Abstract
Sensing from a moving platform is challenging for both man-made machines and animals. Animals' heads jitter during movement, so if the sensors they carry are not stabilized, any spatial estimation might be biased. Flying animals, like bats, seriously suffer from this problem because flapping flight induces rapid changes in acceleration which moves the body up and down. For echolocating bats, the problem is crucial. Because they emit a sound to sense the world, an unstable head means sound energy pointed in the wrong direction. It is unknown how bats mitigate this problem. By tracking the head and body of flying fruit bats, we show that they stabilize their heads, accurately maintaining a fixed acoustic-gaze relative to a target. Bats can solve the stabilization task even in complete darkness using only echo-based information. Moreover, the bats point their echolocation beam below the target and not towards it, a strategy that should result in better estimations of target elevation.
Keywords: bats, echolocation, gaze stabilization, active sensing, sensory perception, tracking
1. Introduction
Sensing on-board a moving platform such as a drone or the vertebrate's head poses serious challenges. Undesired movements of the head might induce uncertainties in the position and heading of the sensors it carries [1,2]. Such uncertainties would be translated into errors in any sensory-based spatial estimation, such as the direction of a sound source or an echo-reflecting object in the egocentric coordinate frame (i.e. the position relative to self). Because the bat must respond correctly to incoming sensory information, for example, by turning towards a prey item or away from a predator, these uncertainties must be dealt with. Flying animals, such as bats, which rely on wing flapping in order to propel themselves, are extremely prone to suffer from this problem, because their movement is characterized by constantly switching between instances of low acceleration (during the up stroke) and instances of high acceleration (during the down stroke [3]). Acceleration changes can be specifically large in bats, because of their large wing-mass to body-mass ratio [3,4].
So far, all examples of stabilizing sensory acquisition come from animals that rely on external visual input in addition to internal feedback provided by the vestibular system. Humans are known to stabilize their gaze while walking, by compensating for the body-induced jitter using head pitch movements [1,2]. This response, known as the vestibulo-collic reflex, has also been observed in other primates [5,6], where it has been hypothesized to be activated only when there is a need to lock the gaze, for example, on a target. In birds, several studies suggested a gaze stabilization mechanism [7–14]. For example, the head bobbing behaviour of pigeons has been extensively studied, and it probably functions to stabilize visual gaze [15]. Furthermore, it has been shown that pigeons were unable to fly towards a perch and crashed on the ground when wearing restrictive collars which prevented them from stabilizing their heads during flight [16].
Echolocating bats mostly rely on sound to perceive their proximal environment. They emit sound signals and use the returning echoes to sense their environment [17]. Bats' sound emission is directional, meaning that the intensity of the sound beam emitted from their mouth (or nostrils) is not equal in all directions but is loudest in front and attenuates to the sides [18,19]. Bats steer their beam in space in order to control and improve information acquisition [20]. They would therefore suffer twice from an unstable sensory gaze: once during echo reception—while their ears are pointing in a wrong direction—and another time during sound emission, where they risk pointing their beam in a suboptimal direction. It has been shown that bats direct their bio-sonar beam towards their prey [20–23], but the mechanism allowing them to keep their beam direction steady is currently unclear. It has been suggested that bats might compensate for flight-induced jitter using head movements, but this has never been shown [20–22]. It is also unclear whether acoustic-echoic information can be used as external input for head stabilization.
We tested how Egyptian fruit bats (Rousettus aegyptiacus) deal with wing flapping induced (vertical and horizontal) gaze jitter. These fruit bats rely on (lingual) echolocation to localize objects such as cave walls or tree branches when avoiding them or when landing on them in the dark, but even in relatively high light levels [24,25]. Unstable head movements induced by wing flapping could thus impede the localization of a branch or a wall, hindering landing. We examined how fruit bats overcome this sensory challenge in flight, and how this affects their vertical beam steering.
2. Results
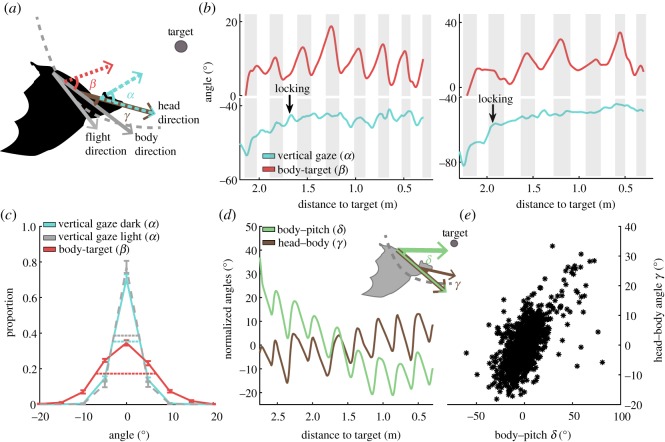
We trained a total of eight Egyptian fruit bats to take off from a fixed point and land on a 20 cm diameter sphere 2.5 m away from the release point in darkness (less than 10−3 lux). We used a high-resolution tracking system (see the electronic supplementary material, figure S1) to track the pitch- and yaw-angles of the bats’ head and body and the head's roll (an average of 46 trials were collected for each bat, but not all bats participated in all experiments, see Material and methods; electronic supplementary material, table S1). We will first focus on flight-pitch (the vertical angle between the flight direction and the horizontal plane (ω), electronic supplementary material, figure S2). This is the angle mostly affected by the wingbeat, and we examined how changes in this angle affect the bats' vertical gaze (α, the angle between the vertical head direction vector and vertical target direction vector; see figure 1a and the electronic supplementary material, table S2 for angle definition). In other words, the vertical gaze reflects the direction of the bat's mouth relative to the target in the vertical plane. In most mouth-emitting bats, the direction of the echolocation beam is the same as the direction of the head. Although Rousettus steer their beams horizontally, using the tongue, they do not do so vertically and thus the vertical gaze (α) that we measured is a good proxy for their beam direction (as we also confirmed below). We defined the vertical gaze relative to the target (and not the horizon), as a main question we focused on is whether bats can maintain the gaze fixed relative to a specific target—a task that would require the use of external sensory input regarding the position of the target. As has been shown in other bat species [3], flapping flight induced substantial jitter in pitch during the approach to landing. We quantified this jitter by measuring the body-to-target angle (β) the angle between the vertical body direction vector and vertical target direction vector (figure 1b, top trace, note the correlation between the body-to-target angle (β)—red line, and the wingbeat—grey shadings). Indeed, the body jittered substantially during flight, which could impede sensory estimations, such as estimating the elevation of the landing target, if the head jittered to the same degree.
Figure 1.
Bats maintain a fixed vertical gaze (α) despite the jitter in body pitch. (a) Schematic showing the angles, which we refer to in this figure including α—the vertical gaze relative to target and β— the body-to-target vertical angle. (b) Vertical gaze (α), wing-movement and body-to-target angle (β) in two exemplary flights. Top trace—body-to-target angle (β red) demonstrates the body jitter during flight. The grey shadings represent half wingbeat cycle (estimated from the wings' elevation, see the electronic supplementary material, figure S11). Bottom—the vertical gaze (α light blue). The y-axis is divided into two parts (with a gap) to increase visibility, but the y-scale of both parts is identical. The arrows show the locking point. (c) The distribution of the vertical gaze (α) in darkness (light blue line) and in light (grey dashed line), and the body-to-target angles (β, red line). The mean and s.e. are presented for all bats (n = 8 for the head and body in dark, n = 3 for light). All distributions were centred around ‘0’ to allow comparison. The horizontal lines represent the width at half height for the different distributions. (d) The angle between the head and the body (γ, brown line, see schematic) was correlated to body pitch (δ, green line; see schematic). A single trial is presented. (e) Head–body angle (γ) versus body pitch (δ)—same as in (d) but for all bats and all trials (n = 6, two bats were excluded owing to insufficient tracking of γ).
Despite this jitter in the body-to-target angle (β), the bats managed to maintain an almost fixed vertical gaze (α) relative to the target (figure 1b, bottom trace, note the straight line, see also the electronic supplementary material, movies S1 and S2). The width of the distribution of the vertical gaze angles (α) was less than twice the width of the body-to-target angle (β) distribution (6° versus 14° on average; figure 1c, the difference in width was significant, p < 10−4, n = 8 bats, paired, two-tailed, t-test, on the group). In order to show that all bats responded similarly, we also ran all statistical tests on the individual level, comparing the standard deviation of the vertical gaze (α) and the body-target (β) angles across trials (within each bat and at the group level). We found that the body-to-target (β) angle standard deviation was significantly larger in all eight bats (p < 10−4, for the group, n = 8, and p < 0.05 for each bat with a false discovery rate (FDR) correction for multiple comparisons; paired, two-tailed, t-test for each bat and for the group). To achieve a fixed heading, the bats moved their head in phase relative to the movement of the body, namely, when the body rotated downwards relative to the horizon (negative pitch (δ), see the electronic supplementary material, figure S2 and table S2), the bat moved its head upwards relative to its body (figure 1d,e; the correlation between pitch (δ) and the head-to-body angle (γ) was significant for all bats, p < 0.0003 for each bat, R = 0.6 ± 0.04, mean ± s.e., six bats were tested, Spearman's rank correlation coefficient, with an FDR correction).
To examine whether there is a difference in head stabilization when visual information is available in addition to acoustic-echoic information, we tested three bats in dim light (approx. 1 lux), which is considered ideal for vision in these bats [24]. As in the dark condition, the bats maintained their vertical gaze (α) fixed towards the target (figure 1c, grey dashed line), and their performance under light and darkness did not differ significantly (p > 0.15, two-sample Kolmogorov–Smirnov test for each of the three bats). The bats flew slightly but significantly faster in the light condition (1.95 ± 0.15 versus 1.70 ± 0.22 m s−1, mean ± s.d. for the three bats p < 0.05 for each of the three bats, two-sample, two-tailed, t-test, with an FDR correction).
Notably, as the bats were fixating their vertical gaze relative to a target positioned at a constantly changing distance (owing to the bat's flight), the bat could not simply control its head movements based on internal vestibular information. To validate this, we shuffled the tracking data from the first and second halves of the flight (electronic supplementary material, figure S3). If the bat was simply moving its head, independently of external feedback, the vertical gaze should have remained locked under this manipulation, but this was not the case, demonstrating that the bats were adjusting head movements based on external feedback. We tested this by shuffling the data and found that the width of the distribution of the non-shuffled vertical gaze angles (α) was significantly narrower than the width of the shuffled angles (12° versus 21°, on average; electronic supplementary material, figure S3, p < 0.05, n = 8 bats, paired, two-tailed, t-test, on the group level). Moreover, an examination of the head's movement in the z-axis revealed complex movements (rather than simple repetitive oscillations; electronic supplementary material, figure S4).
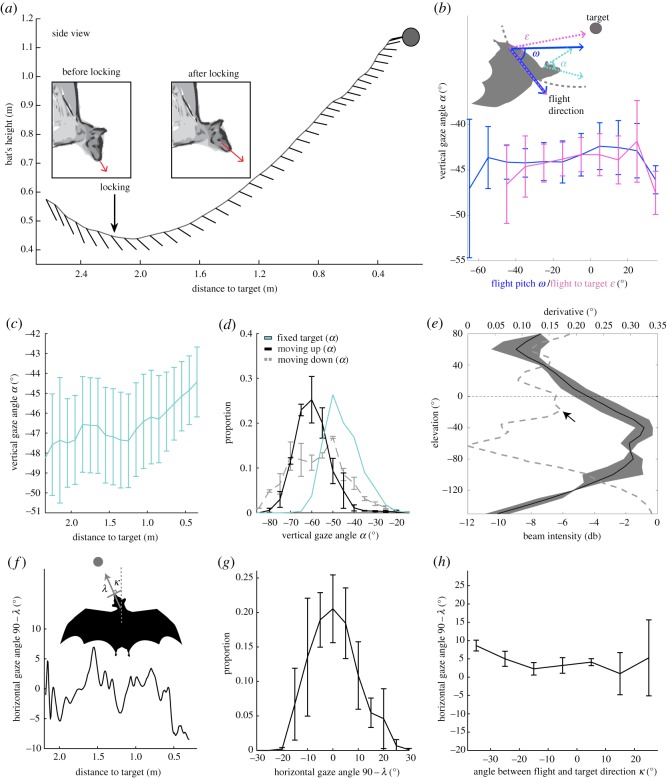
After observing that the bats maintain a fixed vertical gaze (α) relative to the target, we examined their precise preferred vertical heading (figure 2a–c). The bats started off pointing their gaze approximately 55 ± 11° below the target (mean ± s.d.). Around approximately 500 ms after take-off (see arrows in figures 1b and 2a), the bats adjusted their vertical gaze (α) by 10° on average and pointed it approximately 45 ± 6° below the target. Each individual had a slightly different preferred angle, between −51° and −38° (electronic supplementary material, figure S5). We hypothesize that the change, approximately 500 ms after take-off, represents the moment in which the bats ‘locked’ their vertical gaze (α) relative to target after localizing it. After locking, the bats kept their vertical gaze (α) fixed until landing, maintaining a much more stable gaze relative to before (see the electronic supplementary material, table S4 for the definition of locking). The change in the vertical gaze (α) after locking was significant both in terms of the mean angle and its standard deviation, i.e. the bats significantly elevated their gaze (from −55° to −45°) and they kept it more stable after locking (p < 10−4 for the change in the mean angle, paired, two-tailed, t-test for the group, n = 8; and p < 0.05 for the change in standard deviation, paired, two-tailed, t-test for the same group). Importantly, the bats were able to stabilize their vertical gaze (α) independently of flight-pitch (ω). Namely, once they locked onto the target, they maintained their vertical gaze (α) approximately 45° below the target, whether they were ascending or descending (figure 2b; note that while the flight-pitch (ω) varied by 100°, i.e. between −65 and +35°, the vertical gaze (α) only changed by 5°, see also the electronic supplementary material, figure S6).
Figure 2.
Bats locked their vertical gaze (α) below the target and maintain a fixed horizontal gaze directing their head straight towards the target. (a) Schematic shows the movement of the bat and the direction of its gaze (α) at a constant interval along one flight trajectory. Notice the change ca 200 ms after take-off (arrow depicts the locking point). (b) The bats were able to maintain a stable vertical gaze (α) after locking relative to the target (y-axis) independently of their flight-pitch (ω, blue line) and flight relative to the target (ε, pink line; we tested two flight angles, see schematic, the mean and s.e. are presented n = 6, two bats were excluded owing to insufficient flight angle data). (c) Vertical gaze (α) after locking as a function of the bats' distance from landing. The mean and s.e. are presented for all bats (n = 8). (d) Distribution of vertical gaze (α) angles for a target moving upwards or downwards in comparison to the fixed target angle. The mean and s.e. are presented for the two bats that performed the task. The fixed angles' distribution is the mean of all bats. (e) Bats directed the derivative of the beam and not the peak to the target. The mean vertical beam and s.e. for four bats is presented (black). The beams were aligned such that their peak is at −40°. The dashed grey line shows the derivative of the beam. The dashed horizontal line depicts the direction of the target 40° above the peak which falls near the maximum derivative depicted by an arrow. The derivative increases towards the sides of the beam, but pointing that part of the beam would mean very little reflected energy. Note that the derivative of degrees is still degrees because we measure the difference. (f) Top trace: schematic showing the angles which we refer to in this figure (f–h). λ is the angle between the vector connecting the front head marker, the right eye and the horizontal gaze relative to the target depicted by the arrow-head. When 90 − λ is 0, this means pointing straight to the target. κ is the angle between the direction of flight and the direction of the target. Bottom: the bats maintained a fixed horizontal gaze relative to the target, mean (90 − λ) was approximately 0°. A single trial is presented. (g) The distribution of horizontal gaze (90 − λ). The mean of all bats’ distributions (and their s.e.) is presented (n = 4; see also the electronic supplementary material, figure S9 for the angle distribution on the individual level). (h) The bats were able to maintain this fixed horizontal gaze relative to the target (y-axis) independently of their flight azimuth relative to the target (x-axis). (The mean and s.e. are presented, n = 3, one bat was excluded from this analysis owing to insufficient tracking of κ.) (Online version in colour.)
To further examine the bats' ability to maintain a fixed vertical gaze (α), we challenged two bats with a moving target. The target was either elevated by 0.5 m or lowered by 0.5 m, as the bat approached it, mimicking a branch swinging in the wind. The target started moving between approximately 0.4 and 0.5 s after the bat took off, and it stopped moving between approx. 0.5–0.1 s before the bat landed, so the duration of the movement was approx. 0.8 s on average (Material and methods). When the target was moving upwards, the bats’ vertical gaze lagged behind the target, and thus, they managed to maintain a stable vertical gaze (α), but the mean gaze angle was approx. 60° below the target instead of 45° as before (figure 2d, grey line; electronic supplementary material, figure S7). The bats had more difficulties when the target was moving downwards, as can be learnt from the wider distribution of their vertical gaze (α) angles (the width at half height was 29° when moving down versus 8° when the target was fixed), the vertical gaze (α) standard deviation was significantly higher in the downwards moving target compared to the fixed target (p < 0.0001 for each of the two bats; two-sample, two-tailed, t-test, with an FDR correction; figure 2d, black lines). The vertical gaze (α) standard deviation was also significantly higher in the downwards condition compared to the upwards condition (p < 0.05 for each of the two bats; two-sample, two-tailed, t-test with an FDR correction).
Our tracking results showed that the bats directed their vertical gaze (α) and therefore also their bio-sonar beams below the target and not straight at the target. Pointing the beam below the target should reduce the signal-to-noise ratio (SNR) in which they operated, because less energy is reflected back from the target. This in turn should have made it more difficult for them to estimate the position of the target (as sensory estimations usually deteriorate with SNR). A possible explanation for this counterintuitive strategy is that the bats were increasing information about changes in target elevation, by pointing towards the target a part of the beam where echo intensity is expected to vary more as a function of target elevation (i.e. a part of the beam where its vertical derivative is larger). To test this, we flew four bats in the same experimental set-up, but this time, we placed a vertical microphone array behind the target, and we reconstructed their vertical beam (Material and methods; electronic supplementary material, figure S8). Our acoustic estimation of the vertical beam confirmed the visual assessment—we found that the bats pointed the peak of their beam 40 ± 4° below the target (mean ± s.d. and see the electronic supplementary material, table S3 for the estimates for individual bats). We moreover found that indeed, when directing their gaze below the target, the bats were directing to the target a part of the beam where the (energy-to-elevation) derivative was close to the maximum possible (compare black and grey lines in figure 2e).
The discussion so far only considered the vertical gaze (α), but wing flapping can also induce horizontal jitter, which could lead to errors in target-azimuth estimations. We tracked the horizontal gaze (i.e. head azimuth, λ, the angle between the horizontal head direction vector and the target's horizontal direction vector, figure 2f) of four bats, and found that they maintained a fixed horizontal gaze (λ) relative to the target as well (figure 2f,g, and see also the electronic supplementary material, figure S9 for the angle distribution on the individual level). Unlike in the vertical case, the bats pointed their horizontal gaze (λ) straight towards the target (without any offset) even when they were not flying straight towards the target (figure 2h, note that while the flight horizontal angle-to-target (κ) varied by up to 60° (between −35 and +25°) the horizontal gaze (λ) only varied by 8°). Moreover, the bats kept an almost fixed zero head-roll (3 ± 2.1°, mean ± s.d.) as was also reported by a previous study [26,27].
3. Discussion
Flapping flight is detrimental for sensing owing to the noise that is induced by the constantly changing acceleration of the body. Indeed, bats' flapping introduced a substantial jitter in their body-to-target angle (β), which oscillated up and down in synchrony with the wingbeat (figure 1b). Despite this jitter, the bats managed to maintain a fixed vertical gaze (α) relative to a landing target with an error of only 6° (i.e. the mean width of the vertical gaze distribution). They achieved this by moving their head in in phase with the oscillations of the body. After locking on the target, the bats maintained a fixed vertical gaze (α) independently of their flight-pitch (ω). We demonstrate that head stabilization could not be achieved by an automatic gaze control mechanism (e.g. simply oscillating the head at the wingbeat rate), and that it probably requires both internal and external sensory feedback. We also show that bats stabilize their head in light and dark with similar accuracy.
Several previous studies found that animals stabilize their gaze during different tasks such as walking [2,6,9,15], running [28], flying [9–11,13], landing [14] and hovering [8,12], but more relevant to our study, relative to a target, where it has been shown that the fixation occurred after identifying a target. Monkeys, for example, performed pitch and yaw head rotations while scanning the environment, but kept a steady gaze when they focused on a target [28]. In landing experiments, it has been shown that, similar to our bats, lovebirds kept a steady gaze in all axes (pitch, yaw and roll) relative to a target, but more often in the second half of the flight, where there was a need for high precision during landing [14]. Zebra finches used head saccades in order to adjust their horizontal gaze while avoiding an obstacle and going through a window [13]. All of the examples above are based on a visual information, but to the best of our knowledge, this is the first study showing that animals can do so based on non-visual (in this case, acoustic) external sensory information. For an animal that relies on active emission of a sound beam for sensing, this might be crucial.
We hypothesize that the vestibular system together with the neck proprioceptive sensors can provide internal inputs for controlling vertical gaze (α), as has been shown in the case of gaze stabilization in humans [1,2,29]. It would be interesting to investigate the control algorithms executed by the brain to stabilize the head [30] and to examine the performance in a less predictable situation, for example, when the bat must deal with an abrupt perturbation as a result of a sudden wind blow [31].
After locking on the target, the bats directed their gaze (α) 45° below the target on average, maintaining a very fixed vertical gaze (α) independent of the target's elevation. This seems like a suboptimal sensory strategy because most sound energy does not impinge on the target. For comparison, a human holding a flashlight would point it straight towards the target. Instead, the bats pointed a part of the beam that is near the maximum vertical derivative of their beam to the target. This strategy should increase the sensitivity to changes in the target's elevation because when the elevation of the reflecting object changed relative to the bat (for example, owing to changing flight-pitch), this would result in a large change in the reflected energy over consecutive beams. Alternatively, if the bat pointed the peak of its beam towards the target, a change in elevation would not alter the reflected energy at all. In the past, we have shown that Rousettus bats use the horizontal maximum derivative of the beam in order to achieve maximum sensitivity to changes in azimuth [32].
In the case of the horizontal beam, we have previously shown [32] that bats further increased localization by steering the beam alternately to the right and to the left of the object (note that as bats steer the beam horizontally using their tongue, this does not contradict our finding that horizontal gaze is fixed to the target). We did not observe such an alternating beam steering in the vertical axis, and such a strategy would probably be difficult to execute with the tongue. However, note that the bats would still benefit from pointing a large-derivative part of the beam, because even without vertical alteration, they could estimate changes in target elevation by comparing consecutive beams. How bats decipher simultaneous intensity changes in both the vertical and the horizontal angles of an object is an open question. As these bats typically produce sound clicks in pairs (with longer inter-pair intervals and shorter intra-pair intervals [33]), one hypothesis would be that intra-pair intensity differences are used to estimate azimuth while the inter-pair differences are used to estimate elevation. Naturally, bats also use intra- and inter-aural information to localize objects [34], so beam steering only provides part of the necessary information.
Stabilizing the vertical gaze is not only important for controlling the echolocation emission, it is also very important on the reception level. Rousettus bats constantly move their ears, supposedly in order to enhance echo localization [35], and this is also known for many other bat species [36,37]. Such active hearing strategies would probably suffer great inaccuracies if the basis of the ears (i.e. the head) constantly changed its vertical angle.
Interestingly, like human-engineered systems, bats solve the problem of movement-based noise by stabilizing the system at the acquisition level and thus acquiring data with less noise rather than filtering the data post-acquisition. Similarly, when imaging on-board an unstable platform such as a drone, the preferred solution is to use a gimbal that compensates for the jitter induced by the drone rather than to apply image correction algorithms post-acquisition. However, even after stabilizing the head, there was still some angular jitter in the vertical gaze (α) (which probably reflected actual jitter and not only our measurement errors; figure 1b). Post-acquisition neural processing is therefore probably necessary in order to achieve the very high angular accuracy typically exhibited by bats [38,39].
The bats’ inability to maintain a stable gaze when the target was moving suggests that these bats did not evolve an ability to track moving targets, which is reasonable when considering their ecology. Many other bat species track and intercept flying insects using an arsenal of active strategies to do so [20,36]. It is intriguing to imagine how such bats stabilize head movements when performing the rapid manoeuvres, which are required for chasing an insect.
4. Material and methods
(a). Animals
Eight male R. aegyptiacus bats were captured in a cave in central Israel. For identification, each bat's fur was bleached with a different pattern. The bats were held in a large cage (1 × 2 × 2.5 m) in the Zoological garden at Tel Aviv University, Israel. The bats were kept in reversed light cycle (light on: 19:00–08:00), in a temperature of 26° during their subjective day and 23° during their subjective night. The bats' diet included 100 g of diverse fruits (per bat, daily). Water was available ad libitum. The bats were weighed on a weekly basis to monitor their health.
(b). Experiments
Bats were trained to take off from the experimenter's hands and land on a 20 cm diameter styrofoam ball, which was mounted on a 1 m high wooden pole covered with felt in order to reduce the echoes and was positioned 2.5 m away from the release point. Experiments took place in our acoustics flight room (5.5 × 4.5 × 2.5 m) which is acoustically isolated and anechoic. Experiments were performed in darkness so that the bats would rely on echolocation. Eight bats were flown in darkness (less than 10−3 lux), and three of the eight bats were also flown in dim light (approx. 1 lux) to examine whether there is a difference between their performance in dark versus in light (electronic supplementary material, table S1). Moreover, a strong light was flashed into the bats’ eyes just before each release to decrease its visual sensitivity. In addition, in order to reduce the reliance on spatial memory, we randomly moved the target, every few trials, ca 50 cm to the right or left, or changed the bats' release point along the starting line, also ca 50 cm to the right or left. The bats were tracked using a commercial motion-capture system (composed of 16 Raptor E 1280 × 1024 pixels cameras, and four Raptor-12 4096 × 3072 pixels cameras, Motion-Analysis Corp.). Motion was tracked at 200 frames s−1 and with a spatial resolution of less than 1 mm (electronic supplementary material, figure S1C). To enable tracking, spherical reflective facial markers (3X3 Designs Corp.) were glued to the bats using skin bond latex cement (OTSO-BOND Montreal Ostomy Corp.). To track the head and body's vertical angle (pitch), two markers (2.4 mm) were mounted along the head and two (6 mm) along the body, to track the wings, one marker (2.4 mm) was mounted on the wrist of each wing (electronic supplementary material, figure S1A). In order to estimate the bats’ horizontal gaze (azimuth) and roll, we tracked the bats' eyes (no markers added). In addition to the tracking system, a high-speed monochromatic camera, 640 × 480 pixels recorded raw video of the flight at 200 fps (Basler, acA1300-60gmNIR, Basler Corp.). The entire system was triggered by an assistant before the bat took off the experimenter's hand and turned off after the bat landed on the target. A total of 370 trials were collected for the eight bats with a minimum of five trials per bat across conditions (an average of 46 trials per bat, note that not all bats participated in all the conditions, see the electronic supplementary material, table S1). Because the task we used was very simple in terms of flight, the bats only had to fly 2.5 m in a straight line, we glued a 3.1 g, cross-shaped weight (23 mm high and 22 mm wide) on the bat's lower back in order to increase jitter (we glued the cross using skin bond latex cement, OTSO-BOND Montreal Ostomy Corp.). We used the cross in 31% of the trials (it was only used in experiments in the dark not including the beam reconstruction and the moving target experiments).
As a control to the effect of the weight on the bats, we ran 20 trials on average for each bat without weight (electronic supplementary material, figure S10). Because there was no significant difference in the bats’ vertical gaze (α) with and without the weight (p > 0.07 for each bat, two-sample Kolmogorov–Smirnov test), we ended up pooling the data from the two conditions.
In addition, in order to examine the bats' ability to maintain a fixed vertical gaze (α), we challenged two bats with a moving target. After the bat took off, the experimenter pulled the platform up or lowered it down using a pulley system, the target was either elevated by 0.5 m (an average speed of 0.7 m s−1) or lowered by 0.5 m (an average speed of 0.5 m s−1) while the bats were approaching the target. The target was pulled up 0.37 s ± 0.29 after the bat took off and stopped 0.49 s ± 0.25 before the bat landed. The target was pulled down 0.54 s ± 0.32 after the bat took off and stopped 0.06 s ± 0.24 before the bat landed (mean ± s.d. of all bats, n = 2).
(c). Data processing and analysis
The three-dimensional data were reconstructed using the commercial motion-capture software (Cortex 6.23, Motion Analysis). Further analysis was performed in Matlab (Mathworks). Missing tracking samples (segments of up to 25 ms, occurring, for example, when the markers were blocked by the wings) were completed using linear interpolation.
(d). Audio recordings and beam direction estimation (see also the electronic supplementary material, figure S8)
Audio was recorded in all trials with a single microphone (USG Omnidirectional Knowles microphone, Avisoft Bioacoustics) which was mounted on the landing target and was synchronized with the motion-capture system. The microphone was sampled by an A/D converter (USB-6218, National Instruments) sampling at a rate of 51 kHz. The purpose of these recordings was only to validate that the bats used echolocation and thus the low sampling rate was sufficient.
In a set of trials that aimed to estimate the vertical echolocation beam, audio was recorded with a 12-wideband microphone array using the Hm1216 AD converter, and USG Electret Ultrasound Microphones (Avisoft Bioacoustics/Knowles FG). Here, 12 microphones were spread vertically at the end of the flight room (behind the landing target), they were 11 cm apart at the centre of the array and 20 cm apart near the edges, two of the microphones (11–12) were spread on the ground beneath the target, facing up, they were 10 cm apart, thus assuring very high angular resolution and spanning most of the vertical axis of the room (electronic supplementary material, figure S8). With this system, audio was recorded at a sampling rate of 375 kHz.
(e). Statistical analysis (see also the electronic supplementary material, table S1)
Statistical analyses were performed on the group level using paired t-tests except for conditions where we had less than six bats. In those cases, we ran the statistics per bat and corrected for multiple comparisons. In order to show that all bats responded similarly, we also ran all statistical tests on the individual level, using the Benjamini–Hochberg FDR test to correct for multiple comparisons [40,41]. Notably, all individuals were always significant.
To measure the individual jitter, we compared the standard deviation of the vertical gaze (α) and the body-target angle (β) across trials, we used paired, two-tailed, t-test, on the group level and for each bat that participated (eight bats) n = 8.
In order to test if the distribution of body-target angles (β) is wider than the vertical gaze (α) distribution (figure 1c), we estimated the width at half height of the distributions and used a paired, two-tailed, t-test for the group (n = 8).
In order to test whether there was a difference between the bats’ vertical gaze (α) under light and dark conditions (figure 1c), we used the two-sample Kolmogorov–Smirnov test to compare the two distributions of each of the three bats that participated in both conditions.
In order to test the flight speed in dark versus light conditions, we used a two-sample, two-tailed, t-test, for each of the three bats that participated in both conditions.
In order to test the correlation between head body angle (γ) and the body pitch (δ) (figure 1e), we used Spearman's rank correlation coefficient for each bat (six bats).
In order to test the change in the mean angle and the standard deviation of the vertical gaze (α, before and after locking), we used a paired, two-tailed, t-test, for the group (n = 8).
In order to test if there was a significant difference in the vertical gaze standard deviation between the moving down to the fixed target conditions, and between the moving down and moving upward conditions (figure 2d), we used a two-sample, two-tailed, t-test for each of the two bats that participated in both conditions (each bat was tested separately).
In order to test if there is a significant difference between the conditions: with/without weight (electronic supplementary material, figure S10), we used a two-sample Kolmogorov–Smirnov test, for each bat (eight bats).
In order to test the correlation between the tracking system's control angle and the motor's angle (electronic supplementary material, figure S1E1), we used Pearson's correlation coefficient.
In order to test if the distribution of the shuffled vertical angle (α) is wider than the non-shuffled vertical gaze (α) distribution (electronic supplementary material figure S3), we estimated the width at half height of the distributions of each of the eight bats and used a paired, two-tailed, t-test, for the group (n = 8).
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
We thank Sharon Swartz and Kenny Breuer for allowing us to use their video for this paper. We thank Arseny Finkelstein and Ziv Kassner for their comments on the manuscript. We thank Arjan Boonman for assisting in the beam experiments. We thank Mor Taub for designing the bats' illustrations. We thank Sahar HajYahia, Lior Mevorach, Gal Shalev, Lana Nagiev and Noa Raish for training the bats and assisting in the experiments.
Ethics
The experiment was approved by the institutional IACUC committee: permit number 04-18-026. The use of the animals was approved by the National Park Authority.
Data accessibility
Data available from: https://www.dropbox.com/sh/bijo2va7m42l8ez/AADsRZozCTbO-VRT8uOA60u2a?dl=0.
Authors' contributions
O.E. conducted, conceived and designed the experiments, analysed the data and wrote the manuscript. G.K. conceived and designed the experiments. Y.Y. conceived and designed the experiments, analysed the data and wrote the manuscript.
Competing interests
The authors declare no conflict of interest.
Funding
The project was partially funded by ONRG grant no. N62909-16-1-2133. The first author was partially funded by the Dr Alexander Lester and Eva Lester fellowships.
References
- 1.Mulavara AP, Bloomberg JJ. 2003. Identifying head–trunk and lower limb contributions to gaze stabilization during locomotion. J. Vestib Res. 12, 255–269. [PubMed] [Google Scholar]
- 2.Pozzo T, Berthoz A, Lefort L. 1990. Head stabilization during various locomotor tasks in humans. Exp. Brain Res. 82, 97–106. ( 10.1007/BF00230842) [DOI] [PubMed] [Google Scholar]
- 3.Iriarte-Díaz J, Riskin DK, Willis DJ, Breuer KS, Swartz SM. 2011. Whole-body kinematics of a fruit bat reveal the influence of wing inertia on body accelerations. J. Exp. Biol. 214, 1546–1553. ( 10.1242/jeb.037804) [DOI] [PubMed] [Google Scholar]
- 4.Bergou AJ, Swartz SM, Vejdani H, Riskin DK, Reimnitz L, Taubin G, Breuer KS. 2015. Falling with style: bats perform complex aerial rotations by adjusting wing inertia. PLoS Biol. 13, e1002297 ( 10.1371/journal.pbio.1002297) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xiang Y, Yakushin SB, Kunin M, Raphan T, Cohen B. 2008. Head stabilization by vestibulocollic reflexes during quadrupedal locomotion in monkey. J. Neurophysiol. 100, 763–780. ( 10.1152/jn.90256.2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hirasaki E, Kumakura H. 2004. Head movements during locomotion in a gibbon and Japanese macaques. Neuroreport 15, 643–647. ( 10.1097/00001756-200403220-00014) [DOI] [PubMed] [Google Scholar]
- 7.Katzir G, Schechtman E, Carmi N, Weihs D. 2001. Head stabilization in herons. J. Comp. Physiol. A Sensory, Neural, Behav. Physiol. 187, 423–432. ( 10.1007/s003590100210) [DOI] [PubMed] [Google Scholar]
- 8.Videler JJ, Weihs D, Daan S. 1983. Intermittent gliding in the hunting flight of the kestrel, Falco tinnunculus L. J. Exp. Biol. 102, 1–2. [Google Scholar]
- 9.Erichsen JT, Hodos W, Evinger C, Bessette BB, Phillips SJ. 1989. Head orientation in pigeons: postural, locomotor and visual determinants. Brain. Behav. Evol. 33, 268–278. ( 10.1159/000115935) [DOI] [PubMed] [Google Scholar]
- 10.Pete AE, Kress D, Dimitrov MA, Lentink D. 2015. The role of passive avian head stabilization in flapping flight. J. R. Soc. Interface 12, 0508 ( 10.1098/rsif.2015.0508) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kano F, Walker J, Sasaki T, Biro D. 2018. Head-mounted sensors reveal visual attention of free-flying homing pigeons. J. Exp. Biol. 221, jeb183475 ( 10.1242/jeb.183475) [DOI] [PubMed] [Google Scholar]
- 12.Ravi S, Crall JD, McNeilly L, Gagliardi SF, Biewener AA, Combes SA. 2015. Hummingbird flight stability and control in freestream turbulent winds. J. Exp. Biol. 218, 1444–1452. ( 10.1242/jeb.114553) [DOI] [PubMed] [Google Scholar]
- 13.Eckmeier D, Geurten BRH, Kress D, Mertes M, Kern R, Egelhaaf M, Bischof H-J. 2008. Gaze strategy in the free flying zebra finch (Taeniopygia guttata). PLoS ONE 3, e3956 ( 10.1371/journal.pone.0003956) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Quinn D, Kress D, Chang E, Stein A, Wegrzynski M, Lentink D. 2019. How lovebirds maneuver through lateral gusts with minimal visual information. Proc. Natl Acad. Sci. USA 116, 15 033–15 041. ( 10.1073/PNAS.1903422116) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Troje NF, Frost BJ. 2000. Head-bobbing in pigeons: how stable is the hold phase? J. Exp. Biol. 203, 935–940. [DOI] [PubMed] [Google Scholar]
- 16.Warrick DR, Bundle MW, Dial KP. 2002. Bird maneuvering flight: blurred bodies, clear heads. Integr. Comp. Biol. 42, 141–148. ( 10.1093/icb/42.1.141) [DOI] [PubMed] [Google Scholar]
- 17.Griffin D. 1958. Listening in the dark: the acoustic orientation of bats and men. New Haven, CT: Yale University Press. [Google Scholar]
- 18.Yovel Y, Falk B, Moss CF, Ulanovsky N. 2011. Active control of acoustic field-of-view in a biosonar system. PLoS Biol. 9, e1001150 ( 10.1371/journal.pbio.1001150) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kounitsky P, Rydell J, Amichai E, Boonman A, Eitan O, Weiss AJ, Yovel Y. 2015. Bats adjust their mouth gape to zoom their biosonar field of view. Proc. Natl Acad. Sci. USA 112, 6724–6729. ( 10.1073/pnas.1422843112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ghose K, Horiuchi TK, Krishnaprasad PS, Moss CF, Shinoda Y. 2006. Echolocating bats use a nearly time-optimal strategy to intercept prey. PLoS Biol. 4, e108 ( 10.1371/journal.pbio.0040108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matsuta N, Hiryu S, Fujioka E, Yamada Y, Riquimaroux H, Watanabe Y. 2013. Adaptive beam-width control of echolocation sounds by CF-FM bats, Rhinolophus ferrumequinum nippon, during prey-capture flight. J. Exp. Biol. 216, 1210–1218. ( 10.1242/jeb.081398) [DOI] [PubMed] [Google Scholar]
- 22.Motoi K, Sumiya M, Fujioka E, Hiryu S. 2017. Three-dimensional sonar beam-width expansion by Japanese house bats (Pipistrellus abramus) during natural foraging. J. Acoust. Soc. Am. 141, EL439– EL444 ( 10.1121/1.4981934) [DOI] [PubMed] [Google Scholar]
- 23.Ghose K, Moss CF. 2006. Steering by hearing: a bat's acoustic gaze is linked to its flight motor output by a delayed, adaptive linear law. J. Neurosci. 26, 1704–1710. ( 10.1523/JNEUROSCI.4315-05.2006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Danilovich S, Krishnan A, Lee WJ, Borrisov I, Eitan O, Kosa G, Moss CF, Yovel Y. 2015. Bats regulate biosonar based on the availability of visual information. Curr. Biol. 25, R1124–R1125. ( 10.1016/j.cub.2015.11.003) [DOI] [PubMed] [Google Scholar]
- 25.Danilovich S, Yovel Y. 2019. Integrating vision and echolocation for navigation and perception in bats. Sci. Adv. 5, eaaw6503 ( 10.1126/sciadv.aaw6503) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Finkelstein A, Derdikman D, Rubin A, Foerster JN, Las L, Ulanovsky N. 2015. Three-dimensional head-direction coding in the bat brain. Nature 517, 159–164. ( 10.1038/nature14031) [DOI] [PubMed] [Google Scholar]
- 27.Finkelstein A, Las L, Ulanovsky N. 2016. 3-D maps and compasses in the brain. Annu. Rev. Neurosci. 39, 171–196. ( 10.1146/annurev-neuro-070815-013831) [DOI] [PubMed] [Google Scholar]
- 28.Dunbar DC, Badam GL, Hallgrímsson B, Vieilledent S. 2004. Stabilization and mobility of the head and trunk in wild monkeys during terrestrial and flat-surface walks and gallops. J. Exp. Biol. 207, 1027–1042. ( 10.1242/jeb.00863) [DOI] [PubMed] [Google Scholar]
- 29.Goldberg JM, Cullen KE. 2011. Vestibular control of the head: possible functions of the vestibulocollic reflex. Exp. Brain Res. 210, 331–345. ( 10.1007/s00221-011-2611-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bar NS, Skogestad S, Marçal JM, Ulanovsky N, Yovel Y.. 2015. A sensory-motor control model of animal flight explains why bats fly differently in light versus dark. PLoS Biol. 13, e1002046 ( 10.1371/journal.pbio.1002046) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Beatus T, Guckenheimer JM, Cohen I. 2015. Controlling roll perturbations in fruit flies. J. R. Soc. Interface 12, 20150075 ( 10.1098/rsif.2015.0075) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yovel Y, Falk B, Moss CF, Ulanovsky N, Åhlén P-A. 2010. Optimal localization by pointing off axis. Science 327, 701–704. ( 10.1126/science.1183310) [DOI] [PubMed] [Google Scholar]
- 33.Yovel Y, Geva-Sagiv M, Ulanovsky N. 2011. Click-based echolocation in bats: not so primitive after all. J. Comp. Physiol. A Neuroethol. Sensory, Neural. Behav. Physiol. 197, 515–530. ( 10.1007/s00359-011-0639-4) [DOI] [PubMed] [Google Scholar]
- 34.Koay G, Heffner RS, Heffner HE. 1998. Hearing in a megachiropteran fruit bat (Rousettus aegyptiacus). J. Comp. Psychol. 112, 371–382. ( 10.1037/0735-7036.112.4.371) [DOI] [PubMed] [Google Scholar]
- 35.Holland RA, Waters DA. 2005. Echolocation signals and pinnae movement in the fruitbat Rousettus aegyptiacus. Acta Chiropterol. 7, 83–90. ( 10.3161/1733-5329(2005)7[83:ESAPMI]2.0.CO;2) [DOI] [Google Scholar]
- 36.Wohlgemuth MJ, Kothari NB, Moss CF. 2016. Action enhances acoustic cues for 3-D target localization by echolocating bats. PLoS Biol. 14, e1002544 ( 10.1371/journal.pbio.1002544) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yin X, Müller R. 2019. Fast-moving bat ears create informative Doppler shifts. Proc. Natl Acad. Sci. USA 116, 12 270–12 274. ( 10.1073/pnas.1901120116) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lawrence B, Simmons J. 1982. Echolocation in bats: the external ear and perception of the vertical positions of targets. Science 218, 481–483. ( 10.1126/science.7123247) [DOI] [PubMed] [Google Scholar]
- 39.Simmons JA, Kick SA, Lawrence BD, Hale C, Bard C, Escudié B. 1983. Acuity of horizontal angle discrimination by the echolocating bat, Eptesicus fuscus. J. Comp. Physiol. A 153, 321–330. ( 10.1007/BF00612586) [DOI] [Google Scholar]
- 40.Benjamini Y, Yekutieli D. 2001. The control of the false discovery rate under dependency. Ann. Stat. 29, 1165–1188. ( 10.1214/aos/1013699998) [DOI] [Google Scholar]
- 41.Benjamini Y, Hochberg Y. 1995. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B 57, 289–300. ( 10.1111/j.2517-6161.1995.tb02031.x) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data available from: https://www.dropbox.com/sh/bijo2va7m42l8ez/AADsRZozCTbO-VRT8uOA60u2a?dl=0.