Complement-fixing antibodies targeting Plasmodium vivax merozoite surface protein 3α are prevalent in both children and adults with infection, with both immunoglobulin G and M mediating complement fixation. Magnitudes of complement-fixing antibodies are influenced by antigenic region.
Keywords: Complement-fixing antibodies, malaria, Plasmodium vivax, PvMSP3α
Abstract
Background
Complement-fixing antibodies are important mediators of protection against Plasmodium falciparum malaria. However, complement-fixing antibodies remain uncharacterized for Plasmodium vivax malaria. P. vivax merozoite surface protein 3α (PvMSP3α) is a target of acquired immunity and a potential vaccine candidate.
Methods
Plasma from children and adults with P. vivax malaria in Sabah, Malaysia, were collected during acute infection, 7 and 28 days after drug treatment. Complement-fixing antibodies and immunoglobulin M and G (IgM and IgG), targeting 3 distinctive regions of PvMSP3α, were measured by means of enzyme-linked immunosorbent assay.
Results
The seroprevalence of complement-fixing antibodies was highest against the PvMSP3α central region (77.6%). IgG1, IgG3, and IgM were significantly correlated with C1q fixation, and both purified IgG and IgM were capable of mediating C1q fixation to PvMSP3α. Complement-fixing antibody levels were similar between age groups, but IgM was predominant in children and IgG3 more prevalent in adults. Levels of functional antibodies increased after acute infection through 7 days after treatment but rapidly waned by day 28.
Conclusion
Our study demonstrates that PvMSP3α antibodies acquired during P. vivax infection can mediate complement fixation and shows the important influence of age in shaping these specific antibody responses. Further studies are warranted to understand the role of these functional antibodies in protective immunity against P. vivax malaria.
Plasmodium vivax malaria is a major contributor to global malaria burden and remains a challenge for elimination and eradication efforts [1]. Morbidity from malarial infections arises from blood-stage replication, and its control is critical for the prevention of clinical disease [2]. Reduction of blood-stage infection also limits gametocyte formation, thus reducing transmission [3, 4]. The first step in each replication cycle of blood-stage malaria is merozoite invasion of red blood cells. Blood exposure of merozoites to immune mediators and chemical inhibitors make this parasite stage a key therapeutic target [2, 5].
Antibodies are key mediators of malarial immunity. Functional antibodies can fix complement at the merozoite to prevent red blood cell invasion, and this immune mechanism is associated with protection from Plasmodium falciparum malaria [6–9]. In addition, complement-fixing antibodies have been associated with better artemisinin treatment efficacy [10]. Multiple antigen targets of complement-fixing antibodies that mediate protection have been identified in P. falciparum [8], and both immunoglobulin G (IgG) and immunoglobulin M (IgM) antibodies mediate complement fixation to the parasite [6, 9]. However, complement-fixing antibodies were more strongly associated with protection than total IgG or IgM responses [9], suggesting that it is the quality and not only the quantity of the antibody response that is important in protection. A study published in 2018 provided initial evidence for the acquisition of complement-fixing antibodies against P. vivax merozoites [11]. However to our knowledge, no other studies have investigated complement-fixing antibodies targeting P. vivax, and the impact of protein regions and age on complement-fixing antibodies, kinetics of acquisition and decay, and the specific antibody isotypes and subclasses associated with functional complement-fixing antibodies are unknown.
P. vivax merozoite surface protein 3α (PvMSP3α) is a member of the PvMSP3 family characterized by an alanine-rich central domain containing a series of heptad repeats that are predicted to form coiled-coil structures involved in protein-protein interactions [12]. The carboxy terminal (C-terminal) and hydrophilic amino (N-terminal) domains of PvMSP3α are relatively conserved, whereas the central domains (block I and II sequences) are highly polymorphic [13]. In malaria-endemic regions, naturally occurring antibodies to PvMSP3α (C-terminal and central regions) have been associated with protection from P. vivax malaria in different populations [14, 15]. Antibodies that target PvMSP3α are predominately cytophilic IgG1 and IgG3 [14]. These IgG subclasses have strong complement fixation potential [2], suggesting that complement-fixing antibodies may play an important role in immunity targeting PvMSP3. To date, no studies have investigated IgM reactivity against PvMSP3α, and recent work has shown that IgM mediates complement fixation and prevention of clinical P. falciparum [9]. Whether IgM mediates a similar functional complement mechanism in P. vivax malaria remains to be determined.
In the current study, we investigated the induction of antibody-mediated complement fixation after acute P. vivax malaria episodes from a low-malaria-endemic setting in Sabah, Malaysia. To increase our knowledge on the factors influencing development of functional complement-fixing antibodies targeting P. vivax malaria, we investigated the influence of age, antigenic region, and antibody composition of responses on antibody responses in children and adults with P. vivax malaria.
METHODS
Detailed methods are provided in the Supplementary Material.
Ethics Statement
Written and informed consent was obtained from all study participants, with consent obtained from parents or guardians in the case of children. Studies were approved by the Ethics Committee of Menzies School of Health Research and the Medical Research and Ethics Committee, Ministry of Health Malaysia.
Study Cohort
Plasma samples were obtained from patients with polymerase chain reaction (PCR)–confirmed P. vivax malaria with fever, who were seeking treatment and subsequently enrolled on diagnosis in malaria cohort studies in Sabah, Malaysia [16, 17], a region of low P. vivax endemicity [18]. Patients were treated according to hospital guidelines. Uninfected control plasma samples were obtained from visitors or patient’s relatives with no history of fever in the last 48 hours, who were blood film negative by microscopy and confirmed negative for Plasmodium infection by PCR. Samples were grouped as from children (aged ≤15 years) or adults (aged >15 years).
Recombinant Antigens
Proteins representing different regions of PvMSP3α were used: N-terminal (nucleotides 73–309), central region (block I–II; nucleotides 316–2058), and C-terminal (nucleotides 2059–2523). Proteins were PCR amplified from P. vivax (Belem strain) and expressed in a vector containing a C-terminal 6His-Tag [14].
Enzyme-Linked Immunosorbent Assays
Enzyme-linked immunosorbent assays were performed as described elsewhere [6, 8]. Plates were coated with PvMSP3α antigens (0.5 µg/mL), blocked, and incubated with plasma (1:250 dilution). Total IgG was quantified with horseradish peroxidase (HRP)-conjugated sheep anti-human IgG (1:2000 dilution) (Life Technologies). For IgM and IgG subclasses, monoclonal mouse anti-human IgM and IgG antibodies (Life Technologies) and goat polyclonal anti-mouse IgG-HRP (Merck Millipore) were added (1:2000 dilution). For C1q fixation, recombinant C1q (10 µg/mL; Quidel) was added after human plasma, and C1q was detected with 1:2000 dilution rabbit anti-C1q antibodies (Dako) and 1:4000 dilution goat anti-rabbit HRP (Bio-Rad).
Malaria-unexposed control sample were obtained from Australian donors. Positive responses were defined as absorbance greater than the average of 7 malaria-unexposed controls plus 3 standard deviations.
Antibody Purification
Plasma samples from P. vivax–infected patients (n = 5) and malaria-unexposed control samples (n = 15) were pooled separately and immunoglobulins precipitated from each pool using saturated ammonium sulfate, and then dialyzed in phosphate-buffered saline. Purification of IgG and IgM was performed using NAb Protein G Spin Column (Thermo Scientific) according to the manufacturer’s instructions. The IgM fraction refers to non-IgG fraction from the column. Both IgG and IgM fractions were dialyzed overnight in phosphate-buffered saline and concentrated using 10-kDa spin columns (Amicon; Merck Millipore).
Statistical Analysis
All analyses were performed using Stata (version 15.0; StataCorp) and GraphPad Prism (version 7.03) software. Differences in antibody magnitudes between age groups were compared using the Mann-Whitney nonparametric test. For comparisons between antigen regions and follow-up time points, Friedman and Wilcoxon-matched pair tests were used, and χ 2 tests were used to compare seroprevalence between antigen regions.
RESULTS
Prevalence of Complement-Fixing Antibodies in Adults and Children With Acute P. vivax Malaria
Antibodies against PvMSP3α were assessed in 21 uninfected subjects and 52 patients with P. vivax malaria (24 children and 28 adults; Table 1). The median level of parasitemia were similar in adults and children (P = .45).
Table 1.
Demographic Parameters of Individuals Providing Sabah Study Samples
| Patients With Plasmodium vivax Malaria | |||
|---|---|---|---|
| Parameter | Uninfected Individuals (n = 21) | Children (n = 24) | Adults (n = 28) |
| Male sex, no. (%), y | 12 (57) | 14 (58) | 24 (86) |
| Age, median (range), y | 39 (18–67) | 10 (5–15) | 34 (16–57) |
| Parasitemia, median (IQR), iRBCs/µL | 0 | 3518 (1490–9610) | 2470 (2470–7600) |
Abbreviations: IQR, interquartile range; iRBCs, infected red blood cells.
Note: Parasitemia was measured during acute presentation of malaria by blood smear. Uninfected samples are Sabah adults with parasite negative by polymerase chain reaction analysis.
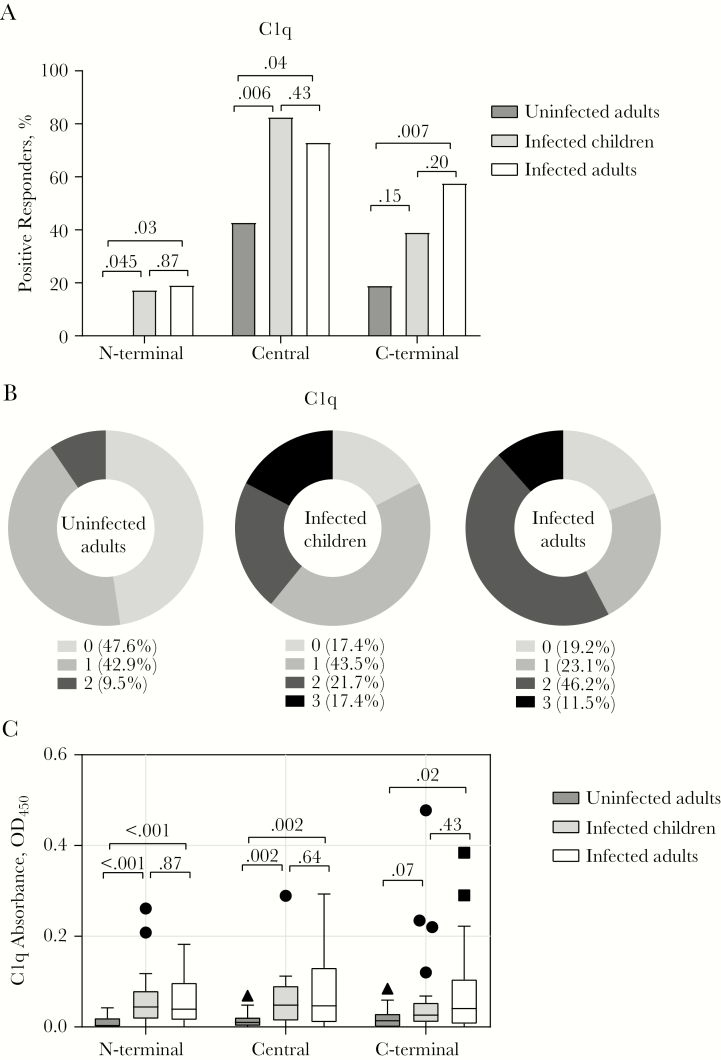
We first assessed antibody-mediated complement fixation, targeting 3 regions of PvMSP3α in children and adults presenting with acute P. vivax malaria, and adults who were PCR negative for P. vivax infection [19] (Table 1). Uninfected adults in this cohort may have been previously exposed to malaria. We quantified the antibody fixation of C1q, the first serum component of the antibody-dependent classic activation pathway (it has been previously demonstrated that C1q fixation is correlated with complement activation and formation of the membrane attack complex [8, 9]). The seroprevalence of C1q-fixing antibodies varied across antigen regions, with the highest overall prevalence against the central region (children, 82.6%; adults, 73.1%), followed by the C-terminal (children, 39.1%, adults, 57.7%) and N-terminal (children,17.4%; adults, 19.2%) regions (Figure 1A and Supplementary Figure 1A).
Figure 1.
Seroprevalence and magnitude of C1q-fixing antibodies targeting Plasmodium vivax merozoite surface protein 3α (PvMSP3α) in children and adults with P. vivax malaria and in uninfected adults. A, Seroprevalence of C1q-fixing antibodies against different regions of PvMSP3α. The positive threshold for seroprevalence was calculated as above the mean plus 3 standard deviations of absorbance detected in malaria-naive Australian donors. Numbers atop brackets are P values; the χ 2 test was used for comparisons between groups. B, Cumulative C1q-fixing antibody responses targeting different regions of PvMSP3α. Data represent the percentage of individuals who are positive for the proteins tested. C, Magnitudes of C1q-fixing antibody responses against different regions of PvMSP3α. For boxplots, the lower and upper lines of boxplot represent the first and third quartiles, the whisker lines correspond to the highest and lowest values no further than 1.5 times the interquartile range, and horizontal lines within boxes indicate medians. Data beyond the whisker lines are treated as outliers, represented as circles, squares and triangles. Numbers atop brackets are P values; the Mann-Whitney test was used for comparisons between groups. Abbreviation: OD450, optical density at 450 nm.
There was no difference in seroprevalence of C1q fixation between infected children and adults (Figure 1A). Compared with uninfected individuals, P. vivax–infected individuals had higher prevalence of C1q-fixing antibodies regardless of age, except for antibody responses in children against the C-terminal domain (Figure 1A). Overall, the majority of infected individuals (82.6% of children and 80.8% of adults) had C1q-fixing antibodies recognizing ≥1 of the PvMSP3α region, compared with only 52.4% of uninfected individuals (Figure 1B). Similarly, the magnitude of C1q-fixing antibodies did not differ between the 2 infected age groups, with both being higher than in uninfected individuals (Figure 1C). Uninfected individuals had higher magnitudes of C1q-fixing antibodies to the central region than malaria-unexposed individuals (Supplementary Figure 1B). The magnitudes of C1q-fixing antibodies were similar across PvMSP3α regions, except that responses for the central region were significantly higher than those for the C-terminus (Supplementary Figure 1B).
Age Dependence of Antibody Composition During Infection
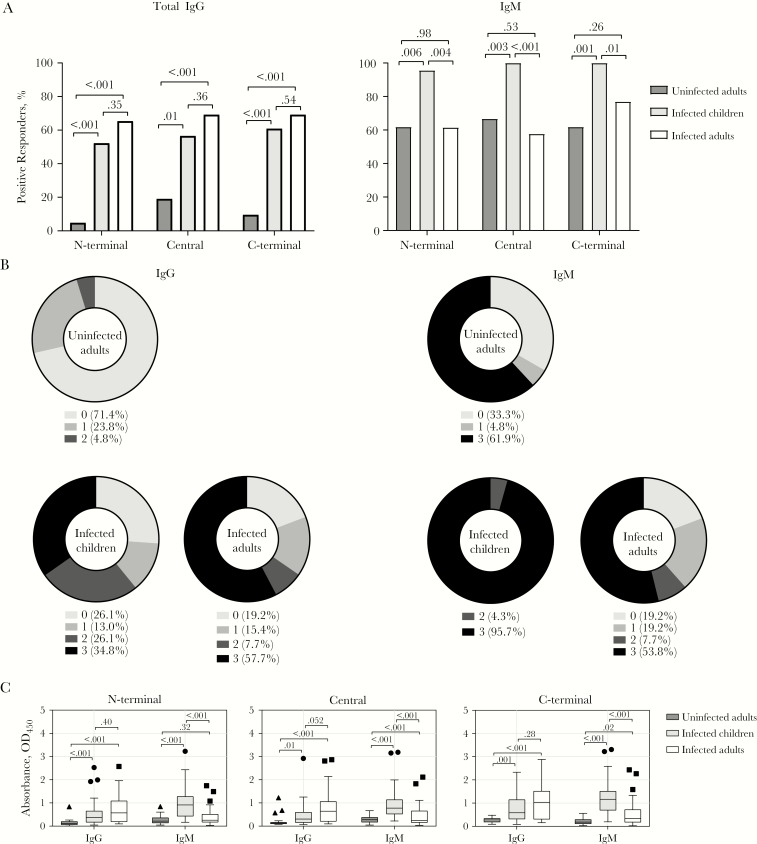
Both IgG and IgM antibodies have C1q-fixing capacity against P. falciparum antigens [6, 9]. To investigate the relative importance of IgG and IgM antibody isotypes in mediating complement fixation on PvMSP3α, we first quantified magnitudes of total IgG (pan–anti-IgG detecting all subclasses) and IgM in P. vivax–infected patients and uninfected individuals. During P. vivax infection, both IgG and IgM seroprevalence was high across all PvMSP3α regions (IgG and IgM in children, 52%–61% and 96%–100%, respectively; in adults, 65%–69% and 58%–77%) (Figure 2A and Supplementary Figure 2A). There was no difference in IgG seroprevalence between infected children and adults, but children had significantly higher IgM seroprevalence (Figure 2A). IgG seroprevalence was significantly lower in uninfected than in infected individuals. There was no overall difference in seroprevalence of IgG and IgM in infected individuals across the PvMSP3α regions (Supplementary Figure 2A). A large proportion of children and adults had IgG and IgM recognition to ≥1 PvMSP3α region (74%–81% for IgG and 81%–96% for IgM) (Figure 2B). The breadth of IgM responses in children was higher than that of IgG, with 95.7% recognizing all 3 PvMSP3α regions as opposed to 34.8% for IgG (χ 2 test, P < .001). In uninfected individuals, IgM recognition to ≥1 PvMSP3α region was also significantly higher than IgG recognition (χ 2 test, P = .01).
Figure 2.
Seroprevalence and magnitude of immunoglobulin G and M (IgG and IgM) antibodies to Plasmodium vivax merozoite surface protein 3α (PvMSP3α) in children and adults with P. vivax malaria and uninfected adults. A, Seroprevalence of IgG and IgM antibodies against different regions of PvMSP3α. The positive threshold for seroprevalence was calculated as above the mean plus 3 standard deviations of absorbance detected in malaria-naive Australian donors. Numbers atop brackets are P values; the χ 2 test was used for comparisons between groups. B, Cumulative IgG and IgM antibody responses targeting different regions of PvMSP3α. Data represent percentage of individuals who are positive for the protein regions tested. C, Magnitudes of IgG and IgM antibody responses against different regions of PvMSP3α. For boxplots, the lower and upper lines of boxplot represent the first and third quartiles, whisker lines correspond to the highest and lowest values no further than 1.5 interquartile range, and horizontal lines within boxes indicate medians. Data beyond the whisker lines are treated as outliers, represented as circles, squares and triangles. Numbers atop brackets are P values; the Mann-Whitney test was used for comparisons between groups. Abbreviation: OD450, optical density at 450 nm.
Similarly, the magnitudes of IgG in infected children and adults were comparable, whereas the magnitude of IgM was significantly higher in children than in adults against all PvMSP3α regions (Figure 2C). Uninfected individuals had lower IgG and IgM than infected individuals, except for IgM to the N-terminal region which was comparable (Figure 2C). However, compared with unexposed controls, uninfected individuals had higher magnitudes of IgG antibodies (to central and C-terminal) and higher magnitudes of IgM antibodies to all regions, suggesting that the uninfected individuals had prior malaria exposure and some levels of maintenance of acquired antibodies (Supplementary Figure 2B). Overall, the magnitudes of both IgG and IgM were highest against C-terminal regions (Supplementary Figure 2B).
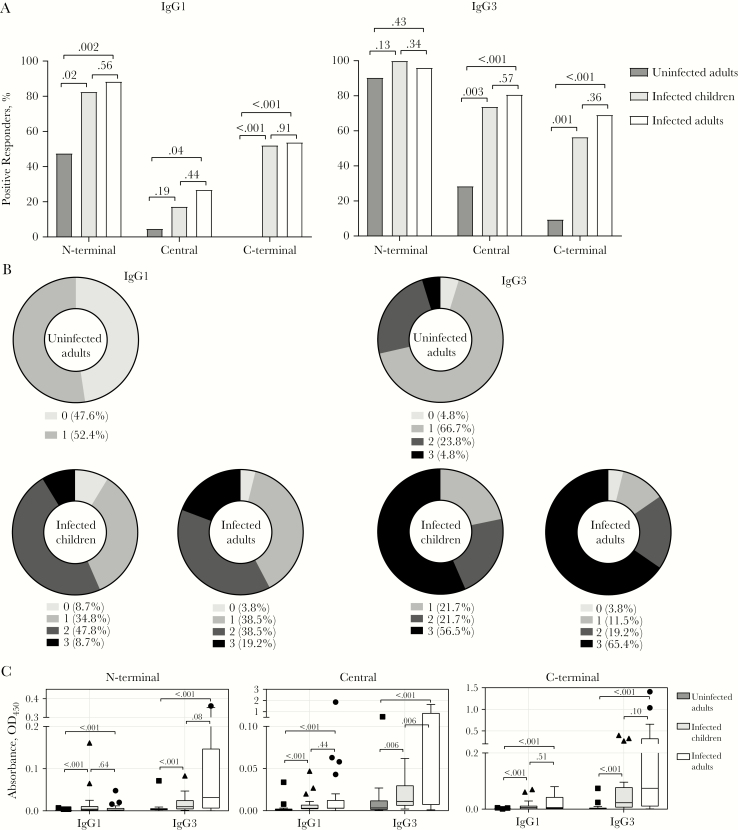
We next quantified the composition of the IgG subclass responses. Consistent with prior studies [14], IgG1 and IgG3 were the dominant antibodies to PvMSP3α (Figure 3) whereas IgG2 and IgG4 responses were below detection limits and therefore were not analyzed further (data not shown). IgG1 and IgG3 seroprevalences were similar between infected children and adults across all regions of PvMSP3α (Figure 3A). Compared with uninfected individuals, seroprevalence in the infected individuals was significantly higher across all PvMSP3α regions, except for IgG3 seroprevalence against the N-terminal region (Figure 3A). Comparisons between regions show that IgG1 seroprevalence was highest against the N-terminal, followed by the C-terminal and central regions (Supplementary Figure 3A). For IgG3, seroprevalence was highest against the N-terminal, followed by the central and C-terminal regions (Supplementary Figure 3A). The majority of infected children and adults had IgG1 and IgG3 recognition to PvMSP3α antigens, with 91%–96% and 96%–100%, respectively, recognizing ≥1 PvMSP3α region (Figure 3B). Antibody recognition to ≥1 PvMSP3α region in uninfected individuals was moderate for IgG1 (52.4%) and high for IgG3 (95.2%) (Figure 3B).
Figure 3.
Seroprevalence and magnitude of immunoglobulin G1 and G3 (IgG1 and IgG3) antibodies to Plasmodium vivax merozoite surface protein 3α (PvMSP3α) in children and adults with P. vivax malaria and uninfected adults. A, Seroprevalence of IgG1 and IgG3 antibodies against different regions of PvMSP3α. The positive threshold for seroprevalence was calculated as above the mean plus 3 standard deviations of absorbance detected in malaria-naive Australian donors. Numbers atop brackets are P values; the χ 2 test was used for comparisons between groups. B, Cumulative IgG1 and IgG3 antibody responses targeting different regions of PvMSP3α. Data represent percentage of individuals who are positive for the proteins tested. C, Magnitudes of IgG1 and IgG3 antibody responses against different regions of PvMSP3α. For boxplots, lower and upper lines of boxplot represent first and third quartiles, whisker lines correspond to the highest and lowest values no further than 1.5 interquartile range, and horizontal lines within boxes indicate medians. Data beyond the whisker lines are treated as outliers, represented as circles, squares and triangles. Numbers atop brackets are P values; the Mann-Whitney test was used for comparisons between groups. Abbreviation: OD450, optical density at 450 nm.
The magnitudes of IgG1 and IgG3 responses in infected children and adults were similar, except for significantly higher IgG3 responses against the central region in adults (Figure 3C). IgG1 and IgG3 responses were significantly higher in infected individuals than in uninfected individuals across all regions (Figure 3C). Compared with unexposed individuals, uninfected Malaysia individuals had higher magnitudes of IgG3 but not IgG1 to all protein regions (Supplementary Figure 3B). There was no difference in the magnitude of IgG1 between PvMSP3α regions (Supplementary Figure 3B). For IgG3, the magnitude was lowest against the N-terminal domain, with similar responses between the central region and the C-terminal domain (Supplementary Figure 3B).
IgG and IgM Mediation of Complement Fixation to PvMSP3
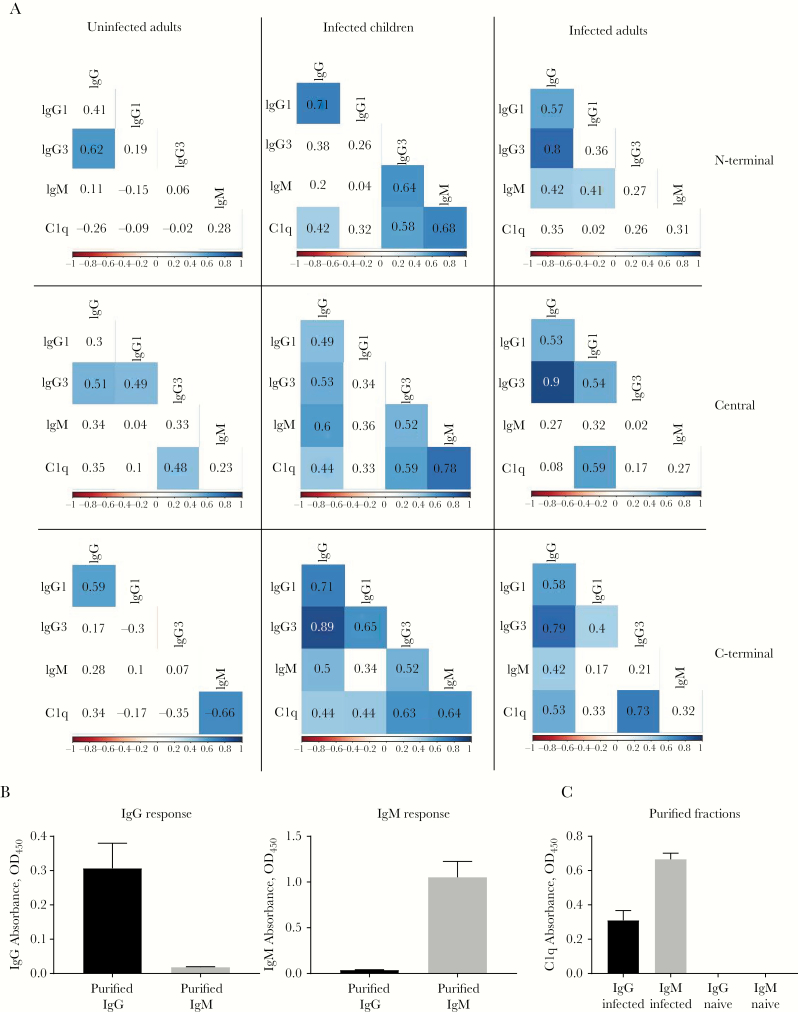
To identify specific antibodies that may mediate complement fixation on PvMSP3α, we assessed correlations between C1q fixation and antibody responses. Total IgG was positively associated with C1q fixation in infected children and adults; the correlations were significant across all PvMSP3α regions in children, whereas only the C-terminus was significantly correlated in adults (Figure 4A). IgG1 responses in children were significantly correlated with C1q fixation against the C-terminal region, whereas elevated levels of IgG3 were associated with all antigenic regions of PvMSP3α. In comparison, significant correlations with C1q fixation were observed for IgG1 only against the central region and for IgG3 only against the C-terminus in infected adults (Figure 4A). No significant correlation was observed between IgG antibodies and C1q fixation in uninfected individuals. IgM was also associated with C1q fixation in children but not in infected adults (Figure 4A). In uninfected adults, IgM response against the C-terminal region had significant correlation with C1q fixation. Overall, IgG1 and IgG3 responses in infected adults, and IgG1, IgG3, and IgM responses in infected children, were correlated with C1q fixation (Supplementary Figure 4).
Figure 4.
Functional C1q-fixing capacity of antibody isotypes. A, Correlations matrixes between immunoglobulin G1, G3, and M (IgG1, IgG3, and IgM) and C1q-fixing antibodies to Plasmodium vivax merozoite surface protein 3α (PvMSP3α) in infected children, infected adults, and uninfected adults. Values represent Spearman correlation coefficients, and blue boxes indicate statistical significance (P < .05). B, Total immunoglobulin G (IgG) and IgM absorbance readings against PvMSP3α central region from purified IgG and IgM fractions in malaria-infected plasma pools. C, C1q fixation absorbance readings against PvMSP3α central region from purified IgG and IgM fractions from malaria-infected (n = 5) and malaria-unexposed (n = 14) plasma pools. Abbreviation: OD450, optical density at 450 nm.
Our results suggest that both IgG and IgM antibodies contribute to complement fixation to PvMSP3α. To test this hypothesis, IgG and IgM were purified from two separate pools of P. vivax–infected (n = 5) and malaria-unexposed individuals (n = 15). The purity of IgG and IgM fractions was confirmed via enzyme-linked immunosorbent assay (Figure 4B). Both IgG and IgM from P. vivax–infected donors showed effective fixation of C1q to the PvMSP3α central region, compared with no C1q fixation in malaria-unexposed donors (Figure 4C).
Increase and Decay of Complement-fixing Antibodies to PvMSP3α After P. vivax Infection
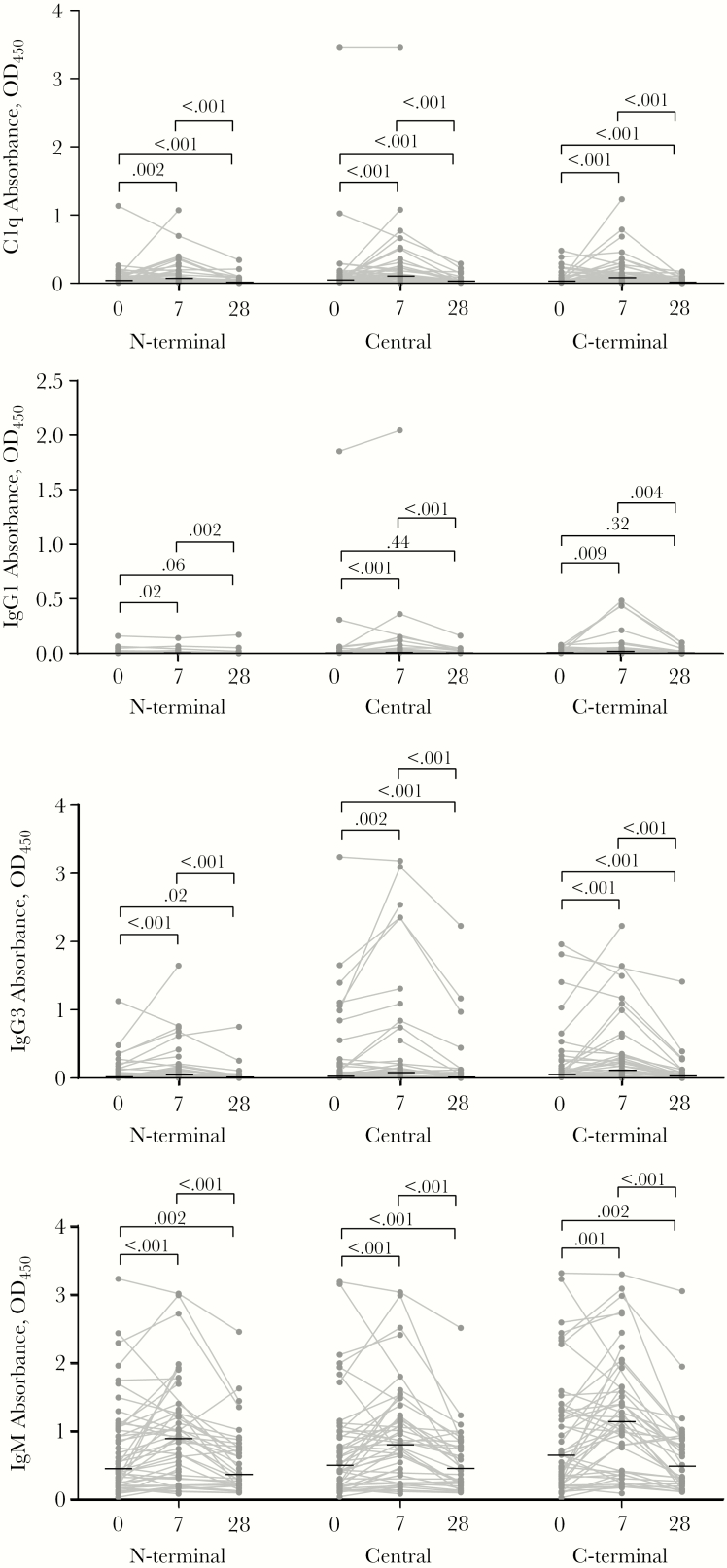
To investigate the acquisition and decay of complement-fixing antibodies after P. vivax infection, we compared antibody levels in Sabah individuals during their infections with levels 7 and 28 days after drug treatment and parasite clearance. Levels of C1q-fixing antibodies were significantly elevated at day 7 after treatment (Figure 5). Antibodies decayed and returned to levels seen at acute infection by day 28 after treatment (Figure 5). The patterns of antibody kinetics for IgG1, IgG3, and IgM were similar to those of C1q-fixing antibodies (Figure 5), although levels of IgG1 were generally low. There was no marked difference in kinetics between infected children and adults (Supplementary Figures 5 and 6). Antibody levels during acute infection (day 0) were not associated with parasitemia (Supplementary Figure 7A). However, IgG (to N-terminal and central region) antibodies at convalescences (day 28) were negatively associated with parasitemia during acute infection (Supplementary Figure 7B).
Figure 5.
Antibody kinetics profile across 28-day follow-up against Plasmodium vivax merozoite surface protein 3α (PvMSP3α). Magnitudes of C1q-fixing antibodies, immunoglobulin G1 G3, and M (IgG1, IgG3, and IgM) against different regions of PvMSP3α were compared between day 0 and days 7 and 28 follow-up. Gray dots and lines represent individual antibody magnitudes over time; horizontal lines, medians. Numbers atop brackets are P values, calculated with the Wilcoxon signed ranked test. Abbreviation: OD450, optical density at 450 nm.
DISCUSSION
Complement-fixing antibodies have been identified as important mediators of immunity against P. falciparum malaria [6, 8, 20], however, our understanding of the targets and acquisition of complement-fixing antibodies against P. vivax is limited. We investigated the acquisition of complement-fixing antibodies targeting 3 regions of a major P. vivax merozoite surface protein, PvMSP3α. Our data show that, during infection, complement-fixing antibodies are prevalent in both children and adults, and the magnitudes of these antibodies differ between antigenic regions of PvMSP3α. The composition of antibody responses was age dependent; IgM was more prominent in children, whereas IgG responses dominated in adults. Furthermore, we established that both IgG and IgM mediated complement fixation to PvMSP3α, suggesting that both isotypes have important roles in functional immunity against P. vivax malaria.
We demonstrated that complement-fixing antibodies are highly prevalent in Sabah patients, especially antibodies to the variable central region. However, the overall prevalence of complement-fixing antibodies is lower than the total for IgG and IgM antibodies. The central region consists of 2 blocks of heptad repeats that have 18 of 25 B-cell epitopes of PvMSP3α, thus providing the most recognition sites for antibody binding on the protein [14], a factor that may increase the induction of complement-fixing antibodies against this region. Differences in the antibody composition targeting each region of PvMSP3α may also influence the magnitude of complement-fixing antibodies. IgG responses against PvMSP3α were mainly IgG1 and IgG3 subclasses, consistent with previous findings [14]. However, the overall balance of IgG1 versus IgG3 was region specific, with IgG1 highest against the conserved N-terminal region, and IgG3 highest against the central region; IgG3 has a higher capacity to bind complement than IgG1 [21].
Consistent with these findings, previous P. falciparum studies show that antigenic regions with polymorphic sequences (eg, merozoite surface protein 1, block 2) tend to induce IgG3, whereas those with conserved regions (eg, merozoite surface protein 1–19) tend to induce IgG1 responses [22, 23]. Other studies of P. falciparum suggest that protein structure can also influence the nature of IgG subclasses responses [24]. Several other factors may also influence the ability of antibodies to mediate complement fixation, including specific epitope of antibody binding [25], epitope distance from cell surfaces [26], levels of nonfunctional interfering antibodies that block complement fixation [27] and glycosylation of binding antibodies [28]. Although more studies are required to elucidate how each of these factors influences complement fixation to Plasmodium antigens, together they may explain why the overall prevalence and magnitude of complement-fixing antibodies is lower than the prevalence/magnitude of total IgG and IgM antibodies.
During P. vivax malaria, there was no difference in the seroprevalence or magnitude of complement-fixing antibodies between infected children and adults. However, the overall composition of the antibody response was age dependent, with higher IgM levels in children and higher IgG3 levels in adults. Both IgG and IgM were correlated with complement-fixing antibodies, and both purified IgG and IgM from P. vivax–infected patients had the capacity to mediate complement fixation to PvMSP3α. The role of IgM in complement fixation against P. vivax is consistent with previous findings in P. falciparum malaria [8, 9, 20, 29], emphasizing the importance of further studies of IgM to elucidate protective immunity against Plasmodium spp. infecting humans.
The age-specific differences in antibody isotype levels could be attributed to different P. vivax infection histories. IgM, with higher levels in children, is thought to be a rapidly induced response that dominates primary infection [30]. Currently, P. vivax transmission in Sabah is undergoing major reductions from the previously higher transmission intensity [31]. As such, children enrolled in our study may not have been previously exposed to P. vivax, and adults were more likely to have had prior infection(s) [32]. Thus, the adult-specific dominance of IgG3 may be due to the induction of antibodies from preexisting B-cell memory.
These findings are consistent with those of a PvMSP1 study in Brazil, where individuals with first malaria episodes mounted higher IgM responses, and IgG responses were higher in previously exposed individuals [33]. Despite the IgM dominance in children, the seroprevalence of IgM was also high in uninfected adults from malaria-endemic Sabah, and the magnitude of PvMSP3α IgM antibodies was significantly higher than detected in malaria-unexposed donors. Although the malaria history and the recency of infection in our uninfected adults is unknown, these data suggest that IgM responses to PvMSP3α are relatively long-lived. Recent studies of antibody responses to P. falciparum merozoites in multiple populations have found that IgM is a prominent component of the response, even in children and adults with high life-time exposure. Furthermore, rather than rapidly decaying after infection, IgM showed kinetics similar to that in IgG [9].
After malaria treatment, complement-fixing IgG and IgM antibodies to PvMSP3α increased rapidly within 7 days but declined to levels seen at acute presentation by day 28. Similar findings in P. falciparum also show that antimerozoite antibodies peaked 7 days after malaria and quickly declined within weeks of infection [34]. In other P. vivax antigens, antibodies against PvMSP1 also seem to be short-lived, with the majority of individuals becoming seronegative 2 months after antimalarial treatment [33]. However, other studies have reported that IgG responses to some antigens are much better maintained [35–41]. Within our population, levels of IgG antibodies at day 28 after infection were negatively associated with parasitemia at acute infection. This finding is consistent with mouse models of malaria, where high parasitemia has been associated with negative consequences for long-lived and mature antibody responses [42]. This observation in human infection with P. vivax warrants further investigation; however the lack of long-term follow-up in our patients precludes the study of the maintenance of antibody responses that may be generated from long-lived cells, and in memory phase immunity.
In P. falciparum infections, complement fixation of antibodies targeting merozoites and sporozoites is a better correlate of protection than total antibody levels, highlighting its significance in malaria immunity [8, 9, 20]. Given the nature of our study, we were not able to define the association of complement fixation with protection from infection or disease. However, we show that PvMSP3α is a target of complement-fixing antibodies, and future studies are warranted to investigate the association between complement-fixing antibodies targeting PvMSP3α and other merozoite antigens and protection from P. vivax infection. Furthermore, evidence of cross-reactivity between other P. vivax and Plasmodium knowlesi antigens has been reported previously in Sabah [43], and we cannot exclude a contribution of PvMSP3α antibody responses from prior infection with P. knowlesi.
In conclusion, our study demonstrates that antibody-mediated complement fixation against PvMSP3α antigen is prevalent in individuals with P. vivax infection living in Sabah, Malaysia, and that the prevalence and magnitudes of complement-fixing antibody responses are dependent on antigenic region. The composition of the antibody response during infection is age dependent, with IgM predominant in children and IgG3 predominant in adults. However, IgG1, IgG3 and IgM antibodies targeting PvMSP3α were all correlated with complement fixation, and both IgG and IgM mediated complement fixation to PvMSP3α. These findings are significant for understanding immunity to P. vivax malaria and for the potential development of vaccines against P. vivax. Indeed, if complement-fixing antibodies targeting P. vivax antigens are shown to be highly protective against P. vivax malaria, vaccines that induce high levels of functional complement-fixing antibodies may lead to increased protection and vaccine efficacy.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. We thank the participants in this study; the clinical and laboratory research staff; the hospital directors at the study sites; the head of Clinical Research Centre Malaysia; Kim Piera, Ammar Azis, Irene Handayuni, and Angelica Tan for providing laboratory and administrative support; and colleagues and staff of the UK Medical Research Council P. knowlesi Monkeybar Project. We also thank the director general of Ministry of Health Malaysia for permission to publish the data.
Disclaimer. The funders had no role in the study design, data collection and interpretation, or the decision to submit the work for publication
Financial support. This work was supported by the Malaysian Ministry of Health (grant BP00500420), the AusAid Asia-Pacific Malaria Elimination Network (grant 108-07), the Australian National Health and Medical Research Council (program grants 1037304 and 1092789, project grant 1045156, senior principal research fellowship 1042072 to N. M. A., project grant 1125656 to M. J. B., career development fellowships 1166753 to F. J. I. F. and 1141632 to M. J. B., early career research fellowships 1088738 to B. E. B.., and 1138860 to M. J. G., and senior research fellowship 1077636 to J. G. B.), Charles Darwin University (PhD scholarship to D. A. O.), Menzies School of Health Research (PhD top-up award to D. A. O.), the National Institutes of Health (grants 1R01AI24710 and R01AI0555994 to M. R. G.), and R01AI116472-01 to T. W. the University of Adelaide (Beacon fellowship to D. W. W.), and Channel 7 Children’s Research Foundation (grant to D. W. W. and M. J. B.).
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Presented in part: First World Malaria Congress, Melbourne, Australia, 1–5 July 2018; Lorne Infection & Immunity Conference, Lorne, Australia, 20–22 February 2019; and Seventh International Conference on Plasmodium vivax Research, Paris, France, 26–28 June 2019.
References
- 1. Robinson LJ, Wampfler R, Betuela I, et al. Strategies for understanding and reducing the Plasmodium vivax and Plasmodium ovale hypnozoite reservoir in Papua New Guinean children: a randomised placebo-controlled trial and mathematical model. PLoS Med 2015; 12:e1001891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Beeson JG, Drew DR, Boyle MJ, Feng G, Fowkes FJ, Richards JS. Merozoite surface proteins in red blood cell invasion, immunity and vaccines against malaria. FEMS Microbiol Rev 2016; 40:343–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Drakeley CJ, Bousema JT, Akim NI, et al. Transmission-reducing immunity is inversely related to age in Plasmodium falciparum gametocyte carriers. Parasite Immunol 2006; 28:185–90. [DOI] [PubMed] [Google Scholar]
- 4. Piper KP, Hayward RE, Cox MJ, Day KP. Malaria transmission and naturally acquired immunity to PfEMP-1. Infect Immun 1999; 67:6369–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Burns AL, Dans MG, Balbin JM, et al. Targeting malaria parasite invasion of red blood cells as an antimalarial strategy. FEMS Microbiol Rev 2019; 43:223–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Boyle MJ, Reiling L, Feng G, et al. Human antibodies fix complement to inhibit Plasmodium falciparum invasion of erythrocytes and are associated with protection against malaria. Immunity 2015; 42:580–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Valmaseda A, Macete E, Nhabomba A, et al. Identifying immune correlates of protection against Plasmodium falciparum through a novel approach to account for heterogeneity in malaria exposure. Clin Infect Dis 2018; 66:586–93. [DOI] [PubMed] [Google Scholar]
- 8. Reiling L, Boyle MJ, White MT, et al. Targets of complement-fixing antibodies in protective immunity against malaria in children. Nat Commun 2019; 10:610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Boyle MJ, Chan JA, Handayuni I, et al. IgM in human immunity to Plasmodium falciparum malaria. Sci Adv in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. O’Flaherty K, Ataide R, Zaloumis SG, et al. The contribution of functional antimalarial immunity to measures of parasite clearance in therapeutic efficacy studies of artemisinin derivatives. J Infect Dis 2019; 220:1178–1187. doi:10.1093/infdis/jiz247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Oyong DA, Kenangalem E, Poespoprodjo JR, et al. Loss of complement regulatory proteins on uninfected erythrocytes in vivax and falciparum malaria anemia. JCI Insight 2018; 3:e124854 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Galinski MR, Corredor-Medina C, Povoa M, Crosby J, Ingravallo P, Barnwell JW. Plasmodium vivax merozoite surface protein-3 contains coiled-coil motifs in an alanine-rich central domain. Mol Biochem Parasitol 1999; 101:131–47. [DOI] [PubMed] [Google Scholar]
- 13. Rayner JC, Corredor V, Feldman D, et al. Extensive polymorphism in the Plasmodium vivax merozoite surface coat protein MSP-3alpha is limited to specific domains. Parasitology 2002; 125:393–405. [DOI] [PubMed] [Google Scholar]
- 14. Lima-Junior JC, Jiang J, Rodrigues-da-Silva RN, et al. B cell epitope mapping and characterization of naturally acquired antibodies to the Plasmodium vivax merozoite surface protein-3α (PvMSP-3α) in malaria exposed individuals from Brazilian Amazon. Vaccine 2011; 29:1801–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Stanisic DI, Javati S, Kiniboro B, et al. Naturally acquired immune responses to P. vivax merozoite surface protein 3α and merozoite surface protein 9 are associated with reduced risk of P. vivax malaria in young Papua New Guinean children. PLoS Negl Trop Dis 2013; 7:e2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Barber BE, William T, Grigg MJ, et al. A prospective comparative study of knowlesi, falciparum, and vivax malaria in Sabah, Malaysia: high proportion with severe disease from Plasmodium knowlesi and Plasmodium vivax but no mortality with early referral and artesunate therapy. Clin Infect Dis 2013; 56:383–97. [DOI] [PubMed] [Google Scholar]
- 17. Grigg MJ, William T, Barber BE, et al. Age-related clinical spectrum of Plasmodium knowlesi malaria and predictors of severity. Clin Infect Dis 2018; 67:350–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rajahram GS, Barber BE, William T, et al. Falling Plasmodium knowlesi malaria death rate among adults despite rising incidence, Sabah, Malaysia, 2010–2014. Emerg Infect Dis 2016; 22:41–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Grigg MJ, William T, Menon J, et al. Efficacy of artesunate-mefloquine for chloroquine-resistant Plasmodium vivax malaria in Malaysia: an open-label, randomized, controlled trial. Clin Infect Dis 2016; 62:1403–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kurtovic L, Behet MC, Feng G, et al. Human antibodies activate complement against Plasmodium falciparum sporozoites, and are associated with protection against malaria in children. BMC Med 2018; 16:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Vidarsson G, Dekkers G, Rispens T. IgG subclasses and allotypes: from structure to effector functions. Front Immunol 2014; 5:520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tongren JE, Drakeley CJ, McDonald SL, et al. Target antigen, age, and duration of antigen exposure independently regulate immunoglobulin G subclass switching in malaria. Infect Immun 2006; 74:257–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cavanagh DR, Dobaño C, Elhassan IM, et al. Differential patterns of human immunoglobulin G subclass responses to distinct regions of a single protein, the merozoite surface protein 1 of Plasmodium falciparum. Infect Immun 2001; 69:1207–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Richards JS, Stanisic DI, Fowkes FJ, et al. Association between naturally acquired antibodies to erythrocyte-binding antigens of Plasmodium falciparum and protection from malaria and high-density parasitemia. Clin Infect Dis 2010; 51:e50–60. [DOI] [PubMed] [Google Scholar]
- 25. Teeling JL, Mackus WJ, Wiegman LJ, et al. The biological activity of human CD20 monoclonal antibodies is linked to unique epitopes on CD20. J Immunol (Baltimore, Md: 1950) 2006; 177:362–71. [DOI] [PubMed] [Google Scholar]
- 26. Cleary KLS, Chan HTC, James S, Glennie MJ, Cragg MS. Antibody distance from the cell membrane regulates antibody effector mechanisms. J Immunol 2017; 198:3999–4011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lilienthal GM, Rahmöller J, Petry J, Bartsch YC, Leliavski A, Ehlers M. Potential of murine IgG1 and human IgG4 to inhibit the classical complement and Fcγ receptor activation pathways. Front Immunol 2018; 9:958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Quast I, Keller CW, Maurer MA, et al. Sialylation of IgG Fc domain impairs complement-dependent cytotoxicity. J Clin Invest 2015; 125:4160–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Behet MC, Kurtovic L, van Gemert GJ, et al. The complement system contributes to functional antibody-mediated responses induced by immunization with Plasmodium falciparum malaria sporozoites. Infect Immun 2018; 86:e00920-17 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tao TW, Uhr JW. Primary-type antibody response in vitro. Science 1966; 151:1096–8. [DOI] [PubMed] [Google Scholar]
- 31. William T, Jelip J, Menon J, et al. Changing epidemiology of malaria in Sabah, Malaysia: increasing incidence of Plasmodium knowlesi. Malar J 2014; 13:390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fornace KM, Herman LS, Abidin TR, et al. Exposure and infection to Plasmodium knowlesi in case study communities in Northern Sabah, Malaysia and Palawan, The Philippines. PLoS Negl Trop Dis 2018; 12:e0006432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Soares IS, da Cunha MG, Silva MN, Souza JM, Del Portillo HA, Rodrigues MM. Longevity of naturally acquired antibody responses to the N- and C-terminal regions of Plasmodium vivax merozoite surface protein 1. Am J Trop Med Hyg 1999; 60:357–63. [DOI] [PubMed] [Google Scholar]
- 34. Kinyanjui SM, Conway DJ, Lanar DE, Marsh K. IgG antibody responses to Plasmodium falciparum merozoite antigens in Kenyan children have a short half-life. Malar J 2007; 6:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mugyenyi CK, Elliott SR, Yap XZ, et al. Declining malaria transmission differentially impacts the maintenance of humoral immunity to Plasmodium falciparum in children. J Infect Dis 2017; 216:887–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Fowkes FJ, McGready R, Cross NJ, et al. New insights into acquisition, boosting, and longevity of immunity to malaria in pregnant women. J Infect Dis 2012; 206:1612–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lim KJ, Park JW, Yeom JS, et al. Humoral responses against the C-terminal region of merozoite surface protein 1 can be remembered for more than 30 years in persons exposed to Plasmodium vivax. Parasitol Res 2004; 92:384–9. [DOI] [PubMed] [Google Scholar]
- 38. Wipasa J, Suphavilai C, Okell LC, et al. Long-lived antibody and B cell memory responses to the human malaria parasites, Plasmodium falciparum and Plasmodium vivax. PLoS Pathog 2010; 6:e1000770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kaneko A, Chaves LF, Taleo G, et al. Characteristic age distribution of Plasmodium vivax infections after malaria elimination on Aneityum Island, Vanuatu. Infect Immun 2014; 82:243–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Longley RJ, Reyes-Sandoval A, Montoya-Díaz E, et al. Acquisition and longevity of antibodies to Plasmodium vivax preerythrocytic antigens in Western Thailand. Clin Vaccine Immunol 2016; 23:117–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Longley RJ, White MT, Takashima E, et al. Naturally acquired antibody responses to more than 300 Plasmodium vivax proteins in three geographic regions. PLoS Negl Trop Dis 2017; 11:e0005888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zander RA, Guthmiller JJ, Graham AC, et al. Type I interferons induce T regulatory 1 responses and restrict humoral immunity during experimental malaria. PLoS Pathog 2016; 12:e1005945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Herman LS, Fornace K, Phelan J, et al. Identification and validation of a novel panel of Plasmodium knowlesi biomarkers of serological exposure. PLoS Negl Trop Dis 2018; 12:e0006457. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.