Abstract
Degenerative changes of the intervertebral disc (IVD) are a leading cause of disability affecting humans worldwide and has been attributed primarily to trauma and the accumulation of pathology during aging. While genetic defects have also been associated with disc degeneration, the precise mechanisms driving the initiation and progression of disease have remained elusive due to a paucity of genetic animal models. Here, we discuss a novel conditional mouse genetic model of endplate-oriented disc herniations in adult mice. Using conditional mouse genetics, we show increased mechanical stiffness and reveal dysregulation of typical gene expression profiles of the IVD in adhesion G-protein coupled receptor G6 (Adgrg6) mutant mice prior to the onset of endplate-oriented disc herniations in adult mice. We observed increased STAT3 activation prior to IVD defects and go on to demonstrate that treatment of Adgrg6 conditional mutant mice with a small molecule inhibitor of STAT3 activation ameliorates endplate-oriented herniations. These findings establish ADGRG6 and STAT3 as novel regulators of IVD endplate and growth plate integrity in the mouse, and implicate ADGRG6/STAT3 signaling as promising therapeutic targets for endplate-oriented disc degeneration.
Author summary
Back pain is a leading cause of disability in humans worldwide and one of the most common culprits of these issues are the consequence of degenerative changes of the intervertebral disc. Here, we demonstrate that conditional loss of the Adgrg6 gene in cartilaginous tissues of the spine results in endplate-oriented disc herniations and degenerative changes of the intervertebral disc in mice. We further establish that these obvious degenerative changes of the disc are preceded by substantial alterations in normal gene expression profiles, including upregulation of pro-inflammatory STAT3 signaling, and increased mechanical stiffness of the intervertebral disc. Increased STAT3 activation is a signal observed in other models of degenerative musculoskeletal tissues. As such, we tested whether systemic treatment with a small-molecule STAT3 inhibitor would protect against the formation of endplate-oriented disc herniations in conditional Adgrg6 mutant mice, and report a significant positive improvement of histopathology in our treatment group. Taken together, we demonstrate a novel conditional model of endplate-oriented disc herniation in mouse. We establish ADGRG6 and STAT3 as novel regulators of endplate integrity of the intervertebral disc in mouse and suggest that modulation of ADGRG6/STAT3 signaling could provide robust disease-modifying targets for endplate-oriented disc degeneration in humans.
Introduction
Spine disorders are one of the most common health issues affecting human populations worldwide, causing a tremendous socio-economic burden. The progression of spine disorders such as low back pain, disc herniation, endplate fracture, and scoliosis are associated with degenerative changes of the intervertebral disc (IVD) [1–4]. Therefore, elucidation of the pathways and signaling important for maintaining spine stability and the development and homeostasis of the IVD tissues is critical for the diagnosis, prevention, and treatment of degenerative spine disorders.
The IVD is a fibrocartilaginous joint that connects two adjacent vertebrae and provides structural stability, flexibility, and cushions axial loading of the spinal column [5]. The disc is composed of a proteoglycan-rich nucleus pulposus, surrounded by a multi-lamellar annulus fibrosus, and situated between the cartilaginous endplates, which provide nutritional flux to the IVD (Fig 1A). Hallmarks of disc degeneration in humans include loss of disc height, reduced proteoglycan staining, and accumulation of markers of fibrosis within the disc. At the same time, the cartilaginous endplate (CEP) may show signs of degeneration and calcification, which further compromises nutrient availability to the inner disc layers [6]. More severe forms of disc degeneration can also result in the herniation of the nucleus pulposus (i) laterally through the annulus fibrosis layer; or (ii) through the cartilaginous endplate into the vertebral body (endplate-oriented). Genetic susceptibility to disc degeneration has been shown to play a major role in disc degeneration [7], with the majority of these findings implicating extracellular matrix components of the disc, matrix metalloproteases, or pro-inflammatory cytokines [8]. Together these data suggest that dysregulation of anabolic and catabolic factors as well as inflammatory signaling may underlie many forms of disc degeneration in humans. However, the molecular regulators and initiating factors for these events remain to be defined.
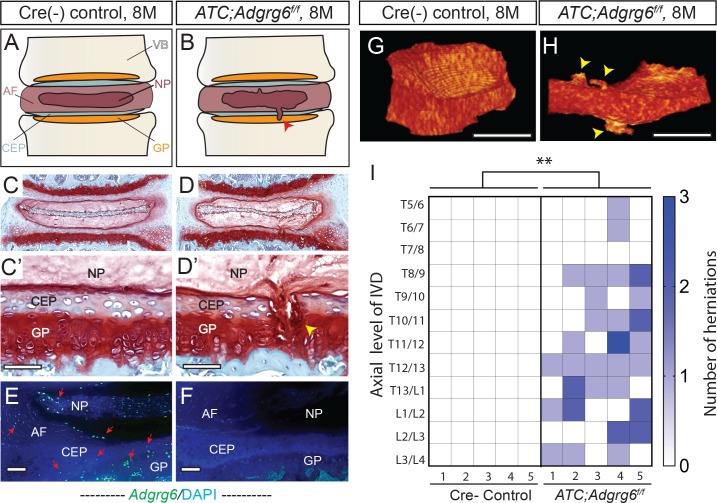
Fig 1. Adult ATC;Adgrg6f/f mutant mice display endplate-oriented herniations of the IVD.
(A and B) Schematic of endplate-oriented herniations (B, red arrowhead) observed in ATC;Adgrg6f/f mutant mice (B), in contrast to a typical wild-type IVD (A) at 8 months of age. (C-D’) Representative midline-sectioned 8-month-old mouse IVDs stained with Safranin-O/Fast green (SO/FG) (induced from P1-P20, n = 4 for each group). (E, F) Adgrg6 riboprobe FISH (green fluorescence) at 8 months (induced from P1-P20, n = 3 for each group). (G, H) Representative reconstructions of contrast-enhanced μCT of Cre (-) control (G) and ATC;Adgrg6f/f mutant (H) IVDs at 8 months of age (induced from E0.5-P20, n = 5 for each group). Endplate-oriented herniations are observed in SO/FG stained sections (D', yellow arrowhead) and by contrast-enhanced μCT (H, yellow arrowheads). (I) Heat map of contrast-enhanced μCT data from five Cre (-) control and five ATC;Adgrg6f/f mutant mouse spines, plotting the axial level of the IVD (left axis) and the number of herniations (right axis) observed in each mouse. (**p≤0.01, two-tailed Student's t Test.) Scale bars: 50μm in (C’, D’), 100μm in (E, F), and 500μm in (G, H). AF- annulus fibrosis, CEP- cartilaginous endplate, GP- growth plate, NP- nucleus pulposus, and VB- vertebral body.
Here, we show that Adgrg6 has a critical role in endplate-oriented herniations of the disc through regulation of STAT3 signaling. ADGRG6 (also called GPR126) is a member of the adhesion G-protein coupled receptor (aGPCR) family of proteins, all of which are thought to have a canonical intercellular signaling function via G-protein coupled signaling, as well as a potential for cell-cell or cell-matrix signaling via the extracellular N-terminal fragment [9]. In zebrafish, adgrg6/gpr126 is critical for the development of cartilaginous tissues of the semicircular canal via regulation extracellular matrix (ECM) gene expression [10], suggesting a role for ADGRG6 in the regulation of cartilaginous tissues. Conditional loss of Adgrg6 in multipotent osteochondral progenitors -giving rise to bone, cartilage, and some connective tissues- of the spine generated postnatal-onset scoliosis, ribcage deformity, and increased incidence of midline clefts in the endplates and annulus fibrosus [11]. Since the development of scoliosis is often linked with IVD deformity [12], we sought to investigate the role of Adgrg6 specifically in cartilaginous tissues of the IVD during embryonic and postnatal development.
To define the role of Adgrg6 we combined conditional mouse genetics, genomic approaches, mechanical assessment of intervertebral disc, and cell biological approaches in chondrogenic cell culture. Together, these studies reveal that ADGRG6 has a conserved role for the maintenance of normal gene expression profiles and regulation of STAT3 signaling in cartilaginous cells. We demonstrate that loss of Adgrg6 leads to increased expression of collagens associated with fibrosis and alteration of the normal biomechanical properties of the IVD, prior to the onset of endplate-oriented herniations. Finally, we demonstrate loss of Adgrg6 leads to increased, ectopic STAT3 activation in the IVD and that blockade of STAT3 activation can decrease the incidence of endplate-oriented herniations in Adgrg6 conditional mutant mice. Taken together, our work establishes Adgrg6 as a novel regulator of IVD endplate and growth plate integrity in the mouse and suggests that modulation of ADGRG6/STAT3 signaling could provide robust disease-modifying targets for endplate-oriented disc degeneration, in humans.
Results
Loss of ADGRG6 in intervertebral discs leads to endplate-oriented herniations in adult mice
We demonstrate that conditional removal of ADGRG6 function in the intervertebral disc (IVD) results in endplate-oriented herniations in adult mice (Fig 1B, 1D and 1D’). We found that Adgrg6 is highly expressed in the growth plate using immunohistochemistry-based in situ hybridization (S1A Fig), but we failed to detect expression in the cortical or trabecular bone in vertebrae of adult mice under these conditions. Using a more sensitive, fluorescent in situ hybridization (FISH) detection method we were also able to detect Adgrg6 expression in cells of the cartilaginous endplate (CEP), annulus fibrosus, and nucleus pulposus (Figs 1E and S1C). To determine the role of ADGRG6 function specifically in these committed chondrogenic lineages of the spine, we utilize an Aggrecan enhancer-driven, Tetracycline-inducible Cre (ATC) transgenic mouse strain [13] (ATC;Adgrg6f/f). Using this inducible Cre-deleter strain we established two experimental groups to address the temporal requirement of ADGRG6 function by induction during embryonic development (from E0.5-P20, prior to IVD specification) or during perinatal development (from P1-P20, after IVD specification) (S1 Table).
Using either induction strategy, we consistently observed endplate-oriented disc herniations [14] in adult mutant mice at 8 months of age (Figs 1 and S2B and S2B’ and S4D). ATC;Adgrg6f/f mutant mice induced during perinatal development (P1-P20) displayed prolapse of the nucleus pulposus material into the growth plate (Figs 1B, 1D and 1D’ and S4D), as did mutant mice recombined during embryonic development (E0.5-P20) (S2B and S2B’ Fig). These data suggest that the formation of these endplate-oriented disc herniations is largely attributed to loss of ADGRG6 function during postnatal development. Interestingly, histological analysis of ATC;Adgrg6f/f mutant IVDs, regardless of timing of induction, did not reveal observable changes in disc height, alterations of overall sulfated-proteoglycan abundance (Safranin-O staining) (Figs 1D and S2 and S4), or any obvious defects of the annulus fibrosis or nucleus pulposus tissues when visualized at the midline of the intervertebral disc at 8 months (Figs 1D and S2C and S4D’). In contrast, endplate-oriented herniations were observed at various spatial levels of the IVD including at the midline (Fig 1D and 1D) and at more lateral sections of the IVD (S2B, S2B’ and S4D Figs), suggesting a unique endplate-driven pathology for this model.
In this way, we found that traditional two-dimensional histological analysis limited our ability to capture the extent and distribution of these endplate-oriented herniations along the spine. To address this, we exploited contrast-enhanced micro-computed tomography (μCT) imaging which allows for a full three-dimensional analysis and segmented visualization of the IVD within the intact mouse spine [15]. Reconstruction and segmentation of the whole IVD in 8-month-old ATC;Adgrg6f/f mutant (Fig 1H and S1 Movie) and Cre (-) control mice (Fig 1G and S2 Movie) (49 discs total; n = 5 mice/genotype) revealed multiple incidences of endplate-oriented disc herniations (yellow arrowheads, Fig 1H). Quantification of the contrast-enhanced μCT imaging indicated from 0–3 herniations present/IVD in ATC;Adgrgf/f mutant mice, while Cre (-) littermate control mice were not observed to display these defects (Fig 1I). Spatial analysis of the intact spine demonstrated that these herniations occurred along the entire axis of the spine (Thoracic (T)5/6—Lumbar (L)3/4), without obvious hotspots. As was demonstrated by histology, we did not observe radial fissures or lateral prolapse of the disc in ATC;Adgrg6f/f mutant mice using contrast-enhanced microCT imaging. This further supports IVD degeneration in this conditional mutant mouse is occurring by endplate-driven mechanisms [14].
In previous studies we demonstrated a clear role for Adgrg6 in the formation of late-onset scoliosis in mouse [11], however the cellular pathogenesis of this process remained unresolved. Interestingly, while ~80% of Col2Cre;Adgrg6f/f mutant mice demonstrated late-onset scoliosis [11], we only observed late-onset scoliosis in ~12% of ATC;Adgrg6f/f mutant mice (S1 Table). One possible explanation is the difference in targeted tissues between the two Cre-deleter strains. Analysis of recombination using β-galactosidase staining of ATC;Rosa-LacZ mice showed nearly complete recombination throughout the IVD and growth plate at weaning (P28) regardless of the timing of induction (S3C and S3D Fig). However, the outer most layers of the annulus fibrosus and the periosteum were not targeted using either strategy (S3A, S3C and S3D Fig). In contrast, the entire IVD as well as periosteum and trabecular bone in the vertebral body is completely recombined at P1 and P28 when crossed to the Col2Cre deleter strain (S3B and S3E Fig). Effective knockdown of Adgrg6 in ATC;Adgrg6f/f mutant mice (induced from E0.5-P20) was further confirmed using FISH analysis of Adgrg6 expression at 8 months (Figs 1F and S1D and S1F) and by qPCR analysis of cDNA derived from IVDs extracted from 1.5 month-old mice (Fig 2O). In situ hybridization analysis showed that Adgrg6 expression was not altered in tissues not recombined by the ATC-deleter strain, such as the periosteum (S1E and S1F Fig). Together these data suggest that the IVD is important for susceptibility of scoliosis, yet additional effectors of spine stability are involved. Importantly, irrespective of the incidence of scoliosis in the thoracic spine in young mice (~12%, observed around P20-P40), ATC;Adgrg6f/f mutant mice consistently exhibited endplate-oriented herniations along the entire axis of the spine in adult mice (100%, n = 5) (Fig 1I). These data demonstrate that ADGRG6 has a unique role in the regulation of endplate-oriented herniations of the adult IVD, in addition to its role in scoliosis.
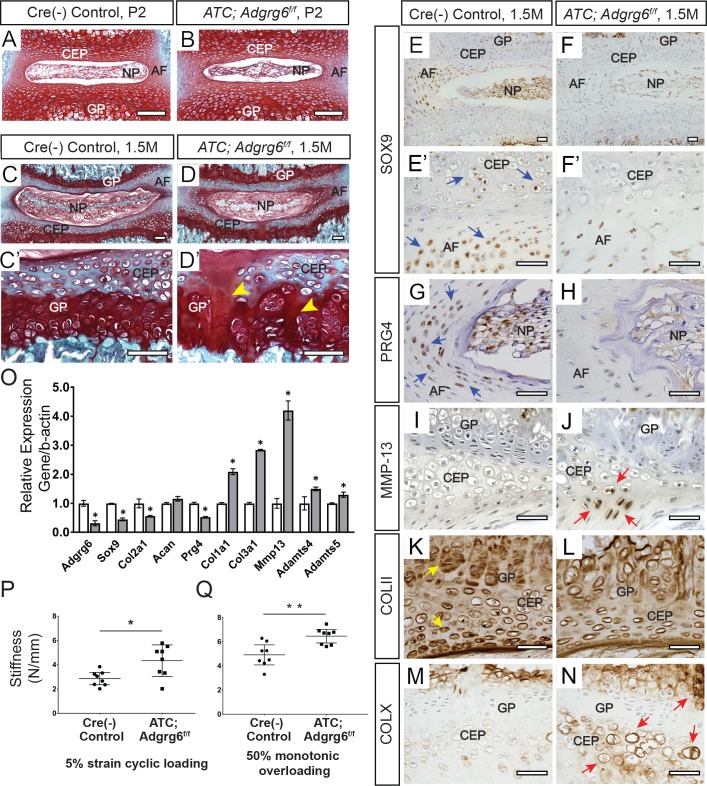
Fig 2. Young ATC;Adgrg6f/f mutant mice display alterations in IVDs consistent with disc degeneration pathology.
(A-D’) Representative IVD tissues stained Safranin-O/Fast green (SO/FG) (Induced from E0.5-P20, n = 3 for each group.) No overt structural defects were observed in ATC;Adgrg6f/f mutant IVDs compared with controls at P2 at midline (A, B) or at 1.5 months of age (C-D’), except for the mild increase of acellular clefts in the CEP and GP at 1.5 months (yellow arrowheads, D’). Section in panel D is a little bit pass midline to show the obvious acellular clefts in CEP and GP. (E-O) IHC (E-N) and qPCR (O) analyses of common markers of degenerative disc in mice at 1.5 months (induced from E0.5-P20). ATC;Adgrg6f/f mutant IVDs display reduced expression of markers of healthy disc: SOX9/Sox9 (blue arrows, E’) and PRG4/Prg4 (blue arrows, G), and mildly reduced COLII/Col2a1 (yellow arrows, K). They also display increased expression of hypertrophic marker COLX/Col10a1 (red arrows, N), extracellular matrix modifying enzymes (MMP-13/Mmp13 (red arrows, J), Adamts4, and Adamts5), fibrosis markers (Col1a1 and Col3a1). (E-N, n = 3 for each group. O, n = 3 biological replicates and representative result is shown. Bars represent mean ± SD. *p≤0.05, two-tailed Student's t Test.) (P, Q) Mechanical testing using 5% strain cyclic loading (stiffness mean w/95% CI, *p < 0.05) (P), and 50% monotonic overloading (stiffness mean w/95% CI, **p < 0.01) (Q), demonstrating increased stiffness in ATC;Adgr6gf/f mutant lumbar IVDs (induced from E0.5-P20, n = 4 for each group, 4 IVDs were analyzed /mouse). Scale bars: 100μm in (A-D’); 50μm in (E-N). AF- annulus fibrosis, CEP- cartilaginous endplate, GP- growth plate, and NP- nucleus pulposus.
Loss of ADGRG6 in the intervertebral discs leads to alterations of gene expression consistent with human disc degeneration pathology
We next sought to understand the molecular mechanism underlying the initiation of endplate-oriented herniations. To guide our analysis, we took cues from molecular changes reported in degenerative IVDs in human [16], which revealed several indicators of degenerative joint disease in ATC;Adgrg6f/f mutant mice at 1.5 months, prior to overt histopathology (Fig 2D and 2D’, induced from E0.5-P20). Using immunohistochemistry (IHC) we observed a consistent reduction in the expression of the transcription factor SOX-9 (SOX9) (Figs 2F and 2F’ and S5B) and proteoglycan 4 (PRG4) (Figs 2H and S5D) in the inner layer of the annulus fibrosus and CEP in these mutant mice. In contrast, the expression of the hypertrophic chondrocyte marker type X collagen (COLX) was increased in the CEP and hypertrophic growth plate in ATC;Adgrg6f/f mutant mice (Figs 2N and S5J). We also observed a mild reduction of type II collagen (COLII) staining in the pericellular matrix of chondrocytes in the CEP, and in the proliferative growth plate in mutant mice (Figs 2L and S5H). We observed mild increases of matrix metalloprotease-13 (MMP-13) expression in the CEP of ATC;Adgrg6f/f mutant mice (Figs 2J and S5F). Analysis of ATC;Adgrg6f/f mutant mice induced after birth (P1-P20) demonstrated similar alterations in protein expression in the IVD at 8 months (S4E–S4L Fig). Alterations of protein expression in the CEP, coupled with the consistency of endplate-oriented herniations in ATC;Adgrg6f/f mutant mice regardless of embryonic or perinatal induction of recombination (Figs 1 and S2 and S4), strongly suggest a postnatal role for ADGRG6 function in the homeostasis of the CEP. These alterations in protein expression were supported by similar changes in gene expression as assayed by qPCR analysis of extracted IVDs (Fig 2O) which demonstrate reduced expression of Col2a1, Sox9 and Prg4 in ATC;Adgrgf/f mutant mice at 1.5 months. Moreover, the expression of several catabolic mediators of degeneration including Mmp13, Adamts4, and Adamts5, as well as markers of fibrosis, Col1a1 and Col3a1, were increased. Together, these alterations of typical gene expression profiles in the IVD are consistent with expression profiles reported for degenerative joint diseases, including degenerative discs [17] and osteoarthritis of articulating joints [18, 19] in humans.
As demonstrated previously, ADGRG6 is not critical for early pattering or the overall morphology of the IVD [11], however it does regulate perinatal and homeostatic processes of the CEP and growth plate (Fig 1D and 1D’ and 2D and 2D’). To underscore this, we show that embryonic recombination of the ATC;Adgrg6f/f mutant mice, which removes Adgrg6 function specifically in cartilaginous tissues, does not affect the specification or overall pattern of the IVD nor lead to obvious alternations of the Safranin-O staining of the disc at P2 (Fig 2B). However, mild morphological differences in nucleus pulposus and delays in midline fusion of the CEP are occasionally observed in embryonically-induced ATC;Adgrg6f/f mutant mice at P2, consistent with our previous findings using the Col2Cre-deleter strain [11], which may reflect a mild developmental delay in these mutant mice. By 1.5 months, we begin to observe a mild increase in acellular clefts in the CEP and growth plate in ATC;Adgrg6f/f mutant mice (7.2±3.4 clefts/IVD; n = 4; p = 0.03) (Fig 2D'), compared to littermate controls (2.5±0.4 clefts/IVD; n = 4) (Fig 2C'). This is consistent with our observations of ATC;Adgrg6f/f mutant mice, recombined perinatally, which also demonstrate acellular clefts in growth plate at 4-months (40%; n = 5) (yellow arrows, S4B Fig). Finally, TUNEL staining demonstrated a mild increase in cell death in ATC;Adgrg6f/f mutant mice within the annulus fibrosus, nucleus pulposus, and CEP compartments of the IVD, less so within vertebral growth plate at 1.5 months (S6B and S6C Fig). Taken together these data support that while ADGRG6 function is important in cartilaginous tissues of the spine it is not a critical factor for overall development of the IVD. In contrast, it seems to have an increasingly important role within the CEP and growth plate for perinatal development and adult homeostasis of these tissues.
Mechanical properties are altered prior to the onset of obvious histopathology in the intervertebral discs of Adgrg6 mutant mice
During the progression of disc degeneration and osteoarthritis-related joint remodeling in humans, increased catabolic factors, inflammatory signaling, coupled with alterations of normal extracellular matrix composition can have deleterious effects on the mechanical properties on these tissues [20]. In order to assess alterations of mechanical properties of ATC;Adgrg6f/f mutant IVDs, we isolated individual lumbar discs (L1/2 and L4/5) for dynamic mechanical testing (16 discs total; n = 4 mice/genotype) from 1.5-month-old mutant and control mice (induced from E0.5-P20). Using micro-indentation we demonstrated a consistent increase in the stiffness (Newton (N)/mm) of ATC;Adgrg6f/f mutant IVDs under 5% strain cyclic loading (Fig 2P; p = 0.0114; mean w/95% CI) and under 50% monotonic overloading (Fig 2Q; p = 0.0026; mean w/95% CI). Stiffening of the IVD is commonly observed with early-onset degenerative changes, which compromises the damage resistance of the tissue [21]. We analyzed proteoglycan quantification in these IVDs by dimethylmethylene blue (DMMB) assay and found no significant alterations comparing mutant and littermate control mice at 1.5 months. Similarly, we observed no alteration in total collagen content in ATC;Adgrg6f/f mutant IVDs by hydroxyproline assay (measured as collagen/wet weight; p = 0.0561; one-tailed t-test). However, we observed increased expression collagens associated with fibrosis in 1.5-month-old mutant IVDs, correlated with a decrease in the expression of typical collagen genes observed in healthy cartilage (Fig 2O). We speculate that, alterations of typical extracellular matrix/collagen gene and protein expression, coupled with increased cell death contribute a constellation of factors leading to the decline in the normal mechanical properties of the IVD in ATC;Adgrg6f/f mutant mice.
Loss of Adgrg6 in the intervertebral disc leads to alterations of collagen gene expression and alterations in ion transport components
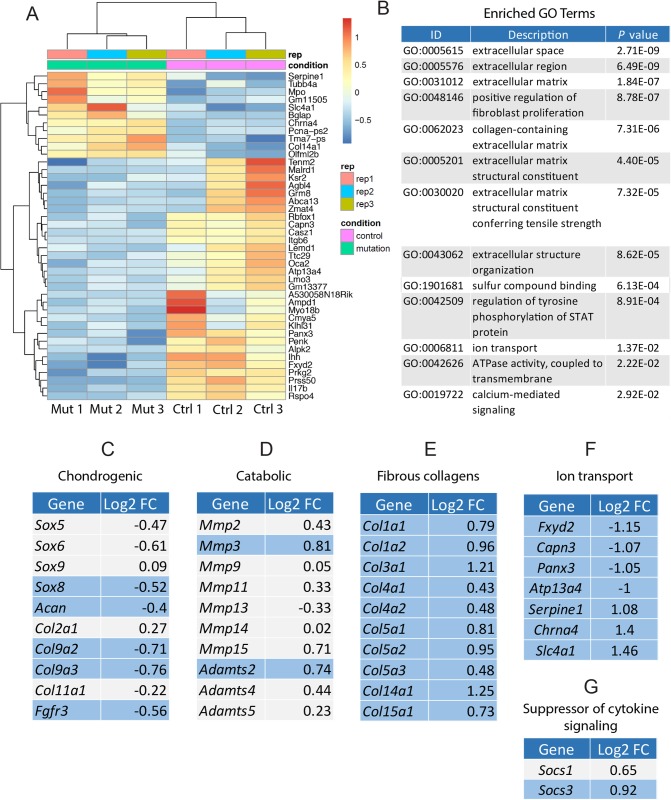
To obtain additional, unbiased insights of the cellular and molecular changes in Adgrg6 conditional mutant IVDs prior to overt histopathology, we applied transcriptomic analysis (RNA-seq) on whole IVDs extracted from mice at P20. To avoid the contamination of untargeted IVD tissue in the ATC;Adgrg6f/f mutant mice (e.g. the outer most annulus fibrosus, S3C and S3D Fig), we choose to use Col2Cre;Adgrg6f/f mutant mice for these studies as this experimental group completely recombines throughout the entire IVD during embryonic development (S3B and S3E Fig). At P20, Col2Cre;Adgrg6f/f mutant mice display no overt histopathology at the midline in any IVD tissues or in the growth plate, akin to Cre(-) littermate control IVDs (S7A and S7B’ Fig). However, in 8-month-old Col2Cre;Adgrg6f/f mutant mice we observe endplate-oriented herniations (S7D’ Fig), analogous to observations in ATC;Adgrg6f/f mutant IVDs (Fig 1). Similarly, we do not observe overt defects of the annulus fibrosus and nucleus pulposus in Col2Cre;Adgrg6f/f mutant IVDs when imaged at the midline of the IVD (S7E Fig). We generated three independent libraries for each genotype (using Col2Cre;Adgrg6f/f mutants and Cre (-) littermate controls) from extracted IVD tissues (T8/9-L4/5), pooled from 2–3 individual mice at P20. We found 884 differential expressed genes with statistical significance (p value < 0.05) (S2 Table), and with a more stringent cut-off adjusted p value <0.05 and fold-change ≥2, we observed 42 differential expressed genes (Fig 3A). Enrichment of pathways and biological processes using gene ontology (GO) terms [22] demonstrated associations with extracellular matrix, positive regulation of fibroblast proliferation, extracellular matrix structural constituent conferring tensile strength, regulation of tyrosine phosphorylation of STAT protein, and ion transport (Fig 3B). Several of the significantly upregulated genes are associated with risk of lumbar disc degeneration and osteoarthritis assayed in humans or animal models, including Aspn, Dkk-3, and Mmp3 [23–27] (S2 Table). We also observed alterations of chondrogenic and catabolic gene expression (Fig 3C and 3D). Surprisingly some of the genes altered in ATC;Adgrg6f/f mutant mice at 1.5 months by qPCR analysis (Fig 2O), such as Sox9, Col2a1 and Mmp13, were not similarly changed in slightly younger Col2Cre;Adgrg6f/f mutant mice (P20) by RNA-Seq analysis. However, in both ATC and Col2Cre conditional Adgrg6 mutant mouse models we observed consistent alteration of collagen expression, including Col1a1, Col3a1 among others at P20 in Col2Cre;Adgrg6f/f mutant mice (Fig 3E) and at 1.5 months in ATC;Adgrg6f/f mutant mice (Fig 2O), resulting in a shift in collagen gene expression, including type II collagen, towards more fibrous collagen gene expression associated with degenerative IVD from both human and mouse models [28–31]. We suggest that increased expression of these collagens may drive increased stiffness of the IVD observed in ATC;Adgrg6f/f mutant mice at 1.5 months (Fig 2P and 2Q) which in turn may contribute to increased susceptibility of endplate-oriented disc herniations in adult mutant mice.
Fig 3. Young Col2Cre;Adgrg6f/f mutant mice display fibrotic-like changes of gene expression and dysregulation of genes associated with ion transport in the IVD.
(A) Heatmap of differentially expressed gene based on RNA-sequencing analysis of IVDs derived from both Col2Cre;Adgrg6f/f mutant (Mut 1–3) and Cre (-) littermates (Ctrl 1–3) at P20. (B) Gene ontology (GO) analysis revealed a suite of differentially expressed genes important for extracellular matrix organization and ion transport. (C-G) RNA-sequencing analysis revealed mild alterations in some chondrogenic (C) and catabolic (D) gene expression, but significantly induced fibrotic gene expression (E) and dysregulation of genes involved in ion transport (F) in the Col2Cre;Adgrg6f/f mutant IVDs at P20. Some genes encode members of the suppressor of cytokine signaling were also upregulated in the mutant IVDs (G). Differential expressed genes with p value < 0.05 were highlighted in blue. Log2FC: gene expression fold changes in Log2 scale.
In addition, we observed gene expression changes in several genes associated with ion transport in the IVD of Col2Cre;Adgrg6f/f mutant mice at P20, including reduced expression of Fxyd2, Capn3, Panx3, and Atp13a4, as well as increased expression of Serpine1, Chrna4, and Slc4a1 (Fig 3F). High osmotic pressure is a characteristic of the IVD [32] and ion channel activity plays a critical role in the regulation of osmotic changes [33, 34]. In agreement, recent analysis of the SM/J isotype mouse model of disc degeneration mice is associated with gene expression changes in ion transport systems [31]. Altogether, our transcriptomic analysis of the IVDs in Col2Cre;Adgrg6f/f mutant mice demonstrated a robust dysregulation of several important pathways and components of the IVD homeostasis, including collagen gene expression, alteration of ion transport components, as well as increased expression of several established catabolic factors prior to the onset of histopathology and disc degeneration. These data suggest that ADGRG6 signaling is a critical regulator of postnatal homeostasis of the CEP and growth plate.
ADGRG6 regulates STAT3 signaling in cartilaginous cells
RNA-Seq analyses also implicate pro-inflammatory signaling is involved in Adgrg6 mutant IVDs. For example, pathways associated with inflammation and activation/phosphorylation of STAT proteins (GO: 0042509—regulation of tyrosine phosphorylation of STAT protein) were altered (Fig 3B) and the expression of suppressor of cytokine signaling (Socs) genes, Socs3 and Socs1 are expressed 1.9 and 1.6 fold higher in Col2Cre;Adgrg6f/f mutant mice respectively (Fig 3G). SOCS3 is known to directly regulate STAT1 (signal transducer and activator of transcription 1) and STAT3 activation [35]. SOCS3 also acts as a negative feedback effector inhibiting IL-6 production which acts to inhibit prolonged IL-6/STAT-3 signaling [36]. Finally, STAT3/IL-6 signaling has been previously implicated in IVD degeneration [37, 38]. For these reasons, we wanted to assay STAT1 and STAT3 activity in the IVD of ATC;Adgrg6f/f mutant mice.
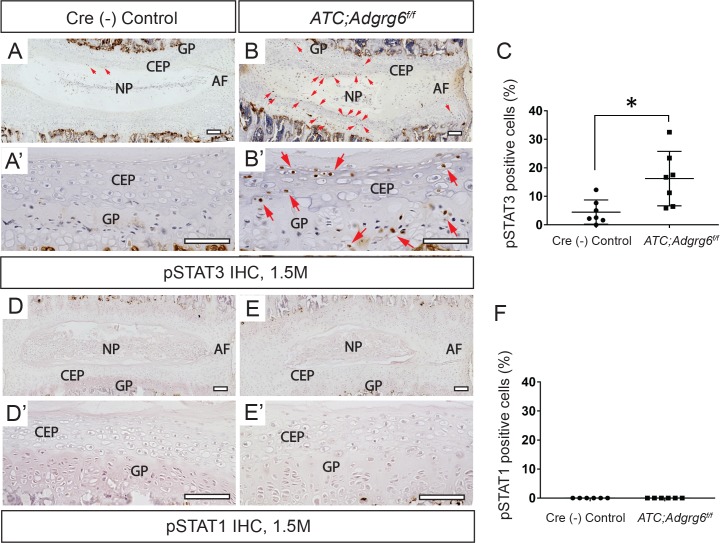
By IHC analysis, we observed a substantial increase in phosphorylated STAT3 (pSTAT3) signal, the active form of STAT3 protein, in the CEP and the growth plate of the ATC;Adgrg6f/f mutant mice at 1.5 months (Fig 4B and 4B’), prior to overt histopathology of the disc. Quantification analysis revealed that 16.7% of cells in the mutant IVD are pSTAT3 positive (n = 3 mice; 2–3 IVDs/mouse; n = 526 cells total), in comparison to 5.2% of the control IVD (n = 3 mice; 2–3 IVDs/mouse; n = 417 cells total) (Fig 4C). Similar analysis using an antibody against pSTAT1 failed to detect any signal in either genotype (Fig 4D–4F). Together these data demonstrate that ADGRG6 regulates STAT3 activation in the CEP and growth plate of the IVD.
Fig 4. ADGRG6 regulates STAT3 signaling in IVDs.
(A-B’) IHC analysis shows increased expression of pSTAT3 (red arrowheads, B, B’) in ATC;Adgrg6f/f mutant mouse IVD at 1.5 months (induced from E0.5-P20, n = 3 for each group.) (D-E’) IHC analysis shows no signal of pSTAT1 in neither Cre(-) nor ATC;Adgrg6f/f mutant mouse IVD at 1.5 months (induced from E0.5-P20, n = 3 for each group.) (C, F) Quantification of positive pSTAT3 (C) and pSTAT1 (F) cells in Cre(-) control or in ATC;Adgrgf/f mutant mouse IVDs. (n = 3 mice for each group, at least two IVDs were scored for each mouse. Dots plot with mean ± SD. *p≤0.05, two-tailed Student's t Test.) Scale bars: 200μm in (A, B), and 50μm in (A’, B’). CEP- cartilaginous endplate, GP- growth plate, and NP- nucleus pulposus.
To better understand the origin of STAT3 activation we analyzed the distribution of macrophages, one of the key immune cells elicited by inflammatory response [39], by checking a well-established macrophage marker CD68 on IVD sections of the ATC;Adgrg6f/f mutant IVD at 1.5 months. We did not observe any accumulation of macrophages at the CEP and growth plate (S8A and S8B Fig) in these mutant mice, demonstrating that the STAT3 activation is not due to peripheral macrophage infiltration into the IVD. We also assayed CD68 on the 8-month-old ATC;Adgrg6f/f mutant IVD, but only observe minor signal at the site of herniation (S8D Fig), indicating that endplate-oriented herniations within the growth plate does not invoke a robust immune response.
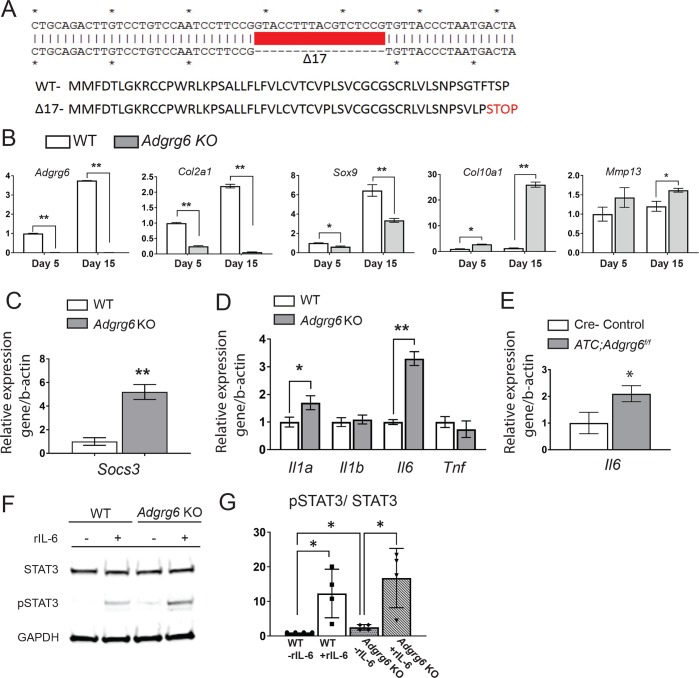
In order to facilitate more mechanistic studies of ADGRG6 function in chondrogenic tissues, we utilized the ATDC5 mouse cell line which can be induced to form cartilage-like tissue in vitro [40]. After 15 days of maturation, the wild type ATDC5 cells robustly express chondrogenesis markers (Col2a1 and Sox9), but not the hypertrophic chondrocyte marker (Col10a1) (Figs 5B and S9), which is analogous to cartilaginous cells in CEP and proliferative growth plate. We utilized lentivirus driven CRISPR-Cas9 technique in ATDC5 cells and engineered a stable INDEL mutant with a homozygous 17-bp deletion in exon 3 of Adgrg6, predicted to generate a frameshift mutation at amino acid Ser49 resulting in a truncated ADGRG6 protein (Adgrg6p.Ser49+3fs) (Fig 5A). The complete reduction of Adgrg6 expression in our clonal Adgrg6 mutant cell line (Adgrg6 KO) suggested a null allele, likely due to non-sense mediated decay of the transcript (Fig 5B). During the course of chondrogenic maturation in unedited ATDC5 cells, Adgrg6 expression increases with similar kinetics as other chondrogenic markers Col2a1, Sox9 and Acan (S9 Fig). Consistent with our observations in vivo (Fig 2E–2N and 2O), we observed reduced expression of chondrogenic markers Sox9 and Col2a1, and mild induction of the catabolic enzyme Mmp13 in Adgrg6 KO cells, demonstrating a common function of Adgrg6 in maintaining gene expression profiles in chondrogenic lineages (Fig 5B). We also observed increased expression of hypertrophic marker Col10a1 in the ATDC5 KO cells (Fig 5B), suggesting precocious maturation and inappropriate initiation of hypertrophy upon loss of ADGRG6 function. Indeed, similar precocious maturation phenotype was observed in Sox9 haploinsufficiency mice [41], and we also found that the expression level of Mef2c, another key regulation of chondrocyte hypertrophy [13, 42], is upregulated in Adgrg6 KO cells (5.2 fold compared with wild type control, p<0.05), which may also contribute to the induced Col10a1 expression. Taken together, our data suggest that Adgrg6 plays a critical role in regulation of normal gene expression profiles in chondrogenic cells. In addition, cleaved-Caspase 3, a key effector of apoptosis, was increased 2-fold in Adgrg6 KO cells after 10-day maturation (S10 Fig), consistent with our observations of increased cell death in ATC;Adgrg6f/f mutant mice IVDs (S6 Fig). Consistent with the RNA-seq analysis of Col2Cre;Adgrg6f/f conditional mutant mice at P20 (Fig 3), we also observed a 5-fold increase in the expression of Socs3 in Adgrg6 KO cells (Fig 5C), while Socs1 was not detectable in either unedited wild-type or Adgrg6 KO ATDC5 cells. Taken together, the strong correlation between these in vivo and in vitro findings suggests a cell autonomous function for ADGRG6 for the regulation of typical gene expression profiles and STAT3 activation in cartilaginous cells.
Fig 5. Adgrg6 regulates gene expression profiles and STAT3 signaling in ATDC5 cell culture.
(A) Schematic of a 17-bp deletion of Adgrg6 from a stable single cell clone of ATDC5 cell line (Adgrg6 KO). (B) qPCR analyses of gene expression in ATDC5 cells at 5 and 15 days of maturation demonstrates decreased expression of markers of healthy disc, Col2a1and Sox9, and increased expression of the hypertrophic marker, Col10a1 and the extracellular matrix modifying enzyme, Mmp13 in Adgrg6 KO cells. (C) qPCR analyses revealed increased expression of Socs3 in Adrgr6 KO cells after 15 days of maturation. (D) qPCR analysis of Il1a, Il1b, Il6 and Tnf in ATDC5 cells maturated for 15 days. (E) qPCR analysis of Il6 expression in 1.5-month-old primary mouse IVDs (induced from E0.5-P20). (B-E, n = 3 biological replicates and representative result is shown. Bars represent mean ± SD. *p≤0.05, **p≤0.01, two-tailed Student's t Test). (F) Representative Western blot of wild type and Adgrg6 KO ATDC5 cell lysates showing stimulation of pSTAT3 staining after treatment with recombinant IL-6 (rIL-6) protein in both cell lines, while Adgrg6 KO cells show a mild constitutive stimulation of pSTAT3 without addition of rIL-6 (n = 4 biological replicates and representative result is shown). (G) Densitometry of the Western blot of wild type and Adgrg6 KO ATDC5 cell with or without IL-6 (rIL-6) treatment. (Each dot represents one biological replicate. Bars plot with mean ± SD. *p≤0.05, two-tailed Student's t Test.)
Cytokines including interleukin-6 (IL-6), IL-1, and Tumor necrosis factor alpha (TNF), are known to induce STAT3 phosphorylation through receptor-associated Janus kinases. pSTAT3 can then translocate to the nucleus to regulate many cellular processes, such as cell growth and apoptosis [43]. To better understand the mechanism of ADGRG6-dependent regulation of STAT3 activation, we assayed a panel of known pro-inflammatory cytokines Il1a, Il1b, Il6 and Tnf in Adgrg6 KO cells maturated for 15 days, observing increased expression of both Il1a and Il6 expression (Fig 5D). Importantly, increased expression of Il6 was also observed in ATC;Adgrg6f/f mutant IVDs at 1.5 months of age (Fig 5E).
IL-6 can not only stimulate the production of catabolic enzymes, but also can suppress the expression of anabolic genes, including Sox9, Col2a1, and Acan [44]. To determine whether increased Il6 expression was coupled with activation of STAT3 upon loss of ADGRG6 function, we assayed the ability of ATDC5 cells to respond to recombinant IL-6 protein (rIL-6) during chondrogenic maturation in vitro. Western blot analysis demonstrated that rIL-6 effectively stimulates increased expression of pSTAT3 in both unedited wild type and Adgrg6 KO cells (Fig 5F and 5G). Interestingly, we revealed a low level of increased pSTAT3 expression in Adgrg6 KO cell lysates that were not stimulated by rIl-6 (Fig 5F and 5G). These in vitro results further demonstrate that ADGRG6 regulates STAT3 activation in cartilaginous cells in a cell autonomous manner through increased paracrine and/or autocrine mediated IL-6 signaling.
STAT3 blockade protects against the formation of endplate-oriented herniations in Adgrg6 mutant mice
To further define the role of STAT3 signaling on the pathogenesis of endplate-oriented disc degeneration, we next tested if systemic inhibition of STAT3 activation could ameliorate these defects in vivo. We choose to utilize the constitutive Col2Cre;Adgrg6f/f conditional mutant mice for this experiment in order to limit stress on the mice and avoid exposure to Dox as potential confounding variable prior to long-term (16-week) treatment with the STAT3 inhibitor, Stattic.
Importantly, these constitutive conditional Col2Cre;Adgrg6f/f mutant mice display no obvious histopathology of IVD and growth plate at P20 (S7B and S7B’ Fig), yet exhibit adult-onset endplate-oriented herniations by histology at 8 months (S7D and S7D’ Fig), while the annulus fibrosus and nucleus pulposus is unremarkable when visualized at the midline (S7E Fig). We set up a treatment regime where in both Cre (-) control and mutant groups would receive Stattic, a nonpeptidic STAT3 inhibitor (25mg/kg, dissolved in DMSO/PBS/Tween-20), [45], or placebo (DMSO/PBS/Tween-20) via i.p. injection for 16 weeks beginning at 1.5 months and imaged at 6 months. IHC staining of pSTAT3 and quantification analysis revealed that after two weeks of Stattic treatment, the percentile of pSTAT3 positive cells was significantly reduced in the Stattic treated mutant mice (Fig 6F and 6G) compared with the placebo treated mutant mice (Fig 6E and 6G), but was not distinguishable from the placebo treated Cre(-) control mice (Fig 6D and 6G), demonstrating efficient inhibition of the STAT3 signaling by systemic Stattic treatment.
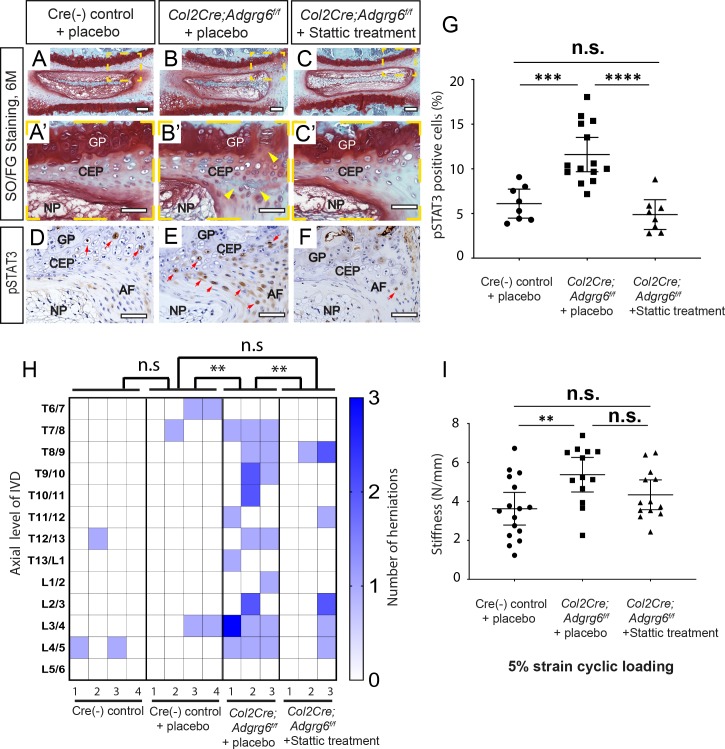
Fig 6. Inhibition of STAT3 by Stattic alleviates the formation of disc herniations and disc stiffness attributed to loss of ADGRG6 function.
(A-C’) Representative Safranin-O/Fast green staining and (D-F) pSTAT3 IHC in medial-sectioned mouse IVD from placebo-treated Cre (-) control (A-A’, D), placebo-treated Col2Cre;Adgrg6f/f mutant (B-B’, E), and Stattic treated Col2Cre;Adgrg6f/f mutant mice (C-C’, F) by the age of 6 months (A-C’) or 2 months (D-F) (n = 3 mice for each group). Col2Cre;Adgrg6f/f mutant mice display defects of the IVD including lesions and clefts in the CEP and GP (yellow arrowheads, B’) and increased, expression of pSTAT3 in CEP and AF (red arrowheads, E), which is reduced by Stattic treatment (C-C’, F). (G) Quantification of positive pSTAT3 cells in Cre (-) control or in Col2Cre;Adgrg6f/f mutant mice mutant mouse IVDs by the age of two months after two weeks of Stattic treatment. (n = 3 mice for each group, two to five IVDs were scored for each mouse. Dots plot with mean ± SD. ***p≤0.001, ****p≤0.0001, One way ANOVA followed by Tukey HSD test. n.s, not significant.) (H) Heat map to represent contrast-enhanced microCT data from 6-month-old mice from three experimental groups: four placebo-treated Cre (-) controls; three placebo-treated Col2Cre;Adgrg6f/f mutants; and three Stattic-treated Col2Cre;Adgrg6f/f mutants. Plotted by the axial level of the IVD (left axis) and the number of herniations (right axis) observed in each mouse. (**p≤0.01, One way ANOVA followed by Tukey HSD test. n.s, not significant.) (I) Mechanical testing on lumbar discs isolated from the same samples imaged in (H) using 5% strain cyclic loading. (Three to five IVDs were analyzed /mouse. Dots plot with mean ± 95% CI. **p≤0.01, One way ANOVA followed by Tukey HSD test. n.s, not significant.) Scale bars: 200μm in (A-C), 50μm in (A’-C’) and (D-F). AF- annulus fibrosis, CEP- cartilaginous endplate, GP- growth plate, and NP- nucleus pulposus.
By contrast-enhanced μCT imaging of the placebo treated Col2Cre;Adgrg6f/f mutant mice at 6 months, we quantified an average of 9.6 herniations/mouse (n = 3 mice; 29 total herniations from 39 total IVDs imaged) (Fig 6H). In this background, Cre (-) littermate controls displayed rare occurrences of endplate-oriented herniations in both the placebo-injected (n = 4 mice; 5 total herniations from 52 total IVDs imaged) and uninjected (n = 4 mice; 3 total herniations from 52 total IVDs imaged) groups (Fig 6H). Importantly, we observed a decrease in the incidence of endplate-herniations in Col2Cre;Adgrg6f/f mutant mice treated with Stattic for 16-weeks (n = 3 mice; 9 total herniations from 39 total IVDs imaged) (Fig 6H). One way ANOVA followed by Tukey HSD test indicated that the mean score for the Stattic-treatment condition ((M)ean = 0.23, SD = 0.54) was significantly different (p<0.01) than the placebo-treated Col2Cre;Adgrg6f/f mutant mice (M = 0.74, SD = 0.56), but did not differ significantly from the placebo-treated Cre (-) control littermates (M = 0.096, SD = 0.09) (S3 Table). Post-hoc analysis shows a medium effect size (Cohen’s δ: 0.7859) comparing the Stattic treatment and placebo Col2Cre;Adgrg6f/f mutant mice (S3 Table). Given that 100% of Col2Cre;Adgrg6f/f mutant mice displayed endplate-oriented herniations at 8 months, an a priori power analysis (assuming effect size 0.5; power of 0.99) suggested that we would need to image 40 discs per group to see a significant effect with a power of 0.99 between any two groups.
To understand if Stattic treatment also improve the biomechanical properties, we isolated lumbar discs (L1/2-5/6) from the same mice after contrast-enhanced μCT imaging for dynamic mechanical testing (n = 4 for placebo-treated Cre (-) controls, 16 discs; n = 3 for placebo-treated Col2Cre;Adgrg6f/f mutants, 13 discs; and n = 3 for Stattic-treated Col2Cre;Adgrg6f/f mutants, 13 discs). We demonstrated that, under 5% strain cyclic loading, placebo-treated Col2Cre;Adgrg6f/f mutant IVDs showed significant increase in the stiffness (5.37±1.43 N/mm) compared with the placebo-treated Cre (-) controls (3.63±1.58 N/mm) (Fig 6I, p<0.01, One way ANOVA followed by Tukey HSD test). Importantly, after 16 weeks of Stattic treatment, the stiffness of the Stattic-treated Col2Cre;Adgrg6f/f mutant IVDs reduced to 4.34±1.27 N/mm, which is not statically significant from neither the placebo-treated Cre (-) control group, nor the placebo-treated Col2Cre;Adgrg6f/f mutant group (Fig 6I, One way ANOVA followed by Tukey HSD test), suggesting a partial rescue of the increased stiffness in the Col2Cre;Adgrg6f/f mutant IVDs after Stattic treatment. We also performed a correlation analysis (Pearson’s r) of the dynamic mechanical testing data and the contrast-enhanced μCT imaging generated from the same lumbar discs in these three experimental group. We found no significant correlation between these variables in individual disc (S12 Fig). This could be due to the limitation that the mechanical testing was only reliable for the lumbar discs [21].
We observed increased pSTAT3 expression in the placebo-treated Col2Cre;Adgrg6f/f mutant mice compared to Cre (-) control mice treated with placebo (p≤0.001) (Fig 6D, 6E and 6G). Importantly, Stattic treatment was observed to decrease the number pSTAT3 positive cells in the IVD of Col2Cre;Adgrg6f/f mutant mice (p≤0.0001) (Fig 6F and 6G), comparable to the incidence observed in Cre (-) littermate control IVDs. Next, we assayed several other markers of degenerative IVD in these Stattic-treatment experimental groups. We found that COLX expression in the CEP was reduced in Stattic-treated Col2Cre;Adgrg6f/f mutant mice compared with the placebo-treatment groups (S11A–S11C’ Fig). In contrast, Stattic treatment had little effect on the general reduction of COLII and SOX9 expression observed in Col2Cre;Adgrg6f/f mutant mice, similar results were observed by qPCR analysis of gene expression in Stattic-treated Adgrg6 KO ATDC5 cells undergoing maturation (S11J Fig). These data suggest that additional effectors of ADGRG6 function, apart from STAT3 signaling, are required for maintenance of normal gene expression profiles in CEP and growth plate.
Taken together, our studies demonstrated that dysregulation of STAT3 signaling is associated with endplate-oriented herniations in the IVD of Adgrg6 conditional mutant mice. Blockade of STAT3 signaling by Stattic treatment can protect against the formation of the endplate-oriented herniations in these mutant mice, potentially through a restoration of normal biomechanical properties of the disc. These results demonstrate that dysregulation of STAT3 signaling can drive endplate-oriented disc herniation, and ADGRG6/STAT3 signaling is a promising therapeutic target for degenerative joint disorders.
Discussion
In this study, we establish for the first time that ADGRG6 signaling acts as a positive regulator of homeostatic mechanisms of the CEP and growth plate. Loss of homeostasis is observed based on global changes in gene expression in the Adgrg6 mutants IVD tissues including: alterations of several anabolic and catabolic factors, increased expression of collagens associated with fibrotic and degenerative cartilage tissues, and increased STAT3 activation. Histological analysis of several of these factors showed altered expression specifically in the CEP and growth plate. We further demonstrate that alterations of protein and gene expression in the IVD and growth plate and increased mechanical stiffening of the discs occur during early postnatal development (P20-1.5M), several months prior to obvious histopathology and endplate-oriented herniations observed in adult mice. We suggest that these transcriptional changes coupled with mild increases in cell death lead to a general stiffening of the IVD, which in turn synergistically contributes to the formation of the endplate-oriented disc herniation in adult mice. Finally, we show that inhibition of STAT3 activation provides a protective effect against the formation of the endplate-oriented disc herniations in ADGRG6 mutant discs and growth plate tissues. This is important given the positive association of increased STAT3 signaling and multiple degenerative joint diseases, including disc degeneration [46], disc herniation [47], and osteoarthritis [48] in humans.
We demonstrate that ADGRG6 acts as a cell autonomous regulator of the STAT3 signaling in vivo and in cartilagenous cell culture. The role in vivo of ADGRG6 appears to be most specific to the CEP and growth plate, as other IVD tissues were not greatly affected even out to 8-months of age. However, a more direct test of the specific role of Adgrg6 in the nucleus pulposus or annulus fibrosus tissues of the IVD is warranted. It will also be interesting to test if blockade of STAT3 signaling has a general protective effect of CEP integrity, not dependent on ADGRG6 function. However, to our knowledge there is not a well-established alternative experimental mouse model of endplate-oriented disc herniations to test this. Finally, it will be important to determine how STAT3 signaling contributes to histopathology observed in other models of disc degeneration including models with lateral herniations and/or pathology of the nucleus pulposus or annulus fibrosus.
The role of ADGRG6 appears to be largely dispensable for embryonic and early postnatal developmental of the IVD, yet is critical for postnatal mechanisms of homeostasis. We demonstrate that ADGRG6 is important for maintaining normal expression of SOX9 in vivo and in chondrogenic ATDC5 cell culture, where we observed reduced expression of Sox9 along with its direct target gene Col2a1 in Adgrg6 KO cells. SOX9 is a master transcription factor for both chondrogenesis during embryonic development [49] and cartilage maintenance during postnatal development [50], in the IVD. Regulation of Adgrg6 and Sox9 was also reported in the cartilaginous semicircular canal which similarly display altered expression of several extracellular matrix genes, as well as, decreased expression of sox9b in adgrg6/gpr126 mutant zebrafish [10]. Genetic ablation of Sox9 in cartilaginous tissues in adult mice leads to reduction of Adgrg6/Gpr126 expression, as well as many pathological similarities of the IVD as we described in this work, including increased cell death and alterations of extracellular matrix gene expression [50]. In contrast, Sox9 mutant mice exhibit more severe IVD defects including decreased disc height and loss of proteoglycan staining. This differences in pathology may be explained by an incomplete or perhaps more gradual loss of SOX9 expression in the conditional Adgrg6 mouse models presented here, which may stimulate undefined compensatory mechanisms [51]. These data suggest that ADGRG6 and SOX9 have some degree of co-regulation in cartilaginous tissues. The identification of factors that govern this regulation and how this mechanistically contributes during homeostasis and disease warrants further investigation.
We previously demonstrated a critical role for Adgrg6 in the formation of scoliosis in young mice (onset at around P20-P40) [11]. Here we demonstrate a novel defect of endplate-oriented disc herniations in both Col2Cre;Adgrg6f/f and ATC;Adgrg6f/f adult mutant mouse (at 6 to 8 months of age). The incidence of scoliosis in Col2Cre;Adgrg6f/f is ~80%, while only ~12% of ATC;Adgrg6f/f mutant mice display spine curvatures. In contrast, endplate-oriented herniations were observed in ~100% of conditional Adgrg6 mutant mouse models. The localization of post-natal onset scoliosis in both models is localized to the thoracic spine, while the formation of endplate-oriented herniations is observed in both thoracic and lumbar sections of the spine. From this, we suggest that the mechanisms promoting the formation of endplate-oriented disc herniations in adult mice are partially independent from the pathogenesis of postnatal-onset idiopathic scoliosis in these mutant mice. However, it is tempting to speculate that alterations of the mechanical properties of the IVD during postnatal development underlie the susceptibility of scoliosis during early postnatal development and may also promote endplate-oriented herniations in adults as well. Additional ongoing studies are seeking understand the cellular pathogenesis underlying Adgrg6-dependent scoliosis and how spine curvature is related to molecular changes in the CEP and growth plate Adgrg6 mutant mice.
Using an innovative contrast-enhanced μCT imaging approach of the intact mouse spine, we for the first time describe the appearance and distribution of the endplate-oriented herniations in mice. Using this approach, we also observed a low frequency of endplate-oriented disc herniations in some Cre(-) control mice suggesting variable genetic susceptibility of this adult-onset pathology in mouse. Standard histology can easily miss subtle defects of the IVD, as we illustrate in adjacent sections from several independent Adgrg6 conditional mutant mice (S2 and S4 and S7 Figs). Given the high resolution of the contrast-enhanced μCT imaging and ease of imaging the intact mouse spine we suggest this technique is a superior method for the identification of age-related defects of the IVD. In the future, it will be interesting to apply comprehensive contrast-enhanced μCT imaging to determine how prevalent endplate defects are in a variety of inbred strains during aging.
Our findings support a model where increased disc stiffness in young Adgrg6 mutant mice proceed the onset of endplate-oriented disc herniations. We demonstrate the upregulation of multiple collagen genes associated with fibrosis in postnatal mice (P20), which is expected to result in disturbed stress distribution of the IVD and concentrate loading at the CEP. This shift in disc mechanics is postulated to increase the risk of CEP fractures, decreases nutrient supply, and the lead to the formation of disc herniations [6, 52–54]. Several studies have shown that different combinations of mechanical loads (e.g. torsion, rotation, and flexion) applied to the disc lead to peak strain locations at both disc-bone interface and in the annulus fibrosus, which can ultimately lead to disc failure [55–57] and suggests that defects in the CEP and growth plate in mice may increase the risk for endplate-oriented disc herniation. Moreover, the adult IVD is thought to be an avascular tissue, as such, its major source of nutrient flux occurs via diffusion through the CEP [58]. As such, CEP sclerosis and reduced nutrient supply in the IVD is correlated with increased expression of collagen genes associated with fibrosis, catabolic enzymes, and expression of COLX [59, 60]. In this way, a viscous cycle comprising increased expression of catabolic enzyme activity, pro-inflammatory factors, and apoptosis, can further exacerbate the integrity of the IVD leading to endplate-oriented herniation during adult development [6, 54]. In conclusion, we suggest that changes of typical collagen expression in the disc coupled with increased expression of other catabolic factors such as MMPs and ADAMTSs, act synergistically to weaken the CEP and growth plate and contribute to the stiffness of the IVD and ultimately increase the susceptibility of the endplate-oriented disc herniation in this mouse model (Fig 7).
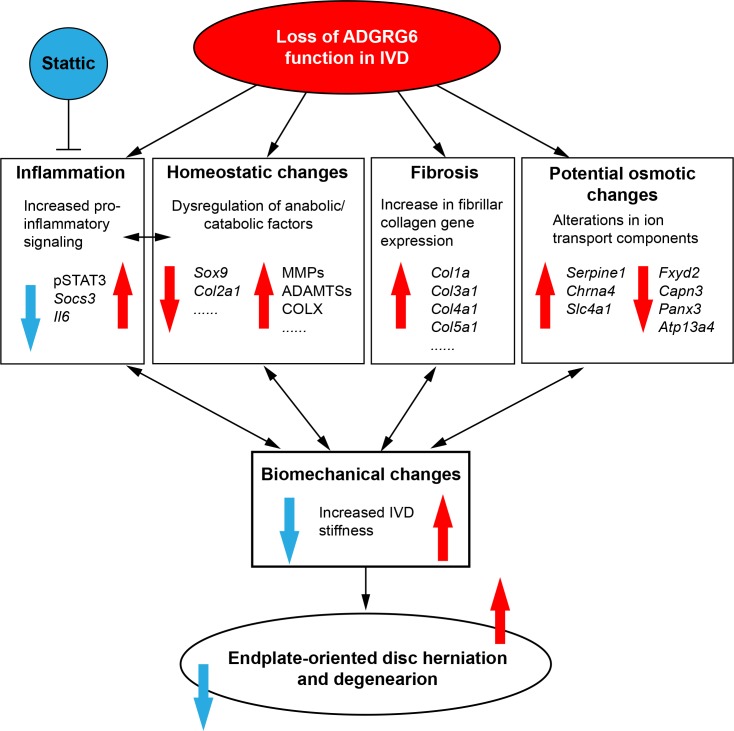
Fig 7. A model of ADGRG6 in regulating IVD integrity.
Loss of ADGRG6 function in cartilaginous tissues of the IVD leads to increased pro-inflammatory singling (especially STAT3 signaling), dysregulation of anabolic and catabolic factors, fibrosis of the disc, and potential osmotic changes of the disc in young mice. These changes synergistically weaken the IVD and result in altered biomechanical changes (increased disc stiffness), and ultimately leads to endplate-oriented disc herniation in adult mice. Inhibition of STAT3 signaling by Stattic treatment can alleviates the formation of disc herniations and partially rescue the disc stiffness upon loss of ADGRG6 function.
We also demonstrate an upregulation of STAT3 signaling in Adgrg6 conditional mutant mice, prior to overt histopathology. Analysis of Adgrg6 KO ATDC5 cells in culture demonstrate that the activation of the IL-6/STAT3/Socs3 pathway is an intrinsic property of cartilaginous cells and that this is regulated by ADGRG6 function. Increased expression of IL-6 and activation of STAT3 (pSTAT3) has been observed in human patients with disc degeneration and disc herniation [38, 61]; circulating IL-6 is positively associated with radiographic osteoarthritis and loss of knee cartilage loss in humans [62]; and IL-6/STAT3 signaling is activated in trauma-induced osteoarthritis [63, 64]. Here we show that systemic inhibition of STAT3 signaling with Stattic [45] acts to alleviate the onset and progression of endplate-oriented herniations of the IVD, which was recently also demonstrated to improve joint remodeling in a post-traumatic osteoarthritis model in mouse [64]. Together these studies underscore the need to further elaborate on the role of STAT3 activation for the initiation and progression of other degenerative joint disorders. Altogether, this study provides multiple supporting evidence of the regulatory role and therapeutic value of ADGRG6/STAT3 signaling for the onset and progression of endplate-specific disc defects.
Materials and methods
Ethics statement
All animal research was conducted according to federal, state, and institutional guidelines and in accordance with protocols approved by Institutional Animal Care and Use Committees at University of Texas at Austin (AUP-2018-00276).
Mouse strains
Mice were housed in standard cages and maintained on a 12-hour light/dark cycle, with rodent chow and water available ad libitum. All mouse strains were described previously, including Adgrg6f/f (Taconic #TF0269) [65]; Rosa26;LacZ (B6;129S-Gt(ROSA)26Sor/J) [66]; ATC [13], and Col2Cre [67]. Doxycycline (Dox) was administered to ATC; Adgrg6f/f mice and littermate controls with two strategies: (i) inducing from embryonic day (E)0.5-postnatal day (P)20 by ad libitum feeding of Dox-chow (Test Diet, 1814469) to plugged isolated females, and supplemented with intraperitoneal (IP) injections of the pregnant dames once/week (10mg Dox/kg body weight) throughout the pregnancy until the pups were weaned at P20; (ii) inducting from P1-P20 by ad libitum feeding of Dox-chow to the mothers after the pups were born, and supplemented with intraperitoneal (IP) injections of the mothers once/week (10mg Dox/kg body weight) until the pups were weaned at P20. ATC; Rosa-LacZf/+ mice were induced with the same strategies. STAT3 inhibitor Stattic (25mg/kg, dissolved in DMSO) or placebo (DMSO/PBS/Tween-20) were administered to Col2Cre; Adgrg6f/f mutant mice or Cre (-) littermate controls via i.p. injection once/week for 16 weeks beginning by the age of 1.5 months. Mice were harvested at P2, P20, 1.5 months, 6 months and 8 months of age.
Analyses of mice
Histological analysis was performed on thoracic spines fixed in 10% neutral-buffered formalin for 3 days at room temperature followed by 1-week decalcification in Formic Acid Bone Decalcifier (Immunocal, StatLab). After decalcification, bones were embedded in paraffin and sectioned at 5μm thickness. Safranin O/Fast Green (SO/FG) and Alcian blue Hematoxylin/Orange G (ABH/OG) staining were performed following standard protocols (Center for Musculoskeletal Research, University of Rochester). Immunohistochemical analyses were performed on paraffin sections with traditional antigen retrieval and colorimetric development methodologies with the following primary antibodies: anti-Collagen II (Thermo Scientific, MS235B), anti-Collagen X (Quartett, 1-CO097-05), anti-SOX9 (Santa Cruz Biotechnology, sc-20095), anti-Lubricin (PRG4) (Abcam, ab28484), anti-MMP-13 (Thermo Scientific, MS-825-P), anti-IL-6 (Abcam, ab6672), and anti-phospho-STAT3 (Cell Signaling, #9145). The Terminal deoxynucleotidyl transferase dUTP Nick-End Labeling (TUNEL) cell death assay was performed on paraffin sections with the In Situ Cell Death Detection Kit, Fluorescein (Roche) according to the manufacturer’s instructions. The b-galactosidase staining was performed on frozen sections as previously described [63]. Spines were harvested and fixed in 4% paraformaldehyde for 2 hours at 4°C and decalcified with 14% EDTA at 4°C for 1 week. Tissues were washed in sucrose gradient, embedded with Tissue-Tek OCT medium, snap-frozen in liquid nitrogen, and sectioned at 10μm with a Thermo Scientific HM 550 cryostat. In situ hybridization using a Digoxygenin-labeled antisense riboprobe for Adgrg6 was performed on 5μm paraffin sections as described previously with modifications [11], and detected with either a chromogenic substrate (BM Purple, Roche) or a tyramine-amplified fluorescent antibody (Perkin Elmer).
Cell culture
ATDC5 cells (Sigma, 99072806) were maintained in DMEM/F-12 (1:1) medium (Gibco, 11330032) supplemented with 5% FBS and 1% penicillin/streptomycin. ATDC5 cells were maturated in DMEM/F-12 (1:1) medium supplemented with 5% FBS, 1% penicillin/streptomycin, 1% ITS premix (Corning, 354352), 50μg/ml ascorbic acid, 10nM dexamethasone, and 10ng/ml TGF-β3 (Sigma, SRP6552) for 5, 10, and 15 days.
Both wild type and Adgrg6 KO ATDC5 cells were treated with 100ng/ml recombinant human IL-6 protein (rIL-6) (R&D System, 206-IL) for 2 hours before protein extraction. Both wild type and Adgrg6 KO ATDC5 cells were treated with 10nM Stattic in maturation medium for 10 days before RNA extraction.
Generation of the Adgrg6 KO cell line
CRISPR reagents were generated to target the 3rd exon of mouse Adgrg6 (ENSMUST00000041168.5) using the following oligos: Adgrg6-g33-fwd ACACCGAGGGTAACACGGAGACGTAAG and Adgrg6-g33-rev AAAACTTACGTCTCCGTGTTACCCTCG and cloned into a lentiviral packing vector (lentiCRISPR v2 was a gift from Feng Zhang (Addgene plasmid # 52961)) along a pCas9_GFP (a gift from Kiran Musunuru (Addgene plasmid # 44719)). Lentiviral particle packaging was in A293T cells using standard 3rd generation approach (https://tronolab.epfl.ch/page-148635-en.html). Human embryonic kidney (HEK) 293T cells (Sigma) were maintained in DMEM supplemented with 10% fetal bovine serum, 2mM GlutaMAX (Life Technologies), 100U/ml penicillin, and 100ug/mL streptomycin at 37°C with 5% CO2 incubation. 293T cells were seeded into 6cm plates (Corning) one day prior to transfection at a density of 2x106 cells per well. 293T cells were transfected using FUGENE 6 (Promega) following the manufacturer’s recommended protocol. For each plate, a total of 0.5ug of each plasmid was used. At 2 and 4 days post transfection, the cell media was collected and filtered with 0.45 μM filter (Corning) and stored at -80°C.
ATDC5 were plated in 6-well plates to 80% confluency and lentiviral transduction was using diluted viral media with 0.1% polybrene (EMD Millipore) for 24 hours followed by selection with 4μg/ml Blastocidin and Puromycin for 5 days post transfection. Serial dilution under selection was used to identify individual clones, expanded colonies were screened for INDEL mutations using Adgrg6-ex3-fwd—TTGACAGTTACTGCTTGATGCCCCC and Adgrg6-ex3-rev- CCCTTGGCAGTCGCTCCACAGAATT primers and amplicons were screen by Sanger sequencing to identify homozygous clones.
RNA isolation and qPCR
Entire intervertebral discs from the thoracic and lumbar spine (T8-L5) of the 1.5-month old ATC; Adgrg6f/f and control mice were isolated in cold PBS, snap frozen and pulverized in liquid nitrogen. Total RNA from intervertebral discs was isolated using the TRIzol Reagent (Invitrogen, 15596026), and cleaned up with the Direct-zol RNA miniprep kit (Zymo Research, Z2070). Total RNA of the cultured ATDC5 cells was isolated using the RNAeasy mini kit (Qiagen, 74104). Reverse transcription was performed using 1μg total RNA with the iScript cDNA synthesis kit (BioRad). Reactions were set up in technical and biological triplicates in a 96 well format on a BioRAD CFX96 real-time PCR detection system, using SYBR green chemistry (SsoAdvanced, BioRad). The PCR conditions were 95°C for 3 min followed by 40 cycles of 95°C for 10s and 58°C for 30s. Gene expression was normalized to b-actin mRNA and relative expression was calculated using the 2-(ΔΔCt) method. All qPCR primers sequences are listed in S4 Table. PCR efficiency was optimized and melting curve analyses of products were performed to ensure reaction specificity.
RNA isolation, library construction and sequencing
Entire intervertebral discs from the thoracic and lumbar spine (T8-L5) of the P20 Col2Cre; Adgrg6f/f and control mice were isolated in cold PBS, snap frozen and pulverized in liquid nitrogen. Total RNA was extracted using Trizol reagent (Invitrogen, CA, USA) following the manufacturer's procedure. The total RNA quantity and purity were analyzed on Bioanalyzer 2100 and RNA 6000 Nano LabChip Kit (Agilent, CA, USA) with RIN number >7.0. Total RNA was subjected to isolate Poly (A) mRNA with poly-T oligo attached magnetic beads (Invitrogen). RNA fragments were reverse-transcribed to create the final cDNA libraries following the NEBNext Ultra RNA Library Prep Kit (Illumina, San Diego, USA), paired-end sequencing was performed. All raw reads are available as GSE128402 in the NCBI Gene Expression Omnibus.
Bioinformatics analysis
Transcripts assembly
Cutadapt [68] and perl scripts in house were used to remove the reads that contained adaptor contamination, low quality bases and undetermined bases. Then sequence quality was verified using FastQC (http://www.bioinformatics.babraham.ac.uk/projects/fastqc/). We used HISAT2 [69] to map reads to the genome of Mus Musculus (GRCm38.88). The mapped reads of each sample were assembled using StringTie [70]. Then, all transcriptomes from biological samples were merged to reconstruct a comprehensive transcriptome using perl scripts and gffcompare. After the final transcriptome was generated, StringTie [71] and Ballgown [70] was used to estimate the expression levels of all transcripts.
Different expression analysis of mRNAs
StringTie [71] was used to perform expression level for mRNAs by calculating FPKM. The differentially expressed mRNAs were selected with log2 (fold change) >1 or log2 (fold change) <-1 and with statistical significance (p value < 0.05) by R package Ballgown [70].
Western blotting
For western blotting analysis, total proteins were extracted from cells with protein extraction buffer [50mM HEPES, 1.5mM EDTA (pH 8.0), 150mM NaCl, 10% glycerol, 1% Triton X-100] supplemented with protease and phosphatase inhibitors (Roche). 10mg of protein from each sample was resolved by 4–15% SDS-polyacrylamide gel electrophoresis and transferred to the nitrocellulose membranes. Western blots were then blocked with LI-COR blocking buffer and incubated overnight with primary antibodies anti-STAT3 (Cell Signaling, #4904), anti-pSTAT3 (Cell Signaling, #9145), and anti-GAPDH (Cell Signaling, #2118) at 4°C with gentle rocking. The next day western blots were detected with the LI-COR Odyssey infrared imaging system.
Contrast-enhanced μCT imaging and segmentation
Samples undergoing contrast-enhanced micro-computed tomography (μCT) were blinded and incubated in a 35%w/v solution Ioversol in PBS (OptiRay 350, Guerbet, St. Louis) supplemented with 1% penicillin–streptomycin at 37C one day prior to scanning. Immediately prior to scanning, the sample was removed from the solution and wrapped in PBS-soaked gauze. These samples were mounted in 2% agarose gels and then scanned using the microCT40 system (Scanco Medical, CH) operating at 6 μm voxel size (45kVp, 177uA, 300 ms integration). Following our previous method for segmentation of murine IVDs [15, 72], the μCT CT data is exported as a DICOM file for further processing. Following an initial median filter (sigma = 0.8, support = 3), bone is then thresholded out, and the soft tissue not part of the IVD was removed by drawing contours around the outer edge of every five transverse slices of the AF and morphed using a linear interpolation. The remaining voxels are designated as the whole disc mask. From the masks of the whole disc, volumes and average attenuations (intensity) are calculated. The volume was determined from the total number of voxels contained within the mask and the attenuation is taken as the average 16-bit grayscale value of the voxels. Visualizations of the μCT were obtained using OsiriX (Pixmeo, Geneva). The volume of the contoured disc was then measured. Endplate defects were defined by three or more consecutive slices that had a rupture in the same area of the endplate. This method was chosen so as to eliminate any potential spatial artifacts that may be misidentified as ruptures.
Mechanical testing
The mechanical properties of the isolated intervertebral discs were determined using dynamic compression on a microindentation system (BioDent 1000; Active Life Scientific, Santa Barbara, CA) with a 2.39 mm non-porous, flat probe [21]. The probe's load cell resolution is 0.001 N, and the system's Piezo actuator resolution is 0.01μm. Each sample was moved into position under the probe tip by gripping the aluminum platen. The indenter tip was aligned over each sample so that the probe covered the entire diameter of the disc. Each disc was first loaded sinusoidally at amplitude of 5% strain with a 10% peak strain at 1 Hz for 20 cycles with a 0.1 N preload. Stiffness for each disc was then calculated by averaging the loading slope of the 20 cycles. After the cyclic tests, the discs were monotonically overloaded to 50% strain at a loading rate of 10% strain per second. The loading slope value was obtained from the linear region of the force displacement curve from all loading curves. These samples were maintained in physiological PBS solution (pH 7.2) during and between trials to simulate the osmotic pressures found in the body and maintain hydration of the IVD.
Quantification of collagen and proteoglycans
The wet weight of each isolated disc was taken after mechanical testing utilizing an analytical balance (A-200DS; Denver Instrument Company, Bohemia, NY). Samples were first digested in papain at 65°C for 18 h. The samples were then centrifuged and the supernatant collected and then plated in triplicate. Proteoglycan content was quantified using the colorimetric dimethyl-methylene blue (DMMB) assay [73] by measuring the absorbance 525nm with chondroitin sulfate from bovine cartilage as standards (Sigma-Aldrich, St. Louis, MO), and then normalized to wet weight of the IVD. The remaining papain-digested lysates were then used for hyproxyproline quantification. The amount of collagen was approximated by assuming that hydroxyproline accounts 1/7 of the mass of collagen. The samples were hydrolyzed in 12 N hydrochloric acid at 120°C for 3 h. The hydrolyzed samples were then plated in triplicates. A chloramine T colorimetric assay [74] and standardized using hydroxyproline by quantifying the absorbance at 560 nm using a plate reader (SpectraMax M2, Molecular Devices, Sunnyvale, CA).
Statistics
Statistical analyses to compared the mutant and control groups were performed using 2-tailed Student’s t-test and one-way ANOVA followed by Turkey HSD test as appropriate (GraphPad Prism 7). Power analysis was done using G*Power. A p value of less than 0.05 is considered statistically significant.
Supporting information
(A, B) In situ hybridizations of Adgrg6 in spine tissue (8 months) using Alkaline phosphatase/BM purple chromogenic developing shows strong Adgrg6 expression (blue stain) in the growth plate (GP) and minor expression in the annulus fibrosis (AF) (red arrowheads) that is mostly abolished in ATC;Adgrg6f/f mutant tissues (B); or using (C, D) tyramine-amplification fluorescence which shows expanded expression throughout the IVD including GP, CEP, AF, and NP, which is mostly diminished in ATC;Adgrg6f/f mutant tissues. Robust expression was detected in periosteum of the long bone tissues in both control and the ATC;Adgrg6f/f mutant moues (E, F, yellow arrows). (Induced from P1-P20, n = 3 for each group.) Scale bars: 200μm in (A-D); 50μm in (E, F). AF- annulus fibrosis, CEP- cartilaginous endplate, GP- growth plate, and NP- nucleus pulposus, Tb- trabecular bone, Cb-cortical bone, P-periosteum.
(TIF)
(A-C) Representative 8-month-old mouse IVDs (induced form E0.5-P20) stained with Safranin-O/Fast green (SO/FG) (n = 3 for controls and n = 6 for mutants). Endplate-oriented disc herniation is indicated with yellow arrows in B and B’. These herniations are very hard to be captured by histological analysis. C is an earlier midline section of an adjacent mutant IVD as shown in B, showing no overt histopathology. Scale bars: 100μm in (A-C); and 50μm in (A’, B’). AF- annulus fibrosis, CEP- cartilaginous endplate, GP- growth plate, and NP- nucleus pulposus.
(TIF)
More robust recombination (blue signal) in CEP, GP, and AF of the IVD was observed in the Col2Cre; Rosa-LacZ mouse (B, E) compared with the ATC; Rosa-LacZ mouse when induced from E0.5-P20 (A, C) and P1-P20 (D). Recombination in periosteum (B, E, red arrows) and the outmost AF layers of the IVD (B, E, black arrows) was observed only in the Col2Cre; Rosa-LacZ mouse but not the ATC; Rosa-LacZ mouse. Scale bars: 100μm in (A-E). CEP- cartilaginous endplate, GP- growth plate, AF- annulus fibrosis, NP- nucleus pulposus, Tb- trabecular bone, and P- periosteum.
(TIF)
(A-D’) Representative 4-month-old (A-B’) or 8-month-old (C-D’) mouse IVDs stained with Safranin-O/Fast green (SO/FG). (Induced from P1-P20. For A-B’, n = 3 for controls and n = 5 for mutants; for C-D’, n = 4 for each group). Minor growth plate erosion is observed by the age of four months in mutant mice (yellow arrowheads, B), while more severe endplate-oriented disc herniations were observed by the age of 8 months (yellow arrowheads, D). (B’) is an earlier midline sections of the same mutant IVD as shown in B. (D’) is a midline section of an adjacent mutant IVD as shown in D, showing no overt histopathology. (E-L) IHC analysis of 8-month-old Cre (-) Control and ATC;Adgrg6f/f mutant mouse IVDs (induced from P1-P20). Several protein markers of IVD health and disease are affected in ATC;Adgrg6f/f mutant IVD including decreased expression of healthy disc markers COLII and SOX9 (G, blue arrows), and increased expression of the hypertrophic marker COLX (F, red arrows) and extracellular matrix modifying enzyme MMP-13 (J, red arrows). (n = 3 for each group.) Scale bars: 100μm in (A-D’); 50μm in (E-L). CEP: cartilaginous endplate, GP: growth plate, AF: annulus fibrosis, and NP: nucleus pulposus.
(TIF)
Large scale images of IHC analysis shown in Fig 2. IHC analysis of common markers of degenerative disc. ATC;Adgrg6f/f conditional mutant IVDs display reduced expression of markers of healthy disc: SOX9 (B), PRG4 (D), and COLII (H); and increased expression of extracellular matrix modifying enzymes MMP-13 (F), hypertrophic marker COLX (J). Scale bars: 100μm in (A-J). AF- annulus fibrosis, CEP- cartilaginous endplate, GP- growth plate, and NP- nucleus pulposus.
(TIF)
(A, B) TUNEL (red fluorescence) staining of 1.5-month-old ATC;Adgrg6f/f mutants (B, white arrows) display increased TUNEL positive cells compared to Cre (-) control (A) mice. (C) Graph of the ratio of TUNEL positive cells to total cells (DAPI) (n = 3 for each group, three to five IVDs were analyzed/mouse. Bars represent mean ± SD. *p≤0.05, two-tailed Student's t Test). AF- annulus fibrosis, CEP- cartilaginous endplate, GP- growth plate, and NP- nucleus pulposus.
(TIF)
(A-B’) Representative medial-sectioned mouse IVDs stained with Alcian blue/Orange G of Cre (-) control (A and A') and Col2Cre;Adgrg6f/f mutant (B, B') mice at P20 (n = 3 for each group). No overt histopathology was observed in mutant mice at this young age. (C-E) Representative mouse IVDs stained with Safranin-O/Fast green (SO/FG) of Cre (-) control (C and C') and Col2Cre;Adgrg6f/f mutant (D, D', and E) mice by the age of 8 months (n = 3 for each group). Endplate-oriented herniations is indicated with yellow arrows. These herniations are very hard to be captured by histological analysis (D is out of the typical plane of section). E is an earlier midline section of the same mutant IVD as shown in D, showing no overt histopathology. Scale bars: 200μm in (A, B) and (C-E), and 50μm in (A’, B’) and (C’, D’). AF- annulus fibrosis, CEP- cartilaginous endplate, GP- growth plate, and NP- nucleus pulposus.
(TIF)
(A-D) IHC analysis of macrophage marker shows no strong signal of CD68 in ATC;Adgrg6f/f mutant mouse IVD at 1.5months (A, B), or 8 months of age (C, D), except for some background signal at the herniation site (red arrow, D). (Induced from E0.5-P20, n = 3 for each group.) Scale bars: 50μm in (A-D). CEP- cartilaginous endplate, GP- growth plate, and NP- nucleus pulposus.
(TIF)
(A) Alcian blue staining on ATDC5 cell culture during the maturation process. (B) Expression profiles of Adgrg6, Col2a1, Acan, and Sox9 during ATDC5 cell maturation. The expression level of Adgrg6 was gradually increased alone with other chondrogenesis markers including Col2a1, Acan, and Sox9. (n = 3 biological replicates and representative result is shown. Bars represent mean ± SD. *p≤0.05, two-tailed Student's t Test). Scale bars: 100μm in A.
(TIF)
(A, B) Immunofluorescence against cleaved-Caspace-3 (green) and DAPI staining (blue) and (C) quantification showing increased apoptosis in Adgrg6 KO cells during maturation (10 days). (n = 3 biological replicates and representative result is shown. Bars represent mean ± SD. *p≤0.05, two-tailed Student's t Test). Scale bars: 50μm in (A, B).
(TIF)
(A-C’) IHC analysis of COLX in 6-month-old Cre (-) control (A, A’) and Col2Cre;Adgrg6f/f mutant mice with (C, C’) or without (B, B’) Stattic treatment. Ectopic COLX expression was observed in CEP of the mutant mice (red arrows, B’), which is rescued after Stattic treatment (C’). (D-I) IHC analysis of COLII and SOX9 in 6-month-old Cre (-) control (A, D) and Col2Cre;Adgrg6f/f mutant mice with (F, I) or without (E, H) Stattic treatment. Reduced COLII and SOX9 expression was observed in Col2Cre;Adgrg6f/f conditional mutant mice compared with Cre (-) control (blue arrows, D, G), but no obvious improvement was observed after Stattic treatment (F, I). (n = 3 for each group). (J) qPCR analyses revealed that Col10a1 and Sox9 expression in Adgrg6 KO ATDC5 cells was partially rescued after Stattic treatment (10nM for 10 days), however the expression of Col2a1 was not significantly changed (n = 3 biological replicates and representative result is shown. Bars represent mean ± SD. *p≤0.05, **p≤0.01, ***p≤0.001, ****p≤0.0001, One way ANOVA followed by Tukey HSD test. n.s, not significant.) Scale bars: 100μm in (A-C), and 50μm in (A’-I). AF- annulus fibrosis, CEP- cartilaginous endplate, GP- growth plate, and NP- nucleus pulposus.
(TIF)
Correlation analysis (Pearson’s r) between number of herniation and disc stiffness were performed on 6-month-old mice from three experimental groups: four placebo-treated Cre (-) controls; three placebo-treated Col2Cre;Adgrgf/f mutants; and three Stattic-treated Col2Cre;Adgrgf/f mutants as shown in Fig 6. Dots plotted by mean ± SD. No correlation was detected.
(TIF)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(MOV)
(MOV)
Acknowledgments
We would like to thank Dr. Fanxin Long and Dr. Veronique Levebre for sharing the Col2Cre and ATC mouse strains respectively. We would like to thank Dr. Francesca Mariani for sharing her protocol for fluorescent in situ hybridization. We thank Dr. John DeGiovanni for helpful advice and suggestion for using Stattic treatment in mice. We would like to thank Drs. John Wallingford and Steve Vokes, and members of the Gray lab for helpful comments on this manuscript prior to submission.
Data Availability
All short read data is uploaded as GSE128402 - Transcriptomic analysis of intervertebral discs isolated from wild type and Adgrg6-deficient mice https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE128402
Funding Statement
Research reported in this publication was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under Award Number R01AR072009-01 (R.S.G.), F32AR073648 (Z.L.), R21AR069804 (S.T.), R01AR063071 (M.J.H) and the National Institute of Child & Human Development P01HD084387 (N.A). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript
References
- 1.Brinjikji W, Diehn FE, Jarvik JG, Carr CM, Kallmes DF, Murad MH, et al. MRI Findings of Disc Degeneration are More Prevalent in Adults with Low Back Pain than in Asymptomatic Controls: A Systematic Review and Meta-Analysis. AJNR Am J Neuroradiol. 2015;36(12):2394–9. Epub 2015/09/12. 10.3174/ajnr.A4498 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sun D, Liu P, Cheng J, Ma Z, Liu J, Qin TJBMD. Correlation between intervertebral disc degeneration, paraspinal muscle atrophy, and lumbar facet joints degeneration in patients with lumbar disc herniation. 2017;18(1):167 10.1186/s12891-017-1522-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dudli S, Ferguson SJ, Haschtmann D. Severity and pattern of post-traumatic intervertebral disc degeneration depend on the type of injury. Spine J. 2014;14(7):1256–64. Epub 2014/03/04. 10.1016/j.spinee.2013.07.488 . [DOI] [PubMed] [Google Scholar]
- 4.Zhu F, Bao H, Yan P, Liu S, Bao M, Zhu Z, et al. Do the disc degeneration and osteophyte contribute to the curve rigidity of degenerative scoliosis? 2017;18(1):128 10.1186/s12891-017-1471-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cortes DH, Elliott DM. The Intervertebral Disc: Overview of Disc Mechanics In: Shapiro IM, Risbud MV, editors. The Intervertebral Disc: Molecular and Structural Studies of the Disc in Health and Disease. Vienna: Springer Vienna; 2014. p. 17–31. [Google Scholar]
- 6.Wong J, Sampson SL, Bell-Briones H, Ouyang A, Lazar AA, Lotz JC, et al. Nutrient supply and nucleus pulposus cell function: effects of the transport properties of the cartilage endplate and potential implications for intradiscal biologic therapy. Osteoarthritis Cartilage. 2019;27(6):956–64. Epub 2019/02/06. 10.1016/j.joca.2019.01.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sambrook PN, MacGregor AJ, Spector TD. Genetic influences on cervical and lumbar disc degeneration: a magnetic resonance imaging study in twins. Arthritis Rheum. 1999;42(2):366–72. Epub 1999/02/20. . [DOI] [PubMed] [Google Scholar]
- 8.Munir S, Rade M, Maatta JH, Freidin MB, Williams FMK. Intervertebral Disc Biology: Genetic Basis of Disc Degeneration. Curr Mol Biol Rep. 2018;4(4):143–50. Epub 2018/11/23. 10.1007/s40610-018-0101-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Patra C, van Amerongen MJ, Ghosh S, Ricciardi F, Sajjad A, Novoyatleva T, et al. Organ-specific function of adhesion G protein-coupled receptor GPR126 is domain-dependent. Proc Natl Acad Sci U S A. 2013;110(42):16898–903. 10.1073/pnas.1304837110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Geng FS, Abbas L, Baxendale S, Holdsworth CJ, Swanson AG, Slanchev K, et al. Semicircular canal morphogenesis in the zebrafish inner ear requires the function of gpr126 (lauscher), an adhesion class G protein-coupled receptor gene. Development. 2013;140(21):4362–74. 10.1242/dev.098061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karner CM, Long F, Solnica-Krezel L, Monk KR, Gray RS. Gpr126/Adgrg6 deletion in cartilage models idiopathic scoliosis and pectus excavatum in mice. Hum Mol Genet. 2015;24(15):4365–73. 10.1093/hmg/ddv170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhu F, Bao H, Yan P, Liu S, Bao M, Zhu Z, et al. Do the disc degeneration and osteophyte contribute to the curve rigidity of degenerative scoliosis? BMC Musculoskeletal Disorders. 2017;18(1):128 10.1186/s12891-017-1471-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dy P, Wang W, Bhattaram P, Wang Q, Wang L, Ballock RT, et al. Sox9 directs hypertrophic maturation and blocks osteoblast differentiation of growth plate chondrocytes. Dev Cell. 2012;22(3):597–609. 10.1016/j.devcel.2011.12.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Adams M, Simms RJ, Abdelhamed Z, Dawe HR, Szymanska K, Logan CV, et al. A meckelin-filamin A interaction mediates ciliogenesis. Hum Mol Genet. 2012;21(6):1272–86. Epub 2011/11/29. 10.1093/hmg/ddr557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin KH, Tang SY. The Quantitative Structural and Compositional Analyses of Degenerating Intervertebral Discs Using Magnetic Resonance Imaging and Contrast-Enhanced Micro-Computed Tomography. Ann Biomed Eng. 2017;45(11):2626–34. Epub 2017/07/27. 10.1007/s10439-017-1891-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yee A, Chan D. Genetic Basis of Intervertebral Disc Degeneration In: Shapiro IM, Risbud MV, editors. The Intervertebral Disc: Molecular and Structural Studies of the Disc in Health and Disease. Vienna: Springer Vienna; 2014. p. 157–76. [Google Scholar]
- 17.Kadow T, Sowa G, Vo N, Kang JD. Molecular basis of intervertebral disc degeneration and herniations: what are the important translational questions? Clin Orthop Relat Res. 2015;473(6):1903–12. Epub 2014/07/16. 10.1007/s11999-014-3774-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tonge DP, Pearson MJ, Jones SW. The hallmarks of osteoarthritis and the potential to develop personalised disease-modifying pharmacological therapeutics. Osteoarthritis Cartilage. 2014;22(5):609–21. Epub 2014/03/19. 10.1016/j.joca.2014.03.004 . [DOI] [PubMed] [Google Scholar]
- 19.Kalb S, Martirosyan NL, Kalani MY, Broc GG, Theodore N. Genetics of the degenerated intervertebral disc. World Neurosurg. 2012;77(3–4):491–501. Epub 2011/11/29. 10.1016/j.wneu.2011.07.014 . [DOI] [PubMed] [Google Scholar]
- 20.Nguyen QT, Jacobsen TD, Chahine NO. Effects of Inflammation on Multiscale Biomechanical Properties of Cartilaginous Cells and Tissues. ACS Biomater Sci Eng. 2017;3(11):2644–56. Epub 2017/11/21. 10.1021/acsbiomaterials.6b00671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu JW, Abraham AC, Tang SY. The high-throughput phenotyping of the viscoelastic behavior of whole mouse intervertebral discs using a novel method of dynamic mechanical testing. J Biomech. 2015;48(10):2189–94. Epub 2015/05/26. 10.1016/j.jbiomech.2015.04.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang da W, Sherman BT, Lempicki RA. Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res. 2009;37(1):1–13. Epub 2008/11/27. 10.1093/nar/gkn923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kizawa H, Kou I, Iida A, Sudo A, Miyamoto Y, Fukuda A, et al. An aspartic acid repeat polymorphism in asporin inhibits chondrogenesis and increases susceptibility to osteoarthritis. Nat Genet. 2005;37(2):138–44. Epub 2005/01/11. 10.1038/ng1496 . [DOI] [PubMed] [Google Scholar]
- 24.Balakrishnan L, Nirujogi RS, Ahmad S, Bhattacharjee M, Manda SS, Renuse S, et al. Proteomic analysis of human osteoarthritis synovial fluid. Clin Proteomics. 2014;11(1):6 Epub 2014/02/19. 10.1186/1559-0275-11-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang Q, Lin S, Liu Y, Yuan B, Harris SE, Feng JQ. Dmp1 Null Mice Develop a Unique Osteoarthritis-like Phenotype. Int J Biol Sci. 2016;12(10):1203–12. Epub 2016/10/22. 10.7150/ijbs.15833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Snelling SJ, Davidson RK, Swingler TE, Le LT, Barter MJ, Culley KL, et al. Dickkopf-3 is upregulated in osteoarthritis and has a chondroprotective role. Osteoarthritis Cartilage. 2016;24(5):883–91. Epub 2015/12/22. 10.1016/j.joca.2015.11.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mahr S, Burmester GR, Hilke D, Gobel U, Grutzkau A, Haupl T, et al. Cis- and trans-acting gene regulation is associated with osteoarthritis. Am J Hum Genet. 2006;78(5):793–803. Epub 2006/04/28. 10.1086/503849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Antoniou J, Steffen T, Nelson F, Winterbottom N, Hollander AP, Poole RA, et al. The human lumbar intervertebral disc: evidence for changes in the biosynthesis and denaturation of the extracellular matrix with growth, maturation, ageing, and degeneration. J Clin Invest. 1996;98(4):996–1003. Epub 1996/08/15. 10.1172/JCI118884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yee A, Lam MP, Tam V, Chan WC, Chu IK, Cheah KS, et al. Fibrotic-like changes in degenerate human intervertebral discs revealed by quantitative proteomic analysis. Osteoarthritis Cartilage. 2016;24(3):503–13. Epub 2015/10/16. 10.1016/j.joca.2015.09.020 . [DOI] [PubMed] [Google Scholar]
- 30.Chen CH, Chiang CJ, Wu LC, Yang CH, Kuo YJ, Tsuang YH, et al. Time course investigation of intervertebral disc degeneration in a rat-tail puncture model. Life Sci. 2016;156:15–20. Epub 2016/05/20. 10.1016/j.lfs.2016.05.020 . [DOI] [PubMed] [Google Scholar]
- 31.Zhang Y, Xiong C, Kudelko M, Li Y, Wang C, Wong YL, et al. Early onset of disc degeneration in SM/J mice is associated with changes in ion transport systems and fibrotic events. Matrix Biol. 2018;70:123–39. Epub 2018/04/13. 10.1016/j.matbio.2018.03.024 . [DOI] [PubMed] [Google Scholar]
- 32.Fearing BV, Hernandez PA, Setton LA, Chahine NO. Mechanotransduction and cell biomechanics of the intervertebral disc. JOR Spine. 2018;1(3). Epub 2018/12/21. 10.1002/jsp2.1026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Erickson GR, Alexopoulos LG, Guilak F. Hyper-osmotic stress induces volume change and calcium transients in chondrocytes by transmembrane, phospholipid, and G-protein pathways. J Biomech. 2001;34(12):1527–35. Epub 2001/11/22. 10.1016/s0021-9290(01)00156-7 . [DOI] [PubMed] [Google Scholar]
- 34.Matta C, Zakany R, Mobasheri A. Voltage-dependent calcium channels in chondrocytes: roles in health and disease. Curr Rheumatol Rep. 2015;17(7):43 Epub 2015/05/20. 10.1007/s11926-015-0521-4 . [DOI] [PubMed] [Google Scholar]
- 35.Carow B, Rottenberg ME. SOCS3, a Major Regulator of Infection and Inflammation. Front Immunol. 2014;5:58 Epub 2014/03/07. 10.3389/fimmu.2014.00058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.O'Reilly S, Ciechomska M, Cant R, Hugle T, van Laar JM. Interleukin-6, its role in fibrosing conditions. Cytokine Growth Factor Rev. 2012;23(3):99–107. Epub 2012/05/09. 10.1016/j.cytogfr.2012.04.003 . [DOI] [PubMed] [Google Scholar]
- 37.Deng X, Zhao F, Kang B, Zhang X. Elevated interleukin-6 expression levels are associated with intervertebral disc degeneration. Exp Ther Med. 2016;11(4):1425–32. 10.3892/etm.2016.3079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Suzuki S, Fujita N, Fujii T, Watanabe K, Yagi M, Tsuji T, et al. Potential Involvement of the IL-6/JAK/STAT3 Pathway in the Pathogenesis of Intervertebral Disc Degeneration. Spine (Phila Pa 1976). 2017;42(14):E817–E24. Epub 2016/11/24. 10.1097/BRS.0000000000001982 . [DOI] [PubMed] [Google Scholar]
- 39.Oishi Y, Manabe I. Macrophages in inflammation, repair and regeneration. Int Immunol. 2018;30(11):511–28. Epub 2018/08/31. 10.1093/intimm/dxy054 . [DOI] [PubMed] [Google Scholar]
- 40.Yao Y, Wang Y. ATDC5: an excellent in vitro model cell line for skeletal development. J Cell Biochem. 2013;114(6):1223–9. Epub 2012/11/30. 10.1002/jcb.24467 . [DOI] [PubMed] [Google Scholar]
- 41.Bi W, Huang W, Whitworth DJ, Deng JM, Zhang Z, Behringer RR, et al. Haploinsufficiency of Sox9 results in defective cartilage primordia and premature skeletal mineralization. Proc Natl Acad Sci U S A. 2001;98(12):6698–703. Epub 2001/05/24. 10.1073/pnas.111092198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Arnold MA, Kim Y, Czubryt MP, Phan D, McAnally J, Qi X, et al. MEF2C transcription factor controls chondrocyte hypertrophy and bone development. Dev Cell. 2007;12(3):377–89. Epub 2007/03/06. 10.1016/j.devcel.2007.02.004 . [DOI] [PubMed] [Google Scholar]
- 43.Garbers C, Aparicio-Siegmund S, Rose-John S. The IL-6/gp130/STAT3 signaling axis: recent advances towards specific inhibition. Curr Opin Immunol. 2015;34:75–82. Epub 2015/03/10. 10.1016/j.coi.2015.02.008 . [DOI] [PubMed] [Google Scholar]
- 44.Kapoor M, Martel-Pelletier J, Lajeunesse D, Pelletier JP, Fahmi H. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat Rev Rheumatol. 2011;7(1):33–42. Epub 2010/12/02. 10.1038/nrrheum.2010.196 . [DOI] [PubMed] [Google Scholar]
- 45.Schust J, Sperl B, Hollis A, Mayer TU, Berg T. Stattic: a small-molecule inhibitor of STAT3 activation and dimerization. Chem Biol. 2006;13(11):1235–42. Epub 2006/11/23. 10.1016/j.chembiol.2006.09.018 . [DOI] [PubMed] [Google Scholar]
- 46.Wuertz K, Haglund L. Inflammatory mediators in intervertebral disk degeneration and discogenic pain. Global Spine J. 2013;3(3):175–84. Epub 2014/01/18. 10.1055/s-0033-1347299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Eskola PJ, Kjaer P, Sorensen JS, Okuloff A, Wedderkopp N, Daavittila I, et al. Gender difference in genetic association between IL1A variant and early lumbar disc degeneration: a three-year follow-up. Int J Mol Epidemiol Genet. 2012;3(3):195–204. Epub 2012/10/11. [PMC free article] [PubMed] [Google Scholar]
- 48.Livshits G, Zhai G, Hart DJ, Kato BS, Wang H, Williams FM, et al. Interleukin-6 is a significant predictor of radiographic knee osteoarthritis: The Chingford Study. Arthritis Rheum. 2009;60(7):2037–45. Epub 2009/07/01. 10.1002/art.24598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Akiyama H, Chaboissier MC, Martin JF, Schedl A, de Crombrugghe B. The transcription factor Sox9 has essential roles in successive steps of the chondrocyte differentiation pathway and is required for expression of Sox5 and Sox6. Genes Dev. 2002;16(21):2813–28. 10.1101/gad.1017802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Henry SP, Liang S, Akdemir KC, de Crombrugghe B. The postnatal role of Sox9 in cartilage. J Bone Miner Res. 2012;27(12):2511–25. 10.1002/jbmr.1696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.El-Brolosy MA, Stainier DYR. Genetic compensation: A phenomenon in search of mechanisms. PLoS Genet. 2017;13(7):e1006780 Epub 2017/07/14. 10.1371/journal.pgen.1006780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Adams MA, McMillan DW, Green TP, Dolan P. Sustained loading generates stress concentrations in lumbar intervertebral discs. Spine (Phila Pa 1976). 1996;21(4):434–8. Epub 1996/02/15. 10.1097/00007632-199602150-00006 . [DOI] [PubMed] [Google Scholar]
- 53.Wilke HJ, Kienle A, Maile S, Rasche V, Berger-Roscher N. A new dynamic six degrees of freedom disc-loading simulator allows to provoke disc damage and herniation. Eur Spine J. 2016;25(5):1363–72. Epub 2016/02/04. 10.1007/s00586-016-4416-5 . [DOI] [PubMed] [Google Scholar]
- 54.Vergroesen PP, Kingma I, Emanuel KS, Hoogendoorn RJ, Welting TJ, van Royen BJ, et al. Mechanics and biology in intervertebral disc degeneration: a vicious circle. Osteoarthritis Cartilage. 2015;23(7):1057–70. Epub 2015/04/02. 10.1016/j.joca.2015.03.028 . [DOI] [PubMed] [Google Scholar]
- 55.Berger-Roscher N, Casaroli G, Rasche V, Villa T, Galbusera F, Wilke HJ. Influence of Complex Loading Conditions on Intervertebral Disc Failure. Spine (Phila Pa 1976). 2017;42(2):E78–E85. Epub 2016/05/18. 10.1097/BRS.0000000000001699 . [DOI] [PubMed] [Google Scholar]
- 56.Veres SP, Robertson PA, Broom ND. The influence of torsion on disc herniation when combined with flexion. Eur Spine J. 2010;19(9):1468–78. Epub 2010/05/04. 10.1007/s00586-010-1383-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yang B, O'Connell GD. Effect of collagen fibre orientation on intervertebral disc torsion mechanics. Biomech Model Mechanobiol. 2017;16(6):2005–15. Epub 2017/07/25. 10.1007/s10237-017-0934-2 . [DOI] [PubMed] [Google Scholar]
- 58.Urban JP, Smith S, Fairbank JC. Nutrition of the intervertebral disc. Spine (Phila Pa 1976). 2004;29(23):2700–9. Epub 2004/11/27. 10.1097/01.brs.0000146499.97948.52 . [DOI] [PubMed] [Google Scholar]
- 59.Smith LJ, Nerurkar NL, Choi KS, Harfe BD, Elliott DM. Degeneration and regeneration of the intervertebral disc: lessons from development. Dis Model Mech. 2011;4(1):31–41. 10.1242/dmm.006403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhao CQ, Wang LM, Jiang LS, Dai LY. The cell biology of intervertebral disc aging and degeneration. Ageing Res Rev. 2007;6(3):247–61. Epub 2007/09/18. 10.1016/j.arr.2007.08.001 . [DOI] [PubMed] [Google Scholar]
- 61.Osuka K, Usuda N, Aoyama M, Yamahata H, Takeuchi M, Yasuda M, et al. Expression of the JAK/STAT3/SOCS3 signaling pathway in herniated lumbar discs. Neurosci Lett. 2014;569:55–8. Epub 2014/04/02. 10.1016/j.neulet.2014.03.045 . [DOI] [PubMed] [Google Scholar]
- 62.Stannus O, Jones G, Cicuttini F, Parameswaran V, Quinn S, Burgess J, et al. Circulating levels of IL-6 and TNF-alpha are associated with knee radiographic osteoarthritis and knee cartilage loss in older adults. Osteoarthritis Cartilage. 2010;18(11):1441–7. Epub 2010/09/08. 10.1016/j.joca.2010.08.016 . [DOI] [PubMed] [Google Scholar]
- 63.Liu Z, Chen J, Mirando AJ, Wang C, Zuscik MJ, O'Keefe RJ, et al. A dual role for NOTCH signaling in joint cartilage maintenance and osteoarthritis. Sci Signal. 2015;8(386):ra71 10.1126/scisignal.aaa3792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Latourte A, Cherifi C, Maillet J, Ea HK, Bouaziz W, Funck-Brentano T, et al. Systemic inhibition of IL-6/Stat3 signalling protects against experimental osteoarthritis. Ann Rheum Dis. 2017;76(4):748–55. 10.1136/annrheumdis-2016-209757 . [DOI] [PubMed] [Google Scholar]
- 65.Mogha A, Benesh AE, Patra C, Engel FB, Schoneberg T, Liebscher I, et al. Gpr126 functions in Schwann cells to control differentiation and myelination via G-protein activation. J Neurosci. 2013;33(46):17976–85. 10.1523/JNEUROSCI.1809-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet. 1999;21(1):70–1. 10.1038/5007 . [DOI] [PubMed] [Google Scholar]
- 67.Long F, Zhang XM, Karp S, Yang Y, McMahon AP. Genetic manipulation of hedgehog signaling in the endochondral skeleton reveals a direct role in the regulation of chondrocyte proliferation. Development. 2001;128(24):5099–108. . [DOI] [PubMed] [Google Scholar]
- 68.Martin M. Cutadapt removes adapter sequences from high-throughput sequencing reads. 2011. 2011;17(1):3%J EMBnet.journal. Epub 2011-08-02. 10.14806/ej.17.1.200 [DOI]
- 69.Kim D, Langmead B, Salzberg SL. HISAT: a fast spliced aligner with low memory requirements. Nature Methods. 2015;12:357 10.1038/nmeth.3317 https://www.nature.com/articles/nmeth.3317#supplementary-information. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Frazee AC, Pertea G, Jaffe AE, Langmead B, Salzberg SL, Leek JT. Ballgown bridges the gap between transcriptome assembly and expression analysis. Nat Biotechnol. 2015;33(3):243–6. 10.1038/nbt.3172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pertea M, Pertea GM, Antonescu CM, Chang T-C, Mendell JT, Salzberg SL. StringTie enables improved reconstruction of a transcriptome from RNA-seq reads. Nature Biotechnology. 2015;33:290 10.1038/nbt.3122, https://www.nature.com/articles/nbt.3122#supplementary-information. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lin KH, Wu Q, Leib DJ, Tang SY. A novel technique for the contrast-enhanced microCT imaging of murine intervertebral discs. J Mech Behav Biomed Mater. 2016;63:66–74. Epub 2016/06/25. 10.1016/j.jmbbm.2016.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sabiston P, Adams ME, Ho YA. Automation of 1,9-dimethylmethylene blue dye-binding assay for sulfated glycosaminoglycans with application to cartilage microcultures. Anal Biochem. 1985;149(2):543–8. Epub 1985/09/01. 10.1016/0003-2697(85)90611-6 . [DOI] [PubMed] [Google Scholar]
- 74.Woessner JF Jr. The determination of hydroxyproline in tissue and protein samples containing small proportions of this imino acid. Arch Biochem Biophys. 1961;93:440–7. Epub 1961/05/01. 10.1016/0003-9861(61)90291-0 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A, B) In situ hybridizations of Adgrg6 in spine tissue (8 months) using Alkaline phosphatase/BM purple chromogenic developing shows strong Adgrg6 expression (blue stain) in the growth plate (GP) and minor expression in the annulus fibrosis (AF) (red arrowheads) that is mostly abolished in ATC;Adgrg6f/f mutant tissues (B); or using (C, D) tyramine-amplification fluorescence which shows expanded expression throughout the IVD including GP, CEP, AF, and NP, which is mostly diminished in ATC;Adgrg6f/f mutant tissues. Robust expression was detected in periosteum of the long bone tissues in both control and the ATC;Adgrg6f/f mutant moues (E, F, yellow arrows). (Induced from P1-P20, n = 3 for each group.) Scale bars: 200μm in (A-D); 50μm in (E, F). AF- annulus fibrosis, CEP- cartilaginous endplate, GP- growth plate, and NP- nucleus pulposus, Tb- trabecular bone, Cb-cortical bone, P-periosteum.
(TIF)
(A-C) Representative 8-month-old mouse IVDs (induced form E0.5-P20) stained with Safranin-O/Fast green (SO/FG) (n = 3 for controls and n = 6 for mutants). Endplate-oriented disc herniation is indicated with yellow arrows in B and B’. These herniations are very hard to be captured by histological analysis. C is an earlier midline section of an adjacent mutant IVD as shown in B, showing no overt histopathology. Scale bars: 100μm in (A-C); and 50μm in (A’, B’). AF- annulus fibrosis, CEP- cartilaginous endplate, GP- growth plate, and NP- nucleus pulposus.
(TIF)
More robust recombination (blue signal) in CEP, GP, and AF of the IVD was observed in the Col2Cre; Rosa-LacZ mouse (B, E) compared with the ATC; Rosa-LacZ mouse when induced from E0.5-P20 (A, C) and P1-P20 (D). Recombination in periosteum (B, E, red arrows) and the outmost AF layers of the IVD (B, E, black arrows) was observed only in the Col2Cre; Rosa-LacZ mouse but not the ATC; Rosa-LacZ mouse. Scale bars: 100μm in (A-E). CEP- cartilaginous endplate, GP- growth plate, AF- annulus fibrosis, NP- nucleus pulposus, Tb- trabecular bone, and P- periosteum.
(TIF)
(A-D’) Representative 4-month-old (A-B’) or 8-month-old (C-D’) mouse IVDs stained with Safranin-O/Fast green (SO/FG). (Induced from P1-P20. For A-B’, n = 3 for controls and n = 5 for mutants; for C-D’, n = 4 for each group). Minor growth plate erosion is observed by the age of four months in mutant mice (yellow arrowheads, B), while more severe endplate-oriented disc herniations were observed by the age of 8 months (yellow arrowheads, D). (B’) is an earlier midline sections of the same mutant IVD as shown in B. (D’) is a midline section of an adjacent mutant IVD as shown in D, showing no overt histopathology. (E-L) IHC analysis of 8-month-old Cre (-) Control and ATC;Adgrg6f/f mutant mouse IVDs (induced from P1-P20). Several protein markers of IVD health and disease are affected in ATC;Adgrg6f/f mutant IVD including decreased expression of healthy disc markers COLII and SOX9 (G, blue arrows), and increased expression of the hypertrophic marker COLX (F, red arrows) and extracellular matrix modifying enzyme MMP-13 (J, red arrows). (n = 3 for each group.) Scale bars: 100μm in (A-D’); 50μm in (E-L). CEP: cartilaginous endplate, GP: growth plate, AF: annulus fibrosis, and NP: nucleus pulposus.
(TIF)
Large scale images of IHC analysis shown in Fig 2. IHC analysis of common markers of degenerative disc. ATC;Adgrg6f/f conditional mutant IVDs display reduced expression of markers of healthy disc: SOX9 (B), PRG4 (D), and COLII (H); and increased expression of extracellular matrix modifying enzymes MMP-13 (F), hypertrophic marker COLX (J). Scale bars: 100μm in (A-J). AF- annulus fibrosis, CEP- cartilaginous endplate, GP- growth plate, and NP- nucleus pulposus.
(TIF)
(A, B) TUNEL (red fluorescence) staining of 1.5-month-old ATC;Adgrg6f/f mutants (B, white arrows) display increased TUNEL positive cells compared to Cre (-) control (A) mice. (C) Graph of the ratio of TUNEL positive cells to total cells (DAPI) (n = 3 for each group, three to five IVDs were analyzed/mouse. Bars represent mean ± SD. *p≤0.05, two-tailed Student's t Test). AF- annulus fibrosis, CEP- cartilaginous endplate, GP- growth plate, and NP- nucleus pulposus.
(TIF)
(A-B’) Representative medial-sectioned mouse IVDs stained with Alcian blue/Orange G of Cre (-) control (A and A') and Col2Cre;Adgrg6f/f mutant (B, B') mice at P20 (n = 3 for each group). No overt histopathology was observed in mutant mice at this young age. (C-E) Representative mouse IVDs stained with Safranin-O/Fast green (SO/FG) of Cre (-) control (C and C') and Col2Cre;Adgrg6f/f mutant (D, D', and E) mice by the age of 8 months (n = 3 for each group). Endplate-oriented herniations is indicated with yellow arrows. These herniations are very hard to be captured by histological analysis (D is out of the typical plane of section). E is an earlier midline section of the same mutant IVD as shown in D, showing no overt histopathology. Scale bars: 200μm in (A, B) and (C-E), and 50μm in (A’, B’) and (C’, D’). AF- annulus fibrosis, CEP- cartilaginous endplate, GP- growth plate, and NP- nucleus pulposus.
(TIF)
(A-D) IHC analysis of macrophage marker shows no strong signal of CD68 in ATC;Adgrg6f/f mutant mouse IVD at 1.5months (A, B), or 8 months of age (C, D), except for some background signal at the herniation site (red arrow, D). (Induced from E0.5-P20, n = 3 for each group.) Scale bars: 50μm in (A-D). CEP- cartilaginous endplate, GP- growth plate, and NP- nucleus pulposus.
(TIF)
(A) Alcian blue staining on ATDC5 cell culture during the maturation process. (B) Expression profiles of Adgrg6, Col2a1, Acan, and Sox9 during ATDC5 cell maturation. The expression level of Adgrg6 was gradually increased alone with other chondrogenesis markers including Col2a1, Acan, and Sox9. (n = 3 biological replicates and representative result is shown. Bars represent mean ± SD. *p≤0.05, two-tailed Student's t Test). Scale bars: 100μm in A.
(TIF)
(A, B) Immunofluorescence against cleaved-Caspace-3 (green) and DAPI staining (blue) and (C) quantification showing increased apoptosis in Adgrg6 KO cells during maturation (10 days). (n = 3 biological replicates and representative result is shown. Bars represent mean ± SD. *p≤0.05, two-tailed Student's t Test). Scale bars: 50μm in (A, B).
(TIF)
(A-C’) IHC analysis of COLX in 6-month-old Cre (-) control (A, A’) and Col2Cre;Adgrg6f/f mutant mice with (C, C’) or without (B, B’) Stattic treatment. Ectopic COLX expression was observed in CEP of the mutant mice (red arrows, B’), which is rescued after Stattic treatment (C’). (D-I) IHC analysis of COLII and SOX9 in 6-month-old Cre (-) control (A, D) and Col2Cre;Adgrg6f/f mutant mice with (F, I) or without (E, H) Stattic treatment. Reduced COLII and SOX9 expression was observed in Col2Cre;Adgrg6f/f conditional mutant mice compared with Cre (-) control (blue arrows, D, G), but no obvious improvement was observed after Stattic treatment (F, I). (n = 3 for each group). (J) qPCR analyses revealed that Col10a1 and Sox9 expression in Adgrg6 KO ATDC5 cells was partially rescued after Stattic treatment (10nM for 10 days), however the expression of Col2a1 was not significantly changed (n = 3 biological replicates and representative result is shown. Bars represent mean ± SD. *p≤0.05, **p≤0.01, ***p≤0.001, ****p≤0.0001, One way ANOVA followed by Tukey HSD test. n.s, not significant.) Scale bars: 100μm in (A-C), and 50μm in (A’-I). AF- annulus fibrosis, CEP- cartilaginous endplate, GP- growth plate, and NP- nucleus pulposus.
(TIF)
Correlation analysis (Pearson’s r) between number of herniation and disc stiffness were performed on 6-month-old mice from three experimental groups: four placebo-treated Cre (-) controls; three placebo-treated Col2Cre;Adgrgf/f mutants; and three Stattic-treated Col2Cre;Adgrgf/f mutants as shown in Fig 6. Dots plotted by mean ± SD. No correlation was detected.
(TIF)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(MOV)
(MOV)
Data Availability Statement
All short read data is uploaded as GSE128402 - Transcriptomic analysis of intervertebral discs isolated from wild type and Adgrg6-deficient mice https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE128402