J. Psychol. 103, 1–15. ( 10.1348/000712610X528305) [DOI] [PubMed] [Google Scholar]
- 15.Sinke C, Halpern JH, Zedler M, Neufeld J, Emrich HM, Passie T. 2012. Genuine and drug-induced synesthesia: a comparison. Conscious. Cogn. 21, 1419–1434. ( 10.1016/j.concog.2012.03.009) [DOI] [PubMed] [Google Scholar]
- 16.Luke DP, Terhune DB. 2013. The induction of synaesthesia with chemical agents: a systematic review. Front. Psychol. 4, 753 ( 10.3389/fpsyg.2013.00753) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Terhune DB, Tai S, Cowey A, Popescu T, Cohen Kadosh R. 2011. Enhanced cortical excitability in grapheme-color synesthesia and its modulation. Curr. Biol. 21, 2006–2009. ( 10.1016/j.cub.2011.10.032) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barnett KJ, Newell FN. 2008. Synaesthesia is associated with enhanced, self-rated visual imagery. Conscious. Cogn. 17, 1032–1039. ( 10.1016/j.concog.2007.05.011) [DOI] [PubMed] [Google Scholar]
- 19.Barnett KJ, Foxe JJ, Molholm S, Kelly SP, Shalgi S, Mitchell KJ, Newell FN. 2008. Differences in early sensory-perceptual processing in synesthesia: a visual evoked potential study. Neuroimage 43, 605–613. ( 10.1016/j.neuroimage.2008.07.028) [DOI] [PubMed] [Google Scholar]
- 20.Wiesenfeld K, Moss F. 1995. Stochastic resonance and the benefits of noise: from ice ages to crayfish and SQUIDs. Nature 373, 33–36. ( 10.1038/373033a0) [DOI] [PubMed] [Google Scholar]
- 21.McNamara B, Wiesenfeld K. 1989. Theory of stochastic resonance. Phys. Rev. A 39, 4854–4869. ( 10.1103/PhysRevA.39.4854) [DOI] [PubMed] [Google Scholar]
- 22.Tomson SN, Narayan M, Allen GI, Eagleman DM. 2013. Neural networks of colored sequence synesthesia. J. Neurosci. 33, 14 098–14 106. ( 10.1523/JNEUROSCI.5131-12.2013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chan JS, van den Bosch JJF, Theves S, Hardt S, Pflanz P, Lötsch J, Kaiser J, Naumer MJ. 2014. Synaesthesia or vivid imagery? A single case fMRI study of visually induced olfactory perception . Multisensory Res. 27, 225–246. ( 10.1163/22134808-00002451) [DOI] [PubMed] [Google Scholar]
- 24.Jones CL, Gray MA, Minati L, Simner J, Critchley HD, Ward J. 2011. The neural basis of illusory gustatory sensations: two rare cases of lexical–gustatory synaesthesia. J. Neuropsychol. 5, 243–254. ( 10.1111/j.1748-6653.2011.02013.x) [DOI] [PubMed] [Google Scholar]
- 25.Dovern A, Fink GR, Fromme ACB, Wohlschläger AM, Weiss PH, Riedl V. 2012. Intrinsic network connectivity reflects consistency of synesthetic experiences. J. Neurosci. 32, 7614–7621. ( 10.1523/JNEUROSCI.5401-11.2012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rouw R, Scholte HS, Colizoli O. 2011. Brain areas involved in synaesthesia: a review. J. Neuropsychol. 5, 214–242. ( 10.1111/j.1748-6653.2011.02006.x) [DOI] [PubMed] [Google Scholar]
- 27.Neufeld J, Sinke C, Zedler M, Dillo W, Emrich HM, Bleich S, Szycik GR. 2012. Disinhibited feedback as a cause of synesthesia: evidence from a functional connectivity study on auditory-visual synesthetes. Neuropsychologia 50, 1471–1477. ( 10.1016/j.neuropsychologia.2012.02.032) [DOI] [PubMed] [Google Scholar]
- 28.Hubbard EM, Arman AC, Ramachandran VS, Boynton GM. 2005. Individual differences among grapheme-color synesthetes: brain-behavior correlations. Neuron 45, 975–985. ( 10.1016/j.neuron.2005.02.008) [DOI] [PubMed] [Google Scholar]
- 29.Banissy MJ, Stewart L, Muggleton NG, Griffiths TD, Walsh VY, Ward J, Kanai R. 2012. Grapheme-color and tone-color synesthesia is associated with structural brain changes in visual regions implicated in color, form, and motion. Cogn. Neurosci. 3, 29–35. ( 10.1080/17588928.2011.594499) [DOI] [PubMed] [Google Scholar]
- 30.Jäncke L, Beeli G, Eulig C, Hänggi J. 2009. The neuroanatomy of grapheme–color synesthesia. Eur. J. Neurosci. 29, 1287–1293. ( 10.1111/j.1460-9568.2009.06673.x) [DOI] [PubMed] [Google Scholar]
- 31.Rouw R, Scholte HS. 2007. Increased structural connectivity in grapheme–color synesthesia. Nat. Neurosci. 10, 792–797. ( 10.1038/nn1906) [DOI] [PubMed] [Google Scholar]
- 32.Zamm A, Schlaug G, Eagleman DM, Loui P. 2013. Pathways to seeing music: enhanced structural connectivity in colored-music synesthesia. Neuroimage 74, 359–366. ( 10.1016/j.neuroimage.2013.02.024) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rouw R, Scholte HS. 2010. Neural basis of individual differences in synesthetic experiences. J. Neurosci. 30, 6205–6213. ( 10.1523/JNEUROSCI.3444-09.2010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weiss PH, Fink GR. 2009. Grapheme-colour synaesthetes show increased grey matter volumes of parietal and fusiform cortex. Brain 132, 65–70. ( 10.1093/brain/awn304) [DOI] [PubMed] [Google Scholar]
- 35.Specht K. 2012. Synaesthesia: cross activations, high interconnectivity, and a parietal hub. Transl. Neurosci. 3, 15–21. ( 10.2478/s13380-012-0007-z) [DOI] [Google Scholar]
- 36.O'Hanlon E, Newell FN, Mitchell K. 2013. Combined structural and functional imaging reveals cortical deactivations in grapheme-color synaesthesia. Front. Psychol. 4, 755 ( 10.3389/fpsyg.2013.00755) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Melero H, Peña-Melián Á, Ríos-Lago M, Pajares G, Hernández-Tamames JA, Álvarez-Linera J. 2013. Grapheme-color synesthetes show peculiarities in their emotional brain: cortical and subcortical evidence from VBM analysis of 3D-T1 and DTI data. Exp. Brain Res. 227, 343–353. ( 10.1007/s00221-013-3514-4) [DOI] [PubMed] [Google Scholar]
- 38.Hänggi J, Wotruba D, Jäncke L. 2011. Globally altered structural brain network topology in grapheme-color synesthesia. J. Neurosci. 31, 5816–5828. ( 10.1523/JNEUROSCI.0964-10.2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rothen N, Meier B, Ward J. 2012. Enhanced memory ability: insights from synaesthesia. Neurosci. Biobehav. Rev. 36, 1952–1963. ( 10.1016/j.neubiorev.2012.05.004) [DOI] [PubMed] [Google Scholar]
- 40.Smilek D, Dixon MJ, Cudahy C, Merikle PM. 2002. Synesthetic color experiences influence memory. Psychol. Sci. 13, 548–552. ( 10.1111/1467-9280.00496) [DOI] [PubMed] [Google Scholar]
- 41.Terhune DB, Wudarczyk OA, Kochuparampil P, Cohen Kadosh R. 2013. Enhanced dimension-specific visual working memory in grapheme–color synesthesia. Cognition 129, 123–137. ( 10.1016/j.cognition.2013.06.009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brang D, Miller LE, McQuire M, Ramachandran VS, Coulson S. 2013. Enhanced mental rotation ability in time-space synesthesia. Cogn. Process. 14, 429–434. ( 10.1007/s10339-013-0561-5) [DOI] [PubMed] [Google Scholar]
- 43.Simner J, Mayo N, Spiller M-J. 2009. A foundation for savantism? Visuo-spatial synaesthetes present with cognitive benefits. Cortex 45, 1246–1260. ( 10.1016/j.cortex.2009.07.007) [DOI] [PubMed] [Google Scholar]
- 44.Brang D, Teuscher U, Ramachandran VS, Coulson S. 2010. Temporal sequences, synesthetic mappings, and cultural biases: the geography of time. Conscious. Cogn. 19, 311–320. ( 10.1016/j.concog.2010.01.003) [DOI] [PubMed] [Google Scholar]
- 45.Ramachandran VS, Hubbard EM. 2001. Synaesthesia – a window into perception, thought and language. J. Conscious. Stud. 8, 3–34. [Google Scholar]
- 46.Yaro C, Ward J. 2007. Searching for Shereshevskii: what is superior about the memory of synaesthetes? Q. J. Exp. Psychol. 60, 681–695. ( 10.1080/17470210600785208) [DOI] [PubMed] [Google Scholar]
- 47.Banissy MJ, Tester V, Muggleton NG, Janik AB, Davenport A, Franklin A, Walsh V, Ward J. 2013. Synesthesia for color is linked to improved color perception but reduced motion perception. Psychol. Sci. 24, 2390–2397. ( 10.1177/0956797613492424) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Banissy MJ, Walsh V, Ward J. 2009. Enhanced sensory perception in synaesthesia. Exp. Brain Res. 196, 565–571. ( 10.1007/s00221-009-1888-0) [DOI] [PubMed] [Google Scholar]
- 49.Brang D, Ramachandran VS. 2010. Visual field heterogeneity, laterality, and eidetic imagery in synesthesia. Neurocase 16, 169–174. ( 10.1080/13554790903339645) [DOI] [PubMed] [Google Scholar]
- 50.Chun CA, Hupé J-M. 2016. Are synesthetes exceptional beyond their synesthetic associations? A systematic comparison of creativity, personality, cognition, and mental imagery in synesthetes and controls. Br. J. Psychol. 107, 397–418. ( 10.1111/bjop.12146) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brang D, Ahn E. 2019. Double-blind study of visual imagery in grapheme-color synesthesia. Cortex 117, 89–95. ( 10.1016/j.cortex.2019.02.025) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Terhune DB, Song SM, Cohen Kadosh R. 2015. Transcranial alternating current stimulation reveals atypical 40 Hz phosphene thresholds in synaesthesia. Cortex 63, 267–270. ( 10.1016/j.cortex.2014.09.006) [DOI] [PubMed] [Google Scholar]
- 53.Blake R, Palmeri TJ, Marois R, Kim C-Y. 2005. On the perceptual reality of synesthetic color. In Synesthesia: perspectives from cognitive neuroscience (eds LC Robertson, N Sagiv), pp. 47–73. New York, NY: Oxford University Press. [Google Scholar]
- 54.Spiller MJ, Jonas CN, Simner J, Jansari A. 2015. Beyond visual imagery: how modality-specific is enhanced mental imagery in synesthesia? Conscious. Cogn. 31, 73–85. ( 10.1016/j.concog.2014.10.010) [DOI] [PubMed] [Google Scholar]
- 55.Bolognini N, Miniussi C, Gallo S, Vallar G. 2013. Induction of mirror-touch synaesthesia by increasing somatosensory cortical excitability. Curr. Biol. 23, R436–R437. ( 10.1016/j.cub.2013.03.036) [DOI] [PubMed] [Google Scholar]
- 56.Bor D, Rothen N, Schwartzman DJ, Clayton S, Seth AK. 2014. Adults can be trained to acquire synesthetic experiences. Scient. Rep. 4, 7089 ( 10.1038/srep07089) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Brang D, Edwards L, Ramachandran VS, Coulson S. 2008. Is the Sky 2? Contextual priming in grapheme-color synaesthesia. Psychol. Sci. 19, 421–428. ( 10.1111/j.1467-9280.2008.02103.x) [DOI] [PubMed] [Google Scholar]
- 58.Brang D, Kanai S, Ramachandran VS, Coulson S. 2010. Contextual priming in grapheme–color synesthetes and yoked controls: 400 msec in the life of a synesthete. J. Cogn. Neurosci. 23, 1681–1696. ( 10.1162/jocn.2010.21486) [DOI] [PubMed] [Google Scholar]
- 59.van Leeuwen TM, den Ouden HE, Hagoort P. 2011. Effective connectivity determines the nature of subjective experience in grapheme-color synesthesia. J. Neurosci. 31, 9879–9884. ( 10.1523/JNEUROSCI.0569-11.2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hubbard EM, Brang D, Ramachandran VS. 2011. The cross-activation theory at 10. J. Neuropsychol. 5, 152–177. ( 10.1111/j.1748-6653.2011.02014.x) [DOI] [PubMed] [Google Scholar]
- 61.Maurer D, Gibson L, Spector F, Bremner A, Lewkowicz D, Spence C. 2012. Infant synaesthesia: new insights into the development of multisensory perception. In Multisensory development (eds AJ Bremner, DJ Lewkowicz, C Spence), pp. 229–250. Oxford, UK: Oxford University Press. [Google Scholar]
- 62.Maurer D. 1993. Neonatal synesthesia: implications for the processing of speech and faces. In Developmental neurocognition: speech and face processing in the first year of life (eds B de Boysson-Bardies, PJ Scania de Schonen, P McNeilage, J Morton), pp. 109–124. Berlin, Germany: Springer. [Google Scholar]
- 63.Bosley HG, Eagleman DM. 2015. Synesthesia in twins: incomplete concordance in monozygotes suggests extragenic factors. Behav. Brain Res. 286, 93–96. ( 10.1016/j.bbr.2015.02.024) [DOI] [PubMed] [Google Scholar]
- 64.Tilot AK, Kucera KS, Vino A, Asher JE, Baron-Cohen S, Fisher SE. 2018. Rare variants in axonogenesis genes connect three families with sound–color synesthesia. Proc. Natl Acad. Sci. USA 115, 3168–3173. ( 10.1073/pnas.1715492115) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Simner J, Bain AE. 2013. A longitudinal study of grapheme-color synesthesia in childhood: 6/7 years to 10/11 years. Front. Hum. Neurosci. 7, 603 ( 10.3389/fnhum.2013.00603) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Blair CD, Berryhill ME. 2013. Synesthetic grapheme-color percepts exist for newly encountered Hebrew, Devanagari, Armenian and Cyrillic graphemes. Conscious. Cogn. 22, 944–954. ( 10.1016/j.concog.2013.06.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ghazanfar AA, Schroeder CE. 2006. Is neocortex essentially multisensory? Trends Cogn. Sci. 10, 278–285. ( 10.1016/j.tics.2006.04.008) [DOI] [PubMed] [Google Scholar]
- 68.Stein BE, Stanford TR. 2008. Multisensory integration: current issues from the perspective of the single neuron. Nat. Rev. Neurosci. 9, 255–266. ( 10.1038/nrn2331) [DOI] [PubMed] [Google Scholar]
- 69.Lund JS, Yoshioka T, Levitt JB. 1993. Comparison of intrinsic connectivity in different areas of macaque monkey cerebral cortex. Cereb. Cortex 3, 148–162. ( 10.1093/cercor/3.2.148) [DOI] [PubMed] [Google Scholar]
- 70.Eckert MA, Walczak A, Ahlstrom J, Denslow S, Horwitz A, Dubno JR. 2008. Age-related effects on word recognition: reliance on cognitive control systems with structural declines in speech-responsive cortex. J. Assoc. Res. Otolaryngol. 9, 252–259. ( 10.1007/s10162-008-0113-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Falchier A, Schroeder CE, Hackett TA, Lakatos P, Nascimento-Silva S, Ulbert I, Karmos G, Smiley JF. 2010. Projection from visual areas V2 and prostriata to caudal auditory cortex in the monkey. Cereb. Cortex 20, 1529–1538. ( 10.1093/cercor/bhp213) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rockland KS, Ojima H. 2003. Multisensory convergence in calcarine visual areas in macaque monkey. Int. J. Psychophysiol. 50, 19–26. ( 10.1016/S0167-8760(03)00121-1) [DOI] [PubMed] [Google Scholar]
- 73.Romei V, Murray MM, Cappe C, Thut G. 2009. Preperceptual and stimulus-selective enhancement of low-level human visual cortex excitability by sounds. Curr. Biol. 19, 1799–1805. ( 10.1016/j.cub.2009.09.027) [DOI] [PubMed] [Google Scholar]
- 74.Feng W, Störmer VS, Martinez A, McDonald JJ, Hillyard SA. 2014. Sounds activate visual cortex and improve visual discrimination. J. Neurosci. 34, 9817–9824. ( 10.1523/JNEUROSCI.4869-13.2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.McDonald JJ, Störmer VS, Martinez A, Feng W, Hillyard SA. 2013. Salient sounds activate human visual cortex automatically. J. Neurosci. 33, 9194–9201. ( 10.1523/JNEUROSCI.5902-12.2013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Campus C, Sandini G, Morrone MC, Gori M. 2017. Spatial localization of sound elicits early responses from occipital visual cortex in humans. Scient. Rep. 7, 10415 ( 10.1038/s41598-017-09142-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Feng W, Störmer VS, Martinez A, McDonald JJ, Hillyard SA. 2017. Involuntary orienting of attention to a sound desynchronizes the occipital alpha rhythm and improves visual perception. Neuroimage 150, 318–328. ( 10.1016/j.neuroimage.2017.02.033) [DOI] [PubMed] [Google Scholar]
- 78.Matusz PJ, Retsa C, Murray MM. 2016. The context-contingent nature of cross-modal activations of the visual cortex. Neuroimage 125, 996–1004. ( 10.1016/j.neuroimage.2015.11.016) [DOI] [PubMed] [Google Scholar]
- 79.Plass J, Ahn E, Towle VL, Stacey WC, Wasade VS, Tao J, Wu S, Issa NP, Brang D. 2019. Joint encoding of auditory timing and location in visual cortex. J. Cogn. Neurosci. 31, 1002–1017. ( 10.1162/jocn_a_01399) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Spierer L, Manuel AL, Bueti D, Murray MM. 2013. Contributions of pitch and bandwidth to sound-induced enhancement of visual cortex excitability in humans. Cortex 49, 2728–2734. ( 10.1016/j.cortex.2013.01.001) [DOI] [PubMed] [Google Scholar]
- 81.Romei V, Gross J, Thut G. 2012. Sounds reset rhythms of visual cortex and corresponding human visual perception. Curr. Biol. 22, 807–813. ( 10.1016/j.cub.2012.03.025) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Romei V, Murray MM, Merabet LB, Thut G. 2007. Occipital transcranial magnetic stimulation has opposing effects on visual and auditory stimulus detection: implications for multisensory interactions. J. Neurosci. 27, 11 465–11 472. ( 10.1523/JNEUROSCI.2827-07.2007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Spence C, Driver J. 1997. Audiovisual links in exogenous covert spatial orienting. Percept. Psychophys. 59, 1–22. ( 10.3758/BF03206843) [DOI] [PubMed] [Google Scholar]
- 84.McDonald JJ, Teder-Sälejärvi WA, Hillyard SA. 2000. Involuntary orienting to sound improves visual perception. Nature 407, 906 ( 10.1038/35038085) [DOI] [PubMed] [Google Scholar]
- 85.Nair A, Brang D. 2019. Inducing synesthesia in non-synesthetes: short-term visual deprivation facilitates auditory-evoked visual percepts. Conscious. Cogn. 70, 70–79. ( 10.1016/j.concog.2019.02.006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Afra P, Funke M, Matsuo F. 2009. Acquired auditory-visual synesthesia: a window to early cross-modal sensory interactions. Psychol. Res. Behav. Manag. 2, 31–37. ( 10.2147/PRBM.S4481) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Jacobs L, Karpik A, Bozian D, Gøthgen S. 1981. Auditory-visual synesthesia sound-induced photisms. Arch. Neurol. 38, 211–216. ( 10.1001/archneur.1981.00510040037005) [DOI] [PubMed] [Google Scholar]
- 88.Mroczko A, Metzinger T, Singer W, Nikolić D. 2009. Immediate transfer of synesthesia to a novel inducer. J. Vision 9, 25 ( 10.1167/9.12.25) [DOI] [PubMed] [Google Scholar]
- 89.Kay CL, Carmichael DA, Ruffell HE, Simner J. 2015. Colour fluctuations in grapheme-colour synaesthesia: the effect of clinical and non-clinical mood changes. Br. J. Psychol. 106, 487–504. ( 10.1111/bjop.12102) [DOI] [PubMed] [Google Scholar]
- 90.Niccolai V, Jennes J, Stoerig P, Van Leeuwen TM. 2012. Modality and variability of synesthetic experience. Am. J. Psychol. 125, 81–94. ( 10.5406/amerjpsyc.125.1.0081) [DOI] [PubMed] [Google Scholar]
- 91.Eagleman DM, Kagan AD, Nelson SS, Sagaram D, Sarma AK. 2007. A standardized test battery for the study of synesthesia. J. Neurosci. Methods 159, 139–145. ( 10.1016/j.jneumeth.2006.07.012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Riggs LA, Karwoski T. 1934. Synaesthesia. Br. J. Psychol. Gen Sect. 25, 29–41. ( 10.1111/j.2044-8295.1934.tb00722.x) [DOI] [Google Scholar]
- 93.Cutietta RA, Haggerty KJ. 1987. A comparative study of color association with music at various age levels. J. Res. Music Educ. 35, 78–91. ( 10.2307/3344984) [DOI] [Google Scholar]
- 94.McDonnell MD, Ward LM. 2011. The benefits of noise in neural systems: bridging theory and experiment. Nat. Rev. Neurosci. 12, 415–426. ( 10.1038/nrn3061) [DOI] [PubMed] [Google Scholar]
- 95.Faisal AA, Selen LPJ, Wolpert DM. 2008. Noise in the nervous system. Nat. Rev. Neurosci. 9, 292–303. ( 10.1038/nrn2258) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Söderlund G, Sikström S, Smart A. 2007. Listen to the noise: noise is beneficial for cognitive performance in ADHD. J. Child Psychol. Psychiatry 48, 840–847. ( 10.1111/j.1469-7610.2007.01749.x) [DOI] [PubMed] [Google Scholar]
- 97.Kurrer C, Schulten K. 1995. Noise-induced synchronous neuronal oscillations. Phys. Rev. E 51, 6213–6218. ( 10.1103/PhysRevE.51.6213) [DOI] [PubMed] [Google Scholar]
- 98.McDonnell MD, Abbott D. 2009. What is stochastic resonance? Definitions, misconceptions, debates, and its relevance to biology. PLoS Comput. Biol. 5, e1000348 ( 10.1371/journal.pcbi.1000348) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Neiman A, Schimansky-Geier L, Cornell-Bell A, Moss F. 1999. Noise-enhanced phase synchronization in excitable media. Phys. Rev. Lett. 83, 4896–4899. ( 10.1103/PhysRevLett.83.4896) [DOI] [Google Scholar]
- 100.Kim S, Park SH, Pyo H-B. 1999. Stochastic resonance in coupled oscillator systems with time delay. Phys. Rev. Lett. 82, 1620–1623. ( 10.1103/PhysRevLett.82.1620) [DOI] [Google Scholar]
- 101.Zhou C, Kurths J, Hu B. 2001. Array-enhanced coherence resonance: nontrivial effects of heterogeneity and spatial independence of noise. Phys. Rev. Lett. 87, 098101 ( 10.1103/PhysRevLett.87.098101) [DOI] [PubMed] [Google Scholar]
- 102.Busch H, Hütt M-T, Kaiser F. 2001. Effect of colored noise on networks of nonlinear oscillators. Phys. Rev. E 64, 021105 ( 10.1103/PhysRevE.64.021105) [DOI] [PubMed] [Google Scholar]
- 103.Lindner B, García-Ojalvo J, Neiman A, Schimansky-Geier L. 2004. Effects of noise in excitable systems. Phys. Rep. 392, 321–424. ( 10.1016/j.physrep.2003.10.015) [DOI] [Google Scholar]
- 104.van Atteveldt N, Murray MM, Thut G, Schroeder CE. 2014. Multisensory integration: flexible use of general operations. Neuron 81, 1240–1253. ( 10.1016/j.neuron.2014.02.044) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ward J, Huckstep B, Tsakanikos E. 2006. Sound-colour synaesthesia: to what extent does it use cross-modal mechanisms common to us all? Cortex 42, 264–280. ( 10.1016/S0010-9452(08)70352-6) [DOI] [PubMed] [Google Scholar]
- 106.Simner J. 2009. Synaesthetic visuo-spatial forms: viewing sequences in space. Cortex 45, 1138–1147. ( 10.1016/j.cortex.2009.07.001) [DOI] [PubMed] [Google Scholar]
- 107.Simner J, Ward J, Lanz M, Jansari A, Noonan K, Glover L, Oakley DA. 2005. Non-random associations of graphemes to colours in synaesthetic and non-synaesthetic populations. Cogn. Neuropsychol. 22, 1069–1085. ( 10.1080/02643290500200122) [DOI] [PubMed] [Google Scholar]
- 108.Smilek D, Callejas A, Dixon MJ, Merikle PM. 2007. Ovals of time: time-space associations in synaesthesia. Conscious. Cogn. 16, 507–519. ( 10.1016/j.concog.2006.06.013) [DOI] [PubMed] [Google Scholar]
- 109.Simner J, Ludwig V. 2009. What colour does that feel? Cross-modal correspondences from touch to colour. Paper presented at 3rd Int. Conf. Synaesthesia and Art (Artecitta), Granada, Spain, 26–29 April 2009.
- 110.Ward J, Banissy MJ, Jonas CN. 2008. Haptic perception and synaesthesia. In Human haptic perception: basics and applications (ed. M Grunwald), pp. 259–265. Berlin, Germany: Springer. [Google Scholar]
- 111.Roseman L, Sereno MI, Leech R, Kaelen M, Orban C, McGonigle J, Feilding A, Nutt DJ, Carhart-Harris RL. 2016. LSD alters eyes-closed functional connectivity within the early visual cortex in a retinotopic fashion. Hum. Brain Mapp. 37, 3031–3040. ( 10.1002/hbm.23224) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Carhart-Harris RL, et al. 2016. Neural correlates of the LSD experience revealed by multimodal neuroimaging. Proc. Natl Acad. Sci. USA 113, 4853–4858. ( 10.1073/pnas.1518377113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Aller M, Giani A, Conrad V, Watanabe M, Noppeney U. 2015. A spatially collocated sound thrusts a flash into awareness. Front. Integr. Neurosci. 9, 16 ( 10.3389/fnint.2015.00016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Shams L, Kamitani Y, Shimojo S. 2002. Visual illusion induced by sound. Cogn. Brain Res. 14, 147–152. ( 10.1016/S0926-6410(02)00069-1) [DOI] [PubMed] [Google Scholar]
- 115.Bolognini N, Convento S, Fusaro M, Vallar G. 2013. The sound-induced phosphene illusion. Exp. Brain Res. 231, 469–478. ( 10.1007/s00221-013-3711-1) [DOI] [PubMed] [Google Scholar]
- 116.Etevenon P, Boissier J. 1972. LSD effects on signal-to-noise ratio and lateralization of visual cortex and lateral geniculate during photic stimulation. Experientia 28, 1338–1340. ( 10.1007/BF01965332) [DOI] [PubMed] [Google Scholar]
- 117.Witthoft N, Winawer J. 2013. Learning, memory, and synesthesia. Psychol. Sci. 24, 258–265. ( 10.1177/0956797612452573) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Spector F, Maurer D. 2011. The colors of the alphabet: naturally-biased associations between shape and color. J. Exp. Psychol. Hum. Percept. Perform. 37, 484–495. ( 10.1037/a0021437) [DOI] [PubMed] [Google Scholar]
- 119.Seron X, Pesenti M, Noël M-P, Deloche G, Cornet J-A. 1992. Images of numbers, or ‘when 98 is upper left and 6 sky blue’. Cognition 44, 159–196. ( 10.1016/0010-0277(92)90053-K) [DOI] [PubMed] [Google Scholar]
- 120.Watson MR, Akins K, Spiker C, Crawford L, Enns JT. 2014. Synesthesia and learning: a critical review and novel theory. Front. Hum. Neurosci. 8, 98 ( 10.3389/fnhum.2014.00098) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Gallant JL, Shoup RE, Mazer JA. 2000. A human extrastriate area functionally homologous to macaque V4. Neuron 27, 227–235. ( 10.1016/S0896-6273(00)00032-5) [DOI] [PubMed] [Google Scholar]
- 122.Heywood CA, Gadotti A, Cowey A. 1992. Cortical area V4 and its role in the perception of color. J. Neurosci. 12, 4056–4065. ( 10.1523/JNEUROSCI.12-10-04056.1992) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Heywood CA, Gaffan D, Cowey A. 1995. Cerebral achromatopsia in monkeys. Eur. J. Neurosci. 7, 1064–1073. ( 10.1111/j.1460-9568.1995.tb01093.x) [DOI] [PubMed] [Google Scholar]
- 124.Zeki SM. 1973. Colour coding in rhesus monkey prestriate cortex. Brain Res. 53, 422–427. ( 10.1016/0006-8993(73)90227-8) [DOI] [PubMed] [Google Scholar]
- 125.Wade A, Augath M, Logothetis N, Wandell B. 2008. fMRI measurements of color in macaque and human. J. Vision 8, 6 ( 10.1167/8.10.6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zeki S, Watson JD, Lueck CJ, Friston KJ, Kennard C, Frackowiak RS. 1991. A direct demonstration of functional specialization in human visual cortex. J. Neurosci. 11, 641–649. ( 10.1523/JNEUROSCI.11-03-00641.1991) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Komatsu H, Ideura Y, Kaji S, Yamane S. 1992. Color selectivity of neurons in the inferior temporal cortex of the awake macaque monkey. J. Neurosci. 12, 408–424. ( 10.1523/JNEUROSCI.12-02-00408.1992) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Motter BC. 1994. Neural correlates of attentive selection for color or luminance in extrastriate area V4. J. Neurosci. 14, 2178–2189. ( 10.1523/JNEUROSCI.14-04-02178.1994) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Goddard E, Mannion DJ, McDonald JS, Solomon SG, Clifford CWG. 2011. Color responsiveness argues against a dorsal component of human V4. J. Vision 11, 3 ( 10.1167/11.4.3) [DOI] [PubMed] [Google Scholar]
- 130.Winawer J, Witthoft N. 2015. Human V4 and ventral occipital retinotopic maps. Vis. Neurosci. 32, E020 ( 10.1017/S0952523815000176) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Seymour K, Clifford CWG, Logothetis NK, Bartels A. 2009. The coding of color, motion, and their conjunction in the human visual cortex. Curr. Biol. 19, 177–183. ( 10.1016/j.cub.2008.12.050) [DOI] [PubMed] [Google Scholar]
- 132.Tootell RBH, Hadjikhani N. 1998. Reply to ‘Has a new color area been discovered’. Nat. Neurosci. 1, 335–336. ( 10.1038/1539) [DOI] [PubMed] [Google Scholar]
- 133.Hadjikhani N, Liu AK, Dale AM, Cavanagh P, Tootell RBH. 1998. Retinotopy and color sensitivity in human visual cortical area V8. Nat. Neurosci. 1, 235–241. ( 10.1038/681) [DOI] [PubMed] [Google Scholar]
- 134.Schein SJ, Desimone R. 1990. Spectral properties of V4 neurons in the macaque. J. Neurosci. 10, 3369–3389. ( 10.1523/JNEUROSCI.10-10-03369.1990) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Desimone R, Schein SJ. 1987. Visual properties of neurons in area V4 of the macaque: sensitivity to stimulus form. J. Neurophysiol. 57, 835–868. ( 10.1152/jn.1987.57.3.835) [DOI] [PubMed] [Google Scholar]
- 136.Schluppeck D, Engel SA. 2002. Color opponent neurons in V1: a review and model reconciling results from imaging and single-unit recording. J. Vision 2, 5 ( 10.1167/2.6.5) [DOI] [PubMed] [Google Scholar]
- 137.Conway BR, Tsao DY. 2009. Color-tuned neurons are spatially clustered according to color preference within alert macaque posterior inferior temporal cortex. Proc. Natl Acad. Sci. USA 106, 18 034–18 039. ( 10.1073/pnas.0810943106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Johnson EN, Hawken MJ, Shapley R. 2008. The orientation selectivity of color-responsive neurons in macaque V1. J. Neurosci. 28, 8096–8106. ( 10.1523/JNEUROSCI.1404-08.2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Allison T, Begleiter A, McCarthy G, Roessler E, Nobre AC, Spencer DD. 1993. Electrophysiological studies of color processing in human visual cortex. Electroencephalogr. Clin. Neurophysiol. Evoked Potentials Sect. 88, 343–355. ( 10.1016/0168-5597(93)90011-D) [DOI] [PubMed] [Google Scholar]
- 140.Takechi H, Onoe H, Shizuno H, Yoshikawa E, Sadato N, Tsukada H, Watanabe Y. 1997. Mapping of cortical areas involved in color vision in non-human primates. Neurosci. Lett. 230, 17–20. ( 10.1016/S0304-3940(97)00461-8) [DOI] [PubMed] [Google Scholar]
- 141.Beauchamp MS, Haxby JV, Jennings JE, DeYoe EA. 1999. An fMRI version of the Farnsworth–Munsell 100-Hue test reveals multiple color-selective areas in human ventral occipitotemporal cortex. Cereb. Cortex 9, 257–263. ( 10.1093/cercor/9.3.257) [DOI] [PubMed] [Google Scholar]
- 142.Schein SJ, Marrocco RT, de Monasterio FM. 1982. Is there a high concentration of color-selective cells in area V4 of monkey visual cortex? J. Neurophysiol. 47, 193–213. ( 10.1152/jn.1982.47.2.193) [DOI] [PubMed] [Google Scholar]
- 143.Roe AW, Chelazzi L, Connor CE, Conway BR, Fujita I, Gallant JL, Lu H, Vanduffel W. 2012. Toward a unified theory of visual area V4. Neuron 74, 12–29. ( 10.1016/j.neuron.2012.03.011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Schiller PH, Lee K. 1991. The role of the primate extrastriate area V4 in vision. Science 251, 1251–1253. ( 10.1126/science.2006413) [DOI] [PubMed] [Google Scholar]
- 145.Poort J, Raudies F, Wannig A, Lamme VAF, Neumann H, Roelfsema PR. 2012. The role of attention in figure-ground segregation in areas V1 and V4 of the visual cortex. Neuron 75, 143–156. ( 10.1016/j.neuron.2012.04.032) [DOI] [PubMed] [Google Scholar]
- 146.Hegdé J, Van Essen DC. 2005. Role of primate visual area V4 in the processing of 3-D shape characteristics defined by disparity. J. Neurophysiol. 94, 2856–2866. ( 10.1152/jn.00802.2004) [DOI] [PubMed] [Google Scholar]
- 147.Kosai Y, El-Shamayleh Y, Fyall AM, Pasupathy A. 2014. The role of visual area V4 in the discrimination of partially occluded shapes. J. Neurosci. 34, 8570–8584. ( 10.1523/JNEUROSCI.1375-14.2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Eagleman DM, Goodale MA. 2009. Why color synesthesia involves more than color. Trends Cogn. Sci. 13, 288–292. ( 10.1016/j.tics.2009.03.009) [DOI] [PubMed] [Google Scholar]
- 149.Abrahamyan A, Clifford CWG, Arabzadeh E, Harris JA. 2015. Low intensity TMS enhances perception of visual stimuli. Brain Stimulat. 8, 1175–1182. ( 10.1016/j.brs.2015.06.012) [DOI] [PubMed] [Google Scholar]
- 150.Antal A, Paulus W. 2008. Transcranial direct current stimulation and visual perception. Perception 37, 367–374. ( 10.1068/p5872) [DOI] [PubMed] [Google Scholar]
- 151.Mulckhuyse M, Kelley TA, Theeuwes J, Walsh V, Lavie N. 2011. Enhanced visual perception with occipital transcranial magnetic stimulation. Eur. J. Neurosci. 34, 1320–1325. ( 10.1111/j.1460-9568.2011.07814.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Ward J, Hoadley C, Hughes JEA, Smith P, Allison C, Baron-Cohen S, Simner J. 2017. Atypical sensory sensitivity as a shared feature between synaesthesia and autism. Scient. Rep. 7, 41155 ( 10.1038/srep41155) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Ward J, Brown P, Sherwood J, Simner J. 2018. An autistic-like profile of attention and perception in synaesthesia. Cortex 107, 121–130. ( 10.1016/j.cortex.2017.10.008) [DOI] [PubMed] [Google Scholar]
- 154.Cytowic RE. 1995. Synesthesia: phenomenology and neuropsychology. Psyche (Stuttg.) 2, 2–10. [Google Scholar]
- 155.Baron-Cohen S, Johnson D, Asher J, Wheelwright S, Fisher SE, Gregersen PK, Allison C. 2013. Is synaesthesia more common in autism? Mol. Autism 4, 40 ( 10.1186/2040-2392-4-40) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Neufeld J, Roy M, Zapf A, Sinke C, Emrich HM, Prox-Vagedes V, Dillo W, Zedler M. 2013. Is synesthesia more common in patients with Asperger syndrome? Front. Hum. Neurosci. 7, 847 ( 10.3389/fnhum.2013.00847) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Hughes JEA, Simner J, Baron-Cohen S, Treffert DA, Ward J. 2017. Is synaesthesia more prevalent in autism spectrum conditions? Only where there is prodigious talent. Multisensory Res. 30, 391–408. ( 10.1163/22134808-00002558) [DOI] [PubMed] [Google Scholar]
- 158.Rubenstein JLR, Merzenich MM. 2003. Model of autism: increased ratio of excitation/inhibition in key neural systems. Genes Brain Behav. 2, 255–267. ( 10.1034/j.1601-183X.2003.00037.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Lee E, Lee J, Kim E. 2017. Excitation/inhibition imbalance in animal models of autism spectrum disorders. Biol. Psychiatry 81, 838–847. ( 10.1016/j.biopsych.2016.05.011) [DOI] [PubMed] [Google Scholar]
- 160.Snijders TM, Milivojevic B, Kemner C. 2013. Atypical excitation–inhibition balance in autism captured by the gamma response to contextual modulation. NeuroImage Clin. 3, 65–72. ( 10.1016/j.nicl.2013.06.015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Gogolla N, LeBlanc JJ, Quast KB, Südhof TC, Fagiolini M, Hensch TK. 2009. Common circuit defect of excitatory-inhibitory balance in mouse models of autism. J. Neurodev. Disord. 1, 172–181. ( 10.1007/s11689-009-9023-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Simmons DR, McKay L, McAleer P, Toal E, Robertson A, Pollick FE. 2007. Neural noise and autism spectrum disorders. Perception 36, 119–120. [Google Scholar]
- 163.Coghlan S, Horder J, Inkster B, Mendez MA, Murphy DG, Nutt DJ. 2012. GABA system dysfunction in autism and related disorders: from synapse to symptoms. Neurosci. Biobehav. Rev. 36, 2044–2055. ( 10.1016/j.neubiorev.2012.07.005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Weinger PM, Zemon V, Soorya L, Gordon J. 2014. Low-contrast response deficits and increased neural noise in children with autism spectrum disorder. Neuropsychologia 63, 10–18. ( 10.1016/j.neuropsychologia.2014.07.031) [DOI] [PubMed] [Google Scholar]
- 165.Sengupta B, Laughlin SB, Niven JE. 2013. Balanced excitatory and inhibitory synaptic currents promote efficient coding and metabolic efficiency. PLoS Comput. Biol. 9, e1003263 ( 10.1371/journal.pcbi.1003263) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Agrawal V, Cowley AB, Alfaori Q, Larremore DB, Restrepo JG, Shew WL. 2018. Robust entropy requires strong and balanced excitatory and inhibitory synapses. Chaos Interdiscip. J. Nonlinear Sci. 28, 103115 ( 10.1063/1.5043429) [DOI] [PubMed] [Google Scholar]
- 167.Robertson CE, Ratai E-M, Kanwisher N. 2016. Reduced GABAergic action in the autistic brain. Curr. Biol. 26, 80–85. ( 10.1016/j.cub.2015.11.019) [DOI] [PubMed] [Google Scholar]
- 168.Tavassoli T, Auyeung B, Murphy LC, Baron-Cohen S, Chakrabarti B. 2012. Variation in the autism candidate gene GABRB3 modulates tactile sensitivity in typically developing children. Mol. Autism 3, 6 ( 10.1186/2040-2392-3-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Edden RAE, Muthukumaraswamy SD, Freeman TCA, Singh KD. 2009. Orientation discrimination performance is predicted by GABA concentration and gamma oscillation frequency in human primary visual cortex. J. Neurosci. 29, 15 721–15 726. ( 10.1523/JNEUROSCI.4426-09.2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Puts NAJ, Edden RAE, Evans CJ, McGlone F, McGonigle DJ. 2011. Regionally specific human GABA concentration correlates with tactile discrimination thresholds. J. Neurosci. 31, 16 556–16 560. ( 10.1523/JNEUROSCI.4489-11.2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Greenhouse I, Noah S, Maddock RJ, Ivry RB. 2016. Individual differences in GABA content are reliable but are not uniform across the human cortex. Neuroimage 139, 1–7. ( 10.1016/j.neuroimage.2016.06.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Lalwani P, Gagnon H, Cassady K, Simmonite M, Peltier S, Seidler RD, Taylor SF, Weissman DH, Polk TA. 2019. Neural distinctiveness declines with age in auditory cortex and is associated with auditory GABA levels. NeuroImage 201, 116033 ( 10.1016/j.neuroimage.2019.116033) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Luo C, et al. 2011. Altered functional connectivity in default mode network in absence epilepsy: a resting-state fMRI study. Hum. Brain Mapp. 32, 438–449. ( 10.1002/hbm.21034) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Pittau F, Grova C, Moeller F, Dubeau F, Gotman J. 2012. Patterns of altered functional connectivity in mesial temporal lobe epilepsy. Epilepsia 53, 1013–1023. ( 10.1111/j.1528-1167.2012.03464.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Waites AB, Briellmann RS, Saling MM, Abbott DF, Jackson GD. 2006. Functional connectivity networks are disrupted in left temporal lobe epilepsy. Ann. Neurol. 59, 335–343. ( 10.1002/ana.20733) [DOI] [PubMed] [Google Scholar]
- 176.Ro T, Farnè A, Johnson RM, Wedeen V, Chu Z, Wang ZJ, Hunter JV, Beauchamp MS. 2007. Feeling sounds after a thalamic lesion. Ann. Neurol. 62, 433–441. ( 10.1002/ana.21219) [DOI] [PubMed] [Google Scholar]
- 177.Dixon MJ, Smilek D, Duffy PL, Zanna MP, Merikle PM. 2006. The role of meaning in grapheme-colour synaesthesia. Cortex 42, 243–252. ( 10.1016/S0010-9452(08)70349-6) [DOI] [PubMed] [Google Scholar]
- 178.Gilbert CD, Li W. 2013. Top-down influences on visual processing. Nat. Rev. Neurosci. 14, 350–363. ( 10.1038/nrn3476) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Gilbert CD, Sigman M. 2007. Brain states: top-down influences in sensory processing. Neuron 54, 677–696. ( 10.1016/j.neuron.2007.05.019) [DOI] [PubMed] [Google Scholar]
- 180.Pecher D. 2001. Perception is a two-way junction: feedback semantics in word recognition. Psychon. Bull. Rev. 8, 545–551. ( 10.3758/BF03196190) [DOI] [PubMed] [Google Scholar]
- 181.Bernstein IH, Bissonnette V, Vyas A, Barclay P. 1989. Semantic priming: subliminal perception or context? Percept. Psychophys. 45, 153–161. ( 10.3758/BF03208050) [DOI] [PubMed] [Google Scholar]
- 182.Bramão I, Faísca L, Forkstam C, Reis A, Petersson KM. 2010. Cortical brain regions associated with color processing: an FMRi study. Open Neuroimaging J. 4, 164 ( 10.2174/1874440001004010164) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Bannert MM, Bartels A. 2013. Decoding the yellow of a gray banana. Curr. Biol. 23, 2268–2272. ( 10.1016/j.cub.2013.09.016) [DOI] [PubMed] [Google Scholar]
- 184.Bannert MM, Bartels A. 2018. Human V4 activity patterns predict behavioral performance in imagery of object color. J. Neurosci. 38, 3657–3668. ( 10.1523/JNEUROSCI.2307-17.2018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Novich S, Cheng S, Eagleman DM. 2011. Is synaesthesia one condition or many? A large-scale analysis reveals subgroups. J. Neuropsychol. 5, 353–371. ( 10.1111/j.1748-6653.2011.02015.x) [DOI] [PubMed] [Google Scholar]
- 186.Deutsch D, Henthorn T, Marvin E, Xu H. 2006. Absolute pitch among American and Chinese conservatory students: prevalence differences, and evidence for a speech-related critical period. J. Acoust. Soc. Am. 119, 719–722. ( 10.1121/1.2151799) [DOI] [PubMed] [Google Scholar]
- 187.Deutsch D, Henthorn T, Dolson M. 1999. Absolute pitch is demonstrated in speakers of tone languages. J. Acoust. Soc. Am. 106, 2267 ( 10.1121/1.427738) [DOI] [Google Scholar]
- 188.Gregersen PK, Kowalsky E, Lee A, Baron-Cohen S, Fisher SE, Asher JE, Ballard D, Freudenberg J, Li W. 2013. Absolute pitch exhibits phenotypic and genetic overlap with synesthesia. Hum. Mol. Genet. 22, 2097–2104. ( 10.1093/hmg/ddt059) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.McIntosh AR, Kovacevic N, Lippe S, Garrett D, Grady C, Jirsa V. 2010. The development of a noisy brain. Arch. Ital. Biol. 148, 323–337. ( 10.4449/aib.v148i3.1225) [DOI] [PubMed] [Google Scholar]
- 190.Skoczenski AM, Norcia AM. 1998. Neural noise limitations on infant visual sensitivity. Nature 391, 697–700. ( 10.1038/35630) [DOI] [PubMed] [Google Scholar]
- 191.Shriki O, Sadeh Y, Ward J. 2016. The emergence of synaesthesia in a neuronal network model via changes in perceptual sensitivity and plasticity. PLoS Comput. Biol. 12, e1004959 ( 10.1371/journal.pcbi.1004959) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Seth AK. 2014. A predictive processing theory of sensorimotor contingencies: explaining the puzzle of perceptual presence and its absence in synesthesia. Cogn. Neurosci. 5, 97–118. ( 10.1080/17588928.2013.877880) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.