The lytic cassette proteins XepA and YomS from Bacillus subtilis prophages have been characterized and it was found that only XepA establishes cytotoxic activity in plaque assays. The crystal structures of both proteins show a unique pentameric assembly, in which YomS adopts a very similar fold to the C-terminal domain of the XepA dumbbell pentamer. The overall architecture of XepA, with the N-terminal domain subunits resembling cytoplasmic membrane-binding C2-domain folds, suggests that any lytic functionality could be based on disruption of the proton motive force of the cytoplasmic membrane, which induces cell lysis.
Keywords: prophage, Virus-X Consortium, lytic enzymes, lytic cassette proteins, Bacillus subtilis, XepA, YomS
Abstract
As part of the Virus-X Consortium that aims to identify and characterize novel proteins and enzymes from bacteriophages and archaeal viruses, the genes of the putative lytic proteins XepA from Bacillus subtilis prophage PBSX and YomS from prophage SPβ were cloned and the proteins were subsequently produced and functionally characterized. In order to elucidate the role and the molecular mechanism of XepA and YomS, the crystal structures of these proteins were solved at resolutions of 1.9 and 1.3 Å, respectively. XepA consists of two antiparallel β-sandwich domains connected by a 30-amino-acid linker region. A pentamer of this protein adopts a unique dumbbell-shaped architecture consisting of two discs and a central tunnel. YomS (12.9 kDa per monomer), which is less than half the size of XepA (30.3 kDa), shows homology to the C-terminal part of XepA and exhibits a similar pentameric disc arrangement. Each β-sandwich entity resembles the fold of typical cytoplasmic membrane-binding C2 domains. Only XepA exhibits distinct cytotoxic activity in vivo, suggesting that the N-terminal pentameric domain is essential for this biological activity. The biological and structural data presented here suggest that XepA disrupts the proton motive force of the cytoplasmatic membrane, thus supporting cell lysis.
1. Introduction
Bacteriophages are viruses that infect bacteria and replicate within their host cells. Prophage DNA remains integrated in the bacterial DNA until the proliferation of new phages is triggered, and ultimately the host cell is lysed to release the phage progeny. Bacteriophages employ a versatile and long-evolved proteome which is an invaluable source of proteins with significant potential biotechnological applications. For example, depolymerases have been explored for antiviral strategies (Hsieh et al., 2017 ▸), whilst tail fibre proteins have been investigated for biosensor development (Denyes et al., 2017 ▸) and as cofactors to increase the specificity of PCR-based DNA amplification (Stefanska et al., 2014 ▸). Phage proteins possess enormous potential as bacterial markers, drug transporters and for vaccine development (Drulis-Kawa et al., 2015 ▸; Plotka et al., 2015 ▸). In addition, the global threat of antibiotic resistance emphasizes the requirement for more novel tools and understanding in order to counteract bacterial infection. Lytic enzymes are increasingly being recognized as highly effective antibacterials (Briers, 2019 ▸). As the multinational Virus-X Consortium (http://virus-x.eu/) has been established to explore the function and structure of novel phage enzymes with biotechnological and biomedical potential, we chose two potentially lytic enzymes from two Bacillus subtilis 168 defective prophages as a starting point for investigations: XepA (PBSX exported protein, also known as XkdY or P31) from the PBSX prophage and YomS from the SPβ prophage (Longchamp et al., 1994 ▸).
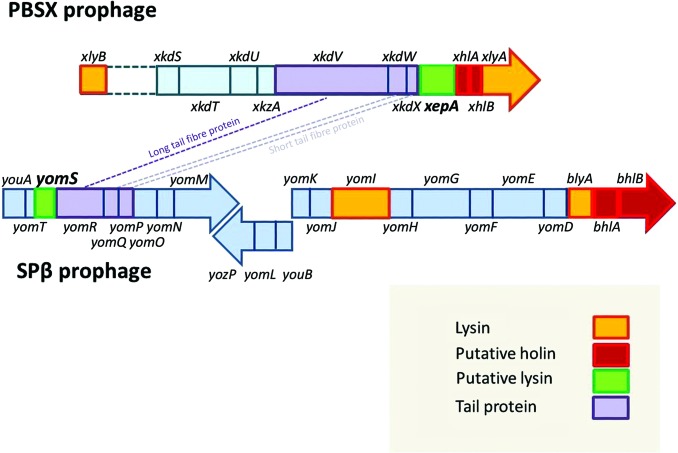
Prophage generation can be induced in B. subtilis by the addition of mitomycin C or thermally. XepA appears 20 min after induction and its concentration increases steadily until cell lysis. It is not present in the mature phage particles, but is exported beyond the cytoplasmic membrane during phage development, suggesting that the protein is involved in cell-wall metabolism or degradation (Mauël & Karamata, 1984 ▸). The genome of both phages, PBSX and SPβ, and their cell-lysis systems (Fig. 1 ▸) have been described previously (Wood et al., 1990 ▸; Lazarevic et al., 1999 ▸). Phage-induced lysis was reported to rely on the holin–lysin dyad, in which holins make the cytoplasmic membrane (CM) permeable and a lysin attacks the host peptidoglycan (PG). All phage lysins can together mostly be described as peptidoglycan hydrolases. However, they have a high diversity and attack different bonds in the PG and thus represent peptidases, amidases and glucosidases, as well as transglycosylases (Smith et al., 2000 ▸; Low et al., 2011 ▸). Although XepA was described as being part of the host cell lysis system of PBSX together with the N-acetylmuramoyl-l-alanine amidase XlyA (also known as P32) and the two putative holins XhlA and XhlB (also known as XpaB), the biological role and molecular mechanism of XepA remain elusive (Longchamp et al., 1994 ▸). The contributions of each member of the lytic system (XepA, XlyA, XhlA and XhlB) were further investigated by variation and deletion of their genes in the PBSX late operon and subsequent expression in B. subtilis (Krogh et al., 1998 ▸). The results showed that the holin XhlA is essential for cell lysis, whereas the other three proteins alone cannot induce cell lysis. XlyA was shown to have a strong affinity for teichoic acid-containing cell walls and to play the major role in the degradation of the host cell wall (Mauël & Karamata, 1984 ▸). More recently, XlyA was also reported to be essential in membrane-vesicle formation in B. subtilis (Toyofuku et al., 2017 ▸). Another gene of a putative member of the PBSX lytic system lies upstream, xlyB (also known as yjpB), which encodes a putative amidase (Smith et al., 2000 ▸).
Figure 1.
Gene organization of the PBSX and SPβ prophage late operons in the area of the lytic systems (red) described. Whereas xepA in prophage PBSX is located in the direct vicinity of the lytic entity (xhlA, xhlB, xlyA), yomS in SPβ is further removed from the blyA, bhlA, bhlB region. The cassettes containing xkdV, xkdW, xkdX and yomR, yomQ, yomP encode structural tail proteins (purple). The xlyB gene is located further upstream in the PBSX genome.
In the SPβ prophage the host cell lysis system consists of the N-acetylmuramoyl-l-alanine amidase BlyA (also known as YomC), BhlA and BhlB as putative holins and YomI (also known as CwlP), which has been described as a virion-associated peptidoglycan hydrolase (Sudiarta et al., 2010 ▸; Rodríguez-Rubio et al., 2013 ▸). YomS is further removed from the genes of the lytic system in the SPβ prophage operon and is located next to the structural tail protein-encoding cassette (Fig. 1 ▸). To date, the only structural detail described for the lytic machinery of both prophages is the X-ray structure of the catalytic domain of XlyA (Low et al., 2011 ▸). In order to elucidate their role in the lytic cassette of B. subtilis prophages, we cloned and produced XepA from the PBSX prophage and YomS from the SPβ prophage. To shed light on the function of XepA and YomS, we concentrated our efforts on gaining high-resolution structural information on the putative lysins, which we now present here together with initial functional studies.
2. Materials and methods
2.1. Cloning, protein production, purification and initial characterization
In addition to XepA and YomS, the lysins XlyA and XlyB were cloned and produced for inclusion in lytic assays. To express the respective genes in Escherichia coli, the coding regions for the xepA, xlyA, xlyB and yomS genes were amplified by PCR with genomic DNA from B. subtilis 168 serving as a template. The gene-specific primers S11669 (gcggatccGTGAAGTATCAATATGAATTTCCTC) and S11670 (gcctgtacaTTATGAAACCGCGGTCCCTTTTAC) for xepA; S11667 (gcggatccGTTAACATTATTCAAGACTTTATTC) and S11668 (gcctgtacaTCAGCTTAATTGCGCTGCGAT) for xlyA; S11665 (gcggatccAGCATTCCAGTAAAGAAAAATTTG) and S11666 (gcctgtacaTTACAGCTTTTCCTCCATCTTC) for xlyB; and S11675 (gcggatccACAGAAACGACTGAAAATGTCG) and S11676 (gcctgtacaTTAACTCACCACAATCCCTTTAAC) for yomS were used in PCR reactions, which introduced a BamHI site into the N-terminal gene sequence and a BsrGI restriction site just behind the stop codon. The respective PCR fragments were cloned in-frame into the rhamnose-inducible vector pJOE5751 (Wegerer et al., 2008 ▸) and resulted in the expression plasmids pHWG1186 for His6-xepA, pHWG1185 for His6-xlyA, pHWG1184 for His6-xlyB and pHWG1189 for His6-yomS.
Deletion mutants of xepA were constructed by PCR amplification using pHWG1186 as a template. The N-terminal xepA domain without the central tunnel region was amplified with the primers S11669 (see above) and S12646 (gcctgtacaTTAACGCACATCCATCTCACCCG) and yielded a 441 bp fragment. The N-terminal xepA domain with the tunnel region was amplified with the primers S11669 (see above) and S12647 (gcctgtacaTTAAGCCTCGACTTTCAGCCGTC), yielding a 520 bp fragment, while for amplification of the C-domain of xepA the primers S12648 (gcggatccGTCAATGAAAAAACGCCTTTACA) and S11670 (see above) were used and resulted in a 325 bp fragment. The respective PCR fragments, which contained additional BamHI and BsrGI restriction sites, respectively, were cloned in-frame into pJOE5751 as mentioned above and resulted in pHWG1319 for His6-xepA without the tunnel region (XepA_N), pHWG1320 for His6-xepA with the tunnel region (XepA_NL) and pHWG1321 for His6-xepA-C-domain (XepA_C). After transformation of the plasmids in E. coli JM109 cells, 15 ml precultures were grown overnight in LB–ampicillin at 37°C. After inoculating a 1 l LB–ampicillin culture with the preculture, the bacteria were grown for 4 h to an OD600 nm of 0.6–0.8. Protein production was induced by the addition of rhamnose to a final concentration of 0.2% at 30°C. The culture was incubated overnight and was subsequently centrifuged at 1300g at 4°C. The pellet was resuspended in lysis buffer (20 mM Tris–HCl pH 7.5) with added protease inhibitor. After sonication (2–15 min, 40%, 4°C) the supernatant was filtered and was subsequently loaded onto a HisTrap HP affinity column, which was subjected to an imidazole-gradient FPLC separation. The protein was dialyzed into 10 mM ammonium bicarbonate buffer pH 7.5 and the molecular weight was verified by ESI-MS and SDS–PAGE. For YomS, selenomethionine (SeMet) needed to be incorporated in the protein sequence for crystallographic structure solution. Accordingly, the YomS expression construct was transformed into the methionine-auxotrophic E. coli strain B834(DE3). SeMet incorporation was performed following the general recommendations (Walden, 2010 ▸). YomS was heterogeneously overexpressed by cultivating the expression strain in mAT/ampicillin medium (de Maré et al., 2005 ▸) supplemented with SeMet to a final concentration of 50 mg l−1. Harvesting, purification and analysis of the SeMet-YomS variant was performed as described for the native proteins.
2.2. Thermal shift assay
Thermal shift assays were performed to identify stabilizing conditions for protein storage and crystallization (Niesen et al., 2007 ▸) using the Durham Screens (Bruce et al., 2019 ▸). Briefly, 1 ml protein solution at approximately 1 mg ml−1 in 10 mM Tris–HCl buffer pH 7.5 was mixed with 4 µl SYPRO Orange dye (5000× in DMSO) and 10 µl was pipetted into a 96-well PCR plate. 10 µl of the screens were added to the wells and the plate was sealed with thermostable film. The plate was centrifuged at 160g at 4°C for 2 min and was subsequently placed in a Real-Time PCR machine for melting-temperature experiments. Data from the thermal shift assay screen were analysed using in-house Microsoft Excel scripts and NAMI (Grøftehauge et al., 2015 ▸). To determine an optimal buffer for the crystallization of XepA and YomS, additional thermal shift assays were performed with a reduced 20-condition screen (Niesen et al., 2007 ▸). In these screens, several buffers at 100 mM with different pH values were used and the sodium chloride and glycerol concentrations were varied. For the small buffer screen the XepA and YomS proteins were diluted to 0.13 mg ml−1 in 10 mM HEPES pH 7.5 and 6.7× SYPRO Orange. 8 µl of a 4× concentration of buffer screen was dispensed into a 96-well PCR plate and 24 µl of the protein/SYPRO Orange mixture was added to each well with the buffer screen. The temperature range for the thermal shift analysis was 25–95°C (1°C steps per minute) and the fluorescence was measured after each increment. In addition, thermal shift assays with all target proteins and a number of glycosides were performed using the same protocol to assess ligand binding.
2.3. Crystallization
Initial crystallization experiments were performed using a range of commercially available crystallization screens. XepA crystallized in several crystal forms. Crystal form I (blocks, ∼0.1 × 0.1 × 0.1 mm) was obtained at 4°C using a protein solution consisting of 15 mg ml−1 XepA in 20 mM bis–Tris–HCl pH 6.5, 50 mM NaCl and was grown in a self-seeded drop with a reservoir consisting of 0.1 M sodium acetate pH 5.0, 6%(w/v) PEG 4000. Co-crystals with a terbium compound (crystal form II; ∼0.03 × 0.03 × 0.2 mm) were obtained by adding 100 µl protein solution (as above) to 0.6 mg of a Tb cluster compound (Crystallophore from Molecular Dimensions; Engilberge et al., 2017 ▸) and setting up self-seeded crystallization experiments at 4°C with the reservoir consisting of 0.1 M sodium acetate pH 5.2, 7%(w/v) PEG 4000. Crystallization experiments were set up in sitting drops in MRC 3-well plates using a Mosquito robot (TTP Labtech). Long needles (crystal form II, ∼0.05 × 0.05 × 0.3 mm) were grown using 0.1 M magnesium acetate, 0.1 M potassium chloride, 12% PEG Smear High (Molecular Dimensions), 0.1 M MES pH 5.5 and 4 mg ml−1 XepA in Tris buffer and were crystallized manually in vapour-diffusion sitting drops. YomS crystals (∼0.03 × 0.2 × 0.3 mm) were obtained at 20°C using 13.4 mg ml−1 YomS in 20 mM MES pH 6.0, 150 mM sodium chloride mixed with an equal volume of 0.1 M cacodylate pH 5.5, 0.2 M ammonium nitrate, 18%(w/v) PEG Smear Low (Molecular Dimensions). Selenomethionine-containing YomS crystals (∼0.03 × 0.1 × 0.2 mm) were grown from 6 mg ml−1 SeMet-YomS under the same conditions by self-seeding techniques.
2.4. Data collection, structure solution and refinement
All crystals were transferred into cryosolution and flash-cooled in liquid nitrogen before data collection (Garman, 2003 ▸). XepA form I crystals were transferred into 0.1 M sodium acetate pH 5.0, 9%(w/v) PEG 4000, 50 mM sodium chloride, 30%(v/v) glycerol. Tb-derivatized XepA form II crystals were soaked in a solution with 0.6 mg terbium cluster added to 10–100 µl cryosolution for approximately 1 min. Native YomS crystals were cryoprotected in 0.1 M sodium cacodylate pH 5.5, 0.2 M ammonium nitrate, 22%(w/v) PEG Smear Low, 25% glycerol, 30 mM sodium chloride. SeMet-YomS crystals were transferred into 0.1 M sodium cacodylate pH 5.5, 0.2 M ammonium nitrate, 20%(w/v) PEG Smear Low, 25% PEG 400. Data were collected using a PILATUS pixel-array detector (Broennimann et al., 2006 ▸) on beamlines I03, I04 and I24 at the Diamond Light Source (DLS), Didcot, England (YomS and XepA), as well as on beamline P13 at EMBL/DESY (SeMet-YomS; Cianci et al., 2017 ▸). All native data were processed using either autoPROC (Vonrhein et al., 2011 ▸) and STARANISO or XDS (Kabsch, 2010 ▸) followed by POINTLESS and AIMLESS as implemented in CCP4 (Winn et al., 2011 ▸) or the xia2 software pipeline (Winter et al., 2013 ▸). The anomalous data were processed in XDS and scaled in XSCALE, and AIMLESS was used to produce an MTZ file that was fed into the CRANK2 pipeline (Pannu et al., 2011 ▸), which uses SHELXC/D/E (Sheldrick, 2010 ▸) for automatic phasing by single-wavelength anomalous diffraction (SAD). For XepA, several data sets were collected at the terbium peak and the two most isomorphous data sets were scaled together to enhance the anomalous signal. The higher resolution native data set from crystal form I was subsequently used for structure refinement (Table 1 ▸). XepA crystal form I and the native crystal form II data were solved employing the SAD solution as a molecular-replacement model with Phaser (McCoy et al., 2007 ▸). In the case of YomS, the structure was determined using a single data set collected at the selenium peak. All structural models were refined against the diffraction data with REFMAC5 (Murshudov et al., 2011 ▸) using local noncrystallographic symmetry restraints when appropriate (Usón et al., 1999 ▸) or with BUSTER (Smart et al., 2012 ▸). All model building and evaluation was performed with Coot (Emsley et al., 2010 ▸). The final models were checked using MolProbity (Chen et al., 2015 ▸). Least-squares superpositions of Cα atoms were performed with RAPIDO (Mosca & Schneider, 2008 ▸) or CCP4mg (McNicholas et al., 2011 ▸). Further crystallographic data are summarized in Table 1 ▸. Coordinates and structure factors have been deposited in the Protein Data Bank with accession codes 6i56 (XepA form I), 6ia5 (XepA form II) and 6i5o (YomS).
Table 1. Data-collection and refinement statistics.
Values in parentheses are for the highest resolution shell.
| XepA (form I) | XepA (Tb derivative) | XepA (form II) | YomS | SeMet-YomS | |
|---|---|---|---|---|---|
| Data collection | |||||
| Beamline | I03, DLS | I04, DLS | I24, DLS | I04, DLS | P13, EMBL/DESY |
| Space group | P212121 | P212121 | P212121 | C2 | C2 |
| Unit-cell parameters | |||||
| a (Å) | 85.81 | 91.34 | 90.61 | 107.00 | 107.83 |
| b (Å) | 106.47 | 126.62 | 126.03 | 52.16 | 49.57 |
| c (Å) | 158.84 | 152.02 | 151.46 | 106.74 | 100.88 |
| β (°) | 90 | 90 | 90 | 95.97 | 92.31 |
| Wavelength (Å) | 0.9795 | 1.649 | 0.9772 | 0.9795 | 0.9795 |
| Resolution (Å) | 29.8–2.12 (2.16–2.12) | 30–2.50 (2.56–2.50) | 96.9–1.88 (2.07–1.88) | 53.08–1.33 (1.36–1.33) | 48.33–2.00 (2.05–2.00) |
| No. of observations | 834395 (46330) | 1040740 (41768) | 1118622 (33451) | 662174 (49107) | 490568 (33696) |
| R merge | 0.148 (2.07) | 0.156 (2.60)† | 0.080 (0.79) | 0.088 (1.40) | 0.098 (0.341)† |
| R p.i.m. | 0.049 (0.676) | 0.053 (1.30)† | 0.024 (0.319) | 0.044 (0.693) | 0.040 (0.141)† |
| 〈I/σ(I)〉 | 11.0 (1.2) | 14.1 (0.8) | 17.2 (1.8) | 9.8 (1.1) | 19.1 (7.4) |
| CC1/2 | 0.998 (0.513) | 0.999 (0.354) | 0.998 (0.683) | 0.998 (0.498) | 0.998 (0.981) |
| Completeness | 1.000 (1.000) | 0.999 (0.997)‡ | 0.771 (0.213)/0.922 (0.616)§ | 0.999 (1.000) | 0.999 (0.985)† |
| Multiplicity | 10.0 (10.2) | 16.9 (9.3)‡ | 11.2 (6.7) | 4.9 (5.0) | 13.5 (12.8) |
| No. of heavy atoms | 5 Tb | 5 Se | |||
| Refinement | |||||
| R work/R free | 0.173/0.221 | 0.172/0.212 | 0.154/0.178 | ||
| No. of atoms | 10657 | 10842 | 4310 | ||
| Ligands | 6 glycerols | 11 glycerols, 22 acetates | None | ||
| No. of waters | 861 | 1026 | 851 | ||
| R.m.s.d., bonds (Å) | 0.013 | 0.007 | 0.016 | ||
| R.m.s.d., angles (°) | 1.68 | 1.43 | 1.90 | ||
| Ramachandran plot | |||||
| Favoured (%) | 98.1 | 97.1 | 98.7 | ||
| Allowed (%) | 100 | 99.2 | 99.2 | ||
R merge within (I +/I −).
Anomalous completeness.
Ellipsoidal/spherical completeness.
2.5. In vivo cytotoxicity assays
XepA, YomS, XlyA and XlyB were dialysed after purification into 20 mM HEPES pH 7.4 for in vivo activity assays. For plaque assays, 10 µl of the proteins at a concentration of 1 mg ml−1 were spotted onto 10 ml LB–agar plates [0.75%(w/v) agar] that contained 500 µl of concentrated bacterial cells. Eight bacterial strains were tested: Bacillus megaterium ATCC 14581, B. subtilis subsp. spizizenii ATCC 6633, B. pumilus KPD 181, B. thuringiensis KPD 114, B. mycoides KPD 15, Micrococcus luteus ATCC 4698, E. coli MG1655 and B. subtilis 168 DSM 23778. Cells were prepared by growing them to an OD600 nm of 0.3 in 25 ml LB. Bacterial cultures were centrifuged at 5000g for 30 min at 4°C and the pellet was washed in 20 mM HEPES pH 7.4. The washed pellet was resuspended in 500 µl HEPES buffer added to the 50°C semi-solid LB–agar. The plates were left for 20 min at room temperature to set before spotting the protein solutions and were subsequently incubated overnight at 37°C. Hen egg-white lysozyme (HEWL) was applied as a positive control, whereas the negative control was HEPES buffer (20 mM, pH 7.4). A summary of the results of the in vivo assays can be found in Table 2 ▸.
Table 2. Summary of the cytotoxic activity of XepA, YomS, XlyA and XlyB on a selected range of bacterial cultures.
Plaque zone: +++, >7 mm; ++, 4–7 mm; +, 1–4 mm; −, none.
| Species | HEWL | Control | XepA | YomS | XlyA | XlyB |
|---|---|---|---|---|---|---|
| B. megaterium ATCC 14581† | +++ | − | ++ | − | + | ++ |
| B. subtilis subsp. spizizenii ATCC 6633† | ++ | − | + | − | − | ++ |
| B. pumilus KPD 181† | +++ | − | + | − | − | ++ |
| B. thuringiensis KPD114† | − | − | ++ | − | ++ | +++ |
| B. mycoides KPD 15† | − | − | ++ | − | − | ++ |
| M. luteus ATCC 4698† | +++ | − | − | − | − | − |
| E. coli MG1655‡ | − | − | − | − | − | − |
| B. subtilis 168 DSM 23778† | +++ | − | + | − | − | ++ |
Gram-positive species.
Gram-negative species.
3. Results
3.1. Sequence analysis
The putative lysins XepA and YomS align very well, with a sequence identity of 38% (54% similarity) covering 85% of the overall sequence (Supplementary Fig. S1). However, neither XepA nor YomS shows significant sequence similarity to the lysins XlyA and XlyB, indicating that these two enzymes have different functionalities and/or mechanisms. In database searches no assigned enzymatic domains could be found for the XepA and YomS sequences. However, YomS corresponds to the C-terminal half of XepA. A sequence search for the N-terminal XepA domain in the SPβ prophage genome in the B. subtilis database SubtiWiki (Michna et al., 2016 ▸) did not identify a protein with similar sequence. Both proteins, XepA and YomS, also align well with the B. subtilis 168 skin element prophage protein YqxG, as found in SubtiWiki and annotated with unknown function. For XepA the sequence identity is 56% (100% cover, 69% similarity), whereas the identity for YomS is 33% (83% cover, 56% similarity). The high sequence identity to B. subtilis YqxG suggests that this protein might have a very similar three-dimensional assembly to the XepA structure described below. The XepA and YomS protein sequences were also screened for transmembrane regions but none were identified, thus indicating that both proteins are soluble components of the lytic system.
3.2. Stability and ligand-binding assays
In addition to XepA and YomS, the lytic enzymes XlyA and XlyB were subjected to thermal shift assays (TSAs). XepA, XlyA and XlyB are significantly more thermostable, with T m values of 63, 68 and 62°C, respectively, compared with only 45°C for YomS. XepA and YomS showed comparable thermal stability over a wide pH range, and in both cases a high salt concentration increases the thermal stability slightly. Hence, a range of commercially available screens were employed for crystallization. Additional TSAs were performed to determine potential binding partners of the proteins. Hen egg-white lysozyme (HEWL), which as an N-acetylmuramide glycanhydrolase attacks the peptidoglycan at the glucose (Vocadlo et al., 2001 ▸), was used as a positive control. It is known that HEWL binds to triacetylchitotriose (GlcNAc)3, and TSAs with 0.05 mM HEWL and (GlcNAc)3 (16 mM) show an increase in the melting temperature, T m, from 68 to 77°C. The same experiments with d-GlcNAc (N-acetyl-d-glucosamine) did not show a stabilization effect. All four proteins were tested for (GlcNAc)3 and d-GlcNAc affinity. A similar stabilization (ΔT m = 2°C) was observed for XlyA and XlyB in TSAs with (GlcNAc)3. For XepA and YomS, however, no stabilizing effect was observed in the presence of these compounds, which suggests that any XepA/YomS lytic action is likely to be based on a different mechanism to the HEWL muramidase activity.
3.3. Crystal structure of XepA
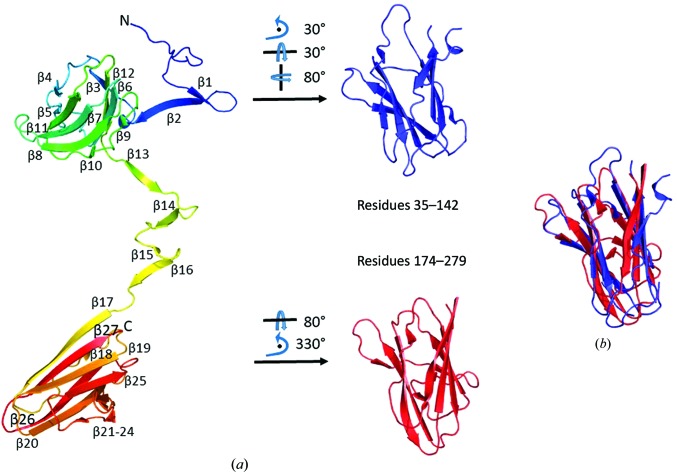
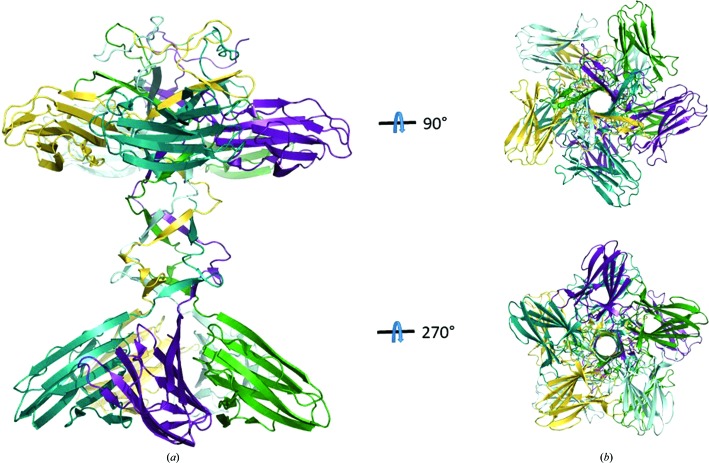
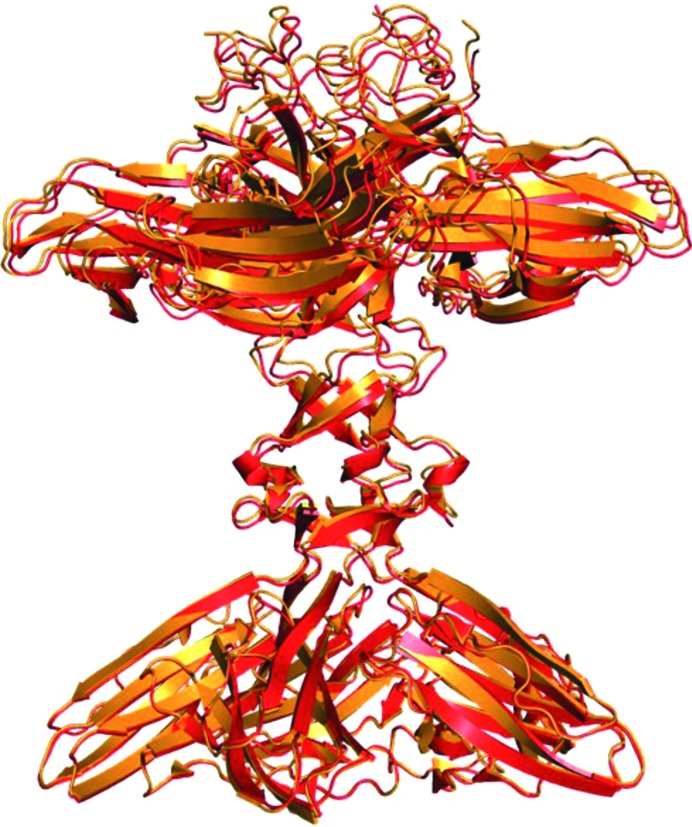
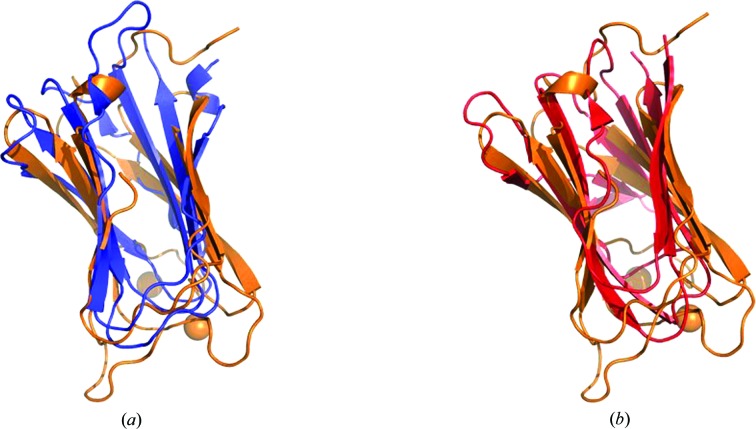
XepA crystallized from several conditions in multiple crystal forms. The crystal structures of the two highest diffracting crystal forms are reported here (Table 1 ▸). In both crystal forms XepA forms two domains of antiparallel 4 + 4 β-sandwich folds (jelly rolls) which are linked by a 30-amino-acid connector (Fig. 2 ▸ a). Superpositions of the N-terminal (residues 35–142) and the C-terminal (residues 174–279) β-sandwiches show that they are similar in fold but have major differences (r.m.s.d. of 2.5 Å; Fig. 2 ▸ b). In the crystal, XepA forms a highly symmetric pentamer (Fig. 3 ▸) with a dumbbell-shaped structure. The N- and C-terminal domain pentamers are discs connected by a tunnel-like linker region, which is about 10 Å wide and 45 Å long. The total length of the molecule is roughly 100 Å. The subunits in the pentameric structure adopt very similar structures, with r.m.s.d.s ranging from 0.4 to 0.9 Å when superimposed. The interactions of the β-sandwich moieties within the N- and C-terminal discs are distinctly different and result in a more planar (N-terminal) and a conical (C-terminal) disc. Comparing the XepA structures in different crystal forms, only minor differences in the overall fold can be observed. A superposition of the whole pentamer of XepA crystal forms I and II resulted in an r.m.s.d. of 0.9 Å. However, using only the C-terminal pentamer disc of the different crystal forms to calculate the superposition matrix (r.m.s.d. of 0.3 Å), the molecule shows a domain motion in the tunnel and N-terminal disc corresponding to a rotation of 2.1° (Fig. 4 ▸). This indicates considerable flexibility between the two discs, which may be important for the protein function. Since the individual chains in each crystal form are very similar, the highest resolution structure is used in the following discussions. The β-sandwich fold in XepA is very similar to a C2-domain fold, which is seen in proteins that interact with the cytoplasmic membrane. A superposition with the C2 domain of Clostridium perfringens α-toxin (PDB entry 2wxt; Naylor et al., 1999 ▸; Vachieri et al., 2010 ▸) is presented in Fig. 5 ▸. The r.m.s.d.s of these superpositions are 2.9 Å for the N-terminal domain and 3.0 Å for the C-terminal domain. However, unlike in the α-toxin C2 domain, no Ca2+ ions were found in XepA.
Figure 2.
Ribbon diagram of the XepA crystal structure. (a) The monomeric unit, which is shown in rainbow colours from blue (N-terminus) to red (C-terminus) with annotation of all strands, reveals two β-sandwich folds that are connected by a linker region. The truncated N-terminal domain (blue) and C-terminal domains (red) are depicted in a reoriented position with the Cα atoms used for least-squares superpositioning. (b) The β-sandwiches can be superimposed with an r.m.s.d. of 2.5 Å.
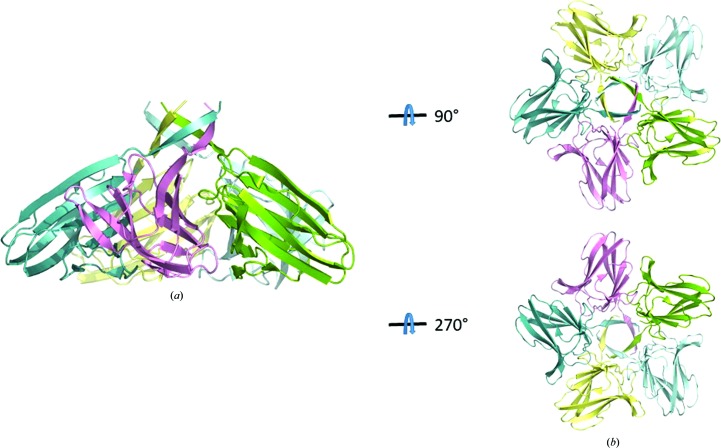
Figure 3.
Ribbon diagram of the crystal structure of the XepA pentamer (a) with each polypeptide chain depicted in a different colour shows a dumbbell-shaped structure in which two discs are connected by a linker region. (b) Top view of the N-terminal domain and bottom view of the C-terminal domain of the XepA pentamer.
Figure 4.
Least-squares superposition of the XepA pentamer in two crystal forms (form I in red and form II in orange). Only the C-terminal pentamer was used to calculate the transformation, which was then applied to the full pentamer. This operation reveals a domain shift that corresponds to a rotation of 2.1° of the N-terminal discs with respect to one another.
Figure 5.
Least-squares superpositions of the β-sandwich folds of XepA with the C2 domain of the α-toxin from C. perfringens (PDB entry 2wxt) in orange: (a) the N-terminal XepA domain in blue (r.m.s.d. on Cα atoms of 2.9 Å), (b) the C-terminal XepA domain in red (r.m.s.d. on Cα atoms of 3.0 Å).
3.4. Crystal structure of YomS
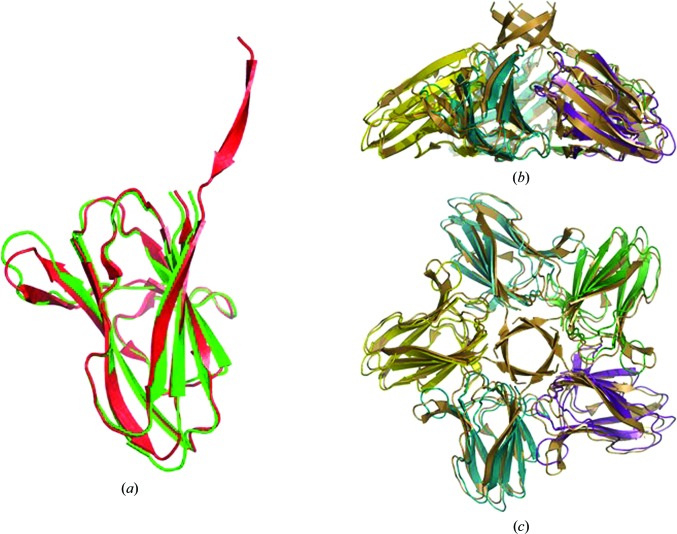
The three-dimensional structure of YomS was determined to a resolution of 1.3 Å by SeMet SAD phasing. YomS adopts an antiparallel β-sandwich fold, which arranges as a pentameric disc similar to the C-terminal domain of XepA (Fig. 6 ▸). In this disc the monomers can be superimposed on each other with an r.m.s.d. of 0.1–0.2 Å. Comparing YomS with the C-terminal domain of XepA shows that the structures are highly conserved, as presented in Fig. 7 ▸. The monomer subunits superimpose with an r.m.s.d. of 0.5–0.6 Å on the XepA C-terminal disc moieties (Fig. 7 ▸ a), whereas the full pentamers superimpose with an r.m.s.d. of 1.2 Å (Fig. 7 ▸ b). Although the sequence identity between XepA and YomS is only 38%, the three-dimensional structures of YomS and the C-terminal XepA domain are highly conserved, supporting the notion that the domains in each protein serve similar functionalities.
Figure 6.
Ribbon diagram of the crystal structure of the YomS homopentamer. (a) Each polypeptide chain is depicted in a different colour. (b) Top view of the YomS pentamer and bottom view of the YomS pentamer.
Figure 7.
Ribbon diagrams of least-squares superpositions of (a) one YomS monomer (red) on the C-terminal domain of an XepA monomer (green; r.m.s.d. of 0.6 Å) and (b) the whole YomS pentamer (brown) on the XepA C-terminal pentameric disc (chains depicted in different colours; r.m.s.d. of 1.2 Å).
3.5. Pentamer interfaces
The individual interfaces within the pentamers of both proteins show extensive electrostatic interactions. The buried surfaces between two XepA chains and two YomS chains amount to approximately 3300 and 1100 Å2, respectively. Rather than being based on one or two key residues, the interfaces are mainly held together by a large number of hydrogen bonds (over 40 for XepA and approximately 20 for YomS) and salt bridges.
3.6. Cytotoxicity
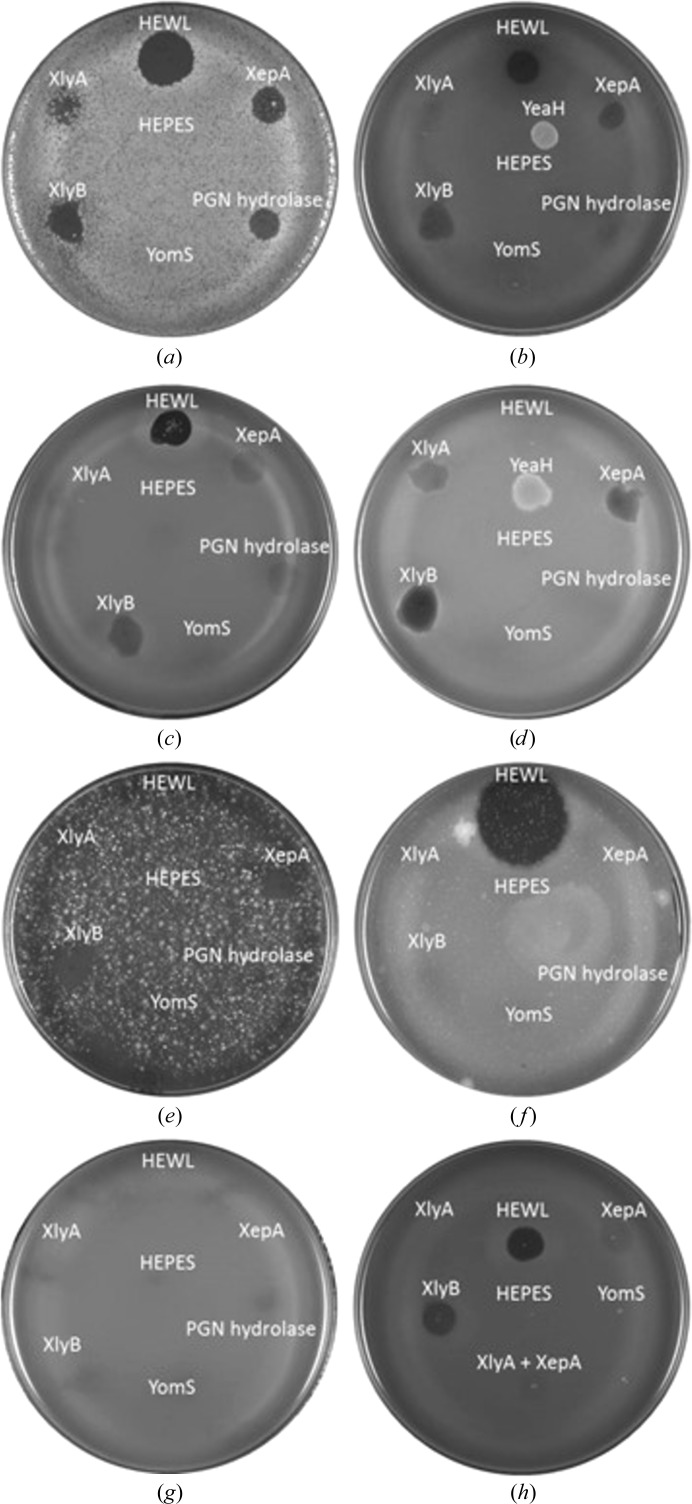
As the target proteins had been described to play a role in the lytic systems of their prophages, they were tested for cytotoxic activity in bacterial plate assays (Fig. 8 ▸). Purified proteins were applied onto mid-log phase bacterial culture plates and incubated overnight at 37°C. HEWL was used as a positive control and HEPES buffer as a negative control. Eight different bacterial cultures (B. megaterium, B. pumilus, B. subtilis subsp. spizizenii, B. thuringiensis, B. mycoides, M. luteus, E. coli and B. subtilis 168) were tested and the results are summarized in Table 2 ▸. The positive control HEWL shows lytic activity with the Gram-positive bacteria B. megaterium, B. pumilus, B. subtilis subsp. spizizenii, M. luteus and B. subtilis 168, whereas XepA and XlyB show plaque formation with the same samples and in addition with B. thuringiensis and B. mycoides. The N-acetylmuramoyl-l-alanine amidase XlyA only shows lysis with B. megaterium and B. thuringiensis. YomS does not exhibit any effects on the tested bacteria. As the crystal structures revealed a high degree of structural similarity of the C-terminal XepA domain and YomS, truncated mutants of XepA were designed and the variants were produced. They included (i) only the N-terminal protein (XepA_N), (ii) the N-terminal domain including the linker region (XepA_NL) and (iii) only the C-terminal domain (XepA_C). In vivo plate assays with these variants and B. megaterium (Supplementary Fig. S2) displayed no activity for XepA_N and XepA_C. XepA_NL shows minimal activity. This clearly indicates that the full-length XepA protein is required for cytotoxic activity.
Figure 8.
Cytotoxicity of target enzymes. Bacterial plate assays of XepA, YomS, XlyA and XlyB. HEWL and HEPES buffer (20 mM, pH 7.4) were used as positive and negative controls, respectively. (a) B. megaterium ATCC 14581, (b) B. subtilis subsp. spizizenii ATCC 6633, (c) B. pumilus KPD 181, (d) B. thuringiensis KPD 114, (e) B. mycoides KPD 15, (f) M. luteus ATCC 4698, (g) E. coli MG 1655, (h) B. subtilis 168 DSM 23778. The activities of the following proteins were also tested: the YeaH protein of unknown function from B. subtilis and PGN hydrolase, a putative lytic enzyme from B. subtilis phage vB_BsuP-Goe1.
4. Discussion
4.1. Ligand binding and biological activity
All prophages rely on a functional lytic system to release their progeny into the host. The enzymes of the lytic system need to target both the cytoplasmic membrane and the peptidoglycan. In our studies, we exposed mainly Gram-positive bacterial cultures, namely different Bacillus species and M. luteus, and the Gram-negative E. coli to four proteins with putative lytic activity. As a positive control, the known lytic enzyme HEWL was tested as an N-acetylmuramide glycanhydrolase. The Gram-negative E. coli did not show any disruption of the bacterial cells, which means that none of the proteins were able to attack the bacterial outer membrane. Interestingly, XepA showed lytic activity against all Bacillus species tested (Table 1 ▸), very similar to the results for XlyB, which was previously identified as an N-acetylmuramoyl-l-alanine amidase. HEWL as a positive control was not active against B. thuringiensis and B. mycoides, but was the only enzyme that shows lytic activity towards M. luteus. XlyA only displays lytic activity towards two bacterial strains: B. megaterium and B. thuringiensis. In contrast, the SPβ prophage YomS protein alone does not display detectable cytotoxic activity against any of the selected bacteria. To fully understand which bacteria are targeted by which lysins, detailed knowledge of the cytoplasmic membrane, the peptidoglycan layer and eventually of the bacterial capsule is required, as these characteristics are species-specific and very diverse. This represents one key to the enzymatic selectivity in lytic systems towards bacteria. Although XlyA and XlyB differ in their lytic activity towards the selected Bacillus strains, both proteins are known N-acetylmuramoyl-l-alanine amidases with a typical lysin composition (Supplementary Fig. S3): a C-terminal cell-wall-binding domain (CBD) and an N-terminal catalytic domain with a Zn2+-dependent active site (Low et al., 2011 ▸). In the thermal shift assays we established that XlyA and XlyB, and also HEWL, are stabilized by (GlcNAc)3 binding, which corresponds to their ability to attack the peptidoglycan layer during the lytic process. XepA and YomS, on the other hand, do not bind the same substrate and hence it is likely that they would employ a different mechanism for any lytic activity. In addition, the crystal structure of the catalytic domain of XlyA (PDB entries 3rdr and 3hmb; Low et al., 2011 ▸) shows no structural similarity to either XepA or YomS. In summary, we show that XepA initiates cell death in vivo, whereas YomS has no effect. We also showed that it is unlikely that XepA attacks the peptidoglycan in the same manner as do the amidases XlyA, XlyB and HEWL.
4.2. From structure to function
XepA forms a remarkable pentameric structure in which two disc-shaped domains are connected by a linker region. As shown above, the C-terminal disc of XepA is very similar to the structure of YomS, and the full-length protein including both domains is essential for cytotoxic activity. Both the XepA and YomS monomeric β-sandwiches adopt a fold resembling the C2 domain, which usually contains about 110 amino acids and 2–3 Ca2+ ions. C2 domains are typically phospholipid-binding and are often involved in cytoplasmic membrane trafficking. Tandem C2 domains are observed in membrane-trafficking proteins, for example synaptotagmins (Xu et al., 2014 ▸). This structural similarity could explain the function of the XepA and YomS β-sandwich structures. Although C2 domains are mostly observed in eukaryotic proteins coupled to enzymatic domains (Cho, 2001 ▸), there are a few prokaryotic examples of C2 domains. The C. perfringens and C. absonum α-toxins consist of C2 domains bound to phospholipase domains (Clark et al., 2003 ▸). The C2 domains in these examples bind Ca2+ ions depending on the cytoplasmic domain, but not all C2 domains are Ca2+-dependent. In the XepA and YomS crystal structures described here no Ca2+ ions were identified. A Ca2+-independent interaction has been described to rely on a lysine-rich cluster of Rabphilin 3A with the cytoplasmic membrane (Guillén et al., 2013 ▸). Multiple lysine residues at the XepA N-terminus (Supplementary Fig. S1) could be involved in similar interactions. As XepA displays two domains with very similar folds, a tandem C2 domain is also possible.
Looking at the three-dimensional structure of XepA with the linker region connecting the N-terminal and C-terminal pentameric discs, it appears possible that the structure may form a tunnel involved in DNA release. However, the absence of a transmembrane domain and the relatively small size of the formed pore discourage this notion. Comparisons with viral portal proteins (Sun et al., 2015 ▸; McElwee et al., 2018 ▸) show that the XepA tunnel has a relatively small diameter of approximately 10 Å, which suggests that it is too small to allow the entry of any DNA molecule (dsDNA, 20 Å; ssDNA, 15 Å). Although it may be conceivable that high flexibility in the XepA linker region may permit a conformational change, for example a rotational movement of the C-terminal and N-terminal discs to open the pore area, this would require a major structural realignment.
The β-sandwich or β-jelly roll is also a common viral capsid-protein motif in non-enveloped viruses (Bamford et al., 2005 ▸). In bacteriophage PRD1, the P31 penton protein is described to have a jelly-roll topology. A pentamer of P31 occupies the vertices in the icosahedral viral capsid shell (Abrescia et al., 2004 ▸) and builds the base of the vertex spikes in PRD1 on pentagon faces. Through their P31 interaction, two other proteins (P5 and P2) allow spike formation (Sokolova et al., 2001 ▸). The P31 jelly rolls are in vertical alignment in these pentamers. A 4 Å resolution X-ray crystal structure of the PRD1 bacteriophage has been deposited in the PDB as entry 1w8x, in which chain N corresponds to P31. Similar penton proteins have been reported in other untailed viruses, but not in Caudovirales, where in most cases the major capsid protein forms hexameric and pentameric capsomers which are arranged on the capsid surface (Fokine & Rossmann, 2014 ▸). Although the sequence similarity to other penton proteins is low, the pentameric assembly of XepA and YomS points towards a similar location in the B. subtilis prophage capsids. However, the orientations of the XepA and YomS jelly rolls in the structures reported here are different, with an approximate angle of 45° to a binding surface. The absence of XepA in the mature phage would be contraindicative to the assumption that it is part of the viral capsid. However, the XepA C-terminal domain and YomS may bind to the capsid in a vertex position according to their fivefold symmetry, stabilizing the virus particle (YomS) and helping the virus particle to escape (XepA). Many phages have additional domains lying on the capsid surface, which can be seen in cryo-EM reconstructions as protrusions. In the tailed bacteriophage φ29, for example, head fibres decorate the phage head (Xiang & Rossmann, 2011 ▸). The fibre bases of φ29 are described as trimers of two small β-barrel subdomains (Xu et al., 2019 ▸). In the tailed bacteriophage T4, the outer capsid proteins Hoc and Soc decorate the capsid surface (Rao & Black, 2010 ▸). As XepA was observed during phage maturation and not in the mature phage, one of its roles might be capsid reinforcement during capsid formation. Cement proteins that reinforce major capsid-protein interactions have been reported to have a similar jelly-roll topology, as shown in the structure of Bordetella bacteriophage BPP-1 (Zhang et al., 2013 ▸).
4.3. Mechanism of cell lysis
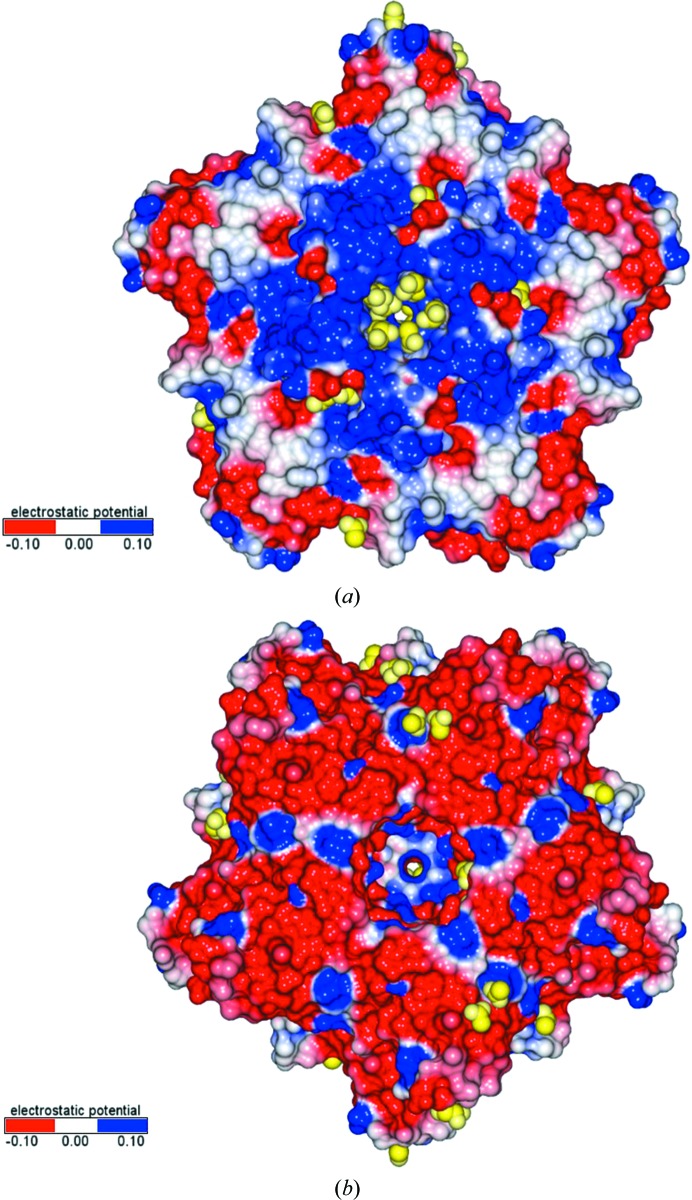
DNA phages can adopt multiple strategies to accomplish host-cell lysis, including the holin-dependent export of lysins, the Sec-mediated export of lysins with signal peptides (as observed in fOg44) and the holin-independent export of lysins with SAR (signal–arrest–release) as observed in coliphage P1 (Catalão et al., 2013 ▸; Fernandes & São-José, 2018 ▸). XepA contains structural domains that are typically associated with cytoplasmic membrane binding. The generally positively charged surface at the XepA N-terminus (Fig. 9 ▸ a) may be able to bind to the negatively charged phosphate moieties of the phospholipid bilayer. Furthermore, the crystal structure of XepA reveals acetate ions from the crystallization solutions that bind on the electropositive surface in the centre of the dumbbell-shaped protein, showing potential phosphate-binding sites. In contrast, the XepA C-terminal surface is almost exclusively electronegatively charged (Fig. 9 ▸ b), which suggests that the same interaction is not feasible between the C-terminal surface and the cytoplasmic membrane.
Figure 9.
Electrostatic surfaces of the XepA pentamer calculated using CCP4mg (McNicholas et al., 2011 ▸): red, −0.10 V e−1; white, 0.00 V e−1; blue, 0.10 V e−1. (a) View of the N-terminal disc, which is mostly positively charged. Acetate ions and glycerol molecules depicted in CPK representation (yellow) bind predominantly in the tube region. (b) View of the C-terminal disc, which shows a prevalently negatively charged surface.
Like all members of the Caudovirales, PBSX and SPβ form an icosahedral head made of hexamers and pentamers of its major coat protein (MCP). Considering the conserved fivefold symmetry in the protein structures described here, it is feasible that the C-terminal pentamer of XepA as well as the structurally very similar YomS may bind at least transiently to the prophage capsid proteins. The XepA pentamer might dock onto the phospholipid membrane with its N-terminal positively charged surface and hence disrupt the proton motive force (PMF) in a similar way to pinholins (Catalão et al., 2013 ▸). Pinholins cause small lesions in the cytoplasmic membrane and in this way activate the host-cell lytic system (Pang et al., 2009 ▸). A major difference to the pinholins is that XepA does not possess a transmembrane domain, since the surface of the linker region is clearly hydrophilic (Supplementary Fig. S4) and thus cannot be integrated into the cytoplasmic membrane.
We hypothesize that XepA may be located on the phage capsid, binding to the cytoplasmic membrane and dissipating the proton motive force of the membrane. XepA could thereby interfere with the host-cell secretion machinery and/or subvert the bacterial lytic system to induce host-cell lysis, as shown previously (Fernandes & São-José, 2018 ▸). Ultimately, XepA supports cell lysis and allows the release of assembled virus particles in the phage lytic cycle.
5. Related literature
The following references are cited in the supporting information for this article: Larkin et al. (2007 ▸) and Robert & Gouet (2014 ▸).
Supplementary Material
PDB reference: YomS, 6i50
PDB reference: XepA, 6i56
PDB reference: 6ia5
Supplementary Tables and Figures. DOI: 10.1107/S2059798319013330/gm5068sup1.pdf
Acknowledgments
We would like to acknowledge I. Edwards for excellent technical help with protein production, N. Czapiewska and P. Neubauer for excellent technical help with the bacterial lysis assays and M. Krupovic for discussions. We are very grateful to the Diamond Light Source and the PETRA synchrotron at DESY and their staff for providing excellent facilities. We thank all of the Virus-X consortium members for their great cooperation.
Funding Statement
This work was funded by European Research Council grant 685778.
References
- Abrescia, N. G. A., Cockburn, J. J. B., Grimes, J. M., Sutton, G. C., Diprose, J. M., Butcher, S. J., Fuller, S. D., San Martín, C., Burnett, R. M., Stuart, D. I., Bamford, D. H. & Bamford, J. K. H. (2004). Nature (London), 432, 68–74. [DOI] [PubMed]
- Bamford, D. H., Grimes, J. M. & Stuart, D. I. (2005). Curr. Opin. Struct. Biol. 15, 655–663. [DOI] [PubMed]
- Briers, Y. (2019). Viruses, 11, 113–115.
- Broennimann, C., Eikenberry, E. F., Henrich, B., Horisberger, R., Huelsen, G., Pohl, E., Schmitt, B., Schulze-Briese, C., Suzuki, M., Tomizaki, T., Toyokawa, H. & Wagner, A. (2006). J. Synchrotron Rad. 13, 120–130. [DOI] [PubMed]
- Bruce, D., Cardew, E., Freitag-Pohl, S. & Pohl, E. (2019). J. Vis. Exp., e58666. [DOI] [PubMed]
- Catalão, M. J., Gil, F., Moniz-Pereira, J., São-José, C. & Pimentel, M. (2013). FEMS Microbiol. Rev. 37, 554–571. [DOI] [PubMed]
- Chen, V. B., Wedell, J. R., Wenger, R. K., Ulrich, E. L. & Markley, J. L. (2015). J. Biomol. NMR, 63, 77–83. [DOI] [PMC free article] [PubMed]
- Cho, W. (2001). J. Biol. Chem. 276, 32407–32410. [DOI] [PubMed]
- Cianci, M., Bourenkov, G., Pompidor, G., Karpics, I., Kallio, J., Bento, I., Roessle, M., Cipriani, F., Fiedler, S. & Schneider, T. R. (2017). J. Synchrotron Rad. 24, 323–332. [DOI] [PMC free article] [PubMed]
- Clark, G. C., Briggs, D. C., Karasawa, T., Wang, X., Cole, A. R., Maegawa, T., Jayasekera, P. N., Naylor, C. E., Miller, J., Moss, D. S., Nakamura, S., Basak, A. K. & Titball, R. W. (2003). J. Mol. Biol. 333, 759–769. [DOI] [PubMed]
- Denyes, J. M., Dunne, M., Steiner, S., Mittelviefhaus, M., Weiss, A., Schmidt, H., Klumpp, J. & Loessner, M. J. (2017). Appl. Environ. Microbiol. 83, e00277-17. [DOI] [PMC free article] [PubMed]
- Drulis-Kawa, Z., Majkowska-Skrobek, G. & Maciejewska, B. (2015). Curr. Med. Chem. 22, 1757–1773. [DOI] [PMC free article] [PubMed]
- Emsley, P., Lohkamp, B., Scott, W. G. & Cowtan, K. (2010). Acta Cryst. D66, 486–501. [DOI] [PMC free article] [PubMed]
- Engilberge, S., Riobé, F., Di Pietro, S., Lassalle, L., Coquelle, N., Arnaud, C.-A., Pitrat, D., Mulatier, J.-C., Madern, D., Breyton, C., Maury, O. & Girard, E. (2017). Chem. Sci. 8, 5909–5917. [DOI] [PMC free article] [PubMed]
- Fernandes, S. & São-José, C. (2018). Viruses, 10, 396. [DOI] [PMC free article] [PubMed]
- Fokine, A. & Rossmann, M. G. (2014). Bacteriophage, 4, e28281. [DOI] [PMC free article] [PubMed]
- Garman, E. (2003). Curr. Opin. Struct. Biol. 13, 545–551. [DOI] [PubMed]
- Grøftehauge, M. K., Hajizadeh, N. R., Swann, M. J. & Pohl, E. (2015). Acta Cryst. D71, 36–44. [DOI] [PMC free article] [PubMed]
- Guillén, J., Ferrer-Orta, C., Buxaderas, M., Pérez-Sánchez, D., Guerrero-Valero, M., Luengo-Gil, G., Pous, J., Guerra, P., Gómez-Fernández, J. C., Verdaguer, N. & Corbalán-García, S. (2013). Proc. Natl. Acad. Sci. USA, 110, 20503–20508. [DOI] [PMC free article] [PubMed]
- Hsieh, P.-F., Lin, H.-H., Lin, T.-L., Chen, Y.-Y. & Wang, J.-T. (2017). Sci. Rep. 7, 4624. [DOI] [PMC free article] [PubMed]
- Kabsch, W. (2010). Acta Cryst. D66, 133–144. [DOI] [PMC free article] [PubMed]
- Krogh, S., Jørgensen, S. T. & Devine, K. M. (1998). J. Bacteriol. 180, 2110–2117. [DOI] [PMC free article] [PubMed]
- Larkin, M. A., Blackshields, G., Brown, N. P., Chenna, R., McGettigan, P. A., McWilliam, H., Valentin, F., Wallace, I. M., Wilm, A., Lopez, R., Thompson, J. D., Gibson, T. J. & Higgins, D. G. (2007). Bioinformatics, 23, 2947–2948. [DOI] [PubMed]
- Lazarevic, V., Düsterhöft, A., Soldo, B., Hilbert, H., Mauël, C. & Karamata, D. (1999). Microbiology, 145, 1055–1067. [DOI] [PubMed]
- Longchamp, P. F., Mauël, C. & Karamata, D. (1994). Microbiology, 140, 1855–1867. [DOI] [PubMed]
- Low, L. Y., Yang, C., Perego, M., Osterman, A. & Liddington, R. (2011). J. Biol. Chem. 286, 34391–34403. [DOI] [PMC free article] [PubMed]
- Maré, L. de, Velut, S., Ledung, E., Cimander, C., Norrman, B., Karlsson, E. N., Holst, O. & Hagander, P. (2005). Biotechnol. Lett. 27, 983–990. [DOI] [PubMed]
- Mauël, C. & Karamata, D. (1984). J. Virol. 49, 806–812. [DOI] [PMC free article] [PubMed]
- McCoy, A. J., Grosse-Kunstleve, R. W., Adams, P. D., Winn, M. D., Storoni, L. C. & Read, R. J. (2007). J. Appl. Cryst. 40, 658–674. [DOI] [PMC free article] [PubMed]
- McElwee, M., Vijayakrishnan, S., Rixon, F. & Bhella, D. (2018). PLoS Biol. 16, e2006191. [DOI] [PMC free article] [PubMed]
- McNicholas, S., Potterton, E., Wilson, K. S. & Noble, M. E. M. (2011). Acta Cryst. D67, 386–394. [DOI] [PMC free article] [PubMed]
- Michna, R. H., Zhu, B., Mäder, U. & Stülke, J. (2016). Nucleic Acids Res. 44, D654–D662. [DOI] [PMC free article] [PubMed]
- Mosca, R. & Schneider, T. R. (2008). Nucleic Acids Res. 36, W42–W46. [DOI] [PMC free article] [PubMed]
- Murshudov, G. N., Skubák, P., Lebedev, A. A., Pannu, N. S., Steiner, R. A., Nicholls, R. A., Winn, M. D., Long, F. & Vagin, A. A. (2011). Acta Cryst. D67, 355–367. [DOI] [PMC free article] [PubMed]
- Naylor, C. E., Jepson, M., Crane, D. T., Titball, R. W., Miller, J., Basak, A. K. & Bolgiano, B. (1999). J. Mol. Biol. 294, 757–770. [DOI] [PubMed]
- Niesen, F. H., Berglund, H. & Vedadi, M. (2007). Nature Protoc. 2, 2212–2221. [DOI] [PubMed]
- Pang, T., Savva, C. G., Fleming, K. G., Struck, D. K. & Young, R. (2009). Proc. Natl Acad. Sci. USA, 106, 18966–18971. [DOI] [PMC free article] [PubMed]
- Pannu, N. S., Waterreus, W.-J., Skubák, P., Sikharulidze, I., Abrahams, J. P. & de Graaff, R. A. G. (2011). Acta Cryst. D67, 331–337. [DOI] [PMC free article] [PubMed]
- Plotka, M., Kaczorowska, A. K., Morzywolek, A., Makowska, J., Kozlowski, L. P., Thorisdottir, A., Skírnisdottir, S., Hjörleifsdottir, S., Fridjonsson, O. H., Hreggvidsson, G. O., Kristjansson, J. K., Dabrowski, S., Bujnicki, J. M. & Kaczorowski, T. (2015). PLoS One, 10, e0137374. [DOI] [PMC free article] [PubMed]
- Rao, V. B. & Black, L. W. (2010). Virol. J. 7, 356–369. [DOI] [PMC free article] [PubMed]
- Robert, X. & Gouet, P. (2014). Nucleic Acids Res. 42, W320–W324. [DOI] [PMC free article] [PubMed]
- Rodríguez-Rubio, L., Martínez, B., Donovan, D. M., Rodríguez, A. & García, P. (2013). Crit. Rev. Microbiol. 39, 427–434. [DOI] [PubMed]
- Sheldrick, G. M. (2010). Acta Cryst. D66, 479–485. [DOI] [PMC free article] [PubMed]
- Smart, O. S., Womack, T. O., Flensburg, C., Keller, P., Paciorek, W., Sharff, A., Vonrhein, C. & Bricogne, G. (2012). Acta Cryst. D68, 368–380. [DOI] [PMC free article] [PubMed]
- Smith, T. J., Blackman, S. A. & Foster, S. J. (2000). Microbiology, 146, 249–262. [DOI] [PubMed]
- Sokolova, A., Malfois, M., Caldentey, J., Svergun, D. I., Koch, M. H. J., Bamford, D. H. & Tuma, R. (2001). J. Biol. Chem. 276, 46187–46195. [DOI] [PubMed]
- Stefanska, A., Kaczorowska, A. K., Plotka, M., Fridjonsson, O. H., Hreggvidsson, G. O., Hjorleifsdottir, S., Kristjansson, J. K., Dabrowski, S. & Kaczorowski, T. (2014). J. Biotechnol. 182–183, 1–10. [DOI] [PubMed]
- Sudiarta, I. P., Fukushima, T. & Sekiguchi, J. (2010). J. Biol. Chem. 285, 41232–41243. [DOI] [PMC free article] [PubMed]
- Sun, L., Zhang, X., Gao, S., Rao, P. A., Padilla-Sanchez, V., Chen, Z., Sun, S., Xiang, Y., Subramaniam, S., Rao, V. B. & Rossmann, M. G. (2015). Nature Commun. 6, 7548. [DOI] [PMC free article] [PubMed]
- Toyofuku, M., Cárcamo-Oyarce, G., Yamamoto, T., Eisenstein, F., Hsiao, C.-C., Kurosawa, M., Gademann, K., Pilhofer, M., Nomura, N. & Eberl, L. (2017). Nature Commun. 8, 481. [DOI] [PMC free article] [PubMed]
- Usón, I., Pohl, E., Schneider, T. R., Dauter, Z., Schmidt, A., Fritz, H. J. & Sheldrick, G. M. (1999). Acta Cryst. D55, 1158–1167. [DOI] [PubMed]
- Vachieri, S. G., Clark, G. C., Alape-Girón, A., Flores-Díaz, M., Justin, N., Naylor, C. E., Titball, R. W. & Basak, A. K. (2010). Acta Cryst. D66, 1067–1074. [DOI] [PubMed]
- Vocadlo, D. J., Davies, G. J., Laine, R. & Withers, S. G. (2001). Nature (London), 412, 835–838. [DOI] [PubMed]
- Vonrhein, C., Flensburg, C., Keller, P., Sharff, A., Smart, O., Paciorek, W., Womack, T. & Bricogne, G. (2011). Acta Cryst. D67, 293–302. [DOI] [PMC free article] [PubMed]
- Walden, H. (2010). Acta Cryst. D66, 352–357. [DOI] [PMC free article] [PubMed]
- Wegerer, A., Sun, T. & Altenbuchner, J. (2008). BMC Biotechnol. 8, 2. [DOI] [PMC free article] [PubMed]
- Winn, M. D., Ballard, C. C., Cowtan, K. D., Dodson, E. J., Emsley, P., Evans, P. R., Keegan, R. M., Krissinel, E. B., Leslie, A. G. W., McCoy, A., McNicholas, S. J., Murshudov, G. N., Pannu, N. S., Potterton, E. A., Powell, H. R., Read, R. J., Vagin, A. & Wilson, K. S. (2011). Acta Cryst. D67, 235–242. [DOI] [PMC free article] [PubMed]
- Winter, G., Lobley, C. M. C. & Prince, S. M. (2013). Acta Cryst. D69, 1260–1273. [DOI] [PMC free article] [PubMed]
- Wood, H. E., Dawson, M. T., Devine, K. M. & McConnell, D. J. (1990). J. Bacteriol. 172, 2667–2674. [DOI] [PMC free article] [PubMed]
- Xiang, Y. & Rossmann, M. G. (2011). Proc. Natl Acad. Sci. USA, 108, 4806–4810. [DOI] [PMC free article] [PubMed]
- Xu, J., Bacaj, T., Zhou, A., Tomchick, D. R., Südhof, T. C. & Rizo, J. (2014). Structure, 22, 269–280. [DOI] [PMC free article] [PubMed]
- Xu, J., Wang, D., Gui, M. & Xiang, Y. (2019). Nature Commun. 10, 2366–2381. [DOI] [PMC free article] [PubMed]
- Zhang, X., Guo, H., Jin, L., Czornyj, E., Hodes, A., Hui, W. H., Nieh, A. W., Miller, J. F. & Zhou, Z. H. (2013). Elife, 2, e01299. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
PDB reference: YomS, 6i50
PDB reference: XepA, 6i56
PDB reference: 6ia5
Supplementary Tables and Figures. DOI: 10.1107/S2059798319013330/gm5068sup1.pdf