Abstract
Patients with chronic granulomatous disease are at increased risk for invasive aspergillosis. Cryptic Aspergillus species are being increasingly recognized as distinct causes of infection in this population. In this study, we describe the first case of Aspergillus udagawae vertebral osteomyelitis in a patient with X-linked chronic granulomatous disease.
Keywords: Aspergillus species, chronic granulomatous disease, osteomyelitis
CASE DESCRIPTION
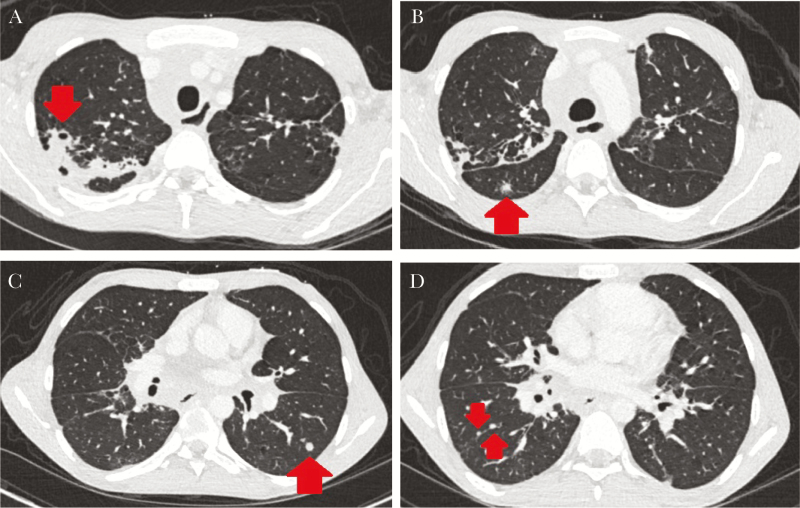
A 23-year-old male with X-linked chronic granulomatous disease (CGD) presented with 4 weeks of back pain. Approximately 15 months prior, he was hospitalized for Burkholderia cenocepacia bacteremia and found to have pulmonary nodules (Figure 1) and a positive bronchial galactomannan. Fungal cultures and polymerase chain reactions (PCR) for Aspergillus fumigatus and fungal organisms from bronchial specimens were negative. He was treated with intravenous (IV) ciprofloxacin for B cenocepacia bacteremia and IV voriconazole for presumed Aspergillus pneumonia. He was discharged on moxifloxacin to complete 3 weeks of therapy for B cenocepacia bacteremia, trimethroprim-sulfamethoxazole for bacterial prophylaxis, and posaconazole for treatment of presumed Aspergillus pneumonia. The patient was nonadherent to antimicrobial therapy and monitoring of posaconazole levels, and he was lost to follow-up.
Figure 1.
Computed tomography of the chest from the patient’s first hospitalization. Red arrows indicate (A) cavitation and consolidation from invasive fungal disease; (B) a “reverse halo sign” characteristic of invasion into a blood vessel by Aspergillus species; (C) an inflammatory nodule; and (D) intrafissural inflammatory lymph nodes.
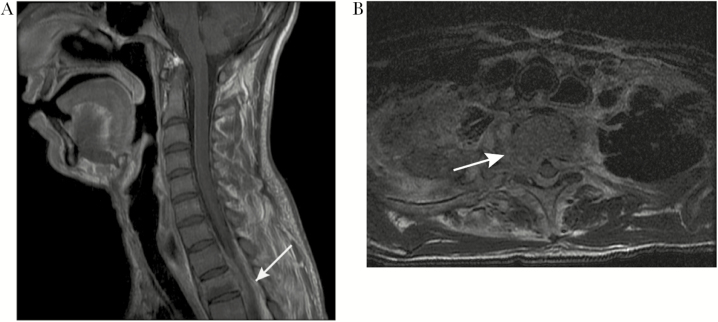
On the day of admission for back pain, the patient denied fevers, incontinence, weakness, and respiratory symptoms. On examination, he had upper thoracic spinal tenderness, bilateral lower extremity clonus, and hyperreflexia in the patellar and Achilles tendons bilaterally. White blood cell count was 13.9 thousand/μL (range, 4.3–10.0) and C-reactive protein was 135 mg/L (range, 0–10). Spinal magnetic resonance imaging (MRI) showed a mass in the epidural space between T1 and T3, with mild cord impingement (Figure 2). He underwent urgent T2 laminectomy with T1–3 posterior spinal instrumentation and fusion (PSIF). Intraoperative specimens were obtained for culture and polymerase chain reaction (PCR) of bacteria, fungi, and acid-fast bacilli. He was started on micafungin, meropenem, and vancomycin for empiric treatment of common pathogens in patients with CGD [1].
Figure 2.
Magnetic resonance imaging of the vertebral spine. (A) shows a sagittal T1 fat saturated postcontrast image of the infiltrative-enhancing lesion centered at the T2 vertebral body associated with contiguous extra-osseous extension involving the epidural levels from T1 to T3 with cord impingement (white arrow). An axial T2 image in (B) demonstrates bony destruction and cortical breakthrough (white arrow).
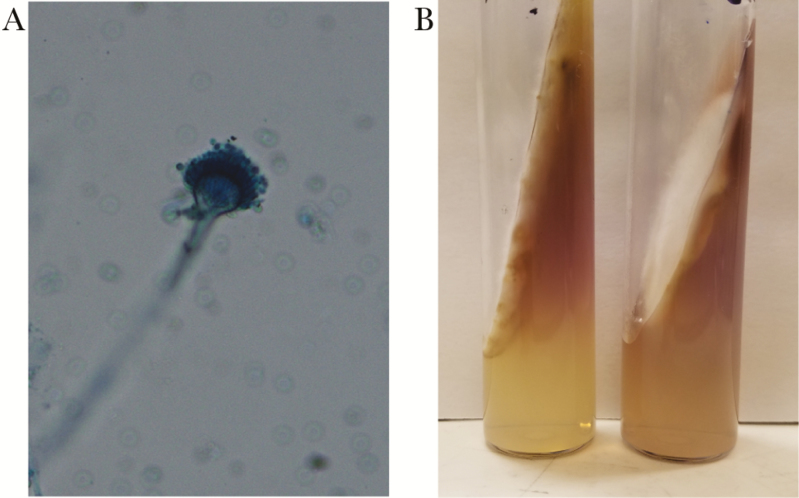
A real-time PCR assay identified Aspergillus species of the Section Fumigati (not A fumigatus) from the epidural mass. This assay uses a fluorescent probe to detect A fumigatus and related species by targeting the deoxyribonucleic acid sequence between 5.3S and 28S ribosomal ribonucleic acid genes (internal transcribed spacer 2 [ITS2]). Lack of binding of a second internal probe specific to A fumigatus was used to exclude this species. The patient was started on liposomal amphotericin B. After 8 days, culture grew the organism shown in Figure 3. Species-level discrimination of Aspergillus udagawae was made by sequencing ITS1, ITS2, 28S, and beta-tubulin genes. Correct species identification was supported by growth at 30°C and 42°C, but not at 50°C, on subculture. The patient’s history of pulmonary nodules and positive bronchial galactomannan suggested possible dissemination (eg, contiguous spread) from a pulmonary source, although this could not be confirmed.
Figure 3.
A cottony white mold was isolated from culture (eSwab specimen of the T2 epidural space) after 8 days of growth on Saboraud Dextrose agar at 30°C. Subculture to potato dextrose agar yielded condida after a further 5–7 days of growth, with lactophenol cotton blue prep revealing structures as shown in (A). The colony remained white and produced a lavender-purple diffusible pigment (B).
DISCUSSION
Aspergillus udagawae was initially isolated from the soil of a Brazilian sugar cane plantation [2], and it is one of several cryptic Aspergillus species recently recognized in patients with CGD and other primary immunodeficiencies [3]. Despite sharing morphologic characteristics with A fumigatus, many cryptic Aspergillus species have distinguishing microbiologic features [3]. For example, in contrast to A fumigatus, A udagawae grows at 10°C but not at temperatures >42°C [2, 4].
Six cases of infectious A udagawae have been reported: 5 cases of invasive pulmonary disease [2], and 1 case of keratitis after nonpenetrating trauma [2, 5]. Neutrophils are critical for the control of invasive aspergillus [6], and 4 of the 5 reported cases of pulmonary aspergillosis occurred in patients with an underlying neutrophil deficiency (CGD in 3 patients, and myelodysplastic syndrome with pancytopenia in 1 patient) [7]. The remaining case was in a woman with type 2 diabetes mellitus [8]. To our knowledge, there are no prior reports of A udagawae osteomyelitis [9].
Retrospective analyses suggest that misclassification of A udagawae as A fumigatus occurs in approximately 11%–16% of cases [7, 10]. Correct identification of Aspergillus species may be critical for selecting empiric antimicrobial therapy, because antifungal minimum inhibitory concentrations (MICs) are often elevated for A udagawae and other cryptic Aspergillus species compared with A fumigatus [3, 7]. In addition, patients with A udagawae may have a longer median duration of infection (35 weeks versus 5.3 weeks) and a higher mortality rate compared with patients with A fumigatus [1, 7].
CONCLUSIONS
After identification of A udagawae, the patient was switched from liposomal amphotericin B to micafungin and posaconazole. One month after his initial PSIF, the patient had increased pain and loosening of hardware on imaging and required a second debridement with additional hardware placement. Drug-susceptibility testing suggested the A udagawae isolated had favorable MICs for both posaconazole (MIC = 0.25 μg/mL) and micafungin (MIC ≤ 0.015 μg/mL). Approximately 6 weeks postoperatively, micafungin was discontinued, and the patient was transitioned to posaconazole monotherapy. Although the patient initially struggled with adherence, his last 3 posaconazole levels were therapeutic (>1 μg/mL) [11]. Follow-up spinal MRI showed stable hardware, resolving epidural disease, and decreased lung opacities. It is anticipated the patient will continue high-dose posaconazole for 3 to 6 months for hardware-associated vertebral osteomyelitis and possible pulmonary infection, followed by long-term posaconazole prophylaxis.
Acknowledgments
We acknowledge Dr. Rangaraj Selvarangan for assistance with development of the polymerase chain reaction assays and Lynda Bui for assistance with phenotypic mycology.
Financial support. This work was funded by the National Institute of Allergy and Infectious Disease T32 Host Defense Training Grant (5T32AI007044-43) (to M. C. S., M. B., and D. M.). S. M. G. was funded by the Robert W. Anderson Endowed Professorship in Medicine.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1. Winkelstein JA, Marino MC, Johnston RB Jr, et al. Chronic granulomatous disease. Report on a national registry of 368 patients. Medicine 2000; 79:155–69. [DOI] [PubMed] [Google Scholar]
- 2. Lamoth F. Aspergillus fumigatus-related species in clinical practice. Front Microbiol 2016; 7:683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Seyedmousavi S, Lionakis MS, Parta M, et al. Emerging Aspergillus species almost exclusively associated with primary immunodeficiencies. Open Forum Infect Dis 2018; 5:ofy213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sugui JA, Vinh DC, Nardone G, et al. Neosartorya udagawae (Aspergillus udagawae), an emerging agent of aspergillosis: how different is it from Aspergillus fumigatus? J Clin Microbiol 2010; 48:220–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Posteraro B, Mattei R, Trivella F, et al. Uncommon Neosartorya udagawae fungus as a causative agent of severe corneal infection. J Clin Microbiol 2011; 49:2357–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mircescu MM, Lipuma L, van Rooijen N, et al. Essential role for neutrophils but not alveolar macrophages at early time points following Aspergillus fumigatus infection. J Infect Dis 2009; 200:647–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Vinh DC, Shea YR, Sugui JA, et al. Invasive aspergillosis due to Neosartorya udagawae. Clin Infect Dis 2009; 49:102–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gyotoku H, Izumikawa K, Ikeda H, et al. A case of bronchial aspergillosis caused by Aspergillus udagawae and its mycological features. Med Mycol 2012; 50:631–6. [DOI] [PubMed] [Google Scholar]
- 9. Dotis J, Roilides E. Osteomyelitis due to Aspergillus species in chronic granulomatous disease: an update of the literature. Mycoses 2011; 54:e686–96. [DOI] [PubMed] [Google Scholar]
- 10. Balajee SA, Nickle D, Varga J, Marr KA. Molecular studies reveal frequent misidentification of Aspergillus fumigatus by morphotyping. Eukaryot Cell 2006; 5:1705–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dekkers BG, Bakker M, van der Elst KC, et al. Therapeutic drug monitoring of posaconazole: an update. Curr Fungal Infect Rep 2016; 10:51–61. [DOI] [PMC free article] [PubMed] [Google Scholar]