Abstract
The incidence of breast cancer ranks first among female malignant tumors that affect women’s health. Epidermal growth factor receptor (EGFR) family overexpression, especially human epidermal receptor2 (HER2), features prominently in breast cancer with a significant relation to poor prognosis. Currently, specific monoclonal antibodies and tyrosine kinase inhibitors (TKIs) are the two HER2 targeting strategies that have successfully improved the prognosis of patients with HER2-positive breast cancer. This paper focuses on three officially approved TKIs for HER2 breast cancer, namely, lapatinib, neratinib and pyrotinib, and systematically reviews the mechanism, safety, efficacy and resistance of these TKIs.
Keywords: HER2-positive breast cancer, lapatinib, neratinib, pyrotinib, tyrosine kinase inhibitors
Introduction
Breast cancer is the most common malignant tumor for women [1]. It has four molecular subtypes based on immunochemistry, including Luminal A, Luminal B, HER2-enriched and Triple negative. Luminal A refers to estrogen receptor (ER) and/or progesterone receptor (PR) positive with Ki-67 less than 14%; Luminal B (HER2+) refers to ER and/or PR with HER2 positive, while Luminal B (HER2-) refers to ER and/or PR, with HER2 negative and Ki-67 more than 14%; HER2-enriched refers to both ER and PR negative and HER2 positive; Triple negative, also called basal-like, refers to ER, PR and HER2 negative [2]. HER2 overexpression or gene amplification represents 15-20% of all breast cancer cases, which is closely related to aggressive phenotypes and poor outcomes [3,4]. In 2018, American Society of Clinical Oncology (ASCO) and College of American Pathologists (CAP) updated the guideline recommendations for HER2 testing in breast cancer and advocated the improvement of the accuracy of HER2 testing by immunohistochemistry (IHC) or in situ hybridization (ISH). HER2 positive criteria were defined as HER2 protein overexpression (IHC, microscopic field of vision > 10% of adjacent homogeneous tumor tissue cell region with complete and intense circumferential membrane staining) or gene amplification (ISH, average HER2 copy number ≥ 6.0 signals/cell or average HER2 copy number ≥ 4.0 signals/cell and HER2/chromosome enumeration probe 17 (CEP17) ratio ≥ 2.0). If indeterminate results appear, a reflex test using an alternative assay (IHC or ISH) is required. If the test results do not conform to other histological tests, they should be repeated. The test results from laboratories should be highly consistent with the validated HER2 test, and the test should be carried out in the laboratories certified by CAP or other authorized institutions [5].
EGFR family
Human epidermal growth factor receptor 2 (HER2/ErbB2/NEU) is a transmembrane protein in human cells encoded by oncogene ERBB2 [6], which is a member of human epidermal growth factor receptor (EGFR/ERB) family of tyrosine kinase receptors, along with epidermal growth factor receptors (EGFR)/HER1/ErbB1, HER3/ErbB3, and HER4/ErbB4.
The molecular structure of EGFR family consists of a large extracellular region, a single spanning transmembrane (TM) domain, an intracellular juxtamembrane (JM) region, a tyrosine kinase domain and a C-terminal regulatory region [7], while HER3 is the only tyrosine kinase-defective receptor [8]. The extracellular domain of EGFR family can bind to 11 ligands, but the ligand of HER2 is still unidentified [9]. In most cases, the combination of extracellular regions and ligands result in receptor-mediated dimerization of EGFRs, but it is widely believed that HER2 undergoes ligand-independent heterodimerization with other 3 EGFR family members because of its constitutively active conformation [10], or homodimerization in the cases of high concentration [11]. Furthermore, because of the lack of tyrosine kinase, HER3 homodimer shows no signaling transition. Besides inactive HER3 homodimer, the signal of HER1, HER2 and HER4 homodimer is weak compared with HER2 heterodimer, and HER2 dimer is formed prior when HER2 is overexpressed [12].
Homodimer and heterodimer formation brings the intracellular domains closer to each other, leading to the asymmetric interaction of intracellular kinase domain between the amino-terminal lobe of one tyrosine kinase and the carboxy-terminal lobe of the other, and promoting the autophosphorylation of the tyrosine kinase domains [7]. Then, several pathways such as PI3K/Akt, MAPK, PLC γ, ERK1/2, JAK/STAT are activated, regulating differentiation, apoptosis, migration, growth and adhesion of normal cells [12]. MAPK and PI3K/Akt are the two main pathways activated by EGFR family, especially HER2 heterodimer, which feature prominently in breast cancer [13]. Activated MAPK pathway promotes relative gene transcription, subsequently improving proliferation, migration, differentiation, angiogenesis and drug resistance of cancer cells [14,15]. And in PI3K/Akt pathway, phosphorylated Akt acts on a series of transcription factors including MDM2, mTOR, p27, GSK3β, BAD, NF-κB, FKHR, enhancing proliferation, survival, and suppressing apoptosis [16,17]. The mechanism of HER2 targeted drugs and EGFR family in breast cancer is summarized in Figure 1.
Figure 1.

Mechanism of HER2 targeted drugs and EGFR family in breast cancer. Trastuzumab, pertuzumab and TDM-1 bind to the juxtamembrane domain of HER2. Lapatinib is reversible TKI of HER1 and HER2, while neratinib and pyrotinib are irreversible HER 1, 2 and 4 inhibitors. These drugs inhibit downstream signals of EGFR family, especially PI3K/Akt and MAPK pathway, improving proliferation, survival, migration, angiogenesis, drug resistance and suppressing apoptosis of cancer cells.
Tyrosine kinase inhibitors (TKIs) targeting HER2
At present, there are two HER2 targeting strategies, namely, specific monoclonal antibodies and TKIs. And five drugs were officially approved by the U.S. Food and Drug Administration (FDA) for the treatment of HER2-positive breast cancer, known as trastuzumab, pertuzumab, trastuzumab emtansine (TDM-1), lapatinib and neratinib. Additionally, the Chinese State Drug Administration has recently authorized a new TKI, pyrotinib, for the treatment of patients with HER2-positive recurrence and metastasis breast cancer. TKI refers to a series of oral small molecular drugs active in promoting apoptosis and inhibiting proliferation of cancer cells. It competitively binds intracellular adenosine triphosphate (ATP) binding domains of EGFR family due to the homological structure of the ATP, resulting in inhibiting tyrosine kinase phosphorylation, subsequently blocking downstream signals [18]. TKI has the advantage of oral administration, multiple targets, and less cardiotoxicity compared with intravenous monoclonal antibodies. In terms of brain metastasis cancer treatment, the efficacy of monoclonal antibodies might be limited in crossing blood-brain-barrier (BBB), while small molecular TKIs, such as lapatinib, are thought to have the permeability through the BBB [19]. This paper reviews the mechanism, safety, efficacy and resistance of these three TKIs, namely, lapatinib, neratinib and pyrotinib. All the HER2 targeted drugs are summarized in Tables 1 and 2.
Table 1.
Summary of HER2 targeted drugs
| Drug | Brand Name | Monoclonal Antibodies | Route of Administration | TKIs | Antibody-drug Conjugate | Target | |||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| HER1 | HER2 | HER3 | HER4 | ||||||
| Trastuzumab | Herceptin | √ | Injection | + | |||||
| Pertuzumab | Perjeta | √ | Injection | + | |||||
| Trastuzumab emtansine | Kadcyla | Injection | √ | + | |||||
| Lapatinib | Tykerb | Oral | √ | + | + | ||||
| Neratinib | Nerlynx | Oral | √ | + | + | + | |||
| Pyrotinib | Irene | Oral | √ | + | + | + | |||
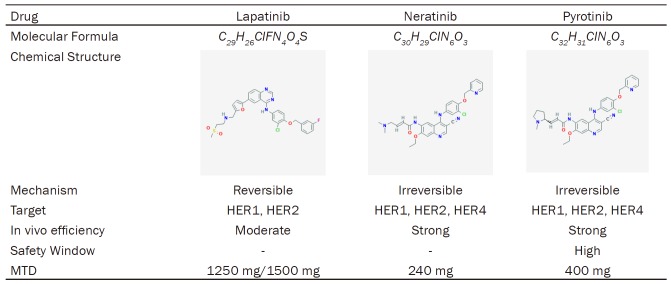
Table 2.
Summary of HER2 Targeted TKIs
Lapatinib
Lapatinib is a TKI with the capacity of reversibly blocking HER1 and HER2. On March 13, 2007, lapatinib in combination with capecitabine obtained the approval of the FDA for HER2-overexpressed/amplified breast cancer patients who previously received therapies including anthracycline, taxane, and trastuzumab [20]. And in February 2010, therapeutic regimen of lapatinib plus letrozole attained the approval of the FDA as a first-line therapeutic option for the post-menopausal women with co-expressing hormone receptors and HER2 metastatic breast cancer.
Mechanism
Lapatinib restricts phosphorylation of HER1 and HER2 by reversibly and competitively inhibiting ATP-binding sites of the intracellular kinase region and subsequently interrupting the downstream signals, namely, Raf, AKT, ERK and PLC γ, resulting in a significant efficacy in inducing the apoptosis and restricting the development and migration of HER2-overexpressing cancer cells [21,22]. But the HER1 expression level is irrelevant to the antineoplastic effect of lapatinib in HER2-overexpressing breast cancer cells [23]. Moreover, drug interaction of lapatinib and trastuzumab combination was discovered in vitro, and lapatinib still plays an antitumor role in cell lines with trastuzumab resistance [22].
Safety
The safety of lapatinib was estimated by several clinical researches in a single dose or in the combination therapy [24-29]. 1250 mg once daily in combination with capecitabine, and 1500 mg once daily in combination with letrozole, were considered as safe and effective dosages of lapatinib. The most frequent adverse events (AEs) are grade I/II, including diarrhea, nausea, fatigue, vomiting, anorexia, rash, and musculoskeletal pain. And grade 3/4 diarrhea is the most severe AE. Furthermore, left ventricular ejection fraction (LVEF) reduction, as well as the elevation of ALT and AST, was observed in patients [24,27], which means a potential hepatic and cardiac toxicity of lapatinib.
Salvage treatment
Lapatinib monotherapy was primarily assessed in some phase II studies, revealing marginal antitumor activity with tolerated toxic effects [25,27-30]. However, stronger efficacy of lapatinib in combination with other antineoplastic drugs was witnessed in some phase III trials. One phase III research (NCT00078572) evaluated lapatinib and capecitabine combination therapy versus capecitabine monotherapy in women with HER2-positive metastatic breast cancer who previously received treatment with anthracycline, a taxane, and trastuzumab [31]. 324 participants were randomized to be administrated with combination therapy (n=163) or monotherapy (n=161). The results illustrated that lapatinib and capecitabine combination therapy extended the time to progression (TTP) versus capecitabine monotherapy (8.4 vs 4.4 months), accompanied with a prolonged progression-free survival (PFS) (8.4 vs 4.1 months) and a favored overall response rate (22% vs 14%). After an additional 75 patients participated in this study by the time enrollment was suspended, the latest analysis of 399 women was carried out [32]. Updated TTP was 6.2 months for combination therapy and 4.3 months for capecitabine alone, and the overall response rate was 24% versus 14%, respectively.
The same therapeutic combination was also evaluated in another phase III trial in patients with progression on trastuzumab treatment, compared with capecitabine alone [33,34]. It was found that there was no dominant difference of median overall survival (OS) between 75 weeks and 64.7 weeks. But the median TTP of lapatinib plus capecitabine (31.3 weeks) was longer than capecitabine monotherapy (18.6 weeks) in patients who received only one previous trastuzumab-based treatment. However, another phase III trial (NCT00820222), CEREBEL, demonstrated there was no differences between lapatinib and trastuzumab both in combination with capecitabine, in terms of occurrence of central nervous system (CNS) metastases [35]. Moreover, EMILIA trial illustrated lapatinib plus capecitabine failed in challenging trastuzumab emtansine with less efficacy and tolerability [36]. Trastuzumab emtansine delayed the time to symptom worsening compared with lapatinib and capecitabine combination arm (7.1 vs 4.6 months), and more patients in trastuzumab emtansine group showed clinically significant improvement than those in combination groups (55.3% vs 49.4%).
In a phase III study (NCT00281658), lapatinib plus paclitaxel dramatically improved median OS (27.8 vs 20.5 months) of patients with HER2 metastasis breast cancer, as well as median PFS (9.7 vs 6.5 months), compared with paclitaxel and placebo [37]. Another phase III trial also showed an improved objective response rate (ORR) (63.3% vs 37.8%), clinical benefit rate (CBR) (69.4% vs 40.5%) and TTP (36.4 vs 25.1 weeks) in patients of lapatinib and paclitaxel combination group versus lapatinib plus placebo [38]. In another phase III trial, improved PFS was observed in lapatinib plus trastuzumab combination treatment as 12.0 weeks versus 8.1 weeks, as well as favored CBR (24.7% vs 12.4%) and OS (51.6 vs 39.0 weeks) compared with lapatinib monotherapy, in HER2-positive metastatic breast cancer patients who had previously progressed on trastuzumab-based therapy [24].
Moreover, lapatinib and letrozole combination therapy was found to have dramatically improved PFS of patients with hormone receptors and HER2 co-expressed metastatic breast cancer, compared with placebo plus letrozole (8.2 vs 3.0 months), as well as CBR (48% vs 29%) [39]. In an ALTERNATIVE trial, 355 patients with HER2 and ER positive breast cancer were randomly administrated with lapatinib, trastuzumab or trastuzumab plus lapatinib, both combined with aromatase inhibitor (AI) [40]. PFS of participants who received trastuzumab plus lapatinib and AI had a longer PFS in contrast with trastuzumab plus AI (11 vs 5.7 months). And PFS of lapatinib plus AI group was also higher that trastuzumab plus AI group (8.3 vs 5.7 months).
Adjuvant treatment
Lapatinib monotherapy as adjuvant treatment versus placebo was estimated in HER2-positive early-stage breast cancer female patients by TEACH research (NCT00374322), a randomized, controlled and multicenter phase III trial [41]. 3147 participants who received prior adjuvant chemotherapy without trastuzumab were administrated with lapatinib (n=1571) or placebo (n=1576). Eventually, only 2490 patients were confirmed HER2-positive after central review, and DSF event is 13% (157 of 1230) in lapatinib group and 17% (208 of 1260) in placebo group (HR 0.82, 95% 0.67-1.00; P=0.04), showing a modest antitumor efficacy. And in ALTTO phase III research (NCT00490139), 8381 HER2-positive early stage breast cancer patients were enrolled and randomly assigned to receive one-year adjuvant treatment of trastuzumab (n=2097), lapatinib (n=2100), trastuzumab plus lapatinib (n=2093), or their sequence (n=2091). Eventually, noninferiority of improving DFS and OS and increased toxicity were found in the combination treatment and the sequence treatment compared with trastuzumab monotherapy [42].
Neoadjuvant treatment
In a non-comparative, randomized, phase II trial, CHERLOB study, neoadjuvant therapy of preoperative chemotherapy plus trastuzumab and lapatinib relatively increased pCR rate (46.7% vs 25% vs 26.3%), in contrast with chemotherapy combined with trastuzumab or lapatinib [43]. Another phase II randomized neoadjuvant study (NCT00429299) estimated the safety and efficacy of letrozole plus lapatinib in contrast with letrozole plus placebo in 92 postmenopausal women with stage II to IIIA hormone receptor-positive/HER2-negative breast cancer. Eventually, neither of the two groups achieved a pathological complete response (pCR), but a higher clinical response rate was concluded out in the letrozole and lapatinib combination group as 70% compared with letrozole plus placebo (63%) [44]. In a randomized, five-arm phase II research, 215 HER2-positive breast cancer patients were enrolled and randomly assigned therapy consisting of trastuzumab, lapatinib, and paclitaxel neoadjuvant treatment, with or without prolongation of anti-HER2 therapy, and with or without endocrine treatment in ER-positive patients. Though there was no divergence in pCR rate between the prolonged exposure groups and the standard groups, ER-negative patients had a superior comprehensive pathological complete response (CpCR) rate than ER-positive patients (63.0 vs 36.1%) [45]. However, in a randomized phase III trial (NCT00567554), GeparQuinto, lapatinib as a neoadjuvant treatment in contrast with trastuzumab both in combination with epirubicin and cyclophosphamide followed by docetaxel, were valued in untreated HER2-positive operable or locally advanced breast cancer patients. pCR rate of lapatinib group was dominantly lower than trastuzumab group as 22.7% to 30.3% respectively [46].
NeoALTTO (NCT00553358), a randomized and multicenter phase III trial, assessed neoadjuvant combination therapy of lapatinib plus trastuzumab in HER2-positive early breast cancer participants [47,48]. 455 eligible women from 23 countries were randomized to receive lapatinib (n=154), trastuzumab (n=149), or lapatinib plus trastuzumab (n=152) at the first 6 weeks and weekly paclitaxel for a further 12 weeks. Then after definitive surgery, lapatinib and trastuzumab dominantly ascended patients’ pCR rate compared with trastuzumab monotherapy and lapatinib monotherapy (51.3% vs 29.5% vs 24.7%), but increased toxicity of grade 3 diarrhea and liver-enzyme alterations. After 3.77 years’ follow-up, 3-year EFS of lapatinib, trastuzumab and combination group was 78%, 76% and 84%, respectively, and 3-year OS was 93%, 90% and 95%, respectively. Though EFS and OS made no difference between treatment groups, longer EFS and OS were observed in participants who came through pCR compared with those who did not.
Neratinib (HKI-272)
Neratinib (HKI-272), whose brand name is Nerlynx, is an irreversible TKI of HER1, HER2, and HER4. On July 17, 2017, neratinib was approved by the FDA as an extended adjuvant treatment for patients with early-stage HER2-overexpressed/amplified breast cancer after surgery and trastuzumab-based adjuvant therapy.
Mechanism
Neratinib inhibits phosphorylation of ErbB family as well as downstream pathways including ERK and Akt, by covalently combining with cysteine residues Cys-773 and Cys-805 of ATP-binding domain of HER1, HER2, and HER4 [49]. The inhibition of downstream signal transduction after neratinib treatment causes reduced phosphorylated retinoblastoma protein and cyclin D1 expression and ascending p27 level, and then arrests the G1-S phase transition, eventually resulting in the down-regulation of cell proliferation [50]. In addition, neratinib can also down-regulate HER2 expression via leading to HSP90 dissociation and subsequently inducing ubiquitylation and endocytic degradation [51]. Moreover, a study found that neratinib can inhibit ATP-binding cassette transporter and thereafter reverse multidrug resistance of cancer cells [52].
Safety
The safety of neratinib was estimated by several trials [53-60] as a single drug or plus other antitumor agents. Maximum tolerated dose (MTD) of neratinib was assessed by dose escalation in these trials, and 240 mg orally once a day was designated as the recommended dosage. Common adverse reactions include diarrhea, nausea, vomiting, fatigue, stomachache, headache, rash, decreased appetite, muscle spasms and dizziness at grade 1 or 2. And diarrhea is the most frequent AE from grade 1 to 3. There was almost no grade 4 AE. Furthermore, elevated AST and ALT level was also observed in a small part of the participants.
Salvage treatment
For patients who received prior trastuzumab and had stage IIIB, IIIC, or IV HER2-positive breast cancer, a phase II trial (NCT00777101) recruited 233 participants and explored the safety and efficacy of neratinib monotherapy versus lapatinib plus capecitabine. In consequence, neratinib monotherapy illustrated no non-inferiority in prolonging PFS and OS compared with lapatinib plus capecitabine [61]. And another phase II study (NCT01494662) investigated the efficacy of neratinib in the treatment of HER2-positive breast cancer patients with brain metastasis and progression on one or more lines of CNS targeted therapy [62]. However, low CNS ORR (8%) and median PFS (1.9 months) were excluded from in neratinib monotherapy. And in NEFERT-T trial (NCT00915018), 479 women patients were randomized to receive neratinib plus paclitaxel or trastuzumab plus paclitaxel. It turned out that neratinib plus paclitaxel did not lengthen the PFS of patients in contrast with trastuzumab plus paclitaxel, but might delay CNS metastasis and reduce the CNS recurrence [63].
Adjuvant treatment
An inspiring result was found in a multicenter, randomized phase III study of extended neratinib adjuvant therapy, ExteNET trial (NCT00878709) [60,64]. 2840 eligible female patients who had early-stage breast cancer and had completed over -2 -year neoadjuvant and adjuvant trastuzumab treatment were finally enrolled and randomly assigned to receive neratinib (n=1420) or placebo (n=1420). Patients received 240 mg neratinib orally once daily for one year, versus placebo. Finally, 2816 patients (1408 in neratinib group, 1408 in placebo group) completed the trial and were included in the safety analysis. After two-year and five-year follow up, neratinib adjuvant therapy revealed an arresting diminution in the recurrence rate of breast cancer compared with placebo group (93.9% vs 91.6% and 90.2% vs 87.7% iDFS, respectively).
Neoadjuvant treatment
An adaptive randomized phase II study, I-SPY-2, explored neoadjuvant of neratinib in high-risk breast cancer [65]. I-SPY-2 is an experimental platform for evaluating the efficacy of new drugs for different breast cancer subtypes in a relatively short period of time, by using data from a small number of patients receiving neoadjuvant chemotherapy. Thus, appropriate drugs would be administrated prior to the subgroup with specific molecular signature. Participants with HER2 overexpressed and hormone receptor-negative breast cancer received 12-week paclitaxel plus 4 cycles of doxorubicin and cyclophosphamide every 2 to 3 weeks, and randomly received neratinib (n=115) or trastuzumab (n=78) in the first 12 weeks. In consequence, pCR rate of neratinib group was 56% (37% to 73%) compared with control group, 33% (11% to 54%). Neratinib and/or trastuzumab plus chemotherapy as neoadjuvant therapy in locally advanced HER2-positive breast cancer was investigated by a phase II study (NCT01008150), NSABP-FB7. 141 patients were enrolled and randomized to be administrated with neratinib, or trastuzumab, or neratinib plus trastuzumab both combined with weekly paclitaxel and followed by doxorubicin and cyclophosphamide. The result was announced at the San Antonio breast cancer symposium in December 2015 [66]. The overall pCR rate was 38.1%, 33.3% and 50.0%, respectively. And pCR rate of HER2-positive and hormone receptor-negative patients were higher at 57.1%, 46.2% and 73.7%, respectively.
Pyrotinib (SHR1258)
Pyrotinib (SHR1258), whose brand name is Irene, is a new generation of anti-HER2 therapeutic target drug developed by Jiangsu Hengrui Pharma. In August 2018, Chinese State Drug Administration first conditionally approved pyrotinib for the combination treatment of capecitabine for patients with HER2-positive advanced or metastatic breast cancer and those who had received anthracycline or taxane chemotherapy previously.
Mechanism
It is a small-molecular irreversible dual pan-ErbB TKI with activity against HER1 (IC50, 5.6 ± 3.9 nM), HER2 (IC50, 8.1 ± 2.3 nM), and HER4 [67]. By covalently binding with ATP binding sites of intracellular kinase regions, the drug inhibits the formation of homologous/heterodimer and auto-phosphorylation of HER family, thus blocking the activation of RAS/RAF/MEK/MAPK, PI3K/AKT signaling pathways and tumor cell cycle in G1 phase and restricting tumor development.
Kai Zhang et al. also found the combination of palbociclib, a CDK4/6 inhibitor, and pyrotinib showed a synergistic effect of proliferation and colony formation suppression both in trastuzumab-sensitive and trastuzumab-resistant cell lines of human HER2-positive breast cancer. The combined treatment promotes RB P70S6K phosphorylation and suppresses AKT and HER3 phosphorylation, inducing G0-G1 cell cycle arrest and apoptosis. Furthermore, in the xenograft model experiment, the combination of palbociclib and pyrotinib corroborate inferior antitumor activity compared with either agent alone without manifest toxicity increase [68].
Preclinical study
The pharmacokinetics of pyrotinib was investigated in two researches [69,70]. After one single oral administration, pyrotinib is absorbed into blood in 1 hour, and reaches peak blood concentrationin 4 hours, while after multiple oral administrations, the plasma concentration gets to a plateau on day 8. Pyrotinib is transported in blood plasma by covalently binding with the amino acid residue Lys190 of human serum albumin. Between the 4th and 12th hour, pyrotinib is practically metabolized into 24 products, but the parent drug still accounts for the largest part in circulation. Among these metabolites, SHR150980 (M1), SHR151468 (M2), and SHR151136 (M5) are the three major substances detected in plasma. The enzyme CYP3A4 features prominently in biotransformation of pyrotinib. The elimination of pyrotinib is quickly processed within 36 hours, and no metabolites can be detected after that. Eventually, pyrotinib and its metabolites are excreted in feces (90.9%) and urine (1.7%) as the intact parent drug.
Clinical study
Hitherto, one phase I and one phase I/II clinical trial about pyrotinib in patients with HER2-positive metastatic breast carcinoma have been completed. The safety, efficacy, pharmacokinetics, and biomarkers of pyrotinib were investigated by the first-in-patients phase I study (NCT01937689) [67]. A total of 38 HER2-positive metastatic breast cancer patients without prior exposure to TKIs were selected to receive pyrotinib orally once daily, in the dosage of 80 mg (n=3), 160 mg (n=8), 240 mg (n=8), 320 mg (n=9), 400 mg (n=8) or 480 mg (n=2). Eventually, the MTD was designated as 400 mg according to the study. The most common AE was diarrhea, followed by nausea, oral ulceration, asthenia, and leukopenia, with the percentage of 44.7%, 13.2%, 13.2%, 10.5% and 10.5%, respectively. And the most severe AE was grade 3 diarrhea, observed in 5 patients, which is dosage limiting. And only 2 patients administered with 480 mg of pyrotinib discontinued the treatment due to diarrhea. Pharmacokinetics analysis illustrated median Tmax (3.00-5.00 hours) and mean t1/2 (11.4-15.9 hours) of pyrotinib after the first dosage intake from 80 mg to 400 mg. The peak of multiple-dose plasma concentration stabilized on day 8, and the exposure was 1.22-1.57 times that of a single dose. Thus, dose dependence rather than major accumulation of pyrotinib exposure was confirmed by these pharmacokinetic results. Meanwhile, the antitumor activity of pyrotinib was primarily assessed in this trial. Two patients in 480 mg who discontinued treatment because of diarrhea were excluded, and the rest 36 patients were enrolled in further analysis of efficacy. The overall response rate was 50.0%, and the median PFS was 35.4 weeks. Interestingly, trastuzumab-naive patients had a considerably higher overall response rate compared with trastuzumab-pretreated patients as 83.3% (10 of 12) versus 33.3% (8 of 24).
In a randomized, open, controlled I/II clinical study, the efficacy and safety of pyrotinib plus capecitabine in contrast with lapatinib plus capecitabine were evaluated in the treatment of HER2-positive recurrent or metastatic breast cancer [71]. 128 participants previously administrated with anthracycline or taxanes (including adjuvant therapy or relapse and metastasis therapy) and no more than 2 lines of chemotherapy after relapse/metastasis were recruited in this trial. Participants were randomized to receive pyrotinib (400 mg once daily) plus capecitabine (1000 mg/m2 twice daily) (n=65) or lapatinib (1250 mg once daily) plus capecitabine (1000 mg/m2 twice daily) (n=63). Gastrointestinal reactions, skin reaction, metabolic and nutritional diseases, hepatobiliary diseases, systemic reactions, blood system diseases were common AEs of combination treatment of pyrotinib and capecitabine, and diarrhea accounts for 96.9%, the largest proportion, followed by palmoplantar erythrodysesthesia (78.5%), vomiting (46.2%), nausea (38.5%), anorexia (32.3%), oral mucositis (29.2%). Grade 3 AE was only diarrhea, whose incidence rate was 13.2%. And no over grade 4 AEs were found. ORR was 78.5% (n=51) of the combination therapy of pyrotinib and capecitabine and 57.1% (n=36) of the combination therapy of lapatinib and capecitabine. PFS of investigators assessment was 18.1 months versus 7.0 months, while the Independent Review Committee (IRC) assessment was 12.6 months and 5.6 months, respectively. Moreover, efficacy outcomes were further analyzed in subgroup based on whether or not trastuzumab had been used in the past. The combination of pyrotinib plus capecitabine revealed a dramatically antitumor efficacy of HER2-positive recurrence or metastasis breast carcinoma.
The result of phase III trial assessing pyrotinib versus placebo both in combination with capecitabine in women with HER2-positive metastatic breast cancer who received prior taxanes and trastuzumab therapy was reported at ASCO in June 2019. Patients were randomly assigned to be administrated with pyrotinib plus capecitabine (n=185) or placebo plus capecitabine (n=94). The median PFS for the combination group was 11.1 months, and that for placebo group was 4.1 months. Furthermore, 71 patients in placebo group whose disease progressed received pyrotinib monotherapy afterwards, revealing single drug response rate of 38.0% and the median PFS of 5.5 months [72].
Additionally, there are many clinical trials underway to further confirm the efficacy and safety of pyrotinib (https://clinicaltrials.gov, http://www.chictr.org.cn) (Table 3).
Table 3.
Clinical trials of pyrotinib in HER2 breast cancer treatment
| NCT Number | Study Type | Trial Arm | Subjects | Patients | Status | Location |
|---|---|---|---|---|---|---|
| NCT01937689 | I | Pyrotinib | 40 | HER2-positive advanced breast cancer | Completed | China |
| NCT02361112 | I | Pyrotinib + capecitabine | 38 | HER2-positive metastatic breast cancer | Completed | China |
| NCT02500199 | I | Pyrotinib | 50 | HER2-positive advanced solid tumors, including breast cancer, non-small cell lung cancer | Recruiting | USA |
| NCT03772353 | I/II | Pyrotinib + letrozole + SHR6390 | 32 | HER2-positive and HR-positive relapsed or metastatic breast cancer | Recruiting | China |
| NCT02422199 | I/II | Pyrotinib + capecitabine vs lapatinib + capecitabine | 128 | HER2-positive metastatic breast cancer | Active, not recruiting | China |
| NCT03805399 | I/II | Pyrotinib + capecitabine | 140 | Luminal androgen receptor subtype triple-negative and HER2 mutation breast cancer | Recruiting | China |
| NCT04001621 | II | Pyrotinib + capecitabine | 100 | HER2-positive advanced breast cancer with Trastuzumab-resistant | Recruiting | China |
| NCT03923179 | II | Pyrotinib + etoposide | 32 | HER2-positive advanced breast cancer | Recruiting | China |
| NCT03993964 | II | Pyrotinib + CDK4/6 Inhibitor | 20 | HER2-positive metastatic breast cancer | Not yet recruiting | China |
| NCT03933982 | II | Pyrotinib + vinorelbine | 30 | HER2-positive CNS metastatic breast cancer | Recruiting | China |
| NCT03919253 | II | Pyrotinib + nab-paclitaxel | 80 | HER2-positive advanced breast cancer | Not yet recruiting | China |
| NCT03735966 | II | Pyrotinib + trastuzumab + docetaxel | 51 | HER2-positive early stage or locally advanced breast cancer | Not yet recruiting | China |
| NCT03923166 | II | Pyrotinib+ capecitabine | 35 | HER2-positive advanced breast cancer | Recruiting | China |
| NCT03691051 | II | Pyrotinib + capecitabine | 102 | HER2-positive metastatic breast cancer | Not yet recruiting | China |
| NCT03997539 | II | Pyrotinib + vinorelbine vs Treatment of Physician’s Choice | 256 | HER2-positive locally advanced or metastatic breast cancer | Not yet recruiting | China |
| NCT03412383 | II | Pyrotinib | 14 | HER2 non-amplified but HER2 mutant metastatic breast cancer | Recruiting | China |
| NCT03876587 | II | Pyrotinib + docetaxel | 79 | HER2-positive metastatic breast cancer | Not yet recruiting | China |
| NCT03910712 | II | Pyrotinib+ trastuzumab + aromatase inhibitor vs trastuzumab + aromatase inhibitor | 250 | HER2-positive and HR-positive metastatic or inoperable locally advanced breast cancer | Not yet recruiting | China |
| NCT03980054 | III | Pyrotinib vs placebo | 1192 | HER2-positive early stage or locally advanced breast cancer | Not yet recruiting | China |
| NCT03080805 | III | Pyrotinib + capecitabine vs Lapatinib + capecitabine | 240 | HER2-positive metastatic breast cancer | Recruiting | China |
| NCT02973737 | III | Pyrotinib + capecitabine vs placebo + capecitabine | 350 | HER2-positive early stage or locally advanced breast cancer | Active, not recruiting | China |
| NCT03588091 | III | Pyrotinib + trastuzumab + docetaxel vs placebo + trastuzumab + docetaxel | 294 | HER2-positive early stage or locally advanced breast cancer | Recruiting | China |
| NCT03863223 | III | Pyrotinib + trastuzumab + docetaxel vs placebo + trastuzumab + docetaxel | 590 | HER2-positive metastatic breast cancer | Not yet recruiting | China |
| NCT03908749 | - | Pyrotinib | 300 | HER2-positive advanced breast cancer | Not yet recruiting | China |
| NCT03947242 | Not Applicable | Pyrotinib + trastuzumab + vinorelbine | 48 | HER2-positive early stage or locally advanced breast cancer | Not yet recruiting | China |
| NCT03847818 | Not Applicable | Pyrotinib + trastuzumab + docetaxel + carboplatin | 268 | HER2-positive early stage or locally advanced breast cancer | Not yet recruiting | China |
| NCT03756064 | Not Applicable | Pyrotinib + trastuzumab + docetaxel + carboplatin vs placebo + trastuzumab + docetaxel + carboplatin | 100 | HER2-positive early stage or locally advanced breast cancer | Not yet recruiting | China |
| ChiCTR1900022844 | I | Pyrotinib + different chemotherapy regimens | 60 | HER2 positive advanced breast cancer | Not yet recruiting | China |
| ChiCTR1900022557 | I | Pyrotinib + chemotherapy | 40 | HER 2-positive and HR low-positive/negative advanced recurrent/metastatic breast cancer | Not yet recruiting | China |
| ChiCTR1800020217 | II | Epirubicin + cyclophosphamide + pyrotinib + sequential docetaxel + trastuzumab + pyrotinib | 60 | HER2-positive early stage or locally advanced breast cancer | Recruiting | China |
| ChiCTR1900023653 | II | Pyrotinib + paclitaxel(albumin-binding) | 55 | HER2 positive advanced breast cancer | Not yet recruiting | China |
| ChiCTR1900022293 | III | Epirubicin + cyclophosphamide + pyrotinib + trastuzumab vs epirubicin + cyclophosphamide + trastuzumab | 210 | Stage I to III HER2-positive breast cancer | Not yet recruiting | China |
| ChiCTR1800020226 | III | Pyrotinib + trastuzumab + carboplatin + docetaxel vs trastuzumab + carboplatin + docetaxel | 336 | HER2-positive early stage or locally advanced breast cancer | Not yet recruiting | China |
| ChiCTR1900021819 | IV | Pyrotinib | 1000 | HER2-positive locally advanced breast cancer | Not yet recruiting | China |
| ChiCTR1900020670 | IV | Pyrotinib + standard treatment | 48 | HER2-positive brain metastatic breast cancer | Not yet recruiting | China |
| ChiCTR1800020449 | IV | Pyrotinib + trastuzumab + paclitaxel + cisplatin | 40 | HER2-positive early stage or locally advanced breast cancer | Not yet recruiting | China |
| ChiCTR1900023152 | IV | Pyrotinib | 60 | HER2 positive advanced breast cancer | Not yet recruiting | China |
Mechanism of resistance
At present, intrinsic and acquired resistance of lapatinib is already found in patients. Multiple genes and pathways function in lapatinib resistance, including EGFR family, PI3K/Akt/mTOR, Ras/Raf/MEK/MAPK, FOXM1/FOXO3a, eEF2/PP2A, autophagy, tumor metabolism and oth- er members of receptor tyrosine kinase family [73]. In a nutshell, activation of compensatory pathways, HER2 tyrosine kinase domain mutation and gene amplification of NIBP (TRAPPC9, trafficking protein particle complex 9) account for the three main mechanisms of lapatinib resistance [74]. Furthermore, phosphorylation-mediated reprogramming of glycolytic activity also features prominently in lapatinib resistance of breast cancer cell lines, and glycolysis inhibitors employment increases the sensitivity of resistant cells [75]. High PTEN or wild-type PIK3CA expression was found in most patients with HER2-positive breast cancers who were administrated with neoadjuvant lapatinib plus trastuzumab and finally achieved pCR [76]. On the contrary, low PTEN and PIK3CA mutations were associated with the resistance of neoadjuvant lapatinib and trastuzumab [77].
In terms of neratinib, Susan Breslin et al. found out that enhanced activity of metabolism enzyme cytochrome P4503A4 leads to neratinib resistance and cross-resistance to trastuzumab, lapatinib and afatinib [78]. One case report found that a HER2 mutant breast cancer patient, who attained HER2 gatekeeper mutation, had induced a neratinib resistance, after neratinib treatment [79]. And Seyhan et al. had identified a collection of genes related to neratinib resistance by using a genome-wide RNAi screen combined with a lethal dose of neratinib, including oncogenesis, transcription factors, cellular ion transport, protein ubiquitination, cell cycle, and genes known to interact with breast cancer-related genes [80].
The expression of RB1CC1, ERBB3, FOXO3a and NR3C1 was also upregulated in HER2 TKI-sensitive breast cancer cell lines after treatment of lapatinib, afatinib and neratinib [81]. And in phase I study of pyrotinib, after analyzing ctDNA and T-primary tumor tissues of 18 patients’ blood samples, the authors suggested that PIK3CA or TP53 mutations in ctDNA were more related to the efficacy of pyrotinib [67,82].
Side effects
Diarrhea is the most common side effect of these three TKI drugs, mostly grade 1 and 2. The mechanism of TKI-induced diarrhea is still unclear, which is different from chemotherapy induced intestinal epithelium injury. One of the hypothesis suggests that TKIs inhibit EGFR downstream signals of intestinal epithelium leading to decreased growth and regeneration [83]. While another hypothesis involved in TKI reversing EGFR negative regulatory in chloride secretion and activates basolateral membrane potassium (K+) channels, resulting in chloride secretory diarrhea [84,85]. At present, management of TKI-induced diarrhea resembles that of chemotherapy induced diarrhea, including patient education, dietary control and pharmacologic management, within valid assessment [86,87]. Rash, nausea, vomiting, anorexia, fatigue and oral ulceration are also common AEs. Moreover, these drugs still have potential hepatic toxicity. Put slightly differently, neratinib can also cause headache and dizziness, and lapatinib might decrease LVEF of heart, and pyrotinib might cause leukopenia.
Conclusion and suggestions for future research
Currently, specific therapies for HER2-overexpression breast cancer include monoclonal antibodies and small molecular TKIs. Trastuzumab is the first monoclonal antibody used in HER2 specific therapy, but increased drug resistance and cardiotoxicity are its shortcomings. TKI has attracted public attention by the advantages of oral administration, multiple targets and low cardiotoxicity. More importantly, it might cross BBB as a potential therapy of HER2-positive breast cancer with CNS metastasis. The FDA has so far approved lapatinib and neratinib, and Chinese State Drug Administration has approved a new TKI, pyrotinib, in HER2 targeted treatment of breast cancer. A series of clinical trials have demonstrated that the TKIs are promising anti-HER2 drugs, especially in the terms of metastatic breast cancer. In the results of the current clinical studies, pyrotinib initially showed decent tolerance, safety and efficacy, though its efficacy needs to be confirmed by further research. Exploring new combination therapy of currently available HER2 targeted drugs, as well as in combination with PI3K inhibitor, AKT inhibitor, mTOR inhibitor, BET inhibitor, CDK4/6 inhibitor, or PD1/PDL1 antibodies, may also discover effective therapy for HER2 positive breast cancer.
Acknowledgements
This study was supported by the Talents Training Program of Third Military Medical University (No. 2017MPRC-18 and No. 2018XLC2004) and the Military Medical Staff Innovation Plan of Southwest Hospital (No. SWH2018BJLC-04).
Disclosure of conflict of interest
None.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Fragomeni SM, Sciallis A, Jeruss JS. Molecular subtypes and local-regional control of breast cancer. Surg Oncol Clin N Am. 2018;27:95–120. doi: 10.1016/j.soc.2017.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abd El-Rehim DM, Pinder SE, Paish CE, Bell JA, Rampaul RS, Blamey RW, Robertson JF, Nicholson RI, Ellis IO. Expression and co-expression of the members of the epidermal growth factor receptor (EGFR) family in invasive breast carcinoma. Br J Cancer. 2004;91:1532–1542. doi: 10.1038/sj.bjc.6602184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wolff AC, Hammond ME, Hicks DG, Dowsett M, McShane LM, Allison KH, Allred DC, Bartlett JM, Bilous M, Fitzgibbons P, Hanna W, Jenkins RB, Mangu PB, Paik S, Perez EA, Press MF, Spears PA, Vance GH, Viale G, Hayes DF American Society of Clinical Oncology; College of American Pathologists. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American society of clinical oncology/college of American pathologists clinical practice guideline update. J. Clin. Oncol. 2013;31:3997–4013. doi: 10.1200/JCO.2013.50.9984. [DOI] [PubMed] [Google Scholar]
- 5.Wolff AC, Hammond MEH, Allison KH, Harvey BE, Mangu PB, Bartlett JMS, Bilous M, Ellis IO, Fitzgibbons P, Hanna W, Jenkins RB, Press MF, Spears PA, Vance GH, Viale G, McShane LM, Dowsett M. Human epidermal growth factor receptor 2 testing in breast cancer: American society of clinical oncology/college of american pathologists clinical practice guideline focused update. Arch Pathol Lab Med. 2018;142:1364–1382. doi: 10.5858/arpa.2018-0902-SA. [DOI] [PubMed] [Google Scholar]
- 6.Mitri Z, Constantine T, O’Regan R. The HER2 receptor in breast cancer: pathophysiology, clinical use, and new advances in therapy. Chemother Res Pract. 2012;2012:743193. doi: 10.1155/2012/743193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferguson KM. Structure-based view of epidermal growth factor receptor regulation. Annu Rev Biophys. 2008;37:353–373. doi: 10.1146/annurev.biophys.37.032807.125829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sierke SL, Cheng K, Kim HH, Koland JG. Biochemical characterization of the protein tyrosine kinase homology domain of the ErbB3 (HER3) receptor protein. Biochem J. 1997;322:757–63. doi: 10.1042/bj3220757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hsu JL, Hung MC. The role of HER2, EGFR, and other receptor tyrosine kinases in breast cancer. Cancer Metastasis Rev. 2016;35:575–588. doi: 10.1007/s10555-016-9649-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Citri A, Yarden Y. EGF-ERBB signalling: towards the systems level. Nat Rev Mol Cell Biol. 2006;7:505–516. doi: 10.1038/nrm1962. [DOI] [PubMed] [Google Scholar]
- 11.Harari D, Yarden Y. Molecular mechanisms underlying ErbB2/HER2 action in breast cancer. Oncogene. 2000;19:6102. doi: 10.1038/sj.onc.1203973. [DOI] [PubMed] [Google Scholar]
- 12.Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol. 2001;2:127–37. doi: 10.1038/35052073. [DOI] [PubMed] [Google Scholar]
- 13.Saini KS, Azim HA, Metzger-Filho O, Loi S, Sotiriou C, de Azambuja E, Piccart M. Beyond trastuzumab: new treatment options for HER2-positive breast cancer. Breast. 2011;20:S20–S27. doi: 10.1016/S0960-9776(11)70289-2. [DOI] [PubMed] [Google Scholar]
- 14.Dhillon AS, Hagan S, Rath O, Kolch W. MAP kinase signalling pathways in cancer. Oncogene. 2007;26:3279–3290. doi: 10.1038/sj.onc.1210421. [DOI] [PubMed] [Google Scholar]
- 15.Gangadhara S, Smith C, Barrett-Lee P, Hiscox S. 3D culture of Her2+ breast cancer cells promotes AKT to MAPK switching and a loss of therapeutic response. BMC Cancer. 2016;16:345. doi: 10.1186/s12885-016-2377-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vivanco I, Sawyers CL. The phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat Rev Cancer. 2002;2:489–501. doi: 10.1038/nrc839. [DOI] [PubMed] [Google Scholar]
- 17.Yuan TL, Cantley LC. PI3K pathway alterations in cancer: variations on a theme. Oncogene. 2008;27:5497–5510. doi: 10.1038/onc.2008.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Traxler P. Tyrosine kinases as targets in cancer therapy-successes and failures. Expert Opin Ther Targets. 2003;7:215–34. doi: 10.1517/14728222.7.2.215. [DOI] [PubMed] [Google Scholar]
- 19.O’Sullivan CC, Davarpanah NN, Abraham J, Bates SE. Current challenges in the management of breast cancer brain metastases. Semin Oncol. 2017;44:85–100. doi: 10.1053/j.seminoncol.2017.06.006. [DOI] [PubMed] [Google Scholar]
- 20.Ryan Q, Ibrahim A, Cohen MH, Johnson J, Ko CW, Sridhara R, Justice R, Pazdur R. FDA drug approval summary: lapatinib in combination with capecitabine for previously treated metastatic breast cancer that overexpresses HER-2. Oncologist. 2008;13:1114–9. doi: 10.1634/theoncologist.2008-0816. [DOI] [PubMed] [Google Scholar]
- 21.Gril B, Palmieri D, Bronder JL, Herring JM, Vega-Valle E, Feigenbaum L, Liewehr DJ, Steinberg SM, Merino MJ, Rubin SD, Steeg PS. Effect of lapatinib on the outgrowth of metastatic breast cancer cells to the brain. J Natl Cancer Inst. 2008;100:1092–1103. doi: 10.1093/jnci/djn216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Konecny GE, Pegram MD, Venkatesan N, Finn R, Yang G, Rahmeh M, Untch M, Rusnak DW, Spehar G, Mullin RJ, Keith BR, Gilmer TM, Berger M, Podratz KC, Slamon DJ. Activity of the dual kinase inhibitor lapatinib (GW572016) against HER-2-overexpressing and trastuzumab-treated breast cancer cells. Cancer Res. 2006;66:1630–1639. doi: 10.1158/0008-5472.CAN-05-1182. [DOI] [PubMed] [Google Scholar]
- 23.Zhang D, Pal A, Bornmann WG, Yamasaki F, Esteva FJ, Hortobagyi GN, Bartholomeusz C, Ueno NT. Activity of lapatinib is independent of EGFR expression level in HER2-overexpressing breast cancer cells. Mol Cancer Ther. 2008;7:1846–50. doi: 10.1158/1535-7163.MCT-08-0168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Blackwell KL, Burstein HJ, Storniolo AM, Rugo H, Sledge G, Koehler M, Ellis C, Casey M, Vukelja S, Bischoff J, Baselga J, O’Shaughnessy J. Randomized study of Lapatinib alone or in combination with trastuzumab in women with ErbB2-positive, trastuzumab-refractory metastatic breast cancer. J. Clin. Oncol. 2010;28:1124–1130. doi: 10.1200/JCO.2008.21.4437. [DOI] [PubMed] [Google Scholar]
- 25.Burstein HJ, Storniolo AM, Franco S, Forster J, Stein S, Rubin S, Salazar VM, Blackwell KL. A phase II study of lapatinib monotherapy in chemotherapy-refractory HER2-positive and HER2-negative advanced or metastatic breast cancer. Ann Oncol. 2008;19:1068–1074. doi: 10.1093/annonc/mdm601. [DOI] [PubMed] [Google Scholar]
- 26.Chien AJ, Munster PN, Melisko ME, Rugo HS, Park JW, Goga A, Auerback G, Khanafshar E, Ordovas K, Koch KM, Moasser MM. Phase I dose-escalation study of 5-day intermittent oral lapatinib therapy in patients with human epidermal growth factor receptor 2-overexpressing breast cancer. J. Clin. Oncol. 2014;32:1472–1479. doi: 10.1200/JCO.2013.52.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gomez HL, Doval DC, Chavez MA, Ang PC, Aziz Z, Nag S, Ng C, Franco SX, Chow LW, Arbushites MC, Casey MA, Berger MS, Stein SH, Sledge GW. Efficacy and safety of lapatinib as first-line therapy for ErbB2-amplified locally advanced or metastatic breast cancer. J. Clin. Oncol. 2008;26:2999–3005. doi: 10.1200/JCO.2007.14.0590. [DOI] [PubMed] [Google Scholar]
- 28.Johnston S, Trudeau M, Kaufman B, Boussen H, Blackwell K, LoRusso P, Lombardi DP, Ben Ahmed S, Citrin DL, DeSilvio ML, Harris J, Westlund RE, Salazar V, Zaks TZ, Spector NL. Phase II study of predictive biomarker profiles for response targeting human epidermal growth factor receptor 2 (HER-2) in advanced inflammatory breast cancer with lapatinib monotherapy. J. Clin. Oncol. 2008;26:1066–1072. doi: 10.1200/JCO.2007.13.9949. [DOI] [PubMed] [Google Scholar]
- 29.Toi M, Iwata H, Fujiwara Y, Ito Y, Nakamura S, Tokuda Y, Taguchi T, Rai Y, Aogi K, Arai T, Watanabe J, Wakamatsu T, Katsura K, Ellis CE, Gagnon RC, Allen KE, Sasaki Y, Takashima S. Lapatinib monotherapy in patients with relapsed, advanced, or metastatic breast cancer: efficacy, safety, and biomarker results from Japanese patients phase II studies. Br J Cancer. 2009;101:1676–1682. doi: 10.1038/sj.bjc.6605343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Blackwell KL, Pegram MD, Tan-Chiu E, Schwartzberg LS, Arbushites MC, Maltzman JD, Forster JK, Rubin SD, Stein SH, Burstein HJ. Single-agent lapatinib for HER2-overexpressing advanced or metastatic breast cancer that progressed on first- or second-line trastuzumab-containing regimens. Ann Oncol. 2009;20:1026–1031. doi: 10.1093/annonc/mdn759. [DOI] [PubMed] [Google Scholar]
- 31.Geyer CE, Forster J, Lindquist D, Chan S, Romieu CG, Pienkowski T, Jagiello-Gruszfeld A, Crown J, Chan A, Kaufman B, Skarlos D, Campone M, Davidson N, Berger M, Oliva C, Rubin SD, Stein S, Cameron D. Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N Engl J Med. 2006;355:2733–43. doi: 10.1056/NEJMoa064320. [DOI] [PubMed] [Google Scholar]
- 32.Kaufman B, Stein S, Casey MA, Newstat BO. Lapatinib in combination with capecitabine in the management of ErbB2-positive (HER2-positive) advanced breast cancer. Biologics. 2008;2:61–5. doi: 10.2147/btt.s1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cameron D, Casey M, Press M, Lindquist D, Pienkowski T, Romieu CG, Chan S, Jagiello-Gruszfeld A, Kaufman B, Crown J, Chan A, Campone M, Viens P, Davidson N, Gorbounova V, Raats JI, Skarlos D, Newstat B, Roychowdhury D, Paoletti P, Oliva C, Rubin S, Stein S, Geyer CE. A phase III randomized comparison of lapatinib plus capecitabine versus capecitabine alone in women with advanced breast cancer that has progressed on trastuzumab: updated efficacy and biomarker analyses. Breast Cancer Res Treat. 2008;112:533–543. doi: 10.1007/s10549-007-9885-0. [DOI] [PubMed] [Google Scholar]
- 34.Cameron D, Casey M, Oliva C, Newstat B, Imwalle B, Geyer CE. Lapatinib plus capecitabine in women with HER-2-positive advanced breast cancer: final survival analysis of a phase III randomized trial. Oncologist. 2010;15:924–934. doi: 10.1634/theoncologist.2009-0181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pivot X, Manikhas A, Żurawski B, Chmielowska E, Karaszewska B, Allerton R, Chan S, Fabi A, Bidoli P, Gori S, Ciruelos E, Dank M, Hornyak L, Margolin S, Nusch A, Parikh R, Nagi F, DeSilvio M, Santillana S, Swaby RF, Semiglazov V. CEREBEL (EGF111438): a phase III, randomized, open-label study of lapatinib plus capecitabine versus trastuzumab plus capecitabine in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer. J. Clin. Oncol. 2015;33:1564–73. doi: 10.1200/JCO.2014.57.1794. [DOI] [PubMed] [Google Scholar]
- 36.Welslau M, Dieras V, Sohn JH, Hurvitz SA, Lalla D, Fang L, Althaus B, Guardino E, Miles D. Patient-reported outcomes from EMILIA, a randomized phase 3 study of trastuzumab emtansine (T-DM1) versus capecitabine and lapatinib in human epidermal growth factor receptor 2-positive locally advanced or metastatic breast cancer. Cancer. 2014;120:642–651. doi: 10.1002/cncr.28465. [DOI] [PubMed] [Google Scholar]
- 37.Guan Z, Xu B, DeSilvio ML, Shen Z, Arpornwirat W, Tong Z, Lorvidhaya V, Jiang Z, Yang J, Makhson A, Leung WL, Russo MW, Newstat B, Wang L, Chen G, Oliva C, Gomez H. Randomized trial of lapatinib versus placebo added to paclitaxel in the treatment of human epidermal growth factor receptor 2-overexpressing metastatic breast cancer. J. Clin. Oncol. 2013;31:1947–53. doi: 10.1200/JCO.2011.40.5241. [DOI] [PubMed] [Google Scholar]
- 38.Di Leo A, Gomez HL, Aziz Z, Zvirbule Z, Bines J, Arbushites MC, Guerrera SF, Koehler M, Oliva C, Stein SH, Williams LS, Dering J, Finn RS, Press MF. Phase III, double-blind, randomized study comparing lapatinib plus paclitaxel with placebo plus paclitaxel as first-line treatment for metastatic breast cancer. J. Clin. Oncol. 2008;26:5544–5552. doi: 10.1200/JCO.2008.16.2578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Johnston S, Pippen J Jr, Pivot X, Lichinitser M, Sadeghi S, Dieras V, Gomez HL, Romieu G, Manikhas A, Kennedy MJ, Press MF, Maltzman J, Florance A, O’Rourke L, Oliva C, Stein S, Pegram M. Lapatinib combined with letrozole versus letrozole and placebo as first-line therapy for postmenopausal hormone receptor-positive metastatic breast cancer. J. Clin. Oncol. 2009;27:5538–5546. doi: 10.1200/JCO.2009.23.3734. [DOI] [PubMed] [Google Scholar]
- 40.Johnston SRD, Hegg R, Im SA, Park IH, Burdaeva O, Kurteva G, Press MF, Tjulandin S, Iwata H, Simon SD, Kenny S, Sarp S, Izquierdo MA, Williams LS, Gradishar WJ. Phase III, randomized study of dual human epidermal growth factor receptor 2 (HER2) blockade with lapatinib plus trastuzumab in combination with an aromatase inhibitor in postmenopausal women with HER2-positive, hormone receptor-positive metastatic breast cancer: ALTERNATIVE. J. Clin. Oncol. 2018;36:741–748. doi: 10.1200/JCO.2017.74.7824. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 41.Goss PE, Smith IE, O’Shaughnessy J, Ejlertsen B, Kaufmann M, Boyle F, Buzdar AU, Fumoleau P, Gradishar W, Martin M, Moy B, Piccart-Gebhart M, Pritchard KI, Lindquist D, Chavarri-Guerra Y, Aktan G, Rappold E, Williams LS, Finkelstein DM. Adjuvant lapatinib for women with early-stage HER2-positive breast cancer: a randomised, controlled, phase 3 trial. Lancet Oncol. 2013;14:88–96. doi: 10.1016/S1470-2045(12)70508-9. [DOI] [PubMed] [Google Scholar]
- 42.Piccart-Gebhart M, Holmes E, Baselga J, de Azambuja E, Dueck AC, Viale G, Zujewski JA, Goldhirsch A, Armour A, Pritchard KI, McCullough AE, Dolci S, McFadden E, Holmes AP, Tonghua L, Eidtmann H, Dinh P, Di Cosimo S, Harbeck N, Tjulandin S, Im YH, Huang CS, Dieras V, Hillman DW, Wolff AC, Jackisch C, Lang I, Untch M, Smith I, Boyle F, Xu B, Gomez H, Suter T, Gelber RD, Perez EA. Adjuvant lapatinib and trastuzumab for early human epidermal growth factor receptor 2-positive breast cancer: results from the randomized phase III adjuvant lapatinib and/or trastuzumab treatment optimization trial. J. Clin. Oncol. 2016;34:1034–1042. doi: 10.1200/JCO.2015.62.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Guarneri V, Frassoldati A, Bottini A, Cagossi K, Bisagni G, Sarti S, Ravaioli A, Cavanna L, Giardina G, Musolino A, Untch M, Orlando L, Artioli F, Boni C, Generali DG, Serra P, Bagnalasta M, Marini L, Piacentini F, D’Amico R, Conte P. Preoperative chemotherapy plus trastuzumab, lapatinib, or both in human epidermal growth factor receptor 2-positive operable breast cancer: results of the randomized phase II CHER-LOB study. J. Clin. Oncol. 2012;30:1989–1995. doi: 10.1200/JCO.2011.39.0823. [DOI] [PubMed] [Google Scholar]
- 44.Guarneri V, Generali DG, Frassoldati A, Artioli F, Boni C, Cavanna L, Tagliafico E, Maiorana A, Bottini A, Cagossi K, Bisagni G, Piacentini F, Ficarra G, Bettelli S, Roncaglia E, Nuzzo S, Swaby R, Ellis C, Holford C, Conte P. Double-blind, placebo-controlled, multicenter, randomized, phase IIb neoadjuvant study of letrozole-lapatinib in postmenopausal hormone receptor-positive, human epidermal growth factor receptor 2-negative, operable breast cancer. J. Clin. Oncol. 2014;32:1050–7. doi: 10.1200/JCO.2013.51.4737. [DOI] [PubMed] [Google Scholar]
- 45.Masuda N, Toi M, Yamamoto N, Iwata H, Kuroi K, Bando H, Ohtani S, Takano T, Inoue K, Yanagita Y, Kasai H, Morita S, Sakurai T, Ohno S. Efficacy and safety of trastuzumab, lapatinib, and paclitaxel neoadjuvant treatment with or without prolonged exposure to anti-HER2 therapy, and with or without hormone therapy for HER2-positive primary breast cancer: a randomised, five-arm, multicentre, open-label phase II trial. Breast Cancer. 2018;25:407–415. doi: 10.1007/s12282-018-0839-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Untch M, Loibl S, Bischoff J, Eidtmann H, Kaufmann M, Blohmer JU, Hilfrich J, Strumberg D, Fasching PA, Kreienberg R, Tesch H, Hanusch C, Gerber B, Rezai M, Jackisch C, Huober J, Kühn T, Nekljudova V, von Minckwitz G German Breast Group (GBG); Arbeitsgemeinschaft Gynäkologische Onkologie-Breast (AGO-B) Study Group. Lapatinib versus trastuzumab in combination with neoadjuvant anthracycline-taxane-based chemotherapy (GeparQuinto, GBG 44): a randomised phase 3 trial. Lancet Oncol. 2012;13:135–44. doi: 10.1016/S1470-2045(11)70397-7. [DOI] [PubMed] [Google Scholar]
- 47.Baselga J, Bradbury I, Eidtmann H, Di Cosimo S, de Azambuja E, Aura C, Gómez H, Dinh P, Fauria K, Van Dooren V, Aktan G, Goldhirsch A, Chang TW, Horváth Z, Coccia-Portugal M, Domont J, Tseng LM, Kunz G, Sohn JH, Semiglazov V, Lerzo G, Palacova M, Probachai V, Pusztai L, Untch M, Gelber RD, Piccart-Gebhart M NeoALTTO Study Team. Lapatinib with trastuzumab for HER2-positive early breast cancer (NeoALTTO): a randomised, open-label, multicentre, phase 3 trial. Lancet. 2012;379:633–640. doi: 10.1016/S0140-6736(11)61847-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.de Azambuja E, Holmes AP, Piccart-Gebhart M, Holmes E, Di Cosimo S, Swaby RF, Untch M, Jackisch C, Lang I, Smith I, Boyle F, Xu B, Barrios CH, Perez EA, Azim HA Jr, Kim SB, Kuemmel S, Huang CS, Vuylsteke P, Hsieh RK, Gorbunova V, Eniu A, Dreosti L, Tavartkiladze N, Gelber RD, Eidtmann H, Baselga J. Lapatinib with trastuzumab for HER2-positive early breast cancer (NeoALTTO): survival outcomes of a randomised, open-label, multicentre, phase 3 trial and their association with pathological complete response. Lancet Oncol. 2014;15:1137–46. doi: 10.1016/S1470-2045(14)70320-1. [DOI] [PubMed] [Google Scholar]
- 49.Wissner A, Mansour TS. The development of HKI-272 and related compounds for the treatment of cancer. Arch Pharm (Weinheim) 2008;341:465–477. doi: 10.1002/ardp.200800009. [DOI] [PubMed] [Google Scholar]
- 50.Rabindran SK, Discafani CM, Rosfjord EC, Baxter M, Floyd MB, Golas J, Hallett WA, Johnson BD, Nilakantan R, Overbeek E, Reich MF, Shen R, Shi X, Tsou HR, Wang YF, Wissner A. Antitumor activity of HKI-272, an orally active, irreversible inhibitor of the HER-2 tyrosine kinase. Cancer Res. 2004;64:3958–65. doi: 10.1158/0008-5472.CAN-03-2868. [DOI] [PubMed] [Google Scholar]
- 51.Zhang Y, Zhang J, Liu C, Du S, Feng L, Luan X, Zhang Y, Shi Y, Wang T, Wu Y, Cheng W, Meng S, Li M, Liu H. Neratinib induces ErbB2 ubiquitylation and endocytic degradation via HSP90 dissociation in breast cancer cells. Cancer Lett. 2016;382:176–185. doi: 10.1016/j.canlet.2016.08.026. [DOI] [PubMed] [Google Scholar]
- 52.Zhao XQ, Xie JD, Chen XG, Sim HM, Zhang X, Liang YJ, Singh S, Talele TT, Sun Y, Ambudkar SV, Chen ZS, Fu LW. Neratinib reverses ATP-binding cassette B1-mediated chemotherapeutic drug resistance in vitro, in vivo, and ex vivo. Mol Pharmacol. 2012;82:47–58. doi: 10.1124/mol.111.076299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wong KK, Fracasso PM, Bukowski RM, Lynch TJ, Munster PN, Shapiro GI, Janne PA, Eder JP, Naughton MJ, Ellis MJ, Jones SF, Mekhail T, Zacharchuk C, Vermette J, Abbas R, Quinn S, Powell C, Burris HA. A phase I study with neratinib (HKI-272), an irreversible pan ErbB receptor tyrosine kinase inhibitor, in patients with solid tumors. Clin Cancer Res. 2009;15:2552–2558. doi: 10.1158/1078-0432.CCR-08-1978. [DOI] [PubMed] [Google Scholar]
- 54.Ito Y, Suenaga M, Hatake K, Takahashi S, Yokoyama M, Onozawa Y, Yamazaki K, Hironaka S, Hashigami K, Hasegawa H, Takenaka N, Boku N. Safety, efficacy and pharmacokinetics of neratinib (HKI-272) in Japanese patients with advanced solid tumors: a Phase 1 dose-escalation study. Jpn J Clin Oncol. 2012;42:278–286. doi: 10.1093/jjco/hys012. [DOI] [PubMed] [Google Scholar]
- 55.Awada A, Dirix L, Manso Sanchez L, Xu B, Luu T, Dieras V, Hershman DL, Agrapart V, Ananthakrishnan R, Staroslawska E. Safety and efficacy of neratinib (HKI-272) plus vinorelbine in the treatment of patients with ErbB2-positive metastatic breast cancer pretreated with anti-HER2 therapy. Ann Oncol. 2013;24:109–116. doi: 10.1093/annonc/mds284. [DOI] [PubMed] [Google Scholar]
- 56.Chow LW, Xu B, Gupta S, Freyman A, Zhao Y, Abbas R, Vo Van ML, Bondarenko I. Combination neratinib (HKI-272) and paclitaxel therapy in patients with HER2-positive metastatic breast cancer. Br J Cancer. 2013;108:1985–1993. doi: 10.1038/bjc.2013.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jankowitz RC, Abraham J, Tan AR, Limentani SA, Tierno MB, Adamson LM, Buyse M, Wolmark N, Jacobs SA. Safety and efficacy of neratinib in combination with weekly paclitaxel and trastuzumab in women with metastatic HER2-positive breast cancer: an NSABP Foundation Research Program phase I study. Cancer Chemother Pharmacol. 2013;72:1205–1212. doi: 10.1007/s00280-013-2262-2. [DOI] [PubMed] [Google Scholar]
- 58.Gandhi L, Bahleda R, Tolaney SM, Kwak EL, Cleary JM, Pandya SS, Hollebecque A, Abbas R, Ananthakrishnan R, Berkenblit A, Krygowski M, Liang Y, Turnbull KW, Shapiro GI, Soria JC. Phase I study of neratinib in combination with temsirolimus in patients with human epidermal growth factor receptor 2-dependent and other solid tumors. J. Clin. Oncol. 2014;32:68–75. doi: 10.1200/JCO.2012.47.2787. [DOI] [PubMed] [Google Scholar]
- 59.Saura C, Garcia-Saenz JA, Xu B, Harb W, Moroose R, Pluard T, Cortes J, Kiger C, Germa C, Wang K, Martin M, Baselga J, Kim SB. Safety and efficacy of neratinib in combination with capecitabine in patients with metastatic human epidermal growth factor receptor 2-positive breast cancer. J. Clin. Oncol. 2014;32:3626–3633. doi: 10.1200/JCO.2014.56.3809. [DOI] [PubMed] [Google Scholar]
- 60.Chan A, Delaloge S, Holmes FA, Moy B, Iwata H, Harvey VJ, Robert NJ, Silovski T, Gokmen E, von Minckwitz G, Ejlertsen B, Chia SK, Mansi J, Barrios CH, Gnant M, Buyse M, Gore I, Smith J 2nd, Harker G, Masuda N, Petrakova K, Zotano AG, Iannotti N, Rodriguez G, Tassone P, Wong A, Bryce R, Ye Y, Yao B, Martin M ExteNET Study Group. Neratinib after trastuzumab-based adjuvant therapy in patients with HER2-positive breast cancer (ExteNET): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2016;17:367–77. doi: 10.1016/S1470-2045(15)00551-3. [DOI] [PubMed] [Google Scholar]
- 61.Martin M, Bonneterre J, Geyer CE Jr, Ito Y, Ro J, Lang I, Kim SB, Germa C, Vermette J, Wang K, Wang K, Awada A. A phase two randomised trial of neratinib monotherapy versus lapatinib plus capecitabine combination therapy in patients with HER2+ advanced breast cancer. Eur J Cancer. 2013;49:3763–3772. doi: 10.1016/j.ejca.2013.07.142. [DOI] [PubMed] [Google Scholar]
- 62.Freedman RA, Gelman RS, Wefel JS, Melisko ME, Hess KR, Connolly RM, Van Poznak CH, Niravath PA, Puhalla SL, Ibrahim N, Blackwell KL, Moy B, Herold C, Liu MC, Lowe A, Agar NY, Ryabin N, Farooq S, Lawler E, Rimawi MF, Krop IE, Wolff AC, Winer EP, Lin NU. Translational breast cancer research consortium (TBCRC) 022: a phase II trial of neratinib for patients with human epidermal growth factor receptor 2-positive breast cancer and brain metastases. J. Clin. Oncol. 2016;34:945–952. doi: 10.1200/JCO.2015.63.0343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Awada A, Colomer R, Inoue K, Bondarenko I, Badwe RA, Demetriou G, Lee SC, Mehta AO, Kim SB, Bachelot T, Goswami C, Deo S, Bose R, Wong A, Xu F, Yao B, Bryce R, Carey LA. Neratinib plus paclitaxel vs trastuzumab plus paclitaxel in previously untreated metastatic ERBB2-positive breast cancer: the NEfERT-T randomized clinical trial. JAMA Oncol. 2016;2:1557–1564. doi: 10.1001/jamaoncol.2016.0237. [DOI] [PubMed] [Google Scholar]
- 64.Martin M, Holmes FA, Ejlertsen B, Delaloge S, Moy B, Iwata H, von Minckwitz G, Chia SKL, Mansi J, Barrios CH, Gnant M, Tomašević Z, Denduluri N, Šeparović R, Gokmen E, Bashford A, Ruiz Borrego M, Kim SB, Jakobsen EH, Ciceniene A, Inoue K, Overkamp F, Heijns JB, Armstrong AC, Link JS, Joy AA, Bryce R, Wong A, Moran S, Yao B, Xu F, Auerbach A, Buyse M, Chan A ExteNET Study Group. Neratinib after trastuzumab-based adjuvant therapy in HER2-positive breast cancer (ExteNET): 5-year analysis of a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2017;18:1688–1700. doi: 10.1016/S1470-2045(17)30717-9. [DOI] [PubMed] [Google Scholar]
- 65.Park JW, Liu MC, Yee D, Yau C, van ‘t Veer LJ, Symmans WF, Paoloni M, Perlmutter J, Hylton NM, Hogarth M, DeMichele A, Buxton MB, Chien AJ, Wallace AM, Boughey JC, Haddad TC, Chui SY, Kemmer KA, Kaplan HG, Isaacs C, Nanda R, Tripathy D, Albain KS, Edmiston KK, Elias AD, Northfelt DW, Pusztai L, Moulder SL, Lang JE, Viscusi RK, Euhus DM, Haley BB, Khan QJ, Wood WC, Melisko M, Schwab R, Helsten T, Lyandres J, Davis SE, Hirst GL, Sanil A, Esserman LJ, Berry DA I-SPY 2 Investigators. Adaptive randomization of neratinib in early breast cancer. N Engl J Med. 2016;375:11–22. doi: 10.1056/NEJMoa1513750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jacobs SA, Robidoux A, Garcia J, et al. NSABP FB-7: a phase II randomized trial evaluating neoadjuvant therapy with weekly paclitaxel (P) plus neratinib (N) or trastuzumab (T) or neratinib and trastuzumab (NþT) followed by doxorubicin and cyclophosphamide (AC) with postoperative T in women with locally advanced HER2-positive breast cancer. Cancer Res. 2016:76. [Google Scholar]
- 67.Ma F, Li Q, Chen S, Zhu W, Fan Y, Wang J, Luo Y, Xing P, Lan B, Li M, Yi Z, Cai R, Yuan P, Zhang P, Li Q, Xu B. Phase I study and biomarker analysis of pyrotinib, a novel irreversible pan-ErbB receptor tyrosine kinase inhibitor, in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer. J. Clin. Oncol. 2017;35:3105–3112. doi: 10.1200/JCO.2016.69.6179. [DOI] [PubMed] [Google Scholar]
- 68.Zhang K, Hong R, Kaping L, Xu F, Xia W, Qin G, Zheng Q, Lu Q, Zhai Q, Shi Y, Yuan Z, Deng W, Chen M, Wang S. CDK4/6 inhibitor palbociclib enhances the effect of pyrotinib in HER2-positive breast cancer. Cancer Lett. 2019;447:130–140. doi: 10.1016/j.canlet.2019.01.005. [DOI] [PubMed] [Google Scholar]
- 69.Meng J, Liu XY, Ma S, Zhang H, Yu SD, Zhang YF, Chen MX, Zhu XY, Liu Y, Yi L, Ding XL, Chen XY, Miao LY, Zhong DF. Metabolism and disposition of pyrotinib in healthy male volunteers: covalent binding with human plasma protein. Acta Pharmacol Sin. 2019;40:980–988. doi: 10.1038/s41401-018-0176-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhu Y, Li L, Zhang G, Wan H, Yang C, Diao X, Chen X, Zhang L, Zhong D. Metabolic characterization of pyrotinib in humans by ultra-performance liquid chromatography/quadrupole time-of-flight mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2016;1033-1034:117–127. doi: 10.1016/j.jchromb.2016.08.009. [DOI] [PubMed] [Google Scholar]
- 71.Ma F, Ouyang Q, Li W, Jiang Z, Tong Z, Liu Y, Li H, Yu S, Feng J, Wang S, Hu X, Zou J, Zhu X, Xu B. Pyrotinib or lapatinib combined with capecitabine in HER2-positive metastatic breast cancer with prior taxanes, anthracyclines, and/or trastuzumab: a randomized, phase II study. J. Clin. Oncol. 2019;37:2610–2619. doi: 10.1200/JCO.19.00108. [DOI] [PubMed] [Google Scholar]
- 72.Jiang Z, Yan M, Hu X, Zhang Q, Ouyang Q, Feng J, Yin Y, Sun T, Tong Z, Wang X, Yao H, Zou J, Zhu X. Pyrotinib combined with capecitabine in women with HER2+ metastatic breast cancer previously treated with trastuzumab and taxanes: a randomized phase III study. J. Clin. Oncol. 2019;37:1001–1001. doi: 10.1200/JCO.19.00108. [DOI] [PubMed] [Google Scholar]
- 73.Shi H, Zhang W, Zhi Q, Jiang M. Lapatinib resistance in HER2+ cancers: latest findings and new concepts on molecular mechanisms. Tumour Biol. 2016 doi: 10.1007/s13277-016-5467-2. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 74.D’Amato V, Raimondo L, Formisano L, Giuliano M, De Placido S, Rosa R, Bianco R. Mechanisms of lapatinib resistance in HER2-driven breast cancer. Cancer Treat Rev. 2015;41:877–883. doi: 10.1016/j.ctrv.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 75.Ruprecht B, Zaal EA, Zecha J, Wu W, Berkers CR, Kuster B, Lemeer S. Lapatinib resistance in breast cancer cells is accompanied by phosphorylation-mediated reprogramming of glycolysis. Cancer Res. 2017;77:1842–1853. doi: 10.1158/0008-5472.CAN-16-2976. [DOI] [PubMed] [Google Scholar]
- 76.Veeraraghavan J, De Angelis C, Mao R, Wang T, Herrera S, Pavlick AC, Contreras A, Nuciforo P, Mayer IA, Forero A, Nanda R, Goetz MP, Chang JC, Wolff AC, Krop IE, Fuqua SAW, Prat A, Hilsenbeck SG, Weigelt B, Reis-Filho JS, Gutierrez C, Osborne CK, Rimawi MF, Schiff R. A combinatorial biomarker predicts pathologic complete response to neoadjuvant lapatinib and trastuzumab without chemotherapy in patients with HER2+ breast cancer. Ann Oncol. 2019 doi: 10.1093/annonc/mdz076. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rimawi MF, De Angelis C, Contreras A, Pareja F, Geyer FC, Burke KA, Herrera S, Wang T, Mayer IA, Forero A, Nanda R, Goetz MP, Chang JC, Krop IE, Wolff AC, Pavlick AC, Fuqua SAW, Gutierrez C, Hilsenbeck SG, Li MM, Weigelt B, Reis-Filho JS, Kent Osborne C, Schiff R. Low PTEN levels and PIK3CA mutations predict resistance to neoadjuvant lapatinib and trastuzumab without chemotherapy in patients with HER2 over-expressing breast cancer. Breast Cancer Res Treat. 2018;167:731–740. doi: 10.1007/s10549-017-4533-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Breslin S, Lowry MC, O’Driscoll L. Neratinib resistance and cross-resistance to other HER2-targeted drugs due to increased activity of metabolism enzyme cytochrome P4503A4. Br J Cancer. 2017;116:620–625. doi: 10.1038/bjc.2016.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hanker AB, Brewer MR, Sheehan JH, Koch JP, Sliwoski GR, Nagy R, Lanman R, Berger MF, Hyman DM, Solit DB, He J, Miller V, Cutler RE, Lalani AS, Cross D, Lovly CM, Meiler J, Arteaga CL. An acquired HER2T798I gatekeeper mutation induces resistance to neratinib in a patient with HER2 mutant-driven breast cancer. Cancer Discovery. 2017;7:575–585. doi: 10.1158/2159-8290.CD-16-1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Seyhan AA, Varadarajan U, Choe S, Liu W, Ryan TE. A genome-wide RNAi screen identifies novel targets of neratinib resistance leading to identification of potential drug resistant genetic markers. Mol Biosyst. 2012;8:1553–1570. doi: 10.1039/c2mb05512k. [DOI] [PubMed] [Google Scholar]
- 81.O’Neill F, Madden SF, Clynes M, Crown J, Doolan P, Aherne ST, O’Connor R. A gene expression profile indicative of early stage HER2 targeted therapy response. Mol Cancer. 2013;12:69. doi: 10.1186/1476-4598-12-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ma F, Zhu W, Guan Y, Yang L, Xia X, Chen S, Li Q, Guan X, Yi Z, Qian H, Yi X, Xu B. ctDNA dynamics: a novel indicator to track resistance in metastatic breast cancer treated with anti-HER2 therapy. Oncotarget. 2016;7:66020–66031. doi: 10.18632/oncotarget.11791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bowen JM. Mechanisms of TKI-induced diarrhea in cancer patients. Curr Opin Support Palliat Care. 2013;7:162–167. doi: 10.1097/SPC.0b013e32835ec861. [DOI] [PubMed] [Google Scholar]
- 84.Van Sebille YZ, Gibson RJ, Wardill HR, Bowen JM. ErbB small molecule tyrosine kinase inhibitor (TKI) induced diarrhoea: chloride secretion as a mechanistic hypothesis. Cancer Treat Rev. 2015;41:646–652. doi: 10.1016/j.ctrv.2015.05.011. [DOI] [PubMed] [Google Scholar]
- 85.Duan T, Cil O, Thiagarajah JR, Verkman AS. Intestinal epithelial potassium channels and CFTR chloride channels activated in ErbB tyrosine kinase inhibitor diarrhea. JCI Insight. 2019;4 doi: 10.1172/jci.insight.126444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hirsh V, Blais N, Burkes R, Verma S, Croitoru K. Management of diarrhea induced by epidermal growth factor receptor tyrosine kinase inhibitors. Curr Oncol. 2014;21:329–336. doi: 10.3747/co.21.2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rugo HS, Di Palma JA, Tripathy D, Bryce R, Moran S, Olek E, Bosserman L. The characterization, management, and future considerations for ErbB-family TKI-associated diarrhea. Breast Cancer Res Treat. 2019;175:5–15. doi: 10.1007/s10549-018-05102-x. [DOI] [PMC free article] [PubMed] [Google Scholar]