Abstract
Microalgal biotechnology has gained considerable importance in recent decades. Applications range from simple biomass production for food and animal feed to valuable products for fuel, pharmaceuticals, health, biomolecules and materials relevant to nanotechnology. There are few reports of the exploration of wider microalgae biodiversity in the literature on high value microalgal compounds, however, because it is believed that there is little to be gained in terms of biomass productivity by examining new strains. Still, without diversity, innovation in biotechnology applications is currently limited. Using microalgal diversity is a very promising way to match species and processes for a specific biotechnological application. In this context, three benthic marine diatom strains (Entomoneis paludosa NCC18.2, Nitzschia alexandrina NCC33, and Staurosira sp NCC182) were selected for their lipid production and growth capacities. Using PAM fluorometry and FTIR spectroscopy, this study investigated the impact of nitrogen repletion and depletion as well as light intensity (30, 100, and 400 μmol.photons.m-2.s-1) on their growth, photosynthetic performance and macromolecular content, with the aim of improving the quality of their lipid composition. Results suggest that under high light and nitrogen limitation, the photosynthetic machinery is negatively impacted, leading cells to reduce their growth and accumulate lipids and/or carbohydrates. However, increasing lipid content under stressful conditions does not increase the production of lipids of interest: PUFA, ARA and EPA production decreases. Culture conditions to optimize production of such fatty acids in these three original strains led to a balance between economic and ecophysiological constraints: low light and no nitrogen limitation led to better photosynthetic capacities associated with energy savings, and hence a more profitable approach.
Introduction
Production of microalgae is gaining interest for the supply of biofuels, feedstock and for added value compounds; several thousand species of microalgae have now been screened for lipid production, including diatoms [1–8]. Diatoms are described as producers of biologically-active compounds: antibiotics, enzyme inhibitors, pharmacologically active compounds and toxins. The most studied molecules are eicosapentaenoic acid (EPA) and arachidonic acid (ARA), which belong to the polyunsaturated fatty acids (PUFA). Fatty acids such as EPA and ARA are considered pharmacologically important for dietetics and therapeutics. They have been used for prophylactic and therapeutic treatment of chronic inflammations (e.g. rheumatism, skin diseases, and inflammation of the mucosa of the gastrointestinal tract) [9]. They are also believed to have a positive effect on cardio-circulatory diseases, coronary heart diseases, atherosclerosis, hypertension, cholesterol and cancer [10]. Fatty acids like saturated fatty acid (SFA) and monounsaturated fatty acid (MUFA) are associated with triacylglycerols (TAG), which are preferred substrates for biodiesel storage production by transesterification [11]. It is essential to identify suitable strains of microalgae for mass cultivation and improve their lipid content. Previous studies have shown that the quantity and quality of lipids can vary as the result of changes in growth condition i.e., nutrient concentrations [12–17], temperature [18–20] and light intensity [12,13,15,19,21]. It is quite common to encounter oil levels reaching 20–50% of microalgal dry weight, although this varies from species to species, and can reach up to 90% of the dry mass when cells are subject to physiological stress conditions, such as nutrient limitation or photo-oxidative stress, or an unfavorable environment [22]. Furthermore, nutrient stress, e.g., nitrogen deprivation, phosphorus starvation, or iron supplementation can also enhance the lipid content in many microalgae species [23–26]. Nitrogen limitation or deprivation is a strategy widely used to elicit this response. Lipid biosynthesis varies within the different diatom species, their growth stages and the environmental parameters [27,28].
Nutrient availability is of considerable importance to growth and primary production in microalgae. For diatoms, typical nutrient limitation in nature involves nitrogen, phosphorus, and silicate [29]. Diatom cells undergoing nutrient limitation change their cellular composition; nitrogen limitation can lead to a decrease in protein content and a relative increase in carbohydrate and/or lipid storage [30,31]. Nitrogen limitation can also result in a decrease in growth rate and photosynthetic efficiency [29,32–34]. The biochemical changes measured in microalgae are linked to changes in physiological parameters and are known to be species-specific. Photosynthetically active radiation can also affect primary production, protein synthesis and other cellular functions [21,35,36].
In the selection of the most adequate species or strains for bioactive lipid production, many parameters need to be considered, such as the ability of microalgae to grow in a wide range of environmental conditions. In this study, three benthic marine diatom species: Entomoneis paludosa NCC18.2, Nitzschia alexandrina NCC33, and Staurosira sp. NCC182, never previously studied except by Cointet et al. (2019) [6], were selected for their high growth capacity, high lipid productivity and/or capacity to produce interesting molecular compounds. The purpose of this study was to examine and characterize their photosynthetic capacity and enhance their lipid productivity for potential future applications. The effects of different light conditions were tested: 30 μmol photon.m-2.s-1 (Low Light: LL); 100 μmol photon.m-2.s-1 (Medium Light: ML) and 400 μmol photons.m-2.s-1 (High Light: HL) in combination with different nitrogen concentrations: 882 μM (N+) and 88.2 μM (N-). The aim of this study is to determine the effects of changing nitrogen concentration and light conditions on growth rate, photosynthetic capacity and intracellular lipid accumulation and composition. Macromolecular composition in relation to culture conditions was evaluated using Fourier transform infrared spectroscopy (FTIR) and photosynthetic capacity obtained from Pulse Amplitude Modulated (PAM) fluorometry measurements, complemented by pigment analysis. Finally, lipid quality was estimated by GC-MS analysis on transesterified lipid extracts to reveal fatty acid (FA) composition and thus to see whether culture conditions impacted production of lipids of interest such as EPA and ARA.
Materials and methods
Diatom cultures and experimental design
The three strains, Entomoneis paludosa NCC18.2, Nitzschia alexandrina NCC33, and Staurosira sp. NCC182 were obtained from the Nantes Culture Collection (NCC). Each strain was grown using artificial seawater medium [37] enriched with F/2 medium major nutrients. This artificial medium made it possible to control the initial amounts of nutrients and other elements (Table 1), thus avoiding the variability of natural seawater composition [37]. Two different media were used in the experiments: N+ and N-, with initial NaNO3 quantities of 882 μM and 88.2 μM, respectively (Table 1). Before inoculation, the medium was sterilized by filtration (0.2 μm) to avoid the nutrient precipitation that often occurs with autoclaving. Culture stocks were acclimated and maintained for 5 weeks in 250-mL Erlenflasks filled with 150 mL medium N+ under low (LL), medium (ML), and high (HL) light conditions: 30 100–400 μmol photons m-2 s-1, respectively. A light-dark cycle was applied (14–10 H), and temperature maintained at 16°C. To prevent pH augmentation due to diatom growth [38], cultures were gently bubbled with continuous sterile filtered air (Sartorius, 0.2 μm PTFE) and aseptic glass delivery tube by air compressor.
Table 1. Artificial seawater medium composition.
| Solutions | Final concentration (μM) | |
|---|---|---|
| Anhydrous salts | NaCl | 336 × 103 |
| Na2SO4 | 28.8 × 103 | |
| KCl | 9.3 × 103 | |
| NaHCO3 | 3.3 × 103 | |
| KBr | 840 | |
| H3BO3 | 48.5 | |
| NaF | 71.5 | |
| Hydrous salts | MgCl2.6H2O | 54.6 × 103 |
| CaCl2.2H2O | 10.5 × 103 | |
| SrCl2.6H2O | 63.8 | |
| Major nutrients | NaH2PO4.H2O | 36.2 |
| NaNO3 | 882 (N+) or 88.2 (N-) | |
| Na2SiO3.9H2O | 106 | |
| Trace metal solution (F/2) | 1 mL | |
| Vitamin solution (F/2) | 1 mL | |
At the start of the experiment, fresh medium was inoculated using cells from stock cultures that were centrifuged (5 min at 3500 g) and washed with N- artificial seawater medium to avoid salt concentration. The initial concentration of cells was set at 30.000 cells.mL-1 in 150 mL sterilized medium for both nitrogen depleted (N-): 88.2 μM, and nitrogen replete (N+): 882 μM (N+) media, and cultures were exposed to the three light conditions: LL, ML and HL. Samples were collected daily for growth rate estimation by cell counting until the cultures reached stationary growth phase. During exponential growth phase, the photosynthetic parameters (maximum PSII quantum efficiency, Fv/Fm and light saturation parameter Ek) were estimated using PAM-fluorometry [39]. Thus, culture volumes necessary to harvest 107 cells were sampled and centrifuged (10 min at 4500 g) for biochemical composition determination: pigment composition by spectrophotometry [40] and protein, carbohydrate and lipid composition by HTSXT-FTIR [6]. The total cells in culture were harvested by filtration (0.7 μm) to estimate DW and perform lipid extraction following Bligh and Dyer [41]. Media free of cells were recuperated during the filtrations to then estimate nutrient composition using a DIONEX ion-chromatography [42].
Nutrients
Before inoculation, 50 mL of fresh medium were sampled. Medium samples were also taken by filtration during the cell harvesting process. In this case, 50 mL of filtered medium were frozen at -80°C for conservation until measurement of nitrogen, phosphate and silica content. Residual phosphate (PO43- μM) and nitrate (NO3- μM) concentrations were analyzed according to the method described by Aminot and Kérouel (2007). After being thawed, samples were centrifuged (10 min at 3000 g) and colorimetric assays were carried out on the supernatant with an AA3 autoanalyzer (SEAL Analytical®), which allows automatic nutrient determination by continuous flow spectrophotometry. Determination of PO43- with this method relies on the reaction of molybdate with antimony to form the phosphomolybdic complex. This complex is then reduced by ascorbic acid to form a blue compound that is measured at λ = 820 nm. Nitrate is initially reduced to NO2 using a cadmium column treated with copper in the presence of two reagents (ammonium chloride and sodium hydroxide). Total NO2 is then determined by reaction with sulfanilamide to produce a diazo that reacts in turn with N-naphtyl-thlenediamine in an acid medium and gives a pink coloration, assayed at λ = 540 nm.
Residual silica (SiO32- μM) was determined by colorimetric assay according to Hansen & Koroleff (1999) [43], based on the formation of silicomolybdic acid, which was then reduced to produce an intense blue color assayed at 870 nm. Nutrient consumption by cells during growth was calculated as the difference between initial and final concentrations for each major nutrient (-NO3-, -PO43-, -SiO32-). Ammonium (NH4+ μM) was also analyzed at the end of the growth period because it is known to be produced by bacteria and can be utilized by diatoms for growth [44]. This nutrient was measured to estimate its potential use by cells and its possible interaction with nitrogen conditions tested. NH4+ was analyzed with the colorimetric indophenol blue method adapted to seawater assayed at 630 nm (Koroleff 1970) directly on cell-free media without freezing [45] because ammonium was unstable in the samples meaning they had to be processed in the shortest time possible [46]. Final NH4+ concentration was used to detect whether production occurred during growth.
Growth rate estimation
Daily triplicate samples of 2 mL were fixed with lugol and counted (n ≥ 300) using a Neubauer hemocytometer and an optical microscope (OLYMPUS CH40, ×400). Following Cointet et al. (2019), maximum cell concentration (A expressed as a log) and latency time (λ in days) were determined by fitting growth kinetics data with a Gompertz model using Matlab software (Eq 1):
| (1) |
with A: maximum cell concentration in the natural logarithm of the biomass; μmax: Maximum growth rate (day− 1); λ: Latency (days).
The relative growth rate (day-1) was calculated following Eq 2, where Nt1 is the number of cells on the first day of the exponential phase (t1), Nt2 is the number of cells at the end of the exponential phase (t2), and T (days) is the interval between t1 and t2.
| (2) |
Determination of photosynthetic parameters
During the exponential growth phase (“mid” sampling), and at the end, when the stationary phase was reached (“end” sampling), 2 mL aliquots of the cultures were sampled for photosynthetic parameter estimations using a Water-PAM fluorometer (cuvette version, Walz GmbH, Effeltrich, Germany). Seven hours after the light was switched on, an aliquot was sampled. It was first dark adapted for 1 hour, and then introduced into the 10 mm quartz glass cuvette of the PAM fluorometer controlled by WinControl-3 software. Measurements were performed on the same amount of Chl a and cultures were diluted in N- or N+ artificial sea water medium depending on the treatment. Maximum PSII quantum efficiency (Eq 3) was then measured using a 600 ms saturating pulse (2500 μmol photons.m-2.s-1):
Where F0 is the minimum fluorescence yield for dark adapted cells, Fm, the maximum fluorescence yield for dark adapted cells during the saturating flash and Fv the variable fluorescence.
| (3) |
To provide detailed information on the overall photosynthetic performance of the microalgae [39], RLCs were constructed using nine 30s incremental irradiance steps (0, 38, 52, 77, 121, 179, 259, 391 and 580 μmol photons m-2s-1) and calculating relative electron transport rate (rETR, Eq 4) through PSII for each level of actinic light [47]:
| (4) |
where PAR was the actinic irradiance (= Photosynthetic Active Radiation from 400 to 700 nm), (F’m-F)/F’m was the effective quantum yield of PSII with F, the fluorescence yield for a given PAR intensity, F’m the maximum fluorescence for a given PAR intensity during the saturating flash and 0.5 was a multiplication factor based on the assumption that 50% of the absorbed quanta are distributed to PSII [47].
rETR value data were fitted using the Eilers and Peeter model [48] in order to obtain the initial slope (α), the light saturation parameter (Ek) and the maximum relative electron transport rate (rETRmax). Ek was derived from rETRmax and α (Eq 5):
| (5) |
Pigment composition
To estimate chlorophyll a (Chl a) and the total amount of carotenoid pigments (Car) per cell, 2 mL of culture were sampled and centrifuged at 11200 g. for 5 min at the end of the growth phase. After supernatant removal, pigments were extracted by adding 2 mL of methanol (99.9%) to the pellet. To remove cell debris, methanol suspension was centrifuged for 5 min at 11200 g. Absorbances at 665, 632, and 480 nm were measured by spectrophotometer (SHIMADZU, UV-1900) on the clean supernatant to calculate pigment content, expressed in pg.cell-1 following [40]:
| (6) |
| (7) |
Lipid analyses
Fourrier-transform infrared spectroscopy (FTIR)
FTIR spectra acquisition was performed according to recommendations of Coat et al. (2014) [49] adapted by Cointet et al. (2019) [6] for benthic diatom strains. The sample preparation and FTIR device are detailed in Cointet et al. FTIR spectra were recorded in transmission mode on 5 μL dried harvested cells (107 cells.mL-1) allowing to quickly obtain their biochemical signatures expressed in terms of total lipids, total proteins and total carbohydrates. Absorbance spectra were collected between 4 000 cm-1 and 700 cm-1 with 30 scans and averaged. The spectra were analyzed by the integral ratios method [50]. The lipid signature was associated with the ester bond (Eb) signal (~1740 cm-1), whereas the carbohydrate signature was associated with the C-O-C signal of the polysaccharides (~1200–900 cm-1) [37] and the protein signature associated with the amide II band (~1540 cm-1) of the N-H of the amides associated with the proteins. To estimate the relative content of lipids, carbohydrates and proteins, their respective integral area was standardized with the total spectrum area and expressed in arbitrary units (a.u.) as recommended by Cointet et al. (2019) [6] (Eq 8).
| (8) |
Where s = lipids (~1740 cm-1) or carbohydrates (~1159 cm-1) or amide II (~1540 cm-1).
Gravimetry
Finally, biomass dry weight was estimated by filtering the remaining algal suspension (47 mm, 0.7 μm pore diameter). Filters with cells were washed using 10 mL of ammonium formiate (68 g.L-1) to remove salt, frozen at -80°C, freeze-dried and weighed (DW in mg). Crude lipid extract (CLE in mg) was estimated following Cointet et al. (2019) and lipid content (LC) was estimated in mg.L-1. Lipid rate (LR) and lipid productivity (LP) were finally calculated as follows:
| (9) |
With CLE and DW expressed in mg
| (10) |
With LC expressed in mg.L-1 and μ in day-1
Fatty acid composition
Fatty acid composition was determined by direct transesterification. A mixture of 800 μL hydrochloric methanol 7.5% and 100 μL chloroform were added to the CLE and heated to 80°C for 5 H. After the reaction was complete, the samples were cooled to room temperature and mixed with 500 μL hexane, which allowed phase separation. The organic phase, which contained fatty acid methyl ester (FAME), was collected, dried using an anhydrous sodium sulfate salt, filtered and evaporated using nitrogen. FAME was analyzed using gas chromatograph mass spectrometry (GC-MS; Hewlett Packard HP 7890 –GC System / Agilent Technologies, Santa Clara, CA, USA) linked to a mass detector (HP 5975C– 70 eV). The sample was injected (1μL injection volume) into a SLB™-5ms column (60 m × 0.25 mm × 0.25 μm). The carrier gas was helium at a flow rate of 1mL.min-1. The injector and detector temperatures were set at 250°C and 280°C, respectively, and the temperature column was programmed with a temperature held at 170°C for 4 min and then increasing to 300°C at 3 °C.min-1. To identify and quantify the FAME, each FA identification was confirmed by comparing mass spectra and retention data with a library build from previous analyses and commercial standards. Chromatogram peak areas were analyzed and quantified using WSEARCH32 software.
Data processing
Each treatment (N+, N-, LL, ML and HL) was done in triplicate for each strain. Data were expressed as the mean of each triplicate ± standard deviation (SD). Two-way ANOVAs with a 5% significant level were carried out after checking normality and homogeneity using Shapiro-Wilk and equal variance tests. Tukey’s least significant tests was used to determine which experimental conditions were significantly different. All statistical analyses were carried out using SigmaPlot software.
Results
Nutrients
Initial major nutrient concentrations (Table 2) were respected for both media (N+ and N-) and for all species. For E. paludosa and N. alexandrina, in N+ conditions, nitrate consumption (NO3-) was higher under HL and ML than under LL (p < 0.001) (Table 3). As expected, under N- conditions, all the NO3- available in the medium (~83 μM) was consumed in HL, ML, and LL. For Staurosira sp., under N+ conditions, the same amount of nitrogen was consumed in LL, HL, and ML (p = 0.30); however, as for the two other species, nitrogen consumption was higher in N+ than in N- (p < 0.05).
Table 2. Initial major nutrient concentrations at the start of the experiment for the different species and culture media (N+ and N-).
| NO3- (μM) | PO43- (μM) | SiO32- (μM) | ||||
|---|---|---|---|---|---|---|
| N+ | N- | N+ | N- | N+ | N- | |
| E. paludosa | 828.54 | 83.56 | 37.31 | 36.31 | 117.08 | 114.89 |
| N. alexandrina | 868.63 | 74.53 | 37.54 | 37.73 | 111.04 | 108.33 |
| Staurosira sp. | 894.66 | 81.62 | 39.59 | 39.34 | 117.70 | 116.87 |
Table 3. Nutrient consumption by the end of the experiment (triplicate mean ± SD) for all species and treatments, with the corresponding two-way ANOVA results (n = 3).
| Species | Statistical results |
Light | Nitrogen | NO3- (μM) |
PO43- (μM) |
NH4+ (μM) |
SiO32- (μM) |
|---|---|---|---|---|---|---|---|
| E. paludosa | LL | N+ | 285.86 ± 57.79 | 19.45 ± 2.55 | 0.08 ± 0.10 | 116.18 ± 0.86 | |
| N- | 83.37 ± 0.04 | 23.50 ± 0.22 | < LOD | 114.51 ± 0.12 | |||
| ML | N+ | 430.89 ± 33.12 | 28.81 ± 0.89 | 2.71 ± 1.43 | 116.84 ± 0.15 | ||
| N- | 83.42 ± 0.04 | 25.45 ± 0.62 | 0.64 ± 0.40 | 114.79 ± 0.18 | |||
| HL | N+ | 458.83 ± 28.47 | 27.61 ± 2.35 | 2.89 ± 0.80 | 116.52 ± 0.61 | ||
| N- | 82.14 ± 1.58 | 22.15 ± 0.83 | 0.50 ± 0.81 | 114.27 ± 0.55 | |||
| Two-way ANOVA | |||||||
| Light conditions | P | <0.001*** | <0.001*** | 0.004** | 0.245 | ||
| N concentration | P | <0.001*** | 0.049 | 0.001* | <0.001*** | ||
| Interaction | P | <0.001*** | <0.001*** | 0.05 | 0.598 | ||
| N. alexandrina | LL | N+ | 212.18 ± 40.72 | 36.46 ± 1.34 | < LOD | 108.29 ± 4.22 | |
| N- | 73.23 ± 1.86 | 36.69 ± 1.14 | < LOD | 108.33 ± 0.02 | |||
| ML | N+ | 322.96 ± 63.24 | 36.73 ± 0.10 | 2.31 ± 1.34 | 111.04 ± 0.01 | ||
| N- | 73.78 ± 0.11 | 34.60 ± 1.91 | < LOD | 108.33 ± 0.01 | |||
| HL | N+ | 334.11 ± 57.97 | 37.09 ± 0.14 | 1.18±2.04 | 111.04 ± 0.01 | ||
| N- | 73.30 ± 1.76 | 35.51 ± 0.59 | < LOD | 108.33 ± 0.01 | |||
| Two-way ANOVA | |||||||
| Light conditions | P | 0.034* | 0.155 | 0.176 | 0.329 | ||
| N concentration | P | <0.001*** | 0.004** | 0.029* | 0.05 | ||
| Interaction | P | 0.035* | 0.031* | 0.176 | 0.321 | ||
| Staurosira sp. | LL | N+ | 82.19 ± 14.57 | 13.91 ± 0.48 | 0.30 ± 0.53 | 117.43 ± 0.42 | |
| N- | 80.72 ± 0.20 | 14.33 ± 0.48 | < LOD | 117.08 ± 0.47 | |||
| ML | N+ | 142.00 ± 54.80 | 18.22 ± 0.98 | < LOD | 117.63 ± 0.16 | ||
| N- | 79.96 ± 0.57 | 16.59 ± 1.35 | < LOD | 117.15 ± 0.63 | |||
| HL | N+ | 156.44 ± 83.17 | 19.89 ± 0.73 | 0.75 ± 1.30 | 116.73 ± 0.99 | ||
| N- | 79.56 ± 2.62 | 19.32 ± 0.40 | 0.35 ± 0.61 | 117.63 ± 0.12 | |||
| Two-way ANOVA | |||||||
| Light conditions | P | 0.30 | <0.001*** | 0.543 | 0.805 | ||
| N concentration | P | 0.033* | 0.145 | 0.383 | 0.931 | ||
| Interaction | P | 0.28 | 0.136 | 0.527 | 0.095 | ||
Significance levels: *** for p < 0.001; ** for p < 0.01 and * for p < 0.05. LOD = Limit of detection.
For E. paludosa, as for nitrogen, more phosphate was consumed in N+ conditions under HL and ML than under LL (p < 0.001). However, the same amount of phosphate was consumed in N+ and N- conditions (p = 0.05). For N. alexandrina, phosphate concentration available in the medium was very low at the end of the experiment (< 5μM) in all tested conditions. These results suggest a phosphate limitation at the end of the experiment in all treatments. However, phosphate consumption was higher under N+ than N- for both HL (p < 0.01) and ML conditions (p < 0.05). For Staurosira sp. less than half of the phosphate concentration available in the medium was consumed. Phosphate consumption decreased with light and was highest under HL whatever the nitrogen conditions (p < 0.001). For all species and conditions tested, silica and ammonium were present in trace amounts at the end of the experiment. All the silica available in the medium was consumed by the end of the experiment for each condition and each species. Presence of a low concentration of NH4+ suggests that a low level of production occurred that did not modify our nitrogen culture conditions.
For E. Paludosa, NO3- and PO43- consumption was impacted by light. Nutrient consumption was more important under HL and ML. For N. alexandrina, consumption was mainly affected by nitrogen concentration. For Staurosira sp., only NO3- consumption was affected by nitrogen concentration, consumption was the same whatever the light level.
Differences observed when results were expressed in μm.cells-1 were the same for each species and treatment.
Growth
For E. paludosa, growth rate (μ) was higher in N+ than N- conditions for ML (p < 0.05) and HL (p < 0.01) (Table 4). In LL, however, no significant difference was detected between N+ and N- (p = 0.77) (Fig 1A). Maximum biomass (A) was the highest under N+ whatever the light level (p < 0.01). Latency phase (λ) was impacted by light level (p < 0.01), being longer for LL, was not significant affected by nitrogen level (p = 0.824). These results can be explained by NO3- and PO43- consumption: more nitrogen was consumed in N+ under HL and ML, allowing higher growth rate and biomass, whereas the LL light intensity was insufficient to allow efficient growth (Table 4), inducing lower nutrient consumption (Table 4).
Table 4. Growth rate (μ in day-1), maximum cell concentration (A in log) and latency time (λ in day) (triplicate mean ± SD) for all species and culture conditions and the corresponding two-way ANOVA results (n = 3).
| Species | Statistical Results |
Light | Nitrogen | μ (day-1) | A (log) | λ (day) |
|---|---|---|---|---|---|---|
| E. paludosa | LL | N+ | 0.29 ± 0.06 | 2.56 ± 0.12 | 2.84 ± 0.47 | |
| N- | 0.30 ± 0.01 | 2.26 ± 0.10 | 2.80 ± 0.71 | |||
| ML | N+ | 0.35 ± 0.01 | 2.48 ± 0.02 | 1.45 ± 0.28 | ||
| N- | 0.30 ± 0.02 | 2.00 ± 0.11 | 1.70 ± 0.65 | |||
| HL | N+ | 0.37 ± 0.01 | 2.61 ± 0.38 | 1.65 ± 0.16 | ||
| N- | 0.28 ± 0.02 | 2.33 ± 0.15 | 1.26 ± 0.60 | |||
| Two-way ANOVA | ||||||
| Light conditions | P | 0.090 | 0.114 | <0.001*** | ||
| N concentration | P | 0.003** | <0.001*** | 0.824 | ||
| Interaction | P | 0,020* | 0.597 | 0.582 | ||
| N. alexandrina | LL | N+ | 0.67 ± 0.02 | 4.62 ± 0.09 | 4.38 ± 0.07 | |
| N- | 0.73 ± 0.02 | 4.65 ± 0.30 | 4.42 ± 0.08 | |||
| ML | N+ | 1.05 ± 0.10 | 4.41 ± 0.17 | 3.45 ± 0.38 | ||
| N- | 0.83 ± 0.11 | 4.61 ± 0.25 | 1.51 ± 0.52 | |||
| HL | N+ | 1.05 ± 0.07 | 4.16 ± 0.16 | 3.56 ± 0.09 | ||
| N- | 1.16 ± 0.13 | 4.40 ± 0.20 | 2.65 ± 0.41 | |||
| Two-way ANOVA | ||||||
| Light conditions | P | <0.001*** | 0.037* | <0.001*** | ||
| N concentration | P | 0.694 | 0.131 | <0.001*** | ||
| Interaction | P | 0.012* | 0.646 | <0.001*** | ||
| Staurosira sp. | LL | N+ | 0.69 ± 0.18 | 2.81 ± 0.96 | 2.65 ± 0.14 | |
| N- | 0.61 ± 0.08 | 1.75 ± 0.13 | 2.60 ± 0.31 | |||
| ML | N+ | 0.46 ± 0.05 | 2.50 ± 0.56 | 1.65 ± 0.50 | ||
| N- | 0.56 ± 0.24 | 2.08 ± 0.05 | 2.66 ± 0.16 | |||
| HL | N+ | 0.49 ± 0.12 | 2.35 ± 0.19 | 2.22 ± 1.51 | ||
| N- | 0.57 ± 0.04 | 1.80 ± 0.47 | 3.37 ± 0.69 | |||
| Two-way ANOVA | ||||||
| Light conditions | P | 0.217 | 0.674 | 0.293 | ||
| N concentration | P | 0.647 | 0.012* | 0.054 | ||
| Interaction | P | 0.497 | 0.499 | 0.296 | ||
Significance levels: *** for p < 0.001; ** for p < 0.01 and * for p < 0.05. Remarkable value in bold: for details see text.
Fig 1. Growth curves expressed in cell.mL-1 of E. paludosa (A), N. alexandrina (B), and Staurosira sp. (C) as a function of time under different light (LL, ML, HL) and nitrogen (N+, N-) conditions.
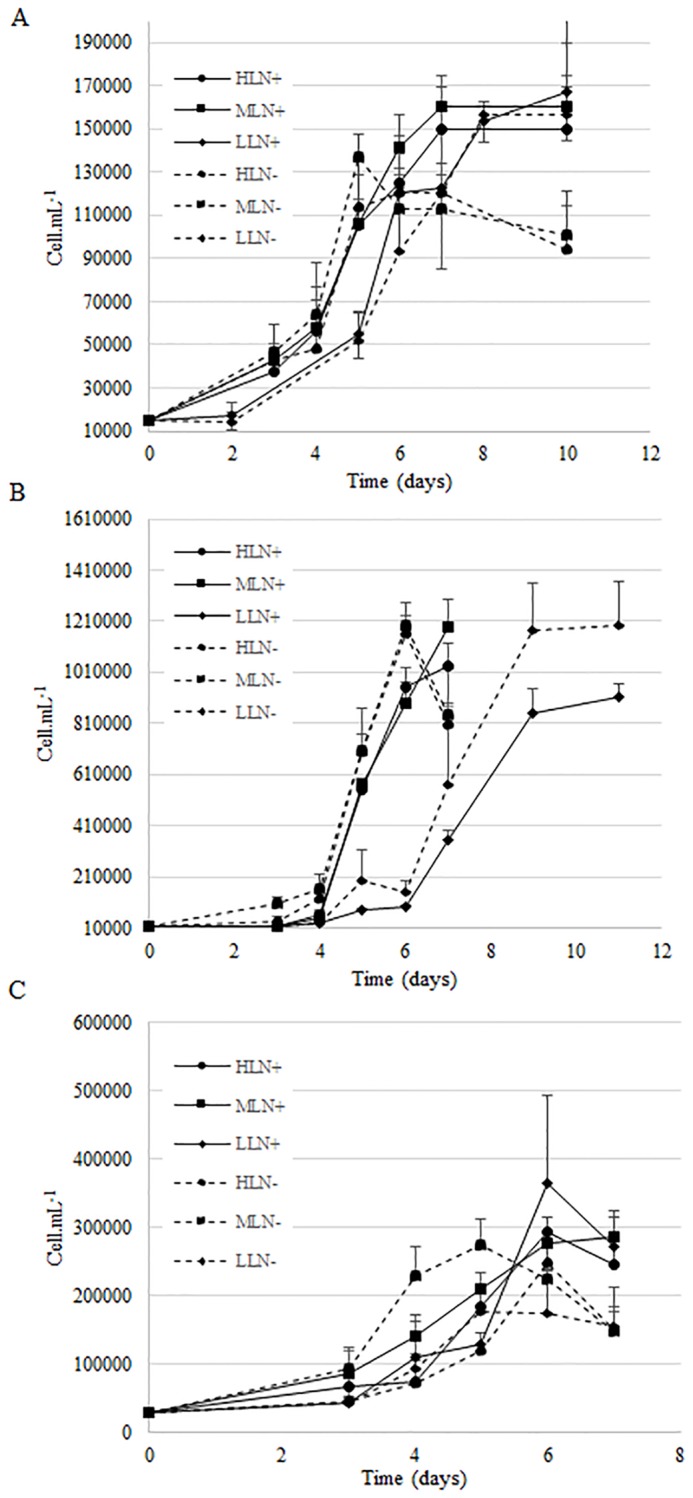
For N. alexandrina, growth rate (μ) increased with light intensity regardless of nitrogen level (p < 0.001). There was a significant difference in maximum biomass (log A) among light levels (p < 0.05), HL and LL showing the lowest and the highest values, respectively (p < 0.05). Conversely, there were no significant differences in growth parameters due to nitrogen conditions (p = 0.646), although a drop in cell concentration in N- medium occurred at the end of the growth in HL and ML (Fig 1B). As for E. paludosa, the latency phase (λ) was longer under LL conditions whatever the nitrogen concentration (p < 0.001). These results showed that the impact of light prevailed over the impact of nitrogen in terms of growth. Even if nitrogen consumption was higher under N+, it did not affect the growth. The other hypothesis was that phosphate limitation occurred in N+ and N- conditions. All the available phosphate was consumed, which could have limited growth in the same way, whatever the nitrogen concentration, and explain the drop observed in N- conditions. Under LL, the low growth led limitation to occur later than under HL and ML, due to slower nutrient consumption.
For Staurosira sp. there was no significant difference for growth rate between light conditions (p = 0.21) and nitrogen concentration (p = 0.64). Mean growth rate was 0.56 ± 0.08 day-1 for all tested conditions. As for E. paludosa, maximum biomass (A) was the highest under N+ condition whatever the light levels (p < 0.05). Contrary to E. paludosa and N. alexandrina, latency phase (λ) was not impacted by culture conditions in this species (p = 0.29) and lasted 2.52 ± 0.79 days in all treatments. As for E. paludosa, more nitrogen was consumed under N+ conditions, explaining the higher biomass obtained. However, no marked trend in growth appeared for Staurosira sp, in contrast to E. paludosa, where growth was more conditioned by N concentration (Fig 1A), and to N. alexandrina, where growth was more conditioned by light (Fig 1B). For Staurosira sp. there was no significant difference in growth except at the end of the growth phase where biomass decreased in N- conditions (Fig 1C).
Photosynthetic performance
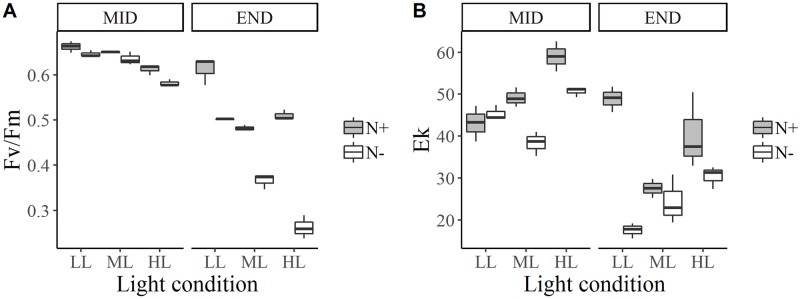
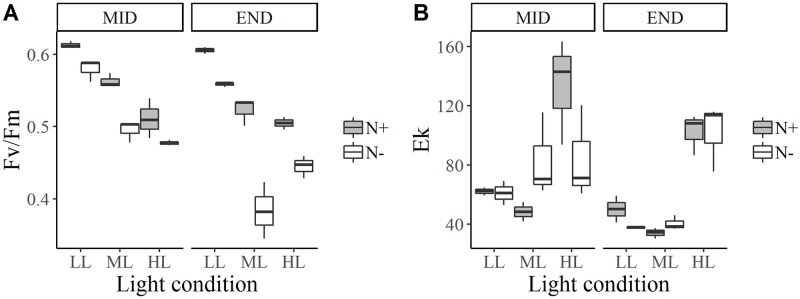
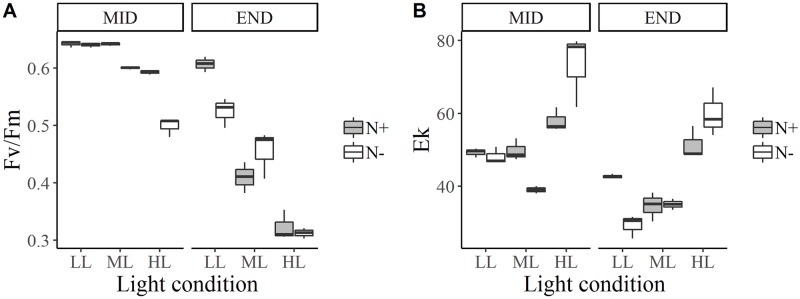
For all species, Fv/Fm was higher in LL photoacclimated cultures (p < 0.001) and higher in N+ medium (p < 0.001) during mid-growth and at the end of growth (Figs 2–4). Fv/Fm was around 0.6 for all species at mid-growth, except for Staurosira sp. under HL, for where Fv/Fm was around 0.5 in N+ condition and 0.4 in N- condition (Fig 4).
Fig 2. Box and whisker plots for E. paludosa photosynthetic parameters: Maximum PSII quantum efficiency, Fv/Fm (A) and maximum light saturation parameter, Ek (B) for each culture condition and for two growth stages: Exponential growth phase (mid) and stationary phase (end).
Fig 4. Box and whisker plots for Staurosira sp. photosynthetic parameters: Maximum PSII quantum efficiency, Fv/Fm (A) and maximum light saturation parameter, Ek (B) for each treatment and for two growth stages: Exponential growth phase (mid-growth) and stationary phase (end of growth).
For E. paludosa (Fig 2A), during mid-growth, Fv/Fm values were all superior to 0.58 ± 0.01. The same pattern was found for N. alexandrina (Fig 3A), where Fv/Fm values were mainly superior to 0.59 ± 0.01. These results indicate that cells were not stressed by the culture conditions during mid-growth, meaning that all major nutrients were available in the medium, even for N. alexandrina for which a limitation by phosphate was suggested (Table 3). For Staurosira sp. (Fig 4A) Fv/Fm values tend to decrease with light and with lack of nitrogen (p < 0.001). The highest Fv/Fm value was obtained under LL and N+ (0.61 ± 0.01) and lowest for HL and N- (0.47 ± 0.01).
Fig 3. Box and whisker plots for N. alexandrina photosynthetic parameters: Maximum PSII quantum efficiency, Fv/Fm (A) and maximum light saturation parameter, Ek (B) for each culture condition and for two growth stages: Exponential growth phase (mid-growth) and stationary phase (end of growth).
In E. paludosa, at the end of the growth phase, Fv/Fm value was lower in N- than N+ in all light levels (p < 0.001); it dropped by 54.89 ± 4.78% for HL, 42.25 ± 3.84% for ML, and 22.38 ± 0.75% for LL. There was a significant interaction between light conditions and nitrogen concentration (p < 0.001). The stronger the light and lower the nitrogen concentration, the lower the Fv/Fm value became. As for growth, these results can be explained by nutrient consumption and demonstrate the effect of nitrogen limitation on cells.
For N. alexandrina (Fig 3A), Fv/Fm decreased between mid and end of growth for all treatments (p < 0.001) and Fv/Fm was higher under LL whatever the nitrogen concentration (p < 0.001), as for E. paludosa. However, no significant difference between N+ and N- was detected for HL or ML (p = 0.62). Even though cell concentration was higher under LL in N- (Fig 1), Fv/Fm value suggested that they were more stressed in N- than N+ conditions (p < 0.01). This can be explained by the accumulation of two nutrient limitations (nitrogen and phosphate) under N-, whereas only phosphate was limited under N+ (Table 3). These nutrient limitations can also explain why no difference between N+ and N- conditions was detected for ML or HL: nitrogen and phosphate limitations could possibly have the same effect on cells and trigger a stress.
For Staurosira sp. (Fig 4A), as for E. paludosa, Fv/Fm dropped between mid-growth and the end of growth, particularly under N- conditions (p < 0.001). The highest Fv/Fm value was found under LL whatever the nitrogen concentration (p < 0.001). These results can be explained by the nitrogen consumption inducing a stressful nitrogen limitation during growth.
The Ek parameter, which is an indicator of the state of cellular photoacclimation was, as expected, always higher under HL (p < 0.001) for all the species, showing that cultures were well photoacclimated. As a result of stress induced by nutrient consumption and growth, this parameter decreased between mid-growth and the end of growth (p < 0.001). This decrease could be induced by autoshading among cells, which implies a decrease in the brightness reaching them. Ek value was higher under HL for Staurosira sp. (133.3 ± 35.8) compared with E. paludosa (59 ± 3.6) and N. alexandrina (65.68 ± 10.74). These results can be explained by the fact that this latter species tends to be more in suspension in the medium than the other which increases the light reaching the cells.
Pigments
For E. paludosa, pigment concentrations, i.e., Chl a and Car, were impacted by both nitrogen (p < 0.001) and light conditions (p < 0.001) (Fig 5A). As expected, Chl a concentration in the cells was higher under LL (p < 0.01) due to their photoacclimation. Chl a concentration was lower in N-, especially for ML and HL (p < 0.001). Chl a content and Fv/Fm followed the same trend: decreasing Fv/Fm (Fig 2A) could be linked to a lower production of Chl a by stressed cells. Car concentration was lower in N- conditions (p < 0.001) and HL (p < 0.01), as for Chl a.
Fig 5. Box and whisker plots for E. paludosa (A), N. alexandrina (B), and Staurosira sp. (C) of Chl a content (pg.cells-1) and Carotenoid content (pg.cells-1) at the end of growth for each culture condition.
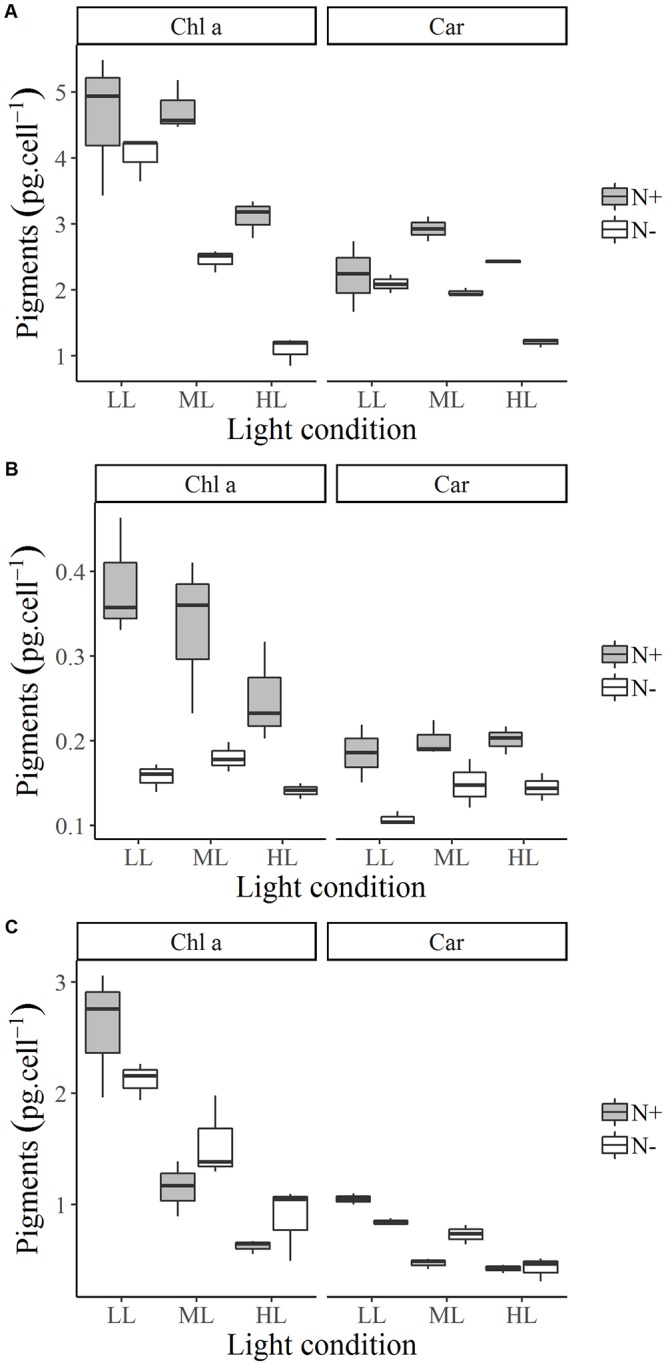
For N. alexandrina, pigment concentrations were affected by nitrogen (p < 0.001) but not by light levels (p = 0.073), although the expected decrease of Chl a with light can be observed (Fig 5B). Chl a and Car concentrations were higher under N+ than N- whatever light conditions (p < 0.001). As for E. paludosa, this tendency can be explained by nutrient limitation and contrasts with Fv/Fm results (which were similar between N+ and N-), as nitrogen limitation induced a halt or decrease in Chl a production. To our knowledge, crossed limitation has not been described in literature: nitrogen or phosphate limitation are addressed separately but neither together. However, on the basis of the current results, we hypothesize that the production of Chl a is mainly impacted by nitrogen limitation. Indeed, nitrogen limitation induces a decrease of RUBISCO which induces an accumulation of ROS leading to cell death and a decrease in Chl a content [51].
Phosphate limitation impacts the photosynthetic machinery in this strain (low Fv/Fm, Table 3), which can be explained by the involvement of phosphate in the synthesis of proteins, phospholipids and ATP that ensure the proper functioning of the photosynthetic apparatus [52].
For Staurosira sp. (Fig 5C), pigment concentrations were impacted by light (p < 0.001) but not by nitrogen (p = 0.70). As seen for E. paludosa, Chl a and Car concentrations were higher under LL (p < 0.001) whatever nitrogen concentration. The absence of differences in Chl a between N+ and N- can be explained by the low differences of nitrogen consumption between these two conditions. Thereby, for this species, Chl a concentration was more impacted by light level.
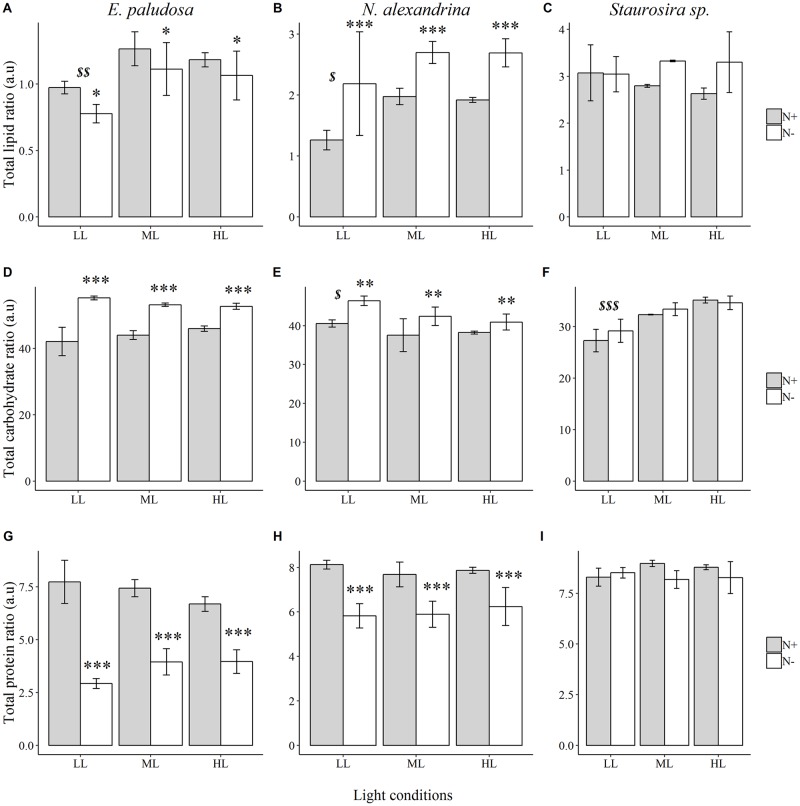
Macromolecular content
Macromolecular contents (lipids, proteins, and carbohydrates) measured using the FTIR method showed that E. paludosa, N. alexandrina, and Staurosira sp. have different biochemical profiles (Fig 6). Total lipid content decreased for E. paludosa grown in nitrogen limiting conditions (p < 0.05), and was also lower under LL (p < 0.05); however, it increased in N. alexandrina in the same nitrogen conditions (p < 0.001) but was still the lowest under LL (p < 0.05). Neither nitrogen (p = 0.06) nor light (p = 0.88) appeared to impact Staurosira sp. total lipid production (Fig 6A–6C). Carbohydrate content increased for both E. paludosa and N. alexandrina under nitrogen limitation (p < 0.001) (Fig 6D and 6E). However, carbohydrate concentration was not impacted by light level in E. paludosa (p = 0.84) but was higher under LL in N. alexandrina (p < 0.05). Protein synthesis for these two species was limited under N- conditions (p < 0.001) (Fig 6G and 6H). No impact of nitrogen concentration on carbohydrate and protein synthesis was found for Staurosira sp (Fig 6F and 6I) and the only effect observed for light was for carbohydrate production under LL (p < 0.001). Results for this species could be explained by the fact that the difference in nitrogen consumption between N+ and N- conditions was very small, indicating little limitation growth by nitrogen, which was also reflected by similar growth parameters (Table 4). E. paludosa and N. alexandrina cultured in stressful conditions remobilize carbon to produce energy storage products (carbohydrates and/or lipids) for acclimation to the altered nutrient conditions. For these two species, cells may produce less storage products (carbohydrates and proteins) when cultured under LL because they are less stressed, as already demonstrated by the high Fv/Fm.
Fig 6. Total lipid (A,B,C), carbohydrate (D,E,F), and protein ratios (G,H,I) of E. paludosa, N. alexandrina, and Staurosira sp. for each treatment.
* p < 0.05;**p < 0.01;***p < 0.001 for nitrogen conditions and $ p < 0.05;$ $p < 0.01;$ $ $p < 0.001 for light conditions.
Biomass and lipid content
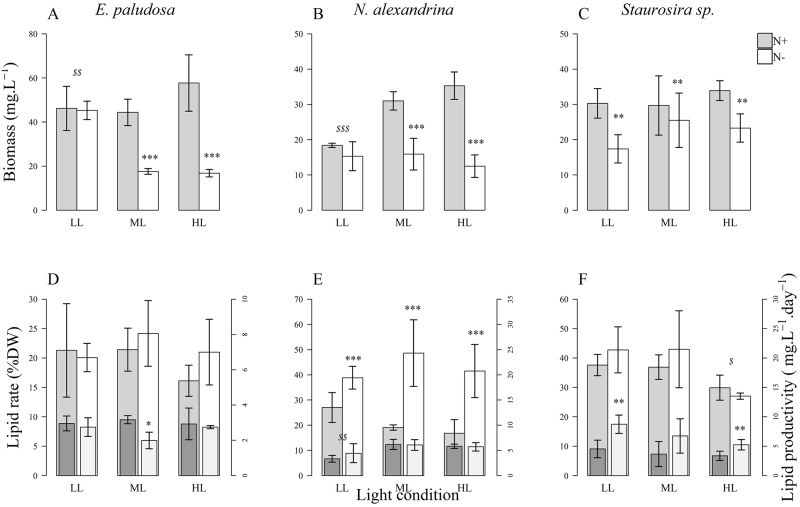
The highest biomass for E. paludosa (57.77 ± 12.86 mg.L-1), N. alexandrina (35.3 ± 3.9 mg.L-1), and Staurosira sp (33.9 ± 0.28 mg.L-1) was achieved under HL and N+ conditions (Fig 7A and 7C). For E. paludosa and N. alexandrina biomass was higher in N+ conditions than N- (p < 0.001) except for LL (p = 0.88). For Staurosira sp. biomass was higher in N+ conditions (p < 0.01) except for ML (p = 0.55). These results were directly linked to the growth parameters (Table 4, Fig 1).
Fig 7. Biomass (A, B, C), lipid rate, and lipid production (D, E, F; high bar = lipid rate; low bar = lipid productivity) obtained at the end of the growth for E. paludosa, N. alexandrina, and Staurosira sp. for each treatment.
* p < 0.05; ** p < 0.01; *** p < 0.001 for nitrogen conditions and $ p < 0.05; $ $ p < 0.01; $ $ $ p < 0.001 for light conditions.
Total lipid contents obtained by gravimetry where not impacted by light (p = 0.30) or nitrogen concentration (p = 0.79) in E. paludosa and corresponded to 20.68 ± 2.62% DW. However, lipid productivity was higher under N+ than N- conditions for ML (p < 0.05). For N. alexandrina total lipid content obtained by gravimetry was impacted by nitrogen concentration (p < 0.001) but not by light (p = 0.63). However, lipid productivity was the lowest under LL (p < 0.05). This confirmed previous observations: N. alexandrina accumulated lipids under nitrogen-limited conditions while E. paludosa did the opposite. Regarding photosynthetic parameters, N. alexandrina was less stressed under LL whatever the nitrogen concentration, explaining a lower lipid production under LL, whereas the highest lipid content for N. alexandrina was 42.99 ± 5.06%, obtained under stressful N- conditions. For Staurosira sp., total lipid content obtained with gravimetry was lower under HL (p < 0.05), but not impacted by nitrogen concentration (p = 0.41). Lipid productivity was higher under N- conditions (p < 0.01). Even though cells were stressed by nitrogen depletion (see Fv/Fm values Fig 4A), this apparently did not induce the production of lipids and/or carbohydrates as seen for the other two species. Staurosira sp. was the species with the highest lipid rate under replete nutrient conditions (36.21 ± 8.42%), whereas N. alexandrina was the only species that accumulated lipids under depleted nutrient conditions (42.99 ± 11.09%).
Fatty acid characteristics
The most abundant saturated fatty acid (SFA) was palmitic acid (C16:0), ranging from 18.1 ± 3.1% to 64.6 ± 6.0% of total FAs for all strains in all treatments. The most abundant unsaturated fatty acid (UFA) was oleic acid (C18:1) for E. paludosa and palmitoleic acid (C16:1) for N. alexandrina and Staurosira sp. (Table 5).
Table 5. FAME composition of E. paludosa, N. alexandrina, and Staurosira sp. for all treatments.
| Fatty acid (% total fatty acid) | E. paludosa | N. alexandrina | Staurosira sp. | ||||||
|---|---|---|---|---|---|---|---|---|---|
| LLN+ | MLN+ | HLN+ | LLN+ | MLN+ | HLN+ | LLN+ | MLN+ | HLN+ | |
| C14 :0 | 10.0 ± 2.1 | 8.0 ± 5.4 | 10.1 ± 1.5 | 0.6 ± 1.1 | 1.5 ± 0.5 | 3.9 ± 1.9 | 1.9 ± 0.7 | 1.7 ± 0.8 | 1.3 ± 0.8 |
| C15 :0 | 0.3 ± 0.4 | 0.5 ± 0.1 | 1.5 ± 1.1 | 0.5 ± 0.2 | 0.5 ± 0.2 | ND | |||
| C16 :1 | 13.6 ± 4.1 | 11.8 ± 8.9 | 9.4 ± 2.6 | 12.7 ± 4.8 | 26.1 ± 2.9 | 22.6 ± 11.6 | 39.7 ± 14.0 | 35.1 ± 10.1 | 23.0 ± 4.6 |
| C16 :0 | 31.7 ± 2.0 | 30.2 ± 1.3 | 36.5 ± 6.0 | 18.1 ± 3.1 | 23.6 ± 0.8 | 24.6 ± 6.4 | 37.7 ± 3.5 | 41.8 ± 2.4 | 47.4 ± 2.1 |
| C16 :2 cj | ND | 1.3 ± 0.5 | 2.9 ± 1.6 | 4.3 ± 5.2 | 4.6 ± 2.9 | 7.4 ± 3.8 | |||
| C18 :2 | 1.0 ± 0.7 | 1.1 ± 1.0 | 0.3 ± 0.4 | ||||||
| C18 :1 | 19.2 ± 5.1 | 36.7 ± 23.2 | 8.9 ± 2.3 | 19.5 ± 15.1 | 9.4 ± 3.6 | 16.1 ± 14.6 | 5.5 ± 2.1 | 3.3 ± 0.4 | 2.8 ± 0.4 |
| C18 :0 | 15.3 ± 5.0 | 5.2 ± 3.5 | 21.1 ± 4.7 | 11.1 ± 1.5 | 10.8 ± 4.4 | 7.7 ± 2.5 | 6.2 ± 3.0 | 9.0 ± 10.2 | 10.9 ± 6.7 |
| C18 :02 cj | ND | ND | 8.8 ± 10.3 | 0.8 ± 1.4 | 1.7 ± 2.7 | 3.8 ± 1.4 | |||
| C20 :5 (EPA) | 1.5 ± 0.4 | 1.7 ± 0.2 | 0.3 ± 0.4 | ||||||
| C20 :4 (ARA) | 10.8 ± 3.0 | 11.7 ± 3.1 | 2.3 ± 2.4 | 1.1 ± 1.6 | ND | ND | |||
| C20 :0 | 1.7 ± 0.5 | 0.9 ± 0.2 | 1.1 ± 0.5 | 0.5 ± 0.2 | 0.6 ± 0.2 | 0.8 ± 0.7 | |||
| C22 :1 | 3.6 ± 1.2 | 3.7 ± 1.9 | 1.6 ± 1.8 | ||||||
| C22 :0 | 2.9 ± 1.4 | 0.8 ± 0.1 | 1.6 ± 1.2 | ||||||
| C24 :0 | 4.4 ± 1.8 | 1.7 ± 0.3 | 1.5 ± 0.2 | 0.5 ± 0.2 | 0.3 ± 0.3 | 1.0 ± 1.0 | |||
| HC | 9.3 ± 3.0 | 7.0 ± 6.6 | 13.7 ± 0.4 | 12.8 ± 5.7 | 7.8 ± 3.4 | 3.6 ± 2.9 | 2.1 ± 1.3 | 1.3 ± 1.0 | 0.5 ± 0.1 |
| UFA | 33.6 ± 5.7 | 49.6 ± 15.2 | 18.6 ± 0.1 | 52.9 ± 4.9 | 52.6 ± 3.6 | 50.0 ± 8.7 | 51.4 ± 7.0 | 44.7 ± 14.0 | 37.0 ± 9.5 |
| MUFA | 32.7 ± 4.8 | 48.5 ± 14.2 | 18.3 ± 0.3 | 40.8 ± 9.6 | 38.0 ± 2.0 | 39.5 ± 4.3 | 45.2 ± 11.9 | 38.4 ± 9.7 | 25.8 ± 4.4 |
| PUFA | 0.8 ± 0.9 | 1.1 ± 1.0 | 0.3 ± 0.4 | 12.2 ± 4.7 | 14.6 ± 2.8 | 10.5 ± 4.4 | 6.2 ± 5.7 | 6.3 ± 4.9 | 11.2 ± 5.1 |
| SFA | 57.0 ± 2.6 | 43.4 ± 8.9 | 67.7 ± 0.3 | 37.4 ± 2.6 | 39.6 ± 4.8 | 45.0 ± 7.0 | 47.4 ± 6.8 | 53.8 ± 12.7 | 61.3 ± 7.6 |
| LLN- | MLN- | HLN- | LLN- | MLN- | HLN- | LLN- | MLN- | HLN- | |
| C14 :0 | 10.8 ± 2.7 | 9.9 ± 2.1 | 8.4 ± 2.4 | 4.6 ± 0.9 | 3.7 ± 1.0 | 3.0 ± 0.8 | 2.9 ± 1.2 | 3.7 ± 1.3 | 3.0 ± 1.1 |
| C15 :0 | 0.9 ± 0.1 | 0.7 ± 0.1 | 1.1 ± 0.9 | 1.0 ± 0.2 | 1.0 ± 0.2 | 0.7 ± 0.1 | 0.3 ± 0.2 | 0.3 ± 0.3 | 0.7 ± 0.3 |
| C16 :1 | 3.2 ± 0.2 | 4.0 ± 0.6 | 6.0 ± 2.1 | 25.2 ± 1.9 | 20.8 ± 3.6 | 25.2 ± 6.4 | 25.0 ± 9.0 | 6.9 ± 2.1 | 19.0 ± 7.0 |
| C16 :0 | 36.6 ± 6.4 | 36.7 ± 6.9 | 33.3 ± 5.7 | 32.5 ± 4.3 | 39.2 ± 3.2 | 35.3 ± 1.2 | 50.9 ± 9.7 | 64.6 ± 6.0 | 58.0 ± 3.7 |
| C16 :2 cj | 5.5 ± 3.9 | 9.5 ± 2.0 | 5.8 ± 1.7 | 4.6 ± 3.8 | 9.5 ± 0.7 | 5.4 ± 2.3 | |||
| C18 :2 | 1.4 ± 1.4 | 1.9 ± 1.8 | 0.9 ± 0.3 | ||||||
| C18 :1 | 11.3 ± 0.0 | 20.1 ± 3.2 | 12.8 ± 9.4 | 4.1 ± 0.6 | 5.7 ± 2.2 | 6.6 ± 2.8 | 4.6 ± 1.4 | 3.0 ± 2.0 | 3.9 ± 1.9 |
| C18 :0 | 13.0 ± 4.0 | 13.5 ± 2.0 | 16.8 ± 4.2 | 8.9 ± 0.5 | 6.6 ± 0.9 | 13.0 ± 2.9 | 5.5 ± 0.9 | 4.6 ± 1.6 | 4.5 ± 0.5 |
| C18 :02 cj | 3.9 ± 1.9 | 4.7 ± 1.3 | 2.5 ± 0.7 | ND | ND | 0.3 ± 0.2 | |||
| C20 :5 (EPA) | 1.0 ± 0.1 | ND | ND | ||||||
| C20 :4 (ARA) | 3.9 ± 0.4 | ND | ND | ||||||
| C20 :0 | 0.7 ± 0.3 | 1.2 ± 0.6 | 0.5 ± 0.5 | 0.8 ± 0.3 | 0.6 ± 0.2 | 0.8 ± 0.3 | 0.3 ± 0.2 | 0.2 ± 0.3 | ND |
| C22 :1 | 1.7 ± 1.3 | 0.8 ± 0.3 | 1.3 ± 0.6 | ||||||
| C22 :0 | 0.7 ± 0.2 | 0.7 ± 0.1 | 0.7 ± 0.2 | ||||||
| C24 :0 | 0.9 ± 0.4 | 0.9 ± 0.1 | 1.0 ± 0.1 | 0.2 ± 0.2 | |||||
| HC | 12.1 ± 1.9 | 18.1 ± 4.0 | 20.1 ± 8.8 | 3.3 ± 1.4 | 2.8 ± 0.9 | 5.0 ± 0.3 | 6.0 ± 1.0 | 7.2 ± 1.6 | 5.0 ± 1.0 |
| UFA | 26.1 ± 13.8 | 26.1 ± 5.7 | 23.9 ± 6.2 | 43.9 ± 11.6 | 41.5 ± 1.2 | 40.5 ± 3.0 | 34.2 ± 9.3 | 19.4 ± 2.6 | 28.3 ± 6.4 |
| MUFA | 25.2 ± 12.7 | 24.2 ± 3.8 | 23.2 ± 6.4 | 38.0 ± 8.4 | 27.4 ± 2.0 | 33.1 ± 3.9 | 29.6 ± 10.4 | 10.0 ± 1.9 | 23.0 ± 5.7 |
| PUFA | 0.9 ± 1.3 | 1.9 ± 1.8 | 0.7 ± 0.2 | 12.6 ± 4.2 | 14.1 ± 1.6 | 7.4 ± 3.2 | 4.6 ± 3.8 | 9.5 ± 0.7 | 5.4 ± 2.3 |
| SFA | 61.7 ± 12.3 | 62.1 ±7.6 | 60.2 ± 4.9 | 46.7 ± 11.0 | 55.7 ± 2.0 | 54.5 ± 2.7 | 59.7 ± 8.4 | 73.4 ± 4.1 | 66.6 ± 5.5 |
Cj: Conjugated, EPA: Eicosapentaenoic acid, ARA: Arachidonic acid, HC: hydrocarbons, UFA: unsaturated fatty acid, MUFA: Monounsaturated fatty acid, PUFA: Polyunsaturated fatty acid, SFA: Saturated fatty acid, ND: Not detected.
N. alexandrina was the only species that produced arachidonic acid (C20:4) and eicosapentaenoic acid (20:5). These fatty acids were mostly produced under N+ and their concentrations were higher under LL and ML compared with HL (p < 0.05). For N. alexandrina and Staurosira sp., under N-, UFA tended to decrease (p < 0.01) and SFA to increase (p < 0.001). E. paludosa produced a significant amount of hydrocarbons (9.81 ± 3.18–18.12 ± 4.02%), which increased under N- conditions as for Staurosira sp. (p < 0.01).
Discussion
Growth and photosynthetic performances
Compared with our previous study [6], the three species examined in the current article show lower growth and biomass. However, this difference could be explained by their previously estimated growth under more favorable conditions, in medium using enriched natural seawater and with continuous light. Continuous light is known to be favorable because diatoms grow faster under longer photoperiods [53]. The use of artificial seawater made it possible to control nutrient concentrations more precisely than in enriched natural seawater, but there can be shortages of essential elements in the basal salt mixture, omission of minor elements present in natural seawater, or contamination of reagent grade salts [54–56], any of which could explain the lower biomass attained in this study.
As expected, growth rate and biomass increase under ML and HL, especially under N+ [15,57]. Latency phase is mostly impacted by light condition and is higher under LL as previously seen in Skeletonema costatum [57], where latency phase was 5 days longer under LL (20 μmol.photon.m-2.s-1) than under HL (340 μmol.photon.m-2.s-1), or ML (100 μmol.photon.m-2.s-1). However, species specific responses occur. For example, growth rate was lower under N- conditions for E. paludosa while it was lower under LL for N. alexandrina and no difference was observed between all tested conditions for Staurosira sp. Nevertheless, the majority trends in the literature show that growth rate normally rises with light intensity up to a certain point where photoinhibition occurs [58–61]. In the present study, only N. alexandrina seems to follow this tendency while E. paludosa and Staurosira sp. seem to have higher resistance to light conditions in terms of growth. Growth patterns of E. paludosa and N. alexandrina showed that under light-limited conditions and whatever the nitrate concentration used, growth curves were similar. This similarity suggests that, at low light intensity, it is light rather than nitrogen availability that is growth limiting. Jauffrais et al. (2015) [62] found the same growth rate for E. paludosa when grown under N+ (1.15 ± 0.06 day-1) and N- (1.13 ± 0.04 day-1). However, growth rates found for this species in the present study are lower; this could be explained by artificial seawater composition in the medium, which differs slightly from this previous study. The artificial medium used by Jauffrais et al. contained more phosphate (72.4 μM) and H3BO3 (178 μM) and less Kbr (12.5 μM) and SrCl2 (37.5 μM).
Photosynthetic efficiency, estimated by the Fv/Fm parameter, decreased for all species during growth, especially under N- conditions compared with N+, except for N. alexandrina. For this latter species, the photosynthetic efficiency decreased similarly under N+ and N-. The general decrease can be explained by the deprivation of nitrogen, known to be the most important element contributing to the dry weight of microalgae cells [63,64]. The low value of the photosynthetic parameter for N. alexandrina under N+ could also be due to phosphate limitation [65]. Regarding the photoacclimation parameter Ek, as expected, lower values were obtained for LL acclimated cells due to their light harvesting complex modification to optimize light capture [66,67]. The variations in both Fv/Fm and Ek, according to light for the three species studied are consistent with Cruz et al. [68] in which the same trend was found for Nitzschia palea under HL (400 μmol.photons.m-2.s-1) and LL (20 μmol.photons.m-2.s-1) treatments. They obtained Ek values of 162.4 under HL and 44.3 under LL. In the present study, similar Ek values were found for Staurosira sp., with an Ek value of 133.36 ± 35.8 under HL and 62.16 ± 2.65 under LL. Ek values under LL for E. paludosa (43.06 ± 4.25) and N. alexandrina (48.6 ± 1.80) were similar to findings of Cruz et al. However, Ek values for these two species under HL were lower than Staurosira sp. Ek value under HL was 65.68 ± 10.74 for E. paludosa and 59 ± 3.6 for N. alexandrina. These results suggest that N. palea and Staurosira sp. have a better adaptation capacity to the stronger light than E. paludosa and N. alexandrina. Cruz et al. [68] found Fv/Fm value to be higher under LL (0.63) than HL (0.55) which is also in accordance with our study. In the same way, Jauffrais et al. (2016) concluded that Fv/Fm reflects photochemical processes dependent on chloroplast reactions that use ATP and reductants provided by photosynthesis. In our study, lower Fv/Fm values obtained under HL were associated with a lesser production of Chl a. It is proven that the decline in pigments due to HL exposure [68,69] or nutrient limitation [70,71] affect photosynthetic activity and thus impact Fv/Fm value.
Under nitrogen [24,29,64,72] and phosphorus limitation [32,73], a decline in cell pigment content often appears and induces chlorosis. This phenomenon leads to a decrease in photosynthetic efficiency as demonstrated in previous studies [70,74,75]. Among these, Zulu at al. (2018) [75] studied the photosynthetic machinery of the diatom Phaeodactylum trinocornutum upon exposure to nitrogen limitation. Degradation of this machinery was induced leading the cells to become chlorotic, the nitrogen pools to shut down and the cellular proteins to decrease. As a result, biomass production was negatively affected as in the current study. Even if cells are nitrogen limited, they continue to consume phosphate. To continue their growth, they must use intracellular inorganic storages, such as Rubisco (known to be N-rich), which stops pigment production [72].
Nutrient consumption is affected by culture conditions. Under replete conditions, nitrate and phosphate consumption are greater under HL and ML than under LL. This expected result is due to slower growth under LL where nutrient consumption is low. However, for E. paludosa, nitrogen and phosphate consumption were mainly affected by light conditions while for N. alexandrina and Staurosira sp., nutrient consumption was mainly affected by nitrogen concentration, whatever the light level. These results suggest that, in some species, increased irradiance results in higher nitrogen and phosphate consumption, as already shown in some other studies [76,77], while for other species, light conditions do not affect nutrient consumption: a relationship not currently described in the literature.
For N. alexandrina, it is important to specify that all the phosphate available in the medium gets consumed whatever the culture conditions in terms of light and nitrate. This similar phosphate consumption could be explained by the fact that several diatom species are able to store phosphate in excess in intracellular pools to be used in case of limitation [78]. This evolutionary advantage allowing the cells to cope with levels of environmental phosphate, which often is the first nutrient to be depleted [79] could also be present in N. alexandrina. with the objective of using microalgal strains in biotechnology, most studies applied light or nutrient stress without verifying the physiological parameter Fv/Fm [13,14,17,26,27,30,80]. If strains cannot bear the culture conditions, their macromolecular content may change. In our case, lipid quantity could be thereby increased, but not necessarily lipid quality. To obtain stable production of a valuable compound it is necessary to ensure the integrity of the photosynthetic machinery. The Ek parameter enables us to verify good cell acclimation and Fv/Fm enables us to confirm their physiological states. It is essential to find culture conditions that sustain good growth and physiological states, in our study these conditions are respected under LL and N+ conditions. Some studies took into account physiological parameters [12,16,58,64] but did not analyze the effects of different light intensity and nutrient limitation simultaneously or used different diatom strains from ours (Pheodactylum tricornutum, Chaetoceros muelleri, Chlorella sp. L1, Monoraphidum dybowskii, Nannochloropsis oceanica, Thalassiosira pseudonana, and Dunaliella tertiolecta).
Macromolecular content
Microalgae cultured in stressful conditions remobilize carbon to produce energy storage products (carbohydrates and/or lipids) for later consumption to cope with the unfavorable conditions and to survive. Nonetheless, this accumulation mechanism is not well understood [81,82]. He et al., (2015) found a 30% decrease in lipid content for Chlorella sp. L1 and Monoraphidium dybowskii cultured under LL intensity (40 μmol.photons.m-2.s-1) compared with cultures grown under HL (400 μmol.photons.m-2.s-1) [58]. The same tendency occurs with the three strains analyzed in the present study, suggesting the same strategy in these diatom strains as in the chlorophytes studied by He et al.: under light starvation conditions (e.g., LL), limited energy is allocated to growth and, with the rising light intensity, more energy is supplied to the synthesis of storage materials (i.e., carbohydrates and lipids). In this study, whereas E. paludosa and N. alexandrina accumulated lipids under the highest light intensities, Staurosira sp. accumulated carbohydrates. However, these observations are not applicable to all diatom species. N. alexandrina shows a decrease in carbohydrate content with light intensity, as demonstrated previously by Vårum et al. [83] for the diatom Skeletonema costatum.
Nitrogen limitation also reduces the ability of microalgae to use carbon fixed during the photosynthetic process. This carbon is normally used for protein synthesis. However, the decline in protein synthesis does not prevent cells from storing energy, which is why a nitrogen deficit, may result in the accumulation of carbohydrates and/or lipids depending on the species [29,36]. Accumulation of carbohydrates under nitrogen limitation has already been reported for E. paludosa by Jauffrais et al., (2015) [62] with a carbohydrate content of 36% under nitrogen-replete conditions and 67% under nitrogen-limited conditions. To our knowledge, only one study exists for Staurosira sp. [84], showing low lipid accumulation under nitrogen depleted conditions: 36% under high N fertilization and 45% under low N fertilization. These results are not consistent with our study, however, probably due to the low difference in nitrogen consumption between N+ and N- conditions: the Staurosira strain used in our study did not seem to be stressed by the low nitrogen level tested and attained a high lipid content–over 25% of its DW and capable of exceeding 40%–regardless of the nitrogen and light conditions.
Fatty acid composition of E. paludosa and Staurosira sp. Were studied here for the first time whereas there are already a few studies concerning the Nitzschia genus [1,5,85,86] and several more concerning diatoms [2,3,5,16,57,85,87–90]. For example, Renaud et al., (1999) studied the gross chemical and fatty acid composition of 18 species including Nitzschia sp. [1]. Lipid content and fatty acid composition found for this diatom species are in accordance with our results, with a production of EPA and ARA occurring under 80 μmol.m-2.s-1 with a 12h/12h photoperiod in F/2 medium. Nitrogen limitation, seems to be the parameter with the greatest impact on the three strains analyzed. This limitation is especially efficient for lipid accumulation in N. alexandrina cultures. However, several detrimental effects of nitrogen limitation on photosynthesis were identified in our study and are in agreement with previous findings [29,70], such as a decrease in Chl a and a 10- to 40-fold protein content decrease, contributing to chlorosis [91]. A consequence of this lack of de novo protein synthesis is a decrease in acetyl CoA carboxylase activity, the first committed step in fatty acid biosynthesis [14,92]. Nitrogen limitation is truly stressful for the cells and can cause them to lose the ability to synthetize and accumulate qualitative fatty acids of economic interest [31]. In our study, N. alexandrina lost the ability to produce ARA and EPA when cultured under nitrogen limitation combined with ML or HL. This finding suggests that if the photosynthetic machinery is impacted, then lipid quality will also be. Moreover, in this study, when cells were stressed, they tended to produce more SFA but less UFA. This trend was previously observed by Yongmanitchai et al. (1991) on Pheodactylum tricornutum [17]. This species produces less EPA and tends to accumulate SFA (C16:0), but also some UFA (C16:1, C18:1), when it grows under nitrogen limitation. Xia et al. (2013) observed a similar trend in Odontella aurita, a species of interest as a feed in aquaculture, with a decrease in EPA (5.6 vs 2.2%) and ARA (12.9 vs 9.0%) under nitrogen limitation and an increase in SFA (25.4 vs 39.0%), which is in accordance with our study [93].
Future prospects for the use of the strains
Diatoms are reported to have a higher lipid content than other algal classes [31]. A literature survey by Griffiths and Harrison of 55 microalgae species in various classes [94] showed that diatoms, as a class, have an average lipid content of 30.6%DW, while they found an average lipid content of 8.6%DW for Cyanobacteria, 23.17% DW for Chlorophyta, 20.5% for Ochrophyta and 23.8% for other classes (Dinophyta, Prasinophyta, Euglenozoa, and Haptophyta). To be considered as an efficient lipid producer in industrial terms, microalgae have to accumulate at least 20% of their dry biomass as lipids [95]. The three strains presented in this study exceed this threshold, making them good candidates for biotechnology applications: E. paludosa and Staurosira sp. could be used for biofuel production because of their high production of SFA and UFA. Fatty acids like EPA and ARA produced by N. alexandrina could find applications in the cosmetic or nutraceutical industries. The main conclusion of this study is in accordance with previous ones [12,16,58]: lipid quantity and quality depend fundamentally on culture conditions, including the light environment and medium composition. Control processing conditions for lipid production at industrial scale has a cost [31]. To be economically sustainable, chosen strains have to be consistent lipid producers under varied environmental conditions rather than strains with higher productivity but only under optimal conditions [31]. Implementation of lipid production processes for microalgae strains has to take into account these economic constraints. Knowing the physiological capacity of strains is a necessary requirement to optimize culture conditions. If strains cannot bear the culture conditions, which can be only assessed by physiological analysis, production of lipids of interest can be made impossible, as seen in this study under HL and N- conditions. If culture conditions are adapted to photosynthetic machinery, long-term lipid production can be assessed. From this point of view, even if nitrogen limitation and HL can enhance lipid content, the strategy would not be advisable for industrial-scale lipid production. For E. paludosa, nitrogen limitation is inefficient for increasing lipid production, especially under LL. Even though growth took longer, biomass was the same under HL or LL, and photosynthetic efficiency remained high. In terms of cost, the use of the LL is of particular interest since E. paludosa produces more UFA and SFA under LL and these conditions diminish the need for high power artificial light that otherwise increase the cost of microalgae production [96]
For large scale production, N. alexandrina should be grown under ML and N+ to have an high, biomass and preserved ARA and EPA production. If grown under higher light and N- medium this strain will lose its ability to produce ARA and EPA and physiological states will be affected which is not desirable for the long-term production of these compounds.
Staurosira sp produces high amounts of lipids under depleted and replete nutrient conditions, but UFA and, particularly, MUFA were preferentially produced under replete conditions while mostly SFA were produced under depleted conditions. This species can also be grown under LL as for E. paludosa. This species could be used for biodiesel production, which in agreement with the study by Huntley et al. [84]. This species should be grown under LL and nutrient-replete conditions to obtain equal proportions of MUFA and SFA compatible with biodiesel.
The three new strains studied here are good candidates for biotechnology applications due to the quantity and quality of their lipid production under simple culture conditions: LL and nitrogen enrichment. Indeed, using low light induces low light energy consumption and using a nitrogen depleted medium induces less maintenance since it’s not necessary to produce these species in a two-stage culture (nitrogen replete and nitrogen depleted). However, to increase lipid content and quality, it would be interesting to test other culture conditions, such as limiting other nutrients like phosphate for example, which is known to increase lipids content in diatoms without damaging photosynthetic machinery as nitrogen limitation does [32,97]. Development of a specific photobioreactor (PBR) [98–101] sustaining biofilm culturing could also be interesting because the three species are benthic and naturally form biofilms. The combination of high quantity and quality lipid production and with low water and light use by these species could represent an ideal solution for new development of sustainable economic activities.
Conclusion
Effects of different nitrogen concentrations and light conditions on Entomoneis paludosa, Nitzschia alexandrina and Staurosira sp. was explored by the evaluation of growth, photosynthetic performance including photosynthetic efficiency, pigment content, macromolecular content (lipids, carbohydrates, proteins), and fatty acid composition. Nitrogen limitation stimulates the accumulation of carbohydrates for Entomoneis paludosa and the accumulation of lipids for Nitzschia alexandrina. An irradiance between 100 and 400 μmol.photons.m-2.s-1 stimulates the accumulation of lipids for Entomoneis paludosa and Nitzschia alexandrina while for Staurosira sp. it stimulates the accumulation of carbohydrates. Under HL and nitrogen-limited conditions, the content of proteins and pigments decline accompanying a decrease in photosynthetic efficiency. This finding supports our main conclusion that the development of a lipid production which must be a compromise between economic and ecophysiological constraints. The selection of strains that fulfill both aspects is the best solution for sustainable production of lipids at an industrial scale. In fact, an increase in lipid level does not mean an increase in the production of lipids of interests, indeed, while PUFAs are economically valuable, under light or nitrogen stress UFAs increase and PUFAs decrease with a halt in EPA and ARA production.
Acknowledgments
We express our sincere thanks to IFREMER (French Institute for the research and exploitation of the Sea) staff, in particular Ewa Lukomska for support and advice on the determination of nutrients.
Abbreviations
- ARA
Arachidonic acid
- CAR
Carotenoids
- Chl a
Chlorophyll a
- Cj
Conjugated
- CLE
Crude lipid extract
- DW
Dry weight
- Eb
Ester bond
- Ek
Photoacclimation parameter
- EPA
Eicosapentaenoic acid
- FA
Fatty acid
- FAME
Fatty acid methyl ester
- Fv/Fm
Maximum quantum efficiency
- HL
High light
- HTSXT-FTIR
Fourrier-transform infrared spectroscopy high-throughput screening extension
- LL
Low light
- LP
Lipid productivity
- LR
Lipid rate
- ML
Middle light
- MUFA
Monounsaturated fatty acid
- ND
Not detected
- PAM
Pulse amplitude modulated
- PSII
Photosystem II
- PUFA
Polyunsaturated fatty acid
- rETR
Relative electron transport rate
- RLC
Rapid light curve
- SFA
Saturated fatty acid
- TAG
Triacylglycerol
- UFA
Unsaturated fatty acid
- μ
Specific growth rate
Data Availability
All 24 files are available from the figshare database https://doi.org/10.6084/m9.figshare.9810389.v3.
Funding Statement
This research was supported under the Atlantic Microalgae research program (AMI), funded by the Région Pays de la Loire, and the BIO-Tide project, itself funded through the 2015–2016 BiodivERsA COFUND call for research proposals, with the national funders BelSPO, FWO, ANR and SNSF.
References
- 1.Renaud S.M., Thinh L.-V., Parry D.L., The gross chemical composition and fatty acid composition of 18 species of tropical Australian microalgae for possible use in mariculture, Aquaculture. 170 (1999) 147–159. [Google Scholar]
- 2.Dunstan G.A., Volkman J.K., Barrett S.M., Leroi J.-M., Jeffrey S.W., Essential polyunsaturated fatty acids from 14 species of diatom (Bacillariophyceae), Phytochemistry. 35 (1993) 155–161. 10.1016/S0031-9422(00)90525-9 [DOI] [Google Scholar]
- 3.Volkman J.K., Jeffrey S.W., Nichols P.D., Rogers G.I., Garland C.D., Fatty acid and lipid composition of 10 species of microalgae used in mariculture, J. Exp. Mar. Biol. Ecol. 128 (1989) 219–240. 10.1016/0022-0981(89)90029-4 [DOI] [Google Scholar]
- 4.d’Ippolito G., Sardo A., Paris D., Vella F.M., Adelfi M.G., Botte P., Gallo C., Fontana A., Potential of lipid metabolism in marine diatoms for biofuel production, Biotechnol. Biofuels. 8 (2015) 28 10.1186/s13068-015-0212-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Joseph M.M., Renjith K.R., John G., Nair S.M., Chandramohanakumar N., Biodiesel prospective of five diatom strains using growth parameters and fatty acid profiles, Biofuels. 8 (2017) 81–89. 10.1080/17597269.2016.1204585 [DOI] [Google Scholar]
- 6.Cointet E., Wielgosz-Collin G., Méléder V., Gonçalves O., Lipids in benthic diatoms: A new suitable screening procedure, Algal Res. 39 (2019) 101425 10.1016/j.algal.2019.101425 [DOI] [Google Scholar]
- 7.Doan T.T.Y., Sivaloganathan B., Obbard J.P., Screening of marine microalgae for biodiesel feedstock, Biomass Bioenergy. 35 (2011) 2534–2544. 10.1016/j.biombioe.2011.02.021 [DOI] [Google Scholar]
- 8.Zhao F., Liang J., Gao Y., Luo Q., Yu Y., Chen C., Sun L., Variations in the total lipid content and biological characteristics of diatom species for potential biodiesel production, Fundam Renew Energy Appl. 6 (2016) 22–26. [Google Scholar]
- 9.Pulz O., Gross W., Valuable products from biotechnology of microalgae, Appl. Microbiol. Biotechnol. 65 (2004) 635–648. 10.1007/s00253-004-1647-x [DOI] [PubMed] [Google Scholar]
- 10.F. Shahidi, C. Barrow, Marine nutraceuticals and functional foods, CRC Press, 2007.
- 11.Rodolfi L., Chini Zittelli G., Bassi N., Padovani G., Biondi N., Bonini G., Tredici M.R., Microalgae for oil: Strain selection, induction of lipid synthesis and outdoor mass cultivation in a low-cost photobioreactor, Biotechnol. Bioeng. 102 (2009) 100–112. 10.1002/bit.22033 [DOI] [PubMed] [Google Scholar]
- 12.Huete-Ortega M., Okurowska K., Kapoore R.V., Johnson M.P., Gilmour D.J., Vaidyanathan S., Effect of ammonium and high light intensity on the accumulation of lipids in Nannochloropsis oceanica (CCAP 849/10) and Phaeodactylum tricornutum (CCAP 1055/1), Biotechnol. Biofuels. 11 (2018) 60 10.1186/s13068-018-1061-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mortensen S.H., Børsheim K.Y., Rainuzzo J., Knutsen G., Fatty acid and elemental composition of the marine diatom Chaetoceros gracilis Schütt. Effects of silicate deprivation, temperature and light intensity, J. Exp. Mar. Biol. Ecol. 122 (1988) 173–185. [Google Scholar]
- 14.Roessler P.G., Effects of silicon deficiency on lipid composition and metabolism in the diatom Cyclotella cryptica 1, J. Phycol. 24 (1988) 394–400. [Google Scholar]
- 15.Shifrin N.S., Chisholm S.W., Phytoplankton lipids: interspecific differences and effects of nitrate, silicate and light-dark cycles 1, J. Phycol. 17 (1981) 374–384. [Google Scholar]
- 16.Gao Y., Yang M., Wang C., Nutrient deprivation enhances lipid content in marine microalgae, Bioresour. Technol. 147 (2013) 484–491. 10.1016/j.biortech.2013.08.066 [DOI] [PubMed] [Google Scholar]
- 17.Yongmanitchai W., Ward O.P., Growth of and omega-3 fatty acid production by Phaeodactylum tricornutum under different culture conditions., Appl Env. Microbiol. 57 (1991) 419–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dempster T.A., Sommerfeld M.R., Effects of environmental conditions on growth and lipid accumulation in Nitzschia communis (Bacillariophyceae), J. Phycol. 34 (1998) 712–721. [Google Scholar]
- 19.Schaub I., Wagner H., Graeve M., Karsten U., Effects of prolonged darkness and temperature on the lipid metabolism in the benthic diatom Navicula perminuta from the Arctic Adventfjorden, Svalbard, Polar Biol. (2017) 1–15. 10.1007/s00300-016-2067-y [DOI] [Google Scholar]
- 20.Sriharan S., Sriharan T., Effects of nutrients and temperature on lipid and fatty acid production in the diatom Hantzshia DI-60, Appl. Biochem. Biotechnol. 24 (1990) 309. [Google Scholar]
- 21.Brown M.R., Dunstan G.A., Norwood S.J., Miller K.A., Effects of harvest stage and light on the biochemical composition of the diatom Thalassiosira pseudonana 1, J. Phycol. 32 (1996) 64–73. [Google Scholar]
- 22.Spolaore P., Joannis-Cassan C., Duran E., Isambert A., Commercial applications of microalgae, J. Biosci. Bioeng. 101 (2006) 87–96. 10.1263/jbb.101.87 [DOI] [PubMed] [Google Scholar]
- 23.Bondioli P., Della Bella L., Rivolta G., Zittelli G.C., Bassi N., Rodolfi L., Casini D., Prussi M., Chiaramonti D., Tredici M.R., Oil production by the marine microalgae Nannochloropsis sp. F&M-M24 and Tetraselmis suecica F&M-M33, Bioresour. Technol. 114 (2012) 567–572. 10.1016/j.biortech.2012.02.123 [DOI] [PubMed] [Google Scholar]
- 24.Converti A., Casazza A.A., Ortiz E.Y., Perego P., Del Borghi M., Effect of temperature and nitrogen concentration on the growth and lipid content of Nannochloropsis oculata and Chlorella vulgaris for biodiesel production, Chem. Eng. Process. Process Intensif. 48 (2009) 1146–1151. 10.1016/j.cep.2009.03.006 [DOI] [Google Scholar]
- 25.Liu Z.-Y., Wang G.-C., Zhou B.-C., Effect of iron on growth and lipid accumulation in Chlorella vulgaris, Bioresour. Technol. 99 (2008) 4717–4722. 10.1016/j.biortech.2007.09.073 [DOI] [PubMed] [Google Scholar]
- 26.Roleda M.Y., Slocombe S.P., Leakey R.J.G., Day J.G., Bell E.M., Stanley M.S., Effects of temperature and nutrient regimes on biomass and lipid production by six oleaginous microalgae in batch culture employing a two-phase cultivation strategy, Bioresour. Technol. 129 (2013) 439–449. 10.1016/j.biortech.2012.11.043 [DOI] [PubMed] [Google Scholar]
- 27.Chen Y.-C., The biomass and total lipid content and composition of twelve species of marine diatoms cultured under various environments, Food Chem. 131 (2012) 211–219. 10.1016/j.foodchem.2011.08.062 [DOI] [Google Scholar]
- 28.Chuecas L., Riley J.P., Component fatty acids of the total lipids of some marine phytoplankton, J. Mar. Biol. Assoc. U. K. 49 (1969) 97 10.1017/S0025315400046439 [DOI] [Google Scholar]
- 29.Berges J.A., Charlebois D.O., Mauzerall D.C., Falkowski P.G., Differential effects of nitrogen limitation on photosynthetic efficiency of photosystems I and II in microalgae, Plant Physiol. 110 (1996) 689–696. 10.1104/pp.110.2.689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Giordano M., Kansiz M., Heraud P., Beardall J., Wood B., McNaughton D., Fourier transform infrared spectroscopy as a novel tool to investigate changes in intracellular macromolecular pools in the marine microalga Chaetoceros muellerii (Bacillariophyceae), J. Phycol. 37 (2001) 271–279. [Google Scholar]
- 31.Hildebrand M., Davis A.K., Smith S.R., Traller J.C., Abbriano R., The place of diatoms in the biofuels industry, Biofuels. 3 (2012) 221–240. 10.4155/bfs.11.157 [DOI] [Google Scholar]
- 32.Geider R.J., La Roche J., Greene R.M., Olaizola M., Response of the photosynthetic apparatus of Phaeodactylum tricornutum (Bacillariophyceae) to nitrate, phosphate, or iron starvation 1, J. Phycol. 29 (1993) 755–766. [Google Scholar]
- 33.Berges J.A., Falkowski P.G., Physiological stress and cell death in marine phytoplankton: induction of proteases in response to nitrogen or light limitation, Limnol. Oceanogr. 43 (1998) 129–135. [Google Scholar]
- 34.Parkhill J., Maillet G., Cullen J.J., Fluorescence‐based maximal quantum yield for PSII as a diagnostic of nutrient stress, J. Phycol. 37 (2001) 517–529. [Google Scholar]
- 35.Vassiliev I.R., Prasil O., Wyman K.D., Kolber Z., Hanson A.K., Prentice J.E., Falkowski P.G., Inhibition of PS II photochemistry by PAR and UV radiation in natural phytoplankton communities, Photosynth. Res. 42 (1994) 51–64. 10.1007/BF00019058 [DOI] [PubMed] [Google Scholar]
- 36.Quigg A., Beardall J., Protein turnover in relation to maintenance metabolism at low photon flux in two marine microalgae, Plant Cell Environ. 26 (2003) 693–703. [Google Scholar]
- 37.Sunda W.G., Huntsman S.A., Relationships among photoperiod, carbon fixation, growth, chlorophyll a, and cellular iron and zinc in a coastal diatom, Limnol. Oceanogr. 49 (2004) 1742–1753. 10.4319/lo.2004.49.5.1742 [DOI] [Google Scholar]
- 38.Dubinsky Z., Rotem J., Relations between algal populations and the pH of their media, Oecologia. 16 (1974) 53–60. 10.1007/BF00345087 [DOI] [PubMed] [Google Scholar]
- 39.Ralph P.J., Gademann R., Rapid light curves: a powerful tool to assess photosynthetic activity, Aquat. Bot. 82 (2005) 222–237. [Google Scholar]
- 40.Ritchie R.J., Consistent sets of spectrophotometric chlorophyll equations for acetone, methanol and ethanol solvents, Photosynth. Res. 89 (2006) 27–41. 10.1007/s11120-006-9065-9 [DOI] [PubMed] [Google Scholar]
- 41.Bligh E.G., Dyer W.J., A rapid method of total lipid extraction and purification, Can. J. Biochem. Physiol. 37 (1959) 911–917. 10.1139/o59-099 [DOI] [PubMed] [Google Scholar]
- 42.Bougaran G., Bernard O., Sciandra A., Modeling continuous cultures of microalgae colimited by nitrogen and phosphorus, J. Theor. Biol. 265 (2010) 443–454. 10.1016/j.jtbi.2010.04.018 [DOI] [PubMed] [Google Scholar]
- 43.Hansen H.P., Koroleff F., Determination of nutrients, Methods Seawater Anal. (1999) 159–228. [Google Scholar]
- 44.Amin S.A., Hmelo L.R., van Tol H.M., Durham B.P., Carlson L.T., Heal K.R., Morales R.L., Berthiaume C.T., Parker M.S., Djunaedi B., Ingalls A.E., Parsek M.R., Moran M.A., Armbrust E.V., Interaction and signalling between a cosmopolitan phytoplankton and associated bacteria, Nature. 522 (2015) 98–101. 10.1038/nature14488 [DOI] [PubMed] [Google Scholar]
- 45.Koroleff F., Direct determination of ammonia in natural waters as indophenol blue, Inf. Tech. Methods Seawater Anal. (1970) 19–22. [Google Scholar]
- 46.A. Aminot, R. Kérouel, Hydrologie des écosystèmes marins: paramètres et analyses, Editions Quae, 2004.
- 47.Beer S., Vilenkin B., Weil A., Veste M., Susel L., Eshel A., Measuring photosynthetic rates in seagrasses by pulse amplitude modulated (PAM) fluorometry, Mar. Ecol. Prog. Ser. 174 (1998) 293–300. [Google Scholar]
- 48.Eilers P., Peeters J., A model for the relationship between light intensity and the rate of photosynthesis in phytoplankton, Ecol. Model. 42 (1988) 199–215. [Google Scholar]
- 49.Coat R., Montalescot V., León E.S., Kucma D., Perrier C., Jubeau S., Thouand G., Legrand J., Pruvost J., Gonçalves O., Unravelling the matrix effect of fresh sampled cells for in vivo unbiased FTIR determination of the absolute concentration of total lipid content of microalgae, Bioprocess Biosyst. Eng. 37 (2014) 2175–2187. 10.1007/s00449-014-1194-5 [DOI] [PubMed] [Google Scholar]
- 50.León E.S., Coat R., Moutel B., Pruvost J., Legrand J., Gonçalves O., Influence of physical and chemical properties of HTSXT-FTIR samples on the quality of prediction models developed to determine absolute concentrations of total proteins, carbohydrates and triglycerides: a preliminary study on the determination of their absolute concentrations in fresh microalgal biomass, Bioprocess Biosyst. Eng. 37 (2014) 2371–2380. 10.1007/s00449-014-1215-4 [DOI] [PubMed] [Google Scholar]
- 51.Brembu T., Mühlroth A., Alipanah L., Bones A.M., The effects of phosphorus limitation on carbon metabolism in diatoms, Philos. Trans. R. Soc. B Biol. Sci. 372 (2017) 20160406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bucciarelli E., Sunda W.G., Influence of CO2, nitrate, phosphate, and silicate limitation on intracellular dimethylsulfoniopropionate in batch cultures of the coastal diatom Thalassiosira pseudonana, Limnol. Oceanogr. 48 (2003) 2256–2265. [Google Scholar]
- 53.Brand L., Guillard R., The effects of continuous light and light intensity on the reproduction rates of twenty-two species of marine phytoplankton, J. Exp. Mar. Biol. Ecol. 50 (1981) 119–132. [Google Scholar]
- 54.Berges J.A., Franklin D.J., Harrison P.J., Evolution of an Artificial Seawater Medium: Improvements in Enriched Seawater, Artificial Water Over the Last Two Decades, J. Phycol. 37 (2001) 1138–1145. 10.1046/j.1529-8817.2001.01052.x [DOI] [Google Scholar]
- 55.McLachlan J., Some considerations of the growth of marine algae in artificial media, Can. J. Microbiol. 10 (1964) 769–782. 10.1139/m64-098 [DOI] [PubMed] [Google Scholar]
- 56.Allen E.J., On the culture of the plankton diatom Thalassiosira grauida Cleve, in artificial sea-water, J. Mar. Biol. Assoc. U. K. 10 (1914) 417–439. 10.1017/S0025315400008225 [DOI] [Google Scholar]
- 57.Guihéneuf F., Mimouni V., Ulmann L., Tremblin G., Environmental factors affecting growth and omega 3 fatty acid composition in Skeletonema costatum. The influences of irradiance and carbon source: Communication presented at the 25ème Congrès Annuel de l’Association des Diatomistes de Langue Francaise (ADLaF), Caen, 25–28 September 2006, Diatom Res. 23 (2008) 93–103. [Google Scholar]
- 58.He Q., Yang H., Wu L., Hu C., Effect of light intensity on physiological changes, carbon allocation and neutral lipid accumulation in oleaginous microalgae, Bioresour. Technol. 191 (2015) 219–228. 10.1016/j.biortech.2015.05.021 [DOI] [PubMed] [Google Scholar]
- 59.Norici A., Bazzoni A.M., Pugnetti A., Raven J.A., Giordano M., Impact of irradiance on the C allocation in the coastal marine diatom Skeletonema marinoi Sarno and Zingone*, Plant Cell Environ. 34 (2011) 1666–1677. 10.1111/j.1365-3040.2011.02362.x [DOI] [PubMed] [Google Scholar]
- 60.Rhee G.-‐ull, Gotham I.J., The effect of environmental factors on phytoplankton growth: Light and the interactions of light with nitrate limitation1, Limnol. Oceanogr. 26 (1981) 649–659. 10.4319/lo.1981.26.4.0649 [DOI] [Google Scholar]
- 61.Solovchenko A.E., Khozin-Goldberg I., Didi-Cohen S., Cohen Z., Merzlyak M.N., Effects of light intensity and nitrogen starvation on growth, total fatty acids and arachidonic acid in the green microalga Parietochloris incisa, J. Appl. Phycol. 20 (2008) 245–251. 10.1007/s10811-007-9233-0 [DOI] [Google Scholar]
- 62.Jauffrais T., Drouet S., Turpin V., Méléder V., Jesus B., Cognie B., Raimbault P., Cosson R.P., Decottignies P., Martin-Jézéquel V., Growth and biochemical composition of a microphytobenthic diatom (Entomoneis paludosa) exposed to shorebird (Calidris alpina) droppings, J. Exp. Mar. Biol. Ecol. 469 (2015) 83–92. [Google Scholar]
- 63.White S., Anandraj A., Bux F., PAM fluorometry as a tool to assess microalgal nutrient stress and monitor cellular neutral lipids, Bioresour. Technol. 102 (2011) 1675–1682. 10.1016/j.biortech.2010.09.097 [DOI] [PubMed] [Google Scholar]
- 64.Jiang Y., Yoshida T., Quigg A., Photosynthetic performance, lipid production and biomass composition in response to nitrogen limitation in marine microalgae, Plant Physiol. Biochem. 54 (2012) 70–77. 10.1016/j.plaphy.2012.02.012 [DOI] [PubMed] [Google Scholar]
- 65.Napoléon C., Raimbault V., Claquin P., Influence of nutrient stress on the relationships between PAM measurements and carbon Incorporation in four phytoplankton species, PLOS ONE. 8 (2013) e66423 10.1371/journal.pone.0066423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wilhelm C., Jungandreas A., Jakob T., Goss R., Light acclimation in diatoms: From phenomenology to mechanisms, Mar. Genomics. 16 (2014) 5–15. 10.1016/j.margen.2013.12.003 [DOI] [PubMed] [Google Scholar]
- 67.Anning T., MacIntyre H.L., Pratt S.M., Sammes P.J., Gibb S., Geider R.J., Photoacclimation in the marine diatom Skeletonema costatum, Limnol. Oceanogr. 45 (2000) 1807–1817. [Google Scholar]
- 68.Cruz S., Serôdio J., Relationship of rapid light curves of variable fluorescence to photoacclimation and non-photochemical quenching in a benthic diatom, Aquat. Bot. 88 (2008) 256–264. 10.1016/j.aquabot.2007.11.001 [DOI] [Google Scholar]
- 69.Behrenfeld M.J., Prasil O., Babin M., Bruyant F., In search of a physiological basis for covariations in light-limited and light-saturated photosynthesis1, J. Phycol. 40 (2004) 4–25. 10.1046/j.1529-8817.2004.03083.x [DOI] [Google Scholar]
- 70.Turpin D.H., Effects of inorganic N availability on algal photosynthesis and carbon metabolism, J. Phycol. 27 (1991) 14–20. [Google Scholar]
- 71.Beardall J., Young E., Roberts S., Approaches for determining phytoplankton nutrient limitation, Aquat. Sci. 63 (2001) 44–69. 10.1007/PL00001344 [DOI] [Google Scholar]
- 72.Alipanah L., Rohloff J., Winge P., Bones A.M., Brembu T., Whole-cell response to nitrogen deprivation in the diatom Phaeodactylum tricornutum, J. Exp. Bot. 66 (2015) 6281–6296. 10.1093/jxb/erv340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mamaeva A., Namsaraev Z., Maltsev Y., Gusev E., Kulikovskiy M., Petrushkina M., Filimonova A., Sorokin B., Zotko N., Vinokurov V., Simultaneous increase in cellular content and volumetric concentration of lipids in Bracteacoccus bullatus cultivated at reduced nitrogen and phosphorus concentrations, J. Appl. Phycol. 30 (2018) 2237–2246. [Google Scholar]
- 74.Beardall J., Berman T., Heraud P., Kadiri M.O., Light B.R., Patterson G., Roberts S., Sulzberger B., Sahan E., Uehlinger U., A comparison of methods for detection of phosphate limitation in microalgae, Aquat. Sci. 63 (2001) 107–121. [Google Scholar]
- 75.Zulu N.N., Zienkiewicz K., Vollheyde K., Feussner I., Current trends to comprehend lipid metabolism in diatoms, Prog. Lipid Res. (2018). [DOI] [PubMed] [Google Scholar]
- 76.Goldman J.C., Dennis J.M. Jr, Effect of large marine diatoms growing at low light on episodic new production, Limnol. Oceanogr. 48 (2003) 1176–1182. [Google Scholar]
- 77.Davis C.O., Continuous culture of marine diatoms under silicate limitation. Ii. Effect of light intensity on growth and nutrient uptake of Skeletonema costatum,2, J. Phycol. 12 (1976) 291–300. 10.1111/j.1529-8817.1976.tb02847.x [DOI] [Google Scholar]
- 78.Cade-Menun B.J., Paytan A., Nutrient temperature and light stress alter phosphorus and carbon forms in culture-grown algae, Mar. Chem. 121 (2010) 27–36. 10.1016/j.marchem.2010.03.002 [DOI] [Google Scholar]
- 79.Lai J., Yu Z., Song X., Cao X., Han X., Responses of the growth and biochemical composition of Prorocentrum donghaiense to different nitrogen and phosphorus concentrations, J. Exp. Mar. Biol. Ecol. 405 (2011) 6–17. 10.1016/j.jembe.2011.05.010 [DOI] [Google Scholar]
- 80.Dean A.P., Sigee D.C., Estrada B., Pittman J.K., Using FTIR spectroscopy for rapid determination of lipid accumulation in response to nitrogen limitation in freshwater microalgae, Bioresour. Technol. 101 (2010) 4499–4507. 10.1016/j.biortech.2010.01.065 [DOI] [PubMed] [Google Scholar]
- 81.Yi Z., Xu M., Di X., Brynjolfsson S., Fu W., Exploring valuable lipids in diatoms, Front. Mar. Sci. 4 (2017). 10.3389/fmars.2017.00017 [DOI] [Google Scholar]
- 82.Olga Sayanova, Virginie Mimouni, Lionel Ulmann, Annick Morant-Manceau, Virginie Pasquet, Schoefs Benoît Napier Johnathan A., Modulation of lipid biosynthesis by stress in diatoms, Philos. Trans. R. Soc. B Biol. Sci. 372 (2017) 20160407 10.1098/rstb.2016.0407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Vårum K.M., Myklestad S., Effects of light, salinity and nutrient limitation on the production of β-1,3-d-glucan and exo-d-glucanase activity in Skeletonema costatum (Grev.) Cleve, J. Exp. Mar. Biol. Ecol. 83 (1984) 13–25. 10.1016/0022-0981(84)90114-X [DOI] [Google Scholar]
- 84.Huntley M.E., Johnson Z.I., Brown S.L., Sills D.L., Gerber L., Archibald I., Machesky S.C., Granados J., Beal C., Greene C.H., Demonstrated large-scale production of marine microalgae for fuels and feed, Algal Res. 10 (2015) 249–265. 10.1016/j.algal.2015.04.016 [DOI] [Google Scholar]
- 85.Kates M., Volcani B.E., Lipid components of diatoms, Biochim. Biophys. Acta BBA—Lipids Lipid Metab. 116 (1966) 264–278. 10.1016/0005-2760(66)90009-9 [DOI] [PubMed] [Google Scholar]
- 86.Chen G., Jiang Y., Chen F., Fatty acid and lipid class composition of the eicosapentaenoic acid-producing microalga, Nitzschia laevis, Food Chem. 104 (2007) 1580–1585. 10.1016/j.foodchem.2007.03.008 [DOI] [Google Scholar]
- 87.Volkman J.K., Eglinton G., Corner E.D.S., Sterols and fatty acids of the marine diatom Biddulphia sinensis, Phytochemistry. 19 (1980) 1809–1813. 10.1016/S0031-9422(00)83818-2 [DOI] [Google Scholar]
- 88.Jiang H., Gao K., Effects of Lowering Temperature During Culture on the Production of Polyunsaturated Fatty Acids in the Marine Diatom Phaeodactylum Tricornutum (bacillariophyceae)1, J. Phycol. 40 (2004) 651–654. 10.1111/j.1529-8817.2004.03112.x [DOI] [Google Scholar]
- 89.Ackman R.G., Jangaard P.M., Hoyle R.J., Brockerhoff H., Origin of marine fatty acids. I. Analyses of the fatty acids produced by the diatom Skeletonema costatum, J. Fish. Res. Board Can. 21 (1964) 747–756. 10.1139/f64-067 [DOI] [Google Scholar]
- 90.Griffiths M.J., van Hille R.P., Harrison S.T.L., Lipid productivity, settling potential and fatty acid profile of 11 microalgal species grown under nitrogen replete and limited conditions, J. Appl. Phycol. 24 (2012) 989–1001. 10.1007/s10811-011-9723-y [DOI] [Google Scholar]
- 91.Plumley F.G., Schmidt G.W., Nitrogen-dependent regulation of photosynthetic gene expression, Proc. Natl. Acad. Sci. 86 (1989) 2678–2682. 10.1073/pnas.86.8.2678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yu E.T., Zendejas F.J., Lane P.D., Gaucher S., Simmons B.A., Lane T.W., Triacylglycerol accumulation and profiling in the model diatoms Thalassiosira pseudonana and Phaeodactylum tricornutum (Baccilariophyceae) during starvation, J. Appl. Phycol. 21 (2009) 669 10.1007/s10811-008-9400-y [DOI] [Google Scholar]
- 93.Xia S., Wan L., Li A., Sang M., Zhang C., Effects of nutrients and light intensity on the growth and biochemical composition of a marine microalga Odontella aurita, Chin. J. Oceanol. Limnol. 31 (2013) 1163–1173. 10.1007/s00343-013-2092-4 [DOI] [Google Scholar]
- 94.Griffiths M.J., Harrison S.T.L., Lipid productivity as a key characteristic for choosing algal species for biodiesel production, J. Appl. Phycol. 21 (2009) 493–507. 10.1007/s10811-008-9392-7 [DOI] [Google Scholar]
- 95.Pessôa M.G., Vespermann K.A.C., Paulino B.N., Barcelos M.C.S., Pastore G.M., Molina G., Newly isolated microorganisms with potential application in biotechnology, Biotechnol. Adv. 37 (2019) 319–339. 10.1016/j.biotechadv.2019.01.007 [DOI] [PubMed] [Google Scholar]
- 96.Amaro H.M., Guedes A.C., Malcata F.X., Advances and perspectives in using microalgae to produce biodiesel, Appl. Energy. 88 (2011) 3402–3410. 10.1016/j.apenergy.2010.12.014 [DOI] [Google Scholar]
- 97.Sharma K.K., Schuhmann H., Schenk P.M., High lipid induction in microalgae for biodiesel production, Energies. 5 (2012) 1532–1553. 10.3390/en5051532 [DOI] [Google Scholar]
- 98.Ozkan A., Kinney K., Katz L., Berberoglu H., Reduction of water and energy requirement of algae cultivation using an algae biofilm photobioreactor, Bioresour. Technol. 114 (2012) 542–548. 10.1016/j.biortech.2012.03.055 [DOI] [PubMed] [Google Scholar]
- 99.Tian X., Liao Q., Zhu X., Wang Y., Zhang P., Li J., Wang H., Characteristics of a biofilm photobioreactor as applied to photo-hydrogen production, Bioresour. Technol. 101 (2010) 977–983. 10.1016/j.biortech.2009.09.007 [DOI] [PubMed] [Google Scholar]
- 100.Schultze L.K., Simon M.-V., Li T., Langenbach D., Podola B., Melkonian M., High light and carbon dioxide optimize surface productivity in a Twin-Layer biofilm photobioreactor, Algal Res. 8 (2015) 37–44. [Google Scholar]
- 101.Silva-Aciares F.R., Riquelme C.E., Comparisons of the growth of six diatom species between two configurations of photobioreactors, Aquac. Eng. 38 (2008) 26–35. 10.1016/j.aquaeng.2007.10.005 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All 24 files are available from the figshare database https://doi.org/10.6084/m9.figshare.9810389.v3.