Abstract
Background
This study evaluated dissolvable microneedle patch (dMNP) delivery of hepatitis B vaccine in rhesus macaques and provides evidence that dMNP delivery elicits seroprotective anti-HBs levels comparable with human seroprotection, potentially useful for hepatitis B birth dose vaccination in resource-constrained regions.
Methods
Sixteen macaques were each vaccinated twice; they were treated in 4 groups, with dMNP delivery of AFV at 24 ± 8 µg (n = 4) or 48 ± 14 µg (n = 4), intramuscular injection of AFV (10 µg; n = 4), or intramuscular injection of AAV (10 µg; n = 4). Levels of antibody to hepatitis B surface antigen (HBsAg) (anti-HBs) and HBsAg-specific T-cell responses were analyzed.
Results
Six of 8 animals with dMNP delivery of AFV had anti-HBs levels ≥10 mIU/mL after the first vaccine dose. After dMNP delivery of AFV, interferon γ, interleukin 2, and interleukin 4 production by HBsAg-specific T cells was detected. A statistically significant positive correlation was detected between anti-HBs levels and cells producing HBsAg-specific interferon γ and interleukin 2 (T-helper 1–type cytokine) and interleukin 4 (T-helper 2–type cytokine) in all anti-HBs–positive animals.
Conclusions
dMNP delivery of AFV can elicit seroprotective anti-HBs levels in rhesus macaques that are correlated with human seroprotection, and it could be particularly promising for birth dose delivery of hepatitis B vaccine in resource-constrained regions.
Keywords: hepatitis B virus, hepatitis B vaccine, microneedle patch, skin vaccination, cellular immune response, adaptive immune response, rhesus macaques
Hepatitis B virus (HBV) infection and its chronic sequelae represent a global public health problem [1]. In 2015, an estimated 257 million people globally were living with chronic HBV infection, and about 887 000 HBV-attributed deaths occurred annually [2]. A primary mode of HBV transmission is from infected mothers to their newborns around birth (perinatal transmission). Perinatal HBV infection is a major risk factor for developing chronic HBV infection [3]. For this reason, the World Health Organization (WHO), the American College of Immunization Practices, and other national immunization programs recommend universal birth dose delivery of monovalent hepatitis B vaccine to infants during the first 24 hours of life, followed by timely completion of the routine hepatitis B vaccine series, because this can prevent 85%–95% of mother-to-child HBV transmission [4].
WHO estimates global routine immunization coverage to be 84% but the average birth dose coverage rate for hepatitis B vaccine is only 39% [2]. Although the WHO Western Pacific Region countries have achieved a high birth dose coverage rate (84%), other HBV-endemic areas have not scaled up birth dose delivery [5], owing to competing priorities for limited resources, the logistics of reaching births in remote areas that lack birth attendants trained to deliver hepatitis B vaccine as a birth dose, limited integration of maternal-child health with expanded immunization programs, and insufficient cold chain systems [4, 6]. More accessible and simpler delivery of the hepatitis B vaccine could facilitate of universal birth dose delivery for hepatitis B vaccine and increase the probability of global elimination of mother-to-child HBV transmission.
Previous studies have shown that microneedle patch (MNP) delivery of many vaccines, including influenza, rotavirus, polio, and measles vaccines, induce potent immune responses [7–11]. Dendritic cells, antigen-presenting cells which recognize foreign microbes and initiate immune responses, are abundant in the skin, and epidermal keratinocytes initiate immune response by releasing cytokines and chemokines against foreign antigens [12–14]. In addition, MNP vaccination offers simplified logistics, including administration by minimally trained personnel, elimination of biohazardous sharps waste, improved thermostability, and small package size [15].
Standard hepatitis B vaccine uses aluminum hydroxide adjuvant. However, the aluminum adjuvant in the hepatitis B vaccine is associated with injection site inflammation [16]. Previous studies have shown that inflammation induced by aluminum adjuvants at the injection site can induce nodule or granuloma formation in humans and animals [17–19], which may make MNP delivery of aluminum adjuvant to skin unacceptable in humans, particularly among newborns.
In the current study, we evaluated dissolvable MNP (dMNP) delivery of adjuvant-free monovalent hepatitis B vaccine (AFV) compared with standard intramuscular injection of aluminum-adjuvanted monovalent hepatitis B vaccine (AAV) and intramuscular injection of AFV in rhesus macaques.
MATERIALS AND METHODS
Hepatitis B Vaccines
Bulk AFV (concentration 2.4 mg/mL) and standard AAV for intramuscular injection were provided by the Serum Institute of India [20].
dMNP and Intramuscular Injection in Rhesus Macaques
The animal use protocol (protocol 2742CHOMONC) and procedures were reviewed and approved by the Institutional Animal Care and Use Committee of the US Centers for Disease Control and Prevention (CDC). dMNPs were fabricated with AFV, as described elsewhere [21]. Before vaccination, each animal was placed in ventral recumbency and sedated with ketamine/xylazine (10 mg/kg and 0.5 mg/kg, respectively) or ketamine hydrochloride (3 mg/kg and 0.075 mg/kg, respectively); depilatory lotion (Nair; Church & Dwight) was used to remove hair from the animals’ backs for dMNP delivery.
The dMNPs were manually applied to the skin with pressure to the center of the patch for 30 seconds to facilitate microneedle insertion. The dMNP remained in place, undisturbed, for 20 minutes before removal. Intramuscular vaccine injections to the vastus lateralis muscle on the leg were performed using a 26-gauge 1/2 hypodermic needle and syringe. Sixteen rhesus macaques were vaccinated in groups of 4 as follows: intramuscular AAV at 10 µg (pediatric dose), intramuscular AFV at 10 µg (10 μg of hepatitis B surface antigen (HBsAg) per 0.5 mL), and AFV delivered by dMNP at an estimated 24 ± 8 μg (dMNP-I) or 48 ± 14 μg (dMNP-II) [21] (Supplementary Materials and Methods). At 8 weeks after vaccination, a second, booster vaccination was performed using the same dose and delivery method each animal had received at initial vaccination. Two dMNP nonresponders (dMNP-II) were vaccinated with a third supplemental dose of intramuscular AAV (10 µg) at 9 weeks after the booster.
Measurement of Antibody to Hepatitis B Surface Antigen (HBsAg)
Prevaccination blood specimens were collected (at –2, –1, and 0 weeks) and used as baseline controls. Levels of antibody to HBsAg (antibody to hepatitis B surface antigen (anti-HBs)) were measured weekly using the VITROS Anti-HBs Reagent Pack (Ortho-Clinical Diagnostics), according to the manufacturer’s instructions. In immunocompetent adults and children, anti-HBs levels ≥10 mIU/mL are considered seroprotective against HBV infection in humans [22].
Interferon γ, Interleukin 5, and Interleukin 4 Enzyme-Linked Immunospot Assay
Isolated peripheral blood mononuclear cells (PBMCs) were resuspended in EmbryoMax 2× freezing medium (Millipore). We performed enzyme-linked immunospot (ELISPOT) assays using 96-well ImmunoSpot kits for human interferon (IFN) γ, interleukin 5 (IL-5), and interleukin 4 (IL-4) (CTL), according to the manufacturer’s instructions. Briefly, PBMCs were washed twice with 5 mL of CTL-Test Medium, the cells were counted with the Countess Automated Cell Counter (Invitrogen), and cell numbers were adjusted to 4 × 106cells/mL. Bulk monovalent AFV was used at 20 μg/mL concentration in CTL-Test Medium. For 24 hours, 2 × 105 cells were stimulated with a final concentration of 1 μg of adjuvant-free HBsAg per well at 37°C in 5% carbon dioxide. Anti-human IFN-γ/IL-5/IL-4 (CTL) detection solution was added, according to the manufacturer’s instructions.
For positive control, cells were stimulated with 50 ng/mL phorbol 12-myristate 13-acetate (Sigma) and 500 ng/mL ionomycin (Sigma), and 1% dimethyl sulfoxide (Sigma) was used as the negative control. All assays were performed in duplicate, and results were averaged. The positive cells (blue-colored spots) on the bottom of the well— HBsAg-specific IFN-γ, interleukin 2 (IL-2), or IL-4 responses—represent secreted cytokines. Cytokine-producing spot-forming cells (SFCs) were scanned and counted with a CTL analyzer. Each vaccination group had a single background mean value that was calculated from triple wells of the negative control. The background mean was then subtracted, and the spot counts were normalized by the number of cells per well. Negative controls had 0 spot-forming cell counts (SFCs)/106 cells for IL-2, IL-4, and IL-5, and 0–5 SFCs/106 cells for IFN-γ in each vaccination group. Positive controls had 100–200 SFCs/106 cells for IL-2, 200–300 SFCs/106 for IL-4, 200–400 SFCs/106 cells for IL-5, and 1500–2500 SFCs/106 cells for IFN-γ. There were no changes in the signal-background ratio over time.
Enzyme-Linked Immunosorbent Assay
Twenty-four hours after stimulation, supernatants were collected from ELISPOT plates and stored at −80°C until testing with enzyme-linked immunosorbent assay (ELISA). The Multi-Analyte ELISA Array kit (Qiagen), containing interleukin 1β (IL-1β), IL-4, IL-6, interleukin 10, 12, and 17A, IFN-γ, tumor necrosis factor (TNF) α, transforming grown factor (TGF) β1, monocyte chemoattractant protein (MCP) 1, macrophage inflammatory protein (MIP) 1α, and MIP-1β, was used according to the manufacturer’s protocol. Levels of protein expression were measured as absorbance at 450 nm using a Biotek ELISA plate reader, and fold changes of cytokines and chemokines were obtained by dividing absorbance value of each cytokine or chemokine by that of prevaccination samples as the baseline controls.
Statistical Analysis
Statistical analyses were performed with GraphPad software (version 7.04). Spearman rank correlation coefficients (r) were calculated using SPSS software (version 21). The statistical significance of r was tested using a 2-tailed t test. Differences were considered significant at P <0.05.
RESULTS
Anti-HBs Responses to Hepatitis B Vaccine in Rhesus Macaques
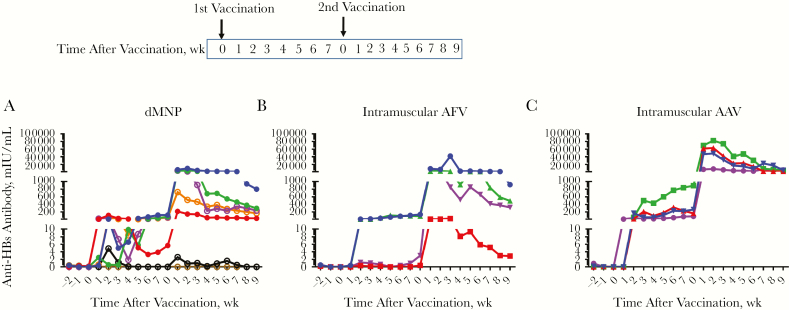
Anti-HBs level kinetics in postvaccination serum samples are shown in Figure 1. Four animals were used for each vaccination group. However, 1 animal that initially received the dMNP-I dose was accidently given intramuscular AAV during the booster vaccine dose. This animal was excluded from further analysis even though the anti-HBs levels after initial dMNP dosing was 490 mIU/mL (data not shown in Figure 1). Three of 4 animals receiving the dMNP-I dose, 2 of 4 receiving the dMNP-II dose, 2 of 4 receiving intramuscular AFV, and all 4 receiving intramuscular AAV had anti-HBs levels ≥10 mIU/mL (range, 13–895 mIU/mL) after the primary vaccination.
Figure 1.
Levels of antibody to hepatitis B surface antigen (anti-HBs) after hepatitis B vaccination of rhesus macaques. Anti-HBs levels after dissolvable microneedle patch (dMNP) vaccination with adjuvant-free hepatitis B vaccine (AFV) (dMNP-I group [dose, 24 ± 8 μg] depicted with closed circles and dMNP-II group [dose, 48 ± 14 μg] with open circles) (A); intramuscular injection of AFV (B); and intramuscular injection of standard aluminum-adjuvanted hepatitis B vaccine (AAV) (C). Anti-HBs levels were analyzed in weekly serum samples after vaccination. Points in the line graphs indicate titers for each animal in each experimental group (n = 7 for dMNP group; n = 4 for both intramuscular AAV and intramuscular AFV groups). Seropositive titers were defined as any detectable anti-HBs level and the threshold as ≥10 mIU/mL. After both initial and booster vaccinations, 5 of 7 animals receiving dMNP vaccine, 3 of 4 receiving intramuscular AFV, and all 4 receiving intramuscular AAV had anti-HBs levels ≥10 mIU/mL.
After the booster dose, anti-HBs levels increased in all vaccinated animals except 2 animals who received the dMNP-II dose. At the end of observation, the anti-HBs levels in 3 animals completing dMNP-I vaccination (dMNP-I group) were 801, 33, and 298 mIU/mL and those in 4 animals receiving dMNP-II vaccination (dMNP-II group) were 236, 167, 0, and 0 mIU/mL. After both initial and booster vaccinations, 5 of 7 animals that received dMNP vaccine, 3 of 4 that received intramuscular AFV, and all 4 that received intramuscular AAV had anti-HBs levels ≥10 mIU/mL (Figure 1). The mean quantitative anti-HBs responses were as follows: 219 mIU/mL in the dMNP groups (n = 7), 434 mIU/mL in the intramuscular AFV group (n = 4), and 4178 mIU/mL in the intramuscular AAV group (n = 4). No adverse reactions were detected at the immunization sites in any animal. The group of 5 animals that received dMNP hepatitis B vaccine with anti-HBs levels ≥10 mIU/mL was too small to separate into the 2 groups; they were treated as a single dMNP group for further analysis.
HBsAg-Specific T-Cell Responses
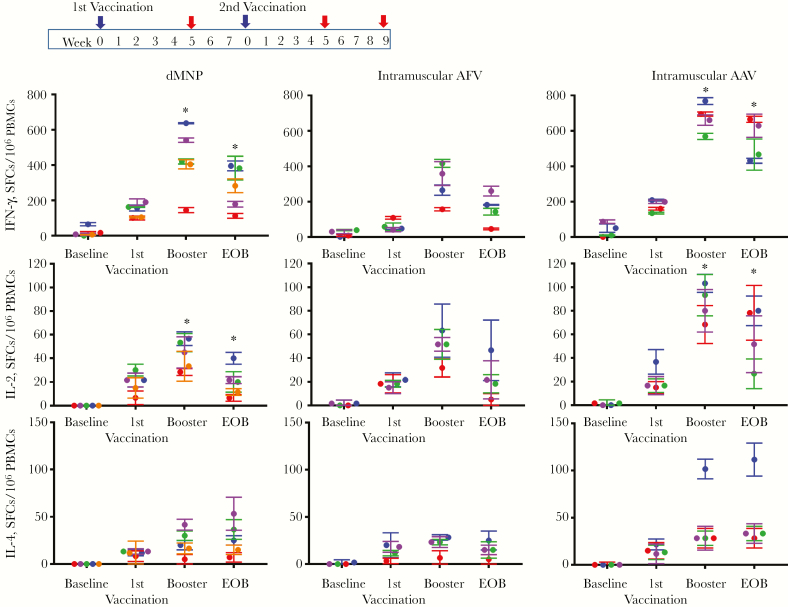
The cellular immune responses were assessed by analyzing HBsAg-specific IFN-γ–, IL-2–, and IL-4–producing T cells by means of ELISPOT analysis. The average of ELISPOT analysis at baseline, 5 weeks after the first vaccination, 5 weeks after the booster, and 9 weeks after the booster (end of observation) are shown in Figure 2. After the initial vaccination, HBsAg-specific IFN-γ– and IL-4–producing T-cell responses in the dMNP group were higher than those in the intramuscular AFV group. In the dMNP group, levels of HBsAg–specific IFN-γ–producing T cells peaked (430 SFCs/106 PBMCs; P < 0.0001) in 5 weeks after the booster dose, and the response was lower than that in the intramuscular AAV group (603 SFCs/106 PBMCs) but higher than that in the intramuscular AFV group (325 SFCs/106 PBMCs). The highest HBsAg-specific IL-2–producing T-cell responses were detected after boosting (P <0.0001), in with similar magnitudes of IL-2 response among the dMNP (43 SFCs/106 PBMCs) and intramuscular AFV (48 SFCs/106 PBMCs) groups but higher responses in the intramuscular AAV group (79 SFCs/106 PBMCs; P < .001).
Figure 2.
Hepatitis B surface antigen (HBsAg)–specific T-cell responses after dissolvable microneedle patch (dMNP) delivery of adjuvant-free hepatitis B vaccine (AFV), intramuscular injection of AFV, and intramuscular injection aluminum-adjuvanted hepatitis B vaccine (AAV). The vaccination schedule is shown in the top panel. The booster (second vaccination) was performed at 8 weeks after the initial vaccination. Frequencies of HBsAg-specific T-helper 1 cells secreting interferon (IFN) γ (A) and interleukin 2 (IL-2) (B) or T-helper 2 cells secreting interleukin 4 (IL-4) (C) were determined with enzyme-linked immunospot assay. T-cell responses were analyzed using samples (red arrows in top panel) obtained from before vaccination (baseline), 5 weeks after the first vaccine dose (identified as 1st vaccination), 5 weeks after the second (booster) immunization, and the end of observation (EOB). Data are expressed as spot-forming cells (SFCs) per 106 peripheral blood mononuclear cells (PBMCs) and different color of dots represent levels from individual animal in each experimental group (Each color represent 5 of 7 animals receiving dMNP, 3 of 4 animals receiving intramuscular AFV and 4 of 4 receiving intramuscular AAV had anti-HBs levels ≥10 mIU/mL). There were statistically significant differences in HBsAg-specific IFN-γ and IL-2 responses between dMNP and intramuscular AAV groups at boosting (P = 0.001 and P < 0.0001, respectively) and EOB (both P < .001). *P < .001.
Five weeks after the booster dose, HBsAg-specific IL-4–producing T-cell responses were similar between the dMNP group (23 SFCs/106 PBMCs) and the intramuscular AFV group (21 SFCs/106 PBMCs) (Figure 2B) (P < .001). However, at the end of observation, HBsAg-specific IL-4–producing T-cell responses increased continuously in the dMNP group (27 SFCs/106 PBMCs; P < .001) but decreased in the intramuscular AFV group (17 SFCs/106 PBMCs; P < .001). Differences in HBsAg-specific IFN-γ and IL-2 responses between dMNP and intramuscular AAV groups after boosting (P = .001 and P < .001, respectively) and at the end of observation (both P < .001) were statistically significant.
Secreted Cytokines and Chemokines in HBsAg-Specific T Cells
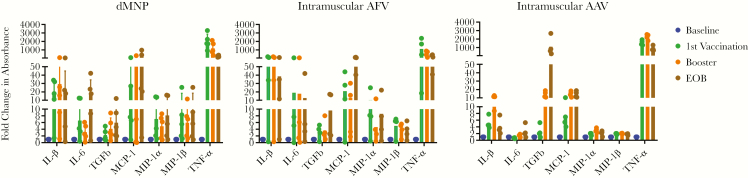
Elevated levels of the proinflammatory cytokines IL-1β, IL-6, TNF-α, and TGF-β and the proinflammatory chemokines MCP-1 (CCL2), MIP-1α (CCL3), and MIP-1β (CCL4) were detected in all 3 groups: dMNP, intramuscular AFV, and intramuscular AAV (Figure 3). Compared with the intramuscular AFV and intramuscular AAV groups, dMNP vaccination produced higher induction of TNF-α (2-way analysis of variance, P = .007) and MIP-1β (P = .002) after the first vaccination, and high MIP-1β expression levels were maintained in the dMNP group until the end of observation. However, TNF-α expression decreased continuously until the end of observation in dMNP and intramuscular AFV groups. Induction of IL-6 (P = .03) after dMNP vaccination peaked at the end of observation, and expression levels were higher than in the intramuscular AAF and AAV groups.
Figure 3.
Secreted cytokines and chemokines in hepatitis B surface antigen (HBsAg)–specific T cells induced by vaccination. Levels of interleukin 1β (IL-1β), interleukin 6 (IL-6), tumor necrosis factor (TNF) α, transforming grown factor (TGF) β, monocyte chemoattractant protein (MCP) 1, macrophage inflammatory protein (MIP) 1α, and MIP-1β were analyzed by means of enzyme-linked immunosorbent assay in supernatants of HBsAg-stimulated cultures of peripheral blood mononuclear cells (PBMCs) after vaccination of macaques with dissolvable microneedle patch (dMNP) delivery of adjuvant-free hepatitis B vaccine (AFV), intramuscular injection of AFV, and standard intramuscular injection of aluminum-adjuvanted hepatitis B vaccine (AAV). The fold change in absorbance of each cytokine was obtained by comparing to absorbance at 450 nm of prevaccination samples, used as baseline controls. The levels of cytokines and chemokines for each animal in the experimental groups are shown at baseline (before vaccination) (blue), 5 weeks after the first vaccine dose (identified as 1st vaccination) (green), 5 weeks after the second (booster) immunization (orange), and at the end of observation (EOB) (brown).
TGF-β expression induced by dMNP (P = .01) and intramuscular AFV increased continuously until the end of observation. MCP-1 expression had increased significantly at the end of observation in the dMNP, intramuscular AFV, and intramuscular AAV groups (P < .001). For the intramuscular AAV group, expression levels of IL-1β (P = .001), TNF-α, TGF-β, MIP-1α (P = .008), and MIP-1β peaked after boosting. Levels of cytokine and chemokines induced by different vaccine delivery strategies were compared at different time points. Statistically significant IL-1β, IL-6, TGF-β, MIP-1α, and MIP-1β responses were detected after boosting and at the end of observation in the dMNP, intramuscular AFV and intramuscular AAV groups (P < .001) (Figure 3).
Correlation Between T-Cell Responses and Anti-HBs Levels
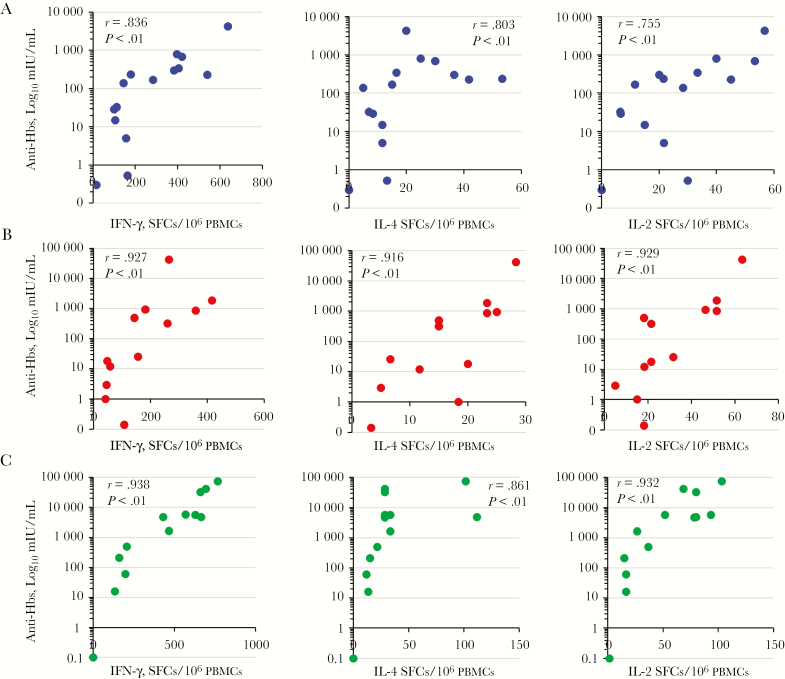
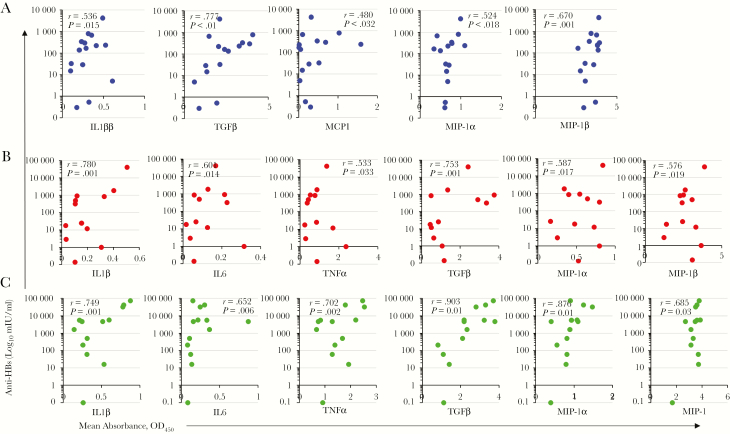
Nonparametric correlation analysis was performed to establish a relationship between the vaccine-induced T-cell and anti-HBs responses. Statistically significant positive correlations were found between IFN-γ–, IL-4–, and IL-2–producing T-cell responses and anti-HBs levels among all vaccination groups (Figure 4). Figure 5 shows the relationship between cytokine and chemokine expression levels in supernatants of HBsAg-stimulated cultures of PBMCs and the anti-HBs levels induced in the dMNP, intramuscular AFV, and intramuscular AAV groups. Statistically significant positive correlations were found between IL-1β, TGF-β, MIP-1α, and MIP-1β expression and anti-HBs levels in all vaccination groups. IL-6 and TNF-α expression was positively correlated with anti-HBs levels in the intramuscular AAV and intramuscular AFV groups. MIP-1 expression was positively correlated with anti-HBs levels only in the dMNP group.
Figure 4.
Correlation between hepatitis B surface antigen (HBsAg)–specific T-cell responses and levels of antibody to HBsAg (anti-HBs) after dissolvable microneedle patch (dMNP) delivery of adjuvant-free hepatitis B vaccine (AFV), intramuscular AFV, and intramuscular aluminum-adjuvanted hepatitis B vaccine (AAV). Spearman correlation tests were performed. Dots represent correlation tests in the dMNP (A), intramuscular AFV (B), and intramuscular AAV (C) groups. Abbreviations: IFN, interferon; IL-2, interleukin 2; IL-4, interleukin 4; PBMCs, peripheral blood mononuclear cells; SFCs, spot-forming cells.
Figure 5.
Correlation between secreted cytokines and chemokines in hepatitis B surface antigen (HBsAg)–specific T cells induced by vaccination and levels of antibody to HBsAg. Spearman correlation tests were performed. Dots represent correlation tests in the group receiving dissolvable microneedle patch delivery of adjuvant-free hepatitis B vaccine (AFV) (A), in the intramuscular AFV group (B), and in the intramuscular aluminum-adjuvanted hepatitis B vaccine group (C). Abbreviations: Abbreviations: IFN, interferon; IL-1β, interleukin 1β; IL-6, interleukin 6; MCP, monocyte chemoattractant protein; MIP, macrophage inflammatory protein; OD450, optical density at 450 nm; TGF, transforming growth factor.
DISCUSSION
In many low- and middle-income settings, HBV infections occur mainly through perinatal or early childhood transmission [23]. The most effective strategy to prevent HBV transmission from mother to child is birth dose delivery of hepatitis B vaccine within a narrow time window after birth, followed by timely delivery of the remaining hepatitis B vaccine series. A birth dose of hepatitis B vaccine followed by 2 more doses of can reduce the prevalence of chronic HBV infection by approximately 85%–90% in infants [4]. However, birth dose vaccination coverage for hepatitis B vaccine lags in a number of HBV-endemic regions, including sub-Saharan Africa, in part owing to lack of trained healthcare personnel to administer hepatitis B vaccine around birth and other logistical challenges [4]. Identification of alternative delivery methods that would overcome these challenges could allow progress toward elimination of new childhood HBV infections.
In the current study, we examined both humoral and cellular immune responses to dMNP delivery of monovalent AFV in rhesus macaques. We used AFV because previous reports suggest that the aluminum adjuvant is reactogenic in skin [16–18], so therefore undesirable for vaccine delivery. Vaccination by dMNP is of particular interest for administering a hepatitis B vaccine birth dose in economically developing countries because this vaccine delivery method can be performed by minimally trained personnel, would reduce hazardous needle waste, and might overcome cold chain challenges. For example, studies have shown that naive human subjects were able to self-administer MNPs after only very brief training [11, 15].
The results of hepatitis B vaccine delivery by dMNP in nonhuman primates could provide more relevant information about the potential efficacy of this delivery method in humans than do experiments in mice. Even though rhesus macaques are not a natural host for HBV infection, they have previously been used to assess immune response to hepatitis B vaccine, and this information was used to establish human dosing for human seroprotective levels ≥10 mIU/mL [24, 25]. dMNP delivery of inactivated polio vaccine has also been tested in rhesus macaques [26]. Similar to HBV, poliovirus does not naturally infect macaques; however, dMNP delivery of inactivated polio vaccine induced immunogenicity in macaques, as measured by neutralizing antibody response.
We found that anti-HBs levels with dMNP hepatitis B vaccine delivery were lower than those with intramuscular AFV or intramuscular AAV in rhesus macaques. However, anti-HBs levels produced by 2 different dMNP doses did not differ significantly. All 4 animals in the dMNP-I group and 2 in the dMNP-II group also produced anti-HBs levels ≥10 mIU/mL (seroprotective levels in human) after the first vaccination (Figure 1). Although 2 animals receiving the dMNP-II dose (48 ± 14 µg) demonstrated no measurable anti-HBs production or induction of cellular immune response, it remains unclear whether these macaques were poor responders or whether the higher dose of vaccine delivered by dMNP negatively affected their ability to produce anti-HBs after antigen exposure. It is possible that the immune systems of these 2 animals were compromised, because when they subsequently received the standard dose (10 μg) of intramuscular AAV as a third vaccination, much lower anti-HBs levels and cellular immune responses were induced compared with responses among the intramuscular AAV group animals after just 2 doses (Supplementary Figures 1 and 2).
We demonstrated that dMNP delivery of AFV can induce antigen-specific cellular immune responses after 1 dose, which increase after a booster dose (Figure 2). Levels of HBsAg-specific IFN-γ– (T-helper 1 [Th1]–type cytokine) and IL-4–producing (T-helper 2 [Th2]–type cytokine) T-cell responses by dMNP delivery were higher than responses to intramuscular AFV delivery. In addition, dMNP delivery induced IFN-γ–, IL-4–, and IL-2–producing T-cell responses that were positively correlated with anti-HBs levels (Figure 4). These results suggest that dMNP-induced antibodies were associated with both antigen-specific Th1 and Th2 responses. dMNP vaccine delivery also induced stronger Th1/Th2 responses than did intramuscular AFV delivery.
We found that high levels of inflammatory cytokines (IL-1β, IL-6, TNF-α, and TGF-β) and chemokines (MCP-1, MIP-1α, and MIP-1β) were induced in response to dMNP delivery (Figure 3). In a previous study, up-regulation of IL-1β, TNF-α, MIP-1α, MIP-2, and MCP-1 were detected in the skin of mice after influenza MNP vaccination [27]. TNF-α and IL-1β have previously been shown to be involved in the migration of Langerhans cells and the subsequent accumulation of dendritic cells in draining lymph nodes during Leishmania major infection [12, 28]. MCP-1, MIP-1α, and MIP-1β are inflammatory chemokines and function on neutrophils, granulocytes, monocytes, immature dendritic cells, and activated T cells by recruiting these effector cells to the site of pathogen entry [29]. A previous study showed that expression of MIP-1α and MIP-1β in activated natural killer and T cells was associated with the secretion of IFN-γ against listeriosis [30]. We found statistically significant positive correlations between MIP-1α (r = 0.594; P = .006), MIP-1β (r = 0.738; P < .01) and HBsAg-specific IFN-γ production with dMNP hepatitis B vaccine delivery. In addition, positive correlations between IL-1β, TGF-β, MIP-1, MIP-1α, and MIP-1β expression and anti-HBs levels in the dMNP group were statistically significant (Figure 5). These results suggest that cytokines and chemokines induced by dMNP hepatitis B vaccine may enhance HBsAg-specific T-cell responses in skin and antigen-presenting B cells [31, 32].
It should be noted that intramuscular vaccination with AAV and AFV were performed using doses of 10 µg of HBsAg, whereas dMNP vaccination was performed using doses of 24 ± 8 µg (dMNP-I) and 48 ± 14 µg (dMNP-II) of bulk, adjuvant-free HBsAg. Comparisons between intramuscular and dMNP groups need to account for different routes of administration and different doses. The current study provides for the first time information on immune responses to dMNP delivery of AVF in rhesus macaques, supporting the hypothesis that dMNP can induce robust anti-HBs and cellular responses to hepatitis B vaccine delivery, even in the absence of aluminum adjuvant. In addition, a limited number of animals were tested in each vaccination group; however, nonhuman primates have been shown to produce the anti-HBs levels that align with anti-HBs levels signifying immune protection in humans, including infants [25, 33].
In conclusion, we showed that delivery of AFV using dMNP induced humoral and cellular immune responses. These included production of anti-HBs levels to human seroprotective levels induced by conventional intramuscular delivery of AAV in humans. dMNP hepatitis B vaccine delivery elicited stronger cellular immune responses than intramuscular AFV, although they were administered at different doses. In addition, proinflammatory cytokine and chemokine secretion was detected in supernatants of HBsAg-stimulated PBMC cultures from the dMNP group. Future translational studies will be needed to improve dMNP hepatitis B vaccine fabrication and delivery and eventually study of hepatitis B vaccination by dMNP in humans. These results suggest that dMNP vaccination elicits an anti-HBs immune response that may be predictive of a seroprotective human response. The data presented in this study provide evidence for dMNP hepatitis B vaccination and possibly for birth dose delivery in HBV-endemic low-resource settings.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Presented in part: 36th Annual Meeting of the American Society for Virology, Madison, Wisconsin, 24–28 June 2017.
Acknowledgments. We thank Donna Bondy (Georgia Institute of Technology) for administrative support, Michael A. Purdy (Division of Viral Hepatitis, U.S. Centers for Disease Control and Prevention [CDC]) for critical reviews, Brianna Skinner (Comparative Medicine Branch, CDC) for veterinary support, and Natasha Khudyakova and Amanda Poe (Division of Viral Hepatitis, CDC) for laboratory analyses of hepatitis B virus markers. We also thank the Serum Institute of India for their generous donation of bulk adjuvant-free vaccine.
Disclaimer. The findings and conclusions in this report are those of the authors and do not necessarily reflect the official position of the US Centers for Disease Control and Prevention (CDC) and the Agency for Toxic Substances and Disease Registry, or the authors’ affiliated institutions. Use of trade names is for identification only and does not imply endorsement by the US Department of Health and Human Services, the Public Health Service, or the CDC.
Financial support. This work was supported by the CDC, the National Science Foundation Graduate Research Fellowship under Grant No. DGE-1650044 (to M. B. P. C.) and the NIH/NIGMS-sponsored Cell and Tissue Engineering (CTEng) Biotechnology Training Program T32GM008433 (to M. B. P. C.).
Potential conflicts of interest. M. R. P. is an inventor of patents that have been or may be licensed to companies developing microneedle-based products, is a paid advisor to companies developing microneedle-based products, and is a founder/shareholder of companies developing microneedle-based products, including Micron Biomedical. These potential conflicts of interest have been disclosed and are being managed by Georgia Institute of Technology and/or Emory University. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. World Health Organization. Global hepatitis report 2017. Geneva, Switzerland: World Health Organization, 2017. [Google Scholar]
- 2. World Health Organization. Hepatitis B vaccines: WHO position paper—July 2017. Wkly Epidemiol Rec 2017; 92:369–92.28685564 [Google Scholar]
- 3. Edmunds WJ, Medley GF, Nokes DJ, Hall AJ, Whittle HC. The influence of age on the development of the hepatitis B carrier state. Proc Biol Sci 1993; 253:197–201. [DOI] [PubMed] [Google Scholar]
- 4. Nelson NP, Easterbrook PJ, McMahon BJ. Epidemiology of hepatitis B virus infection and impact of vaccination on disease. Clin Liver Dis 2016; 20:607–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. World Health Organization. Reported estimates of HepB_BD coverage. 2018. http://apps.who.int/immunization_monitoring/globalsummary/timeseries/tscoveragehepb_bd.html. Accessed 9 July 2018. [Google Scholar]
- 6. GAVI. Hepatitis B vaccine at birth—GAVI responds to MSF. 2013. http://www.gavi.org/library/news/statements/2014/hepatitis-b-vaccine-at-birth-gavi-responds-to-msf/. Accessed 8 November 2017. [Google Scholar]
- 7. Leone M, Mönkäre J, Bouwstra JA, Kersten G. Dissolving microneedle patches for dermal vaccination. Pharm Res 2017; 34:2223–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shin CI, Jeong SD, Rejinold NS, Kim YC. Microneedles for vaccine delivery: challenges and future perspectives. Ther Deliv 2017; 8:447–60. [DOI] [PubMed] [Google Scholar]
- 9. Prausnitz MR. Engineering microneedle patches for vaccination and drug delivery to skin. Annu Rev Chem Biomol Eng 2017; 8:177–200. [DOI] [PubMed] [Google Scholar]
- 10. Marshall S, Sahm LJ, Moore AC. The success of microneedle-mediated vaccine delivery into skin. Hum Vaccin Immunother 2016; 12:2975–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rouphael NG, Paine M, Mosley R, et al. The safety, immunogenicity, and acceptability of inactivated influenza vaccine delivered by microneedle patch (TIV-MNP 2015): a randomised, partly blinded, placebo-controlled, phase 1 trial. Lancet 2017; 390:649–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cumberbatch M, Dearman RJ, Kimber I. Langerhans cells require signals from both tumour necrosis factor-α and interleukin-1β for migration. Immunology 1997; 92:388–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bal SM, Ding Z, van Riet E, Jiskoot W, Bouwstra JA. Advances in transcutaneous vaccine delivery: do all ways lead to Rome? J Control Release 2010; 148:266–82. [DOI] [PubMed] [Google Scholar]
- 14. Jakob T, Udey MC. Epidermal Langerhans cells: from neurons to nature’s adjuvants. Adv Dermatol 1999; 14:209–59. [PubMed] [Google Scholar]
- 15. Arya J, Prausnitz MR. Microneedle patches for vaccination in developing countries. J Control Release 2016; 240:135–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Qin H, Cha SC, Neelapu SS, et al. Vaccine site inflammation potentiates idiotype DNA vaccine-induced therapeutic T cell-, and not B cell-, dependent antilymphoma immunity. Blood 2009; 114:4142–9. [DOI] [PubMed] [Google Scholar]
- 17. Lu F, Hogenesch H. Kinetics of the inflammatory response following intramuscular injection of aluminum adjuvant. Vaccine 2013; 31:3979–86. [DOI] [PubMed] [Google Scholar]
- 18. Munks MW, McKee AS, Macleod MK, et al. Aluminum adjuvants elicit fibrin-dependent extracellular traps in vivo. Blood 2010; 116:5191–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Marsee DK, Williams JM, Velazquez EF. Aluminum granuloma after administration of the quadrivalent human papillomavirus vaccine: report of a case. Am J Dermatopathol 2008; 30:622–4. [DOI] [PubMed] [Google Scholar]
- 20. Shivananda, Somani V, Srikanth BS, Mohan M, Kulkarni PS. Comparison of two hepatitis B vaccines (GeneVac-B and Engerix-B) in healthy infants in India. Clin Vaccine Immunol 2006; 13:661–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Perez Cuevas MB, Kodani M, Choi Y, et al. Hepatitis B vaccination using a dissolvable microneedle patch is immunogenic in mice and rhesus macaques. Bioeng Transl Med 2018; 3:186–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mast EE, Weinbaum CM, Fiore AE, et al. A comprehensive immunization strategy to eliminate transmission of hepatitis B virus infection in the United States: recommendations of the Advisory Committee on Immunization Practices (ACIP). Part II: immunization of adults. MMWR Recomm Rep 2006; 55:1–33; quiz CE1-4. [PubMed] [Google Scholar]
- 23. Stevens CE, Beasley RP, Tsui J, Lee WC. Vertical transmission of hepatitis B antigen in Taiwan. N Engl J Med 1975; 292:771–4. [DOI] [PubMed] [Google Scholar]
- 24. Freed DC, Towne VM, Casimiro DR, Zhao Q, Fu TM. Evaluating functional antibodies in rhesus monkeys immunized with hepatitis B virus surface antigen vaccine with novel adjuvant formulations. Vaccine 2011; 29:9385–90. [DOI] [PubMed] [Google Scholar]
- 25. Reyes-del Valle J, Hodge G, McChesney MB, Cattaneo R. Protective anti-hepatitis B virus responses in rhesus monkeys primed with a vectored measles virus and boosted with a single dose of hepatitis B surface antigen. J Virol 2009; 83:9013–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Edens C, Dybdahl-Sissoko NC, Weldon WC, Oberste MS, Prausnitz MR. Inactivated polio vaccination using a microneedle patch is immunogenic in the rhesus macaque. Vaccine 2015; 33:4683–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. del Pilar Martin M, Weldon WC, Zarnitsyn VG, et al. Local response to microneedle-based influenza immunization in the skin. MBio 2012; 3:e00012–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Arnoldi J, Moll H. Langerhans cell migration in murine cutaneous leishmaniasis: regulation by tumor necrosis factor α, interleukin-1β, and macrophage inflammatory protein-1α. Dev Immunol 1998; 6:3–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Moser B, Loetscher P. Lymphocyte traffic control by chemokines. Nat Immunol 2001; 2:123–8. [DOI] [PubMed] [Google Scholar]
- 30. Dorner BG, Scheffold A, Rolph MS, et al. MIP-1α, MIP-1β, RANTES, and ATAC/lymphotactin function together with IFN-γ as type 1 cytokines. Proc Natl Acad Sci U S A 2002; 99:6181–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Resch TK, Wang Y, Moon SS, et al. Inactivated rotavirus vaccine by parenteral administration induces mucosal immunity in mice. Sci Rep 2018; 8:561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cyster JG. Chemokines and cell migration in secondary lymphoid organs. Science 1999; 286:2098–102. [DOI] [PubMed] [Google Scholar]
- 33. Kamili S, Sozzi V, Thompson G, et al. Efficacy of hepatitis B vaccine against antiviral drug-resistant hepatitis B virus mutants in the chimpanzee model. Hepatology 2009; 49:1483–91. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.