Abstract
Metabolic syndrome is a significant risk factor for the development of hepatocellular carcinoma (HCC). 25-HC (25-hydroxycholesterol) synthesized from cholesterol plays an important role in lipid metabolism. However, the functions and mechanism of 25-HC in HCC remain largely unknown. In this study, we demonstrated that 25-HC promoted HCC cells migration and intrahepatic and extrahepatic metastasis while did not affect the cells proliferation and apoptosis. Mechanistically, the promotive effect of 25-HC was through up-regulation of Toll-like receptor 4 (TLR4) dependent fatty acid binding protein 4 (FABP4). Inhibition of FABP4 hindered 25-HC-induced cells migration and metastasis. Moreover, up-regulation of FABP4 was observed in HCC tissues from database analysis. In summary, our study reveals the effects and mechanism of 25-HC/TLR4/FABP4 axis in promoting HCC metastasis, which provides novel avenue for therapeutic intervention against HCC progression.
Keywords: 25-hydroxycholesterol, hepatocellular carcinoma, metastasis, Toll-like receptor 4, fatty acid binding protein 4
Introduction
Primary liver cancer is the sixth most common malignant tumor and the third largest cause of cancer mortality in the world [1]. Hepatocellular carcinoma (HCC) is the most common histological type of liver cancer. Accumulating evidences have demonstrated that metabolic disorders could raise risks of HCC [2,3].
Dysregulated cholesterol biosynthesis is frequently observed in HCCs, which could induce mitochondrial impairment, confer resistance to apoptosis and support growth of carcinoma [4-6]. Studies have reported that cholesterol intake is an independent risk factor for HCC of which the underlying mechanisms might be the dysregulating expressions of genes involved in inflammation and metabolism and inducing of oncogenic mutations and gene expressions [7,8]. Furthermore, using of statins is associated with the reduced risk of HCC development [9].
25-HC (25-hydroxycholesterol), one form of oxysterol, is synthesized from cholesterol by cholesterol 25-hydroxylase (CH25H) and has been identified as a lipid ligand of integrins and a key regulator of lipid metabolism [10,11]. 25-HC has been demonstrated to inhibit the replication of a wide range of viruses [12-14].
A few studies to date have explored the role of 25-HC in cancers. 25-HC has been found to activate ERα and stimulate cells proliferation in breast and ovarian cancer [15]. In the melanoma tumor micro-environment, 25-HC can inhibit CCR7 expression on DC cells, leading to tumor immune escape and promotion [16]. Wang et al. reported 25-HC promoted lung adenocarcinoma cells migration and invasion [17]. Our previous study also found 25-HC decreased the sensitivity of human gastric cancer cells to 5-fluorouracil and promoted cells invasion via the TLR2/NF-κB signaling pathway [18].
However, the functional role and underlying molecular mechanisms of 25-HC in HCC has not been investigated which prompted us to explore deeply. In this study, we revealed that 25-HC promotes HCC cells migration and metastasis both in vitro and in vivo while has no effects on cells proliferation. The probable mechanism might be the up-regulating of TLR4-depedent FABP4. These findings indicate that 25-HC or FABP4 might act as a potential therapeutic target for HCC.
Materials and methods
Cells and reagents
Human hepatocellular carcinoma cell line HepG2 was purchased from the ATCC and maintained in DMEM medium supplemented with 10% FBS and 1% penicillin-streptomycin. Cells were maintained at 37°C under a humidified 5% CO2 atmosphere. All cell culturing reagents were purchased from Gibco (Shanghai, China).
25-HC was bought from Sigma-Aldrich (Shanghai, China) and dissolved in ethanol as a stock solution. Recombinant human FABP4 was bought from Cloud-Clone Corp. (Houston, TX, USA) and dissolved in PBS. FABP4 inhibitor BMS-309403 was bought from MedChem Express (Shanghai, China) and dissolved in DMSO.
Cell viability measurement and apoptosis analysis
Cell viability was measured by CCK-8 assay kit (Beyotime, Shanghai, China) and cells apoptosis was determined by Annexin V-FITC/PI staining (Lianke Biotech, Co., Ltd., Hangzhou, China) as previously described [19]. For cell viability measurement, the optical density at 450 nm was measured with ultra-microplate reader (EMax; Molecular Devices, Sunnyvale, CA, USA). For cells apoptosis, stained cells were analyzed by flow cytometry (BD FACScan; BD Biosciences, San Jose, CA, USA).
Animal studies
5-6-week-old female BALB/C nude mice were bought from Shanghai Laboratory Animal Company (SLAC; Shanghai, China) and maintained in the animal facility at Zhejiang University (Hangzhou, China). Mice were provided with water and food ad libitum and kept under standard conditions. Current study received ethical approval from the Animal Care and Use Committee of Zhejiang University, and experiments and animal care were performed according to the approved protocols.
Xenograft tumors were generated via the subcutaneous injection of HepG2 cells (2 × 106) into the right flanks of the mice. When the volumes of xenograft tumors reached an average of 100 mm3, mice were randomly divided into PBS group and 25-HC group. For 25-HC group, mice were intraperitoneally injected with 25-HC (10 mg/kg) every 3 days for 20 days. For PBS group, mice were received the same volume of PBS. Tumor size was measured by a slide caliper, and tumor volume was calculated using the formula: V = 1/2 × (length × width2). After 20 days, mice were sacrificed and the tumors were harvested, weighed and proteins were extracted for Western blotting.
To establish the orthotopic HCC mouse models by intrahepatic inoculation of HepG2 cells, mice were anesthetized with 75 mg/kg pentobarbital by intraperitoneal injection. Skin was sterilized with iodophor before 5 × 106 HepG2 cells suspended in 50 μL PBS were injected into right lobes of the liver with a syringe. Afterwards, the injection site was gently pressed with cotton to reduce bleeding and leakage of cell suspensions. Then, the peritoneum and skin were closed with 6-0 sutures. Mice were monitored weekly for their behavior. 3 weeks later, mice were sacrificed by cervical dislocation, livers were removed, tumor nodules in the liver and abdominal cavity were observed, counted and photographed. For FABP4 inhibition, BMS-309403 (45 mg/kg) was intraperitoneally injected twice a week after cells inoculation.
Western blotting
For protein extraction, cells or tumor tissues were washed with PBS twice before lysed with RIPA buffer (Beyotime). Protein concentration was quantified by BCA assay (Cwbiotech, Beijing, China) according to the manufacturer’s instructions. Total proteins (30 μg) were separated by 10% SDS-PAGE gels and transferred to polyvinylidene difluoride (PVDF) membranes. Membranes were probed with primary antibodies against MMP1 (#10371-2-AP), MMP2 (#10373-2-AP), MMP3 (#17873-1-AP), MMP9 (#10375-2-AP), MMP13 (#18165-1-AP), FABP4 (#12802-1-AP), GAPDH (#10494-1-AP) (all bought from Proteintech, Wuhan, China), Bcl-2 (#3498), Bax (#2772), p-Erk1/2 (#4370), Erk1/2 (#4695), p-p38 (#9211), p38 (#9212), p-SAPK/JNK (#4668), SAPK/JNK (#9252), p-PI3K p85/p55 (#4228), PI3K p85 (#4292), p-Akt (#4060), Akt (#4691), p-Stat3 (#9145), Stat3 (#4904), p-NF-κB p65 (#3039), NF-κB p65 (#8242), β-Actin (#8457) (all bought from Cell Signaling Technologies, Danvers, MA, USA), TLR2 (#DF7002), TLR4 (#AF7017), TLR5 (#DF6575), TLR7 (#DF6173) and TLR9 (#DF2970) (all bought from Affinity Biosciences, Jiangsu, China) at 4°C overnight, followed by incubation for 1 hour at room temperature with the HRP-linked goat anti-rabbit secondary antibody. Immunoblotting for β-actin or GAPDH served as loading control. The protein bands were detected with FluorChem E System (ProteinSimple, Santa Clara, CA, USA).
Cell migration assay
Cells migration was assayed with a 24-well 8-μm Transwell chambers (Corning Costar, Corning, NY, USA). Briefly, 2 × 105 HepG2 cells suspended in 200 μL serum-free medium were added to the Transwell inserts in the absence or presence of 25-HC or FABP4 or BMS-309403. The lower chamber filled with 600 μL of medium supplemented with 20% FBS was used as a chemoattractant. Following incubation for 36 hours at 37°C, non-invading cells remaining on the upper surface were removed and cells on the lower surface were fixed with ice-cold methanol and stained with 0.1% crystal violet for 30 min at room temperature. Images were captured and counted under an Olympus CKX41 light microscope (Olympus, Shanghai, China; magnification, × 100).
Reverse transcriptase-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted with the Ultrapure RNA kit (Cwbiotech, Beijing, China) and reverse transcriptase PCR was performed with the HiFiScript cDNA Synthesis kit (Cwbiotech). Quantitative PCR (qPCR) was performed with the iTaq Universal SYBR-Green Supermix (Cwbiotech). The expression level of each target gene (sequences of the primers were listed in Table 1) was normalized to a housekeeping gene encoding β-actin. Relative expression values were calculated from Ct values using the Delta delta CT (2-ΔΔCq) strategy.
Table 1.
The primer sequence of target genes
| Gene Name | Forward | Reverse |
|---|---|---|
| MMP1 | CTCTGGAGTAATGTCACACCTCT | TGTTGGTCCACCTTTCATCTTC |
| MMP2 | CCCACTGCGGTTTTCTCGAAT | CAAAGGGGTATCCATCGCCAT |
| MMP3 | AGTCTTCCAATCCTACTGTTGCT | TCCCCGTCACCTCCAATCC |
| MMP9 | AGACCTGGGCAGATTCCAAAC | CGGCAAGTCTTCCGAGTAGT |
| MMP13 | TCCTGATGTGGGTGAATACAATG | GCCATCGTGAAGTCTGGTAAAAT |
| TLR2 | TTATCCAGCACACGAATACACAG | AGGCATCTGGTAGAGTCATCAA |
| TLR4 | AGACCTGTCCCTGAACCCTAT | CGATGGACTTCTAAACCAGCCA |
| TLR5 | TCCCTGAACTCACGAGTCTTT | GGTTGTCAAGTCCGTAAAATGC |
| TLR7 | TCCTTGGGGCTAGATGGTTTC | TCCACGATCACATGGTTCTTTG |
| TLR9 | CTGCCTTCCTACCCTGTGAG | GGATGCGGTTGGAGGACAA |
| FABP4 | ACTGGGCCAGGAATTTGACG | CTCGTGGAAGTGACGCCTT |
| β-actin | GTATCCTGACCCTGAAGTACC | TGAAGGTCTCAAACATGATCT |
Luciferase reporter assay
The pNF-κB-luc, pAP-1-luc and pSTAT3-TA-luc reporter plasmids were bought from Beyotime. HepG2 cells were seeded in 96-well plate at a density of 2 × 104 per well 1 day prior to transfection. Cells were transient transfected with the luciferase reporter plasmid with Lipofectamine 2000 (Thermo Fisher Scientific, Carlsbad, CA) according to the manufacturer’s instructions. After 24 hours post transfection, cells were treated with 25-HC for the indicated concentrations. 24 hours later, cells were collected, and the luciferase activities were determined with the Bright-Glo luciferase assay system (Promega Corp., Madison, WI).
SiRNA transfection
SiRNA targeting human TLR4, TLR9, FABP4 and scramble siRNA (siNC) were purchased from GenePharma Technologies (Shanghai, China). SiRNA sequences were listed in Table 2. HepG2 cells were transfected with the siRNAs with Lipofectamine 2000 following the manufacturer’s instructions. After transfection for 24 hours, cells were prepared for further experiments. Cells transfection efficiency was determined by Western blotting.
Table 2.
Sequences of siRNA
| Gene | Sense | Antisense |
|---|---|---|
| TLR4 | GCUCACAAUCUUAUCCAAUTT | AUUGGAUAAGAUUGUGAGCTT |
| TLR9 | AGCUUAACCUGUCCUUCAA | UUGAAGGACAGGUUAAGCU |
| FABP4 | UCAAGAGCACCAUAACCUUTT | AAGGUUAUGGUGCUCUUGATT |
| NC | UUCUCCGAACGUGUCACGUTT | ACGUGACACGUUCGGAGAATT |
Database analysis
The mRNA expression of FABP4 in HCC was analyzed from the GEPIA (Gene Expression Profiling Interactive Analysis) database (http://gepia.cancer-pku.cn/). The DNA copy number of FABP4 in HCC was analyzed within the Oncomine database (www.oncomine.org). FABP4 expression was assessed in HCC tissues relative to its expression in normal tissues. EMBL-EBI bioinformatics website (http://www.ebi.ac.uk/gxa/home) was used to determine the expression of FABP4 in hepatocellular carcinoma cell lines. UALCAN database (http://ualcan.path.uab.edu) was used to analyze the expression of FABP4 in various tumor sub-groups based on cancer stages, tumor grade or other clinicopathological features. The correlation between expression of FABP4 and the prognosis of patients with HCC was assessed in OncoLnc (http://www.oncolnc.org).
Statistical analysis
Data are expressed as means ± SEM. Statistical significance was determined by Student’s t-test or one-way analysis of variance (ANOVA) followed by Dunnett’s test. P-value <0.05 was considered as statistical significance.
Results
25-HC has no effects on HepG2 cells proliferation and apoptosis both in vitro and in vivo
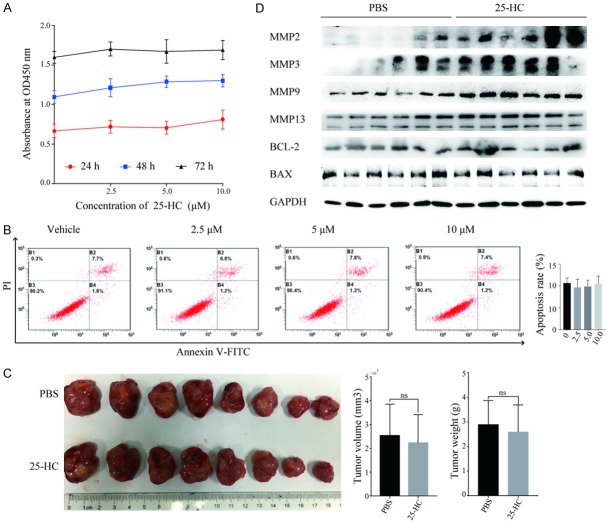
To evaluate the functions of 25-HC on HCC cells, we first performed CCK-8 assay on HepG2 cells. As shown in Figure 1A, the proliferation of HepG2 cells was not affected by 25-HC treatment with varied concentrations from 0-10 μM. Then we detected the cells apoptosis by flowcytometry. Likewise, there was no significant difference between two groups (Figure 1B) (P>0.05). We also assessed the tumor growth in vivo by inoculating HepG2 cells into BALB/C nude mice subcutaneously to establish the xenograft tumor models followed by injecting with PBS or 25-HC intraperitoneally. In accordance with the in vitro results, 25-HC treatment had no effects on cells proliferation as demonstrated by the similar tumor volume and weight (Figure 1C). Furthermore, we detected the protein expressions of Bax, Bcl-2, MMP2, MMP3, MMP9 and MMP13 in xenograft tumor tissues (Figure 1D). Notably, 25-HC treatment significantly increased the MMPs expressions compared with that of PBS group while had relatively less or no effect on the expressions of apoptosis related proteins Bax and Bcl-2. These data indicated that 25-HC has no effects on cells proliferation and apoptosis but promotes HepG2 cells MMPs expression which was related to the cell metastasis.
Figure 1.
25-HC has no effects on HepG2 cells proliferation and apoptosis both in vitro and in vivo. (A) HepG2 cells were treated with the indicated concentrations of 25-HC for 24, 48 or 72 hours and CCK-8 assay was performed. Results are shown as the cells absorbance at 450 nm. (B) HepG2 cells were treated with the indicated concentrations of 25-HC for 48 hours followed by the flow cytometry analysis of cell apoptosis. The percentage of FITC positive cells indicating apoptotic HepG2 cells were calculated and statistically analyzed. (C) Nude mice were inoculated subcutaneously with HepG2 cells (2 × 106) followed by PBS or 25-HC treatment intraperitoneally. The volume of the tumor and the body weight was monitored. Tumors were photographed at the end of the experiment. (D) Proteins of xenograft tumors of two groups were extracted and the expressions of MMP2, MMP3, MMP9, MMP13, Bcl-2 and Bax were determined by Western blotting. Results were obtained from 3 independent experiments (A and B) or 2 independent experiments (C and D) and are expressed as the means ± SEM. Statistical significance in (C) was determined by Student’s t-test. ns, not significant.
25-HC promotes HCC cells migration in vitro and metastasis in vivo
Based on the results in xenograft tumors, Transwell migration assay was performed to validate the effects of 25-HC on HCC cells migration. As shown in Figure 2A, 25-HC markedly increased the number of HepG2 cells migrated through the membrane in a dose-dependent manner. In addition, MMP1, MMP2, MMP3, MMP9 and MMP13 expressions were detected by RT-qPCR and Western blotting, respectively (Figure 2B and 2C). In line with the migration results, 25-HC increased the expressions of the detected MMPs in a dose and time-dependent manner. Taken together, these results demonstrated that 25-HC promotes the migratory ability of HCC cells by regulating MMPs in vitro.
Figure 2.
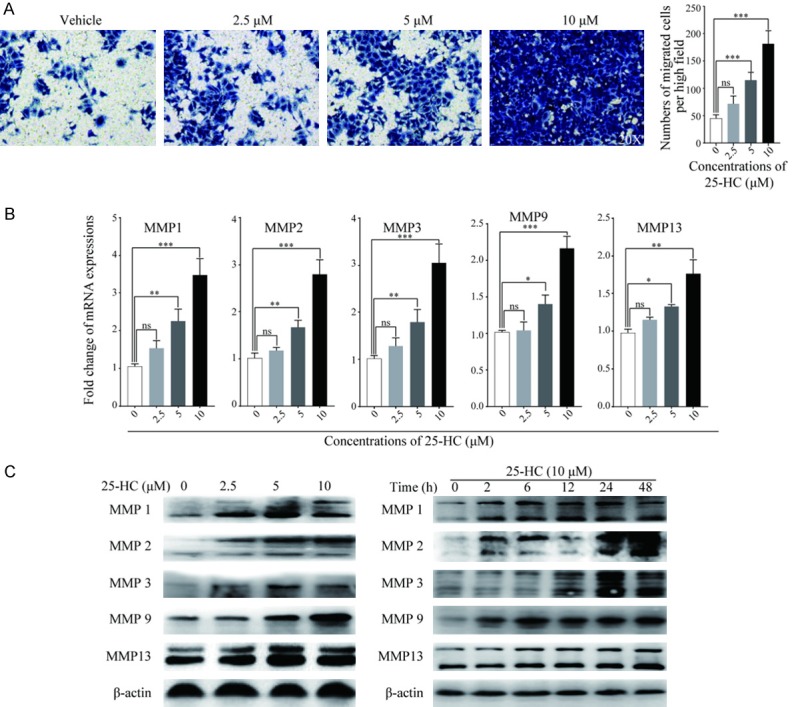
25-HC promotes HepG2 cells migration in vitro. A. Migratory ability of HepG2 cells after treated with 25-HC for 36 hours was determined by Transwell assay. Numbers of cells migrated through the membrane were calculated in five fields per well. B. HepG2 cells were treated with the indicated concentrations of 25-HC for 24 hours before mRNA was extracted and expressions of MMPs were examined by RT-qPCR. C. HepG2 cells were treated with the indicated concentrations of 25-HC for 24 hours or treated with 25-HC at 10 μM for the indicated times before protein was collected to detect the MMPs expressions by Western blotting. Results were obtained from 3 independent experiments and are expressed as the means ± SEM. Statistical significance was determined by one-way ANOVA followed by Dunnett’s test. ns, not significant, *P<0.05, **P<0.01, ***P<0.001.
To further clarify the role of 25-HC in HCC metastasis, HepG2 cells were orthotopically implanted into the livers of nude mice followed by PBS or 25-HC treatment. 3 weeks later, the mice were sacrificed, abdominal and intrahepatic metastasis were observed and recorded (Figure 3A). 25-HC treatment resulted in the more tumors spreading accompanying with the increased body weight of the mice which resulted from the ascitic fluid caused by tumor (Figure 3B). Meanwhile, the mortality rate of mice in the 25-HC group were higher than that in the PBS group (Figure 3C).
Figure 3.
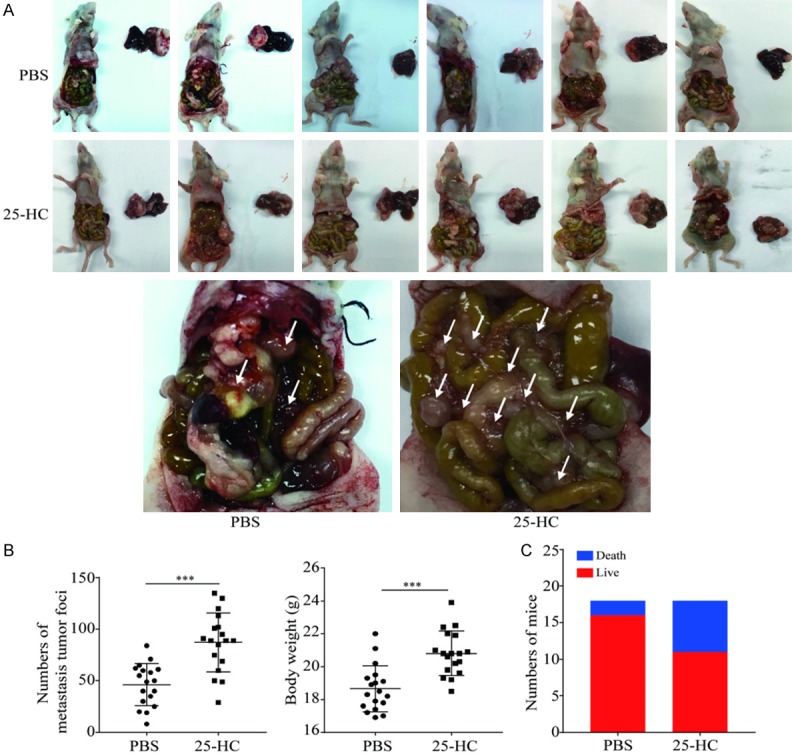
25-HC promotes HepG2 cells intrahepatic and abdominal metastasis. BALB/C nude mice were intrahepatic inoculation of 5 × 106 HepG2 cells followed by intraperitoneally injection with PBS or 25-HC. At the end of the experiment, mice were sacrificed, (A) livers were removed and imaged. (B) The number of abdominal metastasis was counted, and body weight was recorded. (C) The survival of mice for the whole experiment was recorded. Results were obtained from 2 independent experiments and are expressed as the means ± SEM. Statistical significance was determined by Student’s t-test, ***P<0.001.
25-HC enhances the activation of multiple signaling pathways
To get a further understanding of the mechanisms by which 25-HC affected HepG2 cells migration, we next analyzed the phosphorylation level of Erk, p38, SAPK/JNK, PI3K, Akt, Stat3 and NF-κB p65 by Western blotting. To our surprise, 25-HC up-regulated all the phosphorylation level of the detected proteins in a dose- and time-dependent manner (Figure 4A). Then, the activities of NF-κB, activator protein-1 (AP-1) and Stat3 were further determined by luciferase reporter assay. As shown in Figure 4B, 25-HC at 10 μΜ significantly increased the luciferase activity of NF-κB for 4.92-fold, AP-1 for 5.9-fold and Stat3 for 9.7-fold in HepG2 cells. These data indicated that 25-HC promotes HCC migration via the activation of multiple signaling pathways.
Figure 4.
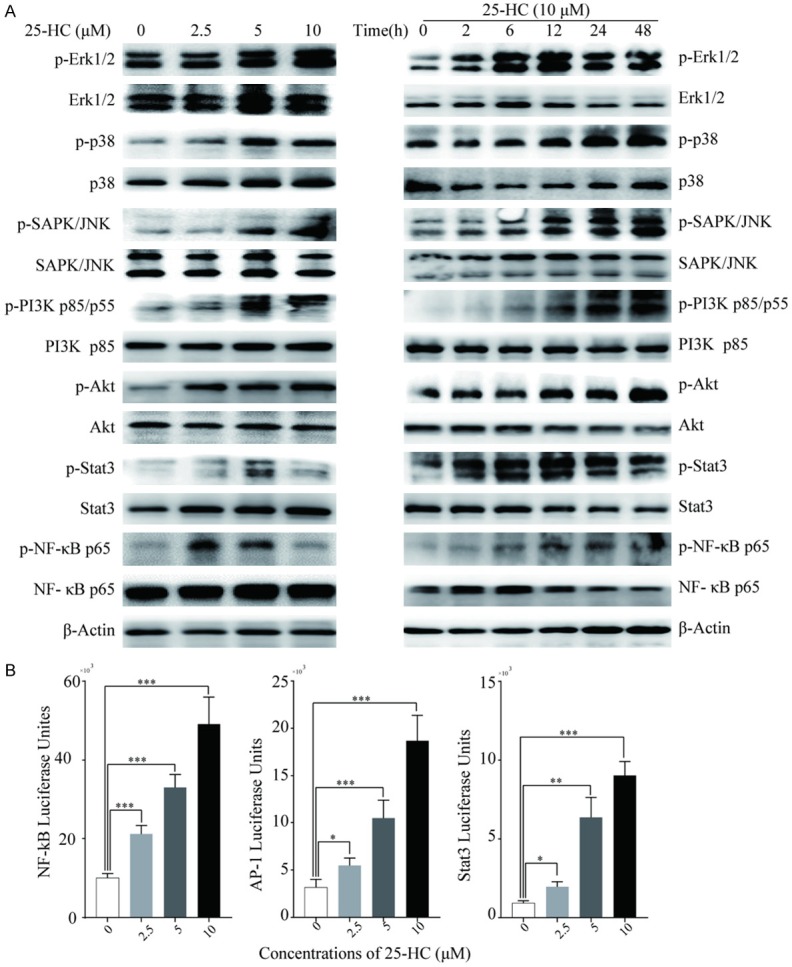
25-HC enhances the activation of multiple signaling pathways. A. HepG2 cells were treated with the indicated concentrations of 25-HC for 24 hours or treated with 25-HC at 10 μM for the indicated times before protein was collected to detect the p-Erk1/2, Erk1/2, p-p38, p38, p-SAPK/JNK, SAPK/JNK, p-Akt, Akt, p-PI3K p85/p55, PI3K p85, p-Stat3, Stat3, p-NF-κB p65, NF-κB p65 expressions by Western blotting. B. HepG2 cells were transfected with pNF-κB-luc, pAP-1-luc and pSTAT3-TA-luc reporter plasmids for 24 hours before cells were treated with the indicated concentrations of 25-HC for 24 hours. The luciferase activities were measured with the Bright-Glo luciferase assay system. Results were obtained from 3 independent experiments and are expressed as the means ± SEM. Statistical significance was determined by one-way ANOVA followed by Dunnett’s test. *P<0.05, **P<0.01, ***P<0.001.
The migration of HepG2 cells induced by 25-HC is partly mediated by TLR4
Our previous study in gastric cancer cells demonstrated that 25-HC could increase TLR2 expression [18]. Thus, we wondered whether the promotive effects of 25-HC on HCC cells migration was related to TLRs expressions. HepG2 cells can express TLR2, 3, 4, 5, 6, 7 and 9 while TLR3 and TLR6 expression was significantly lower [20]. Thus, the expressions of TLRs (TLR2, TLR4, TLR5, TLR7 and TLR9) in HepG2 cells were examined after treated with the indicated concentrations of 25-HC for varied times. Based on the Western blotting results, 25-HC significantly increased the protein expressions while did not affect the mRNA expressions of TLR4 and TLR9 in HepG2 cells (Figure 5A and 5B). Next, we elucidated the role of TLR4 and TLR9 in 25-HC induced cells migration by transfection with the corresponding siRNAs. As shown in Figure 5C and 5D, knockdown of TLR4 in HepG2 cells significantly inhibited the induced MMPs expressions and cells migration, while knockdown of TLR9 had no obvious effects, implying that the promotion of cells migration induced by 25-HC is partly mediated by TLR4.
Figure 5.
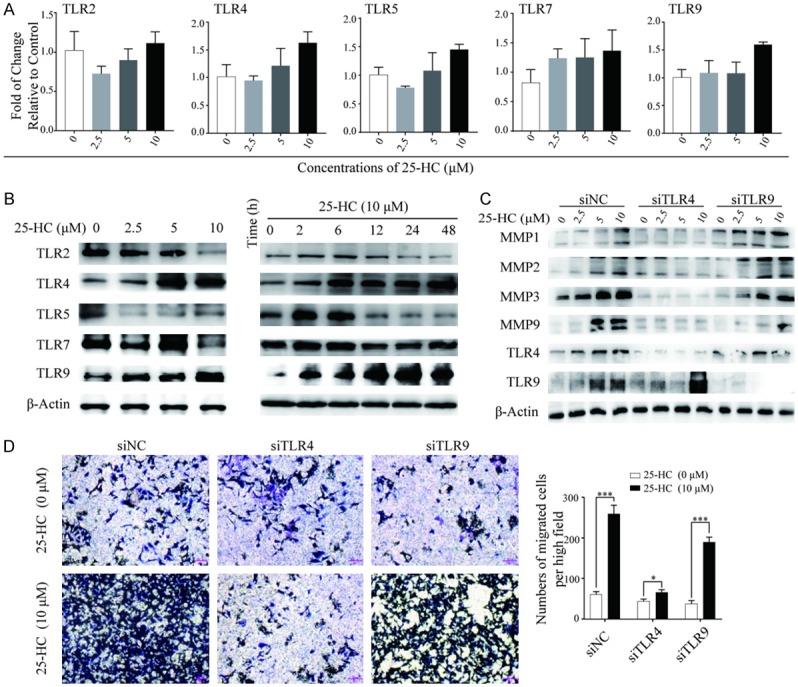
The migration of HepG2 cells induced by 25-HC is partly mediated by the up-regulation of TLR4. (A) HepG2 cells were treated with the indicated concentrations of 25-HC for 24 hours before mRNA was extracted and expressions of TLR2, TLR4, TLR5, TLR7 and TLR9 were examined by RT-qPCR. (B) HepG2 cells were treated with the indicated concentrations of 25-HC for 24 hours or treated with 25-HC at 10 μM for the indicated times before protein was collected to detect the TLR2, TLR4, TLR5, TLR7 and TLR9 expressions by Western blotting. HepG2 cells were transfected with siNC or siTLR4 or siTLR9 for 24 hours, (C) then cells were treated with the indicated concentrations of 25-HC for 24 hours before proteins were collected and the expressions of MMP1, MMP2, MMP3 and MMP9 were determined by Western blotting. (D) Or migratory ability of HepG2 cells after treated with 25-HC for 36 hours was determined by Transwell assay. Results were obtained from 3 independent experiments and are expressed as the means ± SEM. Statistical significance was determined by Student’s t-test. *P<0.05, ***P<0.001.
25-HC increases FABP4 expression via TLR4 to facilitate HepG2 cells migration
To further investigate the mechanisms involved in the 25-HC induced cells migration, we used Fatty Acid Metabolism RT2 Profiler PCR Array to analyze the genes associated with fatty metabolism in HepG2 cells after treated with 25-HC and found that FABP4 was the highest induced. Meanwhile, expressions of FABP4 were validated by RT-qPCR and Western blotting (Figure 6A). As expected, 25-HC dramatically up-regulated FABP4 expression in a dose-dependent manner. Then we treated HepG2 cells with human recombinant FABP4 in vitro, we observed that FABP4 up-regulated MMP1, MMP2, MMP3 and MMP9 expressions accompanying with the increased cells migration (Figure 6B and 6C).
Figure 6.
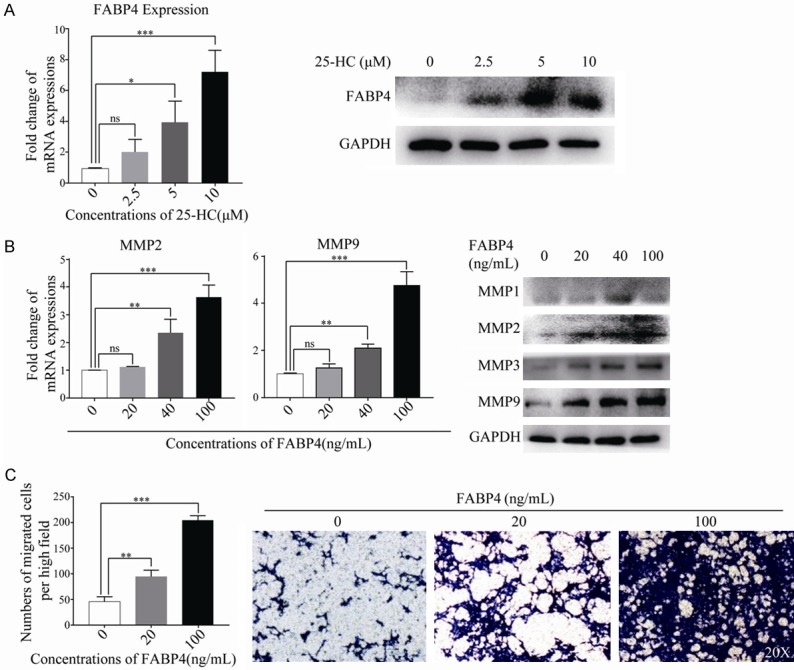
25-HC increases FABP4 expression which could directly enhance HepG2 cells migration. A. HepG2 cells were treated with the indicated concentrations of 25-HC for 24 hours before mRNA or protein was extracted to determine the expression of FABP4 by RT-qPCR or Western blotting, respectively. B. HepG2 cells were treated with the indicated concentrations of FABP4 (0, 20, 40 and 100 ng/mL) for 24 hours before mRNA or protein was extracted. The mRNA expressions of MMP2 and MMP9 were examined by RT-qPCR and the protein expressions of MMP1, MMP2, MMP3, MMP9 were detected by Western blotting, respectively. C. Migration assay of HepG2 cells after stimulation of FABP4 for 36 hours was performed. Results were obtained from 3 independent experiments and are expressed as the means ± SEM. Statistical significance was determined by one-way ANOVA followed by Dunnett’s test. ns, not significant, *P<0.05, **P<0.01, ***P<0.001.
Further, the effects of FABP4 in 25-HC-induced cells migration was detected. First, we measured the effects of FABP4 on HCC cells proliferation by knocking down FABP4 and CCK-8 was performed. As shown in Figure 7A, knocking down FABP4 has no effects on HepG2 cells proliferation. Then the migration assay was carried out, it was obvious that knockdown of FABP4 has no effects on cells migration, while after 25-HC treatment, FABP4 knockdown significantly decreased the number of cells migrated as well as the MMPs expression. Similar to the siRNA results, BMS-309403 (a FABP4 specific inhibitor) treatment also inhibited cells migration induced by 25-HC (Figure 7B). To investigate whether the functions of FABP4 are mediated through TLR4, HepG2 cells were transfected with siRNA against TLR4. Interestingly, knocking down of TLR4 resulted in the decreased FABP4 expression (Figure 7C). These findings indicated that increased FABP4 expression by 25-HC was partly dependent on TLR4 to facilitate HepG2 cells migration.
Figure 7.
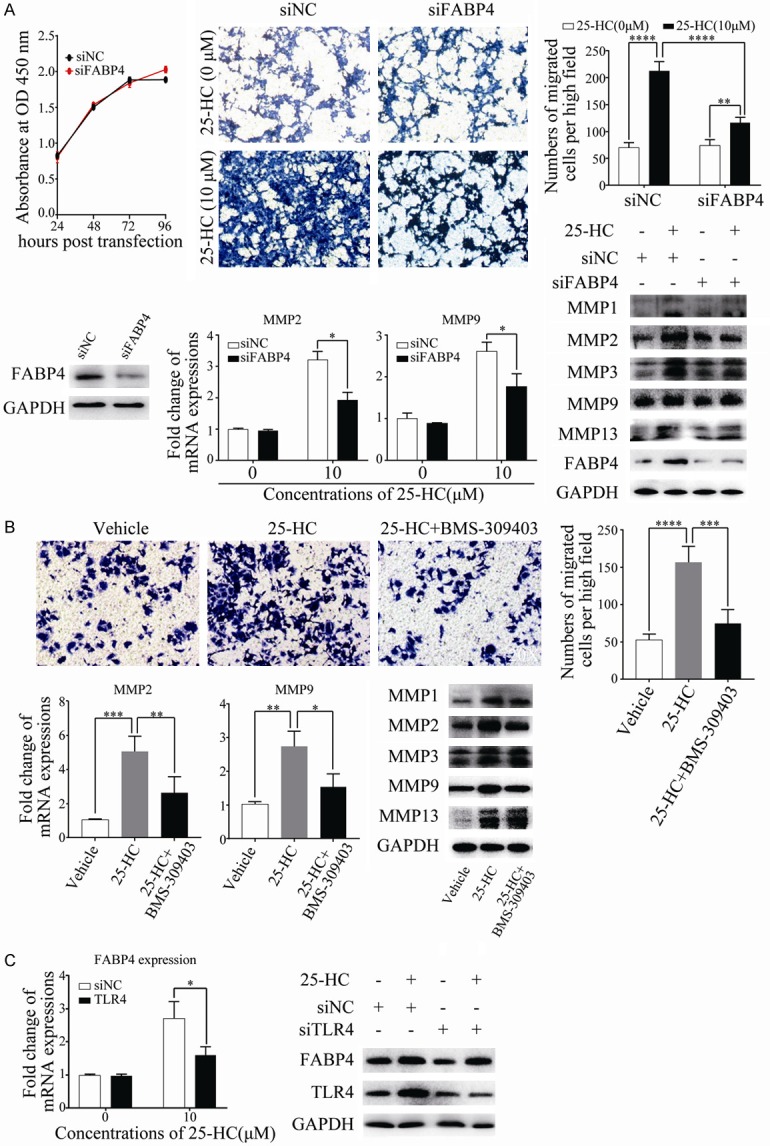
Inhibition of FABP4 decreases the promotive effects of 25-HC on HepG2 cells migration. A. HepG2 cells were transfected with siNC or siFABP4 and CCK-8 assay was performed. Results are shown as the cells absorbance at 450 nm. HepG2 cells were transfected with siNC or siFABP4 for 24 hours before cells were treated with 25-HC at 10 μM. 36 hours later, migratory ability of HepG2 cells was determined by Transwell assay. Or 24 hours later, cells were collected for mRNA or protein extraction to determine the expressions of MMPs by RT-qPCR or Western blotting, respectively. B. HepG2 cells were stimulated with 10 μM 25-HC with or without 30 μM BMS-309403. 36 hours later, migration assay was performed. Or 24 hours later, cells were collected for mRNA or protein extraction to determine the expressions of MMPs by RT-qPCR or Western blotting, respectively. C. HepG2 cells were transfected with siNC or siTLR4 for 24 hours before 25-HC treatment. 24 hours later, the mRNA and protein expression of FABP4 was assessed by RT-qPCR and Western blotting, respectively. Results were obtained from 3 independent experiments and are expressed as the means ± SEM. Statistical significance was determined by Student’s t-test. *P<0.05, **P<0.01, ***P<0.001.
To further examine the role of FABP4 in the 25-HC-promoted HCC cells metastasis, the in vivo experiment was performed by intra-peritoneal injection of BMS-309403 with 25-HC. As shown in Figure 8A, 25-HC treatment induced more tumors spreading which was consistent with the results in Figure 3A. As expected, inhibition of FABP4 by BMS-309403 significantly decreased 25-HC-induced tumor metastasis (P<0.05) (Figure 8B). While, there were no big differences of the body weight.
Figure 8.
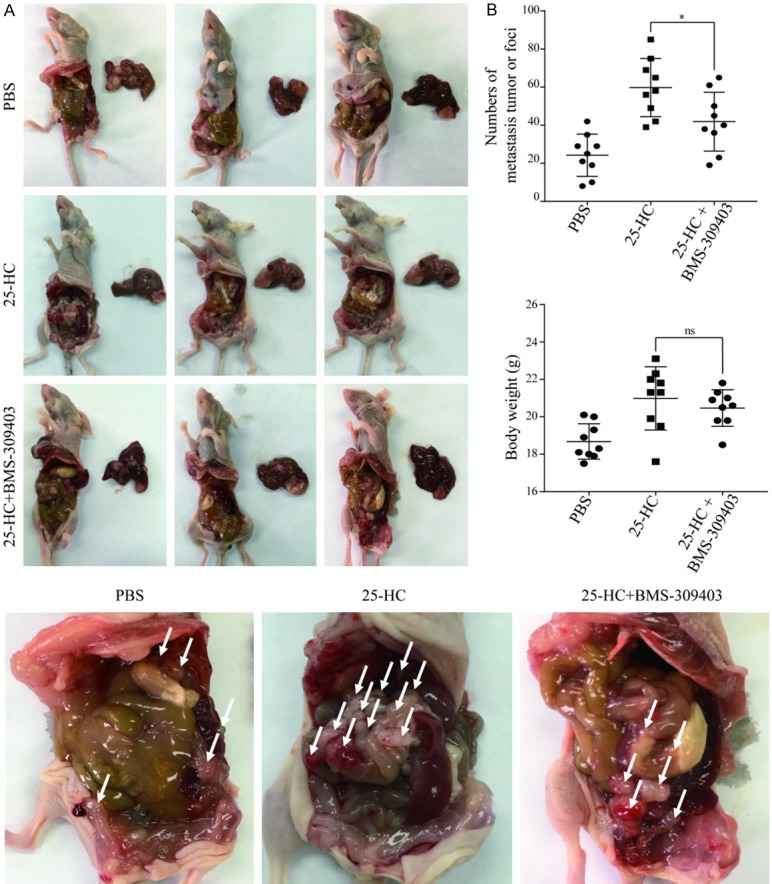
Inhibition of FABP4 decreases the 25-HC-induced HepG2 cells intrahepatic and abdominal metastasis. BALB/C nude mice were intrahepatic inoculation of 5 × 106 HepG2 cells followed by intraperitoneally injection with PBS or 25-HC (10 mg/kg) or 25-HC with BMS-309403 (45 mg/kg). At the end of the experiment, mice were sacrificed, (A) livers were removed and imaged. (B) The number of abdominal metastasis was counted, and body weight was recorded. Results were obtained from 3 independent experiments and are expressed as the means ± SEM. Statistical significance was determined by Student’s t-test, **P<0.01. ns: no significant.
FABP4 was up-regulated in HCC tissues
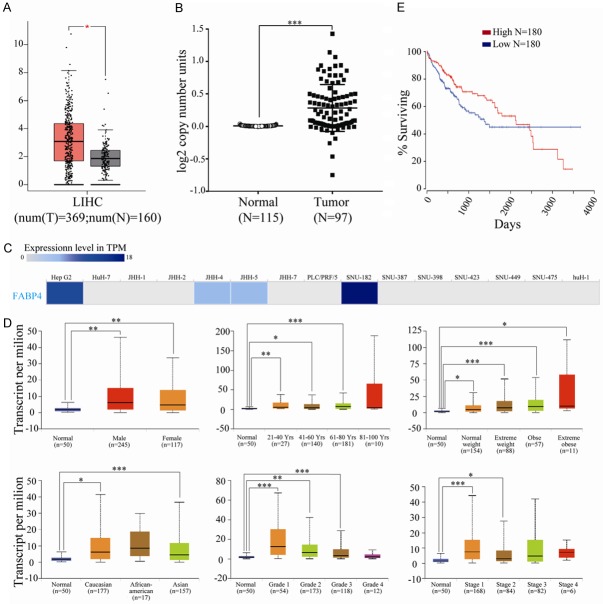
To determine the clinical significance of FABP4 in HCC, we first examined FABP4 mRNA expression in HCC and its normal tissues with GEPIA (Gene Expression Profiling Interactive Analysis) dataset (http://gepia.cancer-pku.cn/). The results indicated that the expression level of FABP4 was higher in liver hepatocellular carcinoma than in normal liver samples (Figure 9A). We also analyzed the DNA copy numbers of FABP4 from TCGA database. Consistently, FABP4 DNA copy number is increased in primary human HCC tissues (Figure 9B). Then EMBL-EBI bioinformatics website (http://www.ebi.ac.uk/gxa/home) was used to determine the expression of FABP4 in hepatocellular carcinoma cell lines, and results indicated that FABP4 was decreased in most cell lines of HCC while increased in HepG2 and SNU-182 cells (Figure 9C). Sub-group analysis of multiple clinic pathological features of HCC samples from the UALCAN consistently showed high transcription of FABP4. The transcription level of FABP4 was significantly higher in HCC patients than healthy people in subgroup analyses based on gender, age, patients’ weight, race, disease stages and tumor grade (Figure 9D). Correlation between expression of FABP4 and the prognosis of patients with HCC was assessed in OncoLnc. Result revealed that expression of FABP4 was not a prognostic factor for HCC (P=0.08, Figure 9E). Furthermore, the FABP4 expression in primary HCC without metastasis, primary HCC with metastasis and the metastatic tumor sites from the in vivo study were also determined. As shown in Supplementary Figure 1, there is a slight increase of FABP4 expression in HCC with metastasis, while the elevation of FABP4 in peritoneal metastatic sites was significantly.
Figure 9.
FABP4 was up-regulated in HCC tissues. (A) The mRNA expression of FABP4 in hepatocellular carcinoma and normal tissues (GEPIA). T, tumor tissues, N, normal tissues. (B) DNA copy number of FABP4 in hepatocellular carcinoma from The Cancer Genome Atlas (TCGA). (C) The expression of FABP4 in hepatocellular carcinoma cell lines (EMBL-EBI). (D) FABP4 transcription in subgroups of patients with hepatocellular carcinoma, stratified based on gender, age, weight, race, tumor grade and cancer stages (UALCAN). (E) Correlation between FABP4 expression and the prognosis of patients with hepatocellular carcinoma (OncoLnc). Data are expressed as the means ± SEM. Statistical significance was determined by Student’s t-test (A and B) or one-way ANOVA followed by Dunnett’s test (D). *P<0.05, **P<0.01, ***P<0.001.
Discussion
HCC is a highly prevalent and lethal cancer. Metabolic syndrome is a significant risk factor for development of HCC [21]. Here, we demonstrate that as one of the oxysterols, 25-HC displays the promotive effects on HCC cells migration and metastasis. Further study reveals that the possible molecular mechanism is the up-regulating of TLR4-dependent FABP4.
Fatty acid binding protein 4 (FABP4), also known as adipocyte-type fatty acid binding protein (A-FABP), is a key adipokine for fatty acid transport. FABP4 has been reported to play important roles in insulin resistance, type 2 diabetes and atherosclerosis [22]. The role of FABP4 in cancer has been extensively studied including breast cancer, colorectal cancer, prostate cancer, bladder cancer and so on [23-27]. In HCC, McKillop et al. reported that the circulating levels of FABP4 increased in patients and in liver tissues of a chemically induced HCC mouse model suffered from a high fat diet. Meanwhile, exogenous FABP4 treatment significantly increases HCC cells proliferation and migration [28]. However, one study reported that FABP4 is down-regulated in HCC tissues and suppresses cells proliferation and invasion, suggesting that FABP4 acts as a tumor suppressor [29], which was inconsistent with our results that we showed exogenous FABP4 enhanced HCC cells migration. This contradictory result requires further research and more clinical samples. More recently, Paradis et al. demonstrated that FABP4 exerts oncogenic effects on hepatoma cell lines by upregulating the angiogenesis gene signature and pathways involved in the cell cycle, leading to increased cell proliferation and migration, and downregulating HIF1 pathway which could be reversed by the FABP4 specific inhibitor BMS-309403 [30].
25-HC is synthesized by cholesterol 25-hydroxylase (CH25H), which is an interferon-stimulated gene and could be up-regulated in macrophages by TLRs activation [31]. In our study, the effect of 25-HC was proved to be partly dependent on TLR4, since knockdown of TLR4 alleviated the promotive effects of 25-HC in HepG2 cells. Till now there was no study reported that 25-HC up-regulates TLRs expressions in macrophages which could be studied further to detect the function of this positive feedback loop.
The prognosis for HCC patients with extrahepatic metastasis is very poor. Thus, studies aimed at understanding cells metastasis associated with metabolic syndrome might find therapeutic applications to improve survival in HCC patients. Our current study offers a therapeutic strategy that targeting 25-HC or FABP4 might reduce HCC metastasis.
Acknowledgements
This work was supported by the grant from the Key Project of Natural Science Foundation of Zhejiang Province (LZ16H160003 to WC).
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Gao C, Fang L, Zhao HC, Li JT, Yao SK. Potential role of diabetes mellitus in the progression of cirrhosis to hepatocellular carcinoma: a cross-sectional case-control study from Chinese patients with HBV infection. Hepatobiliary Pancreat Dis Int. 2013;12:385–393. doi: 10.1016/s1499-3872(13)60060-0. [DOI] [PubMed] [Google Scholar]
- 3.Larsson SC, Wolk A. Overweight, obesity and risk of liver cancer: a meta-analysis of cohort studies. Br J Cancer. 2007;97:1005–1008. doi: 10.1038/sj.bjc.6603932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Che L, Chi W, Qiao Y, Zhang J, Song X, Liu Y, Li L, Jia J, Pilo MG, Wang J, Cigliano A, Ma Z, Kuang W, Tang Z, Zhang Z, Shui G, Ribback S, Dombrowski F, Evert M, Pascale RM, Cossu C, Pes GM, Osborne TF, Calvisi DF, Chen X, Chen L. Cholesterol biosynthesis supports the growth of hepatocarcinoma lesions depleted of fatty acid synthase in mice and humans. Gut. 2019 doi: 10.1136/gutjnl-2018-317581. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dominguez-Perez M, Simoni-Nieves A, Rosales P, Nuno-Lambarri N, Rosas-Lemus M, Souza V, Miranda RU, Bucio L, Uribe Carvajal S, Marquardt JU, Seo D, Gomez-Quiroz LE, Gutierrez-Ruiz MC. Cholesterol burden in the liver induces mitochondrial dynamic changes and resistance to apoptosis. J Cell Physiol. 2019;234:7213–7223. doi: 10.1002/jcp.27474. [DOI] [PubMed] [Google Scholar]
- 6.Bellanti F, Mitarotonda D, Tamborra R, Blonda M, Iannelli G, Petrella A, Sanginario V, Iuliano L, Vendemiale G, Serviddio G. Oxysterols induce mitochondrial impairment and hepatocellular toxicity in non-alcoholic fatty liver disease. Free Radic Biol Med. 2014;75(Suppl 1):S16–17. doi: 10.1016/j.freeradbiomed.2014.10.594. [DOI] [PubMed] [Google Scholar]
- 7.Van Rooyen DM, Larter CZ, Haigh WG, Yeh MM, Ioannou G, Kuver R, Lee SP, Teoh NC, Farrell GC. Hepatic free cholesterol accumulates in obese, diabetic mice and causes nonalcoholic steatohepatitis. Gastroenterology. 2011;141:1393–403. 1403.e1–5. doi: 10.1053/j.gastro.2011.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liang JQ, Teoh N, Xu L, Pok S, Li X, Chu ESH, Chiu J, Dong L, Arfianti E, Haigh WG, Yeh MM, Ioannou GN, Sung JJY, Farrell G, Yu J. Dietary cholesterol promotes steatohepatitis related hepatocellular carcinoma through dysregulated metabolism and calcium signaling. Nat Commun. 2018;9:4490. doi: 10.1038/s41467-018-06931-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Singh S, Singh PP, Singh AG, Murad MH, Sanchez W. Statins are associated with a reduced risk of hepatocellular cancer: a systematic review and meta-analysis. Gastroenterology. 2013;144:323–332. doi: 10.1053/j.gastro.2012.10.005. [DOI] [PubMed] [Google Scholar]
- 10.Guillemot-Legris O, Mutemberezi V, Muccioli GG. Oxysterols in metabolic syndrome: from bystander molecules to bioactive lipids. Trends Mol Med. 2016;22:594–614. doi: 10.1016/j.molmed.2016.05.006. [DOI] [PubMed] [Google Scholar]
- 11.Pokharel SM, Shil NK, Gc JB, Colburn ZT, Tsai SY, Segovia JA, Chang TH, Bandyopadhyay S, Natesan S, Jones JCR, Bose S. Integrin activation by the lipid molecule 25-hydroxycholesterol induces a proinflammatory response. Nat Commun. 2019;10:1482. doi: 10.1038/s41467-019-09453-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shawli GT, Adeyemi OO, Stonehouse NJ, Herod MR. The oxysterol 25-hydroxycholesterol inhibits replication of murine norovirus. Viruses. 2019;11 doi: 10.3390/v11020097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang Y, Song Z, Wang M, Lan M, Zhang K, Jiang P, Li Y, Bai J, Wang X. Cholesterol 25-hydroxylase negatively regulates porcine intestinal coronavirus replication by the production of 25-hydroxycholesterol. Vet Microbiol. 2019;231:129–138. doi: 10.1016/j.vetmic.2019.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li C, Deng YQ, Wang S, Ma F, Aliyari R, Huang XY, Zhang NN, Watanabe M, Dong HL, Liu P, Li XF, Ye Q, Tian M, Hong S, Fan J, Zhao H, Li L, Vishlaghi N, Buth JE, Au C, Liu Y, Lu N, Du P, Qin FX, Zhang B, Gong D, Dai X, Sun R, Novitch BG, Xu Z, Qin CF, Cheng G. 25-hydroxycholesterol protects host against Zika Virus infection and its associated microcephaly in a mouse model. Immunity. 2017;46:446–456. doi: 10.1016/j.immuni.2017.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lappano R, Recchia AG, De Francesco EM, Angelone T, Cerra MC, Picard D, Maggiolini M. The cholesterol metabolite 25-hydroxycholesterol activates estrogen receptor alpha-mediated signaling in cancer cells and in cardiomyocytes. PLoS One. 2011;6:e16631. doi: 10.1371/journal.pone.0016631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Villablanca EJ, Raccosta L, Zhou D, Fontana R, Maggioni D, Negro A, Sanvito F, Ponzoni M, Valentinis B, Bregni M, Prinetti A, Steffensen KR, Sonnino S, Gustafsson JA, Doglioni C, Bordignon C, Traversari C, Russo V. Tumor-mediated liver X receptor-alpha activation inhibits CC chemokine receptor-7 expression on dendritic cells and dampens antitumor responses. Nat Med. 2010;16:98–105. doi: 10.1038/nm.2074. [DOI] [PubMed] [Google Scholar]
- 17.Chen L, Zhang L, Xian G, Lv Y, Lin Y, Wang Y. 25-Hydroxycholesterol promotes migration and invasion of lung adenocarcinoma cells. Biochem Biophys Res Commun. 2017;484:857–863. doi: 10.1016/j.bbrc.2017.02.003. [DOI] [PubMed] [Google Scholar]
- 18.Wang S, Yao Y, Rao C, Zheng G, Chen W. 25-HC decreases the sensitivity of human gastric cancer cells to 5-fluorouracil and promotes cells invasion via the TLR2/NF-kappaB signaling pathway. Int J Oncol. 2019;54:966–980. doi: 10.3892/ijo.2019.4684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang S, Shen Y, Qiu R, Chen Z, Chen Z, Chen W. 18 beta-glycyrrhetinic acid exhibits potent antitumor effects against colorectal cancer via inhibition of cell proliferation and migration. Int J Oncol. 2017;51:615–624. doi: 10.3892/ijo.2017.4059. [DOI] [PubMed] [Google Scholar]
- 20.Xia C, Lu M, Zhang Z, Meng Z, Zhang Z, Shi C. TLRs antiviral effect on hepatitis B virus in HepG2 cells. J Appl Microbiol. 2008;105:1720–1727. doi: 10.1111/j.1365-2672.2008.03896.x. [DOI] [PubMed] [Google Scholar]
- 21.Welzel TM, Graubard BI, Zeuzem S, El-Serag HB, Davila JA, McGlynn KA. Metabolic syndrome increases the risk of primary liver cancer in the United States: a study in the SEER-Medicare database. Hepatology. 2011;54:463–471. doi: 10.1002/hep.24397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Trojnar M, Patro-Malysza J, Kimber-Trojnar Z, Leszczynska-Gorzelak B, Mosiewicz J. Associations between fatty acid-binding protein 4(-) A proinflammatory adipokine and insulin resistance, gestational and type 2 diabetes mellitus. Cells. 2019;8 doi: 10.3390/cells8030227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guaita-Esteruelas S, Bosquet A, Saavedra P, Guma J, Girona J, Lam EW, Amillano K, Borras J, Masana L. Exogenous FABP4 increases breast cancer cell proliferation and activates the expression of fatty acid transport proteins. Mol Carcinog. 2017;56:208–217. doi: 10.1002/mc.22485. [DOI] [PubMed] [Google Scholar]
- 24.Zhao D, Ma Y, Li X, Lu X. microRNA-211 promotes invasion and migration of colorectal cancer cells by targeting FABP4 via PPARgamma. J Cell Physiol. 2019 doi: 10.1002/jcp.28190. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 25.Herroon MK, Rajagurubandara E, Hardaway AL, Powell K, Turchick A, Feldmann D, Podgorski I. Bone marrow adipocytes promote tumor growth in bone via FABP4-dependent mechanisms. Oncotarget. 2013;4:2108–2123. doi: 10.18632/oncotarget.1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boiteux G, Lascombe I, Roche E, Plissonnier ML, Clairotte A, Bittard H, Fauconnet S. A-FABP, a candidate progression marker of human transitional cell carcinoma of the bladder, is differentially regulated by PPAR in urothelial cancer cells. Int J Cancer. 2009;124:1820–1828. doi: 10.1002/ijc.24112. [DOI] [PubMed] [Google Scholar]
- 27.Tang Z, Shen Q, Xie H, Zhou X, Li J, Feng J, Liu H, Wang W, Zhang S, Ni S. Elevated expression of FABP3 and FABP4 cooperatively correlates with poor prognosis in non-small cell lung cancer (NSCLC) Oncotarget. 2016;7:46253–46262. doi: 10.18632/oncotarget.10086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thompson KJ, Austin RG, Nazari SS, Gersin KS, Iannitti DA, McKillop IH. Altered fatty acid-binding protein 4 (FABP4) expression and function in human and animal models of hepatocellular carcinoma. Liver Int. 2018;38:1074–1083. doi: 10.1111/liv.13639. [DOI] [PubMed] [Google Scholar]
- 29.Zhong CQ, Zhang XP, Ma N, Zhang EB, Li JJ, Jiang YB, Gao YZ, Yuan YM, Lan SQ, Xie D, Cheng SQ. FABP4 suppresses proliferation and invasion of hepatocellular carcinoma cells and predicts a poor prognosis for hepatocellular carcinoma. Cancer Med. 2018;7:2629–2640. doi: 10.1002/cam4.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Laouirem S, Sannier A, Norkowski E, Cauchy F, Doblas S, Rautou PE, Albuquerque M, Garteiser P, Sognigbe L, Raffenne J, van Beers BE, Soubrane O, Bedossa P, Cros J, Paradis V. Endothelial fatty liver binding protein 4: a new targetable mediator in hepatocellular carcinoma related to metabolic syndrome. Oncogene. 2019;38:3033–3046. doi: 10.1038/s41388-018-0597-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bauman DR, Bitmansour AD, McDonald JG, Thompson BM, Liang G, Russell DW. 25-Hydroxycholesterol secreted by macrophages in response to Toll-like receptor activation suppresses immunoglobulin a production. Proc Natl Acad Sci U S A. 2009;106:16764–16769. doi: 10.1073/pnas.0909142106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.