Abstract
Introduction
Lumbar interbody fusion is used to treat degenerative lumbar spondylolisthesis with instability. We developed a device that safely expands a percutaneous path through Kambin's triangle and used it via a new technique: percutaneous endoscopic transforaminal lumbar interbody fusion (PETLIF). We report in this study the details and outcomes of this procedure after a one year follow-up.
Methods
Twenty-five patients requiring interbody fusion for degenerative spondylolisthesis of the L4 vertebra were enrolled in this study. The procedure involved percutaneous posterior pedicle screw placement to correct spondylolisthesis. After the exterior of the L5 vertebra superior articular protrusion was shaved with a percutaneous endoscopic drill in order to expand the safe zone, the oval sleeve was inserted through Kambin's triangle and was rotated to expand the disk height and create a path toward the vertebral disk. The interbody cage was inserted against the J-shaped nerve retractor, with the exiting nerve root retracted. Indirect decompression of spinal canal stenosis was expected because the vertebral body spondylolisthesis had been corrected and the interbody distance was expanded. Thus, no direct decompression was performed posterolaterally.
Results
The mean follow-up period, surgery time, and blood loss were 22.7 months, 125.4 min, and 64.8 mL, respectively. The Japanese Orthopaedic Association score improved from 13.3 to 28.0. The Roland-Morris Disability Questionnaire score improved from 10.3 to 3.3. All items were evaluated both preoperatively and one year postoperatively. Bone fusion was observed one year postoperatively in 22 out of 25 patients.
Conclusions
These results demonstrate the feasibility and efficacy of PETLIF for treating degenerative lumbar spondylolisthesis. This minimally invasive procedure is useful and has wide applicability. To obtain safe and favorable results, necessary surgical techniques must be mastered, and surgical equipment, including that for neural monitoring, is required.
Keywords: Percutaneous endoscopic surgery, Lumbar interbody fusion, Degenerative lumbar spondylolisthesis, New surgical technique
Introduction
Degenerative lumbar spondylolisthesis involves symptoms resulting from anteroposterior spondylolisthesis of the lumbar vertebrae occurring because of aging-associated degeneration1). Degenerative spondylolisthesis usually occurs at the L4 level2,3) and in approximately 10% of women aged ≥60 years4). Following conservative management, surgery is an option and has an established efficacy5). Lumbar interbody fusion is performed in cases with high instability, and the mainstream methods include posterior lumbar interbody fusion, as reported in the study by Cloward et al. in 19536), and transforaminal LIF (TLIF), as reported in the study by Harms et al. in 19827). As spinal surgery progressed, TLIF became less invasive. Minimally invasive TLIF (MIS-TLIF), which includes fusion of vertebrae using percutaneous pedicle screws (PPS), was first reported in the study by Foley et al. in 20018). It became a popular procedure, causing a shift toward less invasive surgery. Many studies have shown that MIS-TLIF involves less blood loss, has a shorter recovery time, and requires fewer days in the hospital by comparison with open TLIF9-12). However, in addition to MIS-TLIF, partial laminectomy, facetectomy, and ligamentum flavum dissection, an open incision of the musculature is required to reach the vertebrae.
If the vertebrae are approached posterolaterally via Kambin's triangle, invasive procedures can be avoided13,14). This is a standard route in percutaneous endoscopic discectomy (PED)15-17). If this path can be sufficiently expanded to allow for the passage of an interbody cage of the size used in open TLIF, it may be possible to apply LIF while completely preserving the facet joint. In this study, we developed a surgical device comprising an oval dilator, an oval sleeve, and a J-shaped nerve retractor. This device is capable of securing a percutaneous path large enough to safely insert an interbody cage. We also conceived a new technique, percutaneous endoscopic TLIF (PETLIF). To our knowledge, this is the first report on PETLIF in the treatment of degenerative lumbar spondylolisthesis with instability, using a cage size similar to that used in TLIF. We described here the early clinical results of this new technique.
Materials and Methods
Patients
Twenty-five patients with degenerative lumbar spondylolisthesis (5 men, 20 women; mean age, 68.4 years; standard deviation [SD], 9.1 years) underwent PETLIF at our institution between February 2016 and July 2018. The mean follow-up period was 22.7 (range, 12-30) months. These patients were also diagnosed at our institution. All of them were preoperatively informed about the characteristics, difficulty, and potential complications of the procedure. Written informed consent was obtained from all patients prior to commencement of the study. The study was approved by the ethics committee for clinical research at our institution.
Inclusion criteria
We included patients who were diagnosed with degenerative lumbar spondylolisthesis at the L4 level, with lumbar pain and radicular symptoms caused by intervertebral instability that was associated with spinal canal stenosis. All patients were refractory to conservative treatment and required interbody fusion. Imaging was performed using functional radiography, computed tomography (CT), and magnetic resonance imaging (MRI). Instability was diagnosed if the vertebral slippage was ≥4 mm or if the mobility in flexion or extension was ≥10°2-4).
Evaluation method
The following metrics were used to evaluate the surgery: surgical time, volume of intraoperative blood loss, and volume of postoperative drainage. To evaluate functions before surgery, at hospital discharge, and six months and one year after surgery, we utilized the Japanese Orthopaedic Association (JOA) criteria and the Roland-Morris Disability Questionnaire (RDQ) score to assess the treatment outcomes of patients. The presence or absence of bone fusion was ascertained with CT scans one year postoperatively.
Surgical technique of PETLIF
Surgery was conducted under general anesthesia, with the patient in the prone position. The somatosensory-evoked potentials, electromyography, and free-run electromyography were monitored throughout the procedure using a nerve monitoring system (NVM5Ⓡ; NuVasive, San Diego, USA).
Posterior pedicle screw fixation and spondylolisthesis correction
Firstly, all patients underwent posterior PPS fixation using the IBIS Spinal SystemⓇ (Japan Medical Dynamic Marketing, Tokyo, Japan). A screw, with a diameter of 6.5 mm and length of 40 mm, was then inserted under fluoroscopic guidance. Regarding rod positioning, the L5 set screw was fixed, while the L4 side of the rod was floated (Fig. 1A). The spondylolisthesis of the L4 vertebra was then corrected by tightening the L4 set screw (Fig. 1B).
Figure 1.
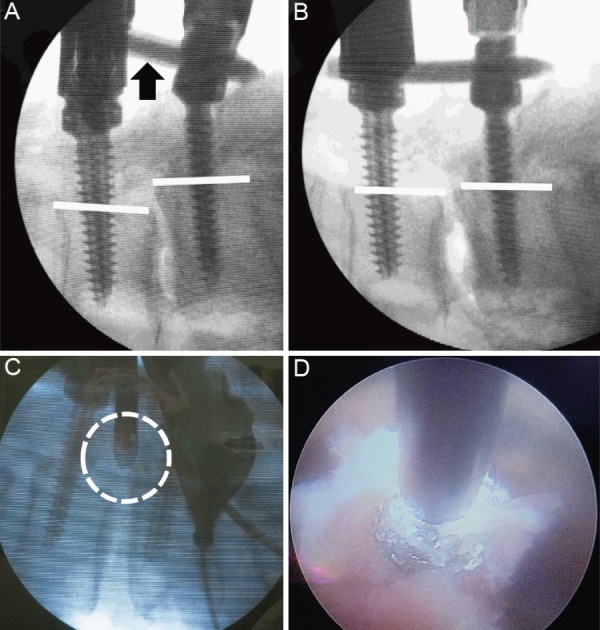
A: With the L4 side of the rod loose (black arrow), the L5 set screw was fixed. B: Correction of the L4 vertebral body spondylolisthesis occurred as the vertebral body and the percutaneous pedicle screws were drawn to the rod; the white line on the back of the vertebral body is almost straight. C: The position of the drill tip was confirmed using imaging (the area encircled by the white dotted line). D: Image showing the shaving of the L5 vertebra superior articular protrusion with a drill using endoscopy.
Expansion of Kambin's triangle
The SpineTIPⓇ transforaminal approach kit (Karl Storz GmbH, Tuttlingen, Germany) endoscopic system was used. To reduce the risk of damaging the exiting nerve root while inserting the cage, the outside of the L5 vertebra superior articular protrusion was shaved with a burr (Nakanishi Primado2Ⓡ total surgical system, Tochigi, Japan) using fluoroscopy and percutaneous endoscopy (Fig. 1C, D).
Harvesting bone for grafting
Bones from the ilium and spinous process were used for grafting and were harvested percutaneously. In the cage, the only filling was the local bone, whereas a mixture of local and artificial bones (hydroxyapatite and tripotassium phosphate composite; PrimaboneⓇ granule size M; 5 g; Japan Medical Dynamic Marketing, Tokyo, Japan) was used for the interbody graft.
Creating a percutaneous path, expanding the interbody distance, and preparing the graft site
New oval dilator, oval sleeve, and J-shaped nerve retractors (Robert Reid Inc., Tokyo, Japan) were used (Fig. 2A, B). After inserting the oval dilator, the oval sleeve, which has a long (10.5 mm) and a short axis (8 mm, outer dimensions), was inserted with the 8-mm cephalocaudal axis. Next, the L5-side set screw of the fixed PPS system was loosened, the oval sleeve was rotated 90° (Fig. 3A), and the interbody distance was expanded to 10.5 mm, which was the length of the longer axis of the oval sleeve (Fig. 3B). The disk was excised via the oval sleeve using curettes and vertebral pulp forceps (Fig. 3C, D).
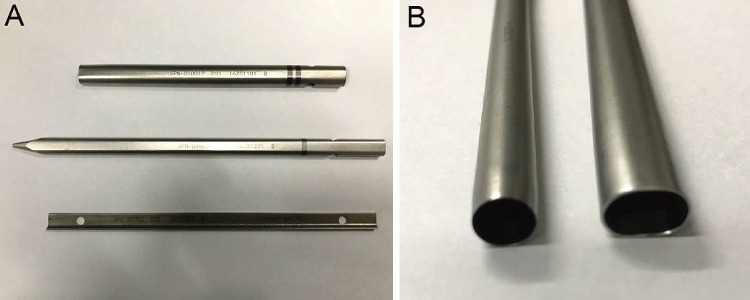
Figure 2.
A: The images in order from top to bottom are of an oval dilator, oval sleeve, and a J-shaped nerve retractor (Robert Reid Inc., Tokyo, Japan). B: The left image is of the oval sleeve for percutaneous endoscopic discectomy, and the right image is of the elliptical sleeve for percutaneous endoscopic transforaminal lumbar interbody fusion. The short axis was set as the same size.
Figure 3.
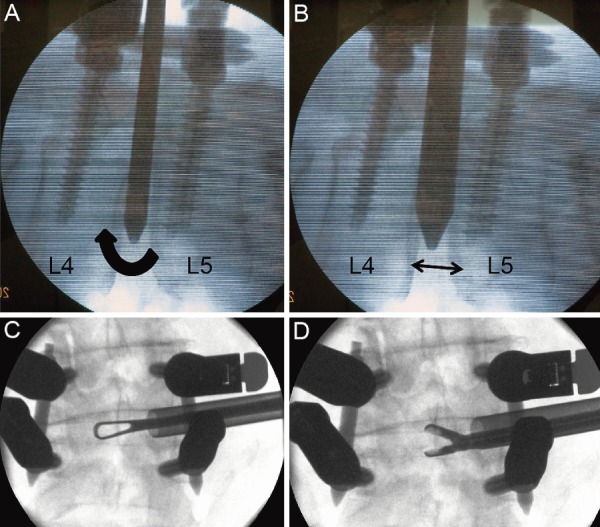
A: The elliptical operating sheath was inserted with the shorter axis cephalocaudal and rotated (in the direction of the black arrow). B: The rotation expanded the interbody distance to 10.5 mm, which was also the length of the longer axis of the operating sheath (black arrow). C and D: A ring curette and vertebral pulp forceps were inserted using a percutaneous endoscopic transforaminal lumbar interbody fusion operating sheath.
Positioning the cage
Graft bone was introduced into the intervertebral space via the oval sleeve (Fig. 4A). Next, a specially designed J-shaped nerve retractor (Robert Reid Inc., Tokyo, Japan) was inserted into the oval sleeve and the oval sleeve was withdrawn. To prevent the exiting nerve root from accidentally encroaching upon the cage insertion route, the J-shaped nerve retractor was kept in the intervertebral space. With the exiting nerve root retracted, the interbody cage was inserted against the J-shaped nerve retractor (Fig. 4B, C). The cage was chosen to match the patient's anatomy and form of his or her vertebral body. A ReyKamⓇ cage (Robert Reid Inc., Tokyo, Japan), measuring 30 × 12 × 9 mm, was inserted in this case (Fig. 4D).
Figure 4.
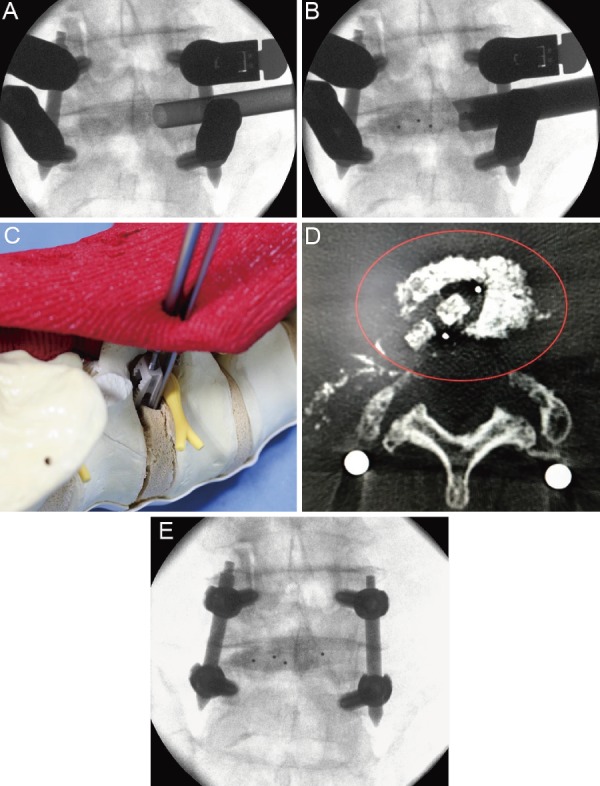
A: The graft bone was introduced into the intervertebral space via the oval sleeve. B and C: The tip of the J-shaped nerve retractor was kept in the intervertebral disk space to prevent the exiting nerve root from straying into the cage entry path, and the cage was inserted along the J-shaped nerve retractor. D: The cage was positioned in the intervertebral space. E: The final task was compression between the vertebral bodies.
Completion of interbody fusion and post-surgery management
Compression was exerted between the vertebral bodies to ensure final bonding (Fig. 4E). The Elliquence Trigger-FlexⓇ bipolar system (Surgi-Max Air, New York, USA) was used to stop any bleeding under percutaneous endoscopic guidance. Finally, a tube drain was installed at the cage insertion site. Regarding the procedure's impact on spinal canal stenosis, indirect decompression was expected because the vertebral body spondylolisthesis was corrected, and the interbody distance was expanded. Consequently, direct decompression was not performed posterolaterally. The patients' activities of daily living were not restricted.
Statistical methods
Continuous variables are presented as mean (SD). Statistical differences were determined using a two-sided paired t-test. All p-values <0.05 were considered significant. Microsoft Excel software (Microsoft, Redmond, WA) was used for analysis.
Results
The mean surgical time, blood loss, and post-surgery drain volume were 125.4 (56.9) min, 64.8 (35.6) mL, and 32.5 (28.6) mL, respectively. Complications occurred in two patients who exhibited a reduction in hip flexion power immediately after surgery that was completely resolved within two weeks. One patient required additional surgery. Although his progress was good after surgery, neural symptoms flared up again one month after surgery because the cage subsided. Thus, posterior decompression was required. The cause was determined to be low compatibility between the surface of the cage and the vertebral body endplate, because the insertion of the cage was rotated about 30° and sinking occurred at an early stage.
The JOA score improved from 13.3 (2.09) preoperatively to 24.9 (1.85; p < 0.001) at discharge, 25.7 (1.43; p < 0.001) six months postoperatively, and 27.3 (1.18; p < 0.001) one year postoperatively. The RDQ score improved from 10.3 (4.56) preoperatively to 10.1 (4.09; p = 0.902) at discharge, 4.8 (2.73; p = 0.002) six months postoperatively, and 3.7 (3.50; p = 0.001) one year postoperatively. One year postoperatively, bone fusion was observed in 22 of the 25 patients. There were no cases of screw loosening.
Case Report
Using imaging, a 67-year-old woman was diagnosed with degenerative spondylolisthesis of the L4 vertebra with instability (Fig. 5A, B). PETLIF was performed (Fig. 5C). At the time of hospital discharge, the patient's lumbar and leg pain had disappeared. Postoperative MRI revealed that her spinal canal had expanded by 176% due to indirect decompression (Fig. 5D).
Figure 5.
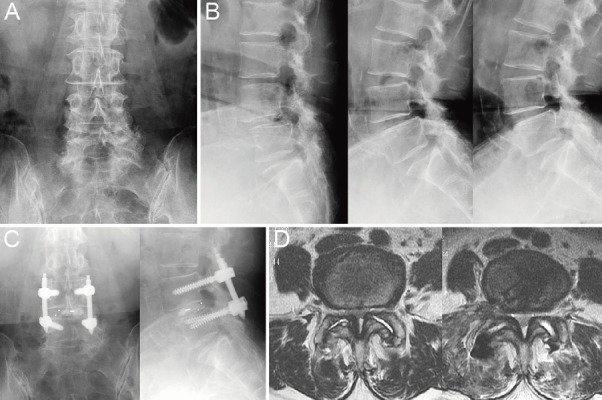
A: A patient with degenerative changes surrounding the L4/L5 facet joint and anterior spondylolisthesis of L4. B: Functional imaging shows intervertebral mobility of 17° and spondylolisthesis of 4 mm. C: X-ray of the lumbar vertebrae immediately after surgery. D: Left, preoperative axial magnetic resonance image (MRI); right, postoperative axial MRI; the spinal canal was expanded from 55.7 mm2 to 92.6 mm2.
Discussion
LIF via Kambin's triangle is a type of MIS-TLIF, which is a microendoscopy-assisted surgery that uses a small incision. Recently, a less invasive percutaneous LIF technique via Kambin's triangle has also been reported18-20). Various reports characteristically state how exiting nerve root injury can be avoided during cage insertion. Wang et al.18) in their study inserted a mesh expandable cage (Spineology's OptiMeshⓇ graft containment, CA, USA) from within a sleeve. This technique is safer because cage insertion is completed via a sleeve using PED techniques, but the strength of fixation remained lower than that of a box-shaped interbody cage using open techniques. On the other hand, there are also reports on the use of a box-shaped interbody cage using open techniques. Nakamura et al.19) used an L-shaped nerve retractor to insert the cage via Kambin's triangle. An L-shaped nerve retractor was inserted into the PED sleeve and the PED sleeve was withdrawn. This technique is the same as PETLIF. Our J-shaped nerve retractor is inserted via a unique oval sleeve; therefore, it differs in size and shape from the L-shaped nerve retractor. By fully covering the cranial side of the cage by this J-shaped retractor, we were able to reduce the risk of having the cage in direct contact with the exiting nerve root. Abbasi et al.20) in their study inserted a guidewire in the middle of the disk from Kambin's triangle and prepared the graft site and inserted the cage via that guidewire. To avoid damage to the exiting nerve root, they used a cage that was shaped to facilitate its avoidance to the exiting nerve root. Whether the exiting nerve root could be retracted safely cannot be confirmed. Most of these reports are about the treatment of lumbar discopathy. However, severe lumbar spondylolisthesis is usually accompanied by disk height narrowing and foraminal stenosis. And in such cases, the Kambin's triangle is also often found to be shrunk which could significantly complicate the perioperative manipulations of the spinal nerves traveling through the triangle and increase the risk of nerve injury. In addition, whether it is safe and effective for spondylolisthesis accompanied by neurologic symptoms has not been adequately verified. There is some novelty about the PETLIF we report in terms of how the risk due to spondylolisthesis is avoided and how the preoperative neurologic symptoms can be improved.
The concepts realized with PETLIF are the 5 points described below.
① Vertebral body spondylolisthesis is corrected by a technique based on the percutaneous pedicle screw (PPS) system.
② By using the oval sleeve, the intervertebral body height is safely and easily increased, thereby expanding the safe area.
③ By temporarily locking the set screws of PPS, the condition is maintained.
④ The interbody cage is safely and accurately set in the proper position.
⑤ Good result is obtained only by indirect decompression for neurologic symptoms of the lower limbs.
For concept ①, the prerequisite is that surgical safety should first be increased. If correction of the spondylolisthesis is inadequate, a change of the surgical modality must be considered. What is most emphasized in this report is the fact that an oval sleeve was developed and used during surgery. It is not the PED sleeve that is already being used worldwide, but an oval sleeve that expands the working area while safety is maintained, making it a novel and unique idea. Introduction of this oval sleeve enabled concepts ② and ③ to be realized safely. For concept ④, in order to install a cage of a size that is adequate to maintain safety, when inserting the cage, the intervertebral body distance needs to be opened and maintained in that state. If those are inadequate, caution is required as there is the risk of stress toward the exiting nerve root increasing even if the nerve retractor is preserved, and the risk of vertebral body endplate injury by the cage. Concept ⑤ is of most importance in lumbar spinal fusion. Maintaining the states acquired in concepts ①-④ is also paramount. To achieve that, presurgical image evaluation to select the proper size and shape of the interbody cage is essential. The cage size is decided based on the position of the exiting nerve, shape of the L5 superior articular process, and height of the interbody using preoperative radiographical assessment (Fig. 6). If the exiting nerve path is anterior, then the safety margin at insertion is wide, and the insertion of a larger cage with a 12-mm diameter becomes possible. In terms of cage height, it is possible to insert a cage with a maximum height of 12 mm in cases with naturally high disk heights, as the safety margin on the caudal side of the Kambin's triangle widens.
Figure 6.
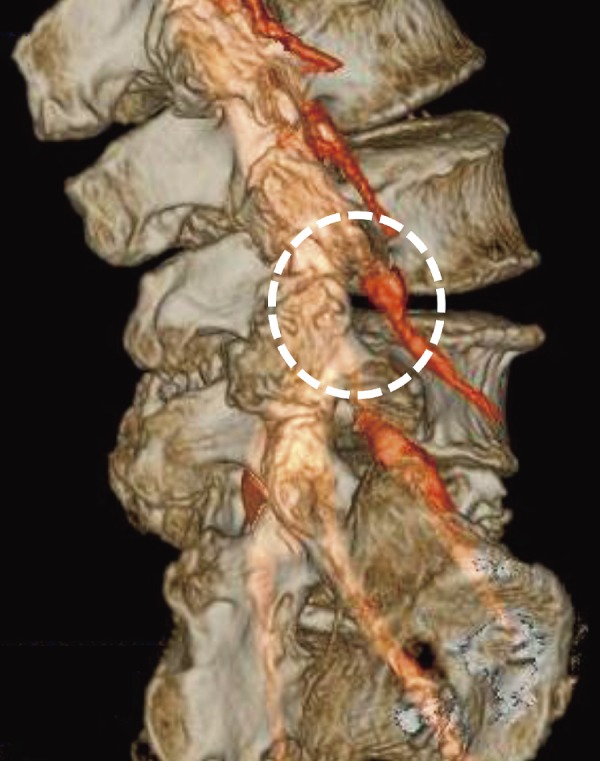
The cage location, confirmed with a multimodality fusion image (the white dotted circle marks the insertion position).
Recently, surgeons have utilized minimally invasive lateral LIF (LLIF) techniques, such as oblique LIF and extreme lateral interbody fusion21-23). However, with LLIF, there are issues with the insertion path, along with the associated damage to major blood vessels, somatic arteries, the greater psoas muscle, lumbar plexus, urinary duct, or internal organs24-26). Although the posterolateral route via Kambin's triangle involves risk of damage to the exiting nerve root, once this is avoided, no risk of complications can arise with the use of LLIF. Furthermore, unlike the cage in LLIF, the cage in PETLIF can be inserted in the same position from either the left or right side. For example, in cases of degenerative scoliosis, the interbody cage can be inserted from the convex side with a wider safety zone. With the LLIF procedure, an indirect decompression effect on degenerative spondylolisthesis has been reported, which does not require direct surgery on the nerve tissue or on the epidural venous plexus27-30). With PETLIF, an improvement in preoperative radicular symptoms was recognized in all cases due to indirect decompression following the insertion of a cage with a height of 9 or 10 mm and correction of vertebral body spondylolisthesis using PPS.
Limitation
The first limitation of this study is that using PETLIF, it is assumed that a cage can be inserted from a widened Kambin's triangle. It is essential to be aware of the increased risk associated with the expansion of the interbody height or the increased risk if the correction of vertebral body spondylolisthesis during surgery is insufficient. In our hospital, we halt any procedure when nerve monitoring shows stress levels on the nerve root, subsequently prompting us to switch to open surgery using TLIF. The possibility of this happening was explained to patients before surgery and their consent was obtained. In this series, two cases were converted to open TLIF. The reason for the conversion was that there was insufficient reduction of spondylolisthesis of the L4. It is important to conduct a thorough preoperative radiographical assessment to ascertain if reduction of the spondylolisthesis is possible. We plan to discuss the dividing line between the indicators of TLIF and PETLIF in a future study.
The second limitation of this study was the small number of cases involved, and the third limitation was that it was not a randomized controlled study. Without comparisons to other surgical methods, the utility of PETLIF cannot be accurately judged. However, we plan to conduct a comparison between PETLIF and existing surgical techniques in the next stage.
Conclusion
This study described a new modified technique, PETLIF, for the treatment of degenerative lumbar spondylolisthesis and also evaluated relevant early clinical results. Intervertebral fusion surgery using Kambin's triangle is a less invasive procedure, but the risk of damaging the exiting nerve root is an important consideration. The safety margin can be increased by enlarging Kambin's triangle under percutaneous endoscopy. To obtain safe and favorable results, proficiency in surgical techniques involved, as well as in terms of surgical equipment, including equipment needed for neural monitoring, is required. This procedure is highly applicable for minimally invasive interbody fusion.
Disclaimer: Manabu Ito is one of the Editors of Spine Surgery and Related Research and on the journal's Editorial Committee. He was not involved in the editorial evaluation or decision to accept this article for publication at all.
Conflicts of Interest: The authors declare that there are no relevant conflicts of interest.
Acknowledgement
We would like to thank Editage (www.editage.com) for English language editing and Publication Support.
References
- 1.Matsunaga S, Sakou T, Morizono Y, et al. Natural history of degenerative spondylolisthesis: pathogenesis and natural course of the slippage. Spine (Phila Pa 1976). 1990;15(11):1204-10. [DOI] [PubMed] [Google Scholar]
- 2.Rosenberg NJ. Degenerative spondylolisthesis. Predisposing factors. J Bone Joint Surg Am. 1975;57(4):467-74. [PubMed] [Google Scholar]
- 3.Herkowitz HN. Spine update: degenerative lumbar spondylolisthesis. Spine (Phila Pa 1976). 1995;20(9):1084-90. [DOI] [PubMed] [Google Scholar]
- 4.Frymoyer JW. Degenerative spondylolisthesis: diagnosis and treatment. J Am Acad Orthop Surg. 1994;2(1):9-15. [DOI] [PubMed] [Google Scholar]
- 5.Weinstein JN, Lurie JD, Tosteson TD, et al. Surgical versus nonsurgical treatment for lumbar degenerative spondylolisthesis. N Engl J Med. 2007;356(22):2257-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cloward RB. The treatment of ruptured lumbar intervertebral disc by vertebral body fusion. III. Method of use of banked bone. J Neurosurg. 1953;10(2):154-68. [DOI] [PubMed] [Google Scholar]
- 7.Harms J, Rolinger H. [A one-stage procedure in operative treatment of spondylolisthesis: dorsal traction-reposition and anterior fusion (author's translation)]. Z Orthop Ihre Grenzgeb. 1982;120(3):343-7. German. [DOI] [PubMed] [Google Scholar]
- 8.Foley KT, Gupta SK, Justis JR, et al. Percutaneous pedicle screw fixation of the lumbar spine. Neurosurg Focus. 2001;10(4):E10. [DOI] [PubMed] [Google Scholar]
- 9.Kim JS, Jung B, Lee SH. Instrumented minimally invasive spinal-transforaminal lumbar interbody fusion (MIS-TLIF); minimum 5-years follow-up with clinical and radiologic outcomes. Clin Spine Surg. 2018;31(6):E302-9. [DOI] [PubMed] [Google Scholar]
- 10.Kim TY, Kang KT, Yoon DH, et al. Effects of lumbar arthrodesis on adjacent segments: differences between surgical techniques. Spine. 2012;37(17):1456-62. [DOI] [PubMed] [Google Scholar]
- 11.Ruetten S, Komp M, Merk H, et al. Full-endoscopic interlaminar and transforaminal lumbar discectomy versus conventional microsurgical technique: a prospective, randomized, controlled study. Spine (Phila Pa 1976). 2008;33(9):931-9. [DOI] [PubMed] [Google Scholar]
- 12.Goldstein C, Macwan K, Sundararajan K, et al. Comparative outcomes of minimally invasive surgery for posterior lumbar fusion: a systematic review. Clin Orthop Relat Res. 2014;472(6):1727-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kambin P, Zhou L. Arthroscopic discectomy of the lumbar spine. Clin Orthop Relat Res. 1997;337:49-57. [DOI] [PubMed] [Google Scholar]
- 14.Kambin P, Sampson S. Posterolateral percutaneous suction-excision of herniated lumbar intervertebral discs. Report of interim results. Clin Orthop Relat Res. 1986;207:37-43. [PubMed] [Google Scholar]
- 15.Yeung AT, Tsou PM. Posterolateral endoscopic excision for lumbar disc herniation: surgical technique, outcome, and complications in 307 consecutive cases. Spine (Phila Pa 1976). 2002;27(7):722-31. [DOI] [PubMed] [Google Scholar]
- 16.Tsou PM, Yeung AT. Transforaminal endoscopic decompression for radiculopathy secondary to intracanal noncontained lumbar disc herniations: outcome and technique. Spine J. 2002;2(1):41-8. [DOI] [PubMed] [Google Scholar]
- 17.Ruetten S, Komp M, Godolias G. An extreme lateral access for the surgery of lumbar disc herniations inside the spinal canal using the full-endoscopic uniportal transforaminal approach-technique and prospective results of 463 patients. Spine (Phila Pa 1976). 2005;30(22):2570-8. [DOI] [PubMed] [Google Scholar]
- 18.Wang MY, Grossman J. Endoscopic minimally invasive transforaminal interbody fusion without general anesthesia: initial clinical experience with 1-year follow-up. Neurosurg Focus. 2016;40(2):E13. [DOI] [PubMed] [Google Scholar]
- 19.Nakamura S, Taguchi M. Full percutaneous lumbar interbody fusion: technical note. J Neurol Surg A. 2017;78(6):601-6. [DOI] [PubMed] [Google Scholar]
- 20.Abbasi H, Abbasi A. Oblique lateral lumbar interbody fusion (OLLIF): technical notes and early results of a single surgeon comparative study. Cureus. 2015;7(10):e351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ozgur BM, Aryan HE, Pimenta L, et al. Extreme lateral interbody fusion (XLIF): a novel surgical technique for anterior lumbar interbody fusion. Spine J. 2006;6(4):435-43. [DOI] [PubMed] [Google Scholar]
- 22.Silvestre C, Mac-Thiong JM, Hilmi R, et al. Complications and morbidities of mini-open anterior retroperitoneal lumbar interbody fusion: oblique lumbar interbody fusion in 179 patients. Asian Spine J. 2012;6(2):89-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fujibayashi S, Hynes RA, Otsuki B, et al. Effect of indirect neural decompression through oblique lateral interbody fusion for degenerative lumbar disease. Spine (Phila Pa 1976). 2015;40(3):E175-82. [DOI] [PubMed] [Google Scholar]
- 24.Cummock MD, Vanni S, Levi AD, et al. An analysis of postoperative thigh symptoms after minimally invasive transpsoas lumbar interbody fusion. J Neurosurg Spine. 2011;15(1):11-8. [DOI] [PubMed] [Google Scholar]
- 25.Rodgers WB, Gerber EJ, Patterson J. Intraoperative and early postoperative complications in extreme lateral interbody fusion: an analysis of 600 cases. Spine (Phila Pa 1976). 2011;36(1):26-32. [DOI] [PubMed] [Google Scholar]
- 26.Uribe JS, Dakwar E, Le TV, et al. Minimally invasive surgery treatment for thoracic spine tumor removal: a mini-open, lateral approach. Spine (Phila Pa 1976). 2010;35(26):S347-54. [DOI] [PubMed] [Google Scholar]
- 27.Castellvi AE, Nienke TW, Marulanda GA, et al. Indirect decompression of lumbar stenosis with transpsoas interbody cages and percutaneous posterior instrumentation. Clin Orthop Relat Res. 2014;472(6):1784-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Elowitz EH, Yanni DS, Chwajol M, et al. Evaluation of indirect decompression of the lumbar spinal canal following minimally invasive lateral transpsoas interbody fusion: radiographic and outcome analysis. Minim Invasive Neurosurg. 2011;54(5-6):201-6. [DOI] [PubMed] [Google Scholar]
- 29.Kepler CK, Sharma AK, Huang RC, et al. Indirect foraminal decompression after lateral transpsoas interbody fusion. J Neurosurg Spine. 2012;16(4):329-33. [DOI] [PubMed] [Google Scholar]
- 30.Oliveira L, Marchi L, Coutinho E, et al. A radiographic assessment of the ability of the extreme lateral interbody fusion procedure to indirectly decompress the neural elements. Spine (Phila Pa 1976). 2010;35(26):S331-7. [DOI] [PubMed] [Google Scholar]