Abstract
Heart failure is associated with profound alterations of energy metabolism thought to play a major role in the progression of this syndrome. SIRT1 is a metabolic sensor of cellular energy and exerts essential functions on energy metabolism, oxidative stress response, apoptosis, or aging. Importantly, SIRT1 deacetylates the peroxisome proliferator-activated receptor gamma co-activator 1α (PGC-1α), the master regulator of energy metabolism involved in mitochondrial biogenesis and fatty acid utilization. However, the exact role of SIRT1 in controlling cardiac energy metabolism is still incompletely understood and conflicting results have been obtained. We generated a cardio-specific inducible model of Sirt1 gene deletion in mice (Sirt1ciKO) to decipher the role of SIRT1 in control conditions and following cardiac stress induced by pressure overload. SIRT1 deficiency induced a progressive cardiac dysfunction, without overt alteration in mitochondrial content or properties. Sixteen weeks after Sirt1 deletion an increase in mitochondrial reactive oxygen species (ROS) production and a higher rate of oxidative damage were observed, suggesting disruption of the ROS production/detoxification balance. Following pressure overload, cardiac dysfunction and alteration in mitochondrial properties were exacerbated in Sirt1ciKO mice. Overall the results demonstrate that SIRT1 plays a cardioprotective role on cardiac energy metabolism and thereby on cardiac function.
Keywords: Sirtuin 1, heart, mitochondria, cardiac function
1. Introduction
Heart failure (HF), defined as the inability of the heart to provide adequate blood flow to meet the needs of the organism, is often the terminal end-point of different chronic cardiovascular diseases (CVD). Despite the advances of pharmaceutical therapeutic approaches over the past two decades, long term survival of HF patients still remains limited. This syndrome is thus a major cause of death worldwide and its prevalence is expected to rise in the ageing world population, prompting the research community to decipher the pathophysiology of HF. It has been demonstrated that HF is associated with profound modulations of energy metabolism of the heart; this altered energetics is thought to play major roles in the progression of the disease [1]. The failing heart is especially characterized by severe dysfunctions of the mitochondria, the main energy producers of the myocardium, and by an important decrease in fatty acid utilization in favor of carbohydrates at least at the beginning of the disorder. These alterations lead to an organ towards a state of depleted energy with lower concentrations of high-energy phosphate compounds (ATP, phosphocreatine) associated with elevated adenosine diphosphate (ADP) in the myocardium that is often described as an “engine out of fuel” [1]. Yet, this metabolic aspect of the disease is still not often taken into consideration by current standard HF therapies due to a lack of therapeutic targets.
The role of sirtuin 1 (SIRT1) in HF pathophysiology and its potential place in future therapies of this syndrome have aroused interest in recent years. This enzyme is a class III histone/protein deacetylase, whose activity depends on intracellular nicotinamide adenine dinucleotide (NAD+) level, presenting SIRT1 as a metabolic sensor of cellular energy [2] linking metabolic status of the cells to the regulation of gene expression. It is part of a family of highly conserved protein modifying enzymes firstly described in yeast. Mammals possess 7 sirtuins (SIRT1-7) and SIRT1 is the closest mammalian ortholog of silent mating type information regulation 2 (SIR2) described in yeast, drosophila melanogaster, and caenorhabditis elegans for its role in life extension in particular under caloric restriction [3,4,5]. SIRT1 deacetylates a large variety of substrates located in the nucleus or in the cytosol including histones, enzymes, transcriptional factors, and cofactors and consequently exerts essential functions in wide-ranging cellular processes such as energy metabolism, oxidative stress response, apoptosis, or aging [6]. It especially regulates energy metabolism by direct interaction with the peroxisome proliferator-activated receptor gamma co-activator 1α (PGC-1α), a master regulator of metabolism [7]. The SIRT1-mediated deacetylation of the latter results in the stimulation of its ability to co-activate a number of transcription factors controlling various facets of energy metabolism like mitochondrial biogenesis and fatty acid utilization [8,9]. The expression of PGC-1α has been shown to be largely reduced in various animal models of HF [10], as well as in cardiac tissue from HF human patients [11], especially in heart failure with reduced ejection fraction [12]. Knowing that a decrease in PGC-1α activity is associated with alterations of mitochondrial biogenesis and functions [13,14], stimulation of the SIRT1/PGC-1α axis could consequently be of great interest to restore cardiac energetics in a therapeutic context of CVD.
It has already been demonstrated that SIRT1 plays important roles in the maintenance of heart function and that it can be protective when the heart is subjected to stress like oxidative injury, hypertrophic stimuli, or ischemia/reperfusion injury (for review see [15]). In rodents, pharmacological activation of SIRT1 in the context of CVD can be beneficial [16,17]. This deacetylase could protect the heart through various mechanisms impacting processes like inflammation, fibrosis, apoptosis, energy metabolism, or calcium homeostasis [16,17,18]; however, the multitude of its targets renders the interpretation of cardiac protection by SIRT1 complex. In HF, the role of SIRT1 is still not perfectly understood. Its level is increased in the early stage of HF in animal models, especially in the nuclear fraction [19,20,21], while a clear reduction in SIRT1 protein content has been reported in advanced HF in rodents and humans [16,17,22,23,24]. Whereas a high SIRT1 content could be considered as an adaptive mechanism to face the increase in cardiac workload, studies have in contrast demonstrated that the increase in SIRT1 protein levels observed in the hypertrophic stage of pressure overload-induced myocardial dysfunction could be harmful [20,21]. Likewise, transgenic mouse models with constitutive high levels of SIRT1 overexpression develop cardiac dysfunctions associated with energy metabolism alterations [19,20,21,25]. Although the vast majority of the studies argue for the beneficial effect of SIRT1 in CVD, these contradictory results highlight the complexity of SIRT1 mechanism of actions in the cardiomyocyte and it seems necessary to clarify the roles of SIRT1 in the heart before considering this enzyme as a therapeutic target in such diseases, especially in HF.
Thus, the aim of the present study was to elucidate the role of SIRT1 under conditions of cardiac stress. For that, we used an original model of cardiac-specific knockout mice inducible in adult by tamoxifen injection.
2. Results
2.1. Cardiac Specific Tamoxifen-Induced Loss of Sirt1 in α-MHC-Cre/Flox Mice
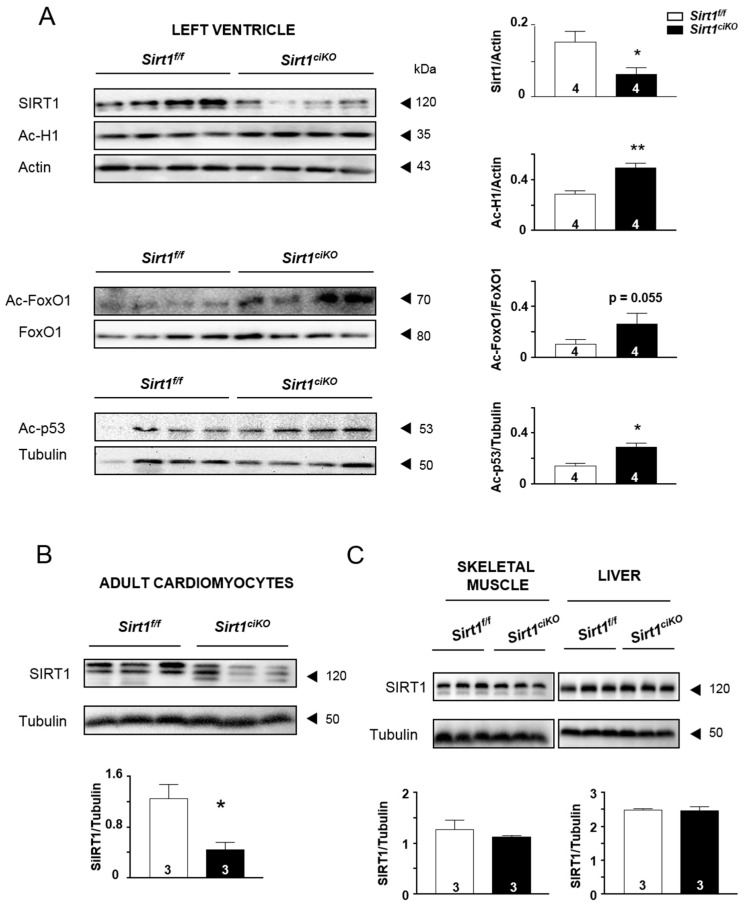
At 8 weeks of age, Sirt1ciKO mice were given tamoxifen to induce exon 4 excision from the floxed Sirt1 alleles. Four weeks later, assessment of SIRT1 protein levels from LV homogenates revealed a reduction of 54 ± 11% in Sirt1ciKO mice in comparison with Sirt1f/f ones (Figure 1A). This was associated with a significantly higher acetylation level of histone H1 (H1) and tumor suppressor p53 protein (p53) as well as a strong trend towards an increase in acetylated forkhead box protein O1 (FoxO1) in Sirt1ciKO mice (p = 0.055). Inasmuch as the cardiomyocytes are not the only cell type encountered in the heart, the SIRT1 level was investigated in isolated cardiomyocytes to prove down-regulation of SIRT1 in these cells. Indeed, the SIRT1 level drastically dropped to 64 ± 8 % in cardiomyocytes isolated from Sirt1ciKO mice heart, although this protein did not completely disappear in this cell population (Figure 1B). Protein levels of SIRT1 in skeletal muscle and liver were similar in Sirt1ciKO and Sirt1f/f groups (Figure 1C). Altogether, the data confirmed the cardiac specificity of Sirt1 deletion in the present animal model and a strong decrease in level/activity of this enzyme even though a small part of the cardiomyocyte population of Sirt1ciKO mice heart escapes the deletion process.
Figure 1.
Cardiac-specific Sirt1 inactivation in adult mice 4 weeks after tamoxifen injection. (A) Protein content of SIRT1 and its downstream acetylated targets (acetylated-Histone H1 (Ac-H1), acetylated-FoxO1 (Ac-FoxO1), and acetylated-p53 (Ac-p53) in left ventricle homogenates. (B) Protein content of SIRT1 in isolated cardiomyocytes. (C) Protein content of SIRT1 in skeletal muscle and liver homogenates. (n = 3 to 4 per experimental group), * p < 0.05; ** p < 0.01 Sirt1f/f versus Sirt1ciKO.
2.2. Sirt1ciKO Mutants Develop a Mild Left Ventricular Systolic Dysfunction
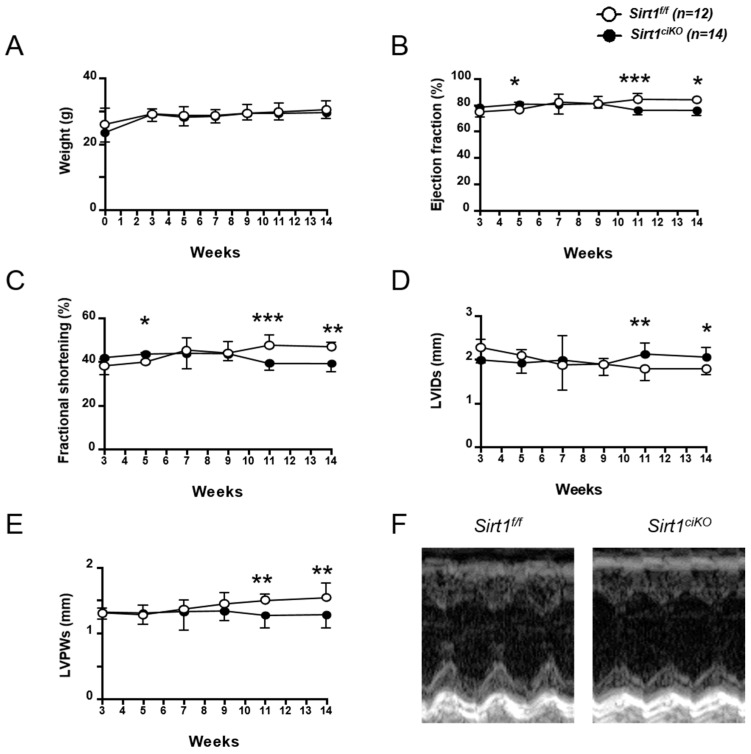
Sirt1ciKO mice body weight was similar to the controls during 14 weeks of observation (Figure 2A). Cardiac function of Sirt1ciKO mice and their control littermates were assessed by serial echocardiography 3, 5, 7, 9, 11, and 14 weeks after the first tamoxifen injection. While echocardiography parameters did not show any difference between control and mutant mice until 9 weeks after Sirt1 deletion, significant decreases in LV ejection fraction (LVEF), fractional shortening (LVFS), and end-systolic left posterior wall thickness (LVPWs), as well as a significant increase in end-systolic left ventricular internal diameter (LVIDs) were observed 11 and 14 weeks after Sirt1 deletion (Figure 2B–F and Table 1). However, these alterations of cardiac systolic function remained moderate after 14 weeks and no significant impact on cardiac output was noticed at this time point (Table 1). After 14 weeks, no difference in diastolic LV parameters was reported between Sirt1ciKO and Sirt1f/f mice (Table 1), and similar heart weight-to-body weight (HW/BW) and heart weight-to-tibia length (HW/TL) ratios in both groups indicated the absence of cardiac hypertrophy in Sirt1ciKO mice (Table 1).
Figure 2.
Progression of body weight and cardiac function of cardiac-specific knockout mice during 14 weeks after Sirt1 deletion induction. (A) Body weight. (B) Left ventricular ejection fraction. (C) Left ventricular fractional shortening. (D) Left ventricular internal dimension at end-systole (LVIDs). (E) Left ventricular posterior wall thickness at end-systole (LVPWs). (F) Representative M-mode images of the left ventricle (14 weeks after induction of Sirt1 deletion). (n = 12 to 14 per experimental group), * p < 0.05; ** p < 0.01; *** p < 0.001 Sirt1f/f versus Sirt1ciKO.
Table 1.
Anatomical and echocardiographic parameters 14 weeks and 11 months after induction of Sirt1 deletion by tamoxifen injection. BW, Body weight. TL, Tibia length. HW, Heart weight. HW/BW, Heart weight-to-body weight ratio. HW/TL, Heart weight-to-tibia length ratio. LW/BW, Lung weight-to-body weight ratio. LW/TL, Lung weight-to-tibia length ratio. KW/BW, Kidney weight-to-body weight ratio. KW/TL, Kidney weight-to-tibia length ratio. HR, Heart rate. IVSd, Interventricular septal thickness at end-diastole. IVSs, Interventricular septal thickness at end-systole. LVIDd, Left ventricular internal dimension at end-diastole. LVIDs, Left ventricular internal dimension at end-systole. LVWPd, Left ventricular posterior wall thickness at end-diastole. LVWPs, Left ventricular posterior wall thickness at end-systole. EDV, Left ventricular telediastolic volume. ESV, Left ventricular telesystolic volume. LVEF, Left ventricular ejection fraction. LVFS, Left ventricular fractional shortening. SV, Stroke volume. LV, left ventricular mass. CO, Cardiac output. ANOVA: ¶ p ≤ 0.05, ¶¶ p ≤ 0.01, ¶¶¶ p ≤ 0.001 for the genotype effect; § p ≤ 0.05, §§ p ≤ 0.01, §§§ p ≤ 0.001 for the aging effect; i p ≤ 0.05, ii p ≤ 0.01 for the interaction effect. Post hoc Newman–Keuls test: * p ≤ 0.05, ** p ≤ 0.01, *** p ≤ 0.001 Sirt1f/f versus Sirt1ciKO (same age); $ p ≤ 0.05, $$ p ≤ 0.01, $$$ p ≤ 0.001 young versus old (same genotype).
| 14 Weeks | 11 Months | 2-Way ANOVA | |||
|---|---|---|---|---|---|
| Sirt1f/f | Sirt1ciKO | Sirt1f/f | Sirt1ciKO | ||
| BW (g) | 30.4 ± 0.4 | 29.9 ± 0.3 | 33.4 ± 1.5 $$ | 29.6 ± 0.6 ** | ¶¶, i |
| TL (mm) | 17.2 ±0.2 | 17.2 ± 0.1 | 18.8 ± 0.3 $$ | 18.6 ± 0.7 $ | §§§ |
| HW (mg) | 143.2 ± 4.4 | 144.8 ± 3.1 | 173.6 ± 3.9 $$ | 162.6 ± 10.4 $ | §§§ |
| HW/BW (mg/g) | 4.7 ± 0.1 | 4.8 ± 0.1 | 5.2 ± 0.2 | 5.5 ± 0.3 $ | §§ |
| HW/TL (mg/mm) | 8.3 ± 0.2 | 8.6 ± 0.1 | 9.2 ± 0.2 | 8.8 ± 0.2 | |
| LW/BW (mg/g) | 4.5 ± 0.1 | 4.9 ± 0.2 | 5.2 ± 0.2 $$ | 5.3 ± 0.1 | §§§ |
| LW/TL (mg/mm) | 8.1 ± 0.2 | 8.6 ± 0.3 | 9.3 ± 0.5 | 8.7 ± 0.4 | / |
| KW/BW (mg/g) | 11.8 ± 0.2 | 12.1 ± 0.3 | 12.6 ± 0.9 | 12.2 ± 0.6 | / |
| KW/TL (mg/mm) | 20.9 ± 0.4 | 21.5 ± 0.5 | 22.2 ± 0.9 | 18.7 ± 1.2 $,* | i |
| HR (bpm) | 506 ± 7 | 498 ± 11 | 542 ± 13 | 549 ± 16 | §§ |
| IVSd (mm) | 1.04 ± 0.04 | 0.93 ± 0.03 | 0.82 ± 0.12 | 0.82 ± 0.07 | §§ |
| IVSs (mm) | 1.64 ± 0.03 | 1.44 ± 0.03 ** | 1.44 ± 0.06 $$ | 1.28 ± 0.03 $,* | ¶¶¶, §§§ |
| LVIDd (mm) | 3.36 ± 0.06 | 3.39 ± 0.06 | 3.82 ± 0.15 $ | 3.81 ± 0.13 $$ | §§§ |
| LVIDs (mm) | 1.78 ± 0.04 | 2.06 ± 0.06 * | 2.09 ± 0.12$ | 2.58 ± 0.12 $$$,*** | ¶¶¶, §§§ |
| LVPWd (mm) | 0.95 ± 0.04 | 0.81 ± 0.05 | 0.81 ± 0.04 | 0.84 ± 0.11 | / |
| LVPWs (mm) | 1.55 ± 0.06 | 1.28 ± 0.06 | 1.53 ± 0.11 | 1.27 ± 0.02 | ¶¶ |
| EDV (mL) | 0.098 ± 0.005 | 0.102 ± 0.006 | 0.142 ± 0.016 $ | 0.14 ± 0.014 $$ | §§§ |
| ESV (mL) | 0.016 ± 0.001 | 0.024 ± 0.002 * | 0.025 ± 0.004 $ | 0.046 ± 0.006 $$$,*** | ¶¶¶, §§§, i |
| LVEF (%) | 84.1 ± 0.5 | 76.3 ± 1.1 * | 82.6 ± 1.4 | 66.7 ± 5 $$$,*** | ¶¶¶, §§§, i |
| LVFS (%) | 46.9 ± 0.6 | 39.4 ± 1 ** | 45.6 ± 1.5 | 32.2 ± 3.3 $$$,*** | ¶¶¶, §§§, ii |
| SV (mL) | 0.083 ± 0.005 | 0.77 ± 0.004 | 0.117 ± 0.012 $$ | 0.094 ± 0.013 $,* | §§ |
| LV (mg) | 109 ± 6 | 94 ± 7 | 98 ± 5 | 102 ± 14 | / |
| CO (mL/min) | 42.0 ± 2.4 | 38.0 ± 2.1 | 63.3 ± 6.9 $$ | 51.1 ± 5.8 $,* | ¶, §§§ |
Eleven months after tamoxifen injection, LV systolic parameters of Sirt1ciKO mice were much more altered. Clear decreases in LVEF and LVSF were observed and LVIDs was increased when mice were deleted for Sirt1 (Table 1). These alterations were associated with an increase in LV end-systolic volume (ESV) and a decrease in cardiac output which is indicative of a cardiac dysfunction (Table 1). At this time point, the mutant mice had significantly lower weight than their littermates (Table 1). Despite alterations of cardiac function, heart weight, HW/BW, and HW/TL of these mice were not significantly different from control ones (Table 1). The comparable lung weight-to-tibia length (LW/TL) ratio between both mice groups suggests that Sirt1ciKO mice did not suffer from congestive heart failure yet (Table 1). These results indicate that this inducible cardiac-specific knockout mouse model developed a cardiac dysfunction with slow progression and, even though SIRT1 is known to regulate many cellular pathways, cardiac pump alterations were still mild 11 months after induction of Sirt1 deletion.
2.3. Mitochondrial Oxidative Capacities are Preserved in Cardiac-Specific Sirt1 Mutant Mice
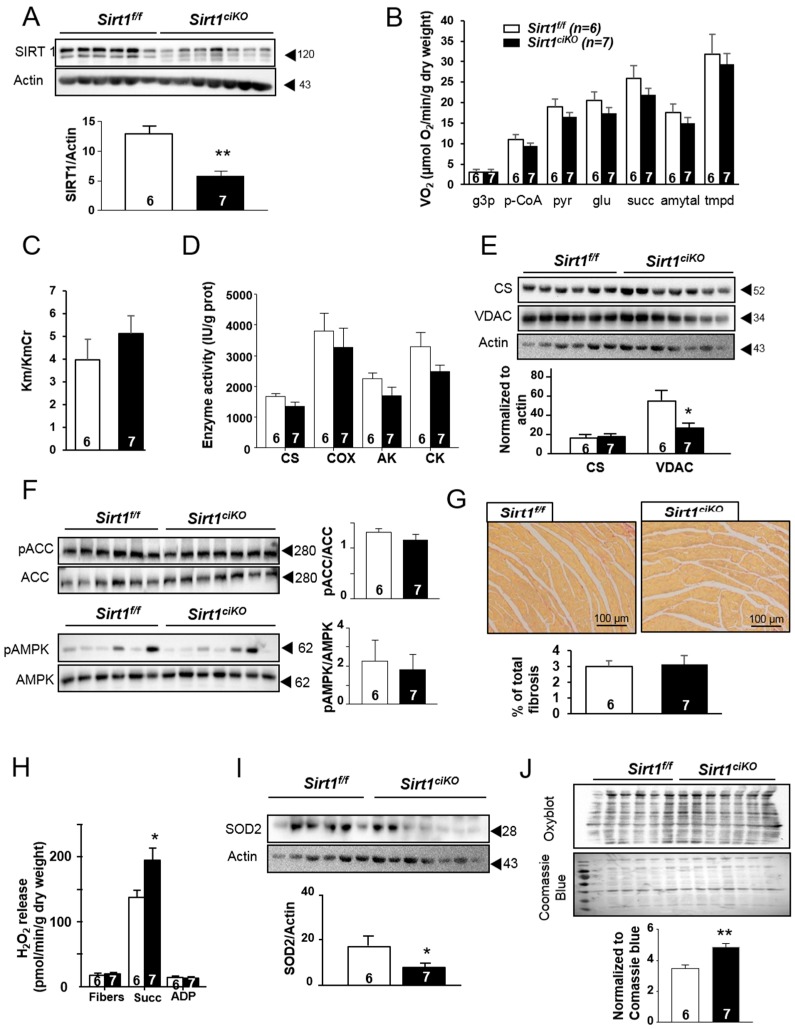
Knowing the role of SIRT1 in cellular energetics and the high reliance of heart function on mitochondrial energy production, the consequences of Sirt1 deletion on the cardiac mitochondria have been assessed. After 16 weeks of Sirt1 deletion, while the SIRT1 level was drastically reduced (Figure 3A), mitochondrial respiration rates after cumulative addition of l-glycerol-3-phosphate, palmitoyl-CoA/carnitine, pyruvate, glutamate, succinate, amytal, and N, N′, N′-tetramethyl-phenylenediamine dihydrochloride (TMPD) were not significantly changed in Sirt1ciKO mice (Figure 3B), thereby indicating no impact of this deletion on mitochondrial substrate preferences and maximal mitochondrial oxidative capacities at this time point. Likewise, the comparable decrease by creatine in the Km ADP for respiration (Km/KmCr ratio) in both groups suggests that mitochondrial creatine kinase in Sirt1ciKO heart was fully functional (Figure 3C). This is associated with unchanged adenylate kinase (AK) and total creatine kinase (CK) enzymatic activity in both types of mice (Figure 3D), which suggests preserved energy transfers within the cardiomyocyte. The activity of citrate synthase (CS), traditionally used as a marker of functional mitochondrial mass, as well as cytochrome c oxidase (COX) activity were unchanged in mutant mice (Figure 3D). In accordance with these results, CS protein level was similar in both groups (Figure 3E). Although voltage-dependent anion channel (VDAC) protein levels showed a significant decrease after 16 weeks of Sirt1 deletion (Figure 3E), the aforementioned results suggest that energy production function of cardiac mitochondria was not markedly altered in the mutants at this stage. A potential compensatory role of AMP-activated protein kinase (AMPK) in the maintenance of mitochondrial oxidative capacities in the Sirt1ciKO mice was not observed as phosphorylation of AMPK (Thr 172) and acetyl-CoA carboxylase (ACC) (Ser 79), used as markers of AMPK activity, were unchanged in comparison with Sirt1f/f (Figure 3F). Of note, no increase in total fibrosis was observed in mutant mice at this time point (Figure 3G).
Figure 3.
Cardiac mitochondrial phenotype of cardiac-specific knockout mice after 16 weeks of Sirt1 deletion. (A) SIRT1 protein content in left ventricle (LV) homogenates. (B) Rate of respiration after successive addition of l-glycerol-3-phosphate (4 mM) (g3p), palmitoyl-CoA, and carnitine (100 µM and 2 mM) (p-CoA), pyruvate (1 mM) (pyr), glutamate (10 mM) (glu), succinate (15 mM) (succ), amytal (1 mM), and N, N′, N′-tetramethyl-phenylenediamine dihydrochloride (TMPD)-ascorbate (0.5 mM). (C) Ratio between Km without creatine and Km with creatine. (D) Citrate synthase (CS), cytochrome c oxidase (COX), adenylate kinase (AK), and creatine kinase (CK) enzymatic activities. (E) Immunoblotting of citrate synthase (CS) and voltage-dependent anion channel (VDAC) in LV homogenates. (F) Immunoblotting of total acetyl-CoA carboxylase (ACC), phosphorylated-ACC (pACC), total AMP-activated protein kinase (AMPK), and phosphorylated-AMPK (pAMPK) in LV homogenates. (G) Representative of fibrosis analysis by Sirius red staining of subequatorial heart sections. (H) Net rate of H2O2 release by the mitochondrial electron transport chain measured following sequential addition of succinate (5 mM) and ADP (1 mM). (I) SOD2 protein content in LV homogenates. (J) Carbonylation of LV proteins. (n = 6 to 7 per experimental group), * p < 0.05; ** p < 0.01 Sirt1f/f versus Sirt1ciKO.
The loss of Sirt1 in the heart during 16 weeks was not without impact on mitochondrial functions since cardiac mitochondria of Sirt1ciKO mice released significantly more H2O2 than control mice when respiration was stimulated by succinate (Figure 3H). This was observed even though the MnSOD2 level was decreased in mutant mice (Figure 3I), indicating a higher propensity for mitochondria to produce superoxide anions during electron transfer within mitochondrial respiratory chain. In accordance with this increase in mitochondrial reactive oxygen species (ROS) production, assessment of protein carbonylation, a consequence of oxidative modifications of proteins by ROS, revealed a higher level of oxidative damage in mutants (Figure 3J), suggesting disruption of the balance between ROS production and detoxification systems.
Even eleven months after tamoxifen injection, when cardiac function alterations were much more pronounced, mitochondrial respiration still did not show any significant difference between Sirt1f/f and Sirt1ciKO mice (Figure 4A). The activity of CS was significantly reduced in Sirt1ciKO heart (Figure 4B) despite the fact that Sirt1ciKO mice exhibited mitochondrial oxidative capacities and CS protein content similar to control mice (Figure 4C). In contrast, COX activity was not impacted by Sirt1 deletion as well as VDAC protein level (Figure 4C). No difference in mitochondrial electron transfer chain complexes between both groups of mice was evidenced by immunoblotting (Figure 4D). Whereas Sirt1 deletion was associated with higher ROS production 16 weeks after tamoxifen injection (Figure 3H), this seemed to be normalized after 11 months since mitochondrial H2O2 release in presence of succinate was not significantly increased in the mutant mice (Figure 4E). Likewise, the MnSOD2 level was not reduced 11 months after induction of Sirt1 deletion (Figure 4F).
Figure 4.
Cardiac mitochondrial phenotype of cardiac-specific knockout mice after 11 months of Sirt1 deletion. (A) Rate of respiration after successive addition of l-glycerol-3-phosphate (4 mM) (g3p), palmitoyl-CoA and carnitine (100 µM and 2 mM) (p-CoA), pyruvate (1 mM) (pyr), glutamate (10 mM) (glu), succinate (15 mM) (succ), amytal (1 mM), and N, N′, N′-tetramethyl-phenylenediamine dihydrochloride (TMPD)-ascorbate (0.5 mM). (B) Citrate synthase (CS) and cytochrome c oxidase (COX) enzymatic activities. (C) Immunoblotting of citrate synthase (CS) and voltage-dependent anion channel (VDAC) in LV homogenates. (D) Total protein content of 5 subunits of oxidative phosphorylation complexes: C-I-20 (complex I (CI)), C-II-30 (complex II (CII)), C-III-Core 2 (complex III (CIII)), C-IV-COXI (complex IV (CIV)), and C-V-α (complex V (CV)). Protein content for CI, CII, CII, and CIV was normalized using CV as internal control. (E) Net rate of H2O2 release by the mitochondrial electron transport chain measured following sequential addition of succinate (5 mM), and ADP (1 mM). (F) SOD2 protein content in LV homogenates. (A–B and E: n = 6 to 7 per experimental group, C–D and F: n = 4 per experimental group), * p < 0.05 Sirt1f/f versus Sirt1ciKO.
In order to confirm that cardiac alterations observed in this model were due to the loss of Sirt1 and not to activation of the Cre-recombinase, the cardiac phenotype of α-MHC-MerCreMer mice treated with the same dose of tamoxifen (40 mg/kg i.p daily during 2 days) was investigated. Sixteen weeks after injection, cardiac functions were normal as judged by LVEF, LVFS, LVIDs, and ESV (Figure 5A–D). Mitochondrial oxidative capacities were not significantly different (Figure 5E) and no sign of fibrosis was evidenced by Sirius red staining (Figure 5F).
Figure 5.
Cardiac phenotype of α-MHC-MerCreMer mice 16 weeks after tamoxifen injection. (A) Left ventricular ejection fraction. (B) Left ventricular fractional shortening. (C) Left ventricular internal dimension at end-systole (LVIDs). (D) Telesystolic LV volume (ESV). (E) Rate of respiration after successive addition of pyruvate (1 mM) (pyr), glutamate (10 mM) (glu), succinate (15 mM) (succ), amytal (1 mM), and TMPD-ascorbate (0.5 mM). (F) Representative pictures of fibrosis analysis by Sirius red staining of subequatorial heart section.
2.4. Sirt1ciKO Mice Are More Sensitive to Cardiac Pressure Overload
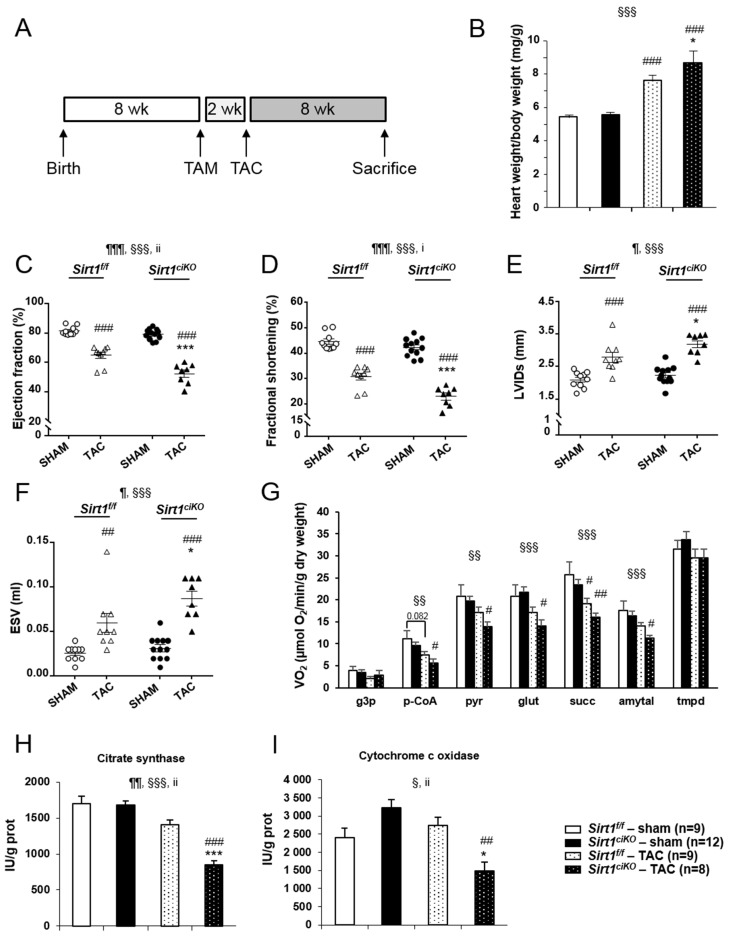
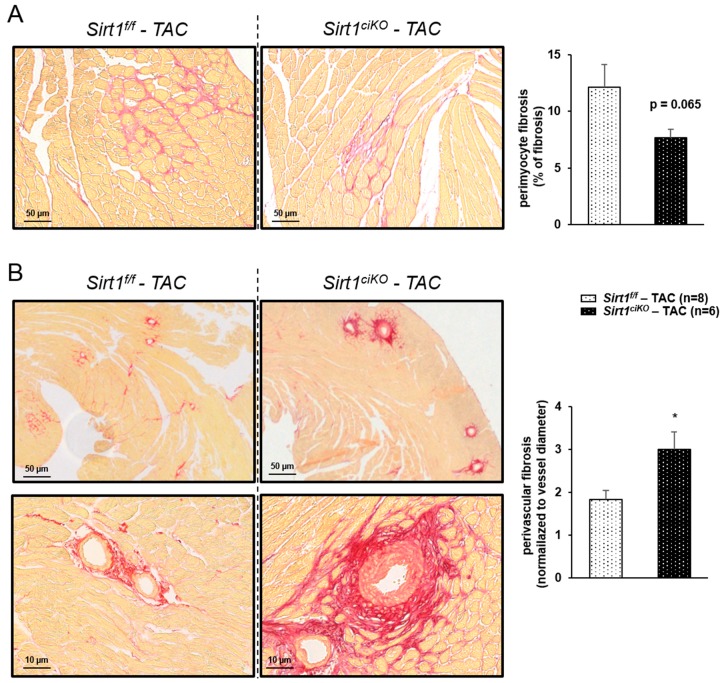
Many studies have indicated that SIRT1 activation could be beneficial when the heart is subjected to various kinds of stress [7]. We thus investigated how cardiac-specific inducible Sirt1 deletion triggered in adults impacted the response to TAC-induced cardiac pressure overload. To do so, mice underwent surgery 2 weeks after tamoxifen injection and were sacrificed 8 weeks later (Figure 6A). At sacrifice, mice of both TAC groups displayed an increase in heart weight compared to their respective sham group, as judged by absolute weight and HW/BW ratio (Table 2). However, the HW/BW ratio after TAC was much higher when mice did not express Sirt1 (Table 2 and Figure 6B). No impact of pressure overload was observed on body and lung weights in any group while TAC induced a decrease in kidney weight independently of the genotype (Table 2). Echocardiography parameters determined just before sacrifice indicate that the decreases in left ventricular ejection fraction (LVEF) and fractional shortening (LVFS) after 8 weeks of TAC were exacerbated by Sirt1 deletion (Table 2 and Figure 6C,D). The clear increases in LVIDs and ESV observed in both TAC groups were also significantly higher in Sirt1ciKO mice (Table 2 and Figure 6E,F). Mitochondrial respiration rates after addition of p-CoA, pyruvate, glutamate succinate, and amytal were reduced by pressure overload (Figure 6G). However, only Sirt1ciKO mice group showed statistically significant decrease in all these parameters when compared with respective sham values. Respiration rates measured from permeabilized cardiac fibers prepared from TAC Sirt1f/f mice exhibited a lower mean value than sham Sirt1f/f mice after the addition of these substrates but a significant difference between these groups was only observed after succinate addition (Figure 6G). Although not significant, respiration rates displayed the same trend towards lower values in TAC Sirt1ciKO group in comparison with TAC Sirt1f/f mice. Analysis of mitochondrial respiration assay combined with large drops of CS and COX activities only in TAC mutant Sirt1ciKO mice (Figure 6H,I) strongly suggests that, in the context of cardiac pressure overload, mitochondrial oxidative capacities were more severely altered when Sirt1 was deleted in cardiomyocytes. Pressure overload is also associated with fibrosis in Sirt1f/f and Sirt1ciKO mice as revealed by Sirius red staining. Perimyocyte interstitial fibrosis was not significantly different in TAC Sirt1ciKO mice compared to TAC controls (Figure 7A), but perivascular fibrosis was significantly more marked in this group than in controls (Figure 7B).
Figure 6.
Cardiac phenotype of cardiac-specific knockout mice after 8 weeks of cardiac pressure overload. (A) Two weeks after tamoxifen injection, mice were subjected to surgery (TAC and sham) and were sacrificed 8 weeks later. (B) Heart weight-to-body weight ratio at sacrifice. (C) Left ventricular ejection fraction just before sacrifice. (D) Left ventricular fractional shortening just before sacrifice. (E) Left ventricular internal dimension at end-systole (LVIDs) just before sacrifice. (F) Telesystolic LV volume (ESV) just before sacrifice. (G) Rate of respiration after successive addition of l-glycerol-3-phosphate (4 mM) (g3p), palmitoyl-CoA and carnitine (100 µM and 2 mM) (p-CoA), pyruvate (1 mM) (pyr), glutamate (10 mM) (glu), succinate (15 mM) (succ), amytal (1 mM), and TMPD-ascorbate (0.5 mM). (H) Citrate synthase (CS) enzymatic activity. (I) Cytochrome c oxidase (COX) enzymatic activity. (n = 8 to 12 per experimental group), ANOVA: ¶ p ≤ 0.05, ¶¶ p ≤ 0.01, ¶¶¶ p ≤ 0.001 for the genotype effect; § p ≤ 0.05, §§ p ≤ 0.01, §§§ p ≤ 0.001 for the TAC effect; i p ≤ 0.05, ii p ≤ 0.01 for the interaction effect. Post hoc Newman–Keuls test: * p ≤ 0.05, ** p ≤ 0.01, *** p ≤ 0.001 Sirt1f/f versus Sirt1ciKO (same surgery); # p ≤ 0.05, ## p ≤ 0.01, ### p ≤ 0.001 sham versus TAC (same genotype).
Table 2.
Anatomical and echocardiographic parameters 8 weeks after induction of pressure overload in Sirt1f/f and Sirt1ciKO mice. BW, Body weight. TL, Tibia length. HW, Heart weight. HW/BW, Heart weight-to-body weight ratio. HW/TL, Heart weight-to-tibia length ratio. LW/BW, Lung weight-to-body weight ratio. LW/TL, Lung weight-to-tibia length ratio. KW/BW, Kidney weight-to-body weight ratio. KW/TL, Kidney weight-to-tibia length ratio. HR, Heart rate. IVSd, Interventricular septal thickness at end-diastole. IVSs, Interventricular septal thickness at end-systole. LVIDd, Left ventricular internal dimension at end-diastole. LVIDs, Left ventricular internal dimension at end-systole. LVWPd, Left ventricular posterior wall thickness at end-diastole. LVWPs, Left ventricular posterior wall thickness at end-systole. EDV, Left ventricular telediastolic volume. ESV, Left ventricular telesystolic volume. LVEF, Left ventricular ejection fraction. LVFS, Left ventricular fractional shortening. SV, Stroke volume. LV, left ventricular mass. CO, Cardiac output. ANOVA: ¶ p ≤ 0.05, ¶¶ p ≤ 0.01, ¶¶¶ p ≤ 0.001 for the genotype effect; § p ≤ 0.05, §§ p ≤ 0.01, §§§ p ≤ 0.001 for the TAC effect; i p ≤ 0.05, ii p ≤ 0.01 for the interaction effect. Post hoc Newman–Keuls test: * p ≤ 0.05, ** p ≤ 0.01, *** p ≤ 0.001 Sirt1f/f vs. Sirt1ciKO (same surgery); $ p ≤ 0.05, $$ p ≤ 0.01, $$$ p ≤ 0.001 Sham vs. TAC (same genotype).
| Sirt1f/f | Sirt1ciKO | 2 Way ANOVA | |||
|---|---|---|---|---|---|
| SHAM | TAC | SHAM | TAC | ||
| BW (g) | 28.3 ± 0.4 | 28.5 ± 0.7 | 28.7 ± 0.5 | 28.7 ± 0.8 | / |
| TL (mm) | 17.6 ±0.1 | 17.8 ± 0.1 | 17.6 ± 0.1 | 18.2 ± 0.1 | / |
| HW (mg) | 153.4 ± 4.4 | 217.7 ± 12.8 $ | 158.9 ± 4.0 | 249.6 ± 24.7 $$$ | §§§ |
| HW/BW (mg/g) | 5.4 ± 0.1 | 7.6 ± 0.3 $$$ | 5.5 ± 0.1 | 8.7 ± 0.7 $$$,* | §§§ |
| HW/TL (mg/mm) | 8.7 ± 0.2 | 12.2 ± 0.7 $$ | 9.0 ± 0.3 | 13.7 ± 1.3$$$ | §§§ |
| LW/BW (mg/g) | 5.2 ± 0.1 | 5.4 ± 0.2 | 5.1 ± 0.1 | 6.2 ± 0.9 | / |
| LW/TL (mg/mm) | 8.3 ± 0.2 | 8.7 ± 0.5 | 8.4 ± 0.2 | 9.9 ± 1.6 | / |
| KW/BW (mg/g) | 12..3 ± 0.3 | 11.2 ± 0.3 $ | 12.1 ± 0.2 | 11.5 ± 0.3 | § |
| KW/TL (mg/mm) | 19.8 ± 0.6 | 17.9 ± 0.6 | 19.7 ± 0.6 | 18.2 ± 0.7 | § |
| HR (bpm) | 542 ± 19 | 541 ± 13 | 514 ± 23 | 543 ± 14 | / |
| IVSd (mm) | 0.72 ± 0.05 | 0.93 ± 0.07 | 0.71 ± 0.04 | 0.83 ± 0.04 | §§ |
| IVSs (mm) | 1.36 ± 0.05 | 1.39 ± 0.07 | 1.34 ± 0.03 | 1.21 ± 0.04 * | / |
| LVIDd (mm) | 3.79 ± 0.09 | 4.03 ± 0.17 | 3.88 ± 0.1 | 4.14 ± 0.11 | § |
| LVIDs (mm) | 2.11 ± 0.08 | 2.79 ± 0.16 $$$ | 2.24 ± 0.08 | 3.19 ± 0.11 $$$,* | ¶, §§§ |
| LVPWd (mm) | 0.71 ± 0.03 | 0.81 ± 0.08 | 0.67 ± 0.03 | 0.87 ± 0.04$ | §§ |
| LVPWs (mm) | 1.25 ± 0.04 | 1.23 ± 0.06 | 1.21 ± 0.04 | 1.14 ± 0.02 | / |
| EDV (mL) | 0.140 ± 0.009 | 0.152 ± 0.014 | 0.149 ± 0.011 | 0.179 ± 0.012 | / |
| ESV (mL) | 0.026 ± 0.003 | 0.061 ± 0.011 $$ | 0.031 ± 0.003 | 0.086 ± 0.008 $$$,* | ¶, §§§ |
| LVEF (%) | 81.7 ± 0.9 | 65.0 ± 2.2 $$$ | 79.5 ± 1.1 | 52.5 ± 2.4 $$$,*** | ¶¶¶, §§§, ii |
| LVFS (%) | 44.5 ± 1.0 | 30.8 ± 1.4 $$$ | 42.4 ± 1.0 | 23.0 ± 1.3 $$$,*** | ¶¶¶, §§§, i |
| SV (mL) | 0.11 ± 0.1 | 0.11 ± 0.01 | 0.12 ±0.01 | 0.09 ± 0.01 | / |
| LV (mg) | 92 ± 4 | 136 ± 17 $$ | 91 ± 5 | 135 ± 10 $$$ | §§§ |
| CO (mL/min) | 61.3 ± 3.9 | 54.3 ± 5.3 | 60 ± 4.6 | 50.8 ± 4 | / |
Figure 7.
Myocardial fibrosis after 8 weeks of pressure overload. (A) Perimyocyte fibrosis analysis by Sirius red staining of subequatorial heart sections. (B) Perivascular fibrosis analysis by Sirius red staining of subequatorial heart sections. (n = 6 to 8 per experimental group), * p < 0.05 Sirt1f/-TACf versus Sirt1ciKO-TAC.
3. Discussion
Despite the extensive study of SIRT1 and its role in the heart over the past twenty years, the consequences of a cardiomyocyte-specific and inducible loss of Sirt1 at adult stage have been poorly addressed. We thus generated a murine model with a truncation of a part of the catalytic domain of Sirt1 (Exon 4) specifically in cardiomyocytes. At baseline, the specific loss of SIRT1 in cardiomyocytes of young adult mice leads to a slight and progressive drop in the left ventricular systolic function. Sixteen weeks after deletion, this cardiac dysfunction was associated with a higher mitochondrial ROS production and marked oxidative damage whereas no alteration in mitochondrial oxidative capacities was noticed. Strikingly, this left ventricular systolic dysfunction gets worse in old KO mice (11 months after tamoxifen injection) even though the mitochondrial propensity to produce more ROS and protein oxidative alterations observed after 16 weeks were not found in these elderly Sirt1 deficient mice. Beyond these results obtained in young and aged mice at basal conditions, we show that the specific loss of Sirt1 in adults exacerbates the cardiac dysfunction induced by pressure overload. This dysfunction is accompanied by more pronounced mitochondrial alterations as well as a more severe perivascular fibrosis.
Sirt1 deletion was induced when mice reached an adult and mature state. This model is attractive since it has been shown that the total and constitutive deficiency of SIRT1 is associated with a very high perinatal mortality and a severe dilated cardiomyopathy when animals survive and reach adult age [26]. Unlike the constitutive deficient models, it makes possible to investigate the consequences of a late loss of functional SIRT1 on heart function and to study the development and progression of HF in the absence of SIRT1. To generate the present specific transgenic mouse line, mice carrying the α-MHC-MerCreMer transgene were used, thereby allowing the expression of a tamoxifen-inducible Cre-recombinase under the control of the mouse alpha-myosin heavy chain promoter (only expressed in the cardiomyocytes of heart). Of note, several studies have reported a cardiac toxicity of the Cre-recombinase depending on the duration and the level of Cre expression [27,28,29]. Thus, mice carrying α-MHC-MerCreMer transgene are likely to develop cardiac dysfunction associated with fibrosis, cell infiltration, and inflammation when treated with repetitive doses of tamoxifen [27,30]. Nevertheless, Lewox and collaborators have demonstrated that a single tamoxifen injection (40 mg/kg) successfully induces robust α-MHC-MerCreMer-dependent recombination without exhibiting any cardiotoxicity. Based on this study and preliminary tests led in our lab (data not shown), a 40 mg/kg/day tamoxifen intraperitoneal injection for 2 consecutive days has been chosen as standard protocol to induce Cre-recombinase activation in adult α-MHC-Cre/flox mice. The total absence of cardiac dysfunction and fibrosis in α-MHC-MerCreMer mice treated with this same dose of tamoxifen confirmed that the alterations observed in the present study were due to the loss of Sirt1 and not to an excessive activation of the Cre-recombinase.
In KO mice under basal conditions, the modest alterations of several cardiac contractile parameters (LVEF, LVFS, IVSs, LVIDs, and LVPWs) suggest a mild ventricular systolic dysfunction. This degradation of cardiac function appears 11 weeks after tamoxifen injection, becomes more important after 11 months of Sirt1 deletion and was not associated with major anatomical changes when compared to control mice. Importantly, the echocardiography analysis revealed that diastolic function is normal and in particular no significant dilatation has been observed during the relaxation of the left ventricle when Sirt1ciKO mice are compared to Sirt1f/f. Interestingly, mice displaying a non-tissue specific complete (homozygous) or partial (heterozygous) constitutive SIRT1 deficiency exhibit a dilated cardiomyopathy at 5 months of age which is not associated with cardiomyocyte hypertrophy [26]; the cross-sectional area of cardiomyocytes was even reduced in this model.
Surprisingly, given that SIRT1 has been extensively studied for its role in energy metabolism [6], the specific cardiac inducible deletion of Sirt1 does not provoke major mitochondrial dysfunction even after 11 months of deletion. This could be explained by the fact that SIRT1 is an enzyme activated by an increase in NAD+ level that is a hallmark of energetic stress. Under standard conditions and in absence of stress, the consequence of the loss of SIRT1 functions could thus be modest, practically imperceptible, and too mild to be evidenced when investigating mitochondrial functions. Moreover, even though the deletion is induced at adult stage, chronic compensatory mechanisms cannot be excluded and could progressively be established, explaining for instance the normalization of mitochondrial ROS production in old mice whereas mitochondria significantly produce more ROS after 16 weeks of Sirt1 deletion. Of note, this increase in ROS production in absence of Sirt1 expression was expected given the role of this enzyme in antioxidant defenses, in particular through FoxOs activation [7]. On the other hand, the mechanism allowing the normalization of these parameters in old mice has not been identified in this study though we could eliminate the hypothesis a compensatory activation of AMPK. The requirement of SIRT1 for AMPK activation via liver kinase B1 (LKB1) deacetylation could explain this absence of AMPK stimulation [31].
In the aforementioned study using constitutive Sirt1 KO mice, cardiac alterations were associated with important mitochondrial alterations such as aberrant mitochondrial structure, lesser mitochondrial content, or reduced mitochondrial-DNA encoded genes [26], suggesting that the myocardium is more damaged in this model. The differences with the present study could be attributed to peripheral disorders caused by a total loss of SIRT1 in other cell types inducing for example a vascular dysfunction that could affect the heart [32,33] or to the roles of SIRT1 in the prenatal and postnatal development of the heart and cardiomyocyte differentiation [34,35,36], thereby inducing mitochondrial defects that will exacerbate cardiac dysfunction. The cardiac specific non-inducible Sirt1 KO murine model developed by Hsu and collaborators in which the expression of the Cre-recombinase is under the control of α-myosin heavy chain promoter (αMHC-Cre) is in line with these hypotheses [37]. Indeed, these cardiac specific KO Sirt1 mice exhibit a normal cardiac phenotype at 3 months of age. This could be explained not only by the fact that Sirt1 ablation is restricted to the heart but also by the low induction of α-MHC expression during the fetal life and early postnatal development in rodents [38]. Therefore, SIRT1 protein level and activity could be sufficiently maintained during prenatal development, thereby giving newborns with normal heart. The expression of Sirt1 would then progressively decline during postnatal development, a period during which α-MHC-Cre expression progressively increases [38,39]. Everything goes as if Sirt1 deletion was progressively induced in the days following birth in this model and the normal cardiac function displayed by these mice at 3 months of age could be due to the slowness of the development of cardiac dysfunction after Sirt1 loss. This is in accordance with the absence of cardiac dysfunction during several weeks after tamoxifen injection in our model. Incidentally, this non-inducible cardiac-specific Sirt1 deletion under α-MHC promoter control makes the mice more sensitive to stress and leads to an increase in oxidative damage in 6-month old mice and to a progressive impaired left ventricle contractility that becomes significant in 12-month old mice [37,40]. This sequence marked by an oxidative stress followed by a more severe degradation of cardiac function in old mice strongly evokes what is observed in our inducible cardiac-specific Sirt1 knockout model, highlighting some similarities between both animal models.
Given the important role of SIRT1 in aging [19,40], particular attention was paid to the inducible cardiac-specific Sirt1 knockout mice at an advanced age. Eleven months after tamoxifen injection, the systolic dysfunction is worsened, as revealed by greater LVIDs and reduced IVSs in comparison with younger mice (14 weeks after tamoxifen injection). Interestingly and as it has been reported in humans [41], old mice show incipient diastolic dysfunction (as shown by higher LVIDd); however, it is not affected by the loss of SIRT1. The slow onset of systolic dysfunction in this model raises questions that will need further investigations. Indeed, although a very slight dysfunction is observed after 11 weeks of Sirt1 ablation, one can wonder to what extent the significant alterations reported in 11-month old mice are the result of small and early cellular homeostasis modifications that slowly impact cardiac function as time goes by, or if they are the consequences of the impairment of phenomena requiring SIRT1 during aging, such as the regulation of endoplasmic reticulum (ER) stress induced by aging [40]. The fact that cardiac dysfunction in elderly mice is not associated with clear degradation in mitochondrial respiration and/or higher oxidative stress is quite unexpected since SIRT1 has largely been described as an important actor of cellular energetics and antioxidant defenses [6]. Nevertheless, the noticeable decrease in CS activity in these old Sirt1ciKO mice suggest perturbations of energy metabolism even though they are not revealed by the assessment of maximal mitochondrial oxidative capacities in permeabilized fibers or mitochondrial protein content. In the light of this study, it seems that alterations of the mitochondria as the main energy producer of the cardiomyocyte would not be the major cause of the cardiac dysfunction following the cardiac-specific deletion of Sirt1 in adults. Additional analysis is required to better understand the profound modulations of energy metabolism in this Sirt1ciKO mice and to determine if other processes could be involved in the establishment of the systolic dysfunction. For instance, SIRT1 is known to be involved in regulation of apoptosis, autophagy, calcium homeostasis, or ER stress [16,42,43], processes that have to be tightly balanced in the healthy heart. Indeed, using a constitutive model of Sirt1 deletion, we have shown recently that SIRT1 is a novel regulatory mechanism for protecting cardiac cells from ER stress [44]. Thus, the potential dysregulation of these processes in this inducible cardiac-specific Sirt1 KO model will be the subject of further studies.
Different models of Sirt1 deficient mice have been described in the past few years and they all displayed an increased sensitivity to cardiac stress when the expression of this protein is reduced or abolished (for review see [7]). Again, in this inducible cardiac-specific Sirt1 KO model, the loss of SIRT1 function exacerbates cardiac dysfunction induced by pressure overload. This result reinforces the idea that SIRT1 plays a protective role when the heart is subjected to a stress. Although a few studies have created doubt regarding the cardioprotective effect of SIRT1 [20,21], the observations made in the present study are perfectly in line with numerous studies showing that SIRT1 is beneficial in the heart facing various stresses such as oxidative stress or ischemia/reperfusion [19,31,37]. As already shown [45], TAC-induced cardiac dysfunction is associated with decreases in mitochondrial respiration rates stimulated by different substrates; these alterations tend to be more pronounced in the absence of SIRT1. The exacerbation of CS and COX activity drop in Sirt1ciKO subjected to TAC clearly demonstrates that SIRT1 is required to maintain cellular energetics under stress conditions. However, although the energy metabolism alterations induced by TAC are more severe in Sirt1ciKO mice, mitochondrial oxidative capacities are not drastically reduced when compared to TAC Sirt1f/f mice, suggesting that the much higher sensitivity to cardiac pressure overload of these mutant mice might be multifactorial.
For instance, it clearly appears that TAC induces fibrosis. It is well known that fibrosis changes tissue properties and negatively affects heart function. Although the loss of Sirt1 was not associated with fibrosis under basal conditions or with higher perimyocyte fibrosis after TAC, the fact that Sirt1ciKO mice heart subjected to TAC developed more perivascular fibrosis could be in part responsible for the hypersensitivity of these mice to pressure overload. The link between SIRT1 and fibrosis has already been studied and it has especially been shown that SIRT1 regulates fibrosis though the TGF-β1/Smad2/3 axis and stimulation of SIRT1 by SRT1720 reduces cardiac fibrosis and improves cardiac function [17]; this could thus be one of the facets of the protective role of SIRT1. In the present genetically modified mice, the specific increase in perivascular fibrosis was unexpected and raises questions. Indeed, concern still persists about how the deletion of Sirt1 in cardiomyocytes results in the higher collagen deposition specifically in the vicinity of the vessels when the heart is stressed. The answer may have to be sought on the side of the impairment of vessel permeability due to some factors released by cardiomyocytes or circulating factors. Indeed, this perivascular fibrosis reminds what was observed and described by Heath and Edwards [46] in pulmonary arterial hypertension in which perivascular fibrosis is preceded by a permeability loss-induced edema. This will need further studies to confirm this mechanism and decipher the role of SIRT1 in the complex interplay between the different cell types of the heart.
Our study also shows that cardiac hypertrophy in response to pressure overload is possible in the absence of SIRT1 even though the hypertrophic role of SIRT1 has been documented [18,20,26,47]. This points out the intricate role of SIRT1 in a process in which it has sometimes been described as a pro-hypertrophic or anti-hypertrophic factor [17,48,49]. This paradoxical effect of SIRT1 in a given process highlights the complexity of its functions that may be modulated according to its activation level. The generation of several models of SIRT1 overexpression illustrates this remark since the phenotype is intimately dependent on the level of SIRT1 [25], thereby highlighting that this deacetylase could act in a dose-effect manner.
Cardiac-specific inducible Sirt1 deletion model proved helpful in deciphering the role of Sirt1 in basal and TAC conditions. Although Sirt1 deletion induced a progressive cardiac dysfunction, this was observed without overt mitochondrial dysfunction. However, the role of SIRT1 in mitochondrial and cardiac function defects was evidenced following pressure overload induced by aortic constriction showing the importance of this pathway in cardioprotection.
4. Materials and Methods
4.1. Animals
Sirt1floxΔE4/floxΔE4 (Sirt1f/f) homozygous mice (kindly provided by David A Sinclair’s group) [36] and heterozygous Myh6-MerCreMer+/− (α-MHC-Cre) mice [50] were crossed to create cardiac-specific and inducible knock-out (Sirt1ciKO) mice (α-MHC-Cre/Sirt1f/f) using Cre-lox technology [51]. Briefly, exon 4 of Sirt1 gene was flanked with two LoxP sites that were recognized and excised by tamoxifen activated Cre recombinase. The cardiac specificity of the deletion was ensured by the fact that the expression of Cre recombinase was under the control of α-MHC. Male Sirt1ciKO mice were injected with tamoxifen (40 mg/kg i.p daily during 2 days) at the age of 8 weeks to induce Sirt1 deletion, thereby generating Sirt1 cardiac-specific inducible mice called Sirt1ciKO in this study. Littermate Sirt1f/f mice not carrying α-MHC-MerCreMer transgene were subjected to the same tamoxifen treatment and were used as control mice. While some of these mice were sacrificed 16 weeks or 11 months after tamoxifen injection with no intervention other than the latter injection, others underwent surgery to induce pressure overload by transverse aortic constriction (TAC) 2 weeks after injection. Anaesthesia was induced by intraperitoneal injection of ketamine (50 mg/kg) and xylazine (8 mg/kg) and silk suture were placed around the aorta using a blunted 27-gauge needle to generate aortic stenosis. Animals were euthanized 8 weeks after surgery by cervical dislocation and hearts were rapidly excised, rinsed in cold calcium-free Krebs solution and weighed. A part of the left ventricle (LV) was immediately used for mitochondrial function assessment and another part was flash frozen in liquid nitrogen for further biochemical determinations. All animal experimental procedures were approved by animal ethics committee of Paris-Sud University, authorized by French government (authorization number: B9201901, APAFIS#2317-2015100615037480 (approved on November 3rd, 2015)) and complied with directive 2010/63/EU of the European Parliament on the protection of animals used for scientific purposes.
4.2. Echocardiography
Echocardiography was done using a 12 MHz transducer (Vivid 7, General Electric Healthcare, General Electric Healthcare, Chicago, IL, USA) under 2.5% isoflurane gas anaesthesia to assess cardiac function. M-mode echocardiography was used to determine left ventricular mass, fractional shortening, and left ventricular ejection fraction.
4.3. Ventricular Cardiomyocyte Isolation
Immediately after sacrifice, the heart from Sirt1f/f or Sirt1ciKO mice four weeks after tamoxifen injection was washed and aorta cannulated in washing buffer (in mM: 113 NaCl, 4.7 KCl, 1.2 MgSO4, 0.6 KH2PO4, 0.6 NaH2PO4, 10 HEPES, 1.6 NaHCO3, 30 Taurine, 20 Glucose) as previously described [52]. The heart was then retrogradely perfused at a constant hydrostatic pressure with digestion buffer (washing buffer added with 80 µg/mL Liberase™ research grade (Roche)) for 6–9 min at 37 °C. The ventricles of the digested heart were excised, shredded into small pieces in the collecting buffer (washing buffer added with 0.2 mM CaCl2 and 5 mg/mL BSA), and gently mechanically shaken to release the cardiomyocytes. After filtration and 10 minutes of decantation, only the cardiomyocyte pellet was kept and resuspended in selecting buffer 1 (washing buffer added with 0.5 mM CaCl2 and 5 mg/mL BSA). After a 10 min period of decantation, a new resuspension/decantation step was carried out in selecting buffer 2 (washing buffer added with 1 mM CaCl2). Finally, the cardiomyocytes pellet obtained was frozen in liquid nitrogen for further analysis.
4.4. Mitochondrial Functional Assays in Permeabilized Cardiac Fibers
Fibers prepared from the left ventricle were permeabilized with saponin as previously described [53] and kept on ice until use in S buffer (in mM: 2.77 CaK2 ethyleneglycol tetraacetic acid (EGTA), 7.23 K2EGTA (100 nM free Ca2+), 6.56 MgCl2 (1 mM free Mg2+), 5.7 Na2ATP, 15 phosphocreatine, 20 taurine, 0.5 dithiothreitol (DTT), 50 K-methane sulfonate (160 mM ionic strength), 20 imidazole, pH 7.1). Measurements aimed at determining mitochondrial parameters were expressed per gram of dry fiber weight.
4.5. Mitochondrial Respiration
Mitochondrial respiratory function was studied in situ in saponin-permeabilized cardiac muscle fibers using a Clarke electrode as previously described (Kuznetsov 2008). A protocol was designed to measure oxygen consumption after successive addition of ADP (2 mM), malate (4 mM), l-glycerol-3-phosphate (4 mM), palmitoyl-CoA and carnitine (100 µM and 2 mM), pyruvate (1 mM), glutamate (10 mM), succinate (15 mM), amytal (an inhibitor of complex I, 1 mM), and the complex IV substrates N, N′, N′-tetramethyl-phenylenediamine dihydrochloride (TMPD)-ascorbate (0.5 mM) (activator of complex IV) to respiration solution (in mM: 2.77 CaK2 ethyleneglycol tetraacetic acid (EGTA), 7.23 K2EGTA (100 nM free Ca2+), 1.38 MgCl2, 3 K2HPO4, 20 taurine, 0.5 dithiothreitol (DTT), 90 K-methane sulfonate and 10 Na-methane sulfonate, 20 imidazole, pH 7.1) at 23 °C. Rates of respiration are given in µmoles O2/min/g dry weight. A second protocol was designed to determine ADP sensitivity of mitochondria and functioning of mitochondrial creatine kinase. For that, respiration was stimulated by the addition of 100 µM ADP in the presence of glutamate (10 mM) and malate (4 mM). Creatine (11 mM) was then added to the chamber and finally the maximal respiration rate (Vmax) was measured by adding 2 mM ADP. The apparent Km values in the presence and absence of creatine were calculated as previously described [39].
4.6. Mitochondrial H2O2 Release
H2O2 released by respiring mitochondria was determined in permeabilized cardiac fibers as previously described [54]. Before measurement, fibers were removed from S buffer and washed in Z1 buffer (in mM: 35 KCl, 1 EGTA, 3 MgCl2, 10 K2HPO4, 10 K-MES, 0.5 mg/mL BSA, pH 7.3 at 4 °C) on ice. Fibers were then incubated in Z2 buffer (in mM: 35 KCl, 1 EGTA, 3 MgCl2, 10 K2HPO4, 10 K-MES, 0.5 mg/mL BSA, pH 7.3 at 37 °C) containing horseradish peroxidase (1.2 U/mL) and Amplex red (20 µM: excitation-emission: 563 to 587 nm) at 37 °C in a fluorescence spectrophotometer (F-2710, Hitachi) under gentle agitation. Baseline fluorescence was measured in the absence of any exogenous respiratory substrates before sequential addition of succinate (5 mM) and ADP (1 mM). Rates of H2O2 production were calculated using a standard curve established under the same experimental conditions.
4.7. Enzyme Activity
Frozen tissue samples were weighed, homogenized (Bertin Precellys 24) in ice-cold buffer (50 mg/mL) containing 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) 5 mM (pH 8.7), EGTA 1 mM, DTT 1 mM, and 0.1% Triton X-100. Activity of citrate synthase (CS) and cytochrome c oxidase (COX) was determined using standard spectrophotometric assays [55,56].
4.8. Immunoblotting
Frozen tissue samples were homogenized (Bertin Precellys 24) in ice cold buffer containing HEPES 50 mM, KCl 50 mM, ethylenediaminetetraacetic acid (EDTA) 1 mM, β-glycerophosphate 5 mM, Triton X-100 0.1%, orthovanadate 1 mM, dithithreitol 1 mM, sodium fluoride 50 mM, Na pyrophosphate 5 mM, phenylmethylsulfonyl fluoride 0.2 mM, and antiprotease cocktail set (Calbiochem 539134). Protein extracts were separated on SDS-polyacrylamide gel (8% to 12%) and then transferred to polyvinylidene difluoride membranes for Western blot. After 1 hour of blocking in PBS containing TWEEN20 (0.1%) and non-fat milk (5%), the membranes were incubated overnight at 4 °C with primary antibody (Table 3). After washing, the membranes were incubated with a secondary antibody coupled with horseradish peroxidase for 1 hour at room temperature and visualized using chemiluminescent substrate (Luminata™ Western Chemiluminescent HRP Substrates, Millipore). Light emission was detected by autoradiography and quantified using an image-analysis system (iBright FL1000, Invitrogen, Waltham, MA, USA).
Table 3.
Antibodies. SIRT1, sirtuin 1. Ac-H1, acetylated histone 1. FoxO1, forkhead box O protein 1. Ac-FoxO1, acetylated forkhead box O protein 1. Ac-p53, acetylated tumor protein 53. CS, citrate synthase. VDAC1, voltage-dependent anion channel 1. ACC, acetyl-CoA carboxylase. pACC, phosphorylated acetyl-CoA carboxylase. AMPK, AMP-activated protein kinase. pAMPK, phosphorylated AMP-activated protein kinase. SOD2, superoxide dismutase 2.
| Antibody | Company | Catalog No | Dilution |
|---|---|---|---|
| SIRT1 | Abcam | ab110304 | 1000 |
| Ac-H1 | Sigma | H7789 | 1000 |
| FoxO1 | Cell signaling | 2880S | 250 |
| Ac-FoxO1 | Santa Cruz | sc49437 | 1000 |
| Ac-p53 | Cell signaling | 2570 | 250 |
| CS | Abcam | ab96600 | 1000 |
| VDAC1 | Cell signaling | 4866 | 1000 |
| ACC | Cell signaling | 3676 | 1000 |
| pACC | Cell signaling | 3661 | 1000 |
| AMPK | Cell signaling | 2532 | 500 |
| pAMPK | Cell signaling | 2531 | 500 |
| SOD2 | Abcam | ab16956 | 500 |
| Total OXPHOS | MitoSciences | MS604 | 250 |
| Actin | Santa Cruz | SC-8432 | 200 |
| Tubulin | Sigma | T6199 | 1000 |
4.9. Histological Analysis
Hearts were fixed in 4% paraformaldehyde, paraffin embedded and serially sectioned (5 μm). Sections were stained with Sirius red. Fibrosis quantification was performed on 3–4 sections (5–10 fields/section) per animal using Image J software.
4.10. Statistical Analysis
For TAC induced-pressure overload, Sirt1f/f or Sirt1ciKO mice were randomly assigned to SHAM and TAC group. All results are expressed as mean ± SEM. Data were analyzed using Statistica software (Statistica, Statsoft Inc., Tulsa, OK, USA). To assess significance, we performed Student’s t test when comparing only 2 groups or two-way ANOVA for independent factors when appropriate for the experimental design followed by Newman–Keuls post-hoc tests to identify significant differences between means. Differences between groups were considered significant if p-value was <0.05.
Acknowledgments
We thank Valérie Domergue, Philippe Mateo and Pauline Robert for preparation of the animals and Claudine Deloménie for her expertise in molecular biology (IFR141-IPSIT). We thank Rodolphe Fischmeister and Ana-Maria Gomez for continuous support. Maryline Moulin’s current address: Université de Paris, BFA, UMR 8251, CNRS, F-75013 Paris, France. Maria-Nieves Sanz’s current address: Department of Cardiovascular Surgery, Inselspital, Bern University Hospital, Bern, Switzerland and Department for BioMedical Research, University of Bern, Bern, Switzerland.
Abbreviations
| HF | Heart failure |
| CVD | Cardiovascular Diseases |
| SIRT | Sirtuin |
| PGC-1α | Peroxisome proliferator-activated receptor gamma coactivator 1α |
| MHC | Myosin heavy chain |
| TAC | Tranverse aortic constriction |
| LV | Left ventricle |
| CS | Citrate synthase |
| COX | Cytochrome c oxidase |
| p53 | Tumour suppressor p53 protein |
| H1 | Histone H1 |
| FoxO1 | Forkhead box protein O1 |
| LVEF | Left ventricular ejection fraction |
| LVFS | Left ventricular fractional shortening |
| LVPWs | End-systolic left ventricular posterior wall thickness |
| LVIDs | End-systolic left ventricular internal diameter |
| ESV | End-systolic volume |
| IVSs | End-systolic interventricular spetal thickness |
| HW | Heart weight |
| BW | Body weight |
| TL | Tibia length |
| AK | Adenylate kinase |
| CK | Creatine kinase |
| VDAC | Voltage-dependent anion channel |
| AMPK | AMP-activated protein kinase |
| ACC | Acetyl-Coa carboxylase |
| ROS | Reactive oxygen species |
| ER | Endoplasmic reticulum |
Author Contributions
Study conception and design: M.-N.S., M.M. (Mathias Mericskay), V.V., R.V.-C., A.G. and J.P.; Acquisition of data: M.-N.S., L.G., M.M. (Maryline Moulin), M.G., C.R.-M., and J.P.; Analysis and interpretation of data: M.-N.S., L.G., M.M. (Maryline Moulin), M.G., C.R.-M., C.L., M.M. (Mathias Mericskay), V.V., R.V.-C., A.G. and J.P.; Drafting of manuscript and critical revision: M.-N.S., L.G., M.M. (Maryline Moulin), M.G., C.R.-M., C.L., M.M. (Mathias Mericskay), V.V., R.V.-C., A.G. and J.P.
Funding
Our laboratory is member of the Laboratory of Excellence LERMIT and is supported by grants from “Fondation pour la Recherche Médicale” (to AG, #DPM20121125546), université Paris-Sud (ERM) and CORDDIM (to RVC, #cod110153). RVC is emeritus scientist at CNRS.
Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Neubauer S. The failing heart--an engine out of fuel. N. Engl. J. Med. 2007;356:1140–1151. doi: 10.1056/NEJMra063052. [DOI] [PubMed] [Google Scholar]
- 2.Li X. SIRT1 and energy metabolism. Acta Biochim. Biophys. Sin. 2013;45:51–60. doi: 10.1093/abbs/gms108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blander G., Guarente L. The Sir2 family of protein deacetylases. Annu. Rev. Biochem. 2004;73:417–435. doi: 10.1146/annurev.biochem.73.011303.073651. [DOI] [PubMed] [Google Scholar]
- 4.Rogina B., Helfand S.L. Sir2 mediates longevity in the fly through a pathway related to calorie restriction. Proc. Natl. Acad. Sci. USA. 2004;101:15998–16003. doi: 10.1073/pnas.0404184101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wood J.G., Rogina B., Lavu S., Howitz K., Helfand S.L., Tatar M., Sinclair D. Sirtuin activators mimic caloric restriction and delay ageing in metazoans. Nature. 2004;430:686–689. doi: 10.1038/nature02789. [DOI] [PubMed] [Google Scholar]
- 6.Tanno M., Kuno A., Horio Y., Miura T. Emerging beneficial roles of sirtuins in heart failure. Basic Res. Cardiol. 2012;107:273. doi: 10.1007/s00395-012-0273-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Matsushima S., Sadoshima J. The role of sirtuins in cardiac disease. Am. J. Physiol. 2015;309:H1375–H1389. doi: 10.1152/ajpheart.00053.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lin J., Handschin C., Spiegelman B.M. Metabolic control through the PGC-1 family of transcription coactivators. Cell Metab. 2005;1:361–370. doi: 10.1016/j.cmet.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 9.Patten I.S., Arany Z. PGC-1 coactivators in the cardiovascular system. Trends Endocrinol. Metab. 2012;23:90–97. doi: 10.1016/j.tem.2011.09.007. [DOI] [PubMed] [Google Scholar]
- 10.Garnier A., Fortin D., Delomenie C., Momken I., Veksler V., Ventura-Clapier R. Depressed mitochondrial transcription factors and oxidative capacity in rat failing cardiac and skeletal muscles. J. Physiol. 2003;551:491–501. doi: 10.1113/jphysiol.2003.045104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sihag S., Cresci S., Li A.Y., Sucharov C.C., Lehman J.J. PGC-1alpha and ERRalpha target gene downregulation is a signature of the failing human heart. J. Mol. Cell. Cardiol. 2009;46:201–212. doi: 10.1016/j.yjmcc.2008.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chaanine A.H., Joyce L.D., Stulak J.M., Maltais S., Joyce D.L., Dearani J.A., Klaus K., Nair K.S., Hajjar R.J., Redfield M.M. Mitochondrial Morphology, Dynamics, and Function in Human Pressure Overload or Ischemic Heart Disease With Preserved or Reduced Ejection Fraction. Circ. Heart Fail. 2019;12:e005131. doi: 10.1161/CIRCHEARTFAILURE.118.005131. [DOI] [PubMed] [Google Scholar]
- 13.Arany Z., Novikov M., Chin S., Ma Y., Rosenzweig A., Spiegelman B.M. Transverse aortic constriction leads to accelerated heart failure in mice lacking PPAR-gamma coactivator 1alpha. Proc. Natl. Acad. Sci. USA. 2006;103:10086–10091. doi: 10.1073/pnas.0603615103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ventura-Clapier R., Garnier A., Veksler V. Transcriptional control of mitochondrial biogenesis: The central role of PGC-1alpha. Cardiovasc. Res. 2008;79:208–217. doi: 10.1093/cvr/cvn098. [DOI] [PubMed] [Google Scholar]
- 15.D’Onofrio N., Servillo L., Balestrieri M.L. SIRT1 and SIRT6 Signaling Pathways in Cardiovascular Disease Protection. Antioxid. Redox. Signal. 2018;28:711–732. doi: 10.1089/ars.2017.7178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gorski P.A., Jang S.P., Jeong D., Lee A., Lee P., Oh J.G., Chepurko V., Yang D.K., Kwak T.H., Eom S.H., et al. Role of SIRT1 in Modulating Acetylation of the Sarco-Endoplasmic Reticulum Ca(2+)-ATPase in Heart Failure. Circ. Res. 2019;124:e63–e80. doi: 10.1161/CIRCRESAHA.118.313865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bugyei-Twum A., Ford C., Civitarese R., Seegobin J., Advani S.L., Desjardins J.F., Kabir G., Zhang Y., Mitchell M., Switzer J., et al. Sirtuin 1 activation attenuates cardiac fibrosis in a rodent pressure overload model by modifying Smad2/3 transactivation. Cardiovasc. Res. 2018;114:1629–1641. doi: 10.1093/cvr/cvy131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alcendor R.R., Kirshenbaum L.A., Imai S., Vatner S.F., Sadoshima J. Silent information regulator 2alpha, a longevity factor and class III histone deacetylase, is an essential endogenous apoptosis inhibitor in cardiac myocytes. Circ. Res. 2004;95:971–980. doi: 10.1161/01.RES.0000147557.75257.ff. [DOI] [PubMed] [Google Scholar]
- 19.Alcendor R.R., Gao S., Zhai P., Zablocki D., Holle E., Yu X., Tian B., Wagner T., Vatner S.F., Sadoshima J. Sirt1 regulates aging and resistance to oxidative stress in the heart. Circ. Res. 2007;100:1512–1521. doi: 10.1161/01.RES.0000267723.65696.4a. [DOI] [PubMed] [Google Scholar]
- 20.Oka S., Alcendor R., Zhai P., Park J.Y., Shao D., Cho J., Yamamoto T., Tian B., Sadoshima J. PPARalpha-Sirt1 complex mediates cardiac hypertrophy and failure through suppression of the ERR transcriptional pathway. Cell Metab. 2011;14:598–611. doi: 10.1016/j.cmet.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oka S., Zhai P., Alcendor R., Park J.Y., Tian B., Sadoshima J. Suppression of ERR targets by a PPARalpha/Sirt1 complex in the failing heart. Cell Cycle. 2012;11:856–864. doi: 10.4161/cc.11.5.19210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pillai J.B., Isbatan A., Imai S., Gupta M.P. Poly(ADP-ribose) polymerase-1-dependent cardiac myocyte cell death during heart failure is mediated by NAD+ depletion and reduced Sir2alpha deacetylase activity. J. Biol. Chem. 2005;280:43121–43130. doi: 10.1074/jbc.M506162200. [DOI] [PubMed] [Google Scholar]
- 23.Lu T.M., Tsai J.Y., Chen Y.C., Huang C.Y., Hsu H.L., Weng C.F., Shih C.C., Hsu C.P. Downregulation of Sirt1 as aging change in advanced heart failure. J. Biomed. Sci. 2014;21:57. doi: 10.1186/1423-0127-21-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Akkafa F., Halil Altiparmak I., Erkus M.E., Aksoy N., Kaya C., Ozer A., Sezen H., Oztuzcu S., Koyuncu I., Umurhan B. Reduced SIRT1 expression correlates with enhanced oxidative stress in compensated and decompensated heart failure. Redox Biol. 2015;6:169–173. doi: 10.1016/j.redox.2015.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kawashima T., Inuzuka Y., Okuda J., Kato T., Niizuma S., Tamaki Y., Iwanaga Y., Kawamoto A., Narazaki M., Matsuda T., et al. Constitutive SIRT1 overexpression impairs mitochondria and reduces cardiac function in mice. J. Mol. Cell. Cardiol. 2011;51:1026–1036. doi: 10.1016/j.yjmcc.2011.09.013. [DOI] [PubMed] [Google Scholar]
- 26.Planavila A., Dominguez E., Navarro M., Vinciguerra M., Iglesias R., Giralt M., Lope-Piedrafita S., Ruberte J., Villarroya F. Dilated cardiomyopathy and mitochondrial dysfunction in Sirt1-deficient mice: A role for Sirt1-Mef2 in adult heart. J. Mol. Cell. Cardiol. 2012;53:521–531. doi: 10.1016/j.yjmcc.2012.07.019. [DOI] [PubMed] [Google Scholar]
- 27.Hall M.E., Smith G., Hall J.E., Stec D.E. Systolic dysfunction in cardiac-specific ligand-inducible MerCreMer transgenic mice. Am. J. Physiol. Heart Circ. Physiol. 2011;301:H253–H260. doi: 10.1152/ajpheart.00786.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Buerger A., Rozhitskaya O., Sherwood M.C., Dorfman A.L., Bisping E., Abel E.D., Pu W.T., Izumo S., Jay P.Y. Dilated cardiomyopathy resulting from high-level myocardial expression of Cre-recombinase. J. Card Fail. 2006;12:392–398. doi: 10.1016/j.cardfail.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 29.Bersell K., Choudhury S., Mollova M., Polizzotti B.D., Ganapathy B., Walsh S., Wadugu B., Arab S., Kuhn B. Moderate and high amounts of tamoxifen in alphaMHC-MerCreMer mice induce a DNA damage response, leading to heart failure and death. Dis. Model. Mech. 2013;6:1459–1469. doi: 10.1242/dmm.010447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lexow J., Poggioli T., Sarathchandra P., Santini M.P., Rosenthal N. Cardiac fibrosis in mice expressing an inducible myocardial-specific Cre driver. Dis. Model. Mech. 2013;6:1470–1476. doi: 10.1242/dmm.010470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang L., Quan N., Sun W., Chen X., Cates C., Rousselle T., Zhou X., Zhao X., Li J. Cardiomyocyte-specific deletion of Sirt1 gene sensitizes myocardium to ischaemia and reperfusion injury. Cardiovasc. Res. 2018;114:805–821. doi: 10.1093/cvr/cvy033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mattagajasingh I., Kim C.S., Naqvi A., Yamamori T., Hoffman T.A., Jung S.B., DeRicco J., Kasuno K., Irani K. SIRT1 promotes endothelium-dependent vascular relaxation by activating endothelial nitric oxide synthase. Proc. Natl. Acad. Sci. USA. 2007;104:14855–14860. doi: 10.1073/pnas.0704329104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maizel J., Xavier S., Chen J., Lin C.H., Vasko R., Goligorsky M.S. Sirtuin 1 ablation in endothelial cells is associated with impaired angiogenesis and diastolic dysfunction. Am. J. Physiol. Heart Circ. Physiol. 2014;307:H1691–H1704. doi: 10.1152/ajpheart.00281.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shin A.N., Han L., Dasgupta C., Huang L., Yang S., Zhang L. SIRT1 increases cardiomyocyte binucleation in the heart development. Oncotarget. 2018;9:7996–8010. doi: 10.18632/oncotarget.23847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sakamoto J., Miura T., Shimamoto K., Horio Y. Predominant expression of Sir2alpha, an NAD-dependent histone deacetylase, in the embryonic mouse heart and brain. FEBS Lett. 2004;556:281–286. doi: 10.1016/S0014-5793(03)01444-3. [DOI] [PubMed] [Google Scholar]
- 36.Cheng H.L., Mostoslavsky R., Saito S., Manis J.P., Gu Y., Patel P., Bronson R., Appella E., Alt F.W., Chua K.F. Developmental defects and p53 hyperacetylation in Sir2 homolog (SIRT1)-deficient mice. Proc. Natl. Acad. Sci. USA. 2003;100:10794–10799. doi: 10.1073/pnas.1934713100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hsu C.P., Zhai P., Yamamoto T., Maejima Y., Matsushima S., Hariharan N., Shao D., Takagi H., Oka S., Sadoshima J. Silent information regulator 1 protects the heart from ischemia/reperfusion. Circulation. 2010;122:2170–2182. doi: 10.1161/CIRCULATIONAHA.110.958033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lompre A.M., Nadal-Ginard B., Mahdavi V. Expression of the cardiac ventricular alpha- and beta-myosin heavy chain genes is developmentally and hormonally regulated. J. Biol. Chem. 1984;259:6437–6446. [PubMed] [Google Scholar]
- 39.Piquereau J., Novotova M., Fortin D., Garnier A., Ventura-Clapier R., Veksler V., Joubert F. Postnatal development of mouse heart: Formation of energetic microdomains. J. Physiol. 2010;588:2443–2454. doi: 10.1113/jphysiol.2010.189670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hsu Y.J., Hsu S.C., Hsu C.P., Chen Y.H., Chang Y.L., Sadoshima J., Huang S.M., Tsai C.S., Lin C.Y. Sirtuin 1 protects the aging heart from contractile dysfunction mediated through the inhibition of endoplasmic reticulum stress-mediated apoptosis in cardiac-specific Sirtuin 1 knockout mouse model. Int. J. Cardiol. 2017;228:543–552. doi: 10.1016/j.ijcard.2016.11.247. [DOI] [PubMed] [Google Scholar]
- 41.Dai D.F., Chen T., Johnson S.C., Szeto H., Rabinovitch P.S. Cardiac aging: From molecular mechanisms to significance in human health and disease. Antioxid. Redox Signal. 2012;16:1492–1526. doi: 10.1089/ars.2011.4179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yamamoto T., Sadoshima J. Protection of the heart against ischemia/reperfusion by silent information regulator 1. Trends Cardiovasc. Med. 2011;21:27–32. doi: 10.1016/j.tcm.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Luo G., Jian Z., Zhu Y., Zhu Y., Chen B., Ma R., Tang F., Xiao Y. Sirt1 promotes autophagy and inhibits apoptosis to protect cardiomyocytes from hypoxic stress. Int. J. Mol. Med. 2019;43:2033–2043. doi: 10.3892/ijmm.2019.4125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Prola A., Silva J.P., Guilbert A., Lecru L., Piquereau J., Ribeiro M., Mateo P., Gressette M., Fortin D., Boursier C., et al. SIRT1 protects the heart from ER stress-induced cell death through eIF2alpha deacetylation. Cell Death Differ. 2016;24:343–356. doi: 10.1038/cdd.2016.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Piquereau J., Moulin M., Zurlo G., Mateo P., Gressette M., Paul J.L., Lemaire C., Ventura-Clapier R., Veksler V., Garnier A. Cobalamin and folate protect mitochondrial and contractile functions in a murine model of cardiac pressure overload. J. Mol. Cell. Cardiol. 2017;102:34–44. doi: 10.1016/j.yjmcc.2016.11.010. [DOI] [PubMed] [Google Scholar]
- 46.Heath D., Edwards J.E. The pathology of hypertensive pulmonary vascular disease; a description of six grades of structural changes in the pulmonary arteries with special reference to congenital cardiac septal defects. Circulation. 1958;18:533–547. doi: 10.1161/01.CIR.18.4.533. [DOI] [PubMed] [Google Scholar]
- 47.Sundaresan N.R., Pillai V.B., Wolfgeher D., Samant S., Vasudevan P., Parekh V., Raghuraman H., Cunningham J.M., Gupta M., Gupta M.P. The deacetylase SIRT1 promotes membrane localization and activation of Akt and PDK1 during tumorigenesis and cardiac hypertrophy. Sci. Signal. 2011;4:ra46. doi: 10.1126/scisignal.2001465. [DOI] [PubMed] [Google Scholar]
- 48.Waldman M., Nudelman V., Shainberg A., Abraham N.G., Kornwoski R., Aravot D., Arad M., Hochhauser E. PARP-1 inhibition protects the diabetic heart through activation of SIRT1-PGC-1alpha axis. Exp. Cell Res. 2018;373:112–118. doi: 10.1016/j.yexcr.2018.10.003. [DOI] [PubMed] [Google Scholar]
- 49.Geng B., Cai Y., Gao S., Lu J., Zhang L., Zou J., Liu M., Yu S., Ye J., Liu P. PARP-2 knockdown protects cardiomyocytes from hypertrophy via activation of SIRT1. Biochem. Biophys. Res. Commun. 2013;430:944–950. doi: 10.1016/j.bbrc.2012.11.132. [DOI] [PubMed] [Google Scholar]
- 50.Sohal D.S., Nghiem M., Crackower M.A., Witt S.A., Kimball T.R., Tymitz K.M., Penninger J.M., Molkentin J.D. Temporally regulated and tissue-specific gene manipulations in the adult and embryonic heart using a tamoxifen-inducible Cre protein. Circ. Res. 2001;89:20–25. doi: 10.1161/hh1301.092687. [DOI] [PubMed] [Google Scholar]
- 51.Feil S., Valtcheva N., Feil R. Inducible Cre mice. Methods Mol. Biol. 2009;530:343–363. doi: 10.1007/978-1-59745-471-1_18. [DOI] [PubMed] [Google Scholar]
- 52.Shioya T. A simple technique for isolating healthy heart cells from mouse models. J. Physiol. Sci. 2007;57:327–335. doi: 10.2170/physiolsci.RP010107. [DOI] [PubMed] [Google Scholar]
- 53.Kuznetsov A.V., Veksler V., Gellerich F.N., Saks V., Margreiter R., Kunz W.S. Analysis of mitochondrial function in situ in permeabilized muscle fibers, tissues and cells. Nat. Protoc. 2008;3:965–976. doi: 10.1038/nprot.2008.61. [DOI] [PubMed] [Google Scholar]
- 54.Piquereau J., Godin R., Deschenes S., Bessi V.L., Mofarrahi M., Hussain S.N., Burelle Y. Protective role of PARK2/Parkin in sepsis-induced cardiac contractile and mitochondrial dysfunction. Autophagy. 2013;9:1837–1851. doi: 10.4161/auto.26502. [DOI] [PubMed] [Google Scholar]
- 55.Wharton D.C., Tzagoloff A. Cytochrome oxidase from beef heart mitochondria. Methods Enzymol. 1967;10:245–250. [Google Scholar]
- 56.Veksler V.I., Kuznetsov A.V., Anflous K., Mateo P., van Deursen J., Wieringa B., Ventura-Clapier R. Muscle creatine kinase-deficient mice. II. Cardiac and skeletal muscles exhibit tissue-specific adaptation of the mitochondrial function. J. Biol. Chem. 1995;270:19921–19929. doi: 10.1074/jbc.270.34.19921. [DOI] [PubMed] [Google Scholar]