Abstract
Background
Since the identification of Zika virus (ZIKV) in Brazil in May 2015, the virus has spread throughout the Americas. However, ZIKV burden in the general population in affected countries remains unknown.
Methods
We conducted a general population survey in the different communities of French Guiana through individual interviews and serologic survey during June–October 2017. All serum samples were tested for anti-ZIKV immunoglobulin G antibodies using a recombinant antigen-based SGERPAxMap microsphere immunoassay, and some of them were further evaluated through anti-ZIKV microneutralization tests.
Results
The overall seroprevalence was estimated at 23.3% (95% confidence interval [CI], 20.9%–25.9%) among 2697 participants, varying from 0% to 45.6% according to municipalities. ZIKV circulated in a large majority of French Guiana but not in the most isolated forest areas. The proportion of reported symptomatic Zika infection was estimated at 25.5% (95% CI, 20.3%–31.4%) in individuals who tested positive for ZIKV.
Conclusions
This study described a large-scale representative ZIKV seroprevalence study in South America from the recent 2015–2016 Zika epidemic. Our findings reveal that the majority of the population remains susceptible to ZIKV, which could potentially allow future reintroductions of the virus.
Keywords: Zika virus, seroprevalence study, general population survey, French Guiana
This study provides a consistent overview of a large-scale representative Zika virus (ZIKV) seroprevalence study in French Guiana, revealing that the majority of the population remains susceptible to ZIKV, which could potentially allow future reintroductions of the virus.
Zika virus (ZIKV) is a flavivirus transmitted by mosquitoes, primarily Aedes aegypti, which also transmits dengue, chikungunya, and yellow fever viruses. It was first isolated in 1947 in African forests, where it circulates between nonhuman primates and sylvatic mosquitoes [1]. ZIKV was considered as an emergent virus with few sporadic cases reported in Africa and Asia until 2007, when a major epidemic occurred in Yap, Federated States of Micronesia [2], followed by one in French Polynesia in 2013 [3, 4]. Subsequently, ZIKV continued to spread in the Pacific region [5] and emerged in South America in early 2015 [6, 7]. During these recent outbreaks, the virus was linked to neurological disorders [8, 9], severe congenital abnormalities, and human birth defects [10–14], leading the World Health Organization to declare a Public Health Emergency of International Concern [15]. Several recent studies have also highlighted that ZIKV can be transmitted through sexual contact or from mother to fetus [13, 16, 17]. In French Guiana, a French overseas department of 260 000 inhabitants that is located in Latin America in the Amazonian forest complex, Ae. aegypti mosquitoes have been responsible for several major dengue fever outbreaks [18, 19] and for the chikungunya outbreak in 2014 [20, 21]. Given the risk of congenital complications, the emergence of ZIKV was particularly concerning for its inhabitants as the territory has the highest fertility rate in the Americas (3.5 children per woman) [22]. During the ZIKV epidemic in French Guiana (January–September 2016), approximately 9700 clinical cases (approximately 4% of the population), with 14 congenital abnormalities including 3 instances of microcephaly, were recorded by local health authorities [23]. A territory-wide active monitoring of pregnant women implemented during the first 4 months of the outbreak also showed that 573 of 3050 (19%) enrolled pregnant women had laboratory evidence of ZIKV infection [22]. However, the ZIKV infection burden remains unclear in the general population. In such a context, population-representative seroprevalence studies provide an opportunity to estimate the underlying burden of infection and to assess the potential for future epidemics of ZIKV in the region.
A number of seroprevalence studies have recently been conducted in affected countries and territories in the Americas among specific subgroups of populations and geographical areas. ZIKV seroprevalence was found to be 63% in patient cohorts and university employees in Salvador, Brazil [24], 73% in a cohort of individuals residing in Pau da Lima community in the Salvador [25]. In Bolivia, ZIKV seroprevalence was estimated in blood donors at 39% in Beni, 21.5% in Santa Cruz de la Sierra and close to 0% in three different highland regions (Cochachamba, La Paz and Tarija) [26]. In Managua, Nicaragua, ZIKV seroprevalence was estimated at 36%, 46%, and 56% among participants of pediatric, household, and adult cohort studies, respectively [27]. In Suriname, seroprevalence rates were estimated at 35.1% and 24.5% in patient cohorts recruited from urban areas and 1 remote village, respectively [28]. In the Caribbean sea, ZIKV seroprevalence was estimated at 42.2% in blood donors in Martinique island [29]. These studies were all performed in a small number of communities or specific population subgroups in a constrained region. It is unclear whether their findings are generalizable to the wider population. No territory-wide study evaluating the impact of ZIKV emergence in the general population has yet been published. Such population-representative studies constitute the most reliable source of information and often estimate seroprevalence rates that are lower but more representative than those obtained in population subgroups [30, 31].
In this context, we conducted a cross-sectional study within the general population of French Guiana in the year following the end of the outbreak, to characterize the seroprevalence of ZIKV and assess its association with sociodemographic and geographical factors.
METHODS
Study Design and Participants
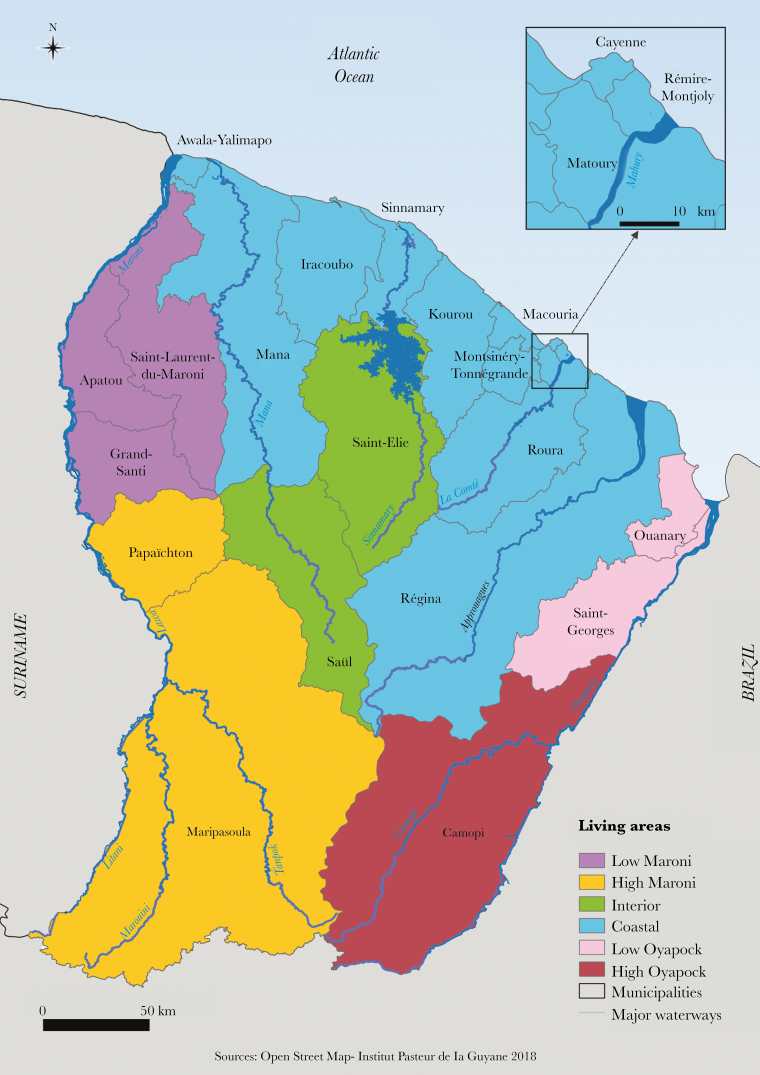
We conducted a cross-sectional population-based study through household interviews and serologic survey during June–October 2017, involving residents located in the 22 municipalities of French Guiana. The territory is composed of 2 main inhabited geographical regions: a central urbanized and coastal strip area along the Atlantic Ocean (“coastal area”) where a large part of the population lives, and 4 distinct remote areas along the Surinamese and Brazilian frontiers (“interior area”) (Figure 1).
Figure 1.
Map of French Guiana with geographical areas.
We estimated the sample size for this survey at 2500 persons distributed in the 5 delimited geographical areas based on a 50% seroprevalence, 95% confidence, 90% power, and a cluster effect. To reach the desired sample size, a total of 1600 households were randomly selected for possible participation in the study from household databases maintained by the Geographic Information and Knowledge Dissemination Unit of the Regional Environment, the planning and housing agency, and the National Institute of Economic and Statistical Information. A stratified simple random sampling method was adopted to select households from the 22 municipalities (strata), allowing an overrepresentation of isolated and small municipalities. Villages from 4 municipalities (Roura, Maripasoula, Regina, and Camopi) were specifically considered in the sample design to ensure that all existing submunicipality areas were adequately represented among the selected households. The distribution of households selected from the 22 strata is presented in Table 1. The global sampling fraction of the households was 1:49, varying from 1:103 to 1:5 according to the municipality.
Table 1.
Description of the Household Selection Process and Weighted Seroprevalence Estimated by Municipality
| Municipality and Submunicipality | Population | No. of Households | No. of Selected Households | No. (%) of Enrolled Households | No. of Enrolled Individuals | Weighted Seroprevalence, % (95% CI) |
|---|---|---|---|---|---|---|
| Cayenne | 57 614 | 21 659 | 210 | 196 (0.9) | 446 | 25.2 (20.2–30.9) |
| Matoury | 32 427 | 10 778 | 180 | 136 (1.3) | 265 | 22.7 (16.5–30.5) |
| Saint-Laurent | 43 600 | 9770 | 180 | 170 (1.7) | 301 | 32.4 (25.7–39.9) |
| Kourou | 26 221 | 8205 | 180 | 167 (2.0) | 294 | 30.1 (23.8–37.3) |
| Rémire-Montjoly | 23 976 | 8117 | 120 | 105 (1.3) | 192 | 13.7 (8.5–20.9) |
| Macouria | 11 719 | 4218 | 80 | 75 (1.8) | 164 | 10.9 (6.8–17.1) |
| Mana | 10 241 | 2297 | 80 | 74 (3.2) | 96 | 16.7 (10.6–25.4) |
| Maripasoula | 11 856 | 1955 | 80 | 74 (3.8) | 145 | 26.7 (16.6–40.0) |
| Maripasoula center area | … | … | 55 | 50 | 77 | 45.6 (30.0–62.0) |
| Twenke-Talhuen village | … | … | 15 | 14 | 33 | 8.4 (2.6–24.0) |
| Antecume-Pata village | … | … | 10 | 10 | 35 | 0 |
| Apatou | 8431 | 1839 | 50 | 45 (2.5) | 62 | 19.1 (10.2–32.9) |
| Grand-Santi | 6969 | 1447 | 30 | 28 (1.9) | 61 | 17.1 (8.3–32.0) |
| Saint-Georges | 4020 | 1208 | 40 | 32 (2.7) | 86 | 27.6 (13.6–47.9) |
| Papaïchton | 7266 | 1150 | 40 | 32 (2.8) | 49 | 22.7 (11.1–40.8) |
| Sinnamary | 2957 | 1092 | 30 | 30 (2.8) | 39 | 37.4 (24.0–53.1) |
| Roura | 3713 | 983 | 50 | 39 (4.0) | 70 | 18.7 (9.7–32.9) |
| Roura main area | … | … | 30 | 26 | 45 | 13.7 (5.1–31.7) |
| Cacao village | … | … | 20 | 13 | 25 | 27.3 (12.2–50.4) |
| Montsinéry-Tonnégrande | 2473 | 898 | 30 | 29 (3.2) | 66 | 10.7 (2.7–33.9) |
| Iracoubo | 1878 | 585 | 30 | 29 (5.0) | 53 | 10.7 (4.8–22.1) |
| Régina | 946 | 401 | 50 | 43 (10.7) | 75 | 12.0 (5.8–23.4) |
| Régina main area | … | … | 40 | 33 | 64 | 13.1 (5.9–26.3) |
| Kaw village | … | … | 10 | 10 | 11 | 5.9 (.8–34.0) |
| Camopi | 1769 | 346 | 50 | 50 (14.5) | 115 | 3.7 (1.3–9.8) |
| Camopi main area | … | … | 34 | 34 | 83 | 5.3 (2.0–13.5) |
| Trois-Sauts village | … | … | 16 | 16 | 32 | 0 |
| Awala | 1379 | 330 | 30 | 28 (8.5) | 60 | 25.5 (10.2–50.7) |
| Saint-Elie | 95 | 143 | 20 | 10 (7.0) | 11 | 0 |
| Ouanary | 165 | 140 | 20 | 5 (3.6) | 13 | 11.3 (3.0–34.5) |
| Saül | 150 | 94 | 20 | 18 (19.2) | 34 | 2.1 (.3–15.3) |
| Total | 259 865 | 77 655 | 1600 | 1415 | 2697 | 23.3 (20.9–25.9) |
Abbreviation: CI, confidence interval.
Procedures and Ethical Considerations
Publicity and information about the survey was provided through the media and contact with local and national authorities. Fieldworker teams including investigators and nurses or medicine residents were trained to visit all households, explain the project objectives, and, when allowed, collect participants’ signatures in a free and informed consent form and carry out the interviews. All members of selected households who were 2–75 years of age were invited to take part in the study during a preliminary face-to-face interview. For all participants <18 years of age, 1 or 2 responsible adults signed the informed consent form. A specific educational-style comic book was designed for children 6–17 years of age to explain, in an understandable way, the nature and objectives of the survey and inform them about the voluntary nature of their participation in the study and their rights to access and rectify their personal information (Supplementary Materials 1).
Data were collected through a standardized questionnaire installed on tablets to register demographics, socioeconomics, and household characteristics. Participants were asked to report the occurrence of a presumptive ZIKV infection, to report the year of Zika presumptive infection, to list the specific associated symptoms (open-ended question), and to specify if they had consulted a doctor or obtained a biological confirmation of their infection. Thereafter, a venous blood sample of 10 mL was collected from each participant, in accordance with current biosafety standards.
The study was recorded on ClinicalTrials.gov (NCT03210363) and approved by the Sud-Ouest & Outre-Mer IV Ethical Research Committee (number CPP17-007a/2017-A00514-49) and by the French Data Protection Authority (number DR-2017–324) responsible for ethical issues and protection of individual data collection.
Laboratory Methods
Blood Sample Collection
Blood samples were collected into 5-mL gold BD Vacutainer SST II advance tubes with gel for serum separation (Becton-Dickinson). Immediately after puncture, samples were stored at 4°C–8°C until centrifugation within 12 hours. Sera were then frozen and stored at –20°C until use at the National Reference Center for arboviruses in Institut Pasteur in French Guiana.
Serologic Diagnosis
All serum samples were tested for anti-ZIKV immunoglobulin G (IgG) antibodies using a recombinant antigen-based SGERPAxMap microsphere immunoassay (MIA) adapted from Beck et al [32]. ZIKV Luminex MIA uses the ZIKV E3 domain as the epitope rather than the whole E protein as in traditional ELISAs. The E3 domain has been shown to limit cross-reactivity between flaviviruses [33–38] (see Supplementary Materials and Methods). In such a way, this assay limits the probability that individuals previously infected with dengue falsely test as ZIKV positive.
Furthermore, some of the samples were evaluated through anti-ZIKV microneutralization tests (MNTs) adapted from Beck et al [32]. Away from infection, as is the case in our study, detection of ZIKV neutralizing antibodies provides good evidence of a contact with ZIKV [39, 40] (Supplementary Materials and Methods).
ZIKV MNT was systematically performed on the first 235 samples to evaluate the correlation between ZIKV MIA and MNT results and to confirm MIA cutoffs.
ZIKV neutralizing antibodies from the MNT were detected in 63 of 65 (96.9%) of samples with an MIA ratio of >2.5, in 26 of 36 (72.2%) of samples with an MIA ratio between 1.5 and 2.5, and in 12 of 134 (9%) of samples with an MIA ratio of <1.5. A sample was considered positive if its MIA ratio was >2.5 and negative for a value <1.5. All samples with an MIA ratio between 1.5 and 2.5 were tested by MNT and considered positive for neutralizing titers >20. MNT was also systematically performed where the MIA ratio was <1.5 for anyone who had reported an arboviral-like infection in the last 2 years (Supplementary Materials 2). Finally, almost a quarter of the samples (22.5%; n = 607) were also tested by anti-ZIKV MNTs.
Statistical Analysis
We use the following notation to describe the study design (Table 1):
i: one of the 22 strata (municipalities)
M i: number of primary sampling units (households) in the ith stratum, i = 1, …, 22
S i: number of primary sampling units (households) selected from the ith stratum, i = 1, …, 22
mi: number of primary sampling units (households) actually enrolled from the ith stratum, i = 1, …, 22
P i: number of individuals living within the ith stratum, i = 1, …, 22 (census data)
p i: number of individuals actually enrolled in the ith stratum, i = 1, …, 22
We considered, that, in each municipality i, the probability of selecting a particular subject was equal to the probability to select his or her household and was (mi/Mi), corresponding to a statistical weight equal to (1/ mi/Mi) =(Mi/mi). This statistical weight indicates the number of people in the population represented by each subject in the sample.
We applied a poststratification adjustment to each of these weights to arrive at the final statistical weight for each subject. This adjustment allowed us to weight the age-sex groups within each municipality to match the distribution in the French Guiana total population. Ten age groups (2–4, 5–9, 10–14, 15–19, 20–24, 25–34, 35–44, 45–54, 55–64 ≥65 years) were used within male and female groups, and for each age-sex subgroup, we applied an adjustment factor cijk, to have a final statistical weight wijk = (Mi/mi) × cijk, where i indexes municipalities, j indexes sex groups, and k indexes age groups.
We constructed a household socioeconomic index combining a multiple correspondence analysis and a hierarchical cluster analysis (Supplementary Materials and Methods). Type of housing, housing equipment such as access to drinking water and electricity, presence of a garden, private swimming pool, refrigerator, air conditioning, internet access, mobile phone, car, boat, type of health insurance, household income, and highest educational level were included to determine and characterize the natural groupings of households regarding socioeconomic levels.
The outcome of interest was the weighted ZIKV seroprevalence estimate, and the associated factors were identified by using a survey-weighted Poisson regression and prevalence ratios (PRs). Any variable having a significant univariate test with a P value cutoff point of 0.25 was selected as a candidate for the multivariate analysis. The strength of the association between selected variables and ZIKV seropositivity was estimated by crude and adjusted PRs with their 95% confidence interval (CI), all PRs excluding 1.0 being considered as significant.
Analyses were carried out using survey capabilities of Stata version 15 statistical software [41] and SPAD 8 [42]. Spatial analyses were performed using QGIS software [43].
RESULTS
In total, 1415 households and 2697 individuals were included from the 27 recruitment areas (Table 1). The mean household size was 1.9 individuals (range, 1–11). The mean age was 34.1 years (range, 2–75 years). Comparison of the sociodemographic characteristics of the study sample to the census data demonstrated an overrepresentation of women (58.9% vs 50% in the general population of French Guiana) and adults >25 years of age (64% vs 53% in French Guiana). These differences were accounted for in the analyses of seroprevalence and risk factors by allocating poststratification weight to each participant.
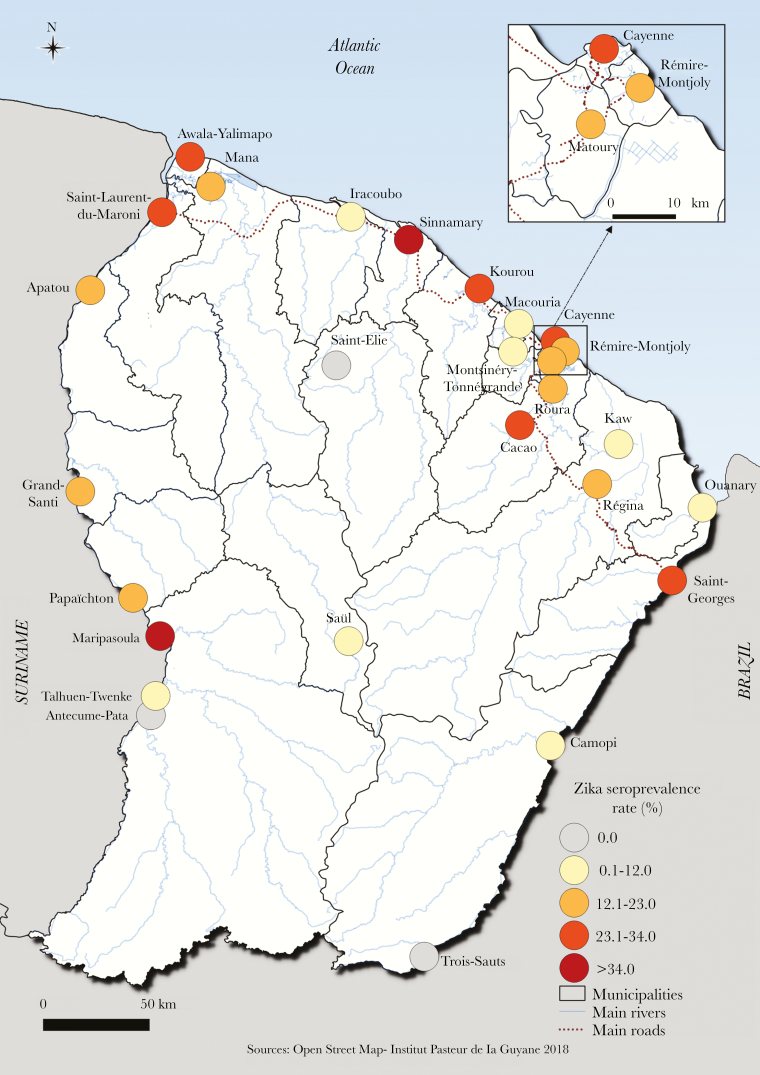
The crude proportion seropositive was 21.6% and the overall weighted seroprevalence of ZIKV antibodies in French Guiana was 23.3% (95% CI, 20.9%–25.9%) (Table 1). The seroprevalence did not differ according to sex (P = .65) or age (P = .47) (Table 2). Serological results in the different geographical areas are shown in Figure 2. While ZIKV circulated in a large part of French Guiana, it barely reached the interior and remote villages located in the most isolated forest areas (Saint-Elie, Saül, Talhuen-Twenke villages, and Camopi) (Table 1). Elevated probabilities of infection were observed in the main population centers in Maroni river (Maripasoula main area: 45.6% [95% CI, 30.0%–62.0%]; Saint-Laurent: 32.4% [95% CI, 25.7%–39.9%]), in the coastal area (Kourou: 30.1% [95% CI, 23.8%–37.3%]; Cayenne: 25.2% [95% CI, 20.2%–30.9%]), and in Low-Oyapock (Saint-Georges: 27.6% [95% CI, 13.6%–47.9%]). Two smaller geographical areas in the coastal area were also strongly impacted (Sinnamary: 37.4% [95% CI, 24.0%–53.1%]; Cacao: 27.3% [95% CI, 12.2%–50.4%]). Living in the northwestern part of the territory, so-called “Low Maroni,” in urban areas and being born in Haiti was significantly associated with being seropositive in both univariate and multivariate analyses (Table 2). Living in a carbet, representing a typical Native American cabin without walls essentially located in the Amazonian forest complex in High Oyapock, interior, and high Maroni villages, was significantly associated with being ZIKV seronegative at univariate level. The seroprevalence estimated in makeshift houses was higher than in individual or collective type of housing. However, this difference was not significant, probably due to small numbers of individuals in this category. Benefiting from universal health coverage or state medical assistance and having a low household income was associated with being seropositive at the univariate level. However, socioeconomic factors and type of housing were no longer significant after adjusting by geographical regions.
Table 2.
Factors Associated With Zika Virus Seropositivity
| Characteristic | Total No. of Tested Individuals | Weighted Prevalence, % (95% CI) |
Crude PR (95% CI) | Pearson P Value | Adjusted PR (95% CI) |
|---|---|---|---|---|---|
| Sex | … | ||||
| Male | 1108 | 22.8 (19.6–26.5) | Ref | .65 | |
| Female | 1589 | 23.8 (21.0–26.8) | 1.04 (.87–1.24) | ||
| Age, y | … | ||||
| 2–4 | 63 | 27.0 (15.2–43.3) | Ref | .47 | |
| 5–14 | 494 | 24.1 (19.1–30.1) | .89 (.50–1.59) | ||
| 15–24 | 413 | 25.9 (21.0–31.6) | .96 (.56–1.66) | ||
| 25–34 | 471 | 23.6 (19.2–28.7) | .87 (.51–1.50) | ||
| 35–44 | 442 | 19.7 (15.8–24.3) | .73 (.43–1.25) | ||
| 45–54 | 362 | 21.2 (16.5–26.8) | .78 (.44–1.39) | ||
| 55–64 | 284 | 18.9 (14.3–24.6) | .70 (.39–1.27) | ||
| ≥65 | 168 | 28.3 (20.4–37.7) | 1.05 (.56–1.95) | ||
| Region of residence | |||||
| Coastal area | 1820 | 22.2 (19.4–25.3) | Ref | .06 | Ref |
| Low Maroni | 424 | 28.8 (23.4–34.8) | 1.29 (1.02–1.64) | 1.41 (1.02–1.95) | |
| High Maroni | 194 | 25.5 (17.2–36.0) | 1.15 (.78–1.70) | 1.51 (.91–2.50) | |
| Low Oyapock | 99 | 27.0 (13.5–46.8) | 1.21 (.64–2.32) | 1.77 (.89–3.52) | |
| High Oyapock | 115 | 3.7 (1.3–9.8) | .16 (.61–.46) | .26 (.94–.75) | |
| Interior | 45 | 1.0 (.1–6.9) | .04 (.01–.33) | .07 (.01–.52) | |
| Type of housing | … | ||||
| Building/collective | 365 | 21.5 (16.4–27.6) | Ref | .05 | |
| Individual | 1768 | 23.8 (20.8–27.2) | 1.11 (.83–1.49) | ||
| Carbet | 213 | 5.9 (1.6–18.9) | .27 (.08–.97) | ||
| Makeshift | 89 | 28.3 (17.7–42.1) | 1.32 (.79–2.19) | ||
| Type of zone | |||||
| Rural | 1304 | 19.4 (16.2–23.1) | Ref | .03 | Ref |
| Urban | 1393 | 24.8 (21.7–28.1) | 1.28 (1.02–1.58) | 1.45 (1.11–1.91) | |
| Birth place | |||||
| French Guiana | 1481 | 23.4 (20.2–27.1) | Ref | <10–2 | Ref |
| Suriname | 213 | 27.0 (20.2–35.1) | 1.15 (.85–1.57) | 1.08 (.75–1.56) | |
| Brazil | 174 | 19.1 (13.3–26.7) | .82 (.56–1.19) | .82 (.55–1.22) | |
| Other South America | 63 | 27.6 (17.7–40.3) | 1.18 (.77–1.80) | 1.18 (.74–1.88) | |
| Haiti | 223 | 34.3 (20.6–42.3) | 1.46 (1.14–1.88) | 1.45 (1.08–1.94) | |
| Caribbean island | 136 | 20.6 (13.5–30.2) | .88 (.58–1.34) | .82 (.51–1.31) | |
| Europe | 349 | 17.0 (12.4–22.8) | .72 (.52–1.01) | .76 (.54–1.08) | |
| Asia | 28 | 4.6 (1.08–17.6) | .20 (.04–.82) | .23 (.05–1.14) | |
| Africa | 22 | 12.4 (3.1–38.6) | .53 (.14–1.97) | .56 (.15–2.15) | |
| Others | 8 | 6.1 (.7–36.6) | .26 (.03–2.04) | .32 (.04–2.50) | |
| Household size | … | ||||
| 1–2 | 536 | 20.0 (16.2–24.5) | Ref | .41 | |
| 3–5 | 1359 | 24.1 (20.7–27.8) | 1.20 (.93–1.55) | ||
| ≥6 | 592 | 24.6 (18.8–31.6) | 1.23 (.88–1.72) | ||
| Household income, € | |||||
| <1000 | 565 | 26.9 (22.2–32.1) | Ref | .10 | Ref |
| 1000–2999 | 833 | 24.5 (19.7–29.9) | .83 (.48–1.41) | .92 (.67–1.25) | |
| 3000–4999 | 285 | 17.7 (12.5–24.3) | .53 (.33–.86) | .72 (.46–1.12) | |
| ≥5000 | 153 | 15.7 (9.4–24.9) | .48 (.24–.94) | .66 (.37–1.16) | |
| Not documented | 861 | 24.0 (19.8–28.8) | .78 (.56–1.10) | .87 (.62–1.22) | |
| Socioeconomic index | |||||
| Low | 233 | 16.3 (10.8–23.9) | Ref | .06 | Ref |
| Intermediate | 908 | 26.5 (21.8–31.8) | 1.63 (1.04–2.53) | 1.48 (.96–2.26) | |
| Elevated | 1291 | 21.4 (18.3–24.9) | 1.31 (.85–2.02) | 1.59 (.97–2.59) | |
| Health insurance status | … | ||||
| General social coverage | 1330 | 20.2 (17.3–23.5) | Ref | <10–2 | |
| Universal health coverage | 1233 | 25.4 (21.5–29.7) | 1.26 (1.00–1.57) | ||
| State medical assistance | 128 | 36.9 (27.6–47.2) | 1.83 (1.34–2.49) | ||
| No healthcare | 6 | 20.1 (2.5–70.8) | .99 (.16–6.94) | ||
Abbreviations: CI, confidence interval; PR, prevalence ratio.
Figure 2.
Spatial distribution of Zika virus seroprevalence, French Guiana.
The proportion of reported symptoms was estimated at 25.5% (95% CI, 20.3%–31.4%) in ZIKV-positive participants vs 3.6% (95% CI, 2.6%–5.0%) in seronegative individuals, resulting in a ZIKV-attributable symptomatic infection rate of 21.9%.
This proportion varied substantially over space from 27.3% in the coastal area to only 12.8% in the High Maroni area, but did not vary significantly by sex (28.6% in ZIKV-positive women vs 21.8% in ZIKV-positive men; P = .22). More than 95% of infected individuals who reported the occurrence of a presumptive ZIKV infection reported a year of occurrence between 2015 and 2017, corresponding to the period of transmission of ZIKV in French Guiana. Three-quarters of them (75.8%) declared that diagnosis was confirmed by a clinician and 54.7% declared that they had a laboratory confirmation. Fever (80.5%) and myalgia (62.4%) were the most frequently reported symptoms in ZIKV-positive participants. However, only rash (38.0%) and arthralgia (33.9%) were significantly more prevalent in ZIKV-positive individuals (Table 3).
Table 3.
Clinical Symptoms Reported, by Zika Virus Infection Status
| Clinical Symptoms | Total (N = 147) | ZIKV-Positive Individuals (n = 96) | ZIKV-Negative Individuals (n = 51) | Pearson P Value | |||
|---|---|---|---|---|---|---|---|
| No. | (%) | No. | (%) | No. | (%) | ||
| Fever | 118 | (81.4) | 77 | (80.5) | 41 | (83.0) | .98 |
| Arthralgia | 84 | (52.2) | 62 | (33.9) | 22 | (61.9) | .01 |
| Myalgia | 93 | (63.1) | 61 | (62.4) | 32 | (64.2) | .92 |
| Rash | 52 | (30.1) | 41 | (38.0) | 11 | (15.3) | .01 |
| Conjunctival hyperemia | 28 | (18.0) | 22 | (21.9) | 6 | (10.6) | .10 |
Abbreviation: ZIKV, Zika virus.
DISCUSSION
Here we have presented a large-scale representative ZIKV seroprevalence study in South America from the recent 2015–2016 Zika epidemic. We found that approximately a quarter of the population of French Guiana became infected during the outbreak. However, there was substantial heterogeneity in infection risk, with communities in the center of the territory hardly affected at all, whereas up to half of the population was infected in urban communities along the coast. Our results were consistent with a recent report from a systematic monitoring of pregnant women in French Guiana that estimated at 19% the proportion of ZIKV-positive women during the first 4 months of the outbreak [25].
Our seroprevalence estimates are slightly lower than most previous estimates from seroprevalence studies in other locations in South America [24–28] and in other parts of the world [2, 34].This highlights the importance of conducting population-representative studies. The previous studies in South America have all consisted of single communities or small numbers of neighboring populations. Therefore, it has not been possible to generalize the findings to the wider country as is the case here, where the entire territory was included in the initial sampling frame. The future risk of ZIKV in the area remains unclear. It has been shown in a study conducted in Thailand that ZIKV can transition to stable endemic circulation [44]. Our findings demonstrate that the majority of the population remains susceptible to ZIKV, which would potentially allow future successful reintroductions of the virus. However, the observed differences in seropositivity across the country may partly reflect differences in the distribution of mosquitoes across the territory. Population movements, economic development, and urbanization have facilitated the geographical expansion of Ae. aegypti and its implantation in almost all inhabited areas of French Guiana, even in villages along the Maroni river up to the Maripasoula main area and inland up to Saül [45, 46]. However, to date no study has reported the presence of Ae. aegypti populations in the most remote villages including Antecume Pata, Twenke-Talhuen, and Camopi [46], where seroprevalence rates varied from 0% to 8%. Furthermore, despite strengthening existing epidemiological surveillance systems [47] and entomo-epidemiological investigations coordinated by local health authorities when a clinical or confirmed case appeared in these areas, no autochthonous transmission of Aedes-transmitted diseases has been identified in these villages.
Differences in vector control activities implemented in the different geographical areas may also have contributed to variability in transmission intensities between municipalities. Indeed, the French Guiana program of surveillance and management of arboviral diseases includes Ae. aegypti density reduction throughout the year, which is intensified during outbreaks. Vector control activities include both indoor and outdoor spatial spraying of deltamethrin against adults and the removal of breeding sites or their treatment with Bacillus thuringiensis var. israelensis–based larvicides [48]. These activities rely on important logistical and human resources that can be in some situations very difficult to apply uniformly in the different geographical areas of French Guiana.
We found that individuals with higher income and benefiting from general health insurance had a reduced risk of infection compared to those with lower income and benefiting from universal health or state medical assistance in the univariate risk factor analysis. However, after correcting for spatial differences in risk, no individual or household-level factor was associated with being seropositive. This suggests that the individual risk of infection is more deeply modulated by different ecoenvironments related to geographic factors, urbanization level, and the related quality of sanitary infrastructures that may have an impact on the extent of vector infestation and, hence, viral circulation.
Whereas the proportion of asymptomatic ZIKV infection in ZIKV-positive participants was estimated in previous general population cross-sectional studies at 43% in French Polynesia [34], 47% in Puerto Rico [49], and 62% in Yap, Micronesia [2], we found that 74.5% (95% CI, 68.5%–79.8%) of the participants with ZIKV IgG did not report symptoms confirming the high proportion of asymptomatic ZIKV infection. Considering that the survey was conducted 8 months after the end of the ZIKV epidemic in French Guiana, recall bias may nonetheless have led to underreporting of symptoms, particularly when there was a considerable time delay between the symptoms’ occurrence and the time of the survey. Furthermore, even if numerous education campaigns may have considerably increased public awareness of health risks related to arboviruses in a large majority of the population, awareness about Zika; cultural, social, and behavioral practices; or previous expositions to diseases and parasites may have affected the reporting of presumptive ZIKV infection.
Our study has other limitations inherent to the study design and cross-reaction issues in the context of co-circulation of related arboviruses.
First, given that individuals without health coverage could not be enrolled in our survey because of restrictions from French legislation, immigrants without health coverage were underrepresented in our sample. Although this population was very small in the majority of the municipalities, some households were excluded in the western border part of the territory, which is known for high levels of immigration, because the adults and household referents did not have health insurance status. Second, sample size calculation was determined to obtain a sufficient point estimate of prevalence but not to study risk factors of infection so that we might lack power to ascertain them. Finally, cross-reaction between viruses of the same family could have affected the interpretation of seroprevalence results. In particular, dengue has circulated in French Guiana for decades. Here we used an assay that minimizes the risk of cross-reaction by relying on the E3 domain of the ZIKV in combination with virus neutralization assay (MNT).We estimated that 9% of those with lower MIA values were still seropositive. This means that the true proportion seropositive may be slightly higher than our estimates; however, as 76.4% of samples had low MIA results, this would only raise the proportion seropositive from 23.3% to 27.1%.
In conclusion, this study is the first to provide a consistent overview of a territory-wide ZIKV seropositivity estimation in the Americas. Given that a huge proportion of ZIKV infections are clinically asymptomatic and that the disease is greatly underreported, our results provide distinctive and useful information per geographical area and population subgroups in a continental area frequently exposed to arbovirus.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Presented in part: 2019 Annual Conference of the International Society for Disease Surveillance, San Diego, California, 29 January–1 February 2019; and Neglected Tropical Diseases Congress: Future Challenges, Dubai, United Arab Emirates, 5–6 December 2018.
Acknowledgments. We thank Laurine Levillayer from the Institut Pasteur of Paris, Dr Paul Brousse from the Cayenne Hospital Center, Dr Katia Le Goaziou from the National Institute of Economic and Statistical Information, and all collaborators and field workers involved in the Arbovirus Epidemiologic Survey (EPI-ARBO) project. We also thank Laetitia Bremand, Bhety Labeau, David Moua, and Marine Rangon from the Arbovirus National Reference Center for their valuable contributions to the laboratory analyses.
Disclaimer. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Financial support. This study was supported by the European Regional Development Fund under EPI-ARBO grant agreement GY0008695; the Regional Health Agency of French Guiana; and the National Center of Spatial Studies. C. Fl. acknowledges financial support from the Centre National d’Etudes Spatiales-Terre solide, Océan, Surfaces Continentales, Atmosphère fund (grant number CNES-TOSCA-4800000720) and funding from Calmette and Yersin allocated by the Pasteur Institut Department of International Affairs. S. C. acknowledges financial support from the AXA Research Fund, the Investissement d’Avenir program, the Laboratoire d’Excellence Integrative Biology of Emerging Infectious Diseases program (grant number ANR-10-LABX-62-IBEID), the Models of Infectious Disease Agent Study of the National Institute of General Medical Sciences, the INCEPTION project (PIA/ANR-16-CONV-0005), and the European Union’s Horizon 2020 research and innovation program under ZIKAlliance grant agreement number 734548.
Potential conflicts of interest. All authors: No reported conflicts of interest.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
REFERENCES
- 1. Dick GW, Kitchen SF, Haddow AJ. Zika virus. I. Isolations and serological specificity. Trans R Soc Trop Med Hyg 1952; 46:509–20. [DOI] [PubMed] [Google Scholar]
- 2. Duffy MR, Chen TH, Hancock WT, et al. . Zika virus outbreak on Yap Island, Federated States of Micronesia. N Engl J Med 2009; 360:2536–43. [DOI] [PubMed] [Google Scholar]
- 3. Ioos S, Mallet HP, Leparc Goffart I, Gauthier V, Cardoso T, Herida M. Current Zika virus epidemiology and recent epidemics. Med Mal Infect 2014; 44:302–7. [DOI] [PubMed] [Google Scholar]
- 4. Jouannic JM, Friszer S, Leparc-Goffart I, Garel C, Eyrolle-Guignot D. Zika virus infection in French Polynesia. Lancet 2016; 387:1051–2. [DOI] [PubMed] [Google Scholar]
- 5. Musso D, Nilles EJ, Cao-Lormeau VM. Rapid spread of emerging Zika virus in the Pacific area. Clin Microbiol Infect 2014; 20:O595–6. [DOI] [PubMed] [Google Scholar]
- 6. Musso D. Zika virus transmission from French Polynesia to Brazil. Emerg Infect Dis 2015; 21:1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pettersson JH-O, Eldholm V, Seligman SJ, et al. . How did Zika virus emerge in the Pacific Islands and Latin America? mBio. 2016; 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Brasil P, Sequeira PC, Freitas AD, et al. . Guillain-Barré syndrome associated with Zika virus infection. Lancet 2016; 387:1482. [DOI] [PubMed] [Google Scholar]
- 9. Cao-Lormeau VM, Blake A, Mons S, et al. . Guillain-Barré syndrome outbreak associated with Zika virus infection in French Polynesia: a case-control study. Lancet 2016; 387:1531–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Driggers RW, Ho CY, Korhonen EM, et al. . Zika virus infection with prolonged maternal viremia and fetal brain abnormalities. N Engl J Med 2016; 374:2142–51. [DOI] [PubMed] [Google Scholar]
- 11. Rasmussen SA, Jamieson DJ, Honein MA, Petersen LR. Zika virus and birth defects—reviewing the evidence for causality. N Engl J Med 2016; 374:1981–7. [DOI] [PubMed] [Google Scholar]
- 12. Cauchemez S, Besnard M, Bompard P, et al. . Association between Zika virus and microcephaly in French Polynesia, 2013-15: a retrospective study. Lancet 2016; 387:2125–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Calvet G, Aguiar RS, Melo ASO, et al. . Detection and sequencing of Zika virus from amniotic fluid of fetuses with microcephaly in Brazil: a case study. Lancet Infect Dis 2016; 16:653–60. [DOI] [PubMed] [Google Scholar]
- 14. Hoen B, Schaub B, Funk AL, et al. . Pregnancy outcomes after ZIKV infection in French territories in the Americas. N Engl J Med 2018; 378:985–94. [DOI] [PubMed] [Google Scholar]
- 15. World Health Organization. Statement on the first meeting of the International Health Regulations (2005) (IHR 2005). Emergency Committee on Zika virus and observed increase in neurological disorders and neonatal malformations. Geneva, Switzerland: WHO, 2016. [Google Scholar]
- 16. Hills SL. Transmission of Zika virus through sexual contact with travelers to areas of ongoing transmission—continental United States, 2016. MMWR Morb Mortal Wkly Rep 2016; 65:215–6. [DOI] [PubMed] [Google Scholar]
- 17. D’Ortenzio E, Matheron S, Yazdanpanah Y, et al. . Evidence of sexual transmission of Zika virus. N Engl J Med 2016; 374:2195–8. [DOI] [PubMed] [Google Scholar]
- 18. L’Azou M, Taurel AF, Flamand C, Quénel P. Recent epidemiological trends of dengue in the French territories of the Americas (2000–2012): a systematic literature review. PLoS Negl Trop Dis 2014; 8:e3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Flamand C, Fritzell C, Prince C, et al. . Epidemiological assessment of the severity of dengue epidemics in French Guiana. PLoS One 2017; 12:e0172267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fritzell C, Raude J, Adde A, Dusfour I, Quenel P, Flamand C. Knowledge, attitude and practices of vector-borne disease prevention during the emergence of a new arbovirus: implications for the control of chikungunya virus in French Guiana. PLoS Negl Trop Dis 2016; 10:e0005081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Flamand C, Fritzell C, Obale P, Quenel P, Raude J. The role of risk proximity in the beliefs and behaviors related to mosquito-borne diseases: the case of chikungunya in French Guiana. Am J Trop Med Hyg 2017; 97:344–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Flamand C, Fritzell C, Matheus S, et al. . The proportion of asymptomatic infections and spectrum of disease among pregnant women infected by Zika virus: systematic monitoring in French Guiana, 2016. Euro Surveill Bull 2017; 22. doi: 10.2807/1560-7917.ES.2017.22.44.17-00102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Santé Publique France. Situation épidémiologique du virus Zika aux Antilles Guyane.2016. http://www.santepubliquefrance.fr/regions/guyane/documents/bulletin-regional/2016/situation-epidemiologique-du-virus-zika-aux-antilles-guyane.-point-au-10-novembre-2016 Accessed 16 August 2019.
- 24. Netto EM, Moreira-Soto A, Pedroso C, et al. . High Zika virus seroprevalence in Salvador, northeastern Brazil limits the potential for further outbreaks. mBio 2017; 8. doi: 10.1128/mBio.01390-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rodriguez-Barraquer I, Costa F, Nascimento EJM, et al. . Impact of preexisting dengue immunity on Zika virus emergence in a dengue endemic region. Science 2019; 363:607–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Saba Villarroel PM, Nurtop E, Pastorino B, et al. . Zika virus epidemiology in Bolivia: a seroprevalence study in volunteer blood donors. PLoS Negl Trop Dis 2018; 12:e0006239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zambrana JV, Bustos Carrillo F, Burger-Calderon R, et al. . Seroprevalence, risk factor, and spatial analyses of Zika virus infection after the 2016 epidemic in Managua, Nicaragua. Proc Natl Acad Sci U S A. 2018; 115:9294–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Langerak T, Brinkman T, Mumtaz N, et al. . Zika virus seroprevalence in urban and rural areas of Suriname, 2017. J Infect Dis 2019; 220:28–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gallian P, Cabié A, Richard P, et al. . Zika virus in asymptomatic blood donors in Martinique. Blood 2017; 129:263–6. [DOI] [PubMed] [Google Scholar]
- 30. Fritzell C, Rousset D, Adde A, Kazanji M, Van Kerkhove MD, Flamand C. Current challenges and implications for dengue, chikungunya and Zika seroprevalence studies worldwide: a scoping review. PLoS Negl Trop Dis 2018; 12:e0006533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Salje H, Paul KK, Paul R, et al. . Nationally-representative serostudy of dengue in Bangladesh allows generalizable disease burden estimates. eLife 2019; 8:e42869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Beck C, Desprès P, Paulous S, et al. . A high-performance multiplex immunoassay for serodiagnosis of flavivirus-associated neurological diseases in horses. Biomed Res Int 2015; 2015:678084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Aubry M, Finke J, Teissier A, et al. . Seroprevalence of arboviruses among blood donors in French Polynesia, 2011–2013. Int J Infect Dis 2015; 41:11–12. [DOI] [PubMed] [Google Scholar]
- 34. Aubry M, Teissier A, Huart M, et al. . Zika virus seroprevalence, French Polynesia, 2014–2015. Emerg Infect Dis 2017; 23:669–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Aubry M, Teissier A, Huart M, et al. . Seroprevalence of dengue and chikungunya virus antibodies, French Polynesia, 2014–2015. Emerg Infect Dis 2018; 24:558–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kama M, Aubry M, Naivalu T, et al. . Sustained low-level transmission of Zika and chikungunya viruses after emergence in the Fiji Islands. Emerg Infect Dis 2019; 25:1535–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kucharski AJ, Kama M, Watson CH, et al. . Using paired serology and surveillance data to quantify dengue transmission and control during a large outbreak in Fiji. eLife 2018; 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Simon O, Acket B, Forfait C, et al. . Zika virus outbreak in New Caledonia and Guillain-Barré syndrome: a case-control study. J Neurovirol 2018; 24:362–8. [DOI] [PubMed] [Google Scholar]
- 39. Swanstrom JA, Plante JA, Plante KS, et al. . Dengue virus envelope dimer epitope monoclonal antibodies isolated from dengue patients are protective against Zika virus. mBio. 2016; 7. doi: 10.1128/mBio.01123-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Collins MH, McGowan E, Jadi R, et al. . Lack of durable cross-neutralizing antibodies against Zika virus from dengue virus infection. Emerg Infect Dis 2017; 23:773–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. StataCorp. Stata statistical software: release 15. College Station, TX: StataCorp LLC, 2017. [Google Scholar]
- 42. SPAD Data Mining, v. 8.0 Software Program. Suresnes, France: Coheris Corp, 2013. [Google Scholar]
- 43. QGIS Development Team. QGIS Geographic Information System. Beaverton, OR: Open; Source Geospatial Foundation, 2009. [Google Scholar]
- 44. Ruchusatsawat K, Wongjaroen P, Posanacharoen A, et al. . Long-term circulation of Zika virus in Thailand: an observational study. Lancet Infect Dis 2019; 19:439–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Fouque F, Carinci R. Aedes aegypti in French Guiana. Some aspects of history, general ecology and vertical transmission of the dengue virus [in French]. Bull Soc Pathol Exot 1996; 89:115–9. [PubMed] [Google Scholar]
- 46. Epelboin Y, Chaney SC, Guidez A, et al. . Successes and failures of sixty years of vector control in French Guiana: what is the next step? Mem Inst Oswaldo Cruz 2018; 113:e170398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Flamand C, Quenel P, Ardillon V, Carvalho L, Bringay S, Teisseire M. The epidemiologic surveillance of dengue-fever in French Guiana: when achievements trigger higher goals. Stud Health Technol Inform 2011; 169:629–33. [PubMed] [Google Scholar]
- 48. Dusfour I, Zorrilla P, Guidez A, et al. . Deltamethrin resistance mechanisms in Aedes aegypti populations from three French overseas territories worldwide. PLoS Negl Trop Dis 2015; 9:e0004226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Lozier MJ, Burke RM, Lopez J, et al. . Differences in prevalence of symptomatic zika virus infection, by age and sex—Puerto Rico, 2016. J Infect Dis 2018; 217:1678–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.