Abstract
The synovium plays a key role in the development of osteoarthritis, as evidenced by pathological changes to the tissue observed in both early and late stages of the disease. One such change is the attachment of cartilage wear particles to the synovial intima. While this phenomenon has been well observed clinically, little is known of the biological effects that such particles have on resident cells in the synovium. The present work investigates the hypothesis that cartilage wear particles elicit a pro-inflammatory response in diseased and healthy human fibroblast-like synoviocytes, like that induced by key cytokines in osteoarthritis. Fibroblast-like synoviocytes from 15 osteoarthritic human donors and a subset of 3 non-osteoarthritic donors were exposed to cartilage wear particles, interleukin-1α or tumor necrosis factor-α for 6 days and analyzed for proliferation, matrix production, and release of pro-inflammatory mediators and degradative enzymes. Wear particles significantly increased proliferation and release of nitric oxide, interleukin-6 and −8, and matrix metalloproteinase-9, −10, and −13 in osteoarthritic synoviocytes, mirroring the effects of both cytokines, with similar trends in non-osteoarthritic cells. These results suggest that cartilage wear particles are a relevant physical factor in the osteoarthritic environment, perpetuating the pro-inflammatory and pro-degradative cascade by modulating synoviocyte behavior at early and late stages of the disease. Future work points to therapeutic strategies for slowing disease progression that target cell-particle interactions.
Keywords: Fibroblast-like Synoviocyte, Synovium, Cartilage Wear Particles, Inflammation, Osteoarthritis
Introduction
The development of inflammation and pathological changes to the synovium is commonly observed in early osteoarthritis (OA) and even preceding degradation of the cartilage, indicating that the synovium may play a key role in progression of this disease1–5. In the inflamed synovium, resident fibroblast-like and macrophage-like synoviocytes (FLS and MLS) release pro-inflammatory factors that further stimulate synoviocyte and chondrocyte production of cytokines and degradative enzymes, leading to cartilage degeneration6. Histological changes to the synovium include a pannus-like thickening and increased cellularity, and patients with even mild synovitis can experience pain and stiffness caused by fibrotic shortening5,7. In addition to these changes in tissue composition, surgeons have observed cartilage particles attached to and embedded within the synovium8.
Cartilage wear particles (CWP) are generated by mechanical and chemical degradation of the articulating cartilage surfaces in the OA environment and released into the surrounding synovial fluid. Trauma to the synovial joint such as meniscal tear, ligament ruptures, or cartilage trauma can also disrupt joint biomechanics, increasing instability and surface roughness, and leading to release of cartilaginous debris7,9. Studies of extracted synovial fluid from healthy and osteoarthritic human knees have observed changes in cartilage particle number and physical descriptors such as size and roughness correlated with grade of disease10,11. In lapine, canine, and equine in vivo studies, injection of cartilage particles into the knee joint led to rapid development of synovitis followed by gradual onset of fibrotic synovium thickening and decreased cartilage thickness, similar to traditional animal models of OA such as ACL transection or meniscal release12–14. Our group has also demonstrated that small CWP (<10 μm diameter) both attach to the cell membrane and are phagocytosed, and stimulate proteinase activity, cellular proliferation, collagen synthesis, and nitric oxide production in bovine FLS monolayer cultures15,16.
As FLS are the predominant cell type in healthy synovium, it is important to understand their contribution in the release of degradative factors that precipitate the development of OA. Most work studying the response of FLS to inflammatory stimuli has been done using rheumatoid arthritis (RA) FLS and has focused on chemical mediators such pro-inflammatory cytokines. In response to interleukin-1α (IL-1), diseased FLS have been shown to increase synthesis of lubricant molecules, cytokines, chemokines, and matrix metalloproteinases (MMPs)17,18. The present study compares the effect of both chemical (cytokine) and physical (CWP) factors in the OA environment on FLS from human donors with and without OA. The results presented here address the hypothesis that CWP produce a similar pro-inflammatory and pro-degradative response in OA FLS compared to those of key cytokines associated with OA. A subset of similarly treated FLS from non-OA donors were analyzed to address the secondary hypothesis that CWP are also a relevant pathological stimulus for synoviocytes at the onset of disease. It is anticipated that by identifying CWP as a relevant pathogenic factor in OA, this work will motivate further research into the mechanisms by which CWP affect cell behavior and point towards therapeutics that target this cell-particle interaction to restore normal synoviocyte function and hinder the progression of OA.
Methods
Isolation and Expansion of Human FLS.
Human synovial tissues were obtained from the medial and lateral femoral condyles of 15 patients with OA (9 female and 6 male of mean age 69 ± 10.6 years old and 83% grade 4 OA) during total knee arthroplasty (IRB AAAQ2703). Individual patient age, gender, and grade of OA, indicated as ‘age-gender-grade’, was as follows: 68-F-4, 65-M-4, 78-M-4, 74-F-4, 86-M-4, 56-F-4, 63-F-4, 80-M-4, 60-F-4, 69-F-3, 60-F-4, 47-M-3, 79-F-4, 66-M-4, 78-F-4. Non-osteoarthritic synovial tissue was obtained from the synovial plica of one patient undergoing arthroscopic surgery to repair a torn ACL (18-M-0) and from a patient undergoing total hip arthroplasty after fracture (88-F-0) (IRB AAAQ2703). An additional healthy donor cell line (38-M-0) was purchased from Cell Applications Inc. (San Diego, CA). Within a few hours of retrieval, synovium was digested in collagenase type II (Worthington Biochemical Corporation, Lakewood, NJ) for 2–3 hours with shaking at 37°C. Resulting cell suspensions were filtered through 40 μm porous nylon mesh (Corning Inc, Corning, NY). Viable cells were counted and plated at 2.64×103 cells/cm2. Cells were expanded in α-Minimum Essential Medium (α-MEM, Life Technologies, Carlsbad, CA) containing 10% FBS, 1% antibiotic-antimycotic and 5 ng/mL fibroblast growth factor-2 (FGF-2, Life Technologies)19 to obtain a homogenous FLS population of sufficient quantity. To create dense synovial monolayers, FLS at passage 3 were plated at 1.25×104 cell/cm2 and cultured as previously described15. Flow cytometry was performed as previously described to confirm that isolated cells maintained the characteristic FLS phenotype20.
Cartilage Wear Particle Preparation and Characterization.
Expired cartilage tissue grafts were obtained from the Musculoskeletal Transplant Foundation (Edison, NJ) and stored at 4 °C until use. As described previously15, cartilage samples were submerged in sterile PBS and manually abraded with waterproof 120 grit sandpaper (McMaster-Carr, Elmhurst, IL). Effort was made to ensure that no bone was abraded during the process, and residual sandpaper grit was removed gravimetrically and via subsequent filtration with 70 μm, 40 μm, and 10 μm porous nylon mesh filters to achieve a pure dispersion of sub-10 μm diameter cartilage wear particles (CWP). An aliquot of the resulting solution was diluted in PBS and counted and sized using a Multisizer 4 Coulter Counter (Beckman Coulter, Brea, CA)21.
Cartilage Wear Particle Treatment.
The dosage of CWP was optimized prior to comparison with cytokines, utilizing high concentrations of particles to yield consistent attachment to FLS in static culture. Concentrations of up to 6.25*106 CWP/mL were cultured for 6 days with confluent FLS monolayers isolated from 4 different OA donors to determine a dosage that would result in a measurable cell response. Using the MTT assay (Sigma-Aldrich, St. Louis, MO) as previously described15, the lowest particle dose that significantly increased metabolic activity was identified for each donor. From these results, 3.125*106 CWP/mL was selected for future experiments to ensure that as many donors as possible responded to the treatment. FLS from 15 OA donors were then treated for 6 days with 10 ng/mL interleukin-1α (IL-1), 10 ng/mL tumor necrosis factor-α (TNF), or 3.125*106 CWP/mL. A subset of available non-OA donors was treated and analyzed in parallel for comparison to diseased FLS. Fluorescent imaging was performed with a custom quasi-3D microscope22, allowing for visualization of CWP stained with 20 μM dichlorotriazinylaminofluorescein (DTAF, Sigma-Aldrich), attached to FLS (34-M) loaded with 20 μM Fura-Red (Life Technologies), from top an side angles to visually confirm attachment of the particles to the cell surface after 6 days in culture. The same dye loading techniques were employed on CWP-treated FLS on glass slides and imaged in 2D to perform counts of particle attachment per individual cell, averaged for 120 cells pooled across three replicate slides.
Biochemistry.
FLS monolayers and media samples were harvested at day 6 and stored at −20°C prior to lyophilization. Monolayers and cartilage particles were digested overnight in 0.5 mg/mL of proteinase K (MP Biomedicals, Santa Ana, CA) in 50 mM Tris-buffered saline containing 1 mM EDTA, 1 mM iodoacetamide and 10 μg/ml pepstatin A (Sigma-Aldrich)23. DNA content was determine using the PicoGreen Kit (Life Technologies). Nitric oxide (NO), hyaluronan (HA) and prostaglandin-E2 (PGE2) released into the media was quantified using the Greiss Reagent for nitrite quantification (Life Technologies), Hyaluronan Quantikine ELISA and the PGE2 Parameter (R&D Systems Inc., Minneapolis, MN), respectively. For all biochemical measurements on particle treated samples, the measured value of an equivalent amount of CWP alone was subtracted out to determine the contribution of the FLS alone. All biochemical products were normalized by respective monolayer DNA for analysis.
Luminex Analysis.
A 25 μl aliquot was taken from the pooled media of non-OA and OA donors from each experimental condition and analyzed in duplicate using a Luminex multiplex human cytokine and chemokine immunoassay kit (EMD Millipore, St. Louis, MO) for IL-6 and IL-8, or a Luminex multiplex human MMP assay kit (R&D Systems) for MMP’s 1, 3, 9, 10, and 13. For xMAP assays, media samples were mixed with anti-chemokine, anti-cytokine, or anti-MMP monoclonal antibody-charged 5.6 μm polystyrene microspheres. Following overnight incubation at 4 °C, streptavidin-phycoerythrin and then a biotinylated polyclonal secondary antibody was added to the samples. The median fluorescence intensity was used to determine the concentration (pg/mL) of each chemokine and cytokine. All media release products were normalized by respective monolayer DNA for analysis.
Statistical Analysis.
Data sets were tested for normality and homogeneity using the Kolmogorov-Smirnov Test and Levene’s test, respectively (R, version 3.3.2). Non-normal and non-homogeneous data were adjusted using a log transformation prior to analysis. For MTT dosage studies, a subset of four donors with four replicates for each particle dose were analyzed separately with a one-way ANOVA and Tukey’s post-hoc test to determine significant differences between dose within each donor. For all monolayer and media analyses (DNA, HA, NO, PGE2, chemokines, cytokines, and MMPs), n = 7–8 replicates were analyzed and then pooled to generate an average value for each donor with a given treatment. OA and non-OA donor groups were analyzed separately using a one-way ANOVA with Tukey’s post-hoc test to determine significant differences between treatments. All one-way ANOVAs were performed as repeated measures to account for variation between donors across treatment groups. Outliers were identified by the ROUT method and when identified in one treatment group, used as an exclusion criterion for that donor in all groups while maintaining a minimum of 6 donors for a given analysis. For ELISA and Luminex assays, the lowest point on the curve was used when samples were below the range of the standard curve for statistical analysis. All statistical analyses were performed in GraphPad Prism 7 with α = 0.05 and data reported as the mean ± standard deviation.
Results
Optimization of Particle Dosage.
Human cartilage wear particles generated from allografts as described had an average diameter of 0.887 ± 0.004 μm and were observed to attach to the FLS cell surface after 6 days in culture (Figure 1). The first four donors listed above were used for dose optimization to equally represent available gender and ages (designated donors A, B, C, and D). Donors A, B, C and D showed a significant increase in metabolic activity over untreated controls at minimal concentrations of 1.25*106 particles/mL, 3.125*106 particles/mL, 6.25*105 particles/mL, and 1.25*106 particles/mL respectively (p = 0.014, 0.0003, 0.0036, and 0.0004, Figure 2a–d). The loading dose of 3.125*106 particles/mL utilized for subsequent studies was found to result in an attachment density of 3.4 ±1.9 particles per individual cell (not shown).
Figure 1.
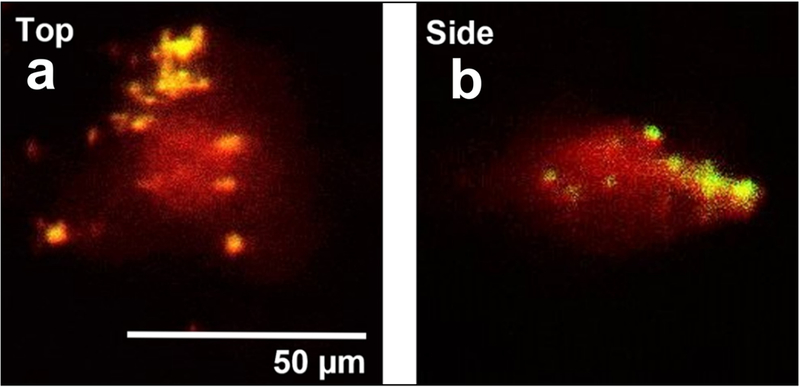
Representative images showing CWP (yellow) attachment to surface of healthy human FLS (34-M) (red) after 6 days in static culture. Top and side views acquired via quasi-3D microscopy.
Figure 2.
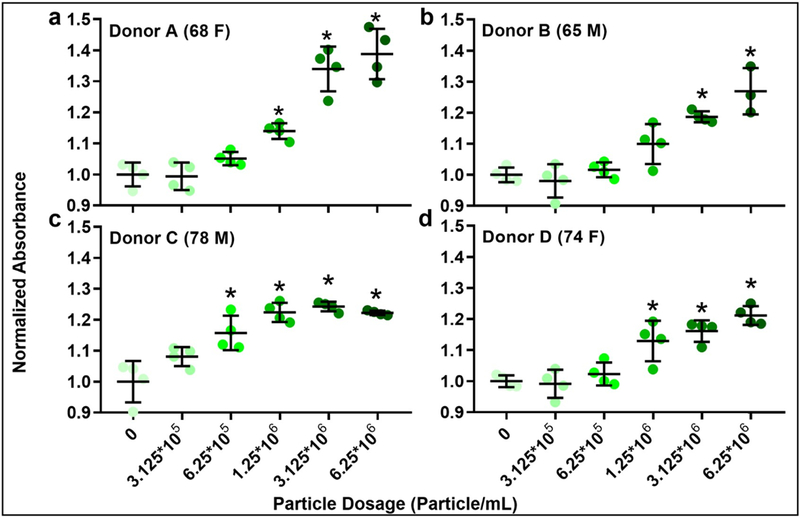
MTT assay for assessing the effect of increasing concentrations of cartilage wear particles on OA FLS from 4 different donors (age and gender as indicated). N = 3–4/group. *p < 0.05 vs. control (0 particle/mL).
Biochemical Analysis.
CWP treatment significantly increased proliferation in OA FLS versus untreated controls (p = 0.001), as quantified by DNA content of the monolayer. DNA was similarly increased with IL-1 and TNF treatments, with significance only in the latter (p = 0.0393, Figure 3). All media release products were normalized by monolayer DNA content for their respective groups. Secretion of the key synovial lubricant hyaluronan (HA) by OA FLS was unaffected by CWP treatment, but significantly increased by both IL-1 and TNF versus untreated controls (p = 0.0001 and 0.0004) and CWP (p = 0.0001 and 0.0004, Figure 4).
Figure 3.
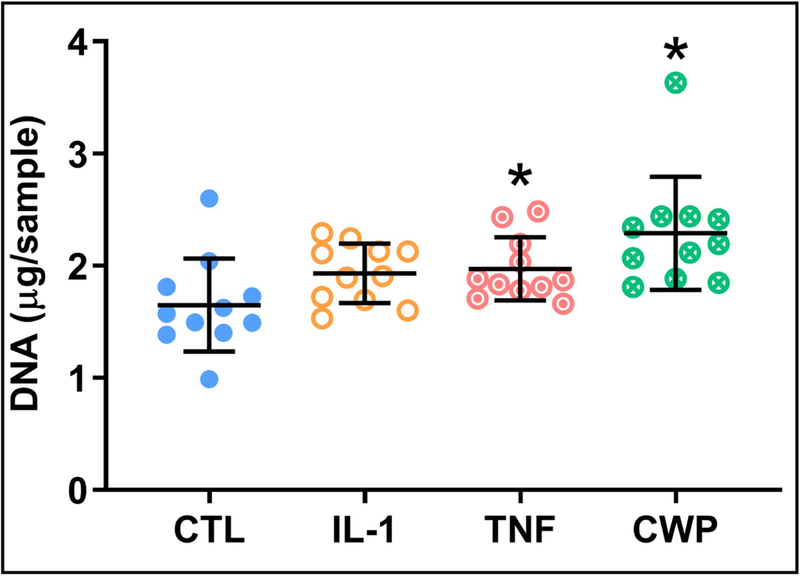
Analysis of DNA content for OA FLS monolayers after 6 days of treatment with IL-1α (IL-1), TNF-α (TNF), or cartilage wear particles (CWP), with untreated controls (CTL). N = 11 donors. *p < 0.05 vs. CTL.
Figure 4.
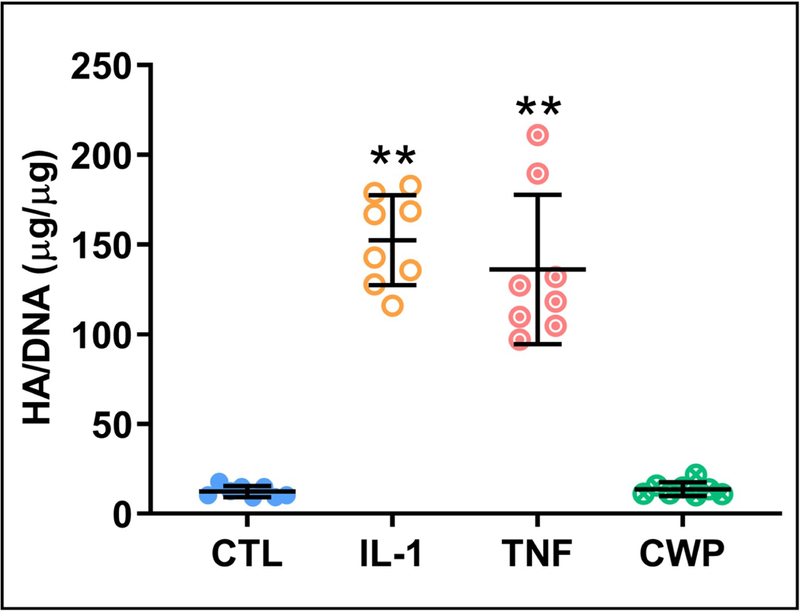
Release of a key synovial fluid lubricant, hyaluronan (HA) (normalized by DNA), from OA FLS monolayers after 6 days of treatment with IL-1α (IL-1), TNF-α (TNF), or cartilage wear particles (CWP), with untreated controls (CTL). N = 8 donors. **p < 0.05 vs. CTL and CWP.
Release of the pro-inflammatory mediator NO by OA FLS was significantly increased with CWP treatment (p =0.0003), and to an even greater degree by IL-1 and TNF, which were significantly higher than controls (p = 0.0001) and CWP (p = 0.0007 and 0.0003, Figure 5a). Similarly, while CWP treatment slightly increased release of the pro-inflammatory mediator PGE2, IL-1 and TNF significantly increased release over both controls (p = 0.0001 and 0.0318) and CWP (p = 0.0001 and 0.032). IL-1 treatment demonstrated the greatest effect on PGE2 synthesis, with a significant increase over TNF-treated levels as well (p = 0.0031, Figure 5b).
Figure 5.
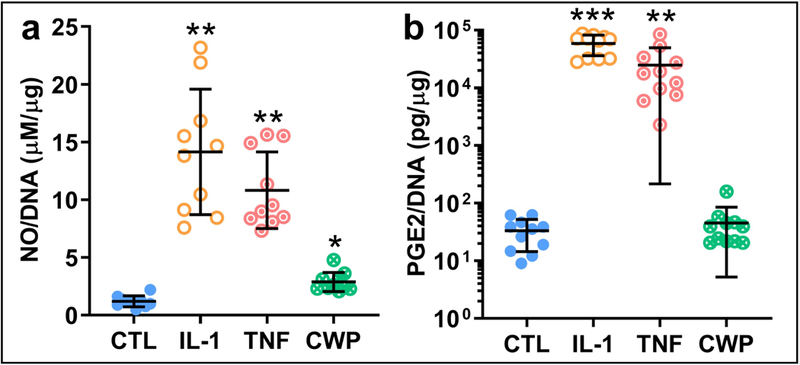
Release of two key inflammatory stress mediators, NO (a) and PGE2 (b) (normalized by DNA), from OA FLS monolayers treated for 6 days with IL-1α (IL-1), TNF-α (TNF), or cartilage wear particles (CWP), with untreated controls (CTL). N = 10 (a) and 11 (b) donors. *p < 0.05 vs. CTL, **p < 0.05 vs. CTL and CWP. ***p < 0.05 vs. CTL, CWP, and TNF.
CWP treatment significantly increased release of both IL-6 and −8 by OA FLS (p = 0.0334 and 0.0285, Figure 6a, b). IL-1 and TNF significantly increased IL-6 versus both controls (p = 0.001 and 0.0022) and CWP (p = 0.0019 and 0.0043, Figure 6a). Similarly, IL-1 and TNF significantly increased IL-8 versus both controls (p = 0.0014 and 0.0013) and CWP (p = 0.0019 and 0.0015, Figure 6b).
Figure 6.
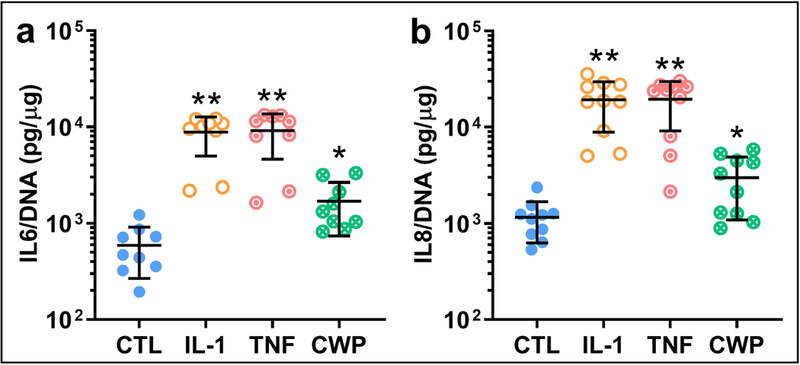
Release of two isoforms of the key pro-inflammatory cytokine interleukin, IL-6 (a) and IL-8 (b) (normalized by DNA), from OA FLS monolayers treated for 6 days with IL-1α (IL-1), TNF-α (TNF), or cartilage wear particles (CWP), with untreated controls (CTL). N = 9 (a) and 10 (b) donors. *p < 0.05 vs. CTL, **p < 0.05 vs. CTL and CWP.
Expression of matrix-metalloproteinases (MMP) followed similar trends to the pro-inflammatory mediators, with CWP increasing expression moderately over controls while IL-1 and TNF treatment resulted in significantly higher increases (Figure 7a–e). CWP treatment resulted in significantly increased release of MMP-9, −10, and −13 versus controls (p = 0.0137, 0.0003, and 0.0059). IL-1 significantly increased release of MMP-1, −3, −9, −10, and −13 versus controls (p = 0.0012, 0.0002, 0.0426, 0.0001, and 0.0192) and CWP (p = 0.0013, 0.0002, 0.0481, 0.0001, and 0.0195). Similarly, TNF significantly increased release of MMP-1, −3, −9, −10, and −13 versus controls (p = 0.0003, 0.0283, 0.0361, 0.001 0.018) and CWP (p = 0.0003, 0.0292, 0.0454, 0.0011, 0.0186). IL-1 was again the more potent cytokine, with significant increases in MMP-1, −3, and −10 versus TNF treatment (p = 0.0061, 0.0025, 0.004, Figure 7a–e).
Figure 7.
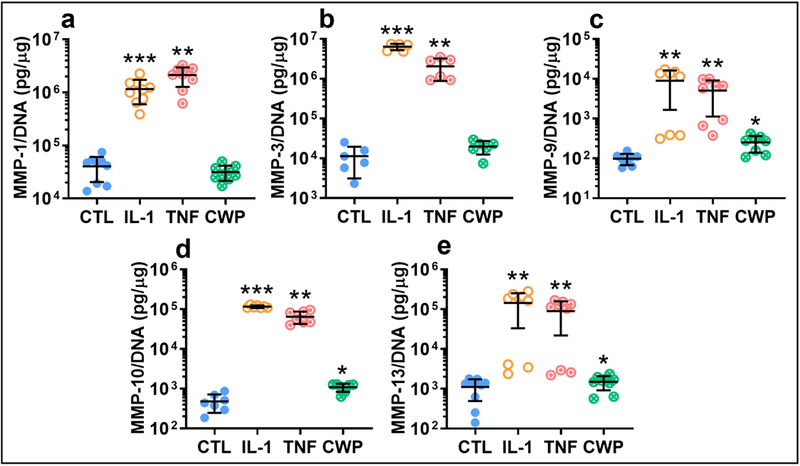
Release of isoforms of the degradative enzyme MMP-1 (a), −3 (b), −9 (c), −10 (d), and −13 (e) (normalized by DNA) from OA FLS monolayers treated for 6 days with IL-1α (IL-1), TNF-α (TNF), or cartilage wear particles (CWP), with untreated controls (CTL). N = 9 (a), 6 (b), 8 (c), 7 (d), and 9 (e) donors. *p < 0.05 vs. CTL, **p < 0.05 vs. CTL and CWP, ***p < 0.05 vs. CTL, CWP, and TNF.
In FLS from a subset of non-OA donors, similar trends to OA FLS were observed for most monolayer and media release measurements, though significance was limited by the small number of available donors (p > 0.1). In non-OA FLS, CWP alone resulted in a slight, though non-significant, increase in DNA compared to controls (Figure 8a). While there were no significant differences in HA synthesis between treated and untreated non-OA FLS, a heightened sensitivity of OA FLS to cytokine treatment was suggested by larger fold-increases in HA over controls, comparing OA and non-OA donor groups for IL-1 (13 vs. 3-fold) and TNF treatments (12 vs. 2-fold) (Figure 8b). Similar though nonsignificant trends were observed in non-OA FLS for both NO and PGE2 release (PGE2 shown as representative, Figure 8c), and production of cytokines (IL-6 shown as representative, Figure 8d). Similar trends to OA FLS were also observed across all MMPS for non-OA donors, while significant differences were observed for MMP-1, with IL-1 significantly higher than controls and CWP treatment (p = 0.0017 and 0.0021, Figure 8e).
Figure 8.
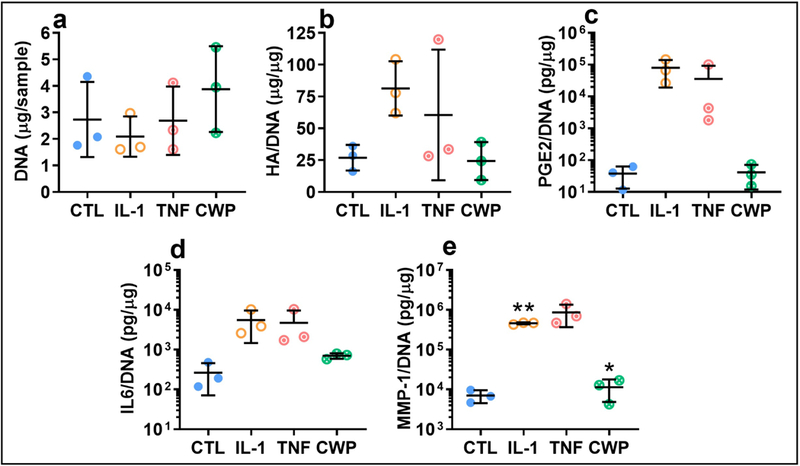
Analysis of key outcome measures in FLS from a set of healthy donors, treated for 6 days with IL-1α (IL-1), TNF-α (TNF), or cartilage wear particles (CWP), with untreated controls (CTL). Similar trends were observed in response to cytokine and particle treatment for monolayer content of DNA (a), and release of hyaluronan (b), inflammatory stress markers (eg. PGE2, c), cytokines (eg. IL-6, d), and MMPs (eg. MMP-1, e). N = 3 donors. *p < 0.05 vs. CTL. **p < 0.05 vs. CTL and CWP.
Discussion
A key role for the synovium in the pathophysiology of OA is indicated by the observation of synovitis in many OA patients even at the earliest stages of disease and often preceding degradation of the articular cartilage itself. Once cartilage begins to degrade, the release of wear particles into the synovial fluid and their attachment to the synovial intima8,10,11. Unlike chemical mediators commonly associated with the disease such as pro-inflammatory cytokines, the cellular response to this physical factor of the OA environment has not been widely studied. The present work demonstrates that treatment of FLS monolayers with CWP increased proliferation and release of pro-inflammatory mediators, chemokines, cytokines, and MMPs. These results support the hypothesis that CWP are a relevant pathologic mediator that elicit a pro-inflammatory response in diseased FLS like that of key cytokines known to contribute to the degradative cascade in the OA environment.
Previous work has characterized CWP from human synovial fluid aspirates that range in size from 5–100 μm diameter25, and correlate varying shape and surface properties to OA disease stage10,11,24. The present work employed a Coulter Counter to isolate and characterize a well-defined distribution of relatively small particles generated by manual abrasion, that represented the lower end of this physiologic range. Previous work in the juvenile bovine model has shown that this size particle interacts directly with FLS by both attachment to the cell surface and phagocytosis15. Future work will characterize the size and shape of wear particles generated in vitro, the influence of these characteristics on the mode of interaction with the cell, and the resulting effect on downstream function. Further experiments will utilize blocking agents, such as cytochalasin to inhibit phagocytosis, to determine the relative contribution of phagocytosis versus membrane attachment in mediating the cellular response to wear particles.
In addition to the macroscopic particles themselves, mechanical wear both in vitro and in vivo may also release protein fragments from the extracellular matrix as well as cellular debris including nucleic acids that can act as chemical stimuli. While previous work in bovine FLS has demonstrated that media conditioned with cartilage particles alone does not elicit the same response as direct interaction15, future work will continue to explore the effect of the entire range of release products generated by cartilage wear on human FLS.
Concentrations of cartilage wear particles in human synovial fluid have been reported at 100–500 particles/mL, but were limited by the collected sample volume and inability to count particles below 5μm diameter25. Relatively little is known therefore, about the physiologic concentrations of wear particles at progressing stages of osteoarthritis or the quantity and distribution of accumulated particles as they attach to the synovial intima. The present work employed a concentration of 3.125*106 particles/mL to represent a supraphysiologic dose that would elicit a timely and measurable response in short-term in vitro culture, in keeping with similar practices when studying cytokines like interleukin, where 10 ng/mL is commonly employed in comparison to the physiologic level of less than 10 pg/mL26–30. Considering estimations of the volume of articular cartilage present in the human knee31, the dosage employed in this study represents only 0.001% of the total cartilage volume being applied to each monolayer. Given the degree of cartilage degeneration observed in late stage OA, which can progress through the full thickness of the tissue, this amount could feasibly degrade into wear particles and accumulate on an area of the synovial intima that corresponds to the cellularity of the monolayers cultured in this study. Accumulation of large numbers of cartilage particles on the synovial intima could be further driven by increased synovial fluid effusion and turnover during OA. Even considering the reported concentrations of 100–500 particles/ml, with estimations of total volume and turnover rates for synovial fluid32,33, millions of particles could accumulate on the synovium over the course of years of continuous cartilage degradation as OA progresses. It will be important for future work to understand the long-term fate of wear particles in the joint, with respect to rates of generation and attachment to the synovial intima versus clearing via phagocytosis and enzymatic breakdown.
Without the benefit of constant mixing during motion and perfusive efflux in the native joint, the high loading concentration employed in vitro was necessary to overcome the low loading efficiency of adding the particles over monolayers in static culture, whereby this high concentration resulted in an average of 3.4 ± 1.9 particles attached to a single cell based on fluorescent imaging and previous analysis in the bovine model15. Similar to previous findings by our group in juvenile bovine FLS15, attachment of these particles increased proliferation in human OA FLS (Figure 3). While previous work reports increased proliferation with IL-1 and TNF as well, this effect may be dependent on specific donor and culture conditions. Studies reporting increases in FLS proliferation with IL-1 are typically derived from OA or RA donors with pooled cell sources that are plated at low density34,35, while those observing a mixed response have used FLS from non-arthritic patients36.
Our previous work in the juvenile bovine model has demonstrated that IL-1 and TNF treatments increase FLS synthesis of hyaluronan (HA), a key lubricating component of synovial fluid15, while others have shown that IL-1 stimulates HA production in FLS from healthy human donors36. While no effect of CWP was observed, the present study confirms this result in non-OA and OA human FLS and demonstrates a trend of higher cytokine-induced HA release with in the latter (Figure 4, Figure 8b), suggesting a heightened sensitivity to cytokines with disease state. In addition to considering the quantity of HA synthesized, the structure of HA found in early and late stage OA may be altered due to production of shorter-chain molecules by FLS or degradation once released into the synovial fluid and should be considered in future work17,37.
CWP treatment increased FLS production of several key pro-inflammatory and degradative factors including NO (Figure 5a), IL-6 and −8, (Figure 6), and MMP-9, −10, and 13 (Figure 7). NO is a catabolic factor upregulated in OA that induce MMP production by FLS and chondrocytes and can also inhibit aggrecan and collagen synthesis38–40. The cytokines IL-6 and IL-8 are also upregulated in OA and induce MMP production41,42. Concentrations of both cytokines have been correlated to disease stage43, patient pain, and size, adhesion, and roughness of cartilage wear particles24. Several matrix-metalloproteinases (MMP) have been identified as potential biomarkers of synovial inflammation in both early and late stage OA, namely MMP-1, 3, 9, 10, and 13. Elevated serum and synovial fluid concentrations of MMP-1 and 3, which target collagen and proteoglycans, have been measured in all stages of OA. Differences in synovial fluid MMP-9 and −13, which target collagen type II and IV, have also been reported between OA patients and healthy volunteers44. Expression of MMP-10, which degrades proteoglycans and collagen, is also increased in OA synovium and cartilage tissue45.
While treatment with the cytokines IL-1 and TNF resulted in larger-fold increases of all the release products analyzed, the ability of CWP to significantly modulate many of these same pro-inflammatory products over control values suggests they may also play a pathologic role in the OA environment. Given the particle-induced increase in release of the pro-inflammatory cytokines IL-6 and IL-8 observed here, possible synergistic effects between CWP and cytokines will be explored in future work seeking to determine relative contributions of these two mediators in the progression of OA.
In non-OA FLS, though statistical power in comparing between treatment groups and with similarly-treated OA FLS was restricted by limited donor availability, the observation of similar trends across all release products analyzed supports the secondary hypothesis that CWP also act as a pathogenic mediator to FLS at the onset of disease, or after acute injury, when cartilage wear particles are first present in the synovial fluid. It is possible that the heightened response of OA FLS to both chemical and physical mediators, such as the larger increases over baseline in HA secretion, are due to an inflammatory phenotype that persists in vitro, as previously demonstrated in OA chondrocytes16, and influences mechanisms of interaction with CWP. Future work will seek a wider collection of non-OA tissues, including from healthy donors without any acute injury or orthopedic pathologies, to more fully elucidate the impact of both chemical and physical mediators on FLS that are representative of early versus late stage OA, as well as further explore the influence of donor age and gender.
The present work employed a biomimetic culture model that allowed for the evaluation of isolated cell types from the synovium of human donors with and without OA, and the comparison of their response to key pro-inflammatory mediators found in the native disease environment. FLS alone were studied first, as they are the primary resident cell type of the synovium, and to isolate the role these cells play in synovial inflammation and the release of degradative factors. The results presented support a key role for FLS by demonstrating that this monoculture model recapitulates the release of pro-inflammatory products observed in OA in the absence of the immune cells found in the native arthritic environment6,46. This simple in vitro model allows for addition of complexity beyond donor disease state and exposure to a variety of chemical and physical inflammatory mediators. Future work will include other isolated cell types such as MLS, which have a more phagocytic role and thus may interact differently with cartilage wear particles of small size.
Our group has shown in bovine FLS that small cartilage wear particles are both phagocytosed and attach to the cell surface15. Integrin engagement may be a key mechanism of this interaction between synoviocytes and small particles as well as larger particles that cannot be phagocytosed15,47–49. While this study focused on relatively small wear particles, future work will investigate FLS and MLS response to a wider range of particle size, as such particle physical characteristics have been correlated to disease progression10,11,24 and may therefore further elucidate the contribution of cellular response to CWP at different stages of OA. By demonstrating the role of cartilage wear particles as a pro-inflammatory mediator of FLS behavior, this work motivates further study into the mechanisms of interaction between synoviocytes and particles. Such work will further the understanding of the role of CWP in OA pathophysiology, and point towards therapeutic interventions that disrupt these mechanisms and restore normal synovial function, thereby mitigating progression of the disease.
Acknowledgements
Additional assistance with critical revision of article draft and approval of final submission was provided by Dr. Andrea R. Tan.
Role of the Funding Sources
Funding for this work was provided by the Orthopedic Scientific Research Foundation, and National Institutes of Health (1R01AR068133, T32 AR059038), and was utilized the in the acquisition of research materials and compensation of graduate research assistants during the course the studies described herein. These sponsors had no role in the conception and design of the study, collection, analysis, or interpretation of the data, nor the decision to submit, preparation, or final approval of the article.
Footnotes
Conflict of Interest
The authors have no conflicts of interest to disclose.
References
- 1.Hügle T & Geurts J What drives osteoarthritis?—synovial versus subchondral bone pathology. Rheumatology 1461–1471 (2016). doi: 10.1093/rheumatology/kew389 [DOI] [PubMed] [Google Scholar]
- 2.Atukorala I et al. Synovitis in knee osteoarthritis: a precursor of disease? Ann. Rheum. Dis. 75, 390–395 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Felson DT et al. Synovitis and the risk of knee osteoarthritis: the MOST Study. Osteoarthritis Cartilage 24, 458–464 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sarmanova A, Hall M, Moses J, Doherty M & Zhang W Synovial changes detected by ultrasound in people with knee osteoarthritis – a meta-analysis of observational studies. Osteoarthritis Cartilage 24, 1376–1383 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wenham CYJ & Conaghan PG The role of synovitis in osteoarthritis. Ther. Adv. Musculoskelet. Dis. 2, 349–359 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bartok B & Firestein GS Fibroblast-like synoviocytes: key effector cells in rheumatoid arthritis. Immunol. Rev. 233, 233–255 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scanzello CR & Goldring SR The role of synovitis in osteoarthritis pathogenesis. Bone 51, 249–257 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lloyd-Roberts GC The Role of Capsular Changes in Osteoarthritis of the Hip Joint. J. Bone Jt. Surg. 35B, 627–642 (1953). [DOI] [PubMed] [Google Scholar]
- 9.Stachowiak GW, Stachowiak GB & Campbell P Application of numerical descriptors to the characterization of wear particles obtained from joint replacements. Proc. Inst. Mech. Eng. [H] 211, 1–10 (1997). [DOI] [PubMed] [Google Scholar]
- 10.Kuster MS, Podsiadlo P & Stachowiak GW Shape of wear particles found in human knee joints and their relationship to osteoarthritis. Rheumatology 37, 978–984 (1998). [DOI] [PubMed] [Google Scholar]
- 11.Podsiadlo P, Kuster M & Stachowiak GW Numerical analysis of wear particles from non-arthritic and osteoarthritic human knee joints. Wear 210, 318–325 (1997). [Google Scholar]
- 12.Evans CH, Mazzocchi RA, Nelson DD & Rubash HE Experimental arthritis induced by intraarticular injection of allogenic cartilaginous particles into rabbit knees. Arthritis Rheum. 27, 200–207 (1984). [DOI] [PubMed] [Google Scholar]
- 13.Chrisman DO, Fessel MJ & Southwick WO Experimental Production of Synovitis and Marginal Articular Exotoses in the Knee Joints of Dogs. Yale J. Biol. Med. 37, 409–417 (1965). [PMC free article] [PubMed] [Google Scholar]
- 14.Kuroki K, Cook CR & Cook JL Subchondral bone changes in three different canine models of osteoarthritis. Osteoarthritis Cartilage 19, 1142–1149 (2011). [DOI] [PubMed] [Google Scholar]
- 15.Silverstein AM et al. Toward understanding the role of cartilage particulates in synovial inflammation. Osteoarthritis Cartilage 25, 1353–1361 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Silverstein AM et al. Transient expression of the diseased phenotype of osteoarthritic chondrocytes in engineered cartilage: PHENOTYPE PERSISTENCE OF OA CHONDROCYTES. J. Orthop. Res. 35, 829–836 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Blewis ME et al. Interactive Cytokine Regulation of Synoviocyte Lubricant Secretion. Tissue Eng. Part A 16, 1329–1337 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Furuzawa-Carballeda J, Macip-Rodríguez PM & Cabral AR Osteoarthritis and rheumatoid arthritis pannus have similar qualitative metabolic characteristics and pro-inflammatory cytokine response. 7 [PubMed] [Google Scholar]
- 19.Sampat SR et al. Growth Factor Priming of Synovium-Derived Stem Cells for Cartilage Tissue Engineering. Tissue Eng. Part A 17, 2259–2265 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alegre-Aguarón E et al. Growth Factor Priming Differentially Modulates Components of the Extracellular Matrix Proteome in Chondrocytes and Synovium-Derived Stem Cells. PLoS ONE 9, e88053 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oungoulian SR et al. Articular Cartilage Wear Characterization With a Particle Sizing and Counting Analyzer. J. Biomech. Eng. 135, 024501 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Qiu J et al. Theoretical Analysis of Novel Quasi-3D Microscopy of Cell Deformation. Cell. Mol. Bioeng. 5, 165–172 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Riesle J, Hollander AP, Langer R, Freed LE & Vunjak‐Novakovic G Collagen in tissue‐engineered cartilage: Types, structure, and crosslinks. J. Cell. Biochem. 71, 313–327 (1998). [DOI] [PubMed] [Google Scholar]
- 24.Wang M, Ketheesan N & Peng Z Investigations of wear particles and selected cytokines in human osteoarthritic knee joints. Proc. Inst. Mech. Eng. [H] 228, 1176–1182 (2014). [DOI] [PubMed] [Google Scholar]
- 25.Hakshur K et al. The effect of hyaluronan injections into human knees on the number of bone and cartilage wear particles captured by bio-ferrography. Acta Biomater. 7, 848–857 (2011). [DOI] [PubMed] [Google Scholar]
- 26.Barakat AF, Elson CJ & Westacott CI Susceptibility to physiological concentrations of IL-1β varies in cartilage at different anatomical locations on human osteoarthritic knee joints. Osteoarthritis Cartilage 10, 264–269 (2002). [DOI] [PubMed] [Google Scholar]
- 27.Elson CJ et al. Cytokines and focal loss of cartilage in osteoarthritis. Rheumatology 37, 106–107 (1998). [DOI] [PubMed] [Google Scholar]
- 28.Pritchard S & Guilak F Effects of interleukin-1 on calcium signaling and the increase of filamentous actin in isolated and in situ articular chondrocytes. Arthritis Rheum. 54, 2164–2174 (2006). [DOI] [PubMed] [Google Scholar]
- 29.Lima EG et al. Differences in Interleukin-1 Response Between Engineered and Native Cartilage. Tissue Eng. Part A 14, 1721–1730 (2008). [DOI] [PubMed] [Google Scholar]
- 30.McNulty AL, Rothfusz NE, Leddy HA & Guilak F Synovial fluid concentrations and relative potency of interleukin-1 alpha and beta in cartilage and meniscus degradation: IL-1 ISOFORMS IN THE JOINT. J. Orthop. Res. 31, 1039–1045 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ateshian GA, Soslowsky LJ & Mow VC Quantitation of articular surface topography and cartilage thickness in knee joints using stereophotogrammetry. J. Biomech. 24, 761–776 (1991). [DOI] [PubMed] [Google Scholar]
- 32.Heilmann H-H, Lindenhayn K & Walther H-U Das Synovia-Volumen gesunder und arthrotischer menschlicher Kniegelenke*. Z. Für Orthop. Ihre Grenzgeb. 134, 144–148 (1996). [DOI] [PubMed] [Google Scholar]
- 33.Brown T, Laurent U & Fraser. Turnover of hyaluronan in synovial joints: elimination of labelled hyaluronan from the knee joint of the rabbit. Exp. Physiol. 76, 125–134 (1991). [DOI] [PubMed] [Google Scholar]
- 34.Gitter BD, Labus JM & Lees SL Characteristics of human synovial fibroblast activation by IL-ifi and TNFa. 5 [PMC free article] [PubMed] [Google Scholar]
- 35.Nishimoto N et al. IL-6 inhibits the proliferation of fibroblastic synovial cells from rheumatoid arthritis patients in the presence of soluble IL-6 receptor. Int. Immunol. 12, 187–193 (2000). [DOI] [PubMed] [Google Scholar]
- 36.Pulkki K The effects of synovial fluid macrophages and interleukin-1 on hyaluronic acid synthesis by normal synovial fibroblasts. Rheumatol. Int. 6, 121–125 (1986). [DOI] [PubMed] [Google Scholar]
- 37.Kosinska MK et al. Articular Joint Lubricants during Osteoarthritis and Rheumatoid Arthritis Display Altered Levels and Molecular Species. PLOS ONE 10, e0125192 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Scher JU, Pillinger MH & Abramson SB Nitric oxide synthases and osteoarthritis. Curr. Rheumatol. Rep. 9, 9–15 (2007). [DOI] [PubMed] [Google Scholar]
- 39.Sellam J & Berenbaum F The role of synovitis in pathophysiology and clinical symptoms of osteoarthritis. Nat. Rev. Rheumatol. 6, 625–635 (2010). [DOI] [PubMed] [Google Scholar]
- 40.Goldring MB & Berenbaum F The Regulation of Chondrocyte Function by Proinflammatory Mediators: Prostaglandins and Nitric Oxide. Clin. Orthop. 427, S37–S46 (2004). [DOI] [PubMed] [Google Scholar]
- 41.Blasioli DJ & Kaplan DL The Roles of Catabolic Factors in the Development of Osteoarthritis. Tissue Eng. Part B Rev. 20, 355–363 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Takahashi A, de Andrés MC, Hashimoto K, Itoi E & Oreffo ROC Epigenetic regulation of interleukin-8, an inflammatory chemokine, in osteoarthritis. Osteoarthritis Cartilage 23, 1946–1954 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wanner J et al. Proteomic profiling and functional characterization of early and late shoulder osteoarthritis. Arthritis Res. Ther. 15, R180 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Heard BJ et al. Matrix metalloproteinase protein expression profiles cannot distinguish between normal and early osteoarthritic synovial fluid. BMC Musculoskelet. Disord. 13, (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Davidson RK et al. Expression profiling of metalloproteinases and their inhibitors in synovium and cartilage. Arthritis Res. 8, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bustamante MF, Garcia-Carbonell R, Whisenant KD & Guma M Fibroblast-like synoviocyte metabolism in the pathogenesis of rheumatoid arthritis. Arthritis Res. Ther. 19, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Evans CH, Mears DC & Cosgrove JL Release of neutral proteinases from mononuclear phagocytes and synovial cells in response to cartilaginous wear particles in vitro. Biochim. Biophys. Acta BBA - Gen. Subj. 677, 287–294 (1981). [DOI] [PubMed] [Google Scholar]
- 48.Sarkissian M & Lafyatis R Integrin Engagement Regulates Proliferation and Collagenase Expression of Rheumatoid Synovial Fibroblasts. J. Immunol. 162, 1772–1779 (1999). [PubMed] [Google Scholar]
- 49.Lowin T & Straub RH Integrins and their ligands in rheumatoid arthritis. Arthritis Res. Ther. 13, 244 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]


