Abstract
The role of interspecific hybridization in evolution is still being debated. Interspecific hybridization has been suggested to facilitate the evolution of ecological novelty, and hence the invasion of new niches and adaptive radiation when ecological opportunity is present beyond the parental species niches. On the other hand, hybrids between two ecologically divergent species may perform less well than parental species in their respective niches because hybrids would be intermediate in performance in both niches. The evolutionary consequences of hybridization may hence be context-dependent, depending on whether ecological opportunities, beyond those of the parental species, do or do not exist. Surprisingly, these complementary predictions may never have been tested in the same experiment in animals. To do so, we investigate if hybrids between ecologically distinct cichlid species perform less well than the parental species when feeding on food either parent is adapted to, and if the same hybrids perform better than their parents when feeding on food none of the species are adapted to. We generated two first-generation hybrid crosses between species of African cichlids. In feeding efficiency experiments we measured the performance of hybrids and parental species on food types representing both parental species niches and additional ‘novel’ niches, not used by either of the parental species but by other species in the African cichlid radiations. We found that hybrids can have higher feeding efficiencies on the ‘novel’ food types but typically have lower efficiencies on parental food types when compared to parental species. This suggests that hybridization can generate functional variation that can be of ecological relevance allowing the access to resources outside of either parental species niche. Hence, we provide support for the hypothesis of ecological context-dependency of the evolutionary impact of interspecific hybridization.
Keywords: adaptive radiation, hybridization, ecological speciation, cichlid fish
1. Introduction
Adaptive radiation is the process whereby an ancestral lineage rapidly diversifies into multiple phenotypically and ecologically differentiated species [1]. This process is thought to be driven by a combination of availability of otherwise underutilized resources (i.e. ecological opportunity), intraspecific competition and divergent natural selection between niches [2]. It is often associated with colonization of a novel adaptive zone, the extinction of previous tenants of an existing adaptive zone or the evolution of a key innovation that provides access to a previously inaccessible adaptive zone, all of which create ecological opportunity [3].
However, only some lineages actually respond to these favourable conditions and diversify, while most lineages seem to remain indifferent [1]. Rapid expansion into and adaptation to a multitude of new niches, as is characteristic in large adaptive radiations [4], requires a large amount of heritable phenotypic variation that is ecologically relevant [5]. One mechanism that can quickly generate high levels of such heritable variation is interspecific hybridization [6–9]. Hybridization can generate novel phenotypes through the combination of alleles that have not segregated before in the same population [10–13], and may release populations from constraining genetic correlations [14], thereby increasing evolvability [15–18] and theoretically facilitating adaptive peak shifts [19]. Unless intrinsic incompatibilities are very strong, such ecological advantages of interspecific hybrids may, at times of ecological opportunity, well outstrip the negative effects associated with intrinsic incompatibilities [20–22].
Hybridization is indeed a common feature in adaptive radiations [13,20]. Some entire radiations started from a hybrid swarm (e.g. Hawaiian silversword plants [23]; cichlid fish [24]), and recurrent hybridization among constituent species is known from many radiations and has been invoked as a facilitator of diversification (e.g. shrubs on Lord Howe Island [25]; Darwin finches [26]; cichlid fish [27,28]; Heliconius butterflies: [29]). Introgressive hybridization can facilitate adaptation and evolutionary success of existing species by increasing their potential for range expansion and rapid adaptation to new or changing environments, and may in this way indirectly trigger diversification in adaptive radiation [30]. Hybridization may also contribute more directly to the build-up of species diversity during adaptive radiations (and elsewhere) if hybrid lineages can establish and persist ecologically and genetically as new species alongside the parental species [9,21,22,31,32]. If performance trade-offs are associated with the respective adaptations of two species, their hybrids are expected to be intermediate in performance and to do less well than do individuals of either parental species in their respective niches. This ecological hybrid performance disadvantage or ecological incompatibility hypothesis is central to ecological speciation theory [1,8,33] and supported by a number of experimental studies (e.g. fish [34–36]; insects [37]).
These apparently contradictory predictions for ecological hybrid performance may be fully compatible and complementary if the influence of hybridization on evolution was dependent on the ecological context (i.e. whether the hybrids have access to ecological opportunities outside the parental species niches [7–9]). Surprisingly, these two sets of predictions for animals have to the best of our knowledge never been tested in the same experiment. Here we set out to do so.
By measuring feeding performance of cichlid fish species hybrids and the corresponding non-hybrids in different ecological contexts, we extend the ecological speciation testing framework. We do not just test the prediction of functional hybrid incompatibility (i.e. ecological hybrid performance disadvantage in parental species niches), but also at the same time the prediction of an ecological hybrid performance advantage in ‘novel’ niches. We test the same first-generation hybrid and non-hybrid individuals on food types representing the niches of their parental species and—depending on the parental species that were crossed—on ‘novel’ food types that neither of the parental species is adapted to. We refer to them as ‘novel’, but whereas one is truly novel to the entire cichlid radiation, we chose the other food types to represent prey types that are rare in the diet of the species that we test here, but that provide the resource basis for entire trophic guilds of other cichlid species in each of the large African lakes [38,39].
To test the ecological context dependency of interspecific hybridization we experimentally test the same first-generation hybrids and non-hybrids on food types representing the niches of the parental species as well as on ‘novel’ food types. This results in three main predictions:
-
1.
The parental species show trade-offs between feeding effectively on the food type they are adapted to and on that of the other species, such that each species performs best on the food type that resembles its natural diet.
-
2.
When tested on the parental species resources, hybrid performance is intermediate between that of both parents on both food types, leading to ecological hybrid performance disadvantage.
-
3.
When tested on ‘novel’ food types, hybrid performance often exceeds that of parental species, resulting in ecological hybrid performance advantage.
All tests were done with fish raised in a common garden environment. We chose two different ecological specialists from the Lake Victoria radiation, the reef zooplanktivore Pundamilia sp. ‘nyererei-like’ [28] and the epilithic algae scraper Neochromis omnicaeruleus [40]. These species differ profoundly in morphology and ecology [41] but are only about 15'000 years divergent [24]. We crossed the same reef zooplanktivore from Lake Victoria also with an omnivorous generalist species from Lake Malawi (Astatotilapia calliptera [42]). These species are about 2.74–4.82 million years divergent [43]. The food types used in the feeding efficiency experiments resembled the food types that the parental species specialize on (i.e. either evasive zooplankton (copepods) or epilithic algae), and other food types (i.e. gammarids and shrimps) that are completely absent from African lakes (gammarids) or are the main food of other species and entire guilds of cichlids in the same radiations (shrimps).
2. Material and methods
(a). Parental species and their first-generation hybrids
All fish used to generate the first-generation hybrid crosses and used in the experiments derived from laboratory populations bred and raised under identical conditions in the research aquarium system at EAWAG. These populations descend from wild individuals caught in Lake Victoria from the islands of Makobe (N. omnicaeruleus) and Python (P. sp. ‘nyererei-like’) and in Lake Malawi from the island of Chizumulu (A. calliptera) (greater than five generations in the laboratory). We will refer to these three species and the two first-generation hybrid crosses as NO for N. omnicaeruleus, PN for P. sp. ‘nyererei-like’ and AC for A. calliptera and we use the corresponding abbreviations for the hybrid crosses, as follows: mother species × father species (i.e. ACxPN and PNxNO).
The crosses differ in the genetic distance and in morphological features between the parental species (for details see electronic supplementary material, appendices 1 and 2). We obtained six independent full-sib first-generation hybrid families of PNxNO and two half-sib (same father) first-generation hybrid families of ACxPN from which several individuals of each family were tested. Individuals of the parental species tested in the experiments derived from several single-species stock tanks, each containing a population lab-bred for multiple generations. To avoid any tank or population effects we tested individuals from two or three single species stock tanks of N. omnicaeruleus, A. calliptera and P. sp. ‘nyererei-like’. All fish were raised in a common garden environment (for details on the breeding and rearing of the parental species and their first-generation hybrids see electronic supplementary material, appendix 3).
(b). Experimental set-up
All individuals used in the experiments were adult males at the age of ten to seventeen months. Females were not tested to avoid confounding effects of sexual dimorphism that may influence feeding efficiency in cichlids [44]. The fish were tested in groups of 7–16 fish in individual experimental tanks after an acclimatization period. At the end of the acclimatization period the fish were fed for habituation on 4 consecutive days each once with one of the four food types (see below) used in subsequent experiments. Prior to the exposure to the different food types the parental species and the hybrid crosses were naive to the presented food types. Two days after the acclimatization phase the alternating experimental schedule started, consisting of one day testing with one of the food types, followed by one day where the fish were not fed. This experimental procedure was continued with each food type (trials with three different food types: algal substitute, gammarids and shrimp) until a sufficient number of successful feeding trials for each individual fish of an entire group had been collected on all three food types. We aimed at getting several successful feeding trials to account for individual variation in feeding efficiency. All fish in a group were tested together to be able to account for possible group effects (see below). Finally, each fish was tested on zooplankton and after one successful trial the fish was sacrificed in accordance with Swiss animal experimentation permits. All fish within an experimental group were tested in the same order on the different food types, and the order alternated between different experimental groups (electronic supplementary material, table S1; for details on the experimental set-up see electronic supplementary material, appendix 4). Altogether a total of 52 fish in five experimental groups were successfully tested on one, several or all food types (electronic supplementary material, table S1). All experimental feeding trials were videotaped and analysed afterwards.
(c). Food types
We used four different food types in individual feeding trials. Two food types resemble the main diet that two of the parental species are specialized on: an algal substitute to resemble firmly attached epilithic algae, the dominant diet of the epilithic algae scraper N. omnicaeruleus and copepods resembling the dominant zooplankton taxon found in Lake Victoria and the main diet of the reef zooplanktivore P. sp. ‘nyererei-like’ [40,45,46]. Furthermore, one of the previously mentioned food types (epilithic algae) and two other food types were ‘novel’ in the sense that—depending on the parental species that were crossed—none of the parental species are adapted to. Although we refer to them as ‘novel’, only one is truly novel to the entire cichlid radiation (gammarids), whereas the other food types (shrimps and algal substitute) represent prey types that are very rare in the diet of all of the test species except the algal substitute for N. omnicaeruleus, but that entire trophic guilds of other cichlid species in each of the large African lakes have adapted to [38,39]. For the cross involving the reef zooplanktivore P. sp. ‘nyererei-like’ and the omnivorous generalist species A. calliptera the algal substitute is a ‘novel’ food type both for the parental species and the hybrids. We chose gammarids to resemble slow moving but powerful evasive benthic prey and shrimps to resemble powerful burst-swimming pelagic prey. The percentage of different food types in the diet of the omnivorous generalist species A. calliptera is not known. Details on the preparation and testing of each food type can be found in the electronic supplementary material, appendix 5.
(d). Statistical analysis
All analyses were conducted using R statistical software, v. 3.5.1 [47]. When normality assumptions were satisfied (Shapiro–Wilk test), parametric statistics were used to analyse the data, otherwise non parametric tests were applied. In all linear mixed models we checked assumptions of normality for the residuals using residual versus predicted plots and normal probability plots. p-Values for all post hoc tests were adjusted for multiple comparisons using a false discovery rate (FDR) of α = 0.05 [48]. We report both raw and FDR-adjusted p-values for the linear mixed models in the tables present in the electronics supplementary material but only the FDR-controlled p-values in the text.
All data are available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.m25t886 [49].
(e). Species differences in feeding efficiency
Feeding efficiency was estimated as the ratio between the amount of consumed food types (weight difference for the algal substitute and number of prey types for gammarids, shrimps or zooplankton) and the total number of feeding bouts (number of unsuccessful and successful attacks). We tested if differences exist between the parental species and their hybrids in the feeding efficiencies on each food type. For food types other than zooplankton, we had repeated measures of an individual's feeding performance; in the majority of cases we obtained an individual's feeding performance from three successful experimental trials. In a few cases this number deviated (from 1 to 5, see electronic supplementary material, table S2) and some individuals were either not tested on all food types or they did not feed on one or several food types.
Feeding efficiency was analysed using linear mixed effect models with the package lme4 in R [50]. The feeding efficiency model included the fixed effects ‘group’ (the three parental species and both first-generation hybrids were each classified as a group), ‘BS’ (body size measured as standard length) and ‘trial length’ (the duration of an experimental trial). Separate tests of feeding efficiency on the four food types were run for each hybrid cross and their parental species (PN/NO/PNxNO and AC/PN/ACxPN). Each model included the random factors ‘individual’ (used only in the models where individual fish were tested repeatedly: algal substitute, gammarids and shrimps), and ‘experimental batch’. The latter accounts for a possible batch-effect as individual fish were tested in different experimental batches. Since each batch was tested over a duration of 2–6 months and at a different time of the year, each group of fish may have experienced slightly different experimental conditions. Furthermore, due to experimental constraints the trial length varied in a few cases significantly between groups tested on several food types, which could influence feeding efficiency as some individual fish were exposed longer to the different experimental treatments. We allowed for an interaction between ‘group’ and ‘BS’ to account for the inter-dependence of the size of the fish and feeding efficiency in each group, as was revealed by preliminary linear models.
To assess the impact of the predictor variables (i.e. the relative-impact value; hereafter RI) in the model we used an AIC-based model averaging approach [51]. The full set of additive models was generated and then the relative importance of each predictor variable was calculated. This can vary on a scale from 0 to 1 and is calculated as the sum of the Akaike weights of the models in which the variable appears. Better models have larger Akaike weights and thus a variable that contributes more to model fit will have a higher relative-importance value (i.e. closer to one). We calculated the differences in feeding efficiency between groups based on the least-squares means and confidence intervals extracted from the feeding efficiency model. For assessing the fit of the model the root mean square error (RMSE) between the predicted and observed values and the AIC were calculated. Normality assumptions were not met in any of the linear mixed models. Hence, we log-transformed the response variable to achieve a fit to a normal error distribution.
(f). Differences in size, weight, trial length, latency and possible trial learning effect
We tested several parameters that may influence feeding efficiency (e.g. allometric effects of size on feeding efficiency) between the parental species and hybrid crosses as well as two behavioural parameters, latency and learning (details are provided in the electronic supplementary material, appendix 6). In general, the parental species and the hybrid crosses did not differ in size or weight when tested for each food type separately with one exception (electronic supplementary material, table S3). There were more cases where the trial length between parental species and their hybrid cross differed when tested for each food type (electronic supplementary material, table S4). Due to some significant differences in size, weight or trial length between the parental species and their hybrid cross, we used the parameters as fixed effects in the linear mixed model. Since size and weight differences were highly correlated and body size can contribute to the ability of using specific food types [52] we only accounted for size as a fixed factor in the model. We further looked at two behavioural parameters, latency to first-feeding and a possible learning effect due to repeated testing of specimens on the four food types.
3. Results
(a). Differences in feeding efficiency between parental species and first-generation hybrids
Parental species and the first-generation hybrids differed significantly in feeding efficiency on several of the different food types, with ‘group’ being the best predictor of feeding efficiency in all models with one exception (RI value across all food types ranged between 0.43 and 1; electronic supplementary material, table S5).
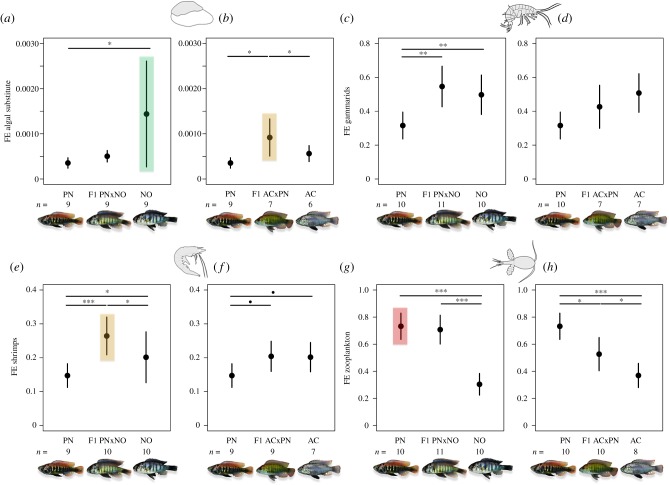
Corroborating our first prediction we found adaptational trade-offs in feeding efficiency between the two specialists, the epilithic algae scraper N. omnicaeruleus and the reef zooplanktivore P. sp. ‘nyererei-like’, on their specific food types. Both specialists were most efficient feeding on the food types that resembled their natural diet (algal substitute: figure 1a; zooplankton: figure 1g,h; electronic supplementary material, table S6).
Figure 1.
Feeding efficiency of two ecological specialists and one generalist cichlid species and their first-generation hybrids reveals an ecological hybrid performance advantage over the parental species when feeding on ‘novel’ food types as well as divergent feeding efficiencies of the two ecological specialists between the food types they are adapted to. Feeding efficiency was measured on four food types: (a,b) algal substitute, (c,d) gammarids, (e,f) shrimps and (g,h) zooplankton. The species P. sp. ‘nyererei-like’ (PN) and N. omnicaeruleus (NO) are ecological specialists (i.e. reef zooplanktivore and epilithic algae scraper, respectively) and A. calliptera (AC) is a generalist omnivore. The feeding efficiency on the respective food types that the parental species are adapted to are highlighted in green for N. omnicaeruleus and in red for P. sp. ‘nyererei-like’ and highlighted in orange are the food types where the hybrid crosses showed a higher feeding efficiency than either parental species. Given on the y-axis are the mean and the standard deviation per group (species or hybrid cross). Feeding efficiency (FE) for each individual was estimated as the ratio between the amount of consumed food types and the total number of feeding bouts. The number of individuals tested on each food type for each group is given at the bottom of each graph. The individuals were each tested multiple times for all food types except for zooplankton. The differences in feeding efficiency between groups were calculated from the least-squares means and confidence intervals of each group extracted from a linear mixed feeding efficiency model (electronic supplementary material table S6). p-Values were adjusted for multiple comparison with the false discovery rate (FDR) method; •p < 0.1, *p < 0.05 and **p < 0.01.
We predicted further that both hybrid crosses would show an intermediate performance on the parental species resources, which would result in an ecological hybrid performance disadvantage in nature if populations were resource limited. Results of our feeding efficiency experiments were largely in agreement with these predictions: When feeding on zooplankton the first-generation hybrid cross ACxPN had a significantly intermediate performance compared to both parental species (figure 1h; electronic supplementary material, table S6). The first-generation hybrid cross PNxNO had a significantly higher performance than the epilithic algae scraper N. omnicaeruleus but did not differ from the specialist reef zooplanktivore P. sp. ‘nyererei-like’ (figure 1g; electronic supplementary material, table S6). When feeding on algal substitute, the first-generation hybrid cross PNxNO was intermediate in performance compared to both parental species (figure 1a; electronic supplementary material, table S6).
Finally, in agreement with our third prediction when tested on ‘novel’ food types—whereby the novelty of the food type depended on the parental species that were crossed—both hybrid crosses outperformed their parental species each on one ‘novel’ food type or were as efficient as the best-performing parental species. In the experiments involving the first-generation hybrid cross PNxNO and the respective parental species two out of the four food types were ‘novel’, namely shrimps and gammarids. When feeding on shrimps the PNxNO hybrids significantly outperformed both parental species (figure 1e; electronic supplementary material, table S6) and when feeding on gammarids both the epilithic algae scraper N. omnicaeruleus and the hybrids significantly outperformed the specialist reef zooplanktivore P. sp. ‘nyererei-like’ (figure 1c; electronic supplementary material, table S6). In the experiments involving the other first-generation hybrid cross ACxPN and the respective parental species, three out of the four food types were ‘novel’, namely shrimps, gammarids and algae: When feeding on algae, the hybrids significantly outperformed both parental species (figure 1b; electronic supplementary material, table S6) and when feeding on shrimps both the omnivorous generalist A. calliptera and the hybrids showed a weak trend towards outperforming the specialist reef zooplanktivore P. sp. ‘nyererei-like’ (figure 1f; electronic supplementary material, table S6). When feeding on gammarids none of the three groups (parental species or their first-generation hybrids) differed significantly in their performance (figure 1d; electronic supplementary material, table S6). Finally, the ecological generalist A. calliptera exhibited no superiority on any of the tested food types (figure 1b,d,f,h; electronic supplementary material, table S6).
In three cases body size and group (i.e. cross type and parental species) had a similar relative-impact value on feeding efficiency (electronic supplementary material, table S5). Body size and group both had large relative impact values in the AC/PN/ACxPN dataset on the efficiency in feeding on algal substitute (‘group’ RI = 0.63; ‘BS’ RI = 0.41) and in both datasets on the efficiency in feeding on gammarids (PN/NO/PNxNO: ‘group’ RI = 0.43, ‘BS’ RI = 0.78; AC/PN/ACxPN: ‘group’ RI = 0.63, ‘BS’ RI = 0.38). In the AC/PN/ACxPN dataset on the algal substitute we found that larger fish (linear regression; r = 0.33, p = 0.008) and particularly larger fish of A. calliptera (r = 0.69, p = 0.002) had a higher feeding efficiency on the algal substitute. When feeding on gammarids in the PN/NO/PNxNO dataset, larger fish in general (independent of species or cross identity) (r = 0.40, p < 0.001) and larger individuals of the epilithic algae scraper N. omnicaeruleus (r = 0.73, p < 0.001) were significantly more efficient. Similarly, in AC/PN/ACxPN experiments larger fish (independent of species or cross identity) were significantly more efficient when feeding on gammarids (r = 0.30 p = 0.011). Finally, although body size was not a strong predictor of feeding efficiency on zooplankton in the AC/PN/ACxPN experiments, there was a significant interaction between body size and group (‘group × BS’ p = 0.009, electronic supplementary material, table S5). This was driven by a significant negative relationship between body size and feeding efficiency on zooplankton in the hybrids (r = −0.71, p = 0.021) and the opposite relationship was observed in the parental species A. calliptera (r = 0.81, p = 0.015).
We further looked at two behavioural parameters, latency and learning. In most cases the parental species and the hybrids did not differ in their latency (i.e. the time in seconds it took an individual to first attack a prey item (e.g. zooplankton, gammarids or shrimps) or take a first bite of the algal substitute). Yet, in two cases the generalist parental species A. calliptera showed a significantly higher latency than the hybrid cross ACxPN or the parental species P. sp. ‘nyererei-like’ in a first attack on a shrimps or taking a first bite of the algal substitute. Also, in one case the hybrid cross PNxNO showed a significantly higher latency than then parental species N. omnicaeruleus in a first attack on shrimps (electronic supplementary material, table S7). Finally, no learning effect was observed on any of the four food types, neither for all individuals of the parental species combined nor for all individuals of the hybrid crosses combined, nor when all individuals were combined into one analysis (electronic supplementary material, table S8).
4. Discussion
Interspecific hybridization can have a variety of consequences for the evolutionary process, including the erosion of existing adaptation or allowing for rapid adaptation to a new situation, constraining or promoting speciation, or leading to the reversal of speciation and loss of species distinctiveness well after speciation [9]. The outcome of hybridization depends on the interactions between the intrinsic viability of the hybrids, the ecological environment including the presence of other species, and on the fitness of hybrids in that ecological context [9]. Theoretical models predict that the ecological performance of hybrids is context-dependent, such that the propensity of hybrids to show ecological performance advantage or disadvantage is largely determined by the ecological context in which hybridization happens [19,31,53] (but see [54] and [55] for non-ecological mechanism). These models predict that ecological hybrid performance advantage requires ecological opportunity beyond the parental species' niches and strong ecological constraint, excluding the parental species from the habitats or resources that lie outside their niche [19,31,53]. This would then facilitate the opportunity for range expansion of hybrids, rapid adaptation to new or changing environments and the invasion of new niches and thereby facilitate the process of adaptive radiation [13,19,20]. Many studies of natural hybridization in animals and plants found that natural hybrids are ecologically distinct from their progenitors (e.g. [27,56–61]). In several plants the ecological divergence of natural hybrids from the parental species is indeed due to the expression of novel ecological functions [61,62], which can cause a fitness advantage of the natural hybrids in the novel ecological niche when compared to their progenitors [62–64]. Experiments with experimental hybrids, even just first-generation hybrids as in our case, help to elucidate if hybridization can initially generate novel functions that are ecologically relevant, a prerequisite for hybrids to possibly escape competition from their progenitors by occupying a novel ecological niche [19,63–66].
Here, we investigated the ecological context-dependency of the consequences of hybridization on individual feeding performance by experimentally testing first-generation hybrids and parental species on food types representing the niches of the parental species as well as on ‘novel’ food types. We found that—consistent with our first prediction and with ecological speciation theory [1,8,33]—the two ecological specialist parental species, the reef zooplanktivore P. sp. ‘nyererei-like’ and the epilithic algae scraper N. omnicaeruleus, both performed best on the food types that resemble their natural diet, zooplankton and epilithic algae, respectively. The third species, the omnivorous A. calliptera, was an ecological generalist, and it showed no superiority on any of the tested food types over either of the other species. Our second prediction, ecological hybrid performance disadvantage when feeding on the parental species resources, was also largely met: In two out of three cases the hybrids did show an intermediate performance on both types of parental species resources, i.e. less good than the specialist. In the third case the hybrids performed equally well as the specialist parental species. Finally, consistent with our third prediction both hybrid crosses outperformed both parental species on a ‘novel’ food type, and were as efficient as the best-performing parental species on the other ‘novel’ food types.
To the best of our knowledge, this is the first experiment in animals that tested and demonstrated ecological context-dependency of ecological performance of interspecific hybrids and their parents. In natural situations where ecological opportunity outside of the parental species niches exists that hybrids with novel phenotypes can tap into, effects of hybridization on diversification can be twofold. First, it may have a direct effect on diversification and speciation (e.g. in adaptive radiations) by instantaneously generating a highly evolvable hybrid population and thereby facilitating peak shifts potentially resulting in the establishment, stabilization and persistence of a hybrid species alongside the parental species [26]. Second, it may indirectly facilitate adaptive radiation by allowing an existing species to rapidly adapt to a changing environment and hence prevent extinction, or to widen its niche and expand its range [67]. Our results also illustrate the importance to explore the functional performance of hybrids in multiple ecological contexts, not just those the parents are adapted to, to better understand the ecological and evolutionary impact of interspecific hybridization.
Our experimental data imply that first-generation hybrids may tap into resources, and hence access potential niche space that is inaccessible for the parental species. We do not know the functional mechanism nor its genetic basis. Heterosis or hybrid vigour could possibly explain part of the feeding efficiency of our first-generation hybrid crosses. Heterosis is only present in first-generation hybrids and has therefore no adaptive significance in subsequent generations [66,68]. Heterosis can affect many different traits, such as physiology (thermal tolerance [66]) or behaviour (boldness/shyness [68]) and its magnitude and outcome (i.e. positive or negative) is dependent on the ecological context and the strength of the ecological contrast tested [66,68]. However, heterosis is unlikely to explain the experimental support for our key directional predictions of context-dependence that we present here, namely that hybrids be intermediate in performance between parental species in parental niches but to outperform both parental species in novel niches.
The adaptive radiations of African cichlids are known for their diverse ecological specializations, which are accompanied by a suite of morphological and behavioural traits that show a complex interplay with feeding performance [69]. These include, for example, ram and suction feeding behaviours [70], body-angle orientation when feeding on epilithic algae [71], the extent of hypertrophied lips [72], variation in tooth morphology [73], the use of vision and/or the cephalic sensory system for prey detection [74] or biting/suction-related differences in head and mouth morphology [46,75]. These empirical and experimental studies suggest that not a single trait but a combination of traits (e.g. locomotor behaviour, sensory system and functional morphology) underlies feeding performance. Furthermore, individual components of morphology and performance can be decoupled, such that different combination of traits may confer the same function [76].
Experimental and experimentally informed simulation studies have shown that hybridization in cichlids can produce functional novelty when there is a many-to-one mapping of form to function [77,78]. This, together with findings that a part of the morphological variation and the major phenotypic axes of first-generation hybrids are retained in later-generation hybrids [17], make it likely that the superior feeding efficiency on the ‘novel’ food types that we document in our first-generation hybrids will be present to some degree also in a fraction of later-generation hybrids. To determine how morphology interrelates with feeding performance-related behaviour in hybrids an integrative approach studying the relationship between functional morphology, behaviour and performance is needed. Such an experiment should ideally be extended to second-generation hybrids.
Another promising direction of research would be experimental evolution in large mesocosms conducted over multiple generations to measure fitness related traits such as growth or survival in hybrids and the parental species [34,36]. Although many studies in fish have used feeding efficiency as a proxy for performance and fitness in the context of ecological speciation, and have shown that morphology can be linked to function, ecological feeding specialization and thus ecological niche [70–72,75], we acknowledge that feeding efficiency captures only one of many possible components of fitness [8] and that other proxies such as growth or survival may more conclusively demonstrate the successful exploitation of an ecological niche because they integrate a larger number of components. Multi-generation hybrid fitness experiments on multiple ecological niches could be particularly interesting if they involved experimental hybrids between ancestral species of adaptive radiations with hybridogenic origins [13,24,79] or between constituent species from adaptive radiations for which recurrent hybridization has been invoked as a facilitator of diversification [25–28]. Multi-generation experiments would also allow to partition fitness variation into effects of heterosis versus novelty. Furthermore, hybrid crosses from a broader phylogenetic scope of parental species [11] and more ecological contrasts than used in our study would be desirable to be able to draw conclusions about the generality of ecological hybrid advantage as a factor in the diversification process. Together with dense QTL mapping or genome-wide association mapping, the genes or gene complexes with phenotypic effects can be detected [13]. These can be compared to the genes from different parental species which segregate in a hybrid populations mosaic genome and may help to assess how the sorting of these segregating genes or gene complexes contribute to adaptive radiations [13,24,79]. Hybridization results in the introduction and reassembling of genetic variation that can both have deleterious or beneficial intrinsic or extrinsic postzygotic effects, and comparative experimental studies are needed to assess how these different effects of hybridization have shaped the genomic architecture of ecology relevant traits in groups of hybridizing species [9,13,22]. Experiments over multiple generations that further vary the strength of the ecological contrast that is being tested and thereby mimic natural systems with fluctuating adaptive landscapes will allow to test how important extrinsic postzygotic isolation is in the speciation process [33–35,80].
Earlier studies on experimental first- and second-generation cichlid hybrids, with similar divergence times between the parental species as in our study, have revealed that hybrids are viable and fertile into higher generations between species several million years divergent [43,81]. Some of these hybrids regularly exhibit novel phenotypes in colour and morphology when compared to their parental species [15,17,43,77,82]. Several of these studies have further suggested that the observed novelties may be co-opted by natural (or sexual) selection since many of the experimental hybrids have been shown to resemble other species known from the cichlid radiations. Also, patterns of morphological diversity found in these hybrids have been shown to predict those observed in extant cichlid radiations [15,17,83].
In conclusion, the data we presented here constitute experimental evidence, lacking so far [57], that functional novelty in cichlid hybrids can be of adaptive relevance in certain ecological contexts and thereby possibly facilitate niche shifts, promote adaptive diversification and—when retained and stabilized in later-generation hybrids—speciation. We provide support for the hypothesis of ecological context dependency of the evolutionary impacts of interspecific hybridization. Our findings corroborate recent theoretical work [19] that in environments with many different and accessible ecological niches, of which only a subset is occupied, transgressive morphology (in our case functional traits) in hybrids can facilitate the process of adaptive radiation through ecological hybrid performance advantage. We suggest that this process was important for the diversification of cichlids of the great lakes of Africa.
Supplementary Material
Acknowledgements
We especially thank Sandro Wanner, who established a first set of feeding efficiency protocols in his Bachelor thesis, which were refined for this study. We thank Andreas Taverna for taking care of the aquarium facilities. We further thank Kotaro Kagawa, Kay Lucek, Jaime Mauricio Anaya-Rojas, Rebecca Best, Catherine E. Wagner and David A. Marques and all the members of the Fish Ecology and Evolution lab for valuable discussions.
Ethics
Fish breeding and experimentation were authorized by the veterinary office of the canton of Lucerne (licence number: LU04/07).
Data accessibility
The complete dataset is deposited on the Dryad Digital Repository: https://doi.org/10.5061/dryad.m25t886 [49].
Authors' contributions
O.M.S. and O.S. conceived the experiment, O.M.S. designed and performed the experiments and processed and analysed the data, and O.M.S. and O.S. wrote the manuscript together.
Competing interests
We declare we have no competing interests.
Funding
This research was supported by a Swiss National Science Foundation (Schweizer Nationalfond) grant no. (31003A-118293) to O.S.
References
- 1.Schluter D. 2000. The ecology of adaptive radiation. Oxford, UK: Oxford University Press. [Google Scholar]
- 2.Losos JB. 2010. Adaptive radiation, ecological opportunity, and evolutionary determinism. Am. Nat. 175, 623–629. ( 10.1086/652433) [DOI] [PubMed] [Google Scholar]
- 3.Yoder JB, et al. 2010. Ecological opportunity and the origin of adaptive radiations. J. Evol. Biol. 23, 1581–1596. ( 10.1111/j.1420-9101.2010.02029.x) [DOI] [PubMed] [Google Scholar]
- 4.Hendry AP, Nosil P, Rieseberg LH. 2007. The speed of ecological speciation. Funct. Ecol. 21, 455–465. ( 10.1111/j.1365-2435.2007.01240.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gavrilets S, Losos JB. 2009. Adaptive radiation: contrasting theory with data. Science 323, 732–737. ( 10.1126/science.1157966) [DOI] [PubMed] [Google Scholar]
- 6.Anderson E, Stebbins GL. 1954. Hybridization as an evolutionary stimulus. Evolution 8, 378–388. ( 10.1111/j.1558-5646.1954.tb01504.x) [DOI] [Google Scholar]
- 7.Lewontin RC, Birch LC. 1966. Hybridization as a source of variation for adaptation to new environments. Evolution 20, 315–336. ( 10.1111/j.1558-5646.1966.tb03369.x) [DOI] [PubMed] [Google Scholar]
- 8.Arnold ML. 2006. Evolution through genetic exchange. Oxford, UK: Oxford University Press. [Google Scholar]
- 9.Abbott R, et al. 2013. Hybridization and speciation. J. Evol. Biol. 26, 229–246. ( 10.1111/j.1420-9101.2012.02599.x) [DOI] [PubMed] [Google Scholar]
- 10.Rieseberg LH, Archer MA, Wayne RK. 1999. Transgressive segregation, adaptation and speciation. Heredity 83, 363–372. ( 10.1038/sj.hdy.6886170) [DOI] [PubMed] [Google Scholar]
- 11.Stelkens R, Seehausen O. 2009. Genetic distance between species predicts novel trait expression in their hybrids. Evolution 63, 884–897. ( 10.1111/j.1558-5646.2008.00599.x) [DOI] [PubMed] [Google Scholar]
- 12.Salzburger W. 2018. Understanding explosive diversification through cichlid fish genomics. Nat. Rev. Genet. 19, 705–717. ( 10.1038/s41576-018-0043-9) [DOI] [PubMed] [Google Scholar]
- 13.Marques DA, Meier JI, Seehausen O. 2019. A combinatorial view on speciation and adaptive radiation. Trends Ecol. Evol. 34, 531–544. ( 10.1016/j.tree.2019.02.008) [DOI] [PubMed] [Google Scholar]
- 14.Grant PR, Grant BR. 1994. Phenotypic and genetic effects of hybridization in Darwin's finches. Evolution 48, 297–316. ( 10.1111/j.1558-5646.1994.tb01313.x) [DOI] [PubMed] [Google Scholar]
- 15.Parsons KJ, Son YH, Albertson RC. 2011. Hybridization promotes evolvability in African cichlids: connections between transgressive segregation and phenotypic integration. Evol. Biol. 38, 306–315. ( 10.1007/s11692-011-9126-7) [DOI] [Google Scholar]
- 16.Renaud S, Alibert P, Auffray J-C. 2012. Modularity as a source of new morphological variation in the mandible of hybrid mice. BMC Evol. Biol. 12, 141 ( 10.1186/1471-2148-12-141) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Selz OM, Lucek K, Young KA, Seehausen O. 2014. Relaxed trait covariance in interspecific cichlid hybrids predicts morphological diversity in adaptive radiations. J. Evol. Biol. 27, 11–24. ( 10.1111/jeb.12283) [DOI] [PubMed] [Google Scholar]
- 18.Mitchell N, Owens GL, Hovick SM, Rieseberg LH, Whitney KD. 2019. Hybridization speeds adaptive evolution in an eight-year field experiment. Sci. Rep. 9, 6746 ( 10.1038/s41598-019-43119-4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kagawa K, Takimoto G. 2017. Hybridization can promote adaptive radiation by means of transgressive segregation. Ecol. Lett. 21, 264–274. ( 10.1111/ele.12891) [DOI] [PubMed] [Google Scholar]
- 20.Seehausen O. 2004. Hybridization and adaptive radiation. Trends Ecol. Evol. 19, 198–207. ( 10.1016/j.tree.2004.01.003) [DOI] [PubMed] [Google Scholar]
- 21.Mallet J. 2007. Hybrid speciation. Nature 446, 279–283. ( 10.1038/nature05706) [DOI] [PubMed] [Google Scholar]
- 22.Schumer M, Rosenthal GG, Andolfatto P. 2018. What do we mean when we talk about hybrid speciation? Heredity 120, 379–382 ( 10.1038/s41437-017-0036-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barrier M, Baldwin BG, Robichaux RH, Purugganan MD. 1999. Interspecific hybrid ancestry of a plant adaptive radiation: allopolyploidy of the Hawaiian Silversword Alliance (Asteraceae) inferred from floral homeotic gene duplications. Mol. Biol. Evol. 16, 1105–1113. ( 10.1093/oxfordjournals.molbev.a026200) [DOI] [PubMed] [Google Scholar]
- 24.Meier JI, Marques DA, Mwaiko S, Wagner CE, Excoffier L, Seehausen O. 2017. Ancient hybridization fuels rapid cichlid fish adaptive radiations. Nat. Commun. 8, 14363 ( 10.1038/ncomms14363) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Papadopulus AST, Price Z, Devaux C, Hipperson H, Smadja CM, Hutton I, Baker WJ, Butlin RK, Savolainen V. 2013. A comparative analysis of the mechanisms underlying speciation on Lord Howe Island. J. Evol. Biol. 26, 733–745. ( 10.1111/jeb.12071) [DOI] [PubMed] [Google Scholar]
- 26.Lamichhaney S, Han F, Webster MT, Andersson L, Grant BR, Grant PR. 2017. Rapid hybrid speciation in Darwin's finches. Science 359, 224–228. ( 10.1126/science.aao4593) [DOI] [PubMed] [Google Scholar]
- 27.Keller I, Wagner CE, Greuter L, Mwaiko S, Selz OM, Sivasundar A, Wittwer S, Seehausen O. 2013. Population genomic signatures of divergent adaptation, gene flow, and hybrid speciation in the rapid radiation of Lake Victoria cichlid fishes. Mol. Ecol. 22, 2848–2863. ( 10.1111/mec.12083) [DOI] [PubMed] [Google Scholar]
- 28.Meier JI, Sousa VC, Marques DA, Selz OM, Wagner CE, Excoffier L, Seehausen O. 2017. Demographic modelling with whole-genome data reveals parallel origin of similar Pundamilia cichlid species after hybridization. Mol. Ecol. 26, 123–141. ( 10.1111/mec.13838) [DOI] [PubMed] [Google Scholar]
- 29.Heliconius Genome Consortium. 2012. Butterfly genome reveals promiscuous exchange of mimicry adaptations among species. Nature 487, 94–98 ( 10.1038/nature11041) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hedrick PW. 2013. Adaptive introgression in animals and comparison to new mutation and standing variation as sources of adaptive variation. Mol. Ecol. 22, 4606–4618 ( 10.1111/mec.12415) [DOI] [PubMed] [Google Scholar]
- 31.Buerkle CA, Morris RJ, Asmussen MA, Rieseberg LH. 2000. The likelihood of homoploid hybrid speciation. Heredity 84, 441–451. ( 10.1046/j.1365-2540.2000.00680.x) [DOI] [PubMed] [Google Scholar]
- 32.Schumer M, Rosenthal GG, Andolfatto P. 2014. How common is homoploid hybrid speciation? Evolution 68, 1553–1560. ( 10.1111/evo.12399) [DOI] [PubMed] [Google Scholar]
- 33.Nosil P. 2012. Ecological speciation Oxford, UK: Oxford University Press. [Google Scholar]
- 34.Martin CH, Wainwright PC. 2013. Multiple fitness peaks on the adaptive landscape drive adaptive radiation in the wild. Science 339, 208–211. ( 10.1126/science.1227710) [DOI] [PubMed] [Google Scholar]
- 35.Arnegard ME, et al. 2014. Genetics of ecological divergence during speciation. Nature 511, 307–311. ( 10.1038/nature13301) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rajkov J, Weber AA-T, Salzburger W, Egger B. 2018. Immigrant and extrinsic hybrid inviability contribute to reproductive isolation between lake and river cichlid ecotypes. Evolution 72, 2553–2564. ( 10.1111/evo.13612) [DOI] [PubMed] [Google Scholar]
- 37.Konuma J, Sota T, Chiba S. 2013. A maladaptive intermediate form: a strong trade-off revealed by hybrids between two forms of a snail-feeding beetle. Ecology 94, 2638–2644. ( 10.1890/12-2041.1) [DOI] [PubMed] [Google Scholar]
- 38.Fryer G, Iles TD. 1972. The cichlid fishes of the great lakes of Africa: their biology and evolution. Edinburgh, UK: Oliver and Boyd. [Google Scholar]
- 39.Witte F, van Oijen MJP. 1990. Taxonomy, ecology and fishery of haplochromine trophic groups. Zool. Verh. Leiden 262, 1–47. [Google Scholar]
- 40.Seehausen O, Bouton N. 1998. The community of rock-dwelling cichlids in Lake Victoria. Bonn. Zool. Beitr. 47, 3–4. [Google Scholar]
- 41.Seehausen O, Lippitsch E, Bouton N, Zwennes H. 1998. Mbipi, the rock-dwelling cichlids of Lake Victoria: description of three new genera and fifteen new species (Teleostei). Ichthyol. Explor. Freshw. 9, 129–228. [Google Scholar]
- 42.Günther A. 1894. Second report on the reptiles, batrachians, and fishes transmitted by Mr H. H. Johnston, C. B., from British Central Africa. Proc. Gen. Meet. Scient. Bus. Zoolog. Societ. Lond. 4, 616–628. [Google Scholar]
- 43.Stelkens RB, Schmid C, Selz O, Seehausen O. 2009. Phenotypic novelty in experimental hybrids is predicted by the genetic distance between species of cichlid fish. BMC Evol. Biol. 9, 283 ( 10.1186/1471-2148-9-283) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tkint T, Verheyen E, De Kegel B, Helsen P, Adriaens D. 2012. Dealing with food and eggs in mouthbrooding cichlids: structural and functional trade-offs in fitness related traits. PLoS ONE 7, e31117 ( 10.1371/journal.pone.0031117) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mwebaza-Ndawula L. 1994. Changes in relative abundance of zooplankton in northern Lake Victoria, East Africa. Hydrobiologia 272, 259–264. ( 10.1007/bf00006526) [DOI] [Google Scholar]
- 46.Bouton N, Seehausen O, van Alphen JJM. 1997. Resource partitioning among rock dwelling haplochromines (Pisces: Cichlidae) from Lake Victoria. Ecol. Freshw. Fish 6, 225–240. ( 10.1111/j.1600-0633.1997.tb00165.x) [DOI] [Google Scholar]
- 47.R Development Core Team, 2008. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; See http://www.R-project.org. [Google Scholar]
- 48.Benjamini Y, Hochberg Y. 1995. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B 57, 289–300. [Google Scholar]
- 49.Selz OM, Seehausen O. 2019. Data from: Interspecific hybridization can generate functional novelty in cichlid fish. Dryad Digital Repository. ( 10.5061/dryad.m25t886) [DOI] [PMC free article] [PubMed]
- 50.Bates D, Kliegl R, Vasishth S, Baayen H. 2015. Parsimonious mixed models. arXiv. (http://arxiv.org/abs/1506.04967)
- 51.Symonds MRE, Moussalli A. 2011. A brief guide to model selection, multimodel inference and model averaging in behavioural ecology using Akaike's information criterion. Behav. Ecol. Sociobiol. 65, 13–21. ( 10.1007/s00265-010-1037-6) [DOI] [Google Scholar]
- 52.Werner EE, Gilliam JF. 1984. The ontogenetic niche and species interactions in size structured populations. Annu. Rev. Ecol. Syst. 15, 393–425. ( 10.1146/annurev.es.15.110184.002141) [DOI] [Google Scholar]
- 53.Duenez-Guzman EA, Mavarez J, Vose MD, Gavrilets S. 2009. Case studies and mathematical models of ecological speciation. 4. Hybrid speciation in butterflies jungle. Evolution 63, 2611–2626. ( 10.1111/j.1558-5646.2009.00756.x) [DOI] [PubMed] [Google Scholar]
- 54.McCarthy EM, Asmussen MA, Anderson WW. 1995. A theoretical assessment of recombinational speciation. Heredity 74, 502–509. ( 10.1038/hdy.1995.71) [DOI] [Google Scholar]
- 55.Blanckaert A, Bank C. 2018. In search of the Goldilocks zone for hybrid speciation. PLoS Genet. 14, e1007613 ( 10.1371/journal.pgen.1007613) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Czypionka T, Cheng J, Pozhitkov A, Nolte A. 2012. Transcriptome changes after genome- wide admixture in invasive sculpins (Cottus). Mol. Ecol. 21, 4797–4810. ( 10.1111/j.1365-294X.2012.05645.x) [DOI] [PubMed] [Google Scholar]
- 57.Genner MJ, Turner GF. 2012. Ancient hybridization and phenotypic novelty within lake Malawi's cichlid fish radiation. Mol. Biol. Evol. 29, 195–206. ( 10.1093/molbev/msr183) [DOI] [PubMed] [Google Scholar]
- 58.Rieseberg LH, et al. 2003. Major ecological transitions in wild sunflowers facilitated by hybridization. Science 301, 1211 ( 10.1126/science.1086949) [DOI] [PubMed] [Google Scholar]
- 59.Gompert Z, Fordyce JA, Forister ML, Shapiro AM, Nice CC. 2006. Homoploid hybrid speciation in an extreme habitat. Science 314, 1923–1925. ( 10.1126/science.1135875) [DOI] [PubMed] [Google Scholar]
- 60.Schliewen UK, Klee B. 2004. Reticulate sympatric speciation in Cameroonian crater lake cichlids. Front. Zool. 1, 1–12. ( 10.1186/1742-9994-1-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Xing F, et al. 2014. Needle morphological evidence of the homoploid hybrid origin of Pinus densata based on analysis of artificial hybrids and the putative parents, Pinus tabuliformis and Pinus yunnanensis. Ecol. Evol. 4, 1890–1902. ( 10.1002/ece3.1062) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Anton KA, Ward JR, Cruzan MB. 2013. Pollinator-mediated selection on floral morphology: evidence for transgressive evolution in a derived hybrid lineage. J. Evol. Biol. 26, 660–673. ( 10.1111/jeb.12083) [DOI] [PubMed] [Google Scholar]
- 63.Donovan LA, Rosenthal DR, Sanchez-Velenosi M, Rieseberg LH, Ludwig F. 2010. Are hybrid species more fit than ancestral parental species in the current hybrid species habitats? J. Evol. Biol. 23, 805–816. ( 10.1111/j.1420-9101.2010.01950.x) [DOI] [PubMed] [Google Scholar]
- 64.Zhao W, Meng J, Wang B, Zhang L, Xu Y, Zeng Q-Y, Li Y, Mao J-F, Wang XR. 2014. Weak crossability barrier but strong juvenile selection supports ecological speciation of the hybrid pine Pinus densata on the Tibetian plateau. Evolution 68, 3120–3133. ( 10.1111/evo.12496) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gross BL, Rieseberg LH. 2005. The ecological genetics of homoploid hybrid speciation. J. Hered. 96, 241–252. ( 10.1093/jhered/esi026) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pereira RJ, Barreto FS, Burton RS. 2013. Ecological novelty by hybridization: experimental evidence for increased thermal tolerance by transgressive segregation in Tigriopus californicus. Evolution 68, 204–215. ( 10.1111/evo.12254) [DOI] [PubMed] [Google Scholar]
- 67.Oziolor EM, Reid NM, Yair S, Lee KM, VerPloeg SG, Bruns PC, Shaw JR, Whitehead A, Matson CW. 2019. Adaptive introgression enables evolutionary rescue from extreme environmental pollution. Science 364, 455–457 ( 10.1126/science.aav4155) [DOI] [PubMed] [Google Scholar]
- 68.Johnson JB, Culumber ZW, Easterling R, Rosenthal GG. 2015. Boldness and predator evasion in naturally hybridizing swordtails (Teleostei: Xiphophorus). Curr. Zool. 61, 596–603. ( 10.1093/czoolo/61.4.596) [DOI] [Google Scholar]
- 69.Seehausen O. 2006. African cichlid fish: a model system in adaptive radiation research. Proc. R. Soc. B 273, 1987–1998. ( 10.1098/rspb.2006.3539) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Higham TE, Hulsey CD, Rican O, Carroll AM. 2007. Feeding with speed: prey capture evolution in cichlids. J. Evol. Biol. 20, 70–78. ( 10.1111/j.1420-9101.2006.01227.x) [DOI] [PubMed] [Google Scholar]
- 71.Rupp MF, Hulsey CD. 2014. Influence of substrate orientation on feeding kinematics and performance of algae grazing Lake Malawi cichlid fishes. J. Exp. Biol. 217, 3057–3066. ( 10.1242/jeb.105080) [DOI] [PubMed] [Google Scholar]
- 72.Henning F, Machado-Schiaffino G, Baumgarten L, Meyer A. 2017. Genetic dissection of adaptive form and function in rapidly speciating cichlid fishes. Evolution 71, 1297–1312. ( 10.1111/evo.13206) [DOI] [PubMed] [Google Scholar]
- 73.Streelman JT, Webb JF, Albertson RC, Kocher TD. 2003. The cusp of evolution and development: a model of cichlid tooth shape diversity. Evol. Dev. 5, 600–608. ( 10.1046/j.1525-142X.2003.03065.x) [DOI] [PubMed] [Google Scholar]
- 74.Schwalbe MAB, Webb JF. 2013. Sensory basis for detection of benthic prey in two Lake Malawi cichlids. Zoology 117, 112–121. ( 10.1016/j.zool.2013.09.003) [DOI] [PubMed] [Google Scholar]
- 75.Bouton N, van Os N, Witte F. 1998. Feeding performance of Lake Victoria rock cichlids: testing predictions from morphology. J. Fish Biol. 53, 118–127 ( 10.1111/j.1095-8649.1998.tb01022.x) [DOI] [Google Scholar]
- 76.Wainwright PC, Alfaro ME, Bolnick DI, Hulsey CD. 2005. Many-to-one mapping of form to function: a general principle in organismal design? Integr. Comp. Biol. 45, 256–262. ( 10.1093/icb/45.2.256) [DOI] [PubMed] [Google Scholar]
- 77.Parnell NF, Hulsey CD, Streelman JT. 2008. Hybridization produces novelty when the mapping of form to function is many to one. BMC Evol. Biol. 8, 122 ( 10.1186/1471-2148-8-122) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Holzman R, Hulsey CD. 2017. Mechanical transgressive segregation and the rapid origin of trophic novelty . Sci. Rep. 7, 40306 ( 10.1038/srep40306) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Malinsky M, Svardal H, Tyers AM, Miska EA, Genner MJ, Turner GF, Durbin R. 2018. Whole-genome sequences of Malawi cichlids reveal multiple radiations interconnected by gene flow. Nat. Ecol. Evol. 2, 1940–1955. ( 10.1038/s41559-018-0717-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.van Rijssel JC, Moser FN, Frei D, Seehausen O. 2018. Prevalence of disruptive selection predicts extent of species differentiation in Lake Victoria cichlids. Proc. R. Soc. B 285, 20172630 ( 10.1098/rspb.2017.2630) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Stelkens R, Young K, Seehausen O. 2009. The accumulation of reproductive incompatibilities in African cichlid fish. Evolution 64, 617–633. ( 10.1111/j.1558-5646.2009.00849.x) [DOI] [PubMed] [Google Scholar]
- 82.Selz OM, Thommen R, Maan ME, Seehausen O. 2014. Behavioural isolation may facilitate homoploid hybrid speciation in cichlid fish. J. Evol. Biol. 27, 275–289. ( 10.1111/jeb.12287) [DOI] [PubMed] [Google Scholar]
- 83.Cooper WJ, Wernle J, Mann K, Albertson RC. 2011. Functional and genetic integration in the skulls of Lake Malawi cichlids. Evol. Biol. 38, 316–334. ( 10.1007/s11692-011-9124-9) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Selz OM, Seehausen O. 2019. Data from: Interspecific hybridization can generate functional novelty in cichlid fish. Dryad Digital Repository. ( 10.5061/dryad.m25t886) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
The complete dataset is deposited on the Dryad Digital Repository: https://doi.org/10.5061/dryad.m25t886 [49].