Abstract
Animal populations will mediate the response of global biodiversity to environmental changes. Population models are thus important tools for both understanding and predicting animal responses to uncertain future conditions. Most approaches, however, are correlative and ignore the individual-level mechanisms that give rise to population dynamics. Here, we assess several existing population modelling approaches and find limitations to both ‘correlative’ and ‘mechanistic’ models. We advocate the need for a standardized mechanistic approach for linking individual mechanisms (physiology, behaviour, and evolution) to population dynamics in spatially explicit landscapes. Such an approach is potentially more flexible and informative than current population models. Key to realizing this goal, however, is overcoming current data limitations, the development and testing of eco-evolutionary theory to represent interactions between individual mechanisms, and standardized multi-dimensional environmental change scenarios which incorporate multiple stressors. Such progress is essential in supporting environmental decisions in uncertain future conditions.
Keywords: individuals, population models, physiology, behaviour, evolution, environmental change
1. Introduction
Animal responses to environmental change have wide-ranging consequences for global biodiversity and ecosystem functioning, through altered species interactions, richness, community composition, and the transfer of energy and nutrients [1]. Yet, much remains unknown about the selective nature of environmental changes and the interactive effects of multiple stressors [2]. An urgent challenge is thus to better understand the mechanisms underpinning animal population responses to environmental change in order to better anticipate the effects of novel future conditions [3].
Disentangling the mechanisms that give rise to population responses is a multifaceted challenge. The urgency of understanding this complexity is likely responsible for the many correlative approaches to ecological forecasting [4]. Yet, such approaches cannot reliably extrapolate outside of the observed environmental range [5,6] and fail to represent key biological and ecological mechanisms that mediate species responses in heterogeneous landscapes [7]. Population dynamics, however, are primarily determined by interactions between individuals with each other and their environment [8]. Accounting for these individual-level mechanisms therefore has the potential to better describe divergent shifts in species abundances and distributions in response to environmental changes.
Multiple stressors often interact with individual-level mechanisms to cause nonlinear population responses and may have additive, exacerbating, or alleviating effects [9]. For instance, many species experience phenological and geographical range shifts consistent with climate changes over time [10], while rapid and widespread declines of other species are being driven by habitat loss and fragmentation, overexploitation, invasive species, and pollution [11]. Honeybee colony collapses across the Northern Hemisphere, for example, have been attributed to the combined spread of invasive parasitic mites, exposure to harmful pesticides [12], climatic changes, and habitat fragmentation [13]. Population responses to environmental changes are thus dependent on individual exposure to multiple stressors in spatially explicit landscapes. Although correlative models often account for heterogeneous environments, they cannot fully represent the interactive effects of multiple stressors at the individual level.
Mechanistic models which incorporate individual-level mechanisms are ideal for generating more informed predictions of population responses to novel environmental changes. However, little progress has been made in developing an approach that is both mechanistic (captures the mechanisms driving population dynamics in spatially explicit landscapes) and general (can be applied to various species and environmental scenarios). Here, we first discuss the importance of individual mechanisms (physiology, behaviour, and evolution) in driving population dynamics and then evaluate the ability of several existing population modelling approaches to predict population responses to novel environmental change. We suggest the need to work towards a standardized mechanistic approach so that individual mechanisms inform predictions at the population level. We then review the availability of quantitative methods for the representation of these individual mechanisms in population models. Finally, we discuss current limitations to developing such an approach and how these could be addressed.
2. Importance of individual-level mechanisms in driving population dynamics
Ecology typically describes individual variation according to species' physiological and behavioural traits [14–16]. Physiology explains the phenotypic plasticity of life-history traits in response to environmental variables. For instance, trade-offs between individual traits (e.g. growth and reproduction) occur in response to changing food availability, quality, and temperature by altering energy acquisition and expenditure [17]. Behaviour then relates individuals of varying physiology to their position in the landscape and interactions with other individuals. Movement is key, as how individuals move across landscapes to fulfil their needs dictate their exposure to adverse conditions (e.g. predation, pollution, drought) [11]. The physiological state of individuals also plays a central role in behavioural mechanisms, for instance, by trading-off high-quality resources for other factors such as finding a mate or avoiding predation.
Plastic effects through altered physiology and behaviour have been widely attributed to population responses under environmental change [18], but genetic effects play an important role for many species [19]. That is, genetic interactions between fitness-related traits and the direction of selection across multiple traits constrain an individual's potential for evolutionary adaptation [20]. Rapid evolutionary change has been shown for a number of taxa exposed to novel environmental conditions [21], short-lived species experiencing rapid changes [22], species unable to disperse to favourable habitats [23], and at landscape scales [24]. Physiology, behaviour, and evolution thus need to be understood together to build a comprehensive understanding of how individuals respond to their environment, and how individual responses translate into population-level effects.
Under future environmental changes, physiology describes the sensitivity of species to stressors, behaviour describes species' exposures to those stressors, and evolution describes the potential variation of individual responses. Interactions between individual mechanisms within the landscape then describe how collective populations either acclimatize to small shifts in environmental conditions, shift their distributions, or decline in response to larger changes. Population ecology has classically understood these individual-level mechanisms using a top-down approach, whereby demographic rates are related to environmental (e.g. temperature) or population-level (e.g. density) variables. More recently, however, mechanistic population models have been developed that use these individual-level mechanisms to predict population-level effects in a bottom-up approach.
3. Existing population modelling approaches
Population modelling approaches are often reviewed in isolation because they integrate different levels of biological organization and ecological scales, but progress in population modelling will rely on a combination of features from different approaches. In this section, we review several modelling approaches commonly used to predict population responses to environmental changes. Most modelling approaches have been developed to answer different ecological or evolutionary questions, and so each method reviewed here is suited to its overarching purpose. Our focus, however, is on their ability to integrate individual-level mechanisms and extrapolate across taxa and environmental scenarios in spatially explicit landscapes, to provide informed predictions under environmental change.
(a). Demographic models
Demographic population models, such as Matrix Population Models (MPMs), have played a key role in the development of ecological and evolutionary theory since their conception [25]. By linking individual variation in species to changes in survival and reproduction rates, MPMs provided a basis for understanding how population dynamics shifted with demographic traits (e.g. birth and death rates, intrinsic growth rate) [26] and population density [27]. Over the last few decades, MPMs have become increasingly powerful with advances in computational and statistical approaches in ecology [28]. Integral projection models (IProjMs), for instance, include both continuous (e.g. mass) and discreet state variables (e.g. life stage) to more accurately represent population structure [29], whereas Integrated Population Models (IPopMs) can combine individual- and population-level data to better estimate the influence of individual variation on demographic rates [30]. Classical demographic models are nevertheless based on statistical relationships between demographic rates and environmental conditions, making them more suited to understanding species dynamics under current environmental conditions than predicting population responses to novel environments in the future [5]. That is, because the representation of demography in response to environmental variables is constrained by the input data, they cannot reliably extrapolate outside of the environmental and/or stressor scenario in which the data were collected. It is also often necessary to parametrize MPMs for different population (e.g. pre- and post-breeding), environmental, or management scenarios because the fundamental relationships between environmental fluctuations, demographic rates, and populations are not integrated [31]. Inclusion of the mechanisms that underpin demographic rates thus allows for the representation of both a greater range of environmental conditions and species traits in MPMs.
Demographic models show improved predictions when incorporating physiological and evolutionary processes [32]. Mechanistic IProjMs, for instance, increasingly combine energy budget models to describe individual life histories [33,34]. Because IProjMs can also account for multiple continuous state variables, trait distributions at the population level can change, either plastically or evolutionarily, according to shifts in individual life cycles and inheritance functions [18]. IProjMs have more recently been combined with IPopMs to provide better estimates of individual-level traits and population-level density dependence from multiple data sources [35]. Still, model predictions are informed by the population data, limiting predictions of population responses to novel environmental conditions in the future for which data do not yet exist. Demographic models are also limited to representing immigration and emigration rates in homogeneous environments, and so cannot incorporate individual-level behavioural decisions in spatially explicit landscapes (table 1).
Table 1.
Summary of modelling approaches typically used in predicting animal population responses to environmental change. Different approaches are categorized according to their ability to describe the individual-level mechanisms (physiology, behaviour, and evolution) that drive population responses to environmental changes in spatially explicit landscapes.
| modelling approach | spatially explicit | vital rates | individual variation | physiology | behaviour | evolution | examples |
|---|---|---|---|---|---|---|---|
| demographic models | |||||||
| matrix population models (MPMs) | N | Y | N | N | N | N | Crouse et al. [36] |
| mechanistic MPMs | N | Y | N | N | N | Y | De Vries & Caswell [37] |
| integrated population models (IPopMs) | N | Y | N | Y | N | Y | Schaub et al. [38] |
| mechanistic IPopMs | N | Y | Y | Y | N | Y | Plard et al. [35] |
| integral projection models (IProjMs) | N | Y | Y | Y | N | Y | Smallegange et al. [33,34], Ozgul et al. [18], Coulson et al. [39] |
| species distribution models (SDMs) | |||||||
| classical SDMs | Y | N | N | N | N | N | Elith & Leathwick [40] |
| process-based SDMs | Y | Y | Y | Y | N | Y | Buckley [41], Kearney et al. [42], Fordham et al. [43] |
| dynamic range models | Y | Y | N | N | Y | N | Zurell et al. [44] |
| individual-based models (IBMs) | |||||||
| classical IBMs | Y | Y | Y | N | Y | N | Liu et al. [45], Becher et al. [46] |
| mechanistic IBMs | Y | Y | Y | Y | Y | Y | Bocedi et al. [47], Galic et al. [48], Johnston et al. [49], Boyd et al. [50] |
(b). Species distribution models
Classical Species Distribution Models (SDMs, also known as niche models, climate envelope models, and habitat models) were developed to better understand the relationships between species distributions and environmental variables in spatially explicit landscapes [40]. Classical SDMs typically infer species’ ecological niches, using statistical relationships, from their distributions across reference landscapes for which abiotic conditions (e.g. temperature, precipitation, soil type) are known. Models are then coupled with environmental change forecasts to project future species distributions [51]. The relative ease of building SDMs makes them popular tools in predicting the distributions of species under climate changes [52,53], conservation planning [54], and invasive species risk assessments [55] at landscape scales. However, the relationships between species abundances and distributions, on which classical SDMs are built, will likely vary outside of the spatial and/or temporal extents of the data to which they were fitted. Projecting population dynamics into the future with classical SDMs is therefore problematic due to the potential for environmental variables and species distributions to covary in novel ways [51]. Future species distributions will also be strongly influenced by species behaviour and landscape factors which limit dispersal of metapopulation dynamics (e.g. habitat fragmentation) [56]. As such, classical SDM predictions in novel environmental conditions are associated with high uncertainty [57]. These limitations of classical SDMs, alongside other caveats, have been reviewed previously [58,59] and has led to the development of process-based SDMs.
Process-based SDMs aim to address the shortcomings of classical SDMs by incorporating additional processes such as demographic rates, physiological and behavioural constraints to movement, connectivity between suitable patches, and population dynamics [60–62]. For a number of species, both correlative and mechanistic SDMs have been developed and often give comparable predictions of future distributions under climate change [61,63]. Other mechanistic SDMs, however, have identified important processes for accurately predicting species abundances and distributions. A mechanistic SDM developed to predict historical changes in the distribution of the mosquito Aedes aegypti across Australia, for instance, found that the incorporation of evolution in egg desiccation resistance was key to predicting species distribution shifts under climate change [42]. Similarly, the range dynamics of the widespread North American lizard, Sceloporus undulates, were better predicted when individual bioenergetics were incorporated in a process-based SDM [41]. Most process-based SDMs, however, focus on processes linked to species demographic rates rather than behaviour.
Dynamic range models (DRMs) have recently been introduced to address the lack of behaviour in SDMs, by incorporating the effects of dispersal on species abundance and distribution alongside population demography [64]. That is, species abundance and distribution data are used to estimate statistical relationships between environmental variables and demographic rates, density dependence, and dispersal rates in a statistical model [64]. There are relatively few examples of operational DRMs, but a recent evaluation of several approaches found DRMs, compared to classical and process-based SDMs, to improve predictions under current climate conditions [44]. However, model results were evaluated using simulated rather than real data, while predictions under climate change scenarios were comparable across models [44]. Pagel & Schurr [64] suggested that the use of mechanistic submodels, for both niche and population dynamics, would increase the predictive power of DRMs under environmental change.
(c). Individual-based models
Individual-based models (IBMs; also known as agent-based models, ABMs) consider individuals and their variation as the fundamental building blocks of ecological systems, while landscapes are often dynamic and characterized by environmental drivers [65]. During model simulations, individuals interact with one another and their environment and make decisions about how to maximize their fitness in a given environment, resulting in emergent predictions at the population level. IBMs can thus describe the bottom-up mechanisms that give rise to population dynamics in novel environmental and management scenarios [8]. Accounting for individual variation explicitly further allows for predictions of population distributions according to individual characteristics across heterogeneous environments. IBMs have thus proven to be particularly useful in addressing land management and conservation scenarios, where the consequences of individual exposures to multiple stressors on species populations can be predicted [3,66]. Despite their many advantages, however, IBMs are far less commonly used for predicting environmental change effects on species abundances and distributions than MPMs and SDMs [3].
A key limitation of IBMs is the need for sufficient, and precise, individual-level data to parametrize species life cycles and behaviours under various environmental scenarios [67]. Data availability at the individual and population level is often limited for different species, and so most IBMs are developed ad hoc with the model's purpose (i.e. species, environmental, and management scenarios) and data availability in mind [68]. IBMs are thus less standardized than demographic models or SDMs and can be time-intensive to develop. IBMs are also not necessarily mechanistic, and demographic rates are widely used to parametrize IBMs. However, demographic models are being increasingly replaced by physiological and behavioural mechanisms which better describe fundamental relationships across species and environmental variables [48–50]. These ‘mechanistic’ IBMs are better able to make predictions outside of the range of environmental conditions for which they were parametrized because the individual-level mechanisms remain unchanged across scenarios.
4. Towards a standardized mechanistic approach in population modelling
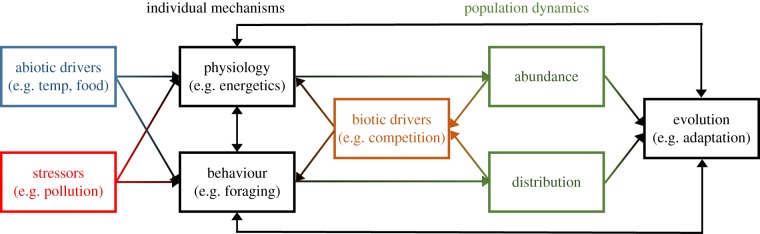
Progress in mechanistic population modelling has been made by integrating individual-level mechanisms in historically correlative or demographic approaches (table 1). Indeed, a common feature of the population modelling approaches reviewed in the previous section is the recent integration of mechanisms to provide better predictive power. However, there is little consensus on how to integrate the full range of mechanisms within population models. There is thus an overarching need to work towards a standardized mechanistic approach across existing population models. Such an approach would consider different individual-level mechanisms (physiology, behaviour, and evolution) and the interactions between them (figure 1). A key benefit to a standardized approach is that current ad hoc development of mechanistic approaches is time-consuming. Also, because population models are typically developed to answer specific questions they are often species and site specific. By integrating fundamental and general eco-evolutionary rules (e.g. thermodynamics and energy conservation, stoichiometry, natural selection), a standardized mechanistic approach would be applicable across taxa and environmental scenarios and have better predictive power under environmental change.
Figure 1.
Conceptual standardized mechanistic approach for predicting animal population dynamics in response to spatially explicit abiotic drivers (blue) and multiple stressors (red). Individual mechanisms (black) interact to drive shifts in population abundance and distribution (green), and biotic drivers (orange) cause feedbacks between population dynamics and individual mechanisms. (Online version in colour.)
5. Mechanistic submodels for representing individual-level mechanisms within population models
Individual mechanisms need to be represented using quantitative submodels in mechanistic population models. Ideally, a toolkit of standardized mechanistic submodels would be available for modellers to integrate into population models and test for different species and scenarios. A synthesis of existing submodels, however, is needed to better understand how these could be linked in a standardized mechanistic population model (figure 1). Here, we review approaches currently used to describe physiological, behavioural, and evolutionary mechanisms at the individual level. While these individual mechanisms interact with one another, the methods to model each often come from disparate fields and so are considered separately in the following section.
(a). Physiology
Phenotypic plasticity is often described using energy budget models (also known as energy allocation, bioenergetics, or biophysical models), which integrate fundamental principles of physiological ecology. Energy budget models represent how individual animals acquire energy from food resources and expend assimilated energy on different life cycle processes in order to maximize Darwinian fitness [69,70]. When food is limited, for instance, r-selected species often allocate energy to reproduction before growth. Because physiological and biochemical properties are widely shared across taxa and/or species, energy budgets also provide a general framework for representing individual life cycles [71]. When coupled with heterogeneous landscapes, energy budgets integrated into population models are useful for predicting population responses to changing resource distributions and temperature regimes [48,49]. However, current energy budget approaches are limited to describing life cycles in response to a small number of abiotic drivers (temperature, resource amount, and energy contents).
Nutrition, together with energy, plays a central role in physiology through the need to maintain nutrient homeostasis [72]. Ecological stoichiometry (ES) is used to investigate environmental effects on the nutrient (carbon, nitrogen, phosphorous) stoichiometry of organisms, and how nutrients flow through individuals and populations [73]. Combinations of energy budget and ES concepts in a unified framework have been suggested to predict the influence of nutrition on animal populations but have not yet been applied within a population model [74]. Similar approaches have been suggested to combine the metabolic theory of ecology and ES [75]. Still, metabolic submodels do not currently integrate mechanisms of acclimatization, adaptation, or genetic plasticity, whereby the expression of physiological traits vary with environmental stress.
(b). Behaviour
Behavioural plasticity plays a central role in the ability of animals to cope with environmental changes [11]. Classical behavioural ecology theories such as optimal foraging, ideal free distribution (IDF), and kin selection provide testable submodels for describing animal behaviour in population models. Yet, most assume that animals will always move in order to optimize their fitness and that they have perfect knowledge of the profitability of their environment [76]. IDF, for example, assumes equilibrium distribution of organisms among patchy resources or habitats [77]. Many animals, however, have shown maladaptive behavioural responses to environmental changes [78], suggesting the need to understand animal behaviour according to trade-offs between an individual's fitness and their position in a rapidly changing environment.
State-space models (SSMs) of animal movement integrate unobserved interactions between individual fitness and environmental variables to better understand movement patterns [79]. Coupling SSMs with robust individual physiology and evolution submodels could thus improve the mechanistic basis for understanding animal abundances and distributions in future conditions. On the other hand, energy budget models coupled with spatially explicit IBMs can be used to understand how animals forage to maximize their fitness in heterogeneous environments [70]. However, the profitability of landscape patches and trade-offs between different environmental variables need to be described [66]. Patch profitability then needs to be linked to the probability of moving, together with movement metrics such as speed, direction, and turning angles [80]. Nutritional ecology has addressed some of these questions through the Geometric Framework, which was developed to understand how individual behaviour (e.g. foraging) responded to changes in the nutritional value (energetic macronutrients, micronutrients, and non-nutritional components) of available food resources [81].
Animal groups are influenced by additional behaviours such as collective decisions and sociality. Many studies have stressed the importance of quorum responses as a key feature of collective decisions at the group level, which are modelled as nonlinear probabilities of an individual choosing a particular action according to the number of individuals already committed to the same decision [82], although this is just one means by which collective decisions are made. In other groups, the age-structure of populations can be critical in group responses to environmental changes, particularly in long-lived species where changes in behaviour can occur faster than evolution [83]. In such cases, the loss of leaders can lead to an overall loss of information from the group [84]. Although animal sociality is an important mechanism driving population responses to environmental change [85], there are currently very few approaches for linking animal culture to behavioural decisions.
(c). Evolution
Evolutionary processes moderate species responses to environmental change via complex eco-evolutionary dynamics [86]. Genetic variation and heritability are often studied at the population level [20], and observations can be used to predict the selection response of a population given single- or multiple-trait heritability and a specified selection pressure [87]. Approaches such as the breeders equation have enabled identification of the genetic and non-genetic components of phenotypic changes in response to novel environments. Demographic processes within populations, however, play a key role in evolutionary change. The mechanistic MPM of de Vries & Caswell [37] addresses this issue by integrating a demographic genetic model which accounts for genotype-stage dynamics and allows for the maintenance of a genetic polymorphism. Adaptive population responses to environmental change, however, rely on interactions between different levels of biological organization in the same way as nonadaptive population responses [88]. That is, evolutionary change at the population level will feedback to a number of mechanisms operating at the individual level ([89], figure 1).
The influence of trait variation on demographic rates and their heritability is increasingly accounted for in population models which integrate evolutionary processes. IProjMs which link demography to trait variation, for instance, can incorporate eco-evolutionary dynamics using statistical relationships between vital rates and environmental variables and estimates of heritability [90]. Likewise, the reaction norm (RN) concept for quantifying genotype–phenotype relationships is typically expressed as simple linear regressions between trait value in the average environment and the change in phenotype across an environmental gradient [91]. While statistical relationships between demographic rates and evolutionary change allow for models to account for the influence of population dynamics on adaptive responses, they cannot describe the fundamental relationships influencing genetic structure [92]. An alternative approach, typically applied to macroevolutionary processes, is the direct representation of alleles coding for a phenotypic trait of individuals that are then inherited by their offspring [47,93]. Although applications of such models have so far been largely theoretical, Coulson et al. [39] recently set out a framework for incorporating developmental and inheritance rules for both genetic and environmental components of a phenotype in IProjMs. Such an approach can predict both plastic and adaptive population responses to environmental change.
6. Current limitations and future directions
Representing how animal population dynamics emerge from interactions between individual mechanisms in spatially explicit landscapes will improve the predictive power of population models. Such mechanistic approaches are potentially more flexible and informative than existing population modelling approaches which rely on correlative relationships and/or ad hoc model development. A number of current limitations, however, need to be overcome before progress in the development of a standardized mechanistic approach in population modelling can be made.
(a). Data availability
A key limitation in population modelling is the availability of data to parametrize, calibrate, and validate models. Historically, SDMs have relied only on presence–absence data, demographic models were built with snapshots of abundance over time, and IBMs have focused on a single well-studied system to fulfil high data needs. A standardized mechanistic approach, however, necessitates data at the individual level for parametrization and the population level for validation. For most species, data are often limiting at one level. For instance, short-lived species are often well studied at the individual level in laboratory conditions and less so at the population and field level (e.g. invertebrates and fish), whereas population data may be available for wild animals but individual-level data are scant (e.g. large mammals). Another limitation is that most empirical studies are conducted over short timescales, while the processes influencing population responses to environmental changes operate over longer timescales.
Individual-based and long-term field studies represent an important resource for the development and evaluation of a standardized mechanistic approach in population modelling [94]. In particular, datasets for diverse species and scenarios will be crucial in testing whether such an approach can identify how different mechanisms influence a population response to different environmental changes. Individual-based studies, for instance, have played a key role in identifying the role of individual variation, age-related fitness, and social structures on population dynamics [95–99]. Still, mechanistic submodels often require more detailed information at the individual level than is recorded in the field. Energy budget models, for example, often require prior knowledge about ingestion, assimilation, growth, and reproduction rates in optimal environmental conditions. An advantage of developing a standardized mechanistic approach in population modelling, however, is in providing a consensus on how to address data gaps using robust statistical techniques and calibration tools (e.g. [66]).
Other promising advances being made in the collection and sharing of data include remote sensing and citizen science projects [100]. For example, satellite tracking technology such as that used in the recently launched International Cooperation for Animal Research Using Space (ICARUS) project [101] can provide valuable data for parametrizing the movements and dispersal ability of individuals. A growing data sharing culture and the growth of freely available online databases such as Add-my-pet [102] and Movebank [103] present another promising source of data for population models. A standardized mechanistic approach, developed and tested for diverse species and scenarios simultaneously, would provide additional consensus on data requirements and availability from diverse sources. Such an approach would also identify key knowledge gaps in physiological, behavioural, and evolutionary ecology which could be addressed through coupled modelling-empirical studies.
(b). Eco-evolutionary theory
Quantitative methods for representing individual mechanisms and the interactions between them as in figure 1 need to be developed and tested. A number of current approaches, based on fundamental eco-evolutionary theory, have been developed to address single mechanisms. A pragmatic way forward, therefore, is to establish which of these competing approaches for representing physiology, behaviour, and evolution can be used within a single framework. Because different approaches have been designed to address different questions, however, components from a variety of approaches may need to be integrated. Using established and extensive datasets for different species and scenarios, as discussed above, provides a way to develop a unified approach by testing their assumptions and predictions. Novel eco-evolutionary theory will likely emerge from such an exercise, because interactions between physiology, behaviour, and evolution need to be accounted for to understand diverse population responses.
(c). Environmental scenarios
There is an overarching need for realistic and multi-dimensional environmental scenarios. Climate forecasts, from a range of Earth system models and for numerous greenhouse gas emission scenarios, are well developed as inputs to population models. A general lack of standardized multiple stressor scenarios, however, limit many population modelling approaches to focusing on the effects of climate changes alone. Multi-dimensional environmental change scenarios would include multiple environmental drivers and stressors and could be developed by integrating key drivers of biodiversity change (e.g. land use, atmospheric CO2 concentration, nitrogen deposition, and climate) using different scenarios generated by global models of climate, vegetation, and land use. Such scenarios could identify how global drivers interacted in the past (e.g. antagonistically or synergistically) to inform more realistic environmental scenarios in the future. Hypothetical scenarios of additional stressors, such as habitat fragmentation, pollution, and invasive species, could be further integrated for projection purposes. Such standardized landscape-scale environmental scenarios will be key to objectively evaluating different modelling approach predictions under environmental change.
7. Concluding remarks
Mechanistic population models are needed to better anticipate, and mitigate, the ecological consequences of future environmental changes. Currently, population models tend to be either ‘correlative’ or ‘mechanistic’. Correlative models assess how current ecological ranges of species will shift or disappear with changing climatic conditions, and provide useful assessments of species' exposure to environmental changes but are limited to extrapolations of historical population patterns into the future. Mechanistic models, on the other hand, provide more robust predictions about a species’ vulnerability to future environmental changes by incorporating individual-level mechanisms but are time- and data-intensive and limited to finer ecological scales compared to correlative approaches. A standardized mechanistic approach is needed for more informed predictions of animal population responses to novel environmental conditions. Progress in predictive population modelling should thus focus on identifying extensive datasets for different species and scenarios for model development and evaluation, the conception of a unified approach for integrating current eco-evolutionary theory to represent individual mechanisms and the interactions between them, and the construction of multidimensional environmental scenarios for informing population predictions in the uncertain future.
Supplementary Material
Acknowledgements
The authors thank the editor and two anonymous reviewers for helpful suggestions which greatly improved the manuscript.
Data accessibility
This article has no additional data.
Authors' contributions
A.S.A.J. conceived the review idea and led the writing of the manuscript. R.J.B., J.W., A.P., L.C.E., E.L.G., and V.L.B. equally contributed ideas, discussions, and to the writing of the manuscript.
Competing interests
We declare no competing interests.
Funding
A.S.A.J. has been financially supported by an NERC fellowship (grant no. NE/N019504/1), V.L.B., R.J.B. & J.W.W. by NERC Scenario studentships (grant no. NE/L002566/1), L.C.E by a BBSRC CASE studentship (grant no. BB/N504129/1), A.P. by an NERC NPIF studentship (grant no. NE/R012229/1), and E.L.G. by a BBSRC grant (grant no. BB/R00580X/1).
References
- 1.Hooper DU, et al. 2012. A global synthesis reveals biodiversity loss as a major driver of ecosystem change. Nature 486, 105 ( 10.1038/nature11118) [DOI] [PubMed] [Google Scholar]
- 2.Dirzo R, Young HS, Galetti M, Ceballos G, Isaac NJ, Collen B. 2014. Defaunation in the Anthropocene. Science 345, 401–406. ( 10.1126/science.1251817) [DOI] [PubMed] [Google Scholar]
- 3.Stillman RA, Railsback SF, Giske J, Berger U, Grimm V. 2015. Making predictions in a changing world: the benefits of individual-based ecology. Bioscience 65, 140–150. ( 10.1093/biosci/biu192) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clark JS, et al. 2001. Ecological forecasts: an emerging imperative. Science 293, 657–660. ( 10.1126/science.293.5530.657) [DOI] [PubMed] [Google Scholar]
- 5.Evans MR. 2012. Modelling ecological systems in a changing world. Phil. Trans. R. Soc. B 367, 181–190. ( 10.1098/rstb.2011.0172) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heikkinen RK, Marmion M, Luoto M. 2012. Does the interpolation accuracy of species distribution models come at the expense of transferability? Ecography 35, 276–288. ( 10.1111/j.1600-0587.2011.06999.x) [DOI] [Google Scholar]
- 7.Moritz C, Agudo R. 2013. The future of species under climate change: resilience or decline? Science 341, 504–508. ( 10.1126/science.1237190) [DOI] [PubMed] [Google Scholar]
- 8.DeAngelis DL, Mooij WM. 2005. Individual-based modeling of ecological and evolutionary processes. Ann. Rev. Ecol. Evol. Syst. 36, 147–168. ( 10.1146/annurev.ecolsys.36.102003.152644) [DOI] [Google Scholar]
- 9.Crain CM, Kroeker K, Halpern BS. 2008. Interactive and cumulative effects of multiple human stressors in marine systems. Ecol. Lett. 11, 1304–1315. ( 10.1111/j.1461-0248.2008.01253.x) [DOI] [PubMed] [Google Scholar]
- 10.Root TL, Price JT, Hall KR, Schneider SH, Rosenzweig C, Pounds JA. 2003. Fingerprints of global warming on wild animals and plants. Nature 421, 57 ( 10.1038/nature01333) [DOI] [PubMed] [Google Scholar]
- 11.Sih A. 2013. Understanding variation in behavioural responses to human-induced rapid environmental change: a conceptual overview. Anim. Behav. 85, 1077–1088. ( 10.1016/j.anbehav.2013.02.017) [DOI] [Google Scholar]
- 12.Goulson D, Nicholls E, Botías C, Rotheray EL. 2015. Bee declines driven by combined stress from parasites, pesticides, and lack of flowers. Science 347, 1255957 ( 10.1126/science.1255957) [DOI] [PubMed] [Google Scholar]
- 13.Danner N, Molitor AM, Schiele S, Härtel S, Steffan-Dewenter I. 2016. Season and landscape composition affect pollen foraging distances and habitat use of honey bees. Ecol. Appl. 26, 1920–1929. ( 10.1890/15-1840.1) [DOI] [PubMed] [Google Scholar]
- 14.Fusco G, Minelli A. 2010. Phenotypic plasticity in development and evolution: facts and concepts. Phil. Trans. R. Soc. B 365, 547–556. ( 10.1098/rstb.2009.0267) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Valladares F, et al. 2014. The effects of phenotypic plasticity and local adaptation on forecasts of species range shifts under climate change. Ecol. Lett. 17, 1351–1364. ( 10.1111/ele.12348) [DOI] [PubMed] [Google Scholar]
- 16.Wong BBM, Candolin U. 2015. Behavioral responses to changing environments. Behav. Ecol. 26, 665–673. ( 10.1093/beheco/aru183) [DOI] [Google Scholar]
- 17.Helmuth B, Kingsolver JG, Carrington E. 2005. Biophysics, physiological ecology, and climate change: does mechanism matter? Annu. Rev. Physiol. 67, 177–201. ( 10.1146/annurev.physiol.67.040403.105027) [DOI] [PubMed] [Google Scholar]
- 18.Ozgul A, Childs DZ, Oli MK, Armitage KB, Blumstein DT, Olson LE, Tuljapurkar S, Coulson T. 2010. Coupled dynamics of body mass and population growth in response to environmental change. Nature 466, 482–485. ( 10.1038/nature09210) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gienapp P, Teplitsky C, Alho JS, Mills JA, Merilä J. 2008. Climate change and evolution: disentangling environmental and genetic responses. Mol. Ecol. 17, 167–178. ( 10.1111/j.1365-294X.2007.03413.x) [DOI] [PubMed] [Google Scholar]
- 20.Hoffmann AA, Sgrò CM. 2011. Climate change and evolutionary adaptation. Nature 470, 479–485. ( 10.1038/nature09670) [DOI] [PubMed] [Google Scholar]
- 21.Whitney KD, Gabler CA. 2008. Rapid evolution in introduced species, ‘invasive traits’ and recipient communities: challenges for predicting invasive potential. Divers. Distrib. 14, 569–580. ( 10.1111/j.1472-4642.2008.00473.x) [DOI] [Google Scholar]
- 22.Kingsolver JG, Massie KR, Ragland GJ, Smith MH. 2007. Rapid population divergence in thermal reaction norms for an invading species: breaking the temperature-size rule. J. Evol. Biol. 20, 892–900. ( 10.1111/j.1420-9101.2007.01318.x) [DOI] [PubMed] [Google Scholar]
- 23.Chevin L-M, Hoffmann AA. 2017. Evolution of phenotypic plasticity in extreme environments. Phil. Trans. R. Soc. B 372, 20160138 ( 10.1098/rstb.2016.0138) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reger J, Lind MI, Robinson MR, Beckerman AP. 2018. Predation drives local adaptation of phenotypic plasticity. Nat. Ecol. Evol. 2, 100–107. ( 10.1038/s41559-017-0373-6) [DOI] [PubMed] [Google Scholar]
- 25.Leslie PH. 1945. On the use of matrices in certain population mathematics. Biometrika 33, 183–212. ( 10.1093/biomet/33.3.183) [DOI] [PubMed] [Google Scholar]
- 26.Cole LC. 1954. The population consequences of life history phenomena. Q Rev. Biol. 29, 103–137. ( 10.1086/400074) [DOI] [PubMed] [Google Scholar]
- 27.MacArthur RH. 1958. Population ecology of some warblers of northeastern coniferous forests. Ecology 39, 599–619. ( 10.2307/1931600) [DOI] [Google Scholar]
- 28.Griffith AB, Salguero-Gómez R, Merow C, McMahon S. 2016. Demography beyond the population. J. Ecol. 104, 271–280. ( 10.1111/1365-2745.12547) [DOI] [Google Scholar]
- 29.Easterling MR, Ellner SP, Dixon PM. 2000. Size-specific sensitivity: applying a new structured population model. Ecology 81, 694–708. ( 10.1890/0012-9658(2000)081[0694:SSSAAN]2.0.CO;2) [DOI] [Google Scholar]
- 30.Schaub M, Abadi F. 2011. Integrated population models: a novel analysis framework for deeper insights into population dynamics. J. Ornithol. 152, 227–237. ( 10.1007/s10336-010-0632-7) [DOI] [Google Scholar]
- 31.Kendall BE, Fujiwara M, Diaz-Lopez J, Schneider S, Voigt J, Wiesner S. 2019. Persistent problems in the construction of matrix population models. Ecol. Modell. 406, 33–43. ( 10.1016/j.ecolmodel.2019.03.011) [DOI] [Google Scholar]
- 32.Gerber LR. 2006. Including behavioral data in demographic models improves estimates of population viability. Front. Ecol. Environ. 4, 419–427. ( 10.1890/1540-9295(2006)4[419:IBDIDM]2.0.CO;2) [DOI] [Google Scholar]
- 33.Smallegange IM, Caswell H, Toorians ME, Roos AM. 2017. Mechanistic description of population dynamics using dynamic energy budget theory incorporated into integral projection models. Methods Ecol. Evol. 8, 146–154. ( 10.1111/2041-210X.12675) [DOI] [Google Scholar]
- 34.Smallegange IM, Ens HM. 2018. Trait-based predictions and responses from laboratory mite populations to harvesting in stochastic environments. J. Anim. Ecol. 87, 893–905. ( 10.1111/1365-2656.12802) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Plard F, Turek D, Grüebler MU, Schaub M. 2019. IPM2: toward better understanding and forecasting of population dynamics. Ecol. Monogr. 89, e01364 ( 10.1002/ecm.1364) [DOI] [Google Scholar]
- 36.Crouse DT, Crowder LB, Caswell H. 1987. A stage-based population model for loggerhead sea turtles and implications for conservation. Ecology 68, 1412–1423. ( 10.2307/1939225) [DOI] [Google Scholar]
- 37.de Vries C, Caswell H. 2019. Stage-structured evolutionary demography: linking life histories, population genetics, and ecological dynamics. Am. Nat. 193, 545–559. ( 10.1086/701857) [DOI] [PubMed] [Google Scholar]
- 38.Schaub M, Gimenez O, Sierro A, Arlettaz R. 2007. Use of integrated modeling to enhance estimates of population dynamics obtained from limited data. Conserv. Biol. 21, 945–955. ( 10.1111/j.1523-1739.2007.00743.x) [DOI] [PubMed] [Google Scholar]
- 39.Coulson T, Kendall BE, Barthold J, Plard F, Schindler S, Ozgul A, Gaillard JM. 2017. Modeling adaptive and nonadaptive responses of populations to environmental change. Am. Nat. 190, 313–336. ( 10.1086/692542) [DOI] [PubMed] [Google Scholar]
- 40.Elith J, Leathwick JR. 2009. Species distribution models: ecological explanation and prediction across space and time. Ann. Rev. Ecol. Evol. Syst. 40, 677–697. ( 10.1146/annurev.ecolsys.110308.120159) [DOI] [Google Scholar]
- 41.Buckley LB. 2008. Linking traits to energetics and population dynamics to predict lizard ranges in changing environments. Am. Nat. 171, E1–E19. ( 10.1086/523949) [DOI] [PubMed] [Google Scholar]
- 42.Kearney M, Porter WP, Williams C, Ritchie S, Hoffmann AA. 2009. Integrating biophysical models and evolutionary theory to predict climatic impacts on species’ ranges: the dengue mosquito Aedes aegypti in Australia. Funct. Ecol. 23, 528–538. ( 10.1111/j.1365-2435.2008.01538.x) [DOI] [Google Scholar]
- 43.Fordham DA, Bertelsmeier C, Brook BW, Early R, Neto D, Brown SC, Ollier S, Araújo MB. 2018. How complex should models be? Comparing correlative and mechanistic range dynamics models. Glob. Change Biol. 24, 1357–1370. ( 10.1111/gcb.13935) [DOI] [PubMed] [Google Scholar]
- 44.Zurell D, et al. 2016. Benchmarking novel approaches for modelling species range dynamics. Glob. Change Biol. 22, 2651–2664. ( 10.1111/gcb.13251) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu C, Sibly RM, Grimm V, Thorbek P. 2013. Linking pesticide exposure and spatial dynamics: an individual-based model of wood mouse (Apodemus sylvaticus) populations in agricultural landscapes. Ecol. Modell. 248, 92–102. ( 10.1016/j.ecolmodel.2012.09.016) [DOI] [Google Scholar]
- 46.Becher MA, Grimm V, Thorbek P, Horn J, Kennedy PJ, Osborne JL. 2014. BEEHAVE: a systems model of honeybee colony dynamics and foraging to explore multifactorial causes of colony failure. J. Appl. Ecol. 51, 470–482. ( 10.1111/1365-2664.12222) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bocedi G, Palmer SCF, Pe'er G, Heikkinen RK, Matsinos YG, Watts K, Travis JM. 2017. RangeShifter: a platform for modelling spatial eco-evolutionary dynamics and species' responses to environmental changes. Methods Ecol. Evol. 5, 388–396. ( 10.1111/2041-210X.12162) [DOI] [Google Scholar]
- 48.Galic N, Grimm V, Forbes VE. 2017. Impaired ecosystem process despite little effects on populations: modeling combined effects of warming and toxicants. Glob. Change Biol. 23, 2973–2989. ( 10.1111/gcb.13581) [DOI] [PubMed] [Google Scholar]
- 49.Johnston ASA, Sibly RM, Thorbek P. 2018. Forecasting tillage and soil warming effects on earthworm populations. J. Appl. Ecol. 55, 1498–1509. ( 10.1111/1365-2664.13096) [DOI] [Google Scholar]
- 50.Boyd R, Roy S, Sibly RM, Thorpe R, Hyder K. 2018. A general approach to incorporating spatial and temporal variation in individual-based models of fish populations with application to Atlantic mackerel. Ecol. Modell. 382, 9–17. ( 10.1016/j.ecolmodel.2018.04.015) [DOI] [Google Scholar]
- 51.Evans TG, Diamond SE, Kelly MW. 2015. Mechanistic species distribution modelling as a link between physiology and conservation. Conserv. Physiol. 3, cov056 ( 10.1093/conphys/cov056) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Barbet-Massin M, Thuiller W, Jiguet F. 2011. The fate of European breeding birds under climate change, land-use and dispersal scenarios. Glob. Change Biol. 18, 881–890. ( 10.1111/j.1365-2486.2011.02552.x) [DOI] [Google Scholar]
- 53.Visconti P, et al. 2016. Projecting global biodiversity indicators under future development scenarios. Conserv. Lett. 9, 5–13. ( 10.1111/conl.12159) [DOI] [Google Scholar]
- 54.Kremen C, et al. 2008. Aligning conservation priorities across taxa in Madagascar with high-resolution planning tools. Science 320, 222–226. ( 10.1126/science.1155193) [DOI] [PubMed] [Google Scholar]
- 55.Gallien L, Douzet R, Pratte S, Zimmermann NE, Thuiller W. 2012. Invasive species distribution models–how violating the equilibrium assumption can create new insights. Global Ecol. Biogeogr. 21, 1126–1136. ( 10.1111/j.1466-8238.2012.00768.x) [DOI] [Google Scholar]
- 56.Miller JA, Holloway P. 2015. Incorporating movement in species distribution models. Progr. Phys. Geogr. 39, 837–849. ( 10.1177/0309133315580890) [DOI] [Google Scholar]
- 57.Thuiller W. 2004. Patterns and uncertainties of species' range shifts under climate change. Glob. Change Biol. 10, 2020–2027. ( 10.1111/j.1365-2486.2004.00859.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ehrlén J, Morris WF. 2015. Predicting changes in the distribution and abundance of species under environmental change. Ecol. Lett. 18, 303–314. ( 10.1111/ele.12410) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lozier JD, Aniello P, Hickerson MJ. 2009. Predicting the distribution of Sasquatch in western North America: anything goes with ecological niche modelling. J. Biogeogr. 36, 1623–1627. ( 10.1111/j.1365-2699.2009.02152.x) [DOI] [Google Scholar]
- 60.Teal LR, van Hal R, van Kooten T, Ruardij P, Rijnsdorp AD. 2012. Bio-energetics underpins the spatial response of North Sea plaice (Pleuronectes platessa L.) and sole (Solea solea L.) to climate change. Glob. Change Biol. 18, 3291–3305. ( 10.1111/j.1365-2486.2012.02795.x) [DOI] [Google Scholar]
- 61.Rougier T, Lassalle G, Drouineau H, Dumoulin N, Faure T, Deffuant G, Rochard E, Lambert P. 2015. The combined use of correlative and mechanistic species distribution models benefits low conservation status species. PloS ONE 10, e0139194 ( 10.1371/journal.pone.0139194) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kearney M, Phillips BL, Tracy CR, Christian KA, Betts G, Porter WP. 2008. Modelling species distributions without using species distributions: the cane toad in Australia under current and future climates. Ecography 31, 423–434. ( 10.1111/j.0906-7590.2008.05457.x) [DOI] [Google Scholar]
- 63.Kearney MR, Wintle BA, Porter WP. 2010. Correlative and mechanistic models of species distribution provide congruent forecasts under climate change. Conserv. Lett. 3, 203–213. ( 10.1111/j.1755-263X.2010.00097.x) [DOI] [Google Scholar]
- 64.Pagel J, Schurr FM. 2012. Forecasting species ranges by statistical estimation of ecological niches and spatial population dynamics. Global Ecol. Biogeogr. 21, 293–304. ( 10.1111/j.1466-8238.2011.00663.x) [DOI] [Google Scholar]
- 65.McLane AJ, Semeniuk C, McDermid GJ, Marceau DJ. 2011. The role of agent-based models in wildlife ecology and management. Ecol. Modell. 222, 1544–1556. ( 10.1016/j.ecolmodel.2011.01.020) [DOI] [Google Scholar]
- 66.van der Vaart E, Johnston ASA, Sibly RM. 2016. Predicting how many animals will be where: how to build, calibrate and evaluate individual-based models. Ecol. Modell. 326, 113–123. ( 10.1016/j.ecolmodel.2015.08.012) [DOI] [Google Scholar]
- 67.Grimm V, Railsback SF. 2005. Individual-based modeling and ecology. Princeton, NJ: Princeton University Press. [Google Scholar]
- 68.Railsback SF, Harvey BC. 2002. Analysis of habitat-selection rules using an individual-based model. Ecology 83, 1817–1830. ( 10.1890/0012-9658(2002)083[1817:aohsru]2.0.co;2) [DOI] [Google Scholar]
- 69.Kooijman SALM. 2010. Dynamic energy budget theory for metabolic organisation. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 70.Sibly RM, et al. 2013. Representing the acquisition and use of energy by individuals in agent-based models of animal populations. Methods Ecol. Evol. 4, 151–161. ( 10.1111/2041-210x.12002) [DOI] [Google Scholar]
- 71.Chipps SR, Wahl DH. 2008. Bioenergetics modeling in the 21st century: reviewing new insights and revisiting old constraints. Trans. Am. Fish. Soc. 137, 298–313. ( 10.1577/T05-236.1) [DOI] [Google Scholar]
- 72.Raubenheimer D, Simpson SJ, Mayntz D. 2009. Nutrition, ecology and nutritional ecology: toward an integrated framework. Funct. Ecol. 23, 4–16. ( 10.1111/j.1365-2435.2009.01522.x) [DOI] [Google Scholar]
- 73.Sterner RW, Elser JJ. 2002. Ecological stoichiometry: the biology of elements from molecules to the biosphere. Princeton, NJ: Princeton University Press. [Google Scholar]
- 74.Sperfeld E, Wagner ND, Halvorson HM, Malishev M, Raubenheimer D. 2017. Bridging ecological stoichiometry and nutritional geometry with homeostasis concepts and integrative models of organism nutrition. Funct. Ecol. 31, 286–296. ( 10.1111/1365-2435.12707) [DOI] [Google Scholar]
- 75.Allen AP, Gillooly JF. 2009. Towards an integration of ecological stoichiometry and the metabolic theory of ecology to better understand nutrient cycling. Ecol. Lett. 12, 369–384. ( 10.1111/j.1461-0248.2009.01302.x) [DOI] [PubMed] [Google Scholar]
- 76.Giraldeau L-A, Caraco T. 2000. Social foraging theory. Princeton, NJ: Princeton University Press. [Google Scholar]
- 77.Fretwell SD, Lucas HL. 1970. On territorial behavior and other factors influencing habitat distribution in birds. Acta Biotheor. 19, 16–36. ( 10.1007/BF01601953) [DOI] [Google Scholar]
- 78.Robertson BA, Rehage JS, Sih A. 2013. Ecological novelty and the emergence of evolutionary traps. Trends Ecol. Evol. 28, 552–560. ( 10.1016/j.tree.2013.04.004) [DOI] [PubMed] [Google Scholar]
- 79.Patterson TA, Thomas L, Wilcox C, Ovaskainen O, Matthiopoulos J. 2008. State–space models of individual animal movement. Trends Ecol. Evol. 23, 87–94. ( 10.1016/j.tree.2007.10.009) [DOI] [PubMed] [Google Scholar]
- 80.Morales JM, Ellner SP. 2002. Scaling up animal movements in heterogeneous landscapes: the importance of behavior. Ecology 83, 2240–2247. ( 10.1890/0012-9658(2002)083[2240:SUAMIH]2.0.CO;2) [DOI] [Google Scholar]
- 81.Simpson SJ, Raubenheimer D, Charleston MA, Clissold FJ. 2010. Modelling nutritional interactions: from individuals to communities. Trends Ecol. Evol. 25, 53–60. ( 10.1016/j.tree.2009.06.012) [DOI] [PubMed] [Google Scholar]
- 82.Sumpter DJT, Pratt SC. 2009. Quorum responses and consensus decision making. Phil. Trans. R. Soc. B 364, 743–753. ( 10.1098/rstb.2008.0204) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Teitelbaum CS, Converse SJ, Fagan WF, Böhning-Gaese K, O'Hara RB, Lacy AE, Mueller T. 2016. Experience drives innovation of new migration patterns of whooping cranes in response to global change. Nat. Commun. 7, 12793 ( 10.1038/ncomms12793) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.McComb K, Moss C, Durant SM, Baker L, Sayialel S. 2001. Matriarchs as repositories of social knowledge in African elephants. Science 292, 491–494. ( 10.1126/science.1057895) [DOI] [PubMed] [Google Scholar]
- 85.Brakes P, et al. 2019. Animal cultures matter for conservation. Science 363, 1032–1034. ( 10.1126/science.aaw3557) [DOI] [PubMed] [Google Scholar]
- 86.Nadeau CP, Urban MC. 2019. Eco-evolution on the edge during climate change. Ecography 42, 1280–1297. [Google Scholar]
- 87.Sinervo B, et al. 2010. Erosion of lizard diversity by climate change and altered thermal niches. Science 2010, 894–899. ( 10.1126/science.1184695) [DOI] [PubMed] [Google Scholar]
- 88.Lynch M, Walsh B. 1998. Genetics and analysis of quantitative traits, vol. 1 Sunderland, MA: Sinauer. [Google Scholar]
- 89.Brunner FS, Deere JA, Egas M, Eizaguirre C, Raeymaekers JA. 2019. The diversity of eco-evolutionary dynamics: comparing the feedbacks between ecology and evolution across scales. Funct. Ecol. 33, 7–12. ( 10.1111/1365-2435.13268) [DOI] [Google Scholar]
- 90.Smallegange IM, Coulson T. 2013. Towards a general, population-level understanding of eco-evolutionary change. Trends Ecol. Evol. 28, 143–148. ( 10.1016/j.tree.2012.07.021) [DOI] [PubMed] [Google Scholar]
- 91.Nussey DH, Wilson AJ, Brommer JE. 2007. The evolutionary ecology of individual phenotypic plasticity in wild populations. J. Evol. Biol. 20, 831–844. ( 10.1111/j.1420-9101.2007.01300.x) [DOI] [PubMed] [Google Scholar]
- 92.Vindenes Y, Langangen Ø. 2015. Individual heterogeneity in life histories and eco-evolutionary dynamics. Ecol. Lett. 18, 417–432. ( 10.1111/ele.12421) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Henry RC, Bocedi G, Travis JMJ. 2013. Eco-evolutionary dynamics of range shifts: elastic margins and critical thresholds. J. Theor. Biol. 321, 1–7. ( 10.1016/j.jtbi.2012.12.004) [DOI] [PubMed] [Google Scholar]
- 94.Clutton-Brock T, Sheldon BC. 2010. Individuals and populations: the role of long-term, individual-based studies of animals in ecology and evolutionary biology. Trends Ecol. Evol. 25, 562–573. ( 10.1016/j.tree.2010.08.002) [DOI] [PubMed] [Google Scholar]
- 95.Coulson T, Catchpole EA, Albon SD, Morgan BJ, Pemberton JM, Clutton-Brock TH, Crawley MJ, Grenfell BT. 2001. Age, sex, density, winter weather, and population crashes in Soay sheep. Science 292, 1528–1531. ( 10.1126/science.292.5521.1528) [DOI] [PubMed] [Google Scholar]
- 96.Nussey DH, Kruuk LEB, Donald A, Fowlie M, Clutton-Brock TH. 2005. The rate of senescence in maternal performance increases with early-life fecundity in red deer. Ecol. Lett. 9, 1342–1350. ( 10.1111/j.1461-0248.2006.00989.x) [DOI] [PubMed] [Google Scholar]
- 97.Bouwhuis S, Charmantier A, Verhulst S, Sheldon BC. 2010. Individual variation in rates of senescence: natal origin effects and disposable soma in a wild bird population. J. Anim. Ecol. 79, 1251–1261. ( 10.1111/j.1365-2656.2010.01730.x) [DOI] [PubMed] [Google Scholar]
- 98.Wilson AJ, Nussey DH, Pemberton JM, Pilkington JG, Morris A, Pelletier F, Clutton-Brock TH, Kruuk LE. 2007. Evidence for a genetic basis of aging in two wild vertebrate populations. Curr. Biol. 17, 2136–2142. ( 10.1016/j.cub.2007.11.043) [DOI] [PubMed] [Google Scholar]
- 99.Matthysen E. 2005. Density-dependent dispersal in birds and mammals. Ecography 28, 403–416. ( 10.1111/j.0906-7590.2005.04073.x) [DOI] [Google Scholar]
- 100.Dickinson JL, Zuckerberg B, Bonter DN. 2010. Citizen science as an ecological research tool: challenges and benefits. Ann. Rev. Ecol. Evol. Syst. 41, 149–172. ( 10.1146/annurev-ecolsys-102209-144636) [DOI] [Google Scholar]
- 101.Wikelski M. 2019. ICARUS: global monitoring with animals. https://www.icarus.mpg.de/en (accessed 5 August 2019).
- 102.Add-my-Pet. 2019. Online database of DEB parameters, implied properties and referenced underlying data. bio.vu.nl/thb/deb/deblab/add_my_pet/ (accessed 24 September 2019).
- 103.Wikelski M, Kays R. 2019. Movebank: archive, analysis and sharing of animal movement data. Hosted by the Max Planck Institute for Animal Behavior. www.movebank.org (accessed 5 August 2019).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
A key limitation in population modelling is the availability of data to parametrize, calibrate, and validate models. Historically, SDMs have relied only on presence–absence data, demographic models were built with snapshots of abundance over time, and IBMs have focused on a single well-studied system to fulfil high data needs. A standardized mechanistic approach, however, necessitates data at the individual level for parametrization and the population level for validation. For most species, data are often limiting at one level. For instance, short-lived species are often well studied at the individual level in laboratory conditions and less so at the population and field level (e.g. invertebrates and fish), whereas population data may be available for wild animals but individual-level data are scant (e.g. large mammals). Another limitation is that most empirical studies are conducted over short timescales, while the processes influencing population responses to environmental changes operate over longer timescales.
Individual-based and long-term field studies represent an important resource for the development and evaluation of a standardized mechanistic approach in population modelling [94]. In particular, datasets for diverse species and scenarios will be crucial in testing whether such an approach can identify how different mechanisms influence a population response to different environmental changes. Individual-based studies, for instance, have played a key role in identifying the role of individual variation, age-related fitness, and social structures on population dynamics [95–99]. Still, mechanistic submodels often require more detailed information at the individual level than is recorded in the field. Energy budget models, for example, often require prior knowledge about ingestion, assimilation, growth, and reproduction rates in optimal environmental conditions. An advantage of developing a standardized mechanistic approach in population modelling, however, is in providing a consensus on how to address data gaps using robust statistical techniques and calibration tools (e.g. [66]).
Other promising advances being made in the collection and sharing of data include remote sensing and citizen science projects [100]. For example, satellite tracking technology such as that used in the recently launched International Cooperation for Animal Research Using Space (ICARUS) project [101] can provide valuable data for parametrizing the movements and dispersal ability of individuals. A growing data sharing culture and the growth of freely available online databases such as Add-my-pet [102] and Movebank [103] present another promising source of data for population models. A standardized mechanistic approach, developed and tested for diverse species and scenarios simultaneously, would provide additional consensus on data requirements and availability from diverse sources. Such an approach would also identify key knowledge gaps in physiological, behavioural, and evolutionary ecology which could be addressed through coupled modelling-empirical studies.
This article has no additional data.