Abstract
Direct venous inoculation of 3.2 × 103 aseptic, purified, cryopreserved, vialed Plasmodium falciparum (Pf) strain NF54 sporozoites, PfSPZ Challenge (NF54), has been used for controlled human malaria infection (CHMI) in the United States, 4 European countries, and 6 African countries. In nonimmune adults, this results in 100% infection rates. We conducted a double-blind, randomized, dose-escalation study to assess the infectivity of the 7G8 clone of Pf (PfSPZ Challenge [7G8]). Results showed dose-dependent infectivity from 43% for 8 × 102 PfSPZ to 100% for 4.8 × 103 PfSPZ. PfSPZ Challenge (7G8) will allow for more complete assessment by CHMI of antimalarial vaccines and drugs.
Keywords: controlled human malaria infection, cryopreservation, humans, Plasmodium falciparum
Infectivity of aseptic, cryopreserved, vialed Plasmodium falciparum 7G8 clone sporozoites is dose-dependent and equivalent to standard NF54 strain sporozoites. Vialed sporozoites from field-adapted strains facilitate controlled human malaria infection studies in endemic and nonendemic areas.
According to World Health Organization (WHO) estimates for 2017, 435 000 people died from malaria, almost all from Plasmodium falciparum (Pf), and an estimated 219 million malaria cases occurred worldwide. Despite recent advances in malaria control, reductions in deaths and cases have stalled since 2015. Development of a vaccine to prevent and ultimately eliminate malaria has been a priority and may now be more urgent because affected communities need new tools to reduce malaria’s impact.
Controlled human malaria infection (CHMI) is a safe and reproducible method for infecting individuals with Pf. Researchers have used CHMI for decades to assess malaria vaccines and drugs for efficacy, often to justify further clinical testing in endemic areas. The most advanced malaria vaccine candidate to date, RTS,S/AS01, relied on CHMI studies to optimize dosing, formulation, and regimen. Recognizing CHMI’s important role in malaria vaccine development, the WHO recently prioritized CHMI optimization [1].
Until approximately a decade ago, CHMI with Pf sporozoites was done by exposure to the bites of 5 Pf-infected, insectary-raised mosquitoes to reliably induce malaria. Disadvantages of CHMI by mosquito bite include requirements for an insectary and entomology expertise, precise timing of mosquito rearing to coordinate with vaccine and drug dosing, and theoretical risk of participant exposure to microorganisms potentially carried by laboratory-raised mosquitoes. Sanaria’s PfSPZ Challenge, composed of aseptic, purified, cryopreserved, vialed injectable PfSPZ was developed to provide an alternative approach to CHMI that would eliminate most of these disadvantages [2]. PfSPZ Challenge facilitated the first CHMI studies at several sites in 6 African countries [3–6].
To date, all CHMIs by parenteral PfSPZ administration used PfSPZ Challenge (NF54), a West African Pf strain [7]. When administered by direct venous inoculation (DVI), 3.2 × 103 PfSPZ infected 78 of 78 malaria-naive adults in the United States and Europe (S. L. H., unpublished observations, 2017–2019) [3, 5, 8], and it has been used in CHMI of 532 volunteers in Equatorial Guinea, Gabon, The Gambia, Kenya, Mali, and Tanzania. Although PfSPZ Challenge (NF54) represents a significant advance, development and characterization of additional strains from diverse geographic areas would help to assess efficacy against heterogeneous parasites in nature. PfSPZ Challenge (7G8), a clone of a Brazilian isolate [9], was chosen for injectable administration because it is culture-adapted and has been used in CHMI by mosquito bite. The 7G8 clone has 22 056 single-nucleotide polymorphisms genome-wide compared with NF54 [10].
This clinical trial assessed safety and infectivity of PfSPZ Challenge (7G8) compared with PfSPZ Challenge (NF54). We aimed to optimize PfSPZ Challenge (7G8) dosing for CHMI and compare this to the standard 3.2 × 103 PfSPZ dose of PfSPZ Challenge (NF54) using the same controlled conditions, because time to first patency and infectivity may differ by strain. These data are essential to confirm PfSPZ Challenge (7G8) infectivity and to ensure safety of future participants who may receive the 7G8 product. The study’s primary objective was to assess safety and reactogenicity of PfSPZ Challenge (7G8) and PfSPZ Challenge (NF54) administered by DVI to malaria-naive adults. Secondary objectives included assessment of infectivity, and time to parasite patency by quantitative polymerase chain reaction (PCR), for 4 increasing doses of PfSPZ Challenge (7G8) by DVI in comparison with the established dose of PfSPZ Challenge (NF54). Primary outcomes included (1) local and systemic solicited and unsolicited reactogenicity for 7 days after PfSPZ Challenge administration and (2) serious adverse events for 56 days. Secondary outcomes included percentage of infectivity of each dose regimen and time to Pf asexual parasitemia after DVI.
METHODS
We conducted a single-center, randomized, controlled human study to assess CHMI with Sanaria’s PfSPZ Challenge (7G8) administered by DVI to malaria-naive adults. Adults from the greater Baltimore, Maryland area were screened following clinical trial educational meetings and informed consent. Screening activities included a study comprehension quiz, medical and medication history, 12-lead electrocardiogram, vital signs, physical exam, and laboratory testing (complete blood count, hemoglobin electrophoresis, random serum glucose, serum creatinine, serum alanine aminotransferase, serum human immunodeficiency virus, serum hepatitis B, serum hepatitis C, serum pregnancy for women of childbearing potential, and urine blood and protein).
Participants meeting inclusion and exclusion criteria (Supplementary Table 1) were enrolled on study day 1 and randomly assigned via an online enrollment module to 1 of 5 groups in a 7:7:9:2:5 ratio to receive PfSPZ Challenge (7G8 or NF54) as follows: (1) 8 × 102 PfSPZ (7G8), (2) 1.6 × 103 PfSPZ (7G8), (3) 3.2 × 103 PfSPZ (7G8), (4) 4.8 × 103 PfSPZ (7G8), and (5) 3.2 × 103 PfSPZ (NF54) (Table 1 and Supplementary Figure 1). The PfSPZ Challenge lots used had similar potency and viability in vitro (Supplementary Table 2). Participants and clinical and laboratory investigators were blinded to group allocation. Baseline venous blood was collected for safety laboratory tests (hemoglobin, white blood cells [WBCs], serum creatinine, serum alanine aminotransferase) and for serology assays. The unblinded research pharmacist prepared each study product by partially submerging PfSPZ cryovials for 30 seconds in a 37ºC water bath and mixing with phosphate-buffered saline containing human serum albumin diluent to a volume of 0.5 mL. Study product appeared clear for all groups. Within 30 minutes of PfSPZ thawing, blinded staff inoculated participants over a few seconds with a 25-gauge needle by DVI with preference for the antecubital vein. Thirty minutes after injection, participants were assessed for vital signs, solicited and unsolicited local and systemic reactogenicity (Supplementary Tables 3 and 4), and given a 4-day symptom diary. Participants were instructed to immediately call investigators for fever or severe reactions, and they were then discharged.
Table 1.
Summary of Study Results by Treatment Group
| Characteristic | 8 × 102 7G8 (N = 7) | 1.6 × 102 7G8 (N = 7) | 3.2 × 103 7G8 (N = 9) | 4.8 × 103 7G8 (N = 2) | 3.2 × 103 Nf54 (N = 5) | All Participants (N = 30) |
|---|---|---|---|---|---|---|
| Male gender (%) | 3 (43) | 5 (71) | 3 (33) | 1 (50) | 3 (60) | 15 (50) |
| Mean age (years) | 30.3 | 31.9 | 33.7 | 33.0 | 33.8 | 32.4 |
| Mean weight (kg) | 85.1 | 95.8 | 82.9 | 81.5 | 67.1 | 83.7 |
| Mean body mass index (kg/m2) | 29.3 | 29.4 | 28.8 | 26.8 | 23.0 | 28.0 |
| Number experiencing any solicited adverse events (%) | 5 (71) | 4 (57) | 5 (56) | 2 (100) | 4 (80) | 20 (67) |
| Number experiencing solicited adverse events with maximum severity of mild (%) | 3 (43) | 3 (43) | 3 (33) | 2 (100) | 2 (40) | 13 (43) |
| Number experiencing solicited adverse events with maximum severity of moderate (%) | 2 (29) | 0 (0) | 2 (22) | 0 (0) | 2 (40) | 6 (20) |
| Number experiencing solicited adverse events with maximum severity of severe (%) | 0 (0) | 1 (14) | 0 (0) | 0 (0) | 0 (0) | 1 (3) |
| Number experiencing solicited systemic adverse events (%) | 4 (57) | 2 (29) | 4 (44) | 2 (100) | 3 (60) | 15 (50) |
| Number experiencing solicited local adverse events (%) | 2 (29) | 2 (29) | 3 (33) | 2 (100) | 3 (60) | 12 (40) |
| Number experiencing malaria symptoms (%) | 1 (14) | 3 (43) | 4 (44) | 2 (100) | 3 (60) | 13 (43) |
| Number Plasmodium falciparum positivea | 3 | 4 | 8 | 2 | 5 | 22 |
| Percentage P falciparum positive (95% confidence interval)a | 43 (10–82) | 57 (18–90) | 89 (52–100) | 100 (16–100) | 100 (48–100) | 73 (54–88) |
| Median time to first P falciparum positive PCR testing in days (Max/Min) | 9 (11/9) | 9 (11/9) | 9 (11/9) | 9 (9/9) | 9 (13/8) | 9 (13/8) |
| Median time to second P falciparum positive PCR testing in days (Max/Min) | 11 (12/11) | 11 (12/11) | 10 (12/10) | 10 (10/10) | 11 (14/9) | 11 (14/9) |
Abbreviations: Max, maximum; Min, minimum; PCR, polymerase chain reaction.
aAll participants who had an initial PCR-positive test also had a subsequent PCR-positive test.
Participants returned to the study clinic 5 days after injection to begin daily monitoring for patent Pf infection (study days 6–19). During these visits, study staff documented participant vital signs, solicited and unsolicited local and systemic reactogenicity (days 1–7 postinjection only), and any spontaneous adverse events with a targeted physical exam as indicated. Study staff collected a 2-mL blood sample for Pf diagnostics using ultrasensitive PCR (uPCR) testing for 18s ribonucleic acid and deoxyribonucleic acid with a sensitivity of 16 parasites/mL using a 50-µL sample [11]. Participants testing positive for Pf (2 positive uPCR tests) provided an additional blood sample for safety laboratory tests, underwent directly observed antimalarial therapy with atovaquone/proguanil 1000/400 mg once daily for 3 days, and returned on study day 29. Participants testing negative through study day 19 continued every other day monitoring for patent Pf infection, vital signs, and adverse events on study days 21, 23, 25, and 27. Four weeks after inoculation on study day 29, study staff (1) collected venous blood for serology assays and malaria diagnostics, (2) recorded vital signs, adverse events, and concomitant medications, and (3) administered first-dose atovaquone/proguanil 1000/400 mg to participants who remained Pf negative. Participants who remained malaria negative completed 2 additional atovaquone/proguanil 1000/400 mg daily doses at home. A final study visit occurred 8 weeks after inoculation on study day 57, when study staff documented vital signs and recorded interim serious adverse events and concomitant medications.
The study was approved by the University of Maryland’s Institutional Review Board and performed according to International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use Good Clinical Practice guidelines and the Declaration of Helsinki. The trial was registered on ClinicalTrials.gov, Identifier NCT02780154, on May 23, 2016.
RESULTS
Sixty participants were assessed for eligibility. Thirty of these were excluded for out-of-range laboratory values (n = 19) or other reasons (n = 5), and some were eligible but elected to not participate (n = 6). Thirty participants were enrolled and randomized to 1 of 5 treatment arms, and all 30 provided data for safety, reactogenicity, and infectivity analysis endpoints. Participant baseline characteristics reflect the Baltimore adult population (Table 1).
Safety and Reactogenicity
Solicited symptoms were separated into “solicited adverse events”, those occurring during the first 7 days after injection, when any reactogenicity associated with the injected PfSPZ and resulting liver stage parasites should have been manifest, and “malaria symptoms” that were attributed to subsequent malaria infection. Of the 30 participants, 20 (67%) experienced solicited systemic and/or local events, 15 (50%) reported systemic events, and 12 (40%) reported local events. For any solicited symptom, 13 (43%) had a maximum severity of mild, 6 (20%) had a maximum severity of moderate, and 1 (3.3%) had a maximum severity of severe (Table 1). For solicited systemic events, 9 participants (30%) reported a maximum severity of mild, 6 (20%) reported a maximum severity of moderate, and 1 (3.3%) reported a severe solicited systemic event. All solicited local events reported were of mild severity. The most common solicited adverse event was headache, reported by 12 participants and had maximum severity of moderate, except for 1 participant who had severe headache related to food poisoning. Thirteen participants (43%) experienced malaria symptoms. None of the 29 documented unsolicited adverse events were deemed related to study product. No serious adverse events occurred.
After study product administration and before malaria diagnosis, the only vital sign abnormality was mild bradycardia in 5 participants. This was documented in all groups except the 1.6 × 103 7G8 group, and it was deemed related to athletic conditioning.
Safety hematology and biochemistry testing on the day of malaria positivity revealed only 3 participants with graded laboratory abnormalities, all mild and not related to study product. One participant in the 8 × 102 7G8 group had elevated WBC due to a viral illness. One participant in the 3.2 × 103 Nf54 group had low WBC related to baseline benign leukopenia. Another participant in the 8 × 102 7G8 group had elevated alanine aminotransferase due to malaria illness.
Infectivity
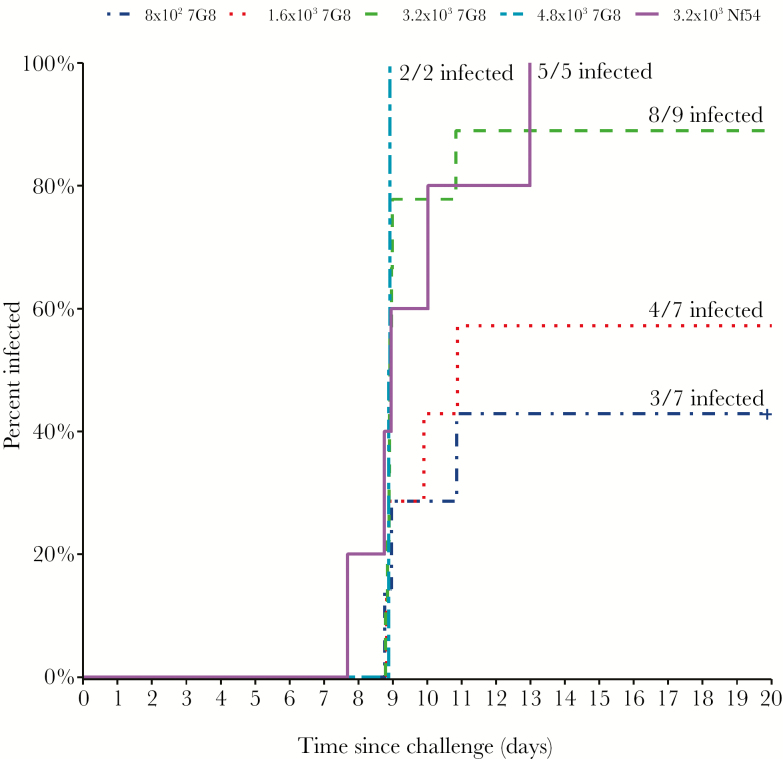
Twenty-two of the 30 participants (73%) were successfully infected with Pf. Infectivity of PfSPZ Challenge (7G8) was dose-dependent: 43% (8 × 102 PfSPZ; n = 7; 95% confidence interval [CI], 10–82); 57% (1.6 × 103 PfSPZ; n = 7; 95% CI, 18–90); 89% (3.2 × 103 PfSPZ; n = 9; 95% CI, 52–100); and 100% (4.8 × 103 PfSPZ; n = 2; 95% CI, 48–100) (Table 1); Pearson correlation coefficient = 0.98. Time-to-infectivity did not differ among the different doses of PfSPZ Challenge (7G8 and NF54) (Table 1 and Figure 1). All 5 participants who received PfSPZ Challenge (NF54) developed Pf parasitemia.
Figure 1.
Time to first ultrasensitive polymerase chain reaction positive testing by study group.
DISCUSSION
Participants infected with different PfSPZ Challenge (7G8) doses and the standard 3.2 × 103 PfSPZ dose of PfSPZ Challenge (Nf54) experienced minimal reactogenicity to injections. Most participants had either no reactogenicity events or only mild events in the 7 days after injection, and no increased reactogenicity occurred in participants receiving the highest doses of PfSPZ. No participant experienced clinically significant laboratory abnormalities or severe malaria. The use of infectious, aseptic, purified, cryopreserved PfSPZ by DVI showed no safety signal and was well tolerated.
Participants were successfully infected with Pf via DVI of aseptic, purified, cryopreserved PfSPZ. PfSPZ Challenge (7G8) infectivity was dose dependent, achieving 89% (8 of 9) infectivity for the 3.2 × 103 PfSPZ dose of 7G8. This dose response was comparable to a study of PfSPZ Challenge (NF54) that showed 7 of 9 (78%) infected with 8 × 102 PfSPZ and 9 of 9 (100%) infected with 3.2 × 103 PfSPZ [5]. The 1 uninfected participant in the 3.2 × 103 PfSPZ 7G8 group was undergoing intense physical endurance training, which has been associated with resistance to CHMI (B. Mordmüller, personal written communication, May 2019).
Time-to-first parasitemia by uPCR did not differ among doses tested, although sample size was small for each group. All 5 participants who received PfSPZ Challenge (NF54) developed Pf parasitemia after a median of 11 days, and time-to-parasitemia for PfSPZ Challenge (7G8) recipients was similar. Time-to parasitemia in this study was comparable to results in controls in a vaccine trial CHMI by mosquito bites with Pf 3D7, a clone of NF54, and Pf 7G8, using the same PCR diagnostic protocol at our institution [12]. In Germany, the time-to-parasitemia by thick blood smear has been similar [2].
Similar to Pf NF54, the Pf 7G8 clone administered by DVI was appropriately infectious for use in future CHMI trials, allowing for testing vaccines and therapeutics against multiple strains. Additional Pf clones have been culture-adapted for CHMI, including NF135.C10 and NF166.C8 [13]. Additional strains may be needed for early testing of malaria vaccines to determine whether they protect against nonhomologous parasites found in nature [14].
CONCLUSIONS
As age, background immunity, nutritional status, and other factors may influence malaria vaccine efficacy, malaria vaccines must also be tested in endemic areas. Over the last 5 years, PfSPZ Challenge by DVI facilitated clinical testing in malaria-endemic areas, including Equatorial Guinea, Gabon, The Gambia, Kenya, Mali, and Tanzania [4, 6, 8, 15] (S. L. H., unpublished observations, 2016–2019). Future CHMI studies may use additional field-adapted strains to support rigorous testing in any geographic location.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Figure 1. Disposition of participants flow chart.
Notes
Acknowledgments. We thank the volunteers from Baltimore, Maryland who participated in this trial. The Division of Microbiology and Infectious Diseases at the National Institute of Allergy and Infectious Diseases, National Institutes of Health (NIH) offered invaluable support and advice. We especially thank the following: Karen Kotloff, the site Principal Investigator for the University of Maryland NIH Vaccine and Treatment Evaluation Unit; Faith Pa’ahana-Brown, Lisa Chrisley, and Aly Kwon for study coordination and regulatory oversight; Brenda Dorsey for Quality Assurance; Panagiota Komninou for data entry; the entire Sanaria Manufacturing team for production of PfSPZ Challenge; and the Sanaria Quality, Regulatory, and Clinical teams for their support. The Emmes Corporation provided database and statistical support.
Financial support. This work was funded in whole or in part with Federal funds from the following: National Institutes of Allergy and Infectious Diseases (NIAID), National Institutes of Health (NIH), Department of Health and Human Services, under Contract HSN272201300022I, Principal Investigator Karen Kotloff, Vaccine and Treatment Evaluation Units; NIH/NIAID Contract HHSN272201500002C, Principal Investigator Marian Ewell, Emmes; and NIAID, NIH Phase II Small Business Innovation Research 2R44AI058375-06A1, Principal Investigator S. L. H. A. A. B. was funded by NIAID Grant K23AI125720.
Potential conflicts of interest. T. L., A. E., A. M., Y. A., T. M., A. G., B. K. L. S., and S. L. H. are employed or contracted by Sanaria, Inc. S. L. H. owns stock in Sanaria Inc. M. B. L., A. A. B., M. A. T., K. S., M. A., B. S., K. E. L., C. V. P., J. K. K., and G. E. P. report NIH funding that supported this research. G. E. P. reports other support for statistical services for products manufactured by Sanaria, and this support did not contribute to this manuscript. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Presented in part: American Society of Tropical Medicine and Hygiene 66th Annual Meeting, November 5–9, 2017, Baltimore, MD.
References
- 1. Laurens MB, Duncan CJ, Epstein JE, et al. A consultation on the optimization of controlled human malaria infection by mosquito bite for evaluation of candidate malaria vaccines. Vaccine 2012; 30:5302–4. [DOI] [PubMed] [Google Scholar]
- 2. Roestenberg M, Bijker EM, Sim BKL, et al. Controlled human malaria infections by intradermal injection of cryopreserved Plasmodium falciparum sporozoites. Am J Trop Med Hyg 2013; 88:5–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gómez-Pérez GP, Legarda A, Muñoz J, et al. Controlled human malaria infection by intramuscular and direct venous inoculation of cryopreserved Plasmodium falciparum sporozoites in malaria-naïve volunteers: effect of injection volume and dose on infectivity rates. Malar J 2015; 14:306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Shekalaghe S, Rutaihwa M, Billingsley PF, et al. Controlled human malaria infection of Tanzanians by intradermal injection of aseptic, purified, cryopreserved Plasmodium falciparum sporozoites. Am J Trop Med Hyg 2014; 91:471–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mordmüller B, Supan C, Sim KL, et al. Direct venous inoculation of Plasmodium falciparum sporozoites for controlled human malaria infection: a dose-finding trial in two centres. Malar J 2015; 14:117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jongo SA, Shekalaghe SA, Church LW, et al. Safety, immunogenicity, and protective efficacy against controlled human malaria infection of Plasmodium falciparum sporozoite vaccine in Tanzanian adults. Am J Trop Med Hyg 2018; 99:338–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Walliker D, Quakyi IA, Wellems TE, et al. Genetic analysis of the human malaria parasite Plasmodium falciparum. Science 1987; 236:1661–6. [DOI] [PubMed] [Google Scholar]
- 8. Dejon-Agobe JC, Ateba-Ngoa U, Lalremruata A, et al. Controlled human malaria infection of healthy lifelong malaria-exposed adults to assess safety, immunogenicity and efficacy of the asexual blood stage malaria vaccine candidate GMZ2. Clin Infect Dis 2018. doi:10.1093/cid/ciy1087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Burkot TR, Williams JL, Schneider I. Infectivity to mosquitoes of Plasmodium falciparum clones grown in vitro from the same isolate. Trans R Soc Trop Med Hyg 1984; 78:339–41. [DOI] [PubMed] [Google Scholar]
- 10. Epstein JE, Paolino KM, Richie TL, et al. Protection against Plasmodium falciparum malaria by PfSPZ vaccine. JCI Insight 2017; 2:e89154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Adams M, Joshi SN, Mbambo G, et al. An ultrasensitive reverse transcription polymerase chain reaction assay to detect asymptomatic low-density Plasmodium falciparum and Plasmodium vivax infections in small volume blood samples. Malar J 2015; 14:520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lyke KE, Ishizuka AS, Berry AA, et al. Attenuated PfSPZ vaccine induces strain-transcending T cells and durable protection against heterologous controlled human malaria infection. Proc Natl Acad Sci U S A 2017; 114:2711–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Langenberg MC, Wammes LJ, McCall MB, et al. Controlled human malaria infection with graded numbers of Plasmodium falciparum NF135.C10- or NF166.C8-infected mosquitoes. Am J Trop Med Hyg 2018; 99:709–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Laurens MB. The promise of a malaria vaccine-are we closer? Annu Rev Microbiol 2018; 72:273–92. [DOI] [PubMed] [Google Scholar]
- 15. Lell B, Mordmüller B, Dejon Agobe JC, et al. Impact of sickle cell trait and naturally acquired immunity on uncomplicated malaria after controlled human malaria infection in adults in Gabon. Am J Trop Med Hyg 2018; 98:508–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.