Abstract
Ecological opportunity is considered a crucial factor for adaptive radiation. Here, we combine genetic, morphological and ecological data to assess species and ecomorphological diversity of Artic charr in six lakes of a catchment in southernmost Greenland, where only charr and stickleback occur. Because the diversity of habitats and resources increases with lake size, we predict a positive association between lake size and the extent of ecomorphological diversity. The largest lake of the catchment harbours the largest Arctic charr assemblage known today. It consists of six genetically differentiated species belonging to five ecomorphs (anadromous, littoral benthic, profundal dwarf, planktivorous, piscivorous), of which the latter comprises two ecomorphologically extremely similar species. Lakes of intermediate size contain two ecomorphologically and genetically distinct species. Small lakes harbour one genetically homogeneous, yet sometimes ecomorphologically variable population. Supporting our prediction, lake size is positively correlated with the extent of ecomorphological specialization towards profundal, pelagic and piscivorous lifestyle. Furthermore, assemblage-wide morphospace increases sharply when more than one genetic cluster is present. Our data suggest that ecological opportunity and speciation jointly determine phenotypic expansion in this charr radiation.
Keywords: adaptive radiation, Arctic charr, ecological opportunity, niche expansion, speciation
1. Introduction
The process of adaptive radiation, often defined as the rapid evolution of several ecologically differentiated species from a common ancestor, is considered an important source of biodiversity [1]. Ecological opportunity (i.e. a wealth of accessible and underutilized resources) is thought to be crucial for adaptive radiation to occur [2,3], however data to test its role are limited [4]. Theoretical models [5,6] and comparative data [7] find that it is the combination of ecological opportunity and intrinsic potential of organisms to speciate that determine the occurrence of adaptive radiation. With increasing isolation and area, speciation rather than immigration governs community assembly [8,9]. Hence, large, newly arisen environments in isolated and competitor-poor regions are promising places for finding adaptive radiations.
Following the ecological theory of adaptive radiation [1], ecological opportunity sets the external bounds within which the radiating taxon expands its resource use and phenotypic variation according to its intrinsic physiological and evolutionary potential. Many studies explored the relationship between ecological opportunity and ecological or phenotypic variation with populations [3,10–12], but only rarely with young species radiations (but see [13]).
Ecological opportunity may drive expansion towards one or multiple ecological niches, which may cause morphological variation to expand towards one [13,14] or multiple [12,15] directions of ecomorphological specializations. In Darwin's finches, the distribution of seed hardness predicted ecomorphological specializations (beak depth) along this single dimension [16]. In contrast, to our knowledge, whether ecological opportunity predicts the extent of expansion towards multiple ecomorphological specializations remains untested.
The Arctic charr (Salvelinus alpinus complex) advances farthest north of all freshwater fish taxa, thereby colonizing many novel, competitor-free environments. Across its subarctic range, it is famous for its diversity of forms, both between and within water bodies (reviewed in [17–19]). The largest sympatric Arctic charr diversity is known from Lake Thingvallavatn, Iceland, harbouring four charr forms that differ in morphology, ecology [20] and, at least for three of them, also in genetics [21]. Generally, the genus Salvelinus shows high sympatric diversity. Lake charr (Salvelinus namaycush) repeatedly evolved up to four sympatric morphs showing adaptations to feeding in different depth zones of large lakes [22]. Currently, the highest number of sympatric charr forms is known from Lake Kronotskoe, Russia, for which seven morphs of Dolly Varden (Salvelinus malma) differing in morphology, ecology and spawning locality have been characterized [23], but whose species status has not been conclusively addressed. Across the genus Salvelinus, it has been repeatedly proposed that the availability of large ecological opportunity in northern lakes was key to their diversification, a hypothesis that has however not formally been tested for charr radiations (see [24] for charr populations).
In this study, we examine the effect of ecological opportunity on ecomorphological diversity in previously unstudied adaptive radiations of Arctic charr in southern Greenland. The Greenlandic ice sheet started to uncover land and lakes in southern Greenland approximately 10 000 years ago [25]. Very few fish taxa (i.e. three-spined stickleback (Gasterosteus aculeatus species complex) and Artic charr (Salvelinus alpinus), and exceptionally Atlantic salmon (Salmo salar) and eel (Anguilla rostratus [26])) colonized freshwaters of Greenland. The scarcity of freshwater fish taxa and the well-known propensity of Artic charr for diversification make Greenlandic lakes an ideal study system to test how ecological opportunity shapes ecomorphological diversity during adaptive radiation.
Here, we assess ecomorphological and species diversity of Artic charr in six lakes of the Equaluit drainage in southern Greenland by combining morphological, ecological and genetic data. In the largest lake, we find the richest Arctic charr assemblage known today, consisting of six species belonging to five distinct ecomorphs, one of which comprises two extremely similar, but genetically distinct species. Using our dataset of lakes containing one to six charr species, we test whether ecological opportunity predicts community-wide phenotypic extremes along several directions of ecological expansion. Furthermore, we test whether ecomorph richness and speciation contribute to morphospace expansion across lakes. We discuss our findings in the context of community assembly by adaptive radiation.
2. Methods
(a). Study system and sampling
Phylogeographic studies show that charr colonized southern Greenland from two glacial refugia, the Atlantic and the Arctic [27]. Previous studies of charr diversity in other Greenlandic lakes found multimodal size distributions, whereby size groups were associated with planktivorous, littoral benthic or piscivorous lifestyle [28–30]. It however remained unclear whether they represented an ontogenetic transition series, as suggested by Riget et al. [29] and Sparholt [30], or different species.
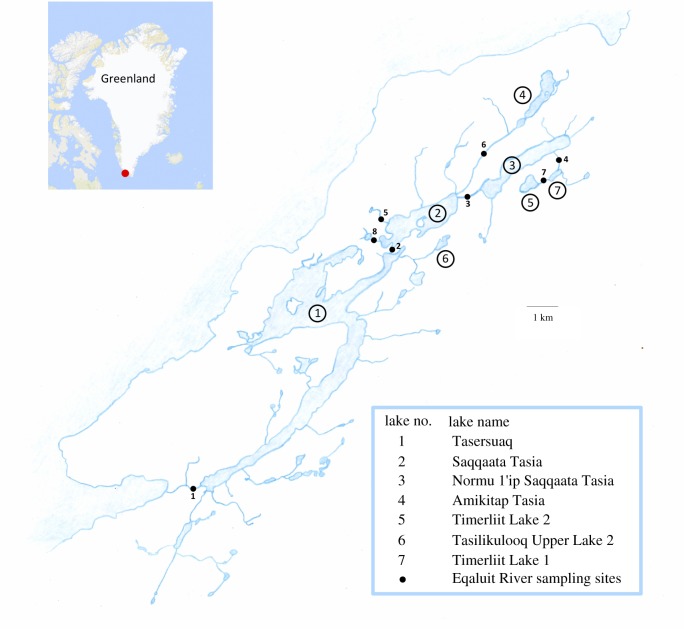
The Eqaluit River in southwestern Greenland is located on the Vatnahverfi peninsula near Qaqortoq, and drains into the Igaliku Fjord (60°45′33″ N, 45°33′54″ W). We sampled charr in seven lakes and eight stream sections of the Eqaluit River in July and August 2014 and 2016 (figure 1; electronic supplementary material, table S1). Surface area of lakes was 0.09–8.93 km2 and maximum depth 1–88 m (electronic supplementary material, table S1). All sampling sites, except Tasilikulooq Upper Lake 2, harboured stickleback besides charr. Benthic and pelagic multi-mesh gillnets were set at various depths, with the number of nets adjusted to lake size (see electronic supplementary material, appendix S1 and table S2 for details). Streams were mostly narrow and shallow, and we fished them by angling, by hand-netting and by hand.
Figure 1.
Map of the Eqaluit drainage in southern Greenland with sampled lakes and stream sections indicated. (Online version in colour.)
Individual fish were carefully removed from the net or hook and immediately photographed in water in an adequately sized photo-cuvette (left side of the fish, grey background). After euthanasia in an overdose of clove oil, fish were individually photographed in air (straightened, left side of the fish, grey background; standard photo). A piece of the right pectoral fin and of dorsal muscle tissue were taken and stored in 100% ethanol for DNA and stable isotope analysis, respectively. We measured standard length (SL) of all fish, fixed them in 4% formalin solution and later incrementally transferred them to 75% ethanol for phenotypic analyses.
(b). Morphology
Our catch revealed an astonishing phenotypic diversity of charr. Guided by studies on other lakes with multiple charr species [18,20,31] and our own unpublished work on charr in Swiss subalpine lakes, we categorized individuals from Greenlandic lakes into six groups exhibiting typical and frequently found morphological and ecological trait combinations in charr, so called ecomorphs (sensu [32]). We recognized five specialist ecomorphs (piscivores, planktivores, littoral benthics, profundal dwarfs and anadromous), as well as generalists. A detailed description of each ecomorph is given in electronic supplementary material, appendix S2, and the distinguishing criteria in electronic supplementary material table S3. Two persons (C.J.D., A.K.K.) independently assigned individuals using cuvette and standard photos. 19% of total catch (13% for lakes, 85% for rivers) were left unassigned, either because they were too young, damaged, phenotypically intermediate or because the two persons did not agree in their assignment. In this paper we use the term ecomorph to refer to these purely morphologically defined groups.
Genetic structure analysis showed that in each of Lakes Tasersuaq and Saqqaata Tasia, the piscivorous ecomorph comprised two distinct genetic clusters. For the comparisons between ecomorphs within lakes, we pooled these two genetic clusters into the piscivorous ecomorph, and assessed their ecological and morphological differences subsequently in a separate analysis.
Sample size of the smallest lake, Timerliit Lake 1, was so low (n = 6) that we excluded it from all analyses, except the genetic structure analysis. For the other lakes, we examined morphological variation for a minimum of 15 individuals per ecomorph (except where our sample sizes were smaller, electronic supplementary material, table S4), choosing individuals randomly within ecomorphs (n = 273 in total). We measured 25 linear distances, including SL, on the preserved fish to the nearest 0.01 mm using a digital calliper. Distances were chosen to reflect body and head shape, including ecologically relevant morphological variation [33] (electronic supplementary material, figure S1). Lateral measurements were taken on the left side of the fish, whenever possible (more than 97% of individuals, more than 99.9% of measurements).
To assess morphological differentiation between ecomorphs within lakes, we performed a PCA using all size-corrected morphological variables (centred and normalized) for each lake separately, and calculated Bhattacharyya distances between any two sympatric ecomorphs in the PC1-2 morphospace of that lake using the R package fpc [34]. Significance was assessed using Hotelling's T-squared test. We performed size correction by taking for each trait residuals from a pooled regression between log-transformed trait and log-transformed SL within each lake separately. For the largest charr assemblage, we also assessed morphological differentiation using body proportions (electronic supplementary material, appendix S9).
Differentiation in body size was assessed by calculating PST, a phenotypic analogue of FST, of SL (measured in the field) for all pairwise ecomorph comparisons within a lake. PST was calculated following Kaeuffer et al. [35] using 1000 resamplings. We compared individual traits between the two small lakes differing in the prevalence of stickleback in linear models using SL, lake and their interaction as independent variables, using only individuals from the overlapping size range (SL < 225 mm).
(c). Stable isotopes
We performed stable isotope analyses of δ15N and a δ13C for 556 individuals from six lakes (see electronic supplementary material, appendix S3 for details). To assess differentiation between sympatric ecomorphs in stable isotopes, we calculated Bhattacharyya distances between any two ecomorphs in the δ13C-δ15N space for each lake assemblage separately using the R package fpc [34], and assessed significance using Hotelling's T-squared test.
Furthermore, we assessed whether the variance in δ15N differed between Tasilikulooq Upper Lake 2 and Timerliit Lake 2 using Levene's test.
(d). Lake habitat and depth
We considered three major lake habitats (littoral, profundal and pelagic) and examined differences in habitat use between ecomorphs within a lake using chi-square tests. All fish caught in benthic nets deeper than 20 m (where light levels were less than 1% of surface light) were assigned to the profundal, those caught in shallower benthic nets to the littoral and those caught in pelagic nets to the pelagic habitat.
Furthermore, we calculated PST of capture depth between any two ecomorphs within a lake with 1000 resampling permutations. We confined this analysis to benthic nets, because these were used in all lakes.
(e). Microsatellite genotyping and genetic data analysis
We genotyped 686 individuals (electronic supplementary material, table S1) at nine microsatellite loci: OMM1228, OMM5151, OMM1329, OMM1236, OMM1211, OMM5146, OMM1302, BX890355, Ssa100 (see electronic supplementary material, appendix S4 for details). To assess genetic structure in the entire Eqaluit catchment, we conducted a hierarchical structure approach with the program Structure [36]. We first ran all fish from all lakes and river stretches together for K = 1–15, with 10 replicates per K, 500 000 burn-in and MCMC steps each, applying the admixture model for correlated allele frequencies. We assessed LnP(D) to evaluate whether K = 1 was most likely. If that was not the case, we determined the best K using the Evanno method [37] as implemented in structure harvester [38]. Second, we ran Structure for each of the genetic clusters obtained in the first step, using the above parameter values. Throughout the manuscript, the term ‘genetic cluster’ refers to the genetic clusters found in this hierarchical Structure analysis.
We calculated pairwise multilocus FST (1000 permutations) for all pairwise ecomorph combinations within each lake. Because of small sample size, we excluded the anadromous ecomorph of Lakes Tasersuaq (n = 5) and Saqqaata Tasia (n = 2) and the littoral benthic ecomorph of Timerliit Lake 2 (n = 6). Because individuals of the piscivorous ecomorph within Lakes Tasersuaq and Saqqaata Tasia belonged to two different genetic clusters, we used the two genetic clusters as separate groups for FST calculation in these lakes. We used Arlequin v. 3.5.1.2 [39] to obtain FSTs, and PGD Spider v. 2.0.7.2 [40] to convert files from Structure to Arlequin format.
Furthermore, we assessed genetic differentiation between different ecomorphs of different lakes belonging to the same genetic cluster, and between populations of the same ecomorph from different lakes belonging to the same genetic cluster. Because planktivorous versus profundal dwarf ecomorphs of Tasersuaq were weakly differentiated, we also performed a locus-by-locus AMOVA (including individual level, 1000 permutations).
We generated a population tree using ecomorphs from each lake as well as samples from different river stretches as populations (see electronic supplementary material, appendix S5).
(f). Ecomorphological comparison of two sympatric genetic clusters of piscivores
In Tasersuaq and Saqqaata Tasia, we tested whether the two genetic clusters of piscivores differed in SL and 24 size-corrected linear traits using Wilcoxon tests. p-values were adjusted for multiple testing using Holm's method [41]. Using the R package ‘adegenet’ [42], we performed a discriminant analysis of principal components on the 24 size corrected traits using the genetic clusters as groups in both lakes separately. The optimal number of PC-axes to retain was determined with the function optim.a.score. We compared medians of capture depth, δ13C and δ15N using Wilcoxon tests, and variances in capture depth using Levene's test between the two genetic groups of piscivores in both lakes separately.
(g). Predicting lake-specific phenotypic extremes
We expect greater ecological specialization and therefore more extreme trait values with increasing ecological opportunity. To test whether ecological opportunity affects single or multiple niches and whether morphological variation would correspondingly expand into one or multiple directions of ecomorphology, we related lake-specific morphological extremes of the major directions of ecomorphological specializations of charr (littoral benthic, profundal dwarf, planktivorous, piscivorous) to proxies of ecological opportunity (maximum lake depth, lake area and lake volume) (see electronic supplementary material, appendix S6 for details).
(h). Contribution of ecomorph richness and speciation to morphospace expansion
To assess whether morphological expansion is associated with ecomorph richness and speciation, we tested whether total morphospace of lake assemblages increased with increasing number of ecomorphs or genetic clusters while correcting for different sample sizes using a resampling approach (see electronic supplementary material, appendix S7 for details).
All analyses, if not stated differently, were performed in R v. 3.2.1 [43].
3. Results
(a). From monomorphic populations to diverse charr communities
The first step of the hierarchical Structure approach for the full dataset including all lakes and river sites revealed seven genetic clusters, none of which was subdivided in subsequent steps (electronic supplementary material, figure S3). The two small isolated lakes each harboured one unique genetic cluster, whereas each of the remaining five clusters were found in several lakes (figure 2). The phylogenetic tree for ecomorphs of different lakes and river sites largely agreed with the Structure results (electronic supplementary material, figure S4). Going from largest to smallest lake, we report differentiation of sympatric ecomorphs in morphology, ecology and genetics.
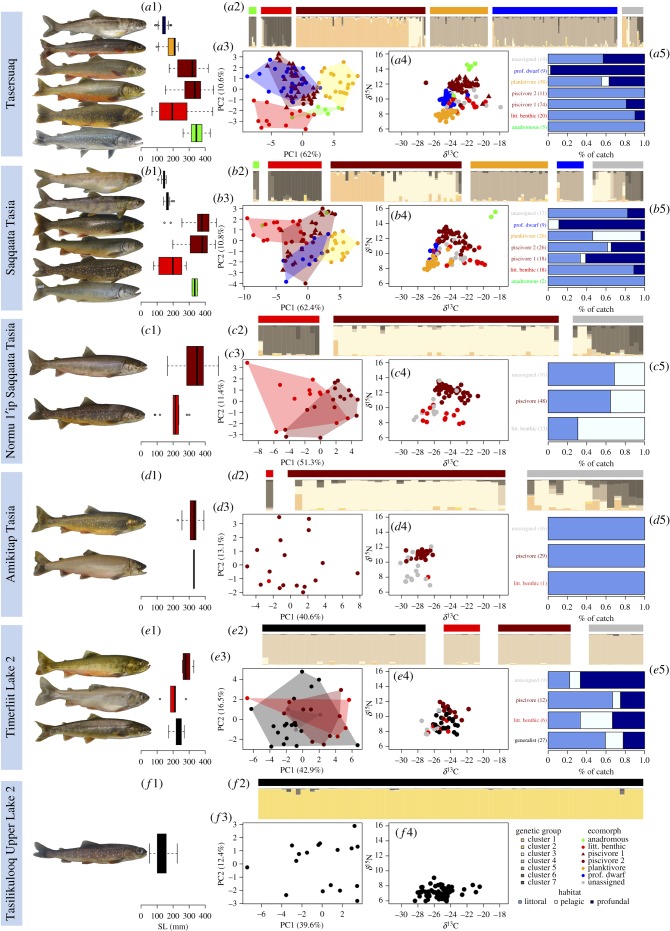
Figure 2.
Overview of the morphological, ecological and genetic diversity of charr in six lakes of the Eqaluit drainage. Lakes are sorted by decreasing lake area from top to bottom. For each lake, a representative of each ecomorph or species within ecomorphs (two species within the piscivorous ecomorph of Tasersuaq and Saqqaata Tasia) is depicted. Horizontal coloured lines above bars indicate phenotypic assignment to ecomorphs; barplots (a2,b2,c2,d2,e2,f2) show the genetic assignment as obtained in the hierarchical Structure analysis across the entire drainage. Variation and differentiation in morphology and ecology among ecomorphs within lakes are represented by box plots of size (a1,b1,c1,d1,e1,f1), PCA of 24 linear measurements (a3,b3,c3,d3,e3,f3), stable isotope space of δ13C and δ15N (a4,b4,c4,d4,e4,f4), and barplots of habitat utilization (a5,b5,c5,d5,e5). For the latter, sample sizes are given in brackets.
Tasersuaq and Saqqaata Tasia harboured the same five specialized ecomorphs (figure 2a,b), none of which was genetically differentiated between these two lakes (all pairwise FST < 0.013, p > 0.05). This and the close proximity and high connectivity of the two lakes (figure 1) are consistent with them being one lake until relatively recently. We therefore describe the charr communities of these two lakes together. Ecomorphs were genetically significantly differentiated (FST) from each other within both lakes, except the planktivore and the profundal dwarf of Saqqaata Tasia were not, potentially due to small sample size (electronic supplementary material, table S5). All ecomorphs were largely assigned to a single genetic cluster, except piscivores comprised of two genetic clusters occurring in both lakes (figure 2a2,b2, see below). Planktivores and profundal dwarfs belonged to the same genetic cluster, yet in Tasersuaq they were significantly genetically differentiated at multiple loci (FST = 0.025, p < 0.001; electronic supplementary material, tables S5 and S6).
All ecomorphs of Tasersuaq and Saqqaata Tasia were significantly differentiated in at least one measure of morphology (size: electronic supplementary material, table S7; morphological PCA: electronic supplementary material, tables S7 and S8), and ecology (stable isotopes: electronic supplementary material, table S9; habitat: electronic supplementary material, tables S9 and S10, appendix S8; figure 2). Unlike other ecomorphs, profundal dwarf and piscivorous ecomorphs were not significantly differentiated in the PCA based on size-corrected residuals (electronic supplementary material, table S7). Their large difference in size may hamper detecting shape differences using residual-based size correction, while they are clearly different when analysing body proportions (electronic supplementary material, appendix S9 and figure S5). One piscivorous individual with extremely low δ13C in Tasersuaq fits into the isotopic space of piscivores from Amikitap Tasia (figure 2), and might be an immigrant from this connected lake at higher elevation.
Given that maintenance of multilocus genetic differentiation in sympatry requires reproductive isolation, we consider significant genetic differentiation among and within ecomorphs within lakes as evidence for them being different species. Hence, we identify six charr species in Tasersuaq/Saqqaata Tasia belonging to five ecologically and morphologically distinct ecomorphs.
In Normu 1'ip Saqqaata Tasia, we found a littoral benthic and a piscivorous ecomorph (figure 2c) that were genetically differentiated, and we therefore consider them as distinct species. The latter ecomorph belonged to the genetic cluster of the rarer piscivore species of Tasersuaq (figure 2). The former was assigned to the same genetic cluster like the anadromous ecomorph of Tasersuaq/Saqqaata Tasia (figure 2), which was however not its closest relative in the NJ tree (electronic supplementary material, figure S4) and was clearly genetically differentiated (FST = 0.08, p = 0.002), suggesting only little gene flow. In Normu 1'ip Saqqaata Tasia, we further caught four individuals belonging to the genetic cluster comprising the littoral benthic ecomorph of Tasersuaq/Saqqaata Tasia. Conversely, in each of Lakes Tasersuaq and Saqqaata Tasia, we caught two individuals of littoral benthic ecomorphology that belonged to the genetic cluster of the littoral benthic ecomorph of Normu 1'ip Saqqaata Tasia (figure 2). This indicates that the three lakes may each host two littoral benthic charr species.
In Amikitap Tasia, we caught the same two ecomorphs with the same genetic assignment as in Normu 1'ip Saqqaata Tasia (only one individual of the littoral benthic ecomorph, figure 2d). The piscivore populations of these two lakes were genetically differentiated from each other and from the population of this genetic cluster in Saqqaata Tasia (pairwise FST = 0.015–0.036, all p < 0.004), but not from that of Tasersuaq (all pairwise FST < 0.005, p > 0.2), suggesting geographical substructure between lakes within this species. According to locals, anadromous charr migrate to Amikitap Tasia for spawning. Consistent with this, we caught there four individuals belonging to the genetic cluster of anadromous charr of Tasersuaq/Saqqaata Tasia. However, we visually assigned one of them to the littoral benthic ecomorph (figure 2), and it also showed littoral benthic isotopic signatures (figure 2d4). It remains to be tested whether anadromous and littoral benthic charr in this lake are different species, or one population exhibiting partial migration.
Timerliit Lake 2 and Tasilikulooq Upper Lake 2 each harboured one genetically unique population (figure 2e,f), which might be explained by their small size and geographical isolation by waterfalls. In Timerliit Lake 2, nonetheless, we found some ecomorphological diversity: a generalist, a littoral benthic and a piscivorous ecomorph. The piscivore was significantly larger (electronic supplementary material, table S7), and had higher δ15N values (electronic supplementary material, table S9) and lower values on morphological PC3 (electronic supplementary material, figure S6) than the other ecomorphs. Yet ecomorphs of that lake were neither significantly differentiated in habitat (electronic supplementary material, tables S9 and S10) nor genetics (electronic supplementary material, table S5). In Tasilikulooq Upper Lake 2, we only found a phenotypically homogeneous generalist population. Charr from this lake had shallower caudal peduncles, narrower upper jaws and different allometric relationships for head length and eye size than those of Timerliit Lake 2 (electronic supplementary material, table S11). Variation in δ15N was significantly larger in the latter than in the former lake (p < 0.001), which might be due to the presence of stickleback in Timerliit Lake 2 favouring piscivory.
The few charr of Timerliit Lake 1 genetically grouped with Timerliit Lake 2 (electronic supplementary material, figure S4), and were visually assigned to the piscivorous ecomorph.
Charr from rivers belonged mainly to the genetic clusters comprising the littoral benthic, anadromous or the widespread piscivore ecomorphs from lakes (electronic supplementary material, figure S7). Based on our criteria for ecomorph assignment (electronic supplementary material, table S3), several individuals in the river stretch nearest to the ocean (site 1, figure 1) were morphologically clearly anadromous. Structure assigned them to the genetic cluster comprising the littoral benthic ecomorph from Tasersuaq/Saqqaata Tasia from which they were genetically differentiated (FST = 0.04 and 0.03, respectively, p < 0.003), while in the NJ tree they grouped with individuals from river site 1 that were assigned to the genetic cluster of the anadromous ecomorph from Tasersuaq/Saqqaata Tasia (electronic supplementary material, figure S4).
(b). Sympatric coexistence of two piscivorous species
The two genetic clusters within the piscivorous ecomorph of Tasersuaq and Saqqaata Tasia hardly differed in morphology or stable isotopes (electronic supplementary material, appendix S10 and figure S8). The cluster found also in two other lakes was caught marginally shallower than that confined to Tasersuaq and Saqqaata Tasia (Tasersuaq: p = 0.052; Saqqaata Tasia: p = 0.081), while variance in capture depth did not significantly differ (p = 0.19 and 0.53, respectively). Because these genetic clusters occur in full sympatry, we consider them from now on as two distinct species.
(c). Ecological opportunity limits planktivorous, profundal and piscivorous ecomorphology
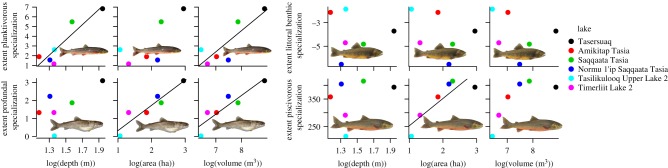
We found a significant positive correlation of the extent of morphological specialization to planktivory (90%-quantile of morphological PC1) with maximum lake depth (p = 0.011) and lake volume (p = 0.035; figure 3). There was no correlation between morphological specialization to littoral benthic lifestyle (10%-quantile of morphological PC1) with any of the proxies of ecological opportunity (all p > 0.48). Morphological specialization towards profundal lifestyle (90%-quantile of morphological PC2) was positively correlated with lake area (p = 0.005) and lake volume (p = 0.011), whereas the proxy for specialization to piscivory (90%-quantile of SL) significantly increased with lake area (p = 0.023; figure 3).
Figure 3.
Ecological opportunity predicts phenotypic extremes for several major directions of ecological specialization. Relationship between lake-specific morphological extremes along the four major directions of ecological specialization in charr, and maximum lake depth, lake area and lake volume. Significant linear relationships are shown. (Online version in colour.)
(d). Large morphospace expansion requires speciation
Assemblage-wide morphospace increased with increasing number of ecomorphs, and genetic clusters in a lake (figure 4; electronic supplementary material, table S12). There was a sharp and significant increase of morphospace from assemblages with a single genetic cluster to those with two genetic clusters, but not from those with a single ecomorph to those with three ecomorphs within a single genetic cluster (electronic supplementary material, table S12). Of the two small lakes harbouring a single genetically homogeneous population each, Timerliit Lake 2, which contains a polymorphic charr population and stickleback, occupied a larger morphospace than Tasilikulooq Upper Lake 2, containing only a monomorphic charr population (electronic supplementary material, table S12).
Figure 4.
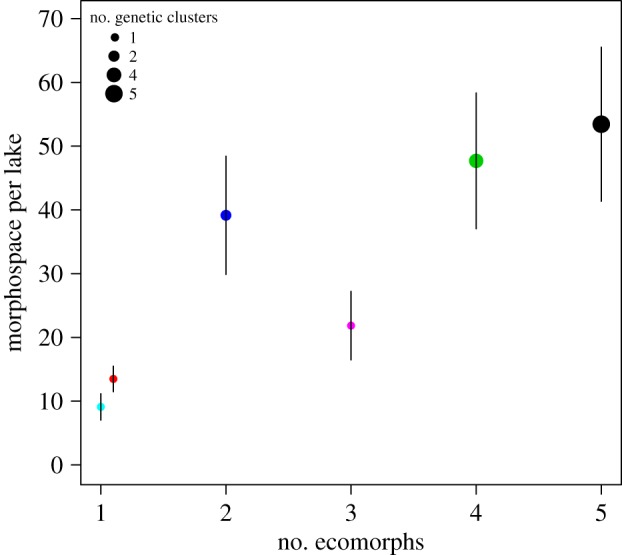
Morphospace of lakes increases with increasing number of ecomorphs and genetic clusters. Per-lake morphospace is the average of 1000 subsamplings ± s.d. Colours correspond to lakes as in figure 3. (Online version in colour.)
4. Discussion
Ecological opportunity is regarded as a key factor for the occurrence and extent of adaptive radiation, both in theory [2,5] and with support from empirical data [7], but the number of tests is limited. In line with this, we find the largest Arctic charr assemblage known today comprising six sympatric species in a large lake in southernmost Greenland, a region providing ample of ecological opportunity with its multitude of deep lakes devoid of other competing salmonid taxa. Two of these six species are ecologically and morphologically highly similar, and only genetic data revealed their presence. Accordingly, clustering methods based on size, stable isotopes or genetic data (nine microsatellite markers) alone all underestimated species richness, exemplifying that assessing species richness in large sympatric radiations requires a multidisciplinary approach [44] (see electronic supplementary material, appendix S11 for a detailed discussion). In intermediately sized lakes of the same catchment, we find two ecomorphologically and genetically distinct charr species, while small lakes harbour a single genetically homogeneous, yet sometimes ecomorphologically variable species. Across the catchment, we find that lake size predicts phenotypic extremes of profundal, pelagic and piscivorous ecomorphology, which is consistent with the prediction that increasing ecological opportunity allows or requires ecomorphological specialization to several different niches simultaneously. Furthermore, we find that a lake's morphospace increases sharply as soon as multiple genetic clusters (but not multiple ecomorphs) are present, providing support for the idea that lack of speciation constrains phenotypic divergence and diversification. We discuss each of these findings in more detail below.
(a). The largest known Arctic charr species assemblage
In the two largest lakes of the Eqaluit catchment, we found six (potentially even seven) charr species belonging to five distinct ecomorphs. This represents the most diverse Arctic charr assemblage known today, which is surprising given the much smaller size of these Greenlandic lakes compared with lakes with large charr assemblages elsewhere [20,23]. Tasersuaq/Saqqaata Tasia may have accumulated many charr species because they have no other salmonids that could compete with charr [45], they are not landlocked (i.e. can be repeatedly colonized from the ocean) and they occur in a contact zone between two glacial lineages of charr (as witnessed in mitochondrial haplotypes [27]), which might promote speciation and adaptive radiation through secondary contact and hybridization [46].
Ecological opportunity may determine both the number of species and their ecological identity by providing a given set of ecological niches. Accordingly, we found that charr species richness increased with lake size, lake size predicted ecomorphological limits of charr assemblages (see below) and ecomorphs present in a lake seemed to be a non-random subset. The two intermediately sized lakes of Eqaluit provided little pelagic or profundal habitat, and we caught there neither planktivorous nor profundal ecomorphs, but only littoral benthics and piscivores. Interestingly, this combination of ecomorphs (littoral benthic, piscivorous) is also common for lakes with two charr ecomorphs in Transbaikalia [47,48]. This may indicate that ecological opportunity causes community assembly of charr to occur in a predictable and nested way, with piscivorous and littoral benthic ecomorphs in small, and additionally profundal, dwarf and planktivorous ecomorphs in larger charr communities.
(b). Ecological opportunity predicts ecomorphological limits of assemblages
Our results are consistent with the hypothesis that ecological opportunity provided by lakes triggers morphological expansion into multiple directions of ecomorphological specialization. The extent of morphological specialization towards planktivory was predicted by maximum lake depth and volume, that towards profundal lifestyle by lake area and volume, and the 90th percentile of body size, consistent with greater specialization to piscivory, by lake area. The patterns for these three specializations might be explained by greater stability and availability of their prey bases (zooplankton, profundal benthos and prey fish (stickleback and small charr), respectively) with increasing ecological opportunity.
In contrast, none of our proxies of ecological opportunity was able to predict morphological specialization towards littoral benthic lifestyle. It is possible that the morphology associated with this lifestyle is also that of the most generalist ecology. Alternatively, given that anadromous populations are most closely related to littoral benthic species (figure 2; electronic supplementary material, figure S7), gene flow with them may prevent local adaptation to the littoral benthic niche.
(c). Evidence for morphological expansion through speciation
We find that assemblage-wide morphospace sharply increases when multiple genetic clusters are present, but not when multiple ecomorphs within a single genetic cluster are present. This suggests that large morphospace expansion is constrained by lack of speciation. To quantitatively test this hypothesis in charr, replicates from different drainage systems will be needed.
Previous studies found smaller morphological ranges of single species assemblages of stickleback and whitefish than of multispecies assemblages [1,49,50], speaking towards niche expansion rather than partitioning of ancestral variation during diversification. Together with our results, this would suggest that speciation plays a crucial role for morphological expansion in adaptive radiations. Phylogenetic studies often find that trait evolution continues [51], whereas lineage diversification slows down in adaptive radiations [1,52], which has been interpreted as evidence for speciation being necessary for adaptive divergence to evolve or persist during adaptive radiation [3]. Hence, speciation may be key for morphological expansion both early in the process of adaptive radiations and in its continuation.
(d). Two cryptic sympatric piscivorous charr species in the largest lakes
The piscivorous ecomorph in the two largest lakes, Tasersuaq and Saqqaata Tasia, consisted of two strongly genetically differentiated species (FST = 0.12 and 0.14, respectively). One was confined to these two lakes, the other occurred in all but the two smallest, most isolated lakes. The latter species was caught slightly shallower than the former, suggesting that this species may not expand its depth range even in deep lakes. The subtle differences in morphology in Tasersuaq, but not in Saqqaata Tasia, and vice versa for stable isotopes, suggest that the two piscivorous species are morphologically cryptic and may not perform obvious ecological niche partitioning detectable by our methods.
Sympatric occurrence of two species belonging to the same ecomorph is not uncommon in charr, but they are usually morphologically distinct and occupy different habitats (e.g. Thingvallavatn [20]; several Russian lakes, reviewed in [19]). To our knowledge, there is no precedence of the extent of morphological and ecological similarity between sympatric charr species despite strong genetic divergence as we show in Tasersuaq/Saqqaata Tasia. However, convergence in niche and associated traits is widespread in large adaptive radiations (e.g. cichlids [53]; Anolis lizards [54]), and is expected from theory [55]. Whether there is a threshold species number when convergence starts remains to be investigated, and probably depends on ecological opportunity, historical contingency, evolvability and time.
(e). Community assembly of species-rich radiations
Compared with some adaptive radiations on geographically isolated archipelagos (e.g. Galapagos, Hawaii), Eqaluit is, like the African Great Lakes, geographically not strongly isolated, but it lies in a region where very few fish species can rapidly colonize freshwater lakes. Accordingly, these tropical archipelagos have isolation-filtered faunas, whereas East African and especially Greenlandic lakes have ecology-filtered faunas. While archipelago radiations mainly accumulated species through allopatric between-island speciation (Darwin's finches [56]; Tetragnatha spiders [57]) and tropical lake radiations through sympatric speciation (East African cichlids [58]), allopatric speciation between drainages and between lakes within drainages, as well as sympatric speciation within lakes, seem plausible for Greenlandic charr. Theoretical models found that dynamic mosaic landscapes with periods of isolation (allowing divergent adaptation) and periods of reconnection (allowing secondary contact, hybrid speciation and reinforcement) generated highest species diversity [59]. The geographical setting of loosely connected drainage systems comprising multiple lakes may predispose the Greenlandic archipelago of lakes to rapidly accumulate exceptionally high sympatric charr species diversity. Given the multitude of lakes along the Greenland coast, this process may have generated hundreds of charr species that remain to be discovered.
Supplementary Material
Acknowledgements
We would like to thank Diego Dagani, Florin Kunz, Michael Haeberli and Philip Dermond for their great help during fieldwork in Greenland. We are thankful to the local sheep farmers of the northern Vatnahverfi Peninsula, especially Isak Lund, Leif Kunuk, Sara and Niels Lund, and Siiku Motzfeldt, for their logistic support during fieldwork. Special thanks go to Corinne Schmid for genotyping all charr from 2014. Thanks to all members of the fish ecology and evolution group of Eawag for discussions.
Ethics
This research was done under research permits no. G-14-028 (J.B.) and G-16-040 (J.B.) from the Naalakkersuisut (Government of Greenland).
Data accessibility
Morphological, ecological and genetic data are available in the Dryad Digital Repository: https://doi.org/10.5061/dryad.q2r87q9 [60].
Authors' contributions
C.J.D. carried out fieldwork, participated in the design of the study, identified ecomorphs, analysed the data with input from A.K.K., J.B. and O.S., and wrote the manuscript together with O.S. and J.B. A.K.K. carried out fieldwork, identified ecomorphs, collected morphological, genetic and isotopic data, contributed to conceptual design and helped drafting the manuscript. J.W. generated stable isotopic data and contributed to conceptual design. J.B. made the sampling design, carried out fieldwork, participated in the design of the study and helped drafting the manuscript. O.S. participated in the design of the study, and wrote the manuscript together with C.J.D. and J.B.
Competing interests
We declare we have no competing interests.
Funding
We thank EAWAG and University of Bern for funding this project.
References
- 1.Schluter D. 2000. The ecology of adaptive radiation. Oxford, UK: Oxford University Press. [Google Scholar]
- 2.Simpson GG. 1949. Tempo and mode in evolution. New York, NY: Columbia University Press. [Google Scholar]
- 3.Yoder JB, et al. 2010. Ecological opportunity and the origin of adaptive radiations. J. Evol. Biol. 23, 1581–1596. ( 10.1111/j.1420-9101.2010.02029.x) [DOI] [PubMed] [Google Scholar]
- 4.Stroud JT, Losos JB. 2016. Ecological opportunity and adaptive radiation. Annu. Rev. Ecol. Evol. Syst. 47, 507–532. ( 10.1146/annurev-ecolsys-121415-032254) [DOI] [Google Scholar]
- 5.Gavrilets S, Vose A. 2005. Dynamic patterns of adaptive radiation. Proc. Natl Acad. Sci. USA 102, 18 040–18 045. ( 10.1073/pnas.0506330102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kagawa K, Takimoto G. 2018. Hybridization can promote adaptive radiation by means of transgressive segregation. Ecol. Lett. 21, 264–274. ( 10.1111/ele.12891) [DOI] [PubMed] [Google Scholar]
- 7.Wagner CE, Harmon LJ, Seehausen O. 2012. Ecological opportunity and sexual selection together predict adaptive radiation. Nature 487, 366–369. ( 10.1038/nature11144) [DOI] [PubMed] [Google Scholar]
- 8.Losos JB, Schluter D. 2000. Analysis of an evolutionary species-area relationship. Nature 408, 847–850. ( 10.1038/35048558) [DOI] [PubMed] [Google Scholar]
- 9.Rosindell J, Phillimore AB. 2011. A unified model of island biogeography sheds light on the zone of radiation. Ecol. Lett. 14, 552–560. ( 10.1111/j.1461-0248.2011.01617.x) [DOI] [PubMed] [Google Scholar]
- 10.Van Valen L. 1965. Morphological variation and width of ecological niche. Am. Nat. 99, 377–389. ( 10.1086/282379) [DOI] [Google Scholar]
- 11.Parent CE, Crespi BJ. 2009. Ecological opportunity in adaptive radiation of Galàpagos endemic land snails. Am. Nat. 174, 898–905. ( 10.1086/646604) [DOI] [PubMed] [Google Scholar]
- 12.Moser FN, van Rijssel JC, Mwaiko S, Meier JI, Ngatunga B, Seehausen O. 2018. The onset of ecological diversification 50 years after colonization of a crater lake by haplochromine cichlid fishes. Proc. R. Soc. B 285, 20180171 ( 10.1098/rspb.2018.0171) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Recknagel H, Elmer KR, Meyer A. 2014. Crater lake habitat predicts morphological diversity in adaptive radiations of cichlid fishes. Evolution 68, 2145–2155. ( 10.1111/evo.12412) [DOI] [PubMed] [Google Scholar]
- 14.Nosil P, Reimchen TE. 2005. Ecological opportunity and levels of morphological variance within freshwater stickleback populations. Biol. J. Linn. Soc. 86, 297–308. ( 10.1111/j.1095-8312.2005.00517.x) [DOI] [Google Scholar]
- 15.Joyce DA, Lunt DH, Bills R, Turner GF, Katongo C, Duftner N, Sturmbauer C, Seehausen O. 2005. An extant cichlid fish radiation emerged in an extinct Pleistocene lake. Nature 435, 90–95. ( 10.1038/nature03489) [DOI] [PubMed] [Google Scholar]
- 16.Schluter D, Grant PR. 1984. Determinants of morphological patterns in communities of Darwin's finches. Am. Nat. 123, 175–196. ( 10.1086/284196) [DOI] [Google Scholar]
- 17.Jonsson B, Jonsson N. 2001. Polymorphism and speciation in Arctic charr. J. Fish Biol. 58, 605–638. ( 10.1111/j.1095-8649.2001.tb00518.x) [DOI] [Google Scholar]
- 18.Klemetsen A. 2010. The charr problem revisited: exceptional phenotypic plasticity promotes ecological speciation in postglacial lakes. Freshw. Rev. 1, 49–74. ( 10.1608/FRJ-3.1.3) [DOI] [Google Scholar]
- 19.Markevich GN, Esin EV. 2018. Evolution of the charrs, genus Salvelinus (Salmonidae). 2. Sympatric inner-lake diversification (ecological peculiarities and evolutionary mechanism illustrated by different groups of fish). J. Ichthyol. 58, 333–352. ( 10.1134/s0032945218030074) [DOI] [Google Scholar]
- 20.Sandlund OT, et al. 1992. The Arctic charr Salvelinus alpinus in Thingvallavatn. Oikos 64, 305–351. [Google Scholar]
- 21.Kapralova KH, Morrissey MB, Kristjánsson BK, Ólafsdóttir GÁ, Snorrason SS, Ferguson MM. 2011. Evolution of adaptive diversity and genetic connectivity in Arctic charr (Salvelinus alpinus) in Iceland. Heredity 106, 472–487. ( 10.1038/hdy.2010.161) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Muir AM, Hansen MJ, Bronte CR, Krueger CC. 2015. If Arctic charr Salvelinus alpinus is ‘the most diverse vertebrate’, what is the lake charr Salvelinus namaycush? Fish Fish. 17, 1194–1207. ( 10.1111/faf.12114) [DOI] [Google Scholar]
- 23.Markevich G, Esin EV, Anisimova L. 2017. Basic description and some notes on the evolution of seven sympatric morphs of Dolly Varden Salvelinus malma from the Lake Kronotskoe basin. Ecol. Evol. 8, 2554–2567. ( 10.1002/ece3.3806) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Recknagel H, Hooker OE, Adams CE, Elmer KR. 2017. Ecosystem size predicts eco-morphological variability in a postglacial diversification. Ecol. Evol. 7, 5560–5570. ( 10.1002/ece3.3013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Young NE, Briner JP. 2015. Holocene evolution of the western Greenland ice sheet: assessing geophysical ice-sheet models with geological reconstructions of ice-margin change. Quat. Sci. Rev. 114, 1–17. ( 10.1016/j.quascirev.2015.01.018) [DOI] [Google Scholar]
- 26.Møller PR, Nielsen J, Knudsen SW, Poulsen JY, Sünksen K, Jørgensen OA. 2010. A checklist of the fish fauna of Greenland waters. Zootaxa 2378, 1. [Google Scholar]
- 27.Brunner PC, Douglas MR, Osinov AO, Wilson CC, Bernatchez L. 2001. Holarctic phylogeography of Arctic charr (Salvelinus alpinus L.) inferred from mitochondrial DNA sequences. Evolution 55, 573–586. ( 10.1111/j.0014-3820.2001.tb00790.x) [DOI] [PubMed] [Google Scholar]
- 28.Riget FF, Jeppesen E, Landkildehus F, Lauridsen TL, Geertz-Hansen P, Christoffersen K, Sparholt H. 2000. Landlocked Arctic charr (Salvelinus alpinus) population structure and lake morphometry in Greenland—is there a connection? Polar Biol. 23, 550–558. ( 10.1007/s003000000120) [DOI] [Google Scholar]
- 29.Riget FF, Nygaard KH, Christensen B. 1986. Population structure, ecological segregation, and reproduction in a population of Arctic char (Salvelinus alpinus) from Lake Tasersuaq, Greenland. Can. J. Fish. Aquat. Sci. 43, 985–992. ( 10.1139/f86-121) [DOI] [Google Scholar]
- 30.Sparholt H. 1985. The population, survival, growth, reproduction and food of Arctic charr, Salvelinus alpinus (L.), in four unexploited lakes in Greenland. J. Fish Biol. 26, 313–330. ( 10.1111/j.1095-8649.1985.tb04270.x) [DOI] [Google Scholar]
- 31.Loewen TN, Gillis DM, Tallman RF. 2009. Ecological niche specialisation inferred from morphological variation and otolith strontium of Arctic charr Salvelinus alpinus (L.), found within open lake systems of Southern Baffin Island, Nunavut, Canada. J. Fish Biol. 75, 1473–1495. ( 10.1111/j.1095-8649.2009.02394.x) [DOI] [PubMed] [Google Scholar]
- 32.Williams EE. 1972. The origin of faunas. Evolution of lizard congeners in a complex island fauna: a trial analysis. Evol. Biol. 6, 47–89. ( 10.1007/978-1-4684-9063-3_3) [DOI] [Google Scholar]
- 33.Adams CE, Huntingford FA. 2002. The functional significance of inherited differences in feeding morphology in a sympatric polymorphic population of Arctic charr. Evol. Ecol. 16, 15–25. ( 10.1023/a:1016014124038) [DOI] [Google Scholar]
- 34.Hennig C.2019. fps: flexible procedures for clustering. R package v. 2.1-11.2. See https://CRAN.R-project.org/package=fpc .
- 35.Kaeuffer R, Peichel CL, Bolnick DI, Hendry AP. 2012. Parallel and nonparallel aspects of ecological, phenotypic, and genetic divergence across replicate population pairs of lake and stream stickleback. Evolution 66, 402–418. ( 10.1111/j.1558-5646.2011.01440.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pritchard JK, Stephens M, Donnelly P. 2000. Inference of population structure from multilocus genotype data. Genetics 155, 945–959. ( 10.1111/j.1471-8286.2007.01758.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Evanno G, Regnaut S, Goudet J. 2005. Detecting the number of clusters of individuals using the software structure: a simulation study. Mol. Ecol. 14, 2611–2620. ( 10.1111/j.1365-294x.2005.02553.x) [DOI] [PubMed] [Google Scholar]
- 38.Earl DA, vonHoldt BM. 2012. STRUCTURE HARVESTER: a website and program for visualizing STRUCTURE output and implementing the Evanno method. Conserv. Genet. Resour. 4, 359–361. ( 10.1007/s12686-011-9548-7) [DOI] [Google Scholar]
- 39.Excoffier L, Lischer HEL. 2010. Arlequin suite v. 3.5: a new series of programs to perform population genetics analyses under Linux and Windows. Mol. Ecol. Resour. 10, 564–567. ( 10.1111/j.1755-0998.2010.02847.x) [DOI] [PubMed] [Google Scholar]
- 40.Lischer HEL, Excoffier L. 2012. PGDSpider: an automated data conversion tool for connecting population genetics and genomics programs. Bioinformatics 28, 298–299. ( 10.1093/bioinformatics/btr642) [DOI] [PubMed] [Google Scholar]
- 41.Holm S. 1979. A simple sequentially rejective multiple test procedure. Scand. J. Stat. 6, 65–70. [Google Scholar]
- 42.Jombart T. 2008. adegenet: a R package for the multivariate analysis of genetic markers. Bioinformatics 24, 1403–1405. ( 10.1093/bioinformatics/btn129) [DOI] [PubMed] [Google Scholar]
- 43.R Core Team. 2015. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 44.Edwards DL, Knowles LL. 2014. Species detection and individual assignment in species delimitation: can integrative data increase efficacy? Proc. R. Soc. B 281, 20132765 ( 10.1098/rspb.2013.2765) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jensen H, Kiljunen M, Knudsen R, Amundsen P-A. 2017. Resource partitioning in food, space and time between Arctic charr (Salvelinus alpinus), brown trout (Salmo trutta) and European whitefish (Coregonus lavaretus) at the southern edge of their continuous coexistence. PLoS ONE 12, e0170582 ( 10.1371/journal.pone.0170582) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Seehausen O. 2004. Hybridization and adaptive radiation. Trends Ecol. Evol. 19, 198–207. ( 10.1016/j.tree.2004.01.003) [DOI] [PubMed] [Google Scholar]
- 47.Gordeeva NV, Alekseyev SS, Matveev AN, Samusenok VP. 2014. Parallel evolutionary divergence in Arctic char Salvelinus alpinus complex from Transbaikalia: variation in differentiation degree and segregation of genetic diversity among sympatric forms. Can. J. Fish. Aquat. Sci. 72, 96–115. ( 10.1139/cjfas-2014-0014) [DOI] [Google Scholar]
- 48.Alekseyev SS, Bajno R, Gordeeva NV, Reist JD, Power M, Kirillov AF, Samusenok VP, Matveev AN. 2009. Phylogeography and sympatric differentiation of the Arctic charr Salvelinus alpinus (L.) complex in Siberia as revealed by mtDNA sequence analysis. J. Fish Biol. 75, 368–392. ( 10.1111/j.1095-8649.2009.02331.x) [DOI] [PubMed] [Google Scholar]
- 49.Harrod C, Mallela J, Kahilainen K. 2010. Phenotype-environment correlations in a putative whitefish adaptive radiation. J. Anim. Ecol. 79, 1057–1068. ( 10.1111/j.1365-2656.2010.01702.x) [DOI] [PubMed] [Google Scholar]
- 50.Vonlanthen P, et al. 2012. Eutrophication causes speciation reversal in whitefish adaptive radiations. Nature 482, 357–363. ( 10.1038/nature10824) [DOI] [PubMed] [Google Scholar]
- 51.Harmon LJ, et al. 2010. Early bursts of body size and shape evolution are rare in comparative data. Evolution 64, 2385–2396. ( 10.1111/j.1558-5646.2010.01025.x) [DOI] [PubMed] [Google Scholar]
- 52.Harmon LJ, Schulte JA II, Larson A, Losos JB. 2003. Tempo and mode of evolutionary radiation in iguanian lizards. Science 301, 961–964. ( 10.1126/science.1084786) [DOI] [PubMed] [Google Scholar]
- 53.Muschick M, Indermaur A, Salzburger W. 2012. Convergent evolution within an adaptive radiation of cichlid fishes. Curr. Biol. 22, 2362–2368. ( 10.1016/j.cub.2012.10.048) [DOI] [PubMed] [Google Scholar]
- 54.Mahler DL, Ingram T, Revell LJ, Losos JB. 2013. Exceptional convergence on the macroevolutionary landscape in island lizard radiations. Science 341, 292–295. ( 10.1126/science.1232392) [DOI] [PubMed] [Google Scholar]
- 55.Scheffer M, van Nes EH. 2006. Self-organized similarity, the evolutionary emergence of groups of similar species. Proc. Natl Acad. Sci. USA 103, 6230–6235. ( 10.1073/pnas.0508024103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Grant PR, Grant BR. 2008. How and why species multiply: the radiation of Darwin's finches . Princeton, NJ: Princeton University Press. [Google Scholar]
- 57.Gillespie RG. 2005. Geographical context of speciation in a radiation of Hawaiian Tetragnatha spiders. J. Arachnol. 33, 313–322. ( 10.1636/05-15.1) [DOI] [Google Scholar]
- 58.Seehausen O, Magalhaes IS. 2010. Geographical mode and evolutionary mechanism of ecological speciation in cichlid fish. In In search of the causes of evolution: from field observations to mechanisms (eds Grant PR, Grant BR), pp. 282–308. Princeton, NJ: Princeton University Press. [Google Scholar]
- 59.Aguilee R, Claessen D, Lambert A. 2012. Adaptive radiation driven by the interplay of eco-evolutionary and landscape dynamics. Evolution 67, 1291–1306. ( 10.1111/evo.12008) [DOI] [PubMed] [Google Scholar]
- 60.Doenz CJ, Krähenbühl AK, Walker J, Seehausen O, Brodersen J. 2019. Ecological opportunity shapes a large Arctic charr species radiation. Dryad Digital Repository. ( 10.5061/dryad.q2r87q9) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Doenz CJ, Krähenbühl AK, Walker J, Seehausen O, Brodersen J. 2019. Ecological opportunity shapes a large Arctic charr species radiation. Dryad Digital Repository. ( 10.5061/dryad.q2r87q9) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
Morphological, ecological and genetic data are available in the Dryad Digital Repository: https://doi.org/10.5061/dryad.q2r87q9 [60].