Abstract
Previous studies showed that intratumoral 27-Hydroxycholesterol (27-HC), a metabolite of cholesterol, promotes growth, invasion and migration of breast cancer cells and that tumor-associated macrophages (TAMs) in breast cancers are closely related to tumor growth and metastatic progression. However, the relationship between 27-HC and TAMs in breast cancer remains unclear. In the present study, we observed that CYP27A1, the 27-HC synthesizing enzyme, was expressed in a much higher level in THP1 monocytes and THP1-derived macrophages than in breast cancer cells, and the promoter of CYP7B1, the degrading enzyme for 27-HC, was highly methylated in breast tumor cells. In addition, THP-1 monocytes and murine bone marrow cells were differentiated toward M2 type macrophages after being co-cultured with breast cancer cells or being exposed to exosomes derived from breast cancer cells. M2 type macrophages produced higher amounts of 27-HC than M0 and M1 type macrophages. 27-HC not only stimulated ER+ cancer cell proliferation as reported, but also promoted the recruitment of CCR2- and CCR5-expressing monocytes by inducing macrophages to express multiple chemokines including CCL2, CCL3 and CCL4. Taken together, our data demonstrate that the hypermethylation of CYP7B1 and recruitment of monocytes likely contribute to the accumulation of 27-Hydroxycholesterol in breast cancer and that the interaction of 27-HC with macrophages further promote the development of breast cancer.
Keywords: 27-Hydroxycholesterol, CYP27A1, CYP7B1, DNA methylation, macrophage, exosome
Introduction
Breast cancer is one of the most commonly diagnosed cancers in women. Epidemiological data suggests that life-style factors, such as increased lipid uptake and decreased physical exercise, contribute to the high morbidity of mammary gland carcinoma [1-3]. Excessive cholesterol either absorbed from fat-rich food or endogenously synthesized is a risk factor for breast cancer [4,5]. Recent studies have identified 27-Hydroxycholesterol (27-HC), a circulating metabolite of cholesterol, the first known endogenous selective estrogen receptor modulator (SERM) [6-8], which is elevated in breast cancer tissues, plays a pathogenic role in driving proliferation [9], invasion and migration of breast cancer cells [10], and thereby establishes a direct link between cholesterol and breast cancer [11]. Since 27-HC can bind to estrogen receptor (ER) and activate proliferative pathways of ER+ breast cancer cells, it might contribute to the drug resistance to anti-estrogen therapy such as aromatase inhibitor [12,13]. Therefore, 27-HC may serve as a new potential target for ER+ breast cancer therapy.
27-HC is synthesized by cytochrome P450 oxidase (CYP27A1) from cholesterol and degraded by oxysterol 7α-hydroxylase (CYP7B1). After being synthesized in the mitochondrial matrix, 27-HC is metabolized in the catabolic pathway of bile acids and bile salts, or transported to the extracellular space to exert various biological functions including cellular signaling, lipid metabolism and vesicle transport in paracrine/intracrine manners [7,14]. Nelson ER et al revealed that 27-HC promotes breast cancer cell growth and metastasis in mouse models of ER+ breast cancer [10,11]. Wu Q et al also demonstrated that dyslipidemia, a medical condition of high cholesterol, increases the risk of breast cancer through 27-HC stimulated cell proliferation in ER-dependent and GDNF-RET-dependent manners and there was 27-HC accumulation in breast cancer tissue [9]. Nelson lab recently showed that 27-HC is a major biochemical mediator of breast cancer metastatic through polymorphonuclear-neutrophils and γδ-T cells in hypercholesterolemia patients [15]. Thus, mounting evidence suggests that 27-HC plays an important role in breast cancer progression. However, the exact source leading to 27-HC accumulation in breast tumor tissues has yet to be identified, although CYP27A1 is detected in macrophages within human benign and malignant mammary tissue [11].
Tumor growth is highly dependent upon tumor microenvironments. The tumor-associated macrophage (TAM) plays important roles in modulating the favorable tumor microenvironment [16-18]. Macrophages are classified into two major subgroups, namely classical inflammatory M1 and alternatively activated M2 macrophages [19,20], based on the expression profiles of distinct cell-surface markers and secreted cytokines and chemokines. M1 type macrophages produce high levels of inducible nitric oxide synthase (iNOS), interleukin (IL) -12 and tumor necrosis factors (TNFs), which tend to kill cancer cells. M2 type macrophages can produce IL-10 and transforming growth factor (TGF)-β to induce anti-inflammatory response, wound healing and pro-tumorigenic properties [21]. TAM closely resembles M2 type macrophage [22,23], not only facilitate tumor growth and angiogenesis [24-26], but also promote tumor invasion and metastasis [17,27-29]. In addition, TAM is able to protect cancer cells from immune elimination [30,31]. Therefore, TAM is one of the major players in the tumor microenvironment [16,22], in which it has become an important target for antitumor therapy [18,32,33].
In the present study, we examined the expression and epigenetic regulation of 27-HC metabolic enzymes CYP27A1 and CYP7B1 in macrophages and breast cancer cells. We observed that both CYP27A1 and CYP7B1 were expressed at a very low level in breast cancer cell lines, particularly CYP7B1 is hypermethylated in ER+ breast cancer cell lines and primary tumor tissues. On the contrary, monocytes and macrophages expressed a high level of CYP27A1 and produced a significant amount of 27-HC. Monocytes differentiated toward M2 type macrophages upon exposure to breast cancer cells and displayed increased production of 27-HC (M2 > M0 or M1). Moreover, 27-HC significantly induced secretion of chemokines in macrophages, which could facilitate recruitment of more monocytes to tumor sites. Therefore, our data demonstrated that the recruitment of monocytes and epigenetic silencing of 27-HC degrading enzymes CYP7B1 are responsible for the 27-HC accumulation in breast cancer, particularly in ER+ breast cancer.
Materials and methods
Cell culture and reagents
MCF-7, MDA-MB-231, THP-1 and 4T1 cell lines were tested to be free of mycoplasma. MDA-MB-231 was maintained in DMEM supplemented with 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin; other cell lines were cultured with RPMI 1640 supplemented with 10% FBS and 1% penicillin/streptomycin at 37°C with 5% CO2. 27-Hydroxycholesterol was purchased from Santa Cruz (sc-358756). β-Estradiol (E2) was bought from Sigma (E2758). Recombinant mouse M-CSF (GM10087M, Genomeditech), LPS (L2880, Sigma), and recombinant mouse IL-4 (AF214-14, Peprotech) were used at a final concentration of 20 ng/ml, 200 ng/ml and 10 ng/ml, respectively. Human 27-HyCL (SY-01096) and mouse 27-HyCL (SY-M0767) ELISA kits were purchased from Shanghai Shuangying Biological Co. Ltd.
Cell proliferation assay
CyQUANT® Cell Proliferation Assay Kit (C7026, Invitrogen) was used to measure cell proliferation. Prior to the assay, MCF-7 cells were cultured in DMEM without phenol red (BC005, Sangon Biotech) supplemented with 5% Charcoal-Filtered Serum (CCS30010.01HT, MRC) for two days to eliminate endogenous estrogen, then treated with 10 nM E2 and serial concentrations of 27-HC after being plated in a 96-well plate overnight at the density of 1 × 104 cells/well for 48 hours or 72 hours. Cell proliferation was determined at the end of each experiment according to the manufacturer’s instruction.
Real Time-PCR analysis
Total RNAs were purified using Trizol reagent (Generay Biotech), and cDNA were reverse-transcribed with RevertAid First stand cDNA synthesis kit (Thermo). Real time PCR was performed using a SYBR green PCR master mix purchased from Roche with specific primers of the target genes. The relative fold changes were calculated by 2-ΔΔCt formula comparing to the control group.
Bisulfite pyrosequencing analysis
Genomic DNA was isolated from breast cancer cell lines and primary tumor tissues using AllPrep DNA/RNA kit (Qiagen, Germany). 1 μg of genomic DNA was bisulfite-converted using EZ DNA Methylation Kit (Zymo Research). Pyrosequencing analysis was performed as described previously [34]. Two PCR products were amplified and sequenced using the PyroMark Q24 instrument (Qiagen) and results were analyzed using PyroMark Q24 software. The heatmap representing percentage methylation status of CpG sites was generated using R package.
Western blot analysis
Cells or tissue samples were lysed with protein lysis buffer (1 M Tris-Hcl, 2.5 M NaCl, 0.2 M NaF, 12.5% C24H39O4 Na, 200 mM Na3VO4, 1% TritonX-100) containing protease inhibitor, cocktail (539131, Milipore) and PMSF (P0100, Solarbio). Total protein lysates were separated by 10% SDS-PAGE and transferred to Pure Nitrocellulose Blotting Membrane (66485, Pall). Blots were blocked in blocking buffer containing 5% milk at room temperature (RT) for 1 hour and then incubated with the respective primary antibodies diluted in PBS containing 1% milk overnight at 4°C. Subsequently, blots were washed by PBS containing 0.5% Tween and incubated with secondary antibodies for 1 hour. Protein expressions were detected with WesternBrightTM ECL (Advansta) by ImageQuant by ChemiDocTM XRS+ (Bio-RAD). Antibodies against human-CYP27A1 (YT1202, Immunoway), CYP27A1 (ab126785, abcam) for the detection of mouse cells or tissues, human-CYP7B1 (TA500050S, clone OT17E5, Origene), MMP-9 (YT1892, Immunoway), CD11b (ab133357, abcam), GAPDH (sc-25778, Santa Cruz), β-Actin (sc-47778, Santa Cruz) were used at 1:1,000 dilutions. The secondary antibodies Goat Anti-Rabbit IgG (31460, Thermo) and Goat Anti-mouse IgG (31430, Thermo) were used at 1:5000 dilutions.
Immunofluorescence
MCF-7, MDA-MB-231 and THP-1 cells or co-cultured cells were cultured on glass cover slips in a 24-well plate for the indicated time period, washed twice with PBS after fixing with 4% paraformaldehyde (PFA) for 30 minutes at RT. The cells were incubated in 1% TritonX-100 for 10 minutes and then blocked with 5% BSA for 1 hour at RT, followed by incubation with anti-CYP27A1, anti-CYP7B1 or anti-CD11b antibody overnight at 4°C, respectively. After removing the primary antibody, the slips were washed with PBS for three times (15 minutes), samples were incubated with fluorescence-labeled second antibodies or phalloidin for 1 hour at RT. After three washes with PBS, DAPI solutions were used for visualizing the nucleus. Images were obtained with the Olympus fluorescence microscope (IX83, Japan).
Isolation of mouse peritoneal macrophages
Thioglycolate solutions (4%) were administered by intraperitoneal injection in experimental mice. The mice were sacrificed by CO2 at day 5 post thioglycolate injection, and then sterilized by soaking in 75% alcohol. The abdominal cavity was gently washed and massaged with 10 ml prewarmed PBS for 2-3 times, the irrigated PBS solutions were collected to 50 ml tubes, and the cells were centrifuged at 1500 rpm for 5 minutes. Macrophages were obtained after treatment with red blood cell lysis buffer (CW0613, CWBIO) to eliminate the red blood cells.
HE and immuno-histochemistry staining
Tissue samples were collected from female Balb/c mice and embedded in paraffin after 4% PFA fixation. Paraffin sections were harvested at 3 μm and stained with hematoxylin & eosin or immunostained with anti-CD11b antibody and the appropriate secondary antibody, following DAB kit instruction (CW0125M, CWBIO). Images of the sections were obtained with the Olympus fluorescence microscope (BX63, Japan).
Measurement of 27-HC
Cells were cultured in a 6-well plate up to 80% confluence. Then, cells were washed twice with PBS and cultured in serum-free media for 24 hours. Supernatants were collected and tested for 27-HC content by 27-HyCL ELISA kit following manufacturer’s protocol. The concentration of 27-HC was measured by a SpectraMax M5 spectrophotometer (Molecular Devices, U.S.) at 450 nm. The total cellular proteins were used to normalize 27-HC content.
Flow cytometric analysis
THP-1 cells were seeded in a 6-well plate at 5 × 105/ml and stimulated with or without the media from breast cancer cells 6-10 hours after seeding (1/10-20 of conditional medium) for 4 days with replacement of medium and stimulators every other day. Cells were collected and fixed in 4% PFA. The fixed cells were incubated in PBS blocking buffer containing 0.5% BSA and stained with CD163-APC antibody (17-1639-41, Thermo) and then, the cells were analyzed by flow cytometry (FACS jazz, BD).
Tumor model
The breast cancer cells (4T1, 1 × 106) were inoculated subcutaneously in the mammary gland fat pad of eight-week old female Balb/c mouse (Chang Zhou Cavens Laboratory Animal Ltd.). Tumor onset and growth was monitored daily for 21 days. Then, mice were sacrificed with CO2 and tumor tissues were harvested for histological analyses and immunostaining as described above. All animal experiments used in this study were conducted following protocols approved by the University Committee on the Use and Care of Animal of Nanchang University.
Statistical analysis
Data were analyzed using the two-tailed Student’s t-test. P < 0.05 was considered statistically significant.
Results
THP-1 monocytes and THP-1 derived macrophages secrete a significant higher amount of 27-HC than breast cancer cells
To test our hypothesis that macrophages contribute to production of 27-HC in breast cancer, we first used the murine 4T1 syngeneic transplant tumor model to verify macrophage infiltration in tumor tissues. Mice injected with 4T1 cells developed visible tumors at the injection sites within 7 days. Tumor-bearing mice exhibited several characteristics including increased ratio of spleen to body weight, increased white blood cell count by 10-50 times along with the tumor growth, and high degrees of macrophage infiltration and distorted internal structure in liver, lung and spleen. The abdominal fat completely faded away 3 weeks after inoculation and all tumor-bearing mice developed hepatic and pulmonary metastases, which are similar to the observations of previous report [35]. Macrophage infiltration was examined by CD11b (a marker of macrophage) immunostaining on tumor sections. The results revealed that there were abundant infiltrating CD11b+ cells in the margin or sub-margin areas of tumor sections (Figure 1A). We also performed CIBERSORT (Cell-type Identification By Estimating Relative Subsets Of RNA Transcripts) to analyze macrophage infiltration in various human breast carcinoma and normal human breast tissues from TCGA data set using the markers established by Aaron M. Newman et al [36]. The results showed that infiltration of M0, M1 and M2 type macrophages in breast cancer samples was higher than that of normal breast samples by chi-square test (Figure 1B). Furthermore, we compared 27-HC secretion between macrophages derived from a human monocytic leukemia cell line (THP-1) and two breast cancer cell lines MCF-7 and MDA-MB-231. As shown in Figure 1C, secretion of 27-HC in both THP-1 monocytes and THP-1 derived macrophages (TDM) was greater than MCF-7 and MDA-MB-231 cells. In addition, we analyzed the relationship between the expression of CYP27A1 and macrophage infiltration in TCGA breast cancer samples by dividing cancer samples into macrophage enriched and non-enriched groups. As showed in Figure 1D, the expression of CYP27A1 was significantly increased in M0, M1 and M2 type macrophage enriched groups compared to the non-enriched group by Bayes t-test. These results indicate that macrophages are likely a major source of 27-HC in breast cancer.
Figure 1.
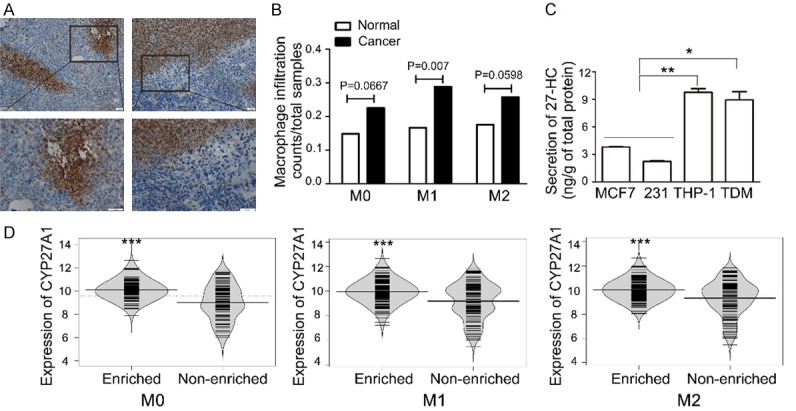
Monocytes/macrophages secrete a higher amount of 27-HC. A. Immunohistochemical staining of CD11b was determined in 4T1-derived breast tumor tissues. B. A meta analyses of macrophage infiltration were performed by chi-square test with 652 breast tumor samples and 114 normal breast tissue samples from TCGA database. C. Secretion of 27-HC was measured by 27-HyCL ELISA kit in breast cancer cells such as MCF-7 and MDA-MB-231 (231), THP-1 cells, and THP-1-derived macrophages (TDM, THP-1 stimulated with 100 nM PMA). *P < 0.05, **P < 0.01 vs MCF-7 or 231, n = 3, the present data are mean ± SEM. D. Expressions of CYP27A1 in different type macrophages in enriched and non-enriched group breast cancer samples in TCGA database.
CYP27A1 expression is significantly higher in THP-1 cells than in breast cancer cells
27-HC contents depend on the expression of both CYP27A1 and CYP7B1. CYP27A1 is mainly expressed in liver, lung and monocytes, while CYP7B1 is ubiquitously expressed in various tissues. 27-HC in blood serum is primarily from the liver, while the local 27-HC level is maintained by various organs themselves. For instance, there is little correlation between the elevated amount of 27-HC in breast cancer tissues and the level of 27-HC in blood serum [9]. Based on our observation that macrophages secrete higher amount of 27-HC than breast cancer cells, we examined the expressions of these two enzymes in MCF-7, MDA-MB-231 and THP-1 by western blot. Both CYP27A1 and CYP7B1 were expressed at a very low level in breast cancer cell lines but abundant in THP-1 (Figure 2A, 2B). Given that the expression of CYP7B1 was decreased in breast cancer cells [9], we paid close attention to the difference of CYP27A1 between macrophage and breast cancer cells. CYP27A1 was barely detected in breast cancer cells, however it is highly expressed in THP-1 cultured alone or cultured with breast cancer cells (Figure 2C). The expression of CYP27A1 was further confirmed by immunofluorescence staining in the co-culture system, in which most fluorescence was observed in THP-1 cells, rather than MCF-7 or MDA-MB-231 cells (co-labeled with phalloidin), though MDA-MB-231 showed greater CYP27A1 expression compared to MCF-7 cells (Figure 2D). In contrast, there were no significant fluorescent differences in CYP7B1 expression in either THP-1 cells or breast cancer cells when they were co-cultured together (Figure 2D). These results indicated that macrophages produce high amount of 27-HC due to increased expression of CYP27A1.
Figure 2.
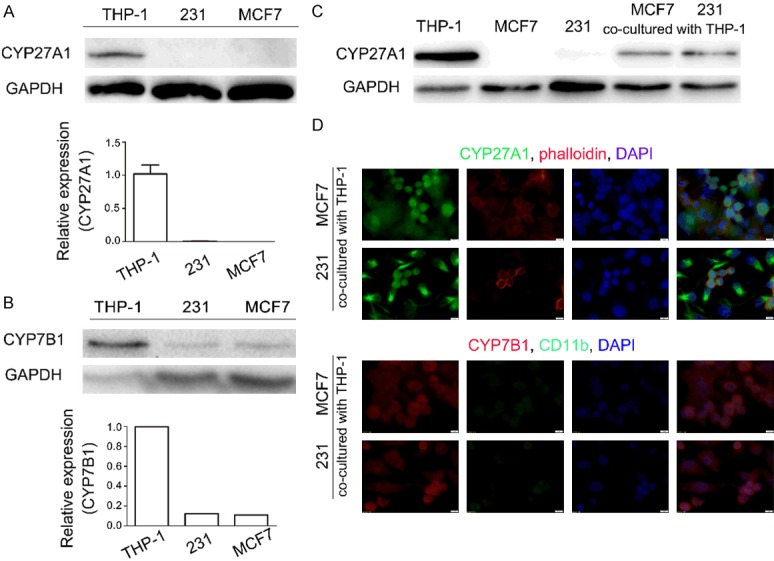
Monocytes/macrophages express high levels of CYP27A1. Expressions of CYP27A1 (A) and CYP7B1 (B) in THP-1 and breast cancer cell lines were determined by western blot. (C) Protein expression of CYP27A1 in the corresponding cells co-cultured with or without THP-1 cells. (D) Immunofluorescence staining with antibodies against CYP27A1, CYP7B1 and CD11b as well as phalloidin which stains actin at 48 hours in co-cultured cells. Scale bars, 5 µm. Each experiment was repeated at least three times.
Conditioned breast cancer media promotes differentiation of THP-1 cells towards M2 type macrophages
Tissue macrophages may arise from two different paths: a portion of them originates during embryonic development to help maintain organ functions [18], and the other is recruited from circulating monocytes in blood by chemokine attractants. Tumor-associated macrophages, which come from circulating monocytes, play important roles in tumor progression [16]. Monocytes will differentiate to the certain subtype in response to stimuli in the tumor microenvironment. We observed that THP-1 became adherent and exhibited pseudopodia formation (Figure 3A) when co-cultured with MCF-7 or MDA-MB-231 for more than 48 hours, suggesting that THP-1 cells were differentiated toward macrophages. Next, we tested the differentiation of THP-1 cells when they were cultured for 48 hours in the conditional media from breast cancer cell cultures or co-cultured with breast cancer cells in transwells, which permit the free exchange of soluble factors but prevent the direct contacts between two cell types. As shown in Figure 3B, under both culture conditions, most cells were spread out and attached to the bottom surface of plates as multinuclear cells with morphology similar to THP-1 cells that were co-cultured with breast cancer cells in a direct contact setting. CD11b, a macrophage marker, and MMP-9, which is secreted by TAMs and known to be involved in tumor proliferation and M2-macrophage differentiation, were used to identify differentiated macrophages. When treated with 100 nM PMA, THP-1 cells began to express CD11b and MMP-9, indicating that the cells were specifically differentiated into macrophages. Conditioned media from MCF-7 and MDA-MB-231 cell cultures could also induce the expression of CD11b and MMP-9 albeit to a less degree (Figure 3C). Marengo et al reported that 27-HC could drive polarization of M2 microphages [37]. In our experiment, we observed that mRNA expression of M2 type macrophage markers, CD163 and IL-10, were increased to 5~60 times in THP-1 cells treated with conditioned media, however, no changes in M1 type marker CD25 expression was observed before or after treatment (Figure 3D). Flow cytometry analysis results indicated that CD163-positive cells were increased by 4.95% and 9.48% in THP-1 cells treated with conditioned media of MCF-7 and MDA-MB-231, respectively (Figure 3E). The expression of monocyte marker, CD14, was also elevated in differentiated THP-1 cells. Since there are differences between blood mononuclear cells and monocytic leukemia cell line THP-1, the expression level of CD14 in TDM is generally debatable [38]. These results indicated that THP-1 cells were likely polarized to M2 type macrophages by stimulatory factors secreted from breast cancer cells.
Figure 3.
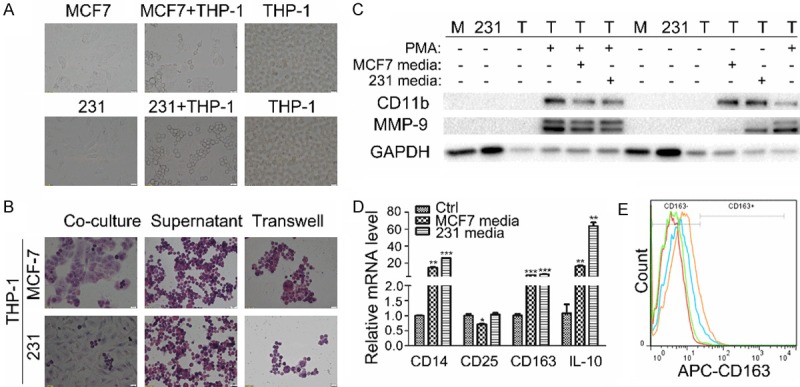
THP-1 cells were polarized to M2 type macrophages by breast cancer media. A. Morphological changes of THP-1 cells were examined when the cells were co-cultured with MCF-7 or MDA-MB-231 for 48 hours. B. HE staining of THP-1 cells was performed under the condition of co-culture, supernatant stimulation, or transwell culture for 48 hours. C. The expressions of CD11b and MMP-9 were detected by western blot analysis in MCF-7 (M), MDA-MB-231 (231) and THP-1 (T) cells differentiated by PMA and conditional media or not. D. The mRNA expressions of CD14, CD25, CD163, IL-10 were detected by q-PCR in THP-1 cells differentiated by conditioned or control media. Human GAPDH was used as housekeeping control gene. E. CD163 expression in THP-1 cells was analyzed using Flow cytometry with or without stimulation of the conditioned media.
We also harvested bone marrow cells from C57Bl/6 mice and treated them with conditioned culture media from murine breast cancer cell line 4T1 (4T1CM) (See the schematic diagram in Figure 4A). We observed that the protein level of CD206, a M2 marker, was significantly increased (Figure 4B) and the mRNA expressions of multiple M2 macrophage markers including CD206, ARG1, PPARγ, IL-13 and IL-10 were markedly induced (Figure 4C) in bone marrow cells stimulated with 4T1CM, further indicating that the soluble factors secreted by breast cancer cells were able to enhance the polarization of M2 type macrophages.
Figure 4.
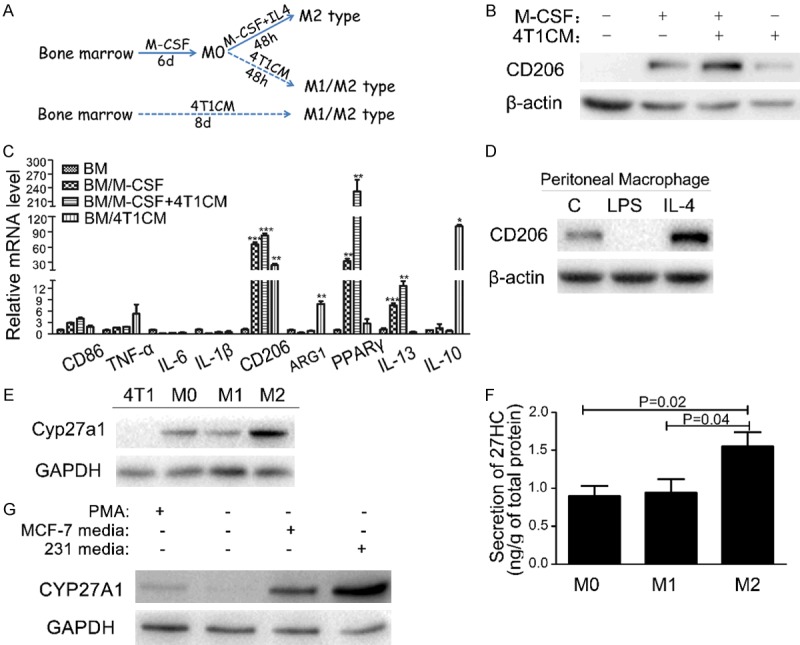
M2 type macrophages are the major sources of 27-HC production. A. Schematic drawing for differentiation of mouse primary bone marrow cells by breast cancer media. B. The expression of CD206 was examined by western blot in bone marrow and differentiated cells. C. The mRNA expression levels of M1 type and M2 type marker genes were detected by q-PCR in bone marrow cells differentiated by M-CSF and 4T1CM. D. The expression of CD206 was detected by western blot in peritoneal macrophages stimulated with LPS or IL-4 for 48 hours. E. Comparison of CYP27A1 expression was performed by western blot in 4T1 cells and different subtypes of macrophages. F. Secretion of 27-HC was measured in mouse peritoneal macrophages stimulated with LPS or IL-4 for 48 hours. The values represent the mean ± SEM, each experiment was repeated at least three times. G. Expression of CYP27A1 was determined by western blot in THP-1 cells stimulated with PMA or conditional media from MCF-7 and MDA-MB-231.
Furthermore, we analyzed the capacity of 27-HC secretions in different subtypes of macrophages. Peritoneal macrophages were polarized to M1 and M2 type by LPS or IL-4, respectively. Elevated expression of M2 marker CD206 was observed in IL-4 treated cells, but not in LPS treated cells, indicating that the differentiation of peritoneal macrophages was successfully induced (Figure 4D). Consistent with expression of CD206, higher expression of CYP27A1 was detected in IL-4 induced M2 type macrophage compared to the untreated peritoneal macrophages and 4T1 breast cancer cells (Figure 4E). In addition, M2 macrophages produced more 27-HC compared to M0 and M1 type (Figure 4F). The same elevation of CYP27A1 expression was observed in THP-1 cells treated with MCF-7 and MDA-MB-231 media (Figure 4G).
Exosomes derived from breast cancer cells promote the polarization of monocytes to type 2 macrophages and 27-HC facilitates the recruitment of monocytes
Recently, it has been reported that 27-HC is present in breast cancer exosomes [39] and 27-HC was correlated with polarization of human macrophages [37]. Therefore, we hypothesized that the M2 type polarization of macrophages in breast cancer might be partially driven by exosomes derived from breast cancer cells. To address this question, we collected exosomes from breast cancer cell cultures by ultrahigh-speed centrifugation [40] and added them into THP-1 cell cultures. Figure 5A illustrates an exosome with a diameter of 52.39 nm shot by transmission electron microscope. After stimulation by exosomes derived from breast tumor cells for 6 days, THP-1 cells were become adherent, multinuclear, and formed pseudopodia as macrophages (Figure 5B). To confirm whether THP-1 cells were polarized into M2 type, the expressions of CD11b, MMP-9, and CD14, CD25, CD163, IL-10 were analyzed by western blot and RT-PCR, respectively. As expected, exosome induced THP-1 to express CD11b and MMP-9 (Figure 5C) and M2 type macrophage markers (Figure 5D), which is similar to the observation in THP-1 cells stimulated by conditioned media from breast cancer cell cultures. We also observed the up-regulated expression of CYP27A1 in THP-1 when treated with exosomes derived from MCF-7 and MDA-MB-231 (Figure 5E).
Figure 5.
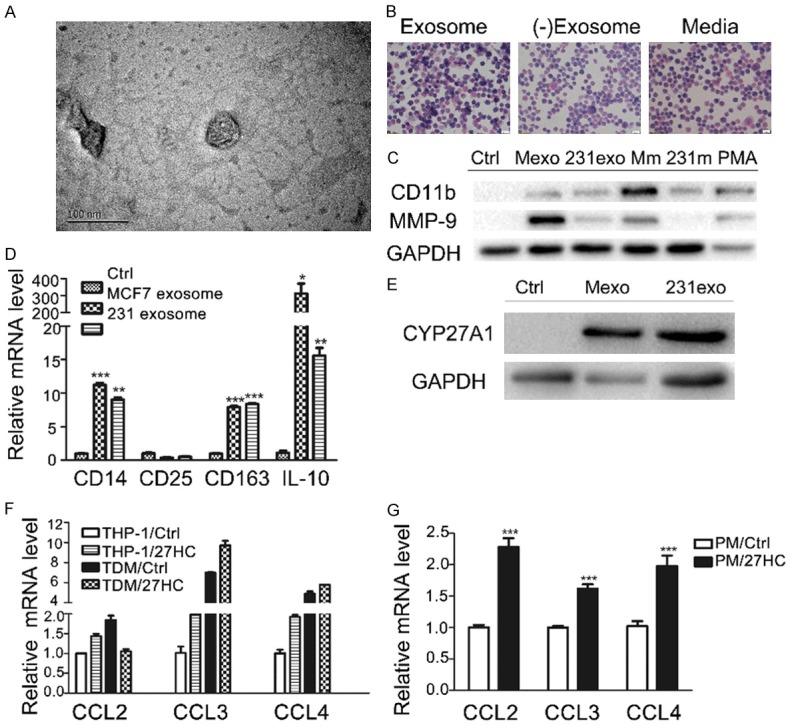
Monocytes were polarized by exosomes derived from breast cancer cells and recruited by 27-HC. A. Morphology of exosomes was determined by NP-2100 transmission electron microscope. Scale bar, 100 nm. B. HE staining of THP-1 cells was performed when cells were treated with breast cancer culture media, exosomes and exosome-depleted media. C. Expressions of CD11b and MMP-9 were detected by western blot in THP-1 cells treated with exosomes, conditional media of MCF-7 (Mm) or MDA-MB-231 (231m) cultures and PMA, respectively. D. Expressions of CD14, CD25, CD163 and IL-10 were examined by q-PCR in THP-1 cells treated with or without exosomes. GAPDH was used as the housekeeping control. E. Expression of CYP27A1 was elevated in THP-1 stimulated by exosomes derived from breast cancer cells. F. The mRNA expressions of chemokines (CCL2, CCL3 and CCL4) were examined by q-PCR in THP-1 and TDM cells (THP-1 treated with 10 nM PMA) stimulated with 1 µM 27-HC for 24 hours. G. The mRNA expressions of CCL2, CCL3, CCL4 were determined by q-PCR in peritoneal macrophages stimulated with 1 µM 27-HC for 24 hours. The values are presented as mean ± SEM (n = 3). *P < 0.05, **P < 0.01, ***P < 0.001 vs control.
It is believed that there are abundant infiltrating macrophages and elevated 27-HC during breast tumor progression. To elucidate the function of elevated 27-HC and determine whether there is a correlation between 27-HC and macrophage infiltration, we first examined the effects of 27-HC on proliferation of breast cancer cell lines such as MCF-7 and MDA-MB-231. 27-HC markedly stimulated the proliferation of ER-positive breast cancer cell line MCF-7 (data not show) as reported. MCP1 (CCL2) and its receptor CCR2 are the major regulators for migration of monocytes. In our experiment, the elevated CCL2 expression and reduced CCR2 expression were observed in TDMs compared to THP-1 cells (data not show). Kim SM et al demonstrated that 27-HC induces macrophages to secrete CCL3 and CCL4, leading to CCR5+ Th1 lymphocyte infiltration [41] and helping attract monocytes through CCL2 [42] in the atherosclerosis area. As shown in Figure 5F, 5G, we observed that the expressions of CCL2, CCL3 and CCL4 were up-regulated by 27-HC in TDMs and mouse peritoneal macrophages. These results indicate that 27-HC may facilitate the recruitment of monocytes to primary tumor sites and further increase 27-HC accumulation in vivo.
CYP7B1 is silenced in breast cancer cells by epigenetic mechanisms
Wu et al reported that the expression of CYP7B1 was down-regulated in human breast cancer tissues [9]. We analyzed the promoter methylation of CYP7B1 in TCGA breast cancer dataset and got a similar result, in which the promoter of CYP7B1 was hypermethylated in LumA, LumB and Her2 subtypes, but not in Basal subtype breast tumors (Figure 6A). Furthermore, the elevated promoter methylation of CYP7B1 is accompanied by decreased expression of CYP7B1 in LumA, LumB and Her2 subtypes of breast cancer patients as compared to normal control and basal subtype tumors (Figure 6B). Next, we designed a bisulfite pyrosequencing assay to investigate the promoter methylation status of CYP7B1 in a panel of breast cancer cell lines. Interestingly, CYP7B1 promoter methylation was mainly observed in ER+ cell lines (BT474, MCF-7 and ZR-75-1), but not in three triple-negative breast cancer (TNBC) cell lines (MDA-MB-468, MDA-MB-231 and Hs578t). The same results were also observed in primary breast tumor tissue samples. CYP7B1 is hypermethylated in 6 out of 8 ER+ breast cancer samples, but not in any of the TNBC samples (n = 10) as compared to normal breast tissue samples (n = 9) (Figure 6C). These results suggested that promoter methylation may downregulate the expression of CYP7B1, resulting in the accumulation of 27-HC in ER+ breast cancer cells. Furthermore, the expression of CYP7B1 was up-regulated by 5-Aza, a demethylating agent, in ER+ breast cancer cell lines such as MCF-7, T47D and BT474 (Figure 6D). All these results indicated that the silencing of CYP7B1 may contribute to the decreased expression of CYP7B1 and increased production of 27-HC in breast cancer tissues.
Figure 6.
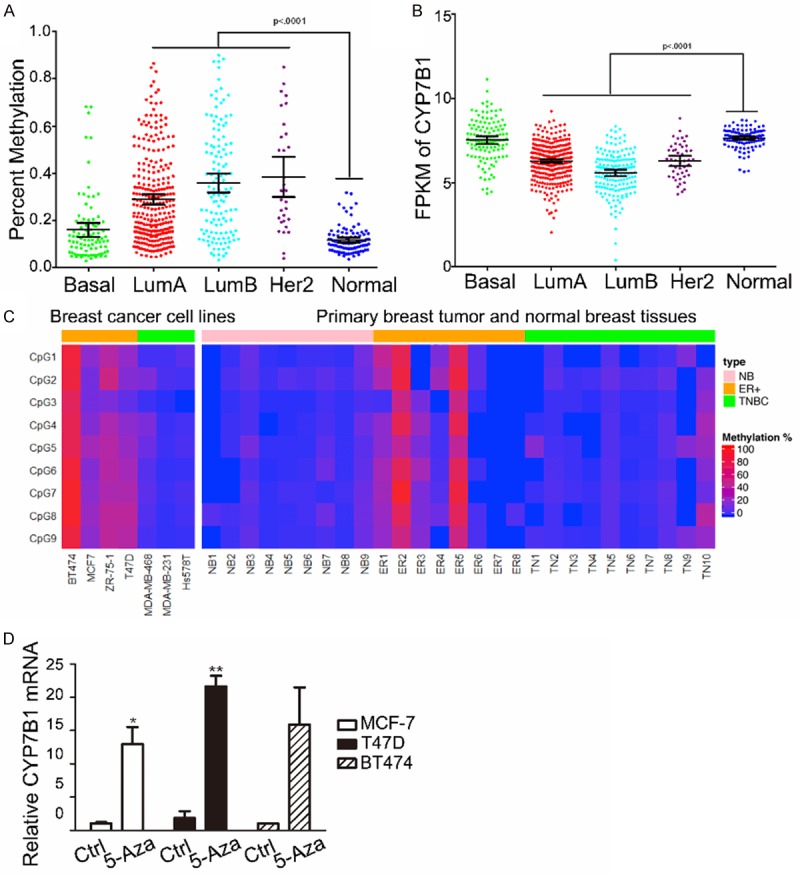
CYP7B1 promoter is hypermethylated in cancer cell lines and human breast cancer tissues. A. Differential CYP7B1 promoter methylation in breast cancer samples from different subtypes such as lumA, lumB or her2 was observed compared with normal human breast tissues. B. The FPKM (Fragments Per Kilobase of transcript per Million mapped reads) of CYP7B1 in different histological subtypes of breast cancers and normal breast tissues were analyze from TCGA RNA-seq data. C. A heatmap of methylation of CYP7B1 was prepared using a bisulfite pyrosequencing assay in breast cancer cell lines and primary breast tumor tissue samples. D. The mRNA expression of CYP7B1 was determined in MCF-7, T47D and BT474 cells treated with or without 2 µM 5-Aza for 48 hours. The values represent the mean ± SEM, *P < 0.05, **P < 0.01 vs control, n = 3.
Discussion
In the present study, we provided evidence that high expression of CYP27A1 in macrophages and hypermethylation of the CYP7B1 promoter in epithelial tumor cells are responsible for the accumulation of 27-HC in breast cancer tissues. We also demonstrated that accumulation of 27-HC aggravates tumor progression not only through promoting cancer cell proliferation but also by affecting the amount and phenotype of macrophages. Therefore, 27-HC serves as an important factor that regulates the complex relationship between macrophages and breast cancer cells.
Several approaches were used in our study to reveal the role of macrophages in the accumulation of 27-HC in breast cancer tissues. First, we showed that the secretion of 27-HC in conditioned medium from THP-1, THP-1-derived macrophages was much higher than breast cancer cell lines. Second, we compared the expression of CYP27A1 and CYP7B1 in THP-1, THP-1-derived macrophages and two breast cancer cell lines in both conventional culture and co-culture systems by western blot and immunofluorescence microscopy. We observed that the expression of the metabolic enzyme CYP27A1 was elevated in macrophages by one order of magnitude compared to breast cancer cells. Taken together, these experiments showed the superiority of macrophages at expressing CYP27A1 and secreting 27-HC.
Macrophages, one of the most flexible cell types with strong plasticity, are classified to several subtypes such as M1, M2a, M2b, etc. We investigated the subtype and function of macrophages in the co-culture system. In our study, monocytes were polarized to M2 type macrophages along with significant increases in CD163 and IL-10 expression when cultured with tumor cells or exposed to tumor cell-derived exosomes, which is consistent with previous observation that most of TAMs are M2 type [23,43]. In addition, the expressions of CD206, ARG1, PPARγ, IL-13 and IL-10 were increased in primary mouse bone marrow cells treated with 4T1-conditioned media, indicating an induction toward type 2 macrophage polarization. Interestingly, M2 type macrophages produce more 27-HC compared to M0 or M1 type macrophages. We also confirmed the ability of 27-HC to increase the number of both ER+ breast cancer cells and macrophages through stimulating growth and attracting infiltration, respectively. It has been reported that 27-HC drives M2 type macrophage differentiation [37]. Therefore, our studies have provided strong evidence to demonstrate that macrophages, especially M2 macrophages, modulate tumor microenvironment through secreting 27-HC.
Exosomes are 30-100 nm membrane vesicles secreted by most cell types, and contain distinct subsets of DNAs, RNAs, proteins and lipids. Exosomes are known to play an important role in modulation of intercellular communication. The relationship between exosomes and tumors has drawn significant attention, especially on its role in aiding tumor metastasis [44]. Interestingly, we also observed that exosomes derived from breast cancer cells could polarize monocytes toward M2 type macrophages with enhanced expression of CD11b, MMP-9, CD163, and IL-10. Thus, exosomes may play a role similar to cytokines in the education of monocytes. Furthermore, it was reported that 27-HC could be detected in breast cancer exosomes [39]. Therefore, it is possible that the low amount of 27-HC produced by breast cancer cells can also exert influences on macrophages’ polarization and function through exosomes.
Previous reports suggest that decreased expression of CYP7B1 in breast cancer cells was the reason for 27-HC accumulation in breast tumor tissues [9]. In the present study, we observed hypermethylation of the CYP7B1 promoter in both ER+ breast cancer cell lines and ER+ primary breast tumor samples. Furthermore, the expression of CYP7B1 in ER+ breast cancer cell lines was up-regulated when they were treated with 5-aza-2-deoxycytidine, a demethylating agent, further confirming the role of promoter methylation in regulating its expression. CYP7B1 catalyzes the degrading reactions of 27-HC, which involve both cholesterol derivatives [45] and ER agonists [46]. It has been shown that activation of PI3K/Akt pathway enhances ER-regulated CYP7B1 promoter activity [46]. It is possible that ER-mediated CYP7B1 up-regulation is a feedback mechanism that counters ER agonist-driven cell proliferation. Therefore, CYP7B1 promoter methylation may prevent ER-mediated regulation of CYP7B1 and lead to accumulation of ER agonists, such as 27-HC, suggesting that the epigenetic therapy could be used to activate CYP7B1 and thus attenuate signaling through ER agonists. Our studies demonstrated that increased promoter methylation may be one of the mechanisms that leads to epigenetic silencing of CYP7B1 in breast cancer cells, particularly in ER+ breast cancers. Despite that hypermethylation was not detected, there are no signs of increased expression in MDA-MB-231 cells, suggesting that other silencing mechanisms may be involved in the ER- breast cancer cells.
Conclusions
Based on our study, we propose the following model as illustrated in Figure 7. The tumor associated macrophages (TAMs) are the main source for local 27-HC production in breast tissues, and the hypermethylation of the CYP7B1 promoter in breast cancer cells further attenuates the degradation of 27-HC in breast cancer cells, resulting in the accumulation of 27-HC in tumor tissues. The elevated 27-HC not only promotes ER+ breast cancer cell proliferation but also induces secretion of chemokines such as CCL2, CCL3 and CCL4 from macrophages, which further attracts monocytes to the primary tumor site. Furthermore, exosomes derived from breast cancer cells significantly polarize the monocytes to M2 type macrophages, which further produce 27-HC to promote the development of the breast cancer when they migrate into tumor tissue.
Figure 7.
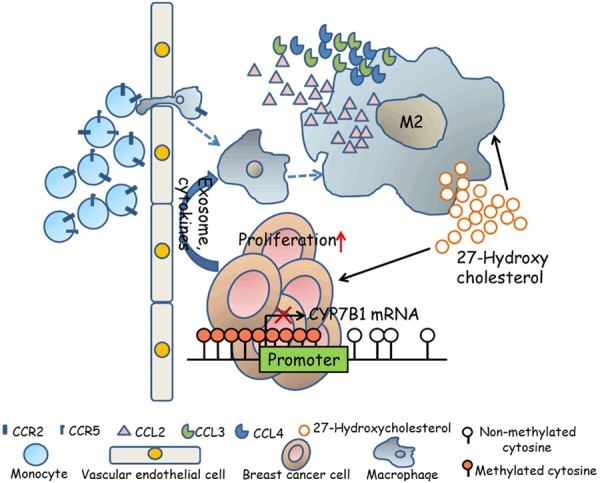
A proposed mechanistic model of 27-HC and M2 type macrophages in the tumor microenvironment. 27-HC secreted by macrophages promotes breast cancer cell proliferation and stimulates macrophages themselves to secrete chemokines, such as CCL2, CCL3 and CCL4, which further promote the CCR2+ and CCR5+ monocytes to migrate to tumor sites. The infiltrated monocytes can be polarized to M2 type macrophages with a stronger 27-HC secreting capacity partly by exosomes derived from breast tumor cells. Breast cancer cells may also contribute to 27-HC accumulation through down-regulating expression of CYP7B1 by hypermethylation in its promoter.
Acknowledgements
The authors would like to thank Dr. Guohuang Fan for the gifts of IL-4 reagent and CD163 antibody and to the member of the Transgenic Mouse Facility in the Institute of Translational Medicine of Nanchang University for their generous help in animal housing and valuable discussion. We also acknowledge support from the National Natural Science Foundation of China (91639106 and 81873659 to H-B. X., 81760140 and 81970256 to K-Y. D), the National Basic Research Program of China (2013CB531103 to H-B. X. and K-Y. D.), Jiangxi Provincial Department of Science and Technology, China (20142BCB24001 to K-Y. D.), and Georgia Cancer Center Startup Fund (to H. S.). H. S. is a Georgia Research Alliance Distinguished Scientist.
Disclosure of conflict of interest
None.
References
- 1.Bianchini F, Kaaks R, Vainio H. Overweight, obesity, and cancer risk. Lancet Oncol. 2002;3:565–74. doi: 10.1016/s1470-2045(02)00849-5. [DOI] [PubMed] [Google Scholar]
- 2.Renehan AG, Roberts DL, Dive C. Obesity and cancer: pathophysiological and biological mechanisms. Arch Physiol Biochem. 2008;114:71–83. doi: 10.1080/13813450801954303. [DOI] [PubMed] [Google Scholar]
- 3.Slattery ML, Hines LH, Lundgreen A, Baumgartner KB, Wolff RK, Stern MC, John EM. Diet and lifestyle factors interact with MAPK genes to influence survival: the breast cancer health disparities study. Cancer Causes Control. 2014;25:1211–1225. doi: 10.1007/s10552-014-0426-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heath V. Lipids: cholesterol metabolite unmasked as a key player in breast cancer. Nat Rev Endocrinol. 2014;10:65. doi: 10.1038/nrendo.2013.254. [DOI] [PubMed] [Google Scholar]
- 5.Llaverias G, Danilo C, Mercier I, Daumer K, Capozza F, Williams TM, Sotgia F, Lisanti MP, Frank PG. Role of cholesterol in the development and progression of breast cancer. Am J Pathol. 2011;178:402–412. doi: 10.1016/j.ajpath.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Umetani M, Shaul PW. 27-Hydroxycholesterol: the first identified endogenous SERM. Trends Endocrinol Metab. 2011;22:130–135. doi: 10.1016/j.tem.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DuSell CD, McDonnell DP. 27-Hydroxycholesterol: a potential endogenous regulator of estrogen receptor signaling. Trends Pharmacol Sci. 2008;29:510–514. doi: 10.1016/j.tips.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DuSell CD, Umetani M, Shaul PW, Mangelsdorf DJ, McDonnell DP. 27-hydroxycholesterol is an endogenous selective estrogen receptor modulator. Mol Endocrinol. 2008;22:65–77. doi: 10.1210/me.2007-0383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu Q, Ishikawa T, Sirianni R, Tang H, McDonald JG, Yuhanna IS, Thompson B, Girard L, Mineo C, Brekken RA, Umetani M, Euhus DM, Xie Y, Shaul PW. 27-Hydroxycholesterol promotes cell-autonomous, ER-positive breast cancer growth. Cell Rep. 2013;5:637–645. doi: 10.1016/j.celrep.2013.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shen Z, Zhu D, Liu J, Chen J, Liu Y, Hu C, Li Z, Li Y. 27-Hydroxycholesterol induces invasion and migration of breast cancer cells by increasing MMP9 and generating EMT through activation of STAT-3. Environ Toxicol Pharmacol. 2017;51:1–8. doi: 10.1016/j.etap.2017.02.001. [DOI] [PubMed] [Google Scholar]
- 11.Nelson ER, Wardell SE, Jasper JS, Park S, Suchindran S, Howe MK, Carver NJ, Pillai RV, Sullivan PM, Sondhi V, Umetani M, Geradts J, McDonnell DP. 27-Hydroxycholesterol links hypercholesterolemia and breast cancer pathophysiology. Science. 2013;342:1094–1098. doi: 10.1126/science.1241908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cruz P, Epunan MJ, Ramirez ME, Torres CG, Valladares LE, Sierralta WD. 27-hydroxycholesterol and the expression of three estrogen-sensitive proteins in MCF7 cells. Oncol Rep. 2012;28:992–998. doi: 10.3892/or.2012.1859. [DOI] [PubMed] [Google Scholar]
- 13.Cruz P, Torres C, Ramirez ME, Epunan MJ, Valladares LE, Sierralta WD. Proliferation of human mammary cancer cells exposed to 27-hydroxycholesterol. Exp Ther Med. 2010;1:531–536. doi: 10.3892/etm_00000084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vesa M, Olkkonen RH. Interactions of oxysterols with membranes and proteins. Mol Aspects Med. 2009;30:123–133. doi: 10.1016/j.mam.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 15.Baek AE, Yu YA, He S, Wardell SE, Chang CY, Kwon S, Pillai RV, McDowell HB, Thompson JW, Dubois LG, Sullivan PM, Kemper JK, Gunn MD, McDonnell DP, Nelson ER. The cholesterol metabolite 27 hydroxycholesterol facilitates breast cancer metastasis through its actions on immune cells. Nat Commun. 2017;8:864. doi: 10.1038/s41467-017-00910-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chanmee T, Ontong P, Konno K, Itano N. Tumor-associated macrophages as major players in the tumor microenvironment. Cancers (Basel) 2014;6:1670–1690. doi: 10.3390/cancers6031670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wyckoff J, Wang W, Lin EY, Wang Y, Pixley F, Stanley ER, Graf T, Pollard JW, Segall J, Condeelis J. A paracrine loop between tumor cells and macrophages is required for tumor cell migration in mammary tumors. Cancer Res. 2004;64:7022–7029. doi: 10.1158/0008-5472.CAN-04-1449. [DOI] [PubMed] [Google Scholar]
- 18.Noy R, Pollard JW. Tumor-associated macrophages: from mechanisms to therapy. Immunity. 2014;41:49–61. doi: 10.1016/j.immuni.2014.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sica A, Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J Clin Invest. 2012;122:787–95. doi: 10.1172/JCI59643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Davies LC, Jenkins SJ, Allen JE, Taylor PR. Tissue-resident macrophages. Nat Immunol. 2013;14:986–995. doi: 10.1038/ni.2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murray PJ, Wynn TA. Protective and pathogenic functions of macrophage subsets. Nat Rev Immunol. 2011;11:723–737. doi: 10.1038/nri3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mantovani A, Sica A. Macrophages, innate immunity and cancer: balance, tolerance, and diversity. Curr Opin Immunol. 2010;22:231–237. doi: 10.1016/j.coi.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 23.Sica A, Larghi P, Mancino A, Rubino L, Porta C, Totaro MG, Rimoldi M, Biswas SK, Allavena P, Mantovani A. Macrophage polarization in tumour progression. Semin Cancer Biol. 2008;18:349–355. doi: 10.1016/j.semcancer.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 24.Guruvayoorappan C. Tumor versus tumor-associated macrophages: how hot is the link? Integr Cancer Ther. 2008;7:90–5. doi: 10.1177/1534735408319060. [DOI] [PubMed] [Google Scholar]
- 25.Burke B, Giannoudis A, Corke KP, Gill D, Wells M, Ziegler-Heitbrock L, Lewis CE. Hypoxia-induced gene expression in human macrophages. Am J Pathol. 2003;163:1233–43. doi: 10.1016/S0002-9440(10)63483-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gocheva V, Wang HW, Gadea BB, Shree T, Hunter KE, Garfall AL, Berman T, Joyce JA. IL-4 induces cathepsin protease activity in tumor-associated macrophages to promote cancer growth and invasion. Genes Dev. 2010;24:241–55. doi: 10.1101/gad.1874010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goswami S, Sahai E, Wyckoff JB, Cammer M, Cox D, Pixley FJ, Stanley ER, Segall JE, Condeelis JS. Macrophages promote the invasion of breast carcinoma cells via a colony-stimulating factor-1/epidermal growth factor paracrine loop. Cancer Res. 2005;65:5278–83. doi: 10.1158/0008-5472.CAN-04-1853. [DOI] [PubMed] [Google Scholar]
- 28.Du R, Lu KV, Petritsch C, Liu P, Ganss R, Passegué E, Song H, VandenBerg S, Johnson RS, Werb Z, Bergers G. HIF1α induces the recruitment of bone marrow-derived vascular modulatory cells to regulate tumor angiogenesis and invasion. Cancer Cell. 2008;13:206–220. doi: 10.1016/j.ccr.2008.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Su S, Liu Q, Chen J, Chen J, Chen F, He C, Huang D, Wu W, Lin L, Huang W, Zhang J, Cui X, Zheng F, Li H, Yao H, Su F, Song E. A positive feedback loop between mesenchymal-like cancer cells and macrophages is essential to breast cancer metastasis. Cancer Cell. 2014;25:605–620. doi: 10.1016/j.ccr.2014.03.021. [DOI] [PubMed] [Google Scholar]
- 30.Mizukami Y, Kono K, Kawaguchi Y, Akaike H, Kamimura K, Sugai H, Fujii H. CCL17 and CCL22 chemokines within tumor microenvironment are related to accumulation of Foxp3+ regulatory T cells in gastric cancer. Int J Cancer. 2008;122:2286–93. doi: 10.1002/ijc.23392. [DOI] [PubMed] [Google Scholar]
- 31.Babiker A, Andersson O, Lund E, Xiu RJ, Deeb S, Reshef A, Leitersdorf E, Diczfalusy U, Björkhem I. Elimination of cholesterol in macrophages and endothelial cells by the sterol 27-hydroxylase mechanism. J Biol Chem. 1997;272:26253–61. doi: 10.1074/jbc.272.42.26253. [DOI] [PubMed] [Google Scholar]
- 32.Tang X, Mo C, Wang Y, Wei D, Xiao H. Anti-tumour strategies aiming to target tumour-associated macrophages. Immunology. 2013;138:93–104. doi: 10.1111/imm.12023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sawa-Wejksza K, Kandefer-Szerszeń M. Tumor-associated macrophages as target for antitumor therapy. Arch Immunol Ther Exp (Warsz) 2018;66:97–111. doi: 10.1007/s00005-017-0480-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Noonepalle SK, Gu F, Lee EJ, Choi JH, Han Q, Kim J, Ouzounova M, Shull AY, Pei L, Hsu PY, Kolhe R, Shi F, Choi J, Chiou K, Huang TH, Korkaya H, Deng L, Xin HB, Huang S, Thangaraju M, Sreekumar A, Ambs S, Tang SC, Munn DH, Shi H. Promoter methylation modulates indoleamine 2,3-dioxygenase 1 induction by activated T cells in human breast cancers. Cancer Immunol Res. 2017;5:330–344. doi: 10.1158/2326-6066.CIR-16-0182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.DuPre SA, Redelman D, Hunter KW. The mouse mammary carcinoma 4T1: characterization of the cellular landscape of primary tumours and metastatic tumour foci. Int J Exp Pathol. 2007;88:351–360. doi: 10.1111/j.1365-2613.2007.00539.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Newman AM, Liu CL, Green MR, Gentles AJ, Feng W, Xu Y, Hoang CD, Diehn M, Alizadeh AA. Robust enumeration of cell subsets from tissue expression profiles. Nat Methods. 2015;12:453–457. doi: 10.1038/nmeth.3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marengo B, Bellora F, Ricciarelli R, Ciucis CD, Furfaro A, Leardi R, Colla R, Pacini D, Traverso N, Moretta A, Pronzato MA, Bottino C, Domenicotti C. Oxysterol mixture and, in particular, 27-hydroxycholesterol drive M2 polarization of human macrophages. Biofactors. 2016;42:80–92. doi: 10.1002/biof.1243. [DOI] [PubMed] [Google Scholar]
- 38.Doherty TM, Daigneault M, Preston JA, Marriott HM, Whyte MK, Dockrell DH. The identification of markers of macrophage differentiation in PMA-stimulated THP-1 cells and monocyte-derived macrophages. PLoS One. 2010;5:e8668. doi: 10.1371/journal.pone.0008668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roberg-Larsen H, Lund K, Seterdal KE, Solheim S, Vehus T, Solberg N, Krauss S, Lundanes E, Wilson SR. Mass spectrometric detection of 27-hydroxycholesterol in breast cancer exosomes. J Steroid Biochem Mol Biol. 2017;169:22–28. doi: 10.1016/j.jsbmb.2016.02.006. [DOI] [PubMed] [Google Scholar]
- 40.Théry C, Amigorena S, Raposo G, Clayton A. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr Protoc Cell Biol. 2006;Chapter 3 doi: 10.1002/0471143030.cb0322s30. Unit 3.22. [DOI] [PubMed] [Google Scholar]
- 41.Kim SM, Kim BY, Lee SA, Eo SK, Yun Y, Kim CD, Kim K. 27-Hydroxycholesterol and 7alpha-hydroxycholesterol trigger a sequence of events leading to migration of CCR5-expressing Th1 lymphocytes. Toxicol Appl Pharmacol. 2014;274:462–470. doi: 10.1016/j.taap.2013.12.007. [DOI] [PubMed] [Google Scholar]
- 42.Kim SM, Lee SA, Kim BY, Bae SS, Eo SK, Kim K. 27-Hydroxycholesterol induces recruitment of monocytic cells by enhancing CCL2 production. Biochem Biophys Res Commun. 2013;442:159–164. doi: 10.1016/j.bbrc.2013.11.052. [DOI] [PubMed] [Google Scholar]
- 43.Mantovani A, Sozzani S, Locati M, Allavena P, Sica A. Macrophage polarization_Tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002;23:549–55. doi: 10.1016/s1471-4906(02)02302-5. [DOI] [PubMed] [Google Scholar]
- 44.Hoshino A, Costa-Silva B, Shen TL, Rodrigues G, Hashimoto A, Tesic Mark M, Molina H, Kohsaka S, Di Giannatale A, Ceder S, Singh S, Williams C, Soplop N, Uryu K, Pharmer L, King T, Bojmar L, Davies AE, Ararso Y, Zhang T, Zhang H, Hernandez J, Weiss JM, Dumont-Cole VD, Kramer K, Wexler LH, Narendran A, Schwartz GK, Healey JH, Sandstrom P, Jørgen Labori K, Kure EH, Grandgenett PM, Hollingsworth MA, de Sousa M, Kaur S, Jain M, Mallya K, Batra SK, Jarnagin WR, Brady MS, Fodstad O, Muller V, Pantel K, Minn AJ, Bissell MJ, Garcia BA, Kang Y, Rajasekhar VK, Ghajar CM, Matei I, Peinado H, Bromberg J, Lyden D. Tumour exosome integrins determine organotropic metastasis. Nature. 2015;527:329–335. doi: 10.1038/nature15756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wicher G, Norlin M. Estrogen-mediated regulation of steroid metabolism in rat glial cells; effects on neurosteroid levels via regulation of CYP7B1-mediated catalysis. J Steroid Biochem Mol Biol. 2015;145:21–27. doi: 10.1016/j.jsbmb.2014.09.022. [DOI] [PubMed] [Google Scholar]
- 46.Tang W, Pettersson H, Norlin M. Involvement of the PI3K/Akt pathway in estrogen-mediated regulation of human CYP7B1: Identification of CYP7B1 as a novel target for PI3K/Akt and MAPK signalling. J Steroid Biochem Mol Biol. 2008;112:63–73. doi: 10.1016/j.jsbmb.2008.08.004. [DOI] [PubMed] [Google Scholar]


