Abstract
In synaesthetes, specific sensory stimuli (e.g. black letters) elicit additional experiences (e.g. colour). Synaesthesia is highly prevalent among individuals with autism spectrum disorder (ASD), but the mechanisms of this co-occurrence are not clear. We hypothesized autism and synaesthesia share atypical sensory sensitivity and perception. We assessed autistic traits, sensory sensitivity and visual perception in two synaesthete populations. In Study 1, synaesthetes (N = 79, of different types) scored higher than non-synaesthetes (N = 76) on the Attention-to-detail and Social skills subscales of the autism spectrum quotient indexing autistic traits, and on the Glasgow Sensory Questionnaire indexing sensory hypersensitivity and hyposensitivity which frequently occur in autism. Synaesthetes performed two local/global visual tasks because individuals with autism typically show a bias towards detail processing. In synaesthetes, elevated motion coherence thresholds (MCTs) suggested reduced global motion perception, and higher accuracy on an embedded figures task suggested enhanced local perception. In Study 2, sequence-space synaesthetes (N = 18) completed the same tasks. Questionnaire and embedded figures results qualitatively resembled Study 1 results, but no significant group differences with non-synaesthetes (N = 20) were obtained. Unexpectedly, sequence-space synaesthetes had reduced MCTs. Altogether, our studies suggest atypical sensory sensitivity and a bias towards detail processing are shared features of synaesthesia and ASD.
This article is part of the discussion meeting issue ‘Bridging senses: novel insights from synaesthesia’.
Keywords: synaesthesia, synesthesia, autism, local/global, visual perception, sensory sensitivity
1. Introduction
Synaesthesia is a mixing of the senses: specific sensory stimuli evoke unusual, additional experiences. For instance, ‘A’ evokes the colour red; music elicits colours; or the word ‘parents’ tastes like apple. Synaesthesia is elicited automatically, is stable over time and the prevalence is estimated at 2–4% of the population [1]. The prevalence of synaesthesia is substantially higher (approx. 20%) among individuals with autism spectrum disorder (ASD) [2,3]. ASD is a neurodevelopmental condition affecting approximately 1% of the population [4] and characterized by deficits in language and social interaction, repetitive behaviour and restricted interests [5]. The remarkably high co-occurrence of synaesthesia and autism—both relatively rare conditions—suggests that the two conditions are related, but the exact nature of the relationship is unknown.
An important hypothesis states that synaesthesia and autism share atypical sensory sensitivity and altered sensory perception [6,7]. In autism research, increased attention to sensory abnormalities (e.g. [8–10]) has resulted in the addition of sensory dysregulation as a diagnostic criterion in the recent DSM-5 [5]. Individuals with ASD frequently experience hypersensitivity or hyposensitivity to the environment, e.g. hypersensitivity to bright lights or strong colours or, to the contrary, seek stimulation by engaging in repetitive behaviours (e.g. fluttering the arms) [5]. Sensory atypicalities can negatively impact daily functioning, as in sensory overload [9], but can also entail enhanced perceptual skills [11–13] or savant abilities (e.g. [14]). Enhanced perception of details (a ‘local bias’) is widespread in autism (e.g. [12,15,16]) and features centrally in several autism theories, e.g. the weak central coherence theory [11] and the enhanced perceptual functioning model [13]. A recent formal meta-analysis on local–global visual processing in ASD, however, revealed no structurally enhanced local visual processing in autism, but concluded that global visual processing requires more time and effort in individuals with autism, especially when incongruent low-level information is present [17].
Synaesthesia is a sensory condition characterized by unusual perception. Sensory atypicalities have been reported for synaesthesia beyond the synaesthetic experiences themselves. These include hypersensitivity of the parvocellular visual system [18], enhanced perception of and sensitivity to colours in colour synaesthetes [19–21] and enhanced tactile sensitivity in tactile synaesthetes [19]. Enhanced colour processing in synaesthetes may come at the cost of reduced motion-processing abilities [22], an example of sensory impairment in synaesthesia which parallels reduced motion processing in autism (e.g. [23,24]). Synaesthesia occurs more frequently among ASD individuals with savant abilities compared to those without these abilities [25] and savant skills often involve synaesthesia [14,26]. Enhanced perception of details is reported for synaesthetes, e.g. for perception of detailed facial features [27] and on visual tasks [7]. Altogether, sensory atypicalities in both synaesthesia and autism form a reason to explore whether atypical sensory processing is shared between the conditions.
A better understanding of the relationship between synaesthesia, autism and sensory atypicalities may elucidate the role of sensory processing in the mechanisms of autism. Deficits in early (multi)sensory processing in autism are suggested to lead to social symptoms [28,29] as these complex cognitive functions rely on proper integration of (multi)sensory cues, for instance, when interpreting speech and simultaneously integrating facial expressions. Care for individuals with autism may benefit from better understanding of (synaesthesia-related) sensory symptoms. Autism and synaesthesia may share other mechanisms: implicit learning, for instance, is altered in both populations [30,31]. Here, however, we focus on sensory processing and explicitly test whether sensory sensitivity and perception in synaesthesia resemble sensory processing in autism.
Several studies have used self-report questionnaires and visual experiments to directly assess autism-related sensory atypicalities in synaesthetes [6,7,32,33]. Ward et al. [6] deployed the Glasgow Sensory Questionnaire (GSQ) which indexes sensory hypersensitivity and hyposensitivity in seven modalities and typically correlates positively with autistic traits [34–36]. Synaesthetes scored intermediate between controls and ASD individuals, qualitatively resembling the ASD pattern. On the autism spectrum quotient (AQ) [37], a self-report questionnaire about autistic traits, synaesthetes scored within the ASD range on the Attention-to-detail subscale but not on other AQ subscales (e.g. social skills). Ward et al. [7] replicated the elevated GSQ and AQ-Attention-to-detail scores for synaesthetes and found dose effects of synaesthesia: having more types of synaesthesia was associated with higher scores supporting a common mechanism of synaesthesia and autism. In Burghoorn et al. [32], autistic traits (AQ-Total) and synaesthesia consistency scores correlated positively in neurotypical individuals extending the autism–synaesthesia relation to neurotypicals. In Mealor et al. [33], synaesthetes scored higher on AQ-Attention-to-detail-derived questions. Hence, self-report questionnaire findings suggest that the synaesthesia–ASD relationship involves perceptual and sensory atypicalities.
Experimental findings from the same studies [7,32] are somewhat mixed. Individuals with autism tend to focus more easily on local than on global visual elements (although they are not necessarily impaired at the global level as reported, for example, by Van der Hallen et al. [15,17]). Ward et al. [7] assessed local/global perceptual abilities in synaesthetes using an embedded figures test and a change blindness test on which individuals with ASD have been reported to perform better [38–42]. The embedded figures test involves identifying a local target shape in a larger complex figure, while the change blindness test involves finding the difference between two alternating pictures (e.g. removal of a small object). On both tests, synaesthetes were more accurate than controls without response time differences, supporting an interpretation of enhanced perception of detail in synaesthetes similar to ASD. In Burghoorn et al. [32], neurotypical individuals performed three visual experiments on local/global perception and were assessed for autistic traits (AQ) and the degree of synaesthesia. Given that AQ-Total and synaesthesia consistency scores were correlated, both measures were expected to relate to enhanced perception of details—individuals with high AQ resemble individuals with ASD [43]. High AQ-Attention-to-detail correlated positively with performance on an embedded figures task and a visual illusions task (two ‘local’ tasks), but not on a motion coherence task (a ‘global’ task). The degree of synaesthesia, however, did not correlate with the performance: only a trend in the visual illusions task resembled the AQ results. In these visual illusions (Müller-Lyer and Ebbinghaus), relatively more focus on local elements reduces susceptibility to the illusion, which is induced by the global Gestalt context of the display. Higher AQ-Attention-to-detail was associated with more veridical perception in the Müller-Lyer illusion in Burghoorn et al. [32], in line with the reduced susceptibility to these illusions in ASD (e.g. [44]). The absence of any correlations of performance with the degree of synaesthesia suggested that the relationship between synaesthesia and perceptual abilities is weaker than the relationship between autistic traits and perception.
Here, we assessed autistic traits, sensory sensitivity and local/global visual perceptual abilities of synaesthetes and related those characteristics to known features of ASD. The same tasks were performed by a large heterogeneous online cohort of synaesthetes (Study 1) and a smaller laboratory cohort of sequence-space synaesthetes (Study 2). Sequence-space synaesthetes perceive sequences such as days of the week, months and/or numbers in a spatial arrangement. We assessed autistic traits (AQ [37]) and sensory sensitivity (Dutch Glasgow Sensory Questionnaire (GSQ) [34,35]). We hypothesized that synaesthetes would score high on AQ-Attention-to-detail and would score more extreme on the GSQ.
Two visual tasks were used to test local/global visual perception in synaesthetes. In a Motion Coherence task, we assessed global motion processing: participants indicate the global direction of motion of a dot display in which a limited number of dots move in a coherent direction (electronic supplementary material, figure S2). Attending to individual dots impairs performance. Individuals with autism generally need approximately 10% more dots to move coherently before perceiving the global motion direction (e.g. [12,15,23,24,45]), although several studies have reported better performance for ASD individuals [46], mixed results depending on whether individuals with ASD or Asperger syndrome were tested [47] or no difference between ASD individuals and controls [48]. On the basis of the majority of studies, however, we expected a similarly decreased performance (higher motion coherence thresholds (MCTs)) for synaesthetes. We replicate one previous study with N = 10 synaesthetes [22] that reported increased MCTs in synaesthetes, and one study with N = 34 synaesthetes in which sequence-space synaesthetes (N = 22) displayed lower MCTs than grapheme-colour synaesthetes (N = 12) and controls (N = 34) [49]. The second experiment was the Leuven Embedded Figures Test [50,51] on which we expected synaesthetes to do better because focusing on local elements is beneficial in this task (electronic supplementary material, figure S3). We explored correlations of performance on these visual tasks with AQ and GSQ scores, and dose effects of synaesthesia.
Our overall approach expands upon Ward et al. [7] in three ways: we added a motion task on which decreased performance of synaesthetes was expected, to rule out stronger motivation of synaesthetes as a potential confounding factor; we added a difficulty manipulation in the embedded figures task, to examine the performance of synaesthetes on this task in more detail, and we used Dutch versions of the AQ and GSQ, replicating earlier studies in another language.
2. Methods Study 1: heterogeneous cohort of online synaesthetes
(a). Participants
Participants were recruited via a nationally advertised crowd-sourcing website about sensory perception and synaesthesia (gno.mpi.nl, [52,53]) and the institute's online recruitment system. One hundred and fifty-nine participants (109 self-reported synaesthetes) completed the online synaesthesia screening questionnaire. Thirty-three people (26 synaesthetes) were excluded because of neuropsychiatric disorders (e.g. ASD, depression), six synaesthetes stopped after the first questionnaire and one synaesthete did not meet our synaesthesia cut-off (consistency tests and classification of synaesthesia types according to Novich et al. [54] are described in the electronic supplementary material). In total, 76 synaesthetes and 43 non-synaesthetes completed the study online. Participants were compensated by entering a raffle for a tablet and/or course credits. The study was approved by the Ethics Committee of the Faculty of Social Sciences (ECSS) at Radboud University, Nijmegen.
Thirty-nine individuals, recruited on campus and via the institute's participant recruitment system, completed the study in the laboratory as part of a perception study [32]. Three individuals were excluded because of neuropsychiatric conditions, and three laboratory participants were synaesthetes, resulting in 33 non-synaesthetes and 3 synaesthetes. Participants were compensated with 12.50 euros or 1.5 course credits. The study was approved by the ECSS at Radboud University, Nijmegen.
Combining online and laboratory participants,1 the total sample included 79 synaesthetes and 76 non-synaesthetes. The groups differed in age (synaesthetes of 36.2 ± 15.1 years; range, 18–72 years; non-synaesthetes of 23.3 ± 6.7 years; range, 18–61 years; t153 = −6.83, p < 0.001). Age was included as a covariate in all group analyses. Gender distribution was similar across groups (synaesthetes, 8M/71F; non-synaesthetes, 15M/61F; , p = 0.092). Synaesthetes predominantly experienced grapheme-colour synaesthesia (N = 59) and sequence-space synaesthesia (SSS) (N = 21); 36 synaesthetes experienced one type of synaesthesia, 26 synaesthetes experienced two types and the remainder (N = 17) three or more types (see electronic supplementary material, figure S1 and table S1 for details). We created three subgroups of synaesthetes to explore whether synaesthesia type influenced the results (e.g. [49]): only grapheme-colour synaesthesia (N = 29), synaesthesias including SSS (N = 21) and ‘other’ types (N = 29). All but three synaesthetes in the sequence-space group had more than one type of synaesthesia (see electronic supplementary material, figure S1).
(b). General procedure
Online participants completed the study in LimeSurvey (https://www.limesurvey.org/). The opening webpage informed participants about the study's purpose and duration and invited them to a synaesthesia consistency test (see electronic supplementary material). Next, participants gave online informed consent. Subsequently, a synaesthesia screening questionnaire (5 min), the AQ (10 min) and the GSQ (10 min) were completed. After completing the GSQ, participants received feedback and clarifications on their AQ and GSQ scores, including a general explanation about the questionnaires, comparisons of their own scores to normative data and the information that the AQ was not a diagnostic instrument. Instructions and weblinks to the motion coherence task (5 min) and embedded figures task (10 min) were provided. Feedback on the performance was given after each task. The entire experiment took approximately 50 min.
Participants in the laboratory had a slightly different task order. They started with the two experimental tasks (and an added visual illusions task reported elsewhere [32]). Instructions were provided on paper and by the researcher. Next, the synaesthesia consistency test, AQ and GSQ were completed. If the allotted laboratory time was insufficient, the GSQ was completed on a voluntary basis at home. At the end of the laboratory session, participants were debriefed on the research purpose and hypothesis.
(c). Questionnaires
(i). Synaesthesia screening questionnaire
Participants reported types of synaesthesia by ticking pre-set boxes and free typing. If synaesthesia was reported, detailed questions on synaesthesia characteristics were presented (e.g. ‘Since when have you experienced synaesthesia?’). Demographic and health-related questions were used to exclude individuals with poor eye-sight, ASD/psychiatric conditions, etc.
(ii). Autism Spectrum Quotient
The AQ assesses autistic traits in non-clinical populations [37] and consists of 50 statements to which individuals agree or disagree on a four-point Likert scale (e.g. ‘I tend to notice details that others do not’, ‘I find social situations easy’). The Dutch version (AQ-NL, [55]) uses the full Likert scale (slightly (dis)agree to definitely (dis)agree) resulting in a minimum score of 50 and a maximum score of 200; a score above 145 is within the ASD range. Aside from the cumulative score (AQ-Total), the AQ has five subscales: Attention to detail, Social skills, Communication, Attention switching and Fantasy. We specifically hypothesized that synaesthetes would score higher on AQ-Attention-to-detail [6,7] and AQ-Total [32].
Data analysis. AQ-Total and the five subscores were subjected to ANCOVAs including Group as a between-subjects factor and age as a covariate of no interest.
(iii). Glasgow Sensory Questionnaire
The GSQ consists of 42 questions (e.g. ‘Do you find certain sounds and/or pitches annoying?’) assessing hypersensitivity and hyposensitivity across seven sensory modalities (Visual, Auditory, Olfactory, Proprioceptive, Gustatory, Tactile and Vestibular). Individuals with ASD or a high AQ typically score higher [35]. We used the Dutch version recently validated by Kuiper et al. [34]. We hypothesized higher total GSQ scores for synaesthetes [6,7].
Data analysis. Total GSQ scores were analysed with an ANCOVA including Group (between-subjects) and age as a covariate of no interest. In an exploratory analysis, hypersensitivity and hyposensitivity subscales for the seven sensory modalities were subjected to a 2 × 7 repeated-measures ANOVA with Group as between-subject factor and age as a covariate of no interest.
(d). Visual tasks
(i). Motion Coherence task
On each 600-ms motion coherence trial, 200 white dots (diameter, 0.15°) moved with a speed of 6° s−1 across an 11.7 by 11.7 cm grey square background (see electronic supplementary material, figure S2). A subset of dots moved in a coherent direction: participants indicated the direction of coherent motion using arrow keys (right, left, up and down). Individual dot lifetime was 60 ms, discouraging the ‘local’ strategy of tracking individual dots ([56]; see Simmons et al. [12] for an overview of MC parameters in ASD studies). Three staircase runs of 60 trials began with 50% of dots moving coherently. After each correct trial, the coherence level for the next trial was reduced logarithmically, dividing by 100.1; if incorrect, the coherence level was multiplied by 100.1, making the task easier. The minimum motion coherence level was close to 0, the maximum 1.0 (100% coherent dots).
The experiment was programmed in HTML 5 (HTML/CSS/JavaScript) running in an Internet browser. The online participants used their home devices to complete the task. As arrow keys were needed for the response, we deduce online participants completed the experiment on a device with a keyboard. For the laboratory participants, the task was run in Google Chrome and displayed on a 24′ BenQ screen (1920 × 1080 resolution) controlled by a Windows 7 Dell computer.
Data analysis. The end scores of the three staircase runs (trials 60, 120, 180) were averaged to obtain an overall MCT for each participant, defined as the percentage of coherent dots necessary to detect the coherent motion.
(ii). Embedded figures task
We implemented the Leuven Embedded Figures Test online [50]. On each trial, a target stimulus was presented above three embedding stimulus contexts (electronic supplementary material, figure S3A), and the participants identified the embedding context containing the target as fast and accurately as possible using their mouse or touchpad. Error rates and reaction times were recorded.
The 16 target stimuli consisted of 3, 4, 6 or 8 lines (electronic supplementary material, figure S3B, see also De-Wit et al. [50], and see the Data Accessibility statement for how to access the complete stimulus set). Half the targets were closed forms, half were open forms; half were symmetric and half asymmetric. The difficulty of target detection was manipulated by modifying the number of target lines that continued into the embedding context (0%, 34%, 64% and 100% of lines on average, electronic supplementary material, figure S3C [50]). The 100% continuous condition was the hardest. Sixteen target stimuli appeared once at each difficulty level, resulting in 64 experimental trials. Participants started with 12 practice trials. Visual feedback was given on every trial (green or red border around chosen context), and after an incorrect response, participants re-tried until succeeding. After trial completion, an arrow appeared allowing the participants to continue to the next trial. The task took approximately 10 min.
Targets and contexts consisted of dark grey line stimuli on a white background (electronic supplementary material, figure S3). The background screen was light grey. The experiment was programmed in HTML 5 (HTML/CSS/JavaScript) running in an Internet browser. The online participants used their home devices to complete the task, and for the laboratory participants, the task was run in Google Chrome, displayed on a 24′ BenQ screen (1920 × 1080 resolution) controlled by a Dell Windows 7 computer.
Data analysis. Error percentages and reaction times were calculated for each subject and stimulus condition. Reaction times to incorrect trials and reaction time outliers of ±2 s.d. from the subject and condition mean were removed. The participants performing more than 2 s.d. away from their group mean on overall error rates or reaction times (RTs) were removed prior to analysis.
3. Results Study 1
All statistical tests were two-sided and with α = 0.05. The descriptive statistics of all main dependent variables are listed in electronic supplementary material, table S2.
(a). Autism Quotient
Our predictions were partly confirmed: synaesthetes (N = 79) scored significantly higher than non-synaesthetes (N = 76) on AQ-Attention-to-detail score (26.7 versus 23.9, F1,152 = 9.46, p = 0.002, figure 1), but not on AQ-Total (110.7 versus 106.0, F1,152 = 2.37, p = 0.126, ).
Figure 1.
Average Autism Quotient subscores for synaesthetes (N = 79) and non-synaesthetes (N = 76). **p < 0.01, *p < 0.05. Detail, AQ-Attention-to-detail; Social, AQ-Social skills; Attention, AQ-Attention switching; Comm., AQ-Communication; Fantasy, AQ-Fantasy. Error bars denote the standard deviation. (Online version in colour.)
We explored group differences for the remaining AQ subscales (figure 1). Synaesthetes scored lower on AQ-Fantasy (18.4 versus 20.0, F1,152 = 5.11, p = 0.025, ) and higher on AQ-Social skills (20.7 versus 19.4, F1,152 = 7.44, p = 0.007, ). No differences were found for AQ-Attention switching or AQ-Communication (all F1,152 < 1, n.s.). Only the difference on AQ-Social skills survived Bonferroni correction for multiple comparisons (corrected α = 0.01).
Several synaesthetes (N = 8) and non-synaesthetes (N = 2) obtained an elevated AQ score of greater than 130 (below the clinical cut-off of 145 but nonetheless elevated). This raises the possibility that our sample included individuals with ASD without a formal diagnosis. To exclude the possibility that our AQ group effects were driven by potential undiagnosed individuals, we repeated the analyses without those 10 individuals. The group effects remained: AQ-Attention-to-detail, F1,142 = 8.33, p = 0.005, ; AQ-Social skills, F1,142 = 6.73, p = 0.010, . Both the effects survived Bonferroni correction for multiple comparisons (corrected α = 0.01).
Dose effects of synaesthesia were found for AQ-Social skills: individuals with more types of synaesthesia scored higher (r79 = 0.243, p = 0.031, 95% CI (0.039, 0.426)). This effect did not survive correction for multiple comparisons across all five subscales of the AQ. The other dose effects were not significant: AQ-Total r79 = 0.154, p = 0.17; AQ-Attention-to-detail r79 = 0.086, p = 0.45; AQ-Communication r79 = 0.052, p = 0.65; AQ-Attention switching r79 = 0.179, p = 0.11 and AQ-Fantasy r79 = −0.090, p = 0.43. This was contrary to our expectations of synaesthesia dose effects on AQ-Attention-to-detail [7].
(b). Glasgow Sensory Questionnaire
As predicted, synaesthetes (N = 74) scored higher than non-synaesthetes (N = 62): 54.3 versus 46.5 (F1,133 = 4.41, p = 0.038, ). Overall, our sample's GSQ scores are higher than those of the normative population (33.5 ± 14.5, [34]), suggesting recruitment bias to our perceptual study. Synaesthetes with high AQ-Total (greater than 130) scored 65.2 ± 22.0 on the GSQ, in line with reported scores for high-AQ, synaesthetic or diagnosed ASD individuals (e.g. [7,34]).
As predicted GSQ scores correlated with AQ-Total across the entire sample (r136 = 0.360, p < 0.001, 95% CI (0.227, 0.498)) and for both groups separately (synaesthetes r74 = 0.351, p = 0.002, 95% CI (0.141, 0.544); non-synaesthetes r62 = 0.329, p = 0.009, 95% CI (0.064, 0.514)). Correlations of AQ-Attention-to-detail and AQ-Social skills with GSQ scores were explored because of group effects on these AQ subscales. For synaesthetes, GSQ score correlated positively with AQ-Attention-to-detail (r74 = 0.460, p < 0.001, 95% CI (0.247, 0.628); AQ-Social r74 = 0.096, n.s.). For non-synaesthetes, the AQ-Attention-to-detail correlation with the GSQ score revealed a trend (r62 = 0.223, p = 0.081, 95% CI (−0.010, 0.406)), while AQ-Social skills correlated positively with the GSQ score (r62 = 0.303, p = 0.017, 95% CI (0.005, 0.568)).
For the GSQ hyper/hyposensitivity and sensory modality subscores, group effects were explored (figure 2). A repeated-measures ANOVA revealed no three-way interaction of Sensitivity (hyper/hypo) by Sensory modality (7 subscales) by Group (F6,798 = 1.56, p = 0.16) and no Sensory modality × Group interaction (F6,798 = 1.57, p = 0.15), suggesting a similar across-modality distribution for both groups. The Sensitivity by Group interaction was significant (F1,133 = 5.13, p = 0.025, ): synaesthetes scored higher on hypersensitivity subscales (F1,133 = 6.91, p = 0.010, ) but not on hyposensitivity subscales (F1,133 = 1.47, n.s.). Exploration of group effects on separate sensory modalities was not justified.
Figure 2.
Average Glasgow Sensory Questionnaire subscores for synaesthetes (N = 74) and non-synaesthetes (N = 62). Vis, visual; Aud, auditory; Gus, gustatory; Olf, olfactory; Tac, tactile; Ves, vestibular; Prop, proprioceptive. Error bars denote the standard deviation. (Online version in colour.)
In both synaesthetes and non-synaesthetes, hypersensitivity and hyposensitivity scores (total scores collapsed over modalities) correlated significantly, r71 = 0.629, p < 0.001, 95% CI (0.448, 0.774) and r59 = 0.697, p < 0.001, 95% CI (0.487, 0.827), respectively. Using Fisher's r to z transforms, we compared the correlation coefficients between the two groups and these did not differ (z = −0.67, p = 0.50).
A marginal dose effect of synaesthesia on GSQ scores (r74 = 0.223, p = 0.056, 95% CI (0.010, 0.453)) suggests that having more types of synaesthesia is associated with a higher score (electronic supplementary material, figure S4A).
(c). Motion Coherence task
Fifty-one synaesthetes and 52 non-synaesthetes completed the motion coherence task.2 One synaesthete and one control completed only two staircase runs but were retained in the analysis. Serious task completion could not be verified for four individuals with an MCT of 1.0, leaving 49 synaesthetes and 50 non-synaesthetes for analysis. Overall, the MCT was 0.40 ± 0.23 indicating high task difficulty [12].
As hypothesized, the MCT was higher in synaesthetes (0.45 versus 0.34, F1,96 = 8.30, p = 0.005, figure 3): synaesthetes needed more coherently moving dots to detect the global direction of motion.
Figure 3.

MCTs for synaesthetes (N = 49) and non-synaesthetes (N = 50). **p < 0.01. Error bars denote the standard deviation. Individual participants' thresholds are indicated by diamonds (synaesthetes) and triangles (non-synaesthetes). (Online version in colour.)
In synaesthetes, the MCT did not correlate significantly with any AQ-(sub)scale (all r49 between (−0.103, 0.137), all p > 0.35), but correlated positively with GSQ scores (r48 = 0.471, p = 0.001, 95% CI (0.241, 0.658)); synaesthetes who scored high on the GSQ as an index of sensory sensitivity performed worse on motion coherence. Correlations with the MCT for the GSQ hypersensitivity and hyposensitivity scores of the synaesthetes were r48 = 0.426, p = 0.003, 95% CI (0.194, 0.624) and r48 = 0.417, p = 0.003, 95% CI (0.175, 0.617), respectively, providing no indication that hypersensitivity scores in particular were related to MCT performance. For controls, the MCT did not correlate with any AQ-(sub)scale (all r50 between (−0.091, 0.079), all p > 0.52) nor GSQ score (r50 = 0.036, n.s.).
There was a significant dose effect of synaesthesia on the MCT (r49 = 0.309, p = 0.031, 95% CI (0.000, 0.595), indicating that individuals with more types of synaesthesia had a higher MCT (poorer task performance, electronic supplementary material, figure S4B).
(d). Embedded figures task
Forty-nine synaesthetes and 70 non-synaesthetes completed the embedded figures task (EFT) with high accuracy (88.5 ± 7.1% correct in 4.3 ± 2.7 s). No one responded unrealistically fast (greater than 15% incorrect responses within less than 1.5 s, see [50]). The reaction time outlier percentage was 4.9% (synaesthetes 5.2%, non-synaesthetes 4.7%). Five synaesthetes and six non-synaesthetes were excluded for performing more than 2 s.d. away from their group mean on overall error rates or RTs. A speed-accuracy trade-off between overall error percentages and overall RTs was present in both groups, synaesthetes: r44 = −0.486, p < 0.001, 95% CI (−0.649, −0.292), non-synaesthetes: r64 = −0.536, p < 0.001, 95% CI (−0.701, −0.326).
We hypothesized that synaesthetes would outperform non-synaesthetes on the EFT. We conducted a multivariate repeated-measures ANOVA with error rates and reaction times as dependent variables and the within-subjects factor Continued lines (0%, 34%, 64% and 100%), the between-subjects factor Group (synaesthetes and non-synaesthetes) and age as a covariate of no interest. There was a main effect of Continued lines (multivariate F6,630 = 12.9, p < 0.001, ) and an interaction of Continued lines with Group, multivariate F6,630 = 2.14, p = 0.047, .
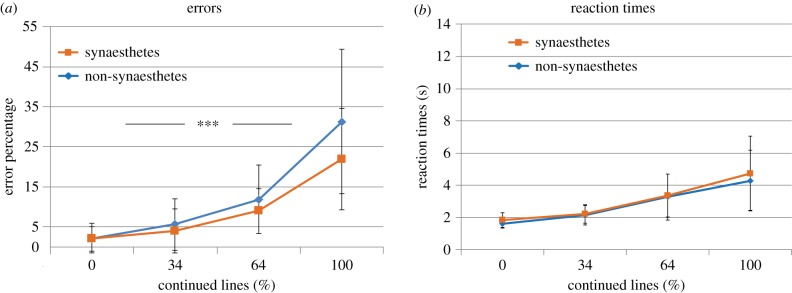
Univariate tests revealed that the error rates (figure 4a) showed a main effect of Continued lines (F3,315 = 26.2, p < 0.001, ), a Group × Continued lines interaction (F3,315 = 3.72, p = 0.026, ) and a marginal effect of Group (F1,105 = 3.79, p = 0.054, ). Follow-up tests on separate levels of Continued lines revealed that synaesthetes made less errors on level 1 (3.7 versus 1.1%, F1,105 = 6.89, p = 0.010, ) and level 4 (28.8 versus 20.1%, F1,105 = 5.78, p = 0.018, ) but not on level 2 or 3 (all F1,105 < 1, n.s.). Thus, synaesthetes outperformed non-synaesthetes on the easiest and hardest difficulty level.
Figure 4.
(a) Embedded figures task error rates and (b) reaction times for synaesthetes (N = 44) and non-synaesthetes (N = 64). ***p < 0.001, **p < 0.01 and *p < 0.05. Error bars denote the standard deviation. The x-axis denotes increasing levels of difficulty. (Online version in colour.)
Univariate tests of reaction times (figure 4b) showed no main effects of Group or Continued lines (F1,105 = 2.88, p = 0.093, ; F3,315 = 0.923, p = 0.361, respectively) and no Group × Continued lines interaction (F3,315 = 0.755, p = 0.417). Increased RTs for synaesthetes in figure 4b are largely explained by age, which correlated with RTs across our entire sample (r108 = 0.605, p < 0.001, 95% CI (0.326, 0.772)). Note that age was a covariate in the analyses to control for age effects and descriptive statistics are plotted in figure 4b (without age correction).
We explored correlations of EFT performance with AQ, GSQ and MCT for overall EFT error rates and RTs. No correlations were found for synaesthetes (all p > 0.11) nor for non-synaesthetes (p > 0.24). There were no significant dose effects of synaesthesia on the synaesthetes' EFT error rates (r44 = −0.072, n.s.) nor reaction times (r44 = −0.012, n.s.).
(e). Subgroup analyses
We explored whether the type of synaesthesia(s) (only grapheme-colour synaesthesia (N = 29), synaesthesias including SSS (N = 21) and ‘other’ types (N = 29)) influenced the results. Age and number of synaesthesias were included as covariates of no interest in all analyses, and we analysed those main dependent variables on which a significant group difference between synaesthetes and non-synaesthetes was found.
Subgroup had a marginal effect on AQ-Attention-to-detail (F2,74 = 2.51, p = 0.088 and ), but differences between pairwise subgroups did not reach significance (scores were 27.8, 27.3 and 25.3, respectively). Subgroup did not affect AQ-Social skills (F2,74 = 0.158, n.s.) nor the GSQ score (F2,69 = 0.823 and p = 0.443). The MCT did differ between the subgroups (F2,44 = 5.26, p = 0.009 and ) with grapheme-colour synaesthetes scoring better than sequence-space (0.25 versus 0.48, F1,23 = 4.40, p = 0.047 and ) and ‘other’ synaesthetes (0.25 versus 0.56, F1,32 = 6.06, p = 0.019 and ). The grapheme-colour subgroup did not perform significantly better than non-synaesthetes (F1,32 = 1.17 and p = 0.284). Given that a dose effect of synaesthesia was present for the MCTs (electronic supplementary material, figure S4B) and the synaesthetes in the grapheme-colour subgroup experienced only one form of synaesthesia, we additionally compared all synaesthetes experiencing grapheme-colour synaesthesia (N = 59) with all other synaesthetes (N = 20) on the motion coherence task and found no difference in performance (F1,44 = 0.343, p = 0.561). This suggests the number of synaesthesia is the main factor driving motion coherence performance. On the embedded figures task, there was no main multivariate effect of subgroup (F4,78 = 1.27, p = 0.287), but there was a marginal multivariate interaction of subgroup with difficulty (F12,234 = 1.70, p = 0.067, ). Pairwise subgroups comparisons were not significant for either the main effect or the interaction.
4. Methods Study 2: sequence-space synaesthesia cohort
(a). Participants
Twenty sequence-space synaesthetes and 21 non-synaesthetes took part in the experiment taking place in the laboratory. Participation followed on a spatial navigation experiment [57]. SSS was verified by means of an online screening questionnaire, a consistency test and a drawing test [57]. The consistency test was a spatial computer task [58], in which participants indicated the location of their synaesthetic experiences [57]. Participants' drawings of days, months and numbers allowed verification of SSS by visual inspection of the drawings’ characteristics. For details on SSS classification, see fig. 7 and the supplementary material of Van Petersen et al. [57].
One synaesthete's SSS did not meet diagnostic criteria and another synaesthete reported autism; one control reported grapheme-colour synaesthesia. Therefore, 18 sequence-space synaesthetes (mean age, 21.9 ± 4.62 years, 2 males) and 20 non-synaesthetes (mean age, 21.6 ± 1.8 years, 2 males) remained in the analyses. Age did not differ between groups (t36 = −0.302, n.s.). Five sequence-space synaesthetes reported additional synaesthesias (e.g. grapheme-colour, day-colour), but the majority experienced only SSS. Participants gave informed written consent prior to participation and were compensated with 15 euros or 1.5 credit points. The study was approved by the ECSS at Radboud University, Nijmegen.
(b). General procedure
Questionnaires and visual tests were identical to Study 1. Participants were invited to the laboratory on the basis of the SSS screening questionnaire and first completed the consistency test, drawing task and spatial navigation task as described in Van Petersen et al. [57]. Next, the motion coherence task and the embedded figures task were completed, running in Google Chrome on a Dell Windows 7 computer with a 24′ BenQ screen (1920 × 1080 resolution). Task settings and instructions were identical to those of Study 1. If time permitted, the AQ and GSQ were completed in the laboratory (participants were paid for 1.5 h); otherwise, participants voluntarily completed the questionnaires at home. One sequence-space synaesthete did not complete the questionnaires. Dose effects of synaesthesia were not considered in Study 2 because participants predominantly experienced only SSS.
5. Results Study 2
All statistical tests were two-sided and with α = 0.05. The descriptive statistics of the main dependent variables are listed in electronic supplementary material, table S3. Additional correlation analyses for the GSQ, motion coherence and embedded figures task are summarized in the electronic supplementary material.
(a). Autism Quotient
Sequence-space synaesthetes scored numerically higher on AQ-Total and AQ-Attention-to-detail (104.6 versus 102.7 and 23.9 versus 22.2, respectively, electronic supplementary material figure S5), resembling the results of Study 1, but group differences were not significant (AQ-Total, t35 = −0.518, n.s.; AQ-Attention-to-detail, t35 = −1.23 and p = 0.23). There were no group differences on any other AQ subscale (all t35 < 1) except for a trend on AQ-Fantasy (synaesthetes, 18.5; non-synaesthetes, 20.1; t35 = 1.67 and p = 0.10).
(b). Glasgow Sensory Questionnaire
As predicted, GSQ scores were higher in sequence-space synaesthetes (47.9 versus 42.6), replicating Study 1, but the group difference was not significant (t35 = −1.03, p = 0.29, electronic supplementary material, figure S6). For an exploratory analysis of hyper/hyposensitivity and sensory modality subscales, see electronic supplementary material.
(c). Motion Coherence task
Questionable data of one control were removed (MCT of 1.0 with fast reaction times), leaving 18 sequence-space synaesthetes and 19 non-synaesthetes for analysis. One synaesthete and one control completed only two staircase runs but were included in the analysis. Overall, the MCT was 0.33 ± 0.17. Contrary to our expectations, sequence-space synaesthetes outperformed non-synaesthetes (figure 5), displaying lower MCTs (27.1 versus 39.2% coherent dots, t35 = 2.26, p = 0.031).
Figure 5.

Motion coherence thresholds for sequence-space synaesthetes (N = 18) and non-synaesthetes (N = 19). *p < 0.05. Error bars denote the standard deviation. Individual participants’ thresholds are indicated by diamonds (synaesthetes) and triangles (non-synaesthetes). (Online version in colour.)
(d). Embedded figures task
Eighteen sequence-space synaesthetes and 20 non-synaesthetes completed the embedded figures task (accuracy 89.5 ± 6.2% in 3.15 ± 1.29 s). No one responded unrealistically fast [50]. The reaction time outlier percentage was 5.6% (synaesthetes, 5.4% versus non-synaesthetes, 5.8%). Two synaesthetes and two non-synaesthetes were excluded for performing more than 2 s.d. away from their group mean on overall error rates or RTs. A speed-accuracy trade-off between overall errors and overall reaction times was significant for sequence-space synaesthetes (r16 = −0.626, p = 0.009 and 95% CI (−0.859, −0.289)), and marginal for non-synaesthetes (r18 = −0.452, p = 0.060 and 95% CI (−0.756, −0.037)).
A multivariate repeated-measures ANOVA with error rates and reaction times as dependent variables was run with within-subject factor Continued lines (0, 34, 64 and 100%) and between-subject factor Group (sequence-space synaesthetes and non-synaesthetes). The multivariate analysis revealed a main effect of Continued lines (F6,192 = 22.85, p < 0.001 and ), but no Continued lines by Group interaction (F6,192 = 0.978 and p = 0.44). Univariate analysis on the error rates (figure 6a) revealed a main effect of Continued lines (F3,96 = 54.7, p < 0.001 and ) and a marginal effect of Group (F1,32 = 3.29, p = 0.079 and ), but no interaction (F3,96 = 1.88, p = 0.17 and ). Thus, errors increased with difficulty; synaesthetes numerically made fewer errors than non-synaesthetes. For univariate reaction times (figure 6b), there was a main effect of Continued lines (F3,96 = 50.9, p < 0.001 and ), no main effect of Group (F1,32 = 0.381, p = 0.541) and no interaction (F3,96 = 0.257 and p = 0.724), indicating reaction times increased with difficulty but did so similarly for both groups.
Figure 6.
(a) Embedded figures task error rates and (b) reaction times for sequence-space synaesthetes (N = 16) and non-synaesthetes (N = 18). Error bars denote the standard deviation. The x-axis denotes increasing levels of task difficulty. (Online version in colour.)
6. Discussion
We hypothesized that synaesthetes would display atypical sensory sensitivity and atypical sensory perception akin to individuals with ASD. We assessed autistic traits, sensory sensitivity and visual perception in synaesthetes. In Study 1, a large heterogeneous group of synaesthetes showed enhanced autistic traits (Autism Quotient), atypical sensory sensitivity (GSQ), decreased global motion processing (Motion Coherence task) and enhanced processing of local elements (embedded figures task). The findings resemble atypical sensory sensitivity and a bias towards local perception as reported for autism, suggesting that synaesthetes share these atypicalities. In Study 2, most findings were replicated qualitatively in sequence-space synaesthetes, but group differences with non-synaesthetes were non-significant; on the motion coherence task, sequence-space synaesthetes unexpectedly performed better than non-synaesthetes. We discuss the results in more detail below.
(a). Questionnaires
On the Autism Quotient, synaesthetes (Study 1) scored higher on the Attention-to-detail subscale, fitting with characteristics of perception in ASD and with previous reports of enhanced AQ-Attention-to-detail in synaesthetes [6,7,33]. The result strengthens the hypothesis of shared atypical perception in synaesthesia and autism. Unexpectedly, synaesthetes also scored higher than non-synaesthetes on AQ-Social skills. In Ward et al. [7], synaesthetes scored significantly higher than controls on ‘AQ-Other’—all AQ subscales except Attention-to-detail. However, as far as we know, a higher synaesthete score on AQ-Social skills specifically has not been reported. This reminds us that the entire synaesthesia phenotype should be considered when looking for commonalities with autism. It is noteworthy that a possible dose effect of synaesthesia emerged only for AQ-Social skills (even though this did not survive corrections for multiple comparisons), suggesting that having more types of synaesthesia increases the vulnerability to specific autism symptomatology [7]. The AQ is a self-report measure of autistic traits and not a diagnostic tool assessing autism. So far, all synaesthete studies have used the AQ: it would be informative if future research would deploy other measures of autism symptomatology such as the Social Responsiveness Scale [59] or structured diagnostic observations or interviews (ADOS [60] or ADI-R [61]).
On the GSQ, synaesthetes (Study 1) obtained a significantly higher total GSQ score, resembling atypical sensory sensitivity to the environment in autism. Synaesthetes specifically scored higher than non-synaesthetes on hypersensitivity subscales, suggesting that atypical sensory sensitivity in synaesthetes mainly manifests as hypersensitivity; in Ward et al. [6], however, both hypersensitivities and hyposensitivities were present. The pattern of responses across the sensory modalities was similar in synaesthetes and non-synaesthetes (e.g. higher subscores for the auditory modality, lower scores for the vestibular modality), and followed patterns expected from previous studies using the GSQ [34–36]. Our high-AQ synaesthetes scored in accordance with the literature on high-AQ individuals (clinical or synaesthetes). Altogether the GSQ responses from our synaesthetes appear reliable and confirm atypical sensory sensitivity in synaesthetes.
In Study 2, the autistic-like profile of the Study 1 synaesthetes was not replicated, although sequence-space synaesthetes did score numerically higher on the AQ and GSQ subscales. One reason for the absence of any group differences could be the smaller sample size of Study 2 (18 versus 79 synaesthetes). Detecting rather subtle effects is more difficult in a smaller population. Second, most participants in Study 2 had only one form of synaesthesia, namely SSS. Because of the dose effects [7], individuals with only one type of synaesthesia may display less autistic-like perception than individuals with multiple types. Indeed, Study 2 synaesthetes experienced on average 1.33 ± 0.59 forms of synaesthesia, a lower ‘dose’ than Study 1 participants (1.89 ± 1.0). In Mealor et al. [33], sequence-space synaesthetes (N = 121) scored higher than grapheme-colour synaesthetes (N = 43) on local/global perceptual questions, and in Ward et al. [6], sequence-space synaesthetes scored numerically higher than grapheme-colour synaesthetes on AQ-Attention-to-detail. These findings suggest studying these characteristics in a larger sample of sequence-space synaesthetes would be relevant.
(b). Visual perception
On the embedded figures task, synaesthetes in Study 1 outperformed non-synaesthetes on accuracy but not on reaction times, similar to Ward et al. [7]. These results mirror enhanced performance of individuals with ASD on this task (e.g. [38,39]), which is the general consensus in the ASD literature even though not all studies—especially those on adults—find that individuals with ASD perform better [12,42,62]. In our study, synaesthetes made fewer errors than non-synaesthetes on the easiest and hardest difficulty levels, where either no target lines (easiest) or all lines (hardest) continued into the embedding context. In the hardest condition, the task required considerable effort (as indicated by an error percentage of approx. 25%, figure 4) and enhanced attention to local elements may have benefitted the synaesthetes. Our hardest difficulty level seems to match best with the difficulty level of the task in Ward et al. [7], with error percentages above 20% and reaction times around 8 s. Together, our studies suggest that synaesthetes benefit the most when the task is hard. It should be noted, however, that in our study, non-synaesthetes also made more errors on the easiest difficulty level, while they did not on the intermediate difficulty levels. A possible explanation may be that non-synaesthetes emphasized speed during the task: in exploratory group comparisons of reaction times, non-synaesthetes were significantly faster than synaesthetes only at difficulty level 1. Emphasis on the speed can, however, not explain why non-synaesthetes made more errors at difficulty level 4, the hardest condition.
Our non-significant Study 2 results on the embedded figures task suggestively corroborate the results of Study 1. Sequence-space synaesthetes performed numerically better than non-synaesthetes on the most difficult task levels, and the group difference with non-synaesthetes was marginal (p = 0.079) at identical reaction times. The similarities with Study 1 can be seen when comparing figures 4 and 6. These findings support the conclusion that synaesthetes outperform non-synaesthetes on the embedded figures task and show enhanced processing of local visual elements.
The increased MCTs for synaesthetes (Study 1) are in line with increased motion thresholds in ASD (e.g. [23,24,45]). Whether individuals with autism specifically show enhanced perception of detail or whether global processing deficits are also present is under discussion [11,13,15]. From a meta-analysis, it appears that global visual processing mostly takes more time and effort for individuals with autism and is especially affected when low-level information is incongruent with the global level [17]. Difficulties with global motion processing, however, have proven rather robust [12,15,63], even though there are exceptions [46,48]. In our task, the short individual dot lifetime (60 ms) forced the participants to use global motion information [56] and ensured that a bias towards local perception (and slower global processing) would result in higher motion thresholds. Moreover, in this task, indeed, the low-level information in many of the individual dots is incongruent with the global direction of motion.
The elevated thresholds for synaesthetes corroborate an earlier study on N = 10 colour synaesthetes [22]. To our knowledge, our study is the first to replicate this finding in a much larger sample of synaesthetes (N = 79). Ward et al. [49] showed that in N = 34 synaesthetes, sequence-space synaesthetes (N = 22) performed better than those without it. In our Study 2, sequence-space synaesthetes also displayed enhanced performance—lower thresholds—on the motion coherence task. It is possible that motion deficits are less pronounced in sequence-space synaesthetes because of the specific type of synaesthetic experiences this entails. We also suggest a role for dose effects of synaesthesia, given that our subgroup analyses revealed that grapheme-colour synaesthetes with only one form of synaesthesia performed better than the other synaesthetes, and individuals with more types of synaesthesia displayed higher MCTs (electronic supplementary material, figure S4B). This figure shows that Study 1 synaesthetes with only one type of synaesthesia (e.g. also the grapheme-colour synaesthesia subgroup) score within the range of Study 1 controls (figure 4). Given that most sequence-space synaesthetes of Study 2 experienced just one type of synaesthesia, their MCTs could be relatively low for that reason. More generally, our motion coherence task comprised fewer trials (180) than previous studies because it was run online; it needed to be fast. Also, our task was rather difficult as indicated by relatively high MCTs compared to earlier studies [12].
Future studies could assess perception in synaesthetes with additional local/global tasks such as visual illusions (e.g. Ebbinghaus and Müller-Lyer illusions) [32,64] or Navon paradigms with hierarchical local/global stimuli [65]. Future studies will have to show whether enhanced processing of detail in synaesthetes is as pronounced as in autism and whether only local biases [17] or additional global processing deficits play a role.
(c). Possible neural mechanisms
Could synaesthesia and autism be explained by a similar underlying neural mechanism? There is evidence of local hyperconnectivity in both synaesthesia [66,67] and ASD (e.g. [68,69]) with reduced long-range connectivity and enhanced recruitment of ventral–occipital areas during performance of visual tasks (e.g. [67,70]). This could explain why synaesthetes and individuals with autism excel at visual tasks in which global contexts, involving long-range feedback, have to be ignored. Relatedly, for both synaesthesia [18,20] and autism [56,71,72], atypical responses in the parvocellular visual system have been reported, which is important for processing spatial detail and colours. Hyperresponsivity of the parvocellular system fits with enhanced perception of details in both synaesthesia and autism and the many forms of colour synaesthesias [1].
Another potential mechanism contributing to both conditions could be a disrupted balance in excitation and inhibition in the brain [73,74]. For autism, excess excitation has been reported with hyper excitability of the cortex leading to increased vulnerability to epilepsy [73]. Grapheme-colour synaesthesia is associated with hyperexcitable visual cortex [74,75]. Cortex that is easily excited leads to higher than normal noise levels during cortical processing, which can disrupt development because cortical selectivity comes about through balanced excitatory and inhibitory processes. Oversensitivity to sensory stimulation can result from hyperexcitable cortex. Relatedly, enhanced local clustering and diminished global brain connectivity has been reported for synaesthesia [66].
Recently, various psychiatric disorders including autism have been interpreted in a predictive coding framework [76–81], which is able to account for a wide variety of autism characteristics. Predictive coding involves active predictions of sensory inputs by the brain (priors), which are updated by actual sensory input that matches or does not match the prediction (prediction error). Predictive coding theories of autism propose that either predictions are too weak [78] or error signals (sensory input) are invariantly interpreted as strong and important [79]. Both accounts predict perception in individuals with autism to be dominated by feedforward, excitatory sensory input. In synaesthete development, hyperexcitable cortex can similarly lead to aberrant weighting of sensory input. Future (modelling) studies could attempt to explain both autism and synaesthesia within the predictive coding framework.
(d). Generalizability and reliability of our findings
Females were overrepresented in all groups in our sample, as often is the case in synaesthesia studies (e.g. [6,7,57]) even though synaesthesia is equally prevalent in men and women [1]. Gender is relevant because AQ is typically higher in males [37,55]. With fewer males in our samples, we may underestimate the absolute elevation of AQ in synaesthetes. Exploring AQ scores across gender in synaesthetes (Study 1), we confirmed that males (N = 8) scored higher than females (N = 71) across all subscales and significantly higher on AQ-Total (123.6 versus 109.3; t77 = 2.58, p = 0.012) and AQ-Social skills (25.5 versus 21.6; t77 = 2.15, p = 0.035).
Details of the recruitment method may limit the generalizability of our study. Many of our participants were recruited via advertisements in popular science media and through the university. It is therefore no surprise that the vast majority of our participants reported high levels of education. In this respect, our sample is not representative of the wider population and future studies could attempt to recruit from a wider variety of locations. Another potential influence of recruitment is that the studies were advertised as related to perception and the senses (especially Study 1). Attracting participants interested in perception may have stacked the deck against finding group differences.
Individual differences in the spatial location of synaesthetic experience may also influence experimental outcomes. Associator synaesthetes experience their synaesthesia ‘in the mind's eye’, while projectors experience their synaesthesia in a location in space, for instance, overlaid on the inducing stimulus [82,83]. Projectors show behavioural and neurobiological differences compared to associators (e.g. [82–85]), including greater reliance on the visual cortex for their synaesthetic experience. Visual atypicalities of the kind studied here may be more pronounced in projector synaesthetes and may influence findings with regard to the relationship between synaesthesia and autism. This is an important area for future research.
Online testing, though important for increasing sample size, also brings its own limitations: there is less direct control of task completion and compliance with instructions. We took the usual precautions to inspect all data gathered online for validity (e.g. reaction time checks on the EFT data and visual inspection of all MCT staircases), and included a second cohort of synaesthetes tested in the laboratory (Study 2) allowing cross-validation. Any noise in the data due to online testing would not facilitate finding group differences, so if anything this has made the findings reported here more conservative.
7. Conclusion
We have presented evidence that synaesthetes have a perceptual style that is characterized by atypical sensitivity to the environment and enhanced attention to details. This pattern resembles perception in autism. We add to the growing body of literature showing synaesthesia and autism share perceptual characteristics and encourage future research on this relation. Understanding why synaesthesia is so prevalent in autism may reveal common mechanisms. Awareness of synaesthesia in ASD may improve therapies: could synaesthesia function as a biomarker facilitating recognition of particular autism subtypes, for instance, individuals with strong hypersensitivities? Differences in functional connectivity patterns in the brain have been linked to autism subtypes with certain brain–behaviour relationships [86]; synaesthesia could, for instance, relate to one of these subtypes. Recognizing synaesthesia in individuals with autism (for instance, by means of a simple questionnaire) can bring clarity about sensory symptoms. Moreover, (multiple) synaesthesia(s) may alert carers to the possibility of autism, aiding ASD diagnosis, for instance, in females where ASD diagnoses are often missed in childhood [87]. In short, awareness of the relationship between ASD and synaesthesia may help to improve the quality of life of individuals with ASD and can direct research efforts into understanding and treating sensory dysregulation in autism.
Supplementary Material
Acknowledgements
We thank Wilbert van Ham of the TSG for online task implementation; Martin E. Johansson, Viola Hollestein and İdil Bostan for their assistance with data coding; Marieke Kuiper for providing the Dutch translation of the Glasgow Sensory Questionnaire; Lee de-Wit for providing the Embedded Figures test stimuli and all participants for their time and effort in participating.
Endnotes
Lab participants were included to increase the number of non-synaesthetes who completed a synaesthesia consistency test to add to the validity of the results. Non-synaesthete results obtained online (N = 43) versus in the laboratory (N = 33) did not differ on any measure except for the reaction times in the EFT (F1,61 = 4.83, p < 0.05). Although laboratory participants responded faster, they did not make any more errors (F1,61 < 1), so this difference cannot explain the synaesthete findings on the EFT errors (see Results of Study 1).
Laboratory participants also performed a version of the motion coherence task with a dot lifetime of 600 ms instead of 60 ms. The order of the 600 and 60 ms conditions was counterbalanced across participants [32]. MC data of participants who completed the 600 ms version first (N = 19 of N = 36; 17 non-synaesthetes; 2 synaesthetes) were excluded to avoid potentially confounding learning effects of the 600 ms condition on performance in the 60 ms condition.
Ethics
All experiments were approved by the Ethics Committee of the Faculty of Social Sciences (ECSS) at Radboud University, Nijmegen. ECSS reference codes: Study 1 online: [ECSW2014-3107-230], Study 1 lab and Study 2: [ECG2012-2711-060]. All participants provided online or written informed consent prior to their participation.
Data accessibility
The raw data and analysis scripts are made available in a data repository, accessible through the following link: http://hdl.handle.net/11633/aab4636d. The complete stimulus set of the embedded figures task is publicly available on Figshare (https://dx.doi.org/10.6084/m9.figshare.3807885, https://dx.doi.org/10.6084/m9.figshare.3807894).
Authors' contributions
T.M.v.L. conceptualized and designed the study, collected and analysed the data, and wrote the paper; E.v.P. recruited/diagnosed SSS and collected and analysed SSS data; F.B. collected Study 1 lab data; M.D. contributed online synaesthesia consistency tests and data processing; R.v.L. contributed to data interpretation; all authors edited the paper and approved the manuscript.
Competing interests
We declare we have no competing interests.
Funding
This study was funded by a Netherlands Organization for Scientific Research (NWO) Veni grant to T.M.v.L. (grant no. 451.14.025) and an NWO Groot Nationaal Onderzoek grant to T.M.v.L. and M.D.
References
- 1.Simner J, Mulvenna C, Sagiv N, Tsakanikos E, Witherby SA, Fraser C, Scott K, Ward J. 2006. Synaesthesia: the prevalence of atypical cross-modal experiences. Perception 35, 1024–1033. ( 10.1068/p5469) [DOI] [PubMed] [Google Scholar]
- 2.Neufeld J, Roy M, Zapf A, Sinke C, Emrich HM, Prox-Vagedes V, Dillo W, Zedler M. 2013. Is synaesthesia more common in patients with Asperger syndrome? Front. Hum. Neurosci. 7, 847 ( 10.3389/fnhum.2013.00847) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baron-Cohen S, Johnson D, Asher J, Wheelwright S, Fisher S, Gregersen P, Allison C. 2013. Is synaesthesia more common in autism? Mol. Autism. 4, 40 ( 10.1186/2040-2392-4-40) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fombonne E. 2002. Epidemiological trends in rates of autism. Mol. Psychiatr. 7, S4–S6. ( 10.1038/sj.mp.4001162) [DOI] [PubMed] [Google Scholar]
- 5.American Psychiatric Association. 2013. Diagnostic and statistical manual of mental disorders, 5th edn Arlington, VA: American Psychiatric Publishing. [Google Scholar]
- 6.Ward J, Hoadley C, Hughes JEA, Smith P, Allison C, Baron-Cohen S, Simner J. 2017. Atypical sensory sensitivity as a shared feature between synaesthesia and autism. Sci. Rep. 7, 41155 ( 10.1038/srep41155) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ward J, Brown P, Sherwood J, Simner J. 2018. An autistic-like profile of attention and perception in synaesthesia. Cortex 107, 121–130. ( 10.1016/j.cortex.2017.10.008) [DOI] [PubMed] [Google Scholar]
- 8.Donaldson CK, Stauder JEA, Donkers FCL. 2017. Increased sensory processing atypicalities in parents of multiplex ASD families versus typically developing and simplex ASD families. J. Autism Dev. Disord. 47, 535–548. ( 10.1007/s10803-016-2888-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pellicano E. 2013. Sensory symptoms in autism: a blooming, buzzing confusion? Child Dev. Perspect. 7, 143–148. ( 10.1111/cdep.12031) [DOI] [Google Scholar]
- 10.Tavassoli T, Miller LJ, Schoen SA, Nielsen DM, Baron-Cohen S. 2014. Sensory over-responsivity in adults with autism spectrum conditions. Autism 18, 428–432. ( 10.1177/1362361313477246) [DOI] [PubMed] [Google Scholar]
- 11.Happé F, Frith U. 2006. The weak coherence account: detail-focused cognitive style in autism spectrum disorders. J. Autism Dev. Disord. 36, 5–25. ( 10.1007/s10803-005-0039-0) [DOI] [PubMed] [Google Scholar]
- 12.Simmons DR, Robertson AE, McKay LS, Toal E, McAleer P, Pollick FE. 2009. Vision in autism spectrum disorders. Vision Res. 49, 2705–2739. ( 10.1016/j.visres.2009.08.005) [DOI] [PubMed] [Google Scholar]
- 13.Mottron L, Dawson M, Soulières I, Hubert B, Burack J. 2006. Enhanced perceptual functioning in autism: an update, and eight principles of autistic perception. J. Autism Dev. Disord. 36, 27–43. ( 10.1007/s10803-005-0040-7) [DOI] [PubMed] [Google Scholar]
- 14.Mottron L, Bouvet L, Bonnel A, Samson F, Burack JA, Dawson M, Heaton P. 2013. Veridical mapping in the development of exceptional autistic abilities. Neurosci. Biobehav. Rev. 37, 209–228. ( 10.1016/j.neubiorev.2012.11.016) [DOI] [PubMed] [Google Scholar]
- 15.Dakin S, Frith U. 2005. Vagaries of visual perception in autism. Neuron 48, 497–507. ( 10.1016/j.neuron.2005.10.018) [DOI] [PubMed] [Google Scholar]
- 16.Bölte S, Holtmann M, Poustka F, Scheurich A, Schmidt L. 2007. Gestalt perception and local–global processing in high-functioning autism. J. Autism Dev. Disord. 37, 1493–1504. ( 10.1007/s10803-006-0231-x) [DOI] [PubMed] [Google Scholar]
- 17.Van der Hallen R, Evers K, Brewaeys K, Van den Noortgate W, Wagemans J. 2015. Global processing takes time: a meta-analysis on local–global visual processing in ASD. Psychol. Bull. 141, 549–573. ( 10.1037/bul0000004) [DOI] [PubMed] [Google Scholar]
- 18.Barnett KJ, Foxe JJ, Molholm S, Kelly SP, Shalgi S, Mitchell KJ, Newell FN. 2008. Differences in early sensory-perceptual processing in synesthesia: a visual evoked potential study. Neuroimage 43, 605–613. ( 10.1016/j.neuroimage.2008.07.028) [DOI] [PubMed] [Google Scholar]
- 19.Banissy MJ, Walsh V, Ward J. 2009. Enhanced sensory perception in synaesthesia. Exp. Brain Res. 196, 565–571. ( 10.1007/s00221-009-1888-0) [DOI] [PubMed] [Google Scholar]
- 20.van Leeuwen TM, Hagoort P, Handel BF. 2013. Real color captures attention and overrides spatial cues in grapheme-color synesthetes but not in controls. Neuropsychologia 51, 1802–1813. ( 10.1016/j.neuropsychologia.2013.06.024) [DOI] [PubMed] [Google Scholar]
- 21.Yaro C, Ward J. 2007. Searching for Shereshevskii: what is superior about the memory of synaesthetes? Q. J. Exp. Psychol. 60, 681–695. ( 10.1080/17470210600785208) [DOI] [PubMed] [Google Scholar]
- 22.Banissy MJ, Tester V, Muggleton NG, Janik AB, Davenport A, Franklin A, Walsh V, Ward J. 2013. Synesthesia for color is linked to improved color perception but reduced motion perception. Psychol. Sci. 24, 2390–2397. ( 10.1177/0956797613492424) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Milne E, Swettenham J, Hansen P, Campbell R, Jeffries H, Plaisted K. 2002. High motion coherence thresholds in children with autism. J. Child Psychol. Psychiatry 43, 255–263. ( 10.1111/1469-7610.00018) [DOI] [PubMed] [Google Scholar]
- 24.Pellicano E, Gibson L, Maybery M, Durkin K, Badcock DR. 2005. Abnormal global processing along the dorsal visual pathway in autism: a possible mechanism for weak visuospatial coherence? Neuropsychologia 43, 1044–1053. ( 10.1016/j.neuropsychologia.2004.10.003) [DOI] [PubMed] [Google Scholar]
- 25.Hughes JEA, Simner J, Baron-Cohen S, Treffert DA, Ward J. 2017. Is synaesthesia more prevalent in autism spectrum conditions? Only where there is prodigious talent. Multisens. Res. 30, 391–408. ( 10.1163/22134808-00002558) [DOI] [PubMed] [Google Scholar]
- 26.Bouvet L, Amsellem F, Maruani A, Tonus-Vic Dupont A, Mathieu A, Bourgeron T, Delorme R, Mottron L. 2019. Synesthesia and autistic features in a large family: evidence for spatial imagery as a common factor. Behav. Brain Res. 362, 266–272. ( 10.1016/j.bbr.2019.01.014) [DOI] [PubMed] [Google Scholar]
- 27.Janik MAB, Susilo T, Rezlescu C, Bray A, Banissy MJ. 2016. Social perception in synaesthesia for colour. Cogn. Neuropsychol. 33, 378–387. ( 10.1080/02643294.2016.1261820) [DOI] [PubMed] [Google Scholar]
- 28.Stevenson RA, Segers M, Ncube BL, Black KR, Bebko JM, Ferber S, Barense MD. 2017. The cascading influence of multisensory processing on speech perception in autism. Autism 22, 609–624. ( 10.1177/1362361317704413) [DOI] [PubMed] [Google Scholar]
- 29.Wallace MT, Stevenson RA. 2014. The construct of the multisensory temporal binding window and its dysregulation in developmental disabilities. Neuropsychologia 64, 105–123. ( 10.1016/j.neuropsychologia.2014.08.005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bankieris KR, Qian T, Aslin RN. 2019. Synesthetes perseverate in implicit learning: evidence from a non-stationary statistical learning task. Q. J. Exp. Psychol. 72, 1771–1779. ( 10.1177/1747021818816285) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Foti F, De Crescenzo F, Vivanti G, Menghini D, Vicari S. 2014. Implicit learning in individuals with autism spectrum disorders: a meta-analysis. Psychol. Med. 45, 897–910. ( 10.1017/S0033291714001950) [DOI] [PubMed] [Google Scholar]
- 32.Burghoorn F, Dingemanse M, Van Lier R, Van Leeuwen TM. 2019. The relation between autistic traits, the degree of synaesthesia, and local/global visual perception. J. Autism Dev. Disord. ( 10.1007/s10803-019-04222-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mealor AD, Simner J, Rothen N, Carmichael DA, Ward J. 2016. Different dimensions of cognitive style in typical and atypical cognition: new evidence and a new measurement tool. PLoS ONE 11, e0155483 ( 10.1371/journal.pone.0155483) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kuiper MW, Verhoeven EW, Geurts HM. 2018. The Dutch Glasgow Sensory questionnaire: psychometric properties of an autism-specific sensory sensitivity measure. Autism 23, 922–932. ( 10.1177/1362361318788065) [DOI] [PubMed] [Google Scholar]
- 35.Robertson AE, Simmons DR. 2013. The relationship between sensory sensitivity and autistic traits in the general population. J. Autism Dev. Disord. 43, 775–784. ( 10.1007/s10803-012-1608-7) [DOI] [PubMed] [Google Scholar]
- 36.Sapey-Triomphe L-A, Moulin A, Sonié S, Schmitz C. 2017. The Glasgow Sensory questionnaire: validation of a French language version and refinement of sensory profiles of people with high autism-spectrum quotient. J. Autism Dev. Disord. 48, 1549–1565. ( 10.1007/s10803-017-3422-8) [DOI] [PubMed] [Google Scholar]
- 37.Baron-Cohen S, Wheelwright S, Skinner R, Martin J, Clubley E. 2001. The autism-spectrum quotient (AQ): evidence from Asperger syndrome/high-functioning autism, males and females, scientists and mathematicians. J. Autism Dev. Disord. 31, 5–17. ( 10.1023/a:1005653411471) [DOI] [PubMed] [Google Scholar]
- 38.Jolliffe T, Baron-Cohen S. 1997. Are people with autism and Asperger syndrome faster than normal on the embedded figures test? J. Child Psychol. Psychiatry 38, 527–534. ( 10.1111/j.1469-7610.1997.tb01539.x) [DOI] [PubMed] [Google Scholar]
- 39.Shah A, Frith U. 1983. An islet of ability in autistic children: a research note. J. Child Psychol. Psychiatry 24, 613–620. ( 10.1111/j.1469-7610.1983.tb00137.x) [DOI] [PubMed] [Google Scholar]
- 40.Smith H, Milne E. 2009. Reduced change blindness suggests enhanced attention to detail in individuals with autism. J. Child Psychol. Psychiatry 50, 300–306. ( 10.1111/j.1469-7610.2008.01957.x) [DOI] [PubMed] [Google Scholar]
- 41.Fletcher-Watson S, Leekam SR, Connolly B, Collis JM, Findlay JM, McConachie H, Rodgers J. 2012. Attenuation of change blindness in children with autism spectrum disorders. Br. J. Dev. Psychol. 30, 446–458. ( 10.1111/j.2044-835X.2011.02054.x) [DOI] [PubMed] [Google Scholar]
- 42.White SJ, Saldaña D. 2011. Performance of children with autism on the embedded figures test: a closer look at a popular task. J. Autism Dev. Disord. 41, 1565–1572. ( 10.1007/s10803-011-1182-4) [DOI] [PubMed] [Google Scholar]
- 43.Cribb SJ, Olaithe M, Di Lorenzo R, Dunlop PD, Maybery MT. 2016. Embedded figures test performance in the broader autism phenotype: a meta-analysis. J. Autism Dev. Disord. 46, 2924–2939. ( 10.1007/s10803-016-2832-3) [DOI] [PubMed] [Google Scholar]
- 44.Happe FG. 1996. Studying weak central coherence at low levels: children with autism do not succumb to visual illusions. A research note. J. Child Psychol. Psychiatry 37, 873–877. ( 10.1111/j.1469-7610.1996.tb01483.x) [DOI] [PubMed] [Google Scholar]
- 45.Spencer J, O'Brien J, Riggs K, Braddick O, Atkinson J, Wattam-Bell J. 2000. Motion processing in autism: evidence for a dorsal stream deficiency. Neuroreport 11, 2765–2767. ( 10.1097/00001756-200008210-00031) [DOI] [PubMed] [Google Scholar]
- 46.Manning C, Tibber MS, Charman T, Dakin SC, Pellicano E. 2015. Enhanced integration of motion information in children with autism. J. Neurosci. 35, 6979–6986. ( 10.1523/jneurosci.4645-14.2015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Spencer JV, O'Brien JMD. 2006. Visual form-processing deficits in autism. Perception 35, 1047–1055. ( 10.1068/p5328) [DOI] [PubMed] [Google Scholar]
- 48.Del Viva MM, Igliozzi R, Tancredi R, Brizzolara D. 2006. Spatial and motion integration in children with autism. Vision Res. 46, 1242–1252. ( 10.1016/j.visres.2005.10.018) [DOI] [PubMed] [Google Scholar]
- 49.Ward J, Rothen N, Chang A, Kanai R. 2016. The structure of inter-individual differences in visual ability: evidence from the general population and synaesthesia. Vision Res. 141, 293–302. ( 10.1016/j.visres.2016.06.009) [DOI] [PubMed] [Google Scholar]
- 50.de-Wit L, Huygelier H, Van der Hallen R, Chamberlain R, Wagemans J. 2017. Developing the Leuven Embedded Figures Test (L-EFT): testing the stimulus features that influence embedding. PeerJ 5, e2862 ( 10.7717/peerj.2862) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Witkin HA, Ottman RK, Raskin E, Karp SA. 1971. A manual for the embedded figures test. Palo Alto, CA: Consulting Psychologists Press. [Google Scholar]
- 52.van Leeuwen TM, Dingemanse M. 2016. Colour associations in synaesthetes and nonsynaesthetes: a large-scale study in Dutch. Perception 45, 333–334. (doi:urn:nbn:nl:ui:22-2066/163052) [Google Scholar]
- 53.Cuskley C, Dingemanse M, Kirby S, van Leeuwen TM. 2019. Cross-modal associations and synesthesia: categorical perception and structure in vowel–color mappings in a large online sample. Behav. Res. Methods. 51, 1651–1675. ( 10.3758/s13428-019-01203-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Novich S, Cheng S, Eagleman DM. 2011. Is synaesthesia one condition or many? A large-scale analysis reveals subgroups. J. Neuropsychol. 5, 353–371. ( 10.1111/j.1748-6653.2011.02015.x) [DOI] [PubMed] [Google Scholar]
- 55.Hoekstra RA, Bartels M, Cath DC, Boomsma DI. 2008. Factor structure, reliability and criterion validity of the autism-spectrum quotient (AQ): a study in Dutch population and patient groups. J. Autism Dev. Disord. 38, 1555–1566. ( 10.1007/s10803-008-0538-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jackson BL, Blackwood EM, Blum J, Carruthers SP, Nemorin S, Pryor BA, Sceneay SD, Bevan S, Crewther DP. 2013. Magno- and parvocellular contrast responses in varying degrees of autistic trait. PLoS ONE 8, e66797 ( 10.1371/journal.pone.0066797) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Van Petersen E, Altgassen M, Van Lier R, Van Leeuwen TM. 2018. Enhanced spatial navigation skills in sequence-space synesthetes. PsyArXiv. ( 10.31234/osf.io/wv76y) [DOI] [PubMed] [Google Scholar]
- 58.Rothen N, Jünemann K, Mealor AD, Burckhardt V, Ward J. 2016. The sensitivity and specificity of a diagnostic test of sequence-space synesthesia. Behav. Res. Methods 48, 1476–1481. ( 10.3758/s13428-015-0656-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Constantino JN, et al. 2003. Validation of a brief quantitative measure of autistic traits: comparison of the social responsiveness scale with the autism diagnostic interview-revised. J. Autism Dev. Disord. 33, 427–433. ( 10.1023/a:1025014929212) [DOI] [PubMed] [Google Scholar]
- 60.Gotham K, Risi S, Pickles A, Lord C. 2006. The autism diagnostic observation schedule: revised algorithms for improved diagnostic validity. J. Autism Dev. Disord. 37, 613–627. ( 10.1007/s10803-006-0280-1) [DOI] [PubMed] [Google Scholar]
- 61.Lord C, Rutter M, Le Couteur A. 1994. Autism diagnostic interview-revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J. Autism Dev. Disord. 24, 659–685. ( 10.1007/bf02172145) [DOI] [PubMed] [Google Scholar]
- 62.Schlooz WAJM, Hulstijn W, van den Broek PJA, van der Pijll ACAM, Gabreëls F, van der Gaag RJ, Rotteveel JJ. 2006. Fragmented visuospatial processing in children with pervasive developmental disorder. J. Autism Dev. Disord. 36, 1025–1037. ( 10.1007/s10803-006-0140-z) [DOI] [PubMed] [Google Scholar]
- 63.Chen Y, Norton DJ, McBain R, Gold J, Frazier JA, Coyle JT. 2012. Enhanced local processing of dynamic visual information in autism: evidence from speed discrimination. Neuropsychologia 50, 733–739. ( 10.1016/j.neuropsychologia.2012.01.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Manning C, Morgan MJ, Allen CTW, Pellicano E. 2017. Susceptibility to Ebbinghaus and Müller-Lyer illusions in autistic children: a comparison of three different methods. Mol. Autism. 8, 16 ( 10.1186/s13229-017-0127-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Navon D. 1977. Forest before trees: the precedence of global features in visual perception. Cogn. Psychol. 9, 353–383. ( 10.1016/0010-0285(77)90012-3) [DOI] [Google Scholar]
- 66.Hänggi J, Wotruba D, Jäncke L. 2011. Globally altered structural brain network topology in grapheme-color synesthesia. J. Neurosci. 31, 5816–5828. ( 10.1523/jneurosci.0964-10.2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tomson SN, Narayan M, Allen GI, Eagleman DM. 2013. Neural networks of colored sequence synesthesia. J. Neurosci. 33, 14 098–14 106. ( 10.1523/jneurosci.5131-12.2013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Just MA, Cherkassky VL, Keller TA, Kana RK, Minshew NJ. 2007. Functional and anatomical cortical underconnectivity in autism: evidence from an fMRI study of an executive function task and corpus callosum morphometry. Cereb. Cortex 17, 951–961. ( 10.1093/cercor/bhl006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Just MA, Keller TA, Malave VL, Kana RK, Varma S. 2012. Autism as a neural systems disorder: a theory of frontal-posterior underconnectivity. Neurosci. Biobehav. Rev. 36, 1292–1313. ( 10.1016/j.neubiorev.2012.02.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ring HA, Baron-Cohen S, Wheelwright S, Williams SC, Brammer M, Andrew C, Bullmore ET. 1999. Cerebral correlates of preserved cognitive skills in autism: a functional MRI study of embedded figures task performance. Brain 122, 1305–1315. ( 10.1093/brain/122.7.1305) [DOI] [PubMed] [Google Scholar]
- 71.Brown AC, Crewther DP. 2017. Autistic children show a surprising relationship between global visual perception, non-verbal intelligence and visual parvocellular function, not seen in typically developing children. Front. Hum. Neurosci. 11, 239 ( 10.3389/fnhum.2017.00239) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sutherland A, Crewther DP. 2010. Magnocellular visual evoked potential delay with high autism spectrum quotient yields a neural mechanism for altered perception. Brain 133, 2089–2097. ( 10.1093/brain/awq122) [DOI] [PubMed] [Google Scholar]
- 73.Rubenstein JLR, Merzenich MM. 2003. Model of autism: increased ratio of excitation/inhibition in key neural systems. Genes Brain Behav. 2, 255–267. ( 10.1046/j.1601-183X.2003.00037.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Terhune DB, Tai S, Cowey A, Popescu T, Cohen KR. 2011. Enhanced cortical excitability in grapheme-color synesthesia and its modulation. Curr. Biol. 21, 2006–2009. ( 10.1016/j.cub.2011.10.032) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Terhune DB, Song SM, Cohen Kadosh R. 2015. Transcranial alternating current stimulation reveals atypical 40 Hz phosphene thresholds in synaesthesia. Cortex 63, 267–270. ( 10.1016/j.cortex.2014.09.006) [DOI] [PubMed] [Google Scholar]
- 76.Powers AR, Mathys C, Corlett PR. 2017. Pavlovian conditioning-induced hallucinations result from overweighting of perceptual priors. Science 357, 596–600. ( 10.1126/science.aan3458) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.van Leeuwen TM, Sauer A, Jurjut A-M, Wibral M, Uhlhaas P, Singer W, Melloni L. 2018. Perceptual phenotypes: perceptual gains and losses in synesthesia and schizophrenia. bioRxiv. ( 10.1101/443846) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pellicano E, Burr D. 2012. When the world becomes ‘too real’: a Bayesian explanation of autistic perception. Trends Cogn. Sci. 16, 504–510. ( 10.1016/j.tics.2012.08.009) [DOI] [PubMed] [Google Scholar]
- 79.Van de Cruys S, Evers K, Van der Hallen R, Van Eylen L, Boets B, de-Wit L, Wagemans J. 2014. Precise minds in uncertain worlds: predictive coding in autism. Psychol. Rev. 121, 649–675. ( 10.1037/a0037665) [DOI] [PubMed] [Google Scholar]
- 80.Seth AK. 2014. A predictive processing theory of sensorimotor contingencies: explaining the puzzle of perceptual presence and its absence in synesthesia. Cogn. Neurosci. 5, 97–118. ( 10.1080/17588928.2013.877880) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.van Leeuwen TM. 2014. Constructing priors in synesthesia. Cogn. Neurosci. 5, 124–126. ( 10.1080/17588928.2014.905520) [DOI] [PubMed] [Google Scholar]
- 82.van Leeuwen TM. 2013. Individual differences in synesthesia. In Oxford handbook of synesthesia (eds Simner J, Hubbard EM), pp. 241–264. Oxford, UK: Oxford University Press. [Google Scholar]
- 83.Dixon MJ, Smilek D, Merikle PM. 2004. Not all synaesthetes are created equal: projector versus associator synaesthetes. Cogn. Affect. Behav. Neurosci. 4, 335–343. ( 10.3758/CABN.4.3.335) [DOI] [PubMed] [Google Scholar]
- 84.Rouw R, Scholte HS. 2007. Increased structural connectivity in grapheme-color synesthesia. Nat. Neurosci. 10, 792–797. ( 10.1038/nn1906) [DOI] [PubMed] [Google Scholar]
- 85.van Leeuwen TM, den Ouden HEM, Hagoort P. 2011. Effective connectivity determines the nature of subjective experience in grapheme-color synesthesia. J. Neurosci. 31, 9879–9884. ( 10.1523/jneurosci.0569-11.2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Easson AK, Fatima Z, McIntosh AR. 2019. Functional connectivity-based subtypes of individuals with and without autism spectrum disorder. Network Neurosci. 3, 344–362. ( 10.1162/netn_a_00067) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Loomes R, Hull L, Mandy WPL. 2017. What is the male-to-female ratio in autism spectrum disorder? A systematic review and meta-analysis. J. Am. Acad. Child Adolesc. Psychiatry 56, 466–474. ( 10.1016/j.jaac.2017.03.013) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw data and analysis scripts are made available in a data repository, accessible through the following link: http://hdl.handle.net/11633/aab4636d. The complete stimulus set of the embedded figures task is publicly available on Figshare (https://dx.doi.org/10.6084/m9.figshare.3807885, https://dx.doi.org/10.6084/m9.figshare.3807894).