Abstract
Context
Noncompliance with thyroxine therapy is the most common cause of poor control of hypothyroidism. An open-label prospective study to compare once-weekly thyroxine (OWT) with standard daily thyroxine (SDT) was undertaken.
Design
Patients taking thyroxine doses of >3 μg/kg/d, with or without normalization of TSH, were included and administered directly observed OWT or nonobserved SDT according to patient preference based on their weight for 6 weeks. Furthermore, patients on OWT were advised to continue the same at home without supervision.
Results
Twenty six of 34 patients on OWT and 7 of 18 patients on SDT achieved a TSH <10 μIU/mL (P < 0.05), and 2 patients from the SDT arm were lost to follow-up. During home treatment, 15 of 25 at 12 weeks and 19 of 23 contactable patients at a median follow-up of 25 months maintained TSH below target. Thyroxine absorption test was unable to predict normalization of TSH at 6 weeks of OWT therapy. No adverse events were seen with OWT-treated patients over the 12-week follow-up period. OWT has significantly higher efficacy (OR = 5.1) than SDT for patients with thyroxine-resistant hypothyroidism and is not associated with side effects.
Conclusion
OWT benefits a majority of patients in the long-term treatment of thyroxine-resistant hypothyroidism, in the real-world setting.
Keywords: poorly controlled hypothyroidism, thyroxine-resistant hypothyroidism, TSH, thyroxine treatment, once-weekly thyroxine
The most common reason for poor control of hypothyroidism is noncompliance to thyroxine, attributed to the inconvenience of taking the medication in a fasting state, waiting for 60 minutes for the next meal or beverage, and avoiding other medications that may interfere with absorption of thyroxine [1]. Directly observed treatment for 5 to 6 weeks is one method to ensure compliance and rule out malabsorption as the cause of poor control of hypothyroidism [2]. Once-weekly thyroxine (OWT) with doses <3 mg/d have been shown to be a safe treatment alternative to standard daily thyroxine (SDT) therapy for treating hypothyroid patients [3–7]. OWT has been recommended by the American Thyroid Association for older adults and patients dependent on caregivers for thyroxine therapy [8]. Still, there are few data regarding performance of OWT for patients who are not able to maintain normal TSH levels with daily therapy. Moreover, very few studies have evaluated the long-term treatment with OWT outside an institution. The current study intended to evaluate the effectiveness of OWT for patients with thyroxine-resistant hypothyroidism and its outcome for patients taking it over the long term at home.
1. Materials and Methods
An open-label prospective study to compare the efficacy and safety of OWT and SDT therapy for patients with thyroxine-resistant hypothyroidism was undertaken. The study was done in the Department of Endocrinology, Government Medical College, Thiruvananthapuram, India, over a 2-year period beginning in February 2017, after institutional ethics committee clearance was obtained. Patients taking levothyroxine >3 μg/kg/d with or without normalization of TSH (considered “thyroxine-resistant hypothyroidism” in this study) were recruited in a consecutive manner from the outpatient clinic. Pregnant or lactating women and patients with comorbid illnesses such as diabetes mellitus, hypertension, coronary artery disease, atrial fibrillation, or any other rhythm disorders of the heart, or history of known malabsorption syndromes, were excluded from the study. Patients taking any medications known to interfere with levothyroxine absorption or metabolism (calcium and iron supplements, antiepileptic agents, antacids, proton pump inhibitors, and H2 blockers) were excluded from the study. The subjects were given the option of OWT or SDT therapy after getting informed consent. Patients who opted for OWT therapy (intervention group) were admitted to the hospital for 1 day for baseline workup and administration of first dose of thyroxine. After an overnight fast, morning pulse rate, blood pressure, electrocardiogram, and a baseline T4 and TSH were estimated apart from routine hematological and biochemical evaluation. OWT dosage was calculated as 1.7 μg/kg/d of thyroxine needed for seven days and rounded off to the closest 50 μg. The tablets were administered in a powdered form under direct observation without spilling, and the medication was visually confirmed to have been swallowed. After the first dose of OWT, pulse rate and blood pressure were closely monitored during hospital stay. T4 and free T4 (fT4) were measured at 2 hours and 24 hours after the first dose of OWT. The subjects were discharged after 24 hours if no adverse effects were noted. Then every week until the sixth dose, OWT was administered on the same weekday and in the same hour as the first dose under supervision in the hospital. Each week the pulse rate and blood pressure were measured before and 2 hours after OWT administration. Serum samples for T3, T4, and TSH were collected before administration of OWT, and samples for T3 and T4 were again collected at 2 hours of administration. At the sixth dose of OWT, free thyroid hormones (fT4 and fT3) were also estimated before and after OWT administration. After the sixth dose patients were advised to continue the procedure at home for another 6 weeks, and T4, fT4, and TSH was estimated at 12 weeks.
Patients who opted for SDT were advised to continue the same dose or a smaller dose that was not less than 1.7 μg/kg/d for 6 weeks, based on the decision of the treating physician. Compliance with therapy was stressed at the initial visit. After a period of 6 weeks patients were reassessed for control of hypothyroidism with serum levels of TSH, T4, and T3 before and 2 hours after thyroxine intake. Hormonal estimations including serum TSH, T4, T3, fT3, and fT4 levels were done via electrochemiluminescence immunoassay. Intra-assay coefficient of variation for TSH was 1.1% to 3%. T4, T3, fT4, and fT3 had coefficients of variation of 1.5% to 3%. Baseline characteristics of both groups were compared via independent t test and χ2 test. Paired t test was done to assess the significance of changes in hormone values after thyroxine treatment. Mann-Whitney U test was used to compare the efficacy of OWT with SDT. P < 0.05 indicated statistical significance. Long-term follow-up of patients was done up to ∼30 months after the start of the study to assess the outcome of continued OWT treatment.
2. Results
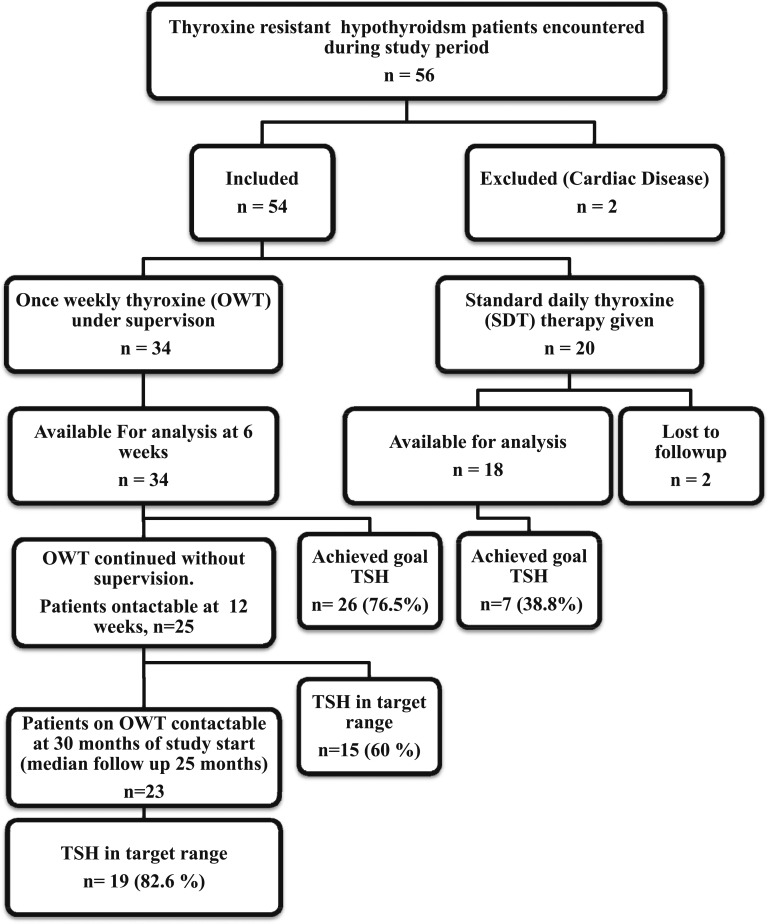
Fifty-six patients with thyroxine-resistant hypothyroidism presented to the department during the study period (Fig. 1). Two subjects who had a history of cardiac disease were excluded from the study. Hence, 54 patients (7 men, 47 women) were included in the study. Thirty-two subjects had autoimmune hypothyroidism, 15 had hypothyroidism as sequela of thyroidectomy, and seven had hypothyroidism from other causes. The median TSH at baseline was 29.7 mIU/mL [interquartile range (IQR), 18.0 to 53.2 mIU/mL]. Thirty subjects (of whom 36 had previous records) had previously documented normalization of TSH levels at some point during treatment of hypothyroidism. All subjects reported compliance and adequate gap of food intake to thyroxine, and none reported any interfering drugs at enrollment. The average reported gap between thyroxine and food or beverage intake was 1.30 ± 0.63 hours. No subjects reported malabsorption symptoms such as diarrhea, weight loss, or steatorrhea. The average daily thyroxine dosage before enrollment was 265.2 (±143.8) μg/d or 4.37 (±2.48) μg/kg/d.
Figure 1.
Flow of patients in the study.
Of the 54 subjects enrolled, 34 opted for a once-weekly regimen, and the rest (20 patients) opted for continuation of daily thyroxine therapy. Two patients from the daily therapy group were lost to follow-up and could not be included in final analysis. Baseline characteristics of both groups are shown in Table 1. The patients who opted for OWT (intervention group) received a mean thyroxine dose of 800 (±177.1) μg/wk (114.28 ± 25.29 μg/d or 1.87 ± 0.17 μg/kg/d).
Table 1.
Baseline Characteristics of Subjects: Comparison Between OWT and SDT Groups
| OWT (Frequency or Mean ± 2 SD or Median ± IQR) | SDT (Frequency or Mean ± 2 SD or Median ± IQR) | P | |
|---|---|---|---|
| Subjects treated, n | 34 | 18 | NA |
| Women, n | 31 | 14 | 0.23 |
| Duration of hypothyroidism, y | 11.2 ± 8.0 | 11.1 ± 7.2 | 0.97 |
| Age, y | 33.2 ± 9.3 | 35.61 ± 12.7 | 0.45 |
| Weight, kg | 61.95 ± 16.2 | 63.11 ± 14.6 | 0.80 |
| Body mass index, kg/m2 | 25.03 ± 5.4 | 29.63 ± 6.6 | 0.13 |
| Pulse rate, beats per min | 76 ± 6.2 | 74 ± 6.3 | 0.32 |
| TSH, median (IQR), mIU/L | 31.3 (17.5 – 53.2) | 36.5 (23.8 – 53.3) | 0.73 |
| T4, μg/dL | 7.5 ± 2.9 | 8.8 ± 7.43 | 0.398 |
| Thyroxine dose given after enrollment, μg/d | 1.8 ± 0.7 | 2.31 ± 0.03 | 0.004 |
Abbreviation: NA, not applicable.
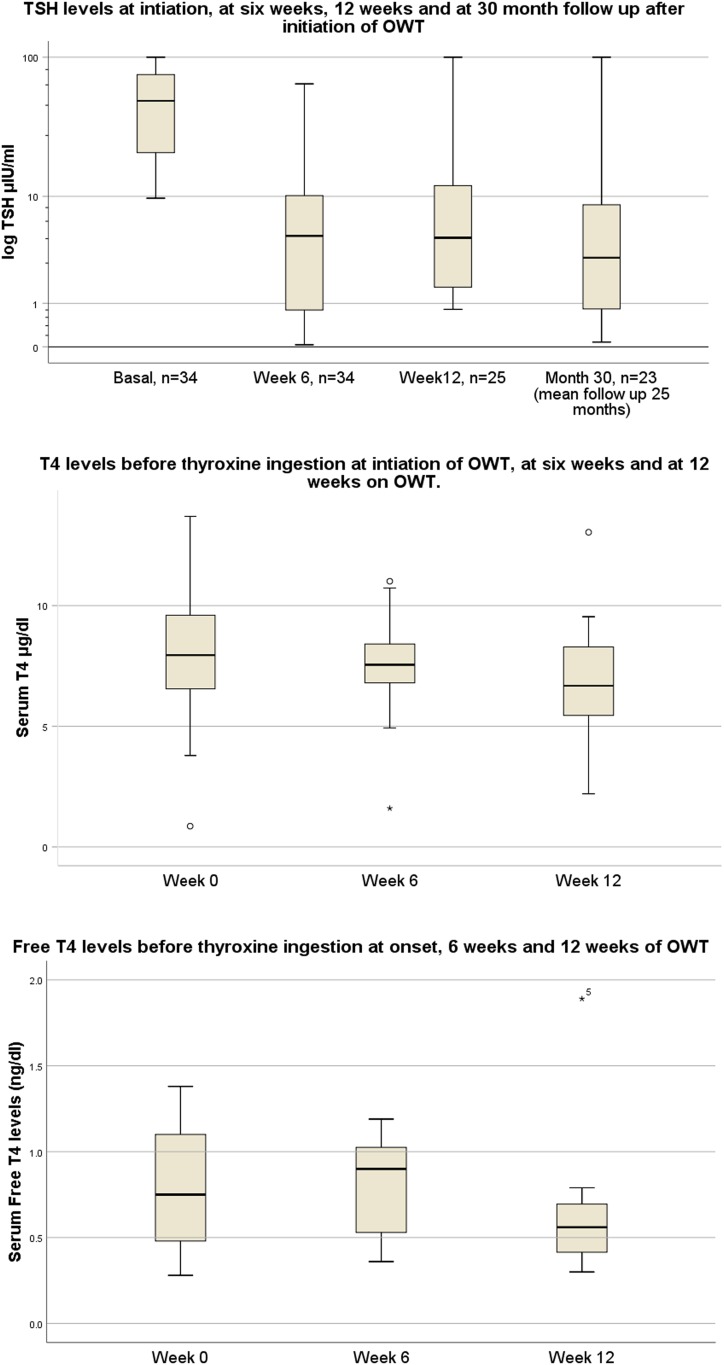
OWT resulted in a TSH <10 mIU/L in 26 of 34 patients after 6 weeks of treatment. Of the 18 patients who took daily treatment, seven improved to a TSH level of <10 mIU/L. On comparison between the groups, OWT was found to be significantly better than SDT, with an OR of 5.1 (P < 0.01) for patients with poorly controlled hypothyroidism in bringing TSH levels below the prespecified cutoff of 10 mIU/L. If a stricter TSH cutoff of 5 mIU/mL is used, a significantly higher number of patients treated with OWT [22 (64%) of 34] achieve the target compared with SDT [6 (33%) of 18] (OR 3.66, P = 0.03). For patients on OWT, the median TSH (IQR) decreased significantly from 26 (13.9 to 49.5) mIU/L at enrollment to 7.84 (1.6 to 14.7) mIU/L at 4 to 6 weeks (P < 0.05 by Mann-Whitney U test) (Fig. 2 and Table 2).
Figure 2.
Serum TSH, T4, and fT4 of patients treated with OWT.
Table 2.
Comparison of Thyroid Hormone Profile Before and 2 hr After Sixth Dose Between Groups
| At Sixth Dose, Before Thyroxine | At Sixth Dose, After Thyroxine | P | ||
|---|---|---|---|---|
| OWT (n = 34) | T4, μg/dL | 7.5 ± 2.1 | 12.9 ± 2.3 | <0.01 |
| T3, ng/dL | 83.61 ± 36 | 86.82 ± 34.4 | 0.89 | |
| fT4, ng/dL | 1.24 ± 1.6 | 1.81 ± 0.87 | <0.01 | |
| fT3, pg/mL | 2.83 ± 0.99 | 3.21 ± 0.80 | 0.03 | |
| TSH, mIU/L | 10.75 ± 13.37 | NA | ||
| SDT (n = 18) | T4, μg/dL | 8.15 ± 3.3 | 10.5 ± 2.9 | 0.018 |
| T3, ng/dL | 108.7 ± 31.0 | 104.5 ± 24.5 | 0.153 | |
| fT4, ng/dL | 1.4 ± 0.9 | 1.76 ± 0.96 | <0.01 | |
| fT3, pg/mL | 3.42 ± 0.94 | 3.69 ± 93 | 0.033 | |
| TSH, mIU/L | 27.2 ± 30.4 | NA |
Abbreviation: NA, not applicable.
Results of thyroxine absorption test were available for 30 patients (Table 3). There was no statistically significant relationship between thyroxine absorption test (measured as total T4 or fT4 rise) result and eventual attainment of the goal TSH level at 4 to 6 weeks of OWT (P = 1.00). After the directly observed treatment with OWT, 26 of 32 patients demonstrated a decrease in TSH to <10 mIU/L, indicating that the efficacy of OWT under strict observation was 77%. One patient from the OWT group whose TSH target could not be achieved admitted to taking antiepileptic medications while being on OWT. Two others who maintained very high levels of TSH on OWT were referred to a gastroenterologist for evaluation for malabsorption syndromes. One of these patients underwent detailed evaluation with upper GI endoscopy, tests for lipid malabsorption, and tests to rule out celiac disease, but no abnormalities were found, whereas the other patient refused detailed gastroenterological evaluation. Of the 25 patients who completed 12 weeks of OWT (including 6 weeks’ self-administration of OWT at home), 15 maintained a TSH <10 mIU/L, indicating that the short-term, real-world efficacy of OWT is likely to be ∼60%.
Table 3.
Association of Thyroxine Absorption Test With the Outcome of OWT
| Achieved Euthyroidism With OWT | Total | P | |||
|---|---|---|---|---|---|
| Yes | No | ||||
| 50% T4 rise 2 hr after OWT dose | Yes | 15 | 6 | 21 | 1.00 |
| No | 7 | 2 | 9 | ||
| Total | 22 | 8 | 30 | ||
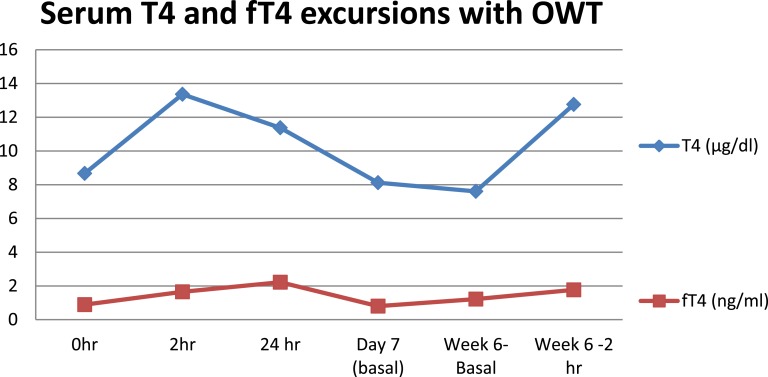
There was no statistically significant change in the pre-OWT T4 and fT4 levels between day 0 and the 6th week of treatment (Fig. 3). Patients who had a T4 below the normal reference range had a significant rise (to the normal reference range) from 3.0 ± 1.26 μg/dL to 7.2 ± 1.9 μg/dL (P < 0.01). Similarly, fT4 levels also rose significantly from 0.49 ± 0.23 ng/mL to 0.79 ± 0.23 ng/mL in those with low fT4 values to start with (P = 0.03). At the sixth dose of OWT, after 2 hours of administration T4 levels averaged 12.7 ± 2.2 µg/dL (Table 2), which was above the upper limit of normal.
Figure 3.
Serum T4 and fT4 excursions of OWT-treated patients at selected time points.
The mean total T3 levels, which were measured only at the sixth week, did not show a statistically significant change 2 hours after OWT administration, and the total T3 values were well within the normal range (Table 2). Free T3 levels showed a 13% increase over preadministration values at 2 hours (sixth week), and this finding was statistically significant (P = 0.03). A statistically significant 7% rise in fT3 levels was also seen for patients administered SDT therapy (P = 0.03). There was no statistically significant change in pulse rate or blood pressure for OWT-administered patients at 2 hours or 24 hours after administration of OWT compared with baseline. Of 32 patients, one experienced palpitation 4 hours after administration of OWT at the sixth dose; an ECG showed sinus tachycardia during the episode. This patient’s TSH was suppressed below the lower limit of normal at that time. Tachycardia subsided and palpitation disappeared with dose reduction in the subsequent week. No other adverse events were observed during the 12-week period.
For long-term follow-up, patients who were treated with OWT were again contacted to gather information about the long-term efficacy of OWT. Of the 34 patients, 23 were contactable, and 17 (77%) of them continued to be on OWT. The median duration of OWT treatment for the 23 contactable patients was 25 months (IQR 17 to 27 months). The median TSH on the last follow-up while the patients were on OWT was 2.97 (IQR 1.01 to 5.88). The last TSH during OWT therapy was below the target of 10 μIU/mL for 19 of the 23 patients (82.6%). Only three of the patients who discontinued OWT were contactable at the last evaluation, all of whom had higher TSH than the goal of 10 μIU/mL. None of the patients reported any incidence of arrhythmias, anxiety, or bone fracture while on OWT during this follow-up period.
3. Discussion
The current study evaluates the efficacy and safety of OWT therapy for patients with thyroxine-resistant hypothyroidism. In this study, a weight-related dose was used in each case, in contrast to many earlier studies where fixed doses were used [2, 3, 9]. Previous studies report that serum levels of levothyroxine are at or near their peak 2 hours after administration of an oral dose [3–6, 9–14]. This study showed that although the total T4 levels peak at 2 hours, the fT4 level is at maximum 24 hours after thyroxine ingestion.
The current study revealed that once-weekly levothyroxine reduced TSH to goal levels in a significantly higher percentage of patients with thyroxine-resistant hypothyroidism than in the daily therapy group (OR 5.1; P < 0.05). Seventy-seven percent of patients had a near normalization of TSH within 6 weeks of OWT therapy when the treatment was directly observed. These results were similar to those of the studies by Bornschein et al. [4] and Grebe et al. [5]. Sixty percent of patients on OWT had near normal TSH at the 12th week (with the last 6 weeks of treatment nonsupervised). Similar studies are summarized in Table 4. In the current study, except one patient in OWT arm (with iatrogenic thyrotoxicosis), none reported adverse events. Bornschein et al. also did not observe any adverse effects during OWT therapy. The corresponding T3 and fT3 levels assessed for thyrotoxicosis in OWT before and after sixth dose revealed no significant changes in T3 but a small but significant rise (of 13%) in fT3 levels. A similar significant rise (7%) was present in the SDT therapy group also, indicating no excess risk of drug-induced thyrotoxicosis in the OWT group in comparison with the SDT group. Also, there was no corresponding rise in pulse rate in any subjects. It was also found that preadministration levels of T4 and fT4 just before the sixth dose were comparable (P = 0.42 and 0.06, respectively) and within the normal reference range in both groups. The mildly higher T4 and fT4 levels in the control group despite higher TSH could be explained by irregular thyroxine intake, or by the fall of T4 to low levels in the presence of tissue euthyroidism even at the end of dosing interval for the OWT group. The mechanisms by which OWT can achieve euthyroidism have been elaborated by Grebe et al. [5]. They found that after the weekly dose of levothyroxine, there was a threefold increase in fT4, a 50% increase in the metabolically inactive form reverse T3, and only a 25% increase in fT3. At the end of the dosing interval, these values fell; fT4 was 30% lower, fT3 was 15% lower, and rT3 was 18% lower, suggesting that at the end of the weekly treatment, conversion of T4 to T3 increased, and conversion to rT3 decreased due to deiodinase activity. This mechanism may be responsible for the maintenance of euthyroidism while a patient is on OWT. Another likely mechanism that may be responsible for maintaining euthyroidism with weekly dosage is that a higher-than-usual level of thyroxine, when given intermittently, may suppress the thyroid antibody levels [15]. In the current study crushed thyroxine tablets were used, which may also have increased the efficacy of OWT, as was seen in an earlier study [16]. Still, the most important factor likely to contribute to the success of OWT is the improvement in compliance, which is not volunteered by the patients.
Table 4.
Summary of Notable Studies on Once-Weekly Thyroxine Therapy
| First Author, Year | Design, Sample Size, Country | Dosage of Weekly Thyroxine and Duration | Result | Adverse Events | Remarks |
|---|---|---|---|---|---|
| Bornschein et al., 2012 [4] | Randomized crossover study, 12 patients, included adequately treated hypothyroid patients (Brazil) | 7 times the daily dose once weekly, duration with OWT 6 wk, duration with daily thyroxine 6 wk for each patient | Weekly dose as effective as daily dose in controlling TSH. T4 levels at 2 hr of administration high after weekly dose but T3 levels not different from daily regimen. | No difference in heart rate, aortic ejection time between OWT and daily regimen | Cardiovascular safety established |
| Walker et al., 2013 [3] | Uncontrolled, open label, 23 patients | 1.69 ± 0.2 μg/wk, duration 4 wk | No increase in fT3 noted. At 4 wk of OWT, TSH levels decreased from baseline for 75% of patients. | None | Nil |
| Included poorly controlled hypothyroid patients (United Kingdom) | |||||
| Grebe et al., 1997 [5] | Randomized crossover design, N = 12 patients (Mayo Clinic, USA) | 7 times the daily dosage | Decreased TSH in OWT group. Low T4 before next weekly dose compared with daily thyroxine therapy. fT3 values 25% elevated after OWT. | No cardiac or other side effects as assessed by ECG and echocardiogram | Nil |
| Rangan et al., 2007 [7] | Case report of 2 poorly controlled hypothyroid patients (United Kingdom) | 1000 μg and 750 μg for one patient each | Both patients had normalization of TSH values after 4 wk of OWT. | One patient (on 1000 μg and body weight of 42 kg) had suppression of TSH levels to 0.05 mIU/L | Nil |
Earlier studies considered 2-hour rapid levothyroxine testing as a marker for normal absorption [4, 6, 17]. In the current study we found that the response to OWT treatment could not be predicted based on the T4 or fT4 rise by >50% 2 hours after administration of the first dose of OWT. The reason for this finding could be the infrequent sampling after thyroxine in the thyroxine absorption test protocol used in most studies and this study, whereby the peak value, and time at peak of fT4 and T4 may have been missed. The T4 levels were seen to peak at 2 hours after OWT, whereas fT4 levels peaked at 24 hours, unlike in previous studies.
Most previous studies were done with patients with controlled hypothyroidism or had a small number of patients with uncontrolled hypothyroidism [18, 19]. The merit of the current study is that it included thyroxine-resistant patients and had a comparison group and a longer follow-up of 12 weeks, including a 6 weeks of home OWT therapy. Walker et al. [3] included 23 uncontrolled hypothyroid patients and found a decrease in TSH from baseline in 75% of patients, but there was no control group for comparison, nor was the percentage of patients achieving goal TSH mentioned. The current study had a thyroxine-resistant control group and a follow-up of the study subjects on home OWT therapy for >2 years, assessing its real-world benefit. Limitations of the current study include absence of fT4 kits in a few instances during the study due to lack of continuous resource supplies for the project.
4. Conclusion
OWT is an effective and safe alternative for long-term use by patients with poor control of hypothyroidism or apparent thyroxine resistance when compared with a daily dose of thyroxine. About three quarters of such patients can achieve normalization of TSH levels with directly observed OWT. This study reports follow-up of patients on OWT for ≤2 years. In the real world OWT is likely to achieve control of hypothyroidism in most cases. Thyroxine absorption test does not reliably predict the outcome of OWT therapy. No serious adverse events are seen with OWT.
Acknowledgments
The authors hereby acknowledge the support extended by Dr. Remla A, senior scientific officer, Indian Institute of Diabetes, Trivandrum, in supervision of biochemical measurements and data management. Mr. Jayakumar P, biochemist, Government Medical College, Thiruvananthapuram, is hereby acknowledged for his help in biochemical investigations and data analysis for this manuscript. Dr. Seena TP (physiological assistant), Mr. Sajeev S, Mrs. Arya Suresh, Mrs. Arya Sugesh, and Mr. Sudi Sisupalan (research assistants) in Department of Endocrinology, Government Medical College, Thiruvananthapuram) are acknowledged for their roles in data collection and tabulation.
Financial Support: The authors thank the State Board of Medical Research, Kerala, for funding this study.
Clinical Trial Information: Clinical Trials Registry–India no. CTRI/2017/06/008855 (registered 16 June 2017).
Glossary
Abbreviations:
- fT4
free T4
- IQR
interquartile range
- OWT
once-weekly thyroxine
- SDT
standard daily thyroxine
Additional Information
Disclosure Summary: The authors have nothing to disclose.
Data Availability: The datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
References and Notes
- 1. Vermeire E, Hearnshaw H, Van Royen P, Denekens J. Patient adherence to treatment: three decades of research. A comprehensive review. J Clin Pharm Ther. 2001;26(5):331–342. [DOI] [PubMed] [Google Scholar]
- 2. Lips DJ, van Reisen MT, Voigt V, Venekamp W. Diagnosis and treatment of levothyroxine pseudomalabsorption. Neth J Med. 2004;62(4):114–118. [PubMed] [Google Scholar]
- 3. Walker JN, Shillo P, Ibbotson V, Vincent A, Karavitaki N, Weetman AP, Wass JA, Allahabadia A. A thyroxine absorption test followed by weekly thyroxine administration: a method to assess non-adherence to treatment. Eur J Endocrinol. 2013;168(6):913–917. [DOI] [PubMed] [Google Scholar]
- 4. Bornschein A, Filho GP, Graf H, Gisah A, Carvalh D. Treating primary hypothyroidism with weekly doses of levothyroxine: a randomized, single-blind, crossover study. Arq Bras Endocrinol Metab. 2012;56(4):250–258. [DOI] [PubMed] [Google Scholar]
- 5. Grebe SKG, Cooke RR, Ford HC, Fagerström JN, Cordwell DP, Lever NA, Purdie GL, Feek CM. Treatment of hypothyroidism with once weekly thyroxine. J Clin Endocrinol Metab. 1997;82(3):870–875. [DOI] [PubMed] [Google Scholar]
- 6. Van Wilder N, Bravenboer B, Herremans S, Vanderbruggen N, Velkeniers B. Pseudomalabsorption of levothyroxine: a challenge for the endocrinologist in the treatment of hypothyroidism. Eur Thyroid J. 2017;6(1):52–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rangan S, Tahrani AA, Macleod AF, Moulik PK. Once weekly thyroxine treatment as a strategy to treat non-compliance. Postgrad Med J. 2007;83(984):e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jonklaas J, Bianco AC, Bauer AJ, Burman KD, Cappola AR, Celi FS, Cooper DS, Kim BW, Peeters RP, Rosenthal MS, Sawka AM; American Thyroid Association Task Force on Thyroid Hormone Replacement. Guidelines for the treatment of hypothyroidism: prepared by the american thyroid association task force on thyroid hormone replacement. Thyroid. 2014;24(12):1670–1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Payer J, Sladekova K, Kinova S, Cesnakova Z, Killinger Z, Krizko M, Klimes I, Langer P. Autoimmune thyroiditis with severe hypothyroidism resistant to the treatment with high peroral doses of thyroxine: case report. Endocr Regul. 2000;34(4):189–193. [PubMed] [Google Scholar]
- 10. Srinivas V, Oyibo SO. Levothyroxine pseudomalabsorption and thyroxine absorption testing with use of high-dose levothyroxine: case report and discussion. Endocr Pract. 2010;16(6):1012–1015. [DOI] [PubMed] [Google Scholar]
- 11. Sun GE, Pantalone KM, Faiman C, Gupta M, Olansky L, Hatipoglu B. The clinical utility of free thyroxine in oral levothyroxine absorption testing. Endocr Pract. 2014;20(9):925–929. [DOI] [PubMed] [Google Scholar]
- 12. Vita R, Benvenga S. Tablet levothyroxine (L-T4) malabsorption induced by proton pump inhibitor; a problem that was solved by switching to L-T4 in soft gel capsule. Endocr Pract. 2014;20(3):e38–e41. [DOI] [PubMed] [Google Scholar]
- 13. Livadariu E, Valdes-Socin H, Burlacu MC, Vulpoi C, Daly AF, Beckers A. Pseudomalabsorption of thyroid hormones: case report and review of the literature. Ann Endocrinol (Paris). 2007;68(6):460–463. [DOI] [PubMed] [Google Scholar]
- 14. Goichot B, Vinzio S, Luca F, Sirlin X, Sapin R, Schlienger JL. In vivo evidence for a direct ultra-fast negative feedback of thyroxine on TSH secretion in humans: a case of L-thyroxine pseudomalabsorption. Clin Endocrinol (Oxf). 2007;67(6):952–953. [DOI] [PubMed] [Google Scholar]
- 15. Altunta F, Uysal AR, Erol C. Twice weekly LT4 for the treatment of primary hypothyroidism. Turk J Endocrinol Metab. 2004;1:25–34. [Google Scholar]
- 16. Yamamoto T. Tablet formulation of levothyroxine is absorbed less well than powdered levothyroxine. Thyroid. 2003;13(12):1177–1181. [DOI] [PubMed] [Google Scholar]
- 17. Balla M, Jhingan RM, Rubin DJ. Rapid levothyroxine absorption testing: a case series of nonadherent patients. Int J Endocrinol Metab. 2015;13(4):e31051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rajput R, Pathak V. The effect of daily versus weekly levothyroxine replacement on thyroid function test in hypothyroid patients at a tertiary care centre in Haryana. Eur Thyroid J. 2017;6(5):250–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wasoori SC, Naik MH. Abstract # 1182. Presented at: AACE Annual Scientific and Clinical Congress; May 25–29; Orlando, FL. [Google Scholar]