Abstract
The activating receptor natural killer group 2, member D (NKG2D) is involved in both innate and adaptive immunities, and functions as a “master switch” in determining the activation status of natural killer (NK) cells. NKG2D binds to a diverse family of ligand molecules, which are only expressed at low levels in normal cells but can be upregulated by a cellular stress response. The NKG2D-NKG2D ligand (NKG2DL) pathway has been considered to be promising target for immunotherapy because of the selective expression of “stress-induced ligands” on tumor cells and the strong NK cell activating potency of NKG2D. Diverse strategies that are aimed at targeting the NKG2D pathway for cancer therapy are based on a thorough understanding of this mechanism, as well as that of NKG2D-mediated cancer immunity. In this review, we summarize the major findings regarding the antitumor immune response mediated by the NKG2D receptor and its ligands, and discuss the potential clinical applications of targeting the NKG2D/NKG2DL pathway for immunotherapy in cancer patients.
Keywords: Cancer immunotherapy, NKG2D, NKG2D ligands, immunity
Introduction
Cancer immunotherapy is now considered a pillar of cancer treatment, alongside surgery, chemotherapy, and radiation; the primary focus of immunotherapy is the adaptive immune system [1]. However, growing evidence has shown that some cancers are preferentially attacked by natural killer (NK) cells. NK cells constitute a key part of the innate immune system that plays a vital role in antitumor and antiviral mechanisms [2]. In recent years, NK cells have shown promise for cancer immunotherapy owing to their unique ability to identify and kill transformed cells without any prior sensitization. Thus, research focusing on the antitumor immune effect of NK cells has become one of the hotspots of tumor immunology.
NK cells possess a variety of activating and inhibitory receptors, and the functional outcome of NK cell activity is strictly governed by the complex integration of signals between the activating and inhibitory receptors that bind to stress-regulated molecules (Figure 1A) [3]. Among the activating receptors, the natural killer group 2, member D (NKG2D) receptor - which is involved in both innate and adaptive immunities and functions as a “master switch” in determining the activation status of NK cells (Figure 1B) - was first identified in 1991 [4]. Its function was subsequently reported in 1999 [5]. Studies have demonstrated that NKG2D is expressed not only in NK cells, but also in other immune cells, such as natural killer T (NKT) cells, activated or memory CD8+ TCR-αβ T cells, a small subset of CD4+ T cells, approximately 25% of spleen TCR-γδ T cells, and activated murine macrophages [6]. NKG2D, via binding to NKG2D ligands (NKG2DLs), plays an important role in the immune response, including immune surveillance, antimicrobial immune response, and antitumor effects [7]. Moreover, it has been reported that cells from epithelial and lymphoid malignancies escape immune surveillance in NKG2D-deficient mice. Even though many studies on NKG2D and its ligands have been reported, elucidation of their mechanisms still requires further investigation. In this review, we summarize the major findings regarding the antitumor immune response mediated by the NKG2D receptor and its ligands, and discuss the potential clinical applications of targeting the NKG2D/NKG2DL pathway for immunotherapy in cancer patients.
Figure 1.
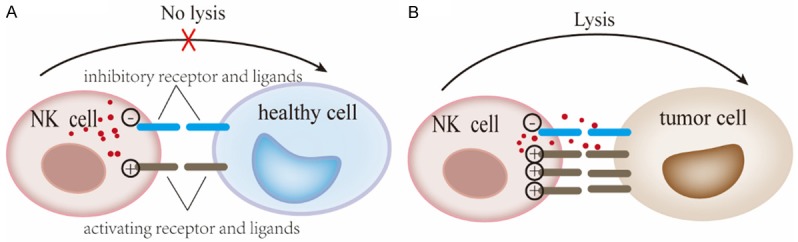
Activation of NK cells by the NKG2D/NKG2DL pathway. A. The balance of signals mediated by activation and inhibition of specific receptors determines the activation of NK cells. B. Tumor cells overexpress NKG2DLs that can be recognized by the NKG2D receptor, which function as a “master switch” and elicits NKG2D-mediated cytotoxicity. This figure was created using Adobe Illustrator.
NKG2D and its ligands
NKG2D and its signaling pathway
In humans, NKG2D, also known as killer cell lectin-like receptor subfamily K, member 1 (Klrk1), is encoded by the NK gene complex [8]. NKG2D is a type II lectin-like transmembrane activating receptor, implicated in the regulation of NK cell function [9]. The NKG2D molecule consists of two β-sheets, two α-helices, and four disulfide bonds. Figure 2 describes the signaling pathway mediated by the NKG2D/NKG2DL axis. The activation of NKG2D differs from that of other activating receptors on NK cells because its intracellular segment has no signaling element [10]. Moreover, NKG2D has two distinct isoforms (NKG2D-S: the short isoform and NKG2D-L: the long isoform) that are produced as a result of alternative splicing. NKG2D-S and NKG2D-L interact with the adaptor, DNAX-activating protein of 10 kDa (DAP10) or KARAP (also known as adaptor DNAX-activating protein of 12 kDa, DAP12) signaling subunits [11]. The NKG2D-L homodimer interacts with transmembrane DAP10 in the cell membrane for activating intracellular signaling, which recruits other molecules essential for the downstream signaling pathway and induces a series of cytotoxic responses when triggered [12,13]. DAP10 is a membrane protein which contains a Tyr-X-X-Met (YXXM) motif in its cytoplasmic domain, and which recruits and activates the p85 subunit of phosphatidylinositol 3-kinase (PI3K) by mediating the tyrosine phosphorylation of YXXM motifs by Src family tyrosine kinases when NKG2D is cross-linked with its ligands [12]. Activation of PI3K is required for the phosphorylation of the downstream anti-apoptotic kinase, Akt, and initiating the extracellular signal-regulated kinase (ERK) 1/2 MAP kinase bypass, both of which are compulsory for mobilization of Ca2+ mediated by NKG2D, cell-mediated cytotoxicity, and the cell survival pathway. Moreover, asparagine in the YXXM motif can also recruit the transfer protein, growth factor receptor-bound protein 2 (Grb2) to initiate MAP kinase bypass [10], thereby activating NK cells. In T cell receptor signaling, Grb2 seems to have the ability to recruit and activate the Ras-Sos pathway [8]. Recent studies have shown that NKG2D-S can interact with DAP10, as well as transiently associate with the immunoreceptor tyrosine-based activation motif (ITAM)-containing DAP12 [14] - depending on the activation status of the mouse cells - which then activates spleen tyrosine kinase (Syk) and zeta-chain-associated protein kinase 70 (ZAP70) [15,16]. Unlike DAP10, which is involved in mediating cytotoxicity, DAP12 is mainly involved in interferon-gamma (IFN-γ) production [17,18]. However, human NKG2D cannot interact with DAP12 [19] because humans only express the NKG2D-L isoform, while mice express both the NKG2D-L and NKG2D-S isoforms [20].
Figure 2.
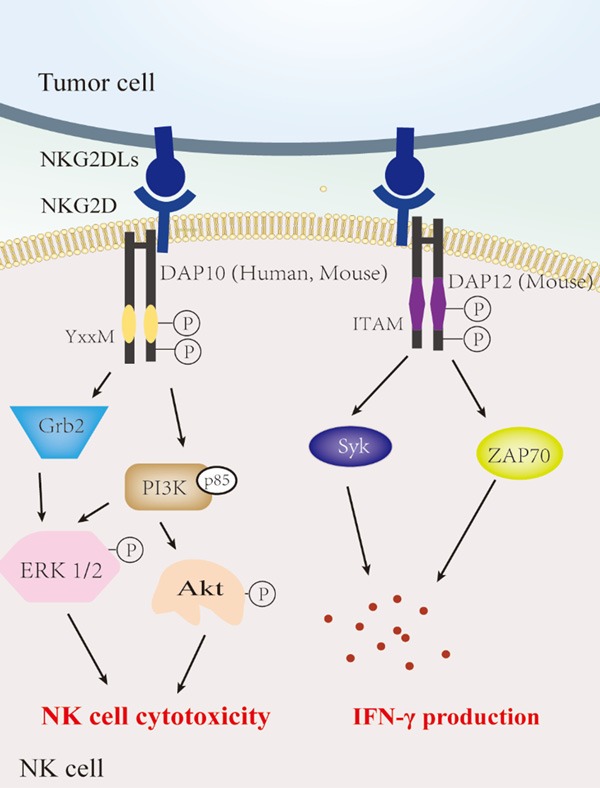
NKG2D/NKG2DL interaction activates NK cells. The activating receptor NKG2D can directly bind to a diverse family of ligand molecules expressed on the surface of tumor cells. In NK cells, NKG2D interacts with either DAP10 (both human and mouse) or DAP12 (mouse only) and induces cytotoxicity and IFN-γ production. DAP10/YXXM-mediated signaling regulates NK cell cytotoxicity via the Grb2 and PI3K pathway, while the NKG2D-DAP12 complex is involved in IFN-γ production through the Syk and ZAP70 pathway. NKG2D, Natural killer group 2, member D; NKG2DLs, NKG2D ligands. This figure was created using Adobe Illustrator.
NKG2D ligands
Humans have two main types of ligands that bind to NKG2D [21-23], a family of class-I chain-related proteins A and B (MICA and MICB) and a family of six cytomegaloviral unique long 16 (UL16)-binding proteins (ULBP 1-6). In mice, five different retinoic acid early transcript 1 (RAET1) isoforms (RAET1 α, β, γ, δ, and ε), three different histocompatibility H60 isoforms (H60 a, b, and c), and one murine UL16-binding protein-like transcript 1 (MULT1) have been identified [24-26].
The structures of NKG2DLs in humans and mice are similar (Figure 3). The ligands can be classified into three different classes based on their structure. MICA and MICB are transmembrane proteins with three extracellular domains that are analogous to the α1-α3 domains of the major histocompatibility class Ia (MHC Ia) proteins. Other human NKG2DLs and all mouse NKG2DLs contain two domains that are analogous to the α1 and α2 domains of MHC Ia proteins, but lack the α3-like domain [27]. ULBP-1, -2, -3, and -6 are glycosylphosphatidylinositol (GPI)-anchoring receptors, while ULBP-4 and -5 have a transmembrane domain and a cytoplasmic tail [28]. Notably, stressed and malignant transformed cells have been found to express higher levels MICA and MICB on their surface than those of conventional MHC class I molecule [8,29,30].
Figure 3.
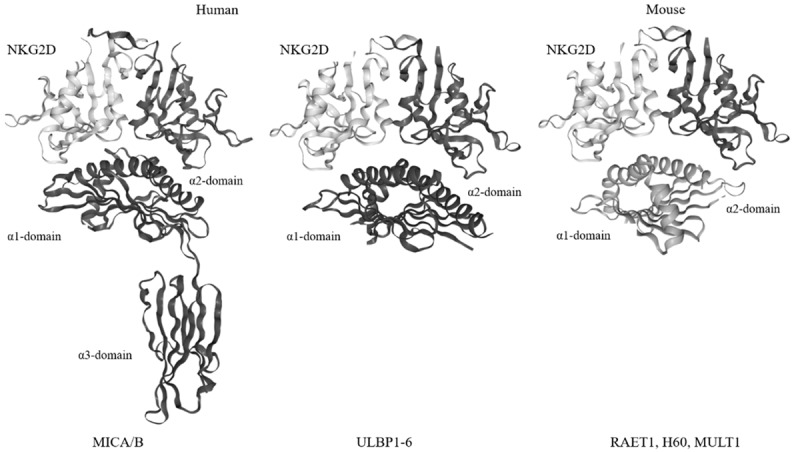
The structure of NKG2DLs. Humans have two main types of ligands for NKG2D (left panel): a family of class-I chain-related proteins A and B (MICA and MICB) and a family of six cytomegaloviral UL16-binding proteins (ULBP 1-6). Mouse NKG2DLs are mainly RAET1 isoforms, H60 families, and MULT1 (right panel). The structure of NKG2DLs in humans and mice is similar. The ligands can be classified into three general structures. MICA and MICB are transmembrane proteins with three extracellular domains analogous to the α1-α3 domains of MHC Ia proteins. The remaining human NKG2DLs and all mouse NKG2DLs contain two domains analogous to α1 and α2 of MHC Ia proteins but lack the α3-like domain. The structures (Protein Data Bank codes) of MICA-human NKG2D (1HYR), ULBP3-human NKG2D (1KCG), and (RAE-1β)-mouse NKG2D (4PP8) are shown.
The dual function of NKG2D/NKG2DL in tumor immunity
The significance of NKG2D/NKG2DL pathway regulation in controlling tumor progression and immune recognition has been demonstrated in numerous experimental animal models and clinical data [31]. These studies highlighted the controversy over the role of NKG2D and its ligands in tumor immunity and the potential significance of the NKG2D/NKG2DL pathway in the treatment of cancer (Figure 4) [32].
Figure 4.
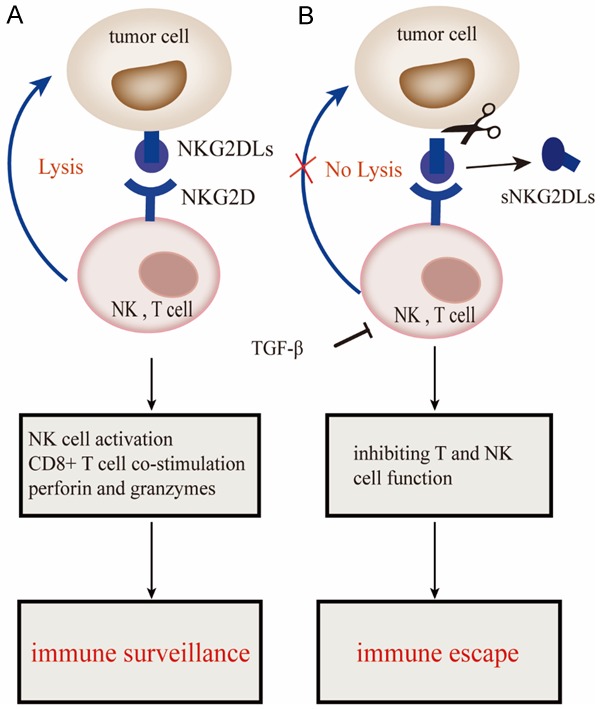
The dual functions of NKG2D/NKG2DL in tumor immunity. A. Induction of NKG2DLs on tumor cells induces immune surveillance via binding to NKG2D receptors expressed on NK and T cells. B. Failure to kill tumor cells due to the shedding of NKG2DLs by metalloproteinases. TGF-β allows immune surveillance escape by inhibiting T and NK cell function. NKG2D, Natural killer group 2, member D; sNKG2DLs, soluble NKG2D ligands. This figure was created using Adobe Illustrator.
NKG2D/NKG2DL-mediated immune surveillance
The function of NKG2D differs between NK cells and other immune cells. Studies have demonstrated that activating signals mediated by the NKG2D/NKG2DL pathway can override the signals induced by the inhibitory receptors, thereby allowing NKG2D to acts as a “master switch” for activating NK cells [33]. Thus, the NKG2D signaling pathway can mediate a direct killing effect in NK cells. Nevertheless, its effect is controversial in CD8+ T cells. Seminal work by Raulet [34] indicated that NKG2D functions as a costimulatory molecule in CD8+ T cells, thereby resulting in enhanced CD8+ T cell function [30,35].
NKG2D is a multifunctional receptor that can directly bind to a diverse family of ligand molecules expressed on the surface of target cells without antigen presentation, thereby resulting in the activation or synergistic stimulation of immune effectors [36] and the subsequent release of preformed granules containing cytolytic proteins such as perforin and granzymes that are mediate a killing effect in tumor cells [37]. Experimental evidence indicates that NKG2D-mediated immune responses play a critical role in tumor surveillance, and that the NKG2D pathway can modulate tumorigenesis and tumor progression, which is particularly significant for inhibiting tumor cell metastasis.
The expression of NKG2DLs on the surface of tumor cells is induced by transcriptional upregulation due to cellular or genomic stress, and while it is usually expressed in most epithelial-derived tumor cells, such as ovarian cancer, colon cancer, and leukemia, it is rarely detected in healthy adult tissues [38,39]. Moreover, when cells are exposed to DNA-damaging agents, specific cytokines, or cell proliferation agents, the expression of these ligands increases [40]. Increasing evidence has confirmed that the NKG2D/NKG2DL pathway is essential for the development of many malignancies. In healthy individuals, MICA/B expression has been detected in some normal tissues such as gastrointestinal epithelial cells, but the expression levels are low and in many cases rare. NKG2DLs are upregulated when cells undergo malignant transformation or when they are exposed to other forms of stress such as oxidative stress and viral infection.
NKG2D/NKG2DL-mediated immune escape
NK cells also play an essential role in the escape of tumor cells from immune surveillance via NKG2D/NKG2DLs. Pooled data from clinical trials show the association between NKG2D expression and cancer cells, as evidenced by the significantly higher expression of NKG2D in mononuclear cells in patients with early gastric cancer compared with that observed in advanced gastric cancer [41]. Further, patients with malignant tumors exhibit a decrease in the percentage of NKG2D-positive NK cells. Thus, decreased NKG2D expression in NK cells may be one of the critical mechanisms underlying NK cell dysfunction in cancer. The downregulation of NKG2D may be due to the production of soluble NKG2DLs by tumor cells that regulates not only expression of the NKG2D receptor in immune cells, but also the amount of NKG2DLs in tumor cells. Soluble NKG2DLs, produced via proteolytic shedding from the surface of cancer cells by the activity of a disulfide isomerase (ERp5) and several proteases belonging to the a disintegrin and metalloprotease (ADAM) and matrix metalloproteinase (MMP) families, operate as immunosuppressive molecules that mediate malignant tumor cell evasion from the immune recognition pathway in advanced cancers [42,43]. Research has demonstrated that high serum concentrations of soluble NKG2DLs [44,45] may suppress tumor immunity and NK cell activity via downregulation of NKG2D expression or proteolytic shedding of MICA/B, thereby contributing to an escape from the tumor immunosurveillance machinery [42,46]. Previous studies have reported that high expression of soluble NKG2DLs is correlated with the tumor node metastasis (TNM) stage in patients with breast cancer [47], and with poor clinical outcomes for multiple cancer types, including melanoma, neuroblastoma, prostate cancer, kidney cancer, multiple myeloma, and chronic lymphocytic leukemia [42,48-52]. Moreover, accumulating data suggest that soluble ligands for NKG2D can limit the efficacy of the immune checkpoint blockade [53].
Zhang et al. [54] engineered transgenic adenocarcinomas in a mouse prostate model (TRAMP) to investigate the response of soluble NKG2DLs to a murine CTLA-4-targeting antibody. Increased levels of soluble MIC were associated with poor efficacy with respect to antibody therapy and reduced survival of these animals. However, co-administration of an sMIC-neutralizing antibody along with the anti-CTLA4 antibody could improve the clinical response and alleviate treatment-induced colitis in animals [54]. Accordingly, increased serum levels of soluble NKG2DLs may not only serve as novel indicators of solid cancers, but can also provide a potential evasion mechanism by which cancer cells escape from NKG2D-mediated immune cell attack.
In addition to soluble NKG2DLs, cytokines and regulatory cells within the tumor microenvironment participate in the NKG2D-mediated tumor escape mechanism. The proinflammatory cytokine, IFN-γ downregulates MICA and ULBP expression and impairs NKG2D-mediated cytolysis by NK cells in melanoma and glioma cells [55]. In addition to IFN-γ, the cytokine transforming growth factor-β (TGF-β), an immunosuppressant secreted by tumors of different histotypes, aids the immune evasion by inhibiting T and NK cell function. These results were also supported by the finding that the expression of NKG2DLs and NKG2D receptors in immune cells is downregulated by TGF-β treatment [56]. Eisele et al. [57] found that TGF-β production is increased during tumor growth and malignant progression, and it selectively mediates the transcription of MICA, ULBP2, and ULBP4, while the mRNA and cell surface expression of MICB, ULBP1, and ULBP3 remain unaffected. The function of TGF-β not only involves disruption of the NKG2D/NKG2DL recognition system on NK cells, but also involves mediating NKG2DLs expression in CD8+ T cells [58,59]. Therefore, cytokines are involved in the regulation of immune responses and play an important role in tumor immunotherapy.
Targeting NKG2D/NKG2DL for cancer immunotherapy
Tumor cells expressing high levels of NKG2DLs can be effectively eliminated by NK cells, but the NKG2DL levels decreased in late-stage tumors, and thus, the induced immune response is weak. Therefore, strategies that focus on enhancing NKG2D expression in immune cells, increasing the expression of NKG2DLs in tumor cells, and eliminating soluble NKG2DLs could effectively activate the antitumor immune response (Figure 5).
Figure 5.
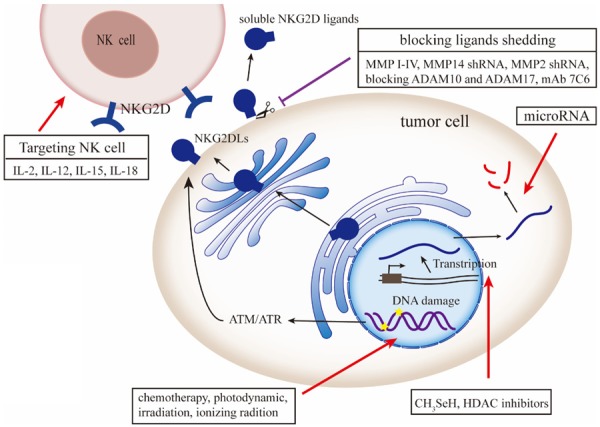
Strategies for targeting the NKG2D/NKG2DL axis for cancer immunotherapy. A variety of cytokines, such as IL-2, -12, -15, and -18, can modulate the function of NK cells. Strategies, such as CH3SeH HDAC, microRNA, and targeting post-transcriptional mechanisms, also play an important role in NKG2D-mediated cytotoxicity by regulating the NKG2DL expression on the tumor cell. Chemotherapy, irradiation and ionizing radiation, and current cancer therapeutic modalities induce DNA damage and upregulate the expression of NKG2DLs in cancer via the ATM/ATR pathway. Small molecule inhibitors blocking MMP and ADAM were developed to decrease shedding of soluble NKG2DLs. NKG2DL, NKG2D ligands; MMP, matrix metalloprotease; ADAM, a disintegrin and metalloprotease; HDAC, histone deacetylase. This figure was created using Adobe Illustrator.
Targeting NKG2D expression
The therapeutic effects of NK-based immunotherapy can be achieved by upregulating NKG2D expression in immune cells (Table 1). Thus, identifying and understanding the role of multiple transcription factors that regulate NKG2D expression on the cell surface through its ligands is of utmost importance. A variety of soluble common γ chain-related cytokines, such as IL-2, IL-12, IL-18, and IL-15 [60], have been reported to positively or negatively modulate the expression of NKG2D on the cell surface, and have been widely used in antitumor treatments [16]. IL-2, a crucial cytokine in cancer therapy, enhances NK cell cytotoxicity by increasing NKG2D expression in CD16+ NK cells in patients with malignancies [61]. However, Anderson et al. [62] reported that in normal mice, high-dose rIL-2 therapy (600,000 IU) induced systemic side effects, such as vascular leak syndrome, fever, hypotension, and hepatocyte necrosis due to activation of the vascular endothelium. Though lowering the IL-2 dose can ameliorate the side effects, but it also reduces the efficacy of immunotherapy. In contrast to the limited antitumor efficacy of IL-2 alone, a low dose of IL-2 (100 U/ml) combined with IL-18 (75 ng/ml) enhanced NKG2D expression without causing IL-2-related toxicity in cancer patients [63]. Moreover, mutant forms of IL-2 with reduced affinity for IL-2Rα can also minimize the many IL-2 related side effects. A fusion protein composed of an IL-2Rα-deficient IL-2 and orthopoxvirus major histocompatibility complex class I-like protein (OMCP) selectively activated IL-2 signaling only in NKG2D-bearing cells, and decreased the growth and viability of both solid and liquid tumors [64]. Studies have shown that NKG2D expression was increased and IFN-γ production was partially recovered by overnight treatment with IL-15 - a cytokine with structural similarity to IL-2 - following tumor exposure [65]. These data suggest that IL-15 may also have a therapeutic potential for the treatment of solid tumors.
Table 1.
Strategies targeting the NKG2D/NKG2DL pathway for cancer immunotherapy
| Targeting | Agent | Function | NK cell functions | Ref |
|---|---|---|---|---|
| NKG2D expression | ||||
| Cytokines (IL-2, -18, and -15) | ↑ NKG2D receptor | ↑ Cytotoxicity | [61-64] | |
| NKG2D ligands | ||||
| CH3SeH | ↑ MICA, MICB | ↑ Cytotoxicity | [66] | |
| ↑ ULBP2 mRNA | ||||
| EGFR activation | ↑ MICA, ULBP2 | ↑ Cytotoxicity | [67] | |
| miR-20a | ↓ MICA, MICB | ↓ Cytotoxicity | [72-75] | |
| miR-34a | both induce and reduce MICB | ↑ IFN-γ | [77,78] | |
| HDAC inhibitor Entinostat | ↑ MICA, MICB | ↑ Cytotoxicity | [80] | |
| ↑ NKG2D receptor | ||||
| Romidepsin | ↑ MICA, MICB | ↑ Cytotoxicity | [81,82] | |
| TMZ or IR | ↑ MICA, MICB, ULBP2, RAE-1, MULT-1 | n.d. | [83] | |
| Cisplatin | ↑ MICA, MICB | ↑ Cytotoxicity | [84] | |
| MG132 | ↑ MICB | ↑ Cytotoxicity | [85] | |
| Bortezomib and ionizing radiation | ↑ MICB, ULBP1 | ↑ Cytotoxicity | [86] | |
| Hp | ↑ MICB, ULBP1, ULBP2, ULBP3 | ↑ Cytotoxicity | [87] | |
| HPPH | ↑ MICA | ↑ Cytotoxicity | [88] | |
| soluble NKG2D ligands | ||||
| MMPI-IV + IL-15 | ↑ MICA, MICB, ULBP2, ULBP3 | ↑ Cytotoxicity | [94] | |
| ↓ sMICA | ||||
| MMP14 shRNA | ↓ sMICA | ↑ Cytotoxicity | [95] | |
| MMP2 shRNA | ↓ sMICA | n.d. | [52] | |
| Blocking ADAM10 and ADAM17 | ↑ ULBP2 | ↑ IFN-γ | [98,99] | |
| ↓ sULPB2 | ||||
| mAb 7C6 | ↑ MICA, MICB | ↑ Cytotoxicity | [101] | |
| ↓ sMICA | ||||
↑ increase; ↓ decrease; CH3SeH, metabolite methylselenol; TMZ, Temozolomide; IR, irradiation; MMP, matrix metalloprotease; HDAC, histone deacetylase; MMPI-IV, MMP-2/MMP-9 inhibitor IV; Hp, hematoporphyrin; HPPH, 2-[1-hexyloxyethyl]-2-devinyl pyropheophor-bide-a; ADAM, a disintegrin and metalloprotease; mAb 7C6, monoclonal antibodies (mAbs) that bind to the a3 domain; n.d., not done.
Targeting NKG2D ligand expression
Other than therapies that target NKG2D expression, recent studies have indicated that post-transcriptional mechanisms may also regulate surface expression of NKG2DL in tumor cells (Table 1). Many studies have identified several pathways that participate in the regulation of NKG2DLs. For example, methylselenol (CH3SeH)-generating selenium compounds triggered immune activation through the induction of MICA and MICB transcription, at both the transcriptional and post-transcriptional levels, and inhibited soluble ULBP2-mediated immunosuppression [66]. The increased expression of NKG2DLs in response to environmental perturbation could be more readily be attributed to the activation of the epidermal growth factor receptor (EGFR) pathway. EGFR activation resulted in cellular re-localization of the AU-rich element RNA-binding (AUF1) protein, which typically destabilizes the NKG2DL mRNA by targeting AU-rich elements conserved within the 3’ end of most human NKG2D ligand genes [67]. The E2F family transcription factor-mediated pathway plays a vital role in regulating the cell cycle and may directly activate Raet1 genes in cancer cell lines and may be involved in the proliferation of normal cells [68]. Oncogenes, such as BCR/ABL, are known to directly control MICA and ULPB expression [69,70]. Moreover, the mRNA levels of NKG2DLs could be regulated by microRNA (miRNA) under specific circumstances via targeting their 3’-untranslated region (3’-UTR). Previous studies have identified a group of endogenous cellular miRNA [71], such as miR-20a, miR-93, miR-106a, miR-373, and miR-520d, which could regulate MICA and MICB expression by targeting the 3’-UTR sites in MICA and MICB mRNA in human cancer cells as well as in normal cells, such as human foreskin fibroblasts and human umbilical vein endothelial cells. Members of the miR-17-92 cluster, especially miR-20a, reportedly decrease the expression of MICA and MICB by targeting the 3’-UTR region and downregulate ULBP2 expression via the MAPK/ERK pathway in ovarian tumors, glioma, and normal breast cell lines [72-75]. MicroRNA-34a (miR-34a) reportedly acts as a tumor suppressor in multiple tumors [76]; however, the role of miR-34a in the regulation of NKG2DLs expression is controversial. miR-34a could induce MICB expression by upregulating ataxia telangiectasia and Rad3-related (ATR) protein kinase in hepatocytes and hepatocellular carcinoma (HCC) cells that have low E2F1 levels, while on the other hand, it could also decrease MICB expression via downregulating the transcription factor E2F1 in HCC cells [77,78]. Taken together, further studies on the post-transcriptional regulation mechanisms of NKG2DL expression might provide new insights into the development of novel cancer treatments.
Considering the evidence that NKG2DLs expression could be stress-induced, drugs that act as DNA damaging agents, proteasome inhibitors, or histone deacetylase (HDAC) inhibitors (HDACIs) could be used as antitumor agents.
In recent years, at least 18 HDACs have been identified with different functions. Several HDACs are overexpressed in tumor cells [79]. HDACIs are therefore considered as promising anticancer drugs owing to their ability to regulate NKG2DL expression in tumor cells. Zhu et al. [80] found that entinostat, a selective HDACI, can not only enhance the expression of NKG2DLs MICA and MICB in human cancer cells, but can also simultaneously increase the expression of the activating receptor, NKG2D in human NK cells, indicating the potential of entinostat to improve the efficacy of NK cell activity against solid tumors such as carcinomas and osteosarcomas. Romidepsin, a cyclic peptide HDACI, reportedly enhanced NK cell cytotoxicity in vitro and in vivo, and significantly increased MICA/B expression in acute lymphoblastic leukemia and non-Hodgkin lymphoma cells [81,82]. However, these HDACIs did not increase the expression of NKG2DLs in mononuclear cells from healthy volunteers, thereby indicating the specificity of HDACI-mediated repression of NKG2DL expression in tumor cells.
Mounting evidence has shown that chemotherapy and irradiation (IR) can affect NKG2DL expression. Temozolomide (TMZ), an alkylating agent that induces DNA damage, reportedly induced the expression of NKG2DLs in vitro and in vivo in a variety of murine and human glioblastoma models [83]. Moreover, patients treated with TMZ and IR had increased levels of NKG2DLs [83]. Studies show that cisplatin-based adjuvant chemotherapy might enhance NK cell-mediated cytotoxicity through upregulating the expression of MICA and MICB in non-small cell lung cancer (NSCLC) cells via the ataxia-telangiectasia-mutated (ATM)- and Rad3-associated protein kinase (ATR) pathways [84]. Additionally, MG132 [85], a proteasome inhibitor, can upregulate the expression of MICB, cause DNA damage, and activate key molecules in the DNA damage response pathway. Combined treatment with bortezomib (a potent proteasome inhibitor used as the first-line treatment for multiple myeloma) and ionizing radiotherapy could upregulate the expression levels of NKG2DLs, increase the sensitivity of NK92 cells to myeloma cells, and enhance the NK cell-mediated anti-tumor immune response, compared with bortezomib alone [86].
Photodynamic therapy (PDT) has been approved by the Food and Drug Administration (FDA) as a clinical anticancer modality for the treatment of various types of malignancies. It is suggested that NK cells can be activated through PDT-mediated immune responses. In addition, mRNA levels of the ULBP1 and ULBP2 in the SNU-1 human gastric tumor cell line, and the MICA/B, ULBP1, ULBP2, and ULBP3 in the SW-900 human lung cancer cell line increased after treatment with PDT - using sublethal doses of hematoporphyrin (Hp) - leading to increased susceptibility of cancer cells to NK cells [87]. MICA expression was significantly induced in human colon carcinoma Colo205 cells and murine CT26 tumors after PDT treatment with a second-generation photosensitizer, 2-[1-hexyloxyethyl]-2-devinyl pyropheophor-bide-a (HPPH), and the induction of MICA was associated with an increased NK cell killing effect. However, in contrast to the upregulation of MICA, PDT treatment did not result in increased expression of either MICB or any of the ULBP family members [88].
Targeting soluble NKG2D ligands
Clearance of soluble NKG2DLs or inhibition of NKG2DL shedding can also have therapeutic effects (Table 1). MMPs and ADAM are involved in the shedding of NKG2DLs, such as MICA, MICB, and ULBP. Pharmacological inhibition of either MMPs and/or ADAM reduced the level of released NKG2DLs, increased cell surface expression, and reversed their immunosurveillance escape properties. MMPs are expressed in nearly all human cancers and play a crucial role in promoting tumor angiogenesis, growth, and metastasis. Increased MMP expression is reported to be strongly associated with tumor aggressiveness, stage, and patient prognosis [89,90]. Overexpression of MMP-3 has been shown to promote mammary carcinogenesis and induce spontaneous disease progression [91]. MMP-2 levels detected in serum and cancer tissue could be used as indicators of the severity of breast cancer invasion and tumor size [92]. Shiraishi et al. found that MMP-9 expression was inversely associated with NKG2DL (MICA/B, ULBP-2 and -3) expression in vitro, and MMP inhibitors could restore the expression of NKG2DLs in clinical gastric tumor samples, thereby improving their susceptibility to NK cells in vitro [93]. Therefore, nearly every member of the MMP family has become an attractive target for development of therapeutics. Treatment of lung adenocarcinoma (ADC)-Coco cells with the MMP-2/MMP-9 inhibitor IV (MMPI-IV) led to improved NK cell-dependent cytotoxicity, mediated mainly by NKG2D [94]. MMP14 can mediate MICA shedding, and its expression in MICA-positive tumor cells regulated the sensitivity of tumor cells to NK cell killing. Short hairpin RNA (shRNA) suppression of MMP14 expression blocked the MICA shedding independent of ADAMs [95]. Moreover, MMP2 shRNA could significantly suppress MICA proteolytic shedding in renal cell carcinoma, suggesting that MMP is involved in the proteolytic release of soluble MICA, which contributes to tumor escape from immune surveillance machinery [52]. Furthermore, inhibitors targeting ADAM have also been reported to have antitumor effects. The ADAM family has been implicated in the proteolytic shedding process of membrane-associated proteins and is, therefore, associated with the regulation of key cellular signaling pathways in the tumor microenvironment [96]. ADAM 17 (also known as tumor necrosis factor-alpha converting enzyme, TACE) is involved in various biological processes, including tumorigenesis, invasiveness, and tumor growth in vitro and in vivo [97]. ADAM10 promotes glioma cell migration and modulates the immunogenicity of glioblastoma-initiating cells (GICs). Previous studies have indicated that the expression of ADAM17 in glioma is approximately 4.8-fold higher than that in normal human brain tissue. The results reported by Wolpert et al. showed that blocking ADAM10 and ADAM17 function with specific pharmacological inhibitors or gene silencing through small interfering RNAs (siRNAs) enhanced the ULBP2 expression on the tumor cell surface [98]. Moreover, the concentration of soluble ULBP2 (sULBP2) in the serum decreased, while the mRNA level of ULBP2 remained unchanged. Therefore, inhibition of ADAM10 and ADAM17 led to enhanced immune recognition by NK cells in a ULBP2-dependent manner [98]. In addition, two hydroxamate compounds, LT4 and MN8, which are specific inhibitors of ADAM10 and ADAM17, were synthesized to verify whether inhibition of ADAM10 in Hodgkin lymphoma (HL) cells could restore the activation of the NKG2D-dependent anti-lymphoma T cell response [99]. The results showed that the new LT4 and MN8 compounds could reduce the shedding of NKG2DLs and enhance the binding of the NKG2D receptor. In addition, HL cells exposed to these inhibitors showed increased sensitivity to NKG2D-dependent cell killing exerted by NK and γδ T cells. However, only nonspecific inhibitors are available at present; therefore, this therapeutic option will not be available in the near future.
In addition to small molecule inhibitors, antibodies targeting the site of proteolytic shedding in a highly specific manner (the membrane-proximal MICA and MICB α3 domains) were designed to prevent loss of MICA and MICB from the cell surface [100]. These antibodies increased the density of the stimulatory MICA and MICB ligands on the surface of tumor cells, inhibited tumor growth, and induced NK-mediated immunity in a humanized mouse model [101]. However, Deng et al. found that shedding MULT1, a high-affinity NKG2D ligand in mice, enhanced antitumor immune responses by promoting NK cell activation and tumor rejection in vivo [102]. These inconsistent results may be due to a lower affinity of soluble MICA and MICB for the NKG2D receptor than MULT1 [21], which may also explain why the shed MICA and MICB do not stimulate an immune response. As MICA and MICB are widely expressed in various human cancers, the development of antibodies targeting MICA and MICB holds considerable importance for the treatment of both solid and hematological malignancies [27,103-105]. These antibodies could be used in combination with established treatments, such as radiotherapy and chemotherapy, which upregulate NKG2DL expression, along with a drug delivery system to enhance the delivery of toxic payloads to tumor cells, or with other immunotherapies to activate immune cells and overcome the protective antitumor immunity.
Conclusion
In summary, the NKG2D/NKG2DL pathway involves multiple effector cell types for controlling tumor progression. The function of NK cell inhibitory receptors often dominates that of activated receptors, thereby preventing autologous cells from being killed by NK cells. However, the activation signal mediated by the NKG2D receptor can bypass the traditional inhibitory signaling pathway and is, therefore, not subject to SHP phosphorylation. Numerous studies on the NKG2D/NKG2DL axis have revealed the complexity of the system, and in the past several decades, we have gained new insight into the various aspects of the NKG2D/NKG2DL pathway in tumor development, and a wider understanding of tumor immune escape from NKG2D-mediated immune recognition. Moreover, an advanced understanding of the NKG2D axis has provided support for the use of NKG2D/NKG2DL in cancer immunotherapy. Diverse strategies targeting multilayered regulation mechanisms of NKG2D-mediated cancer immunity can be employed in cancer treatment, including the regulation of NKG2D expression and its ligands, and the clearance of soluble NKG2DL shedding from the surface of tumor cells.
Significant progress has been made in NKG2D-mediated immunotherapy; however, NK cell targeting of tumors and specifically accumulating NK cells in or around the tumor are essential to ensure maximum killing effects. Moreover, therapy mediated by monoclonal antibodies and cytokines based on the NKG2D/NKG2DL axis signaling pathway might have side effects. In short, it is necessary to develop optimized and individualized treatment strategies to fully utilize the antitumor function of the NKG2D/NKG2DL axis. Further studies are needed to identify new immunological therapy agents and to open new avenues with the aim of restoring or improving the antitumor immune response.
Acknowledgements
This work was supported by the National Natural Science Foundation of China [NSFC nos. 61727823, 61775178, 61705177, and 61875159].
Disclosure of conflict of interest
None.
References
- 1.Rotte A, Jin JY, Lemaire V. Mechanistic overview of immune checkpoints to support the rational design of their combinations in cancer immunotherapy. Ann Oncol. 2018;29:71–83. doi: 10.1093/annonc/mdx686. [DOI] [PubMed] [Google Scholar]
- 2.Zhang Q, Bi J, Zheng X, Chen Y, Wang H, Wu W, Wang Z, Wu Q, Peng H, Wei H, Sun R, Tian Z. Blockade of the checkpoint receptor TIGIT prevents NK cell exhaustion and elicits potent anti-tumor immunity. Nat Immunol. 2018;19:723–732. doi: 10.1038/s41590-018-0132-0. [DOI] [PubMed] [Google Scholar]
- 3.Caligiuri MA. Human natural killer cells. Blood. 2008;112:461–469. doi: 10.1182/blood-2007-09-077438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Houchins JP, Yabe T, McSherry C, Bach FH. DNA sequence analysis of NKG2, a family of related cDNA clones encoding type II integral membrane proteins on human natural killer cells. J Exp Med. 1991;173:1017–1020. doi: 10.1084/jem.173.4.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bauer S, Groh V, Wu J, Steinle A, Phillips JH, Lanier LL, Spies T. Activation of NK cells and T cells by NKG2D, a receptor for stress-inducible MICA. Science. 1999;285:727–729. doi: 10.1126/science.285.5428.727. [DOI] [PubMed] [Google Scholar]
- 6.Ogasawara K, Lanier LL. NKG2D in NK and T cell-mediated immunity. J Clin Immunol. 2005;25:534–540. doi: 10.1007/s10875-005-8786-4. [DOI] [PubMed] [Google Scholar]
- 7.Vivier E, Tomasello E, Baratin M, Walzer T, Ugolini S. Functions of natural killer cells. Nat Immunol. 2008;9:503–510. doi: 10.1038/ni1582. [DOI] [PubMed] [Google Scholar]
- 8.Obeidy P, Sharland AF. NKG2D and its ligands. Int J Biochem Cell Biol. 2009;41:2364–2367. doi: 10.1016/j.biocel.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 9.Yabe T, McSherry C, Bach FH, Fisch P, Schall RP, Sondel PM, Houchins JP. A multigene family on human chromosome 12 encodes natural killer-cell lectins. Immunogenetics. 1993;37:455–460. doi: 10.1007/BF00222470. [DOI] [PubMed] [Google Scholar]
- 10.Upshaw JL, Arneson LN, Schoon RA, Dick CJ, Billadeau DD, Leibson PJ. NKG2D-mediated signaling requires a DAP10-bound Grb2-Vav1 intermediate and phosphatidylinositol-3-kinase in human natural killer cells. Nat Immunol. 2006;7:524–532. doi: 10.1038/ni1325. [DOI] [PubMed] [Google Scholar]
- 11.Diefenbach A, Tomasello E, Lucas M, Jamieson AM, Hsia JK, Vivier E, Raulet DH. Selective associations with signaling proteins determine stimulatory versus costimulatory activity of NKG2D. Nat Immunol. 2002;3:1142–1149. doi: 10.1038/ni858. [DOI] [PubMed] [Google Scholar]
- 12.Wu J, Song Y, Bakker AB, Bauer S, Spies T, Lanier LL, Phillips JH. An activating immunoreceptor complex formed by NKG2D and DAP10. Science. 1999;285:730–732. doi: 10.1126/science.285.5428.730. [DOI] [PubMed] [Google Scholar]
- 13.Call ME, Wucherpfennig KW, Chou JJ. The structural basis for intramembrane assembly of an activating immunoreceptor complex. Nat Immunol. 2010;11:1023–1029. doi: 10.1038/ni.1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lanier LL, Corliss BC, Wu J, Leong C, Phillips JH. Immunoreceptor DAP12 bearing a tyrosine-based activation motif is involved in activating NK cells. Nature. 1998;391:703–707. doi: 10.1038/35642. [DOI] [PubMed] [Google Scholar]
- 15.Jelencic V, Lenartic M, Wensveen FM, Polic B. NKG2D: a versatile player in the immune system. Immunol Lett. 2017;189:48–53. doi: 10.1016/j.imlet.2017.04.006. [DOI] [PubMed] [Google Scholar]
- 16.Duan S, Guo W, Xu Z, He Y, Liang C, Mo Y, Wang Y, Xiong F, Guo C, Li Y, Li X, Li G, Zeng Z, Xiong W, Wang F. Natural killer group 2D receptor and its ligands in cancer immune escape. Mol Cancer. 2019;18:29. doi: 10.1186/s12943-019-0956-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gilfillan S, Ho EL, Cella M, Yokoyama WM, Colonna M. NKG2D recruits two distinct adapters to trigger NK cell activation and costimulation. Nat Immunol. 2002;3:1150–1155. doi: 10.1038/ni857. [DOI] [PubMed] [Google Scholar]
- 18.Zompi S, Hamerman JA, Ogasawara K, Schweighoffer E, Tybulewicz VL, Di Santo JP, Lanier LL, Colucci F. NKG2D triggers cytotoxicity in mouse NK cells lacking DAP12 or Syk family kinases. Nat Immunol. 2003;4:565–572. doi: 10.1038/ni930. [DOI] [PubMed] [Google Scholar]
- 19.Wu J, Cherwinski H, Spies T, Phillips JH, Lanier LL. DAP10 and DAP12 form distinct, but functionally cooperative, receptor complexes in natural killer cells. J Exp Med. 2000;192:1059–1068. doi: 10.1084/jem.192.7.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jiang X, Wang J, Deng X, Xiong F, Ge J, Xiang B, Wu X, Ma J, Zhou M, Li X, Li Y, Li G, Xiong W, Guo C, Zeng Z. Role of the tumor microenvironment in PD-L1/PD-1-mediated tumor immune escape. Mol Cancer. 2019;18:10. doi: 10.1186/s12943-018-0928-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Raulet DH, Gasser S, Gowen BG, Deng W, Jung H. Regulation of ligands for the NKG2D activating receptor. Annu Rev Immunol. 2013;31:413–441. doi: 10.1146/annurev-immunol-032712-095951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cosman D, Mullberg J, Sutherland CL, Chin W, Armitage R, Fanslow W, Kubin M, Chalupny NJ. ULBPs, novel MHC class I-related molecules, bind to CMV glycoprotein UL16 and stimulate NK cytotoxicity through the NKG2D receptor. Immunity. 2001;14:123–133. doi: 10.1016/s1074-7613(01)00095-4. [DOI] [PubMed] [Google Scholar]
- 23.Bahram S, Bresnahan M, Geraghty DE, Spies T. A second lineage of mammalian major histocompatibility complex class I genes. Proc Natl Acad Sci U S A. 1994;91:6259–6263. doi: 10.1073/pnas.91.14.6259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lazarova M, Steinle A. The NKG2D axis: an emerging target in cancer immunotherapy. Expert Opin Ther Targets. 2019;23:281–294. doi: 10.1080/14728222.2019.1580693. [DOI] [PubMed] [Google Scholar]
- 25.Dhar P, Wu JD. NKG2D and its ligands in cancer. Curr Opin Immunol. 2018;51:55–61. doi: 10.1016/j.coi.2018.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Takada A, Yoshida S, Kajikawa M, Miyatake Y, Tomaru U, Sakai M, Chiba H, Maenaka K, Kohda D, Fugo K, Kasahara M. Two novel NKG2D ligands of the mouse H60 family with differential expression patterns and binding affinities to NKG2D. J Immunol. 2008;180:1678–1685. doi: 10.4049/jimmunol.180.3.1678. [DOI] [PubMed] [Google Scholar]
- 27.Zhang J, Basher F, Wu JD. NKG2D ligands in tumor immunity: two sides of a coin. Front Immunol. 2015;6:97. doi: 10.3389/fimmu.2015.00097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carapito R, Bahram S. Genetics, genomics, and evolutionary biology of NKG2D ligands. Immunol Rev. 2015;267:88–116. doi: 10.1111/imr.12328. [DOI] [PubMed] [Google Scholar]
- 29.Chavez-Blanco A, Chacon-Salinas R, Dominguez-Gomez G, Gonzalez-Fierro A, Perez-Cardenas E, Taja-Chayeb L, Trejo-Becerril C, Duenas-Gonzalez A. Viral inhibitors of NKG2D ligands for tumor surveillance. Expert Opin Ther Targets. 2016;20:1375–1387. doi: 10.1080/14728222.2016.1202928. [DOI] [PubMed] [Google Scholar]
- 30.Gonzalez S, Lopez-Soto A, Suarez-Alvarez B, Lopez-Vazquez A, Lopez-Larrea C. NKG2D ligands: key targets of the immune response. Trends Immunol. 2008;29:397–403. doi: 10.1016/j.it.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 31.Smyth MJ, Swann J, Cretney E, Zerafa N, Yokoyama WM, Hayakawa Y. NKG2D function protects the host from tumor initiation. J Exp Med. 2005;202:583–588. doi: 10.1084/jem.20050994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guerra N, Tan YX, Joncker NT, Choy A, Gallardo F, Xiong N, Knoblaugh S, Cado D, Greenberg NM, Raulet DH. NKG2D-deficient mice are defective in tumor surveillance in models of spontaneous malignancy. Immunity. 2008;28:571–580. doi: 10.1016/j.immuni.2008.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Watzl C. The NKG2D receptor and its ligands-recognition beyond the “missing self”? Microbes Infect. 2003;5:31–37. doi: 10.1016/s1286-4579(02)00057-6. [DOI] [PubMed] [Google Scholar]
- 34.Raulet DH. Roles of the NKG2D immunoreceptor and its ligands. Nat Rev Immunol. 2003;3:781–790. doi: 10.1038/nri1199. [DOI] [PubMed] [Google Scholar]
- 35.Jamieson AM, Diefenbach A, McMahon CW, Xiong N, Carlyle JR, Raulet DH. The role of the NKG2D immunoreceptor in immune cell activation and natural killing. Immunity. 2002;17:19–29. doi: 10.1016/s1074-7613(02)00333-3. [DOI] [PubMed] [Google Scholar]
- 36.Voskoboinik I, Whisstock JC, Trapani JA. Perforin and granzymes: function, dysfunction and human pathology. Nat Rev Immunol. 2015;15:388–400. doi: 10.1038/nri3839. [DOI] [PubMed] [Google Scholar]
- 37.Lopez-Soto A, Gonzalez S, Smyth MJ, Galluzzi L. Control of metastasis by NK cells. Cancer Cell. 2017;32:135–154. doi: 10.1016/j.ccell.2017.06.009. [DOI] [PubMed] [Google Scholar]
- 38.Waldhauer I, Steinle A. NK cells and cancer immunosurveillance. Oncogene. 2008;27:5932–5943. doi: 10.1038/onc.2008.267. [DOI] [PubMed] [Google Scholar]
- 39.Lopez-Soto A, Huergo-Zapico L, Acebes-Huerta A, Villa-Alvarez M, Gonzalez S. NKG2D signaling in cancer immunosurveillance. Int J Cancer. 2015;136:1741–1750. doi: 10.1002/ijc.28775. [DOI] [PubMed] [Google Scholar]
- 40.Prajapati K, Perez C, Rojas LBP, Burke B, Guevara-Patino JA. Functions of NKG2D in CD8 (+) T cells: an opportunity for immunotherapy. Cell Mol Immunol. 2018;15:470–479. doi: 10.1038/cmi.2017.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Saito H, Osaki T, Ikeguchi M. Decreased NKG2D expression on NK cells correlates with impaired NK cell function in patients with gastric cancer. Gastric Cancer. 2012;15:27–33. doi: 10.1007/s10120-011-0059-8. [DOI] [PubMed] [Google Scholar]
- 42.Chitadze G, Lettau M, Bhat J, Wesch D, Steinle A, Furst D, Mytilineos J, Kalthoff H, Janssen O, Oberg HH, Kabelitz D. Shedding of endogenous MHC class I-related chain molecules A and B from different human tumor entities: heterogeneous involvement of the “a disintegrin and metalloproteases” 10 and 17. Int J Cancer. 2013;133:1557–1566. doi: 10.1002/ijc.28174. [DOI] [PubMed] [Google Scholar]
- 43.Huergo-Zapico L, Gonzalez-Rodriguez AP, Contesti J, Gonzalez E, Lopez-Soto A, Fernandez-Guizan A, Acebes-Huerta A, de Los Toyos JR, Lopez-Larrea C, Groh V, Spies T, Gonzalez S. Expression of ERp5 and GRP78 on the membrane of chronic lymphocytic leukemia cells: association with soluble MICA shedding. Cancer Immunol Immunother. 2012;61:1201–1210. doi: 10.1007/s00262-011-1195-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jinushi M, Takehara T, Tatsumi T, Hiramatsu N, Sakamori R, Yamaguchi S, Hayashi N. Impairment of natural killer cell and dendritic cell functions by the soluble form of MHC class I-related chain A in advanced human hepatocellular carcinomas. J Hepatol. 2005;43:1013–1020. doi: 10.1016/j.jhep.2005.05.026. [DOI] [PubMed] [Google Scholar]
- 45.Song H, Kim J, Cosman D, Choi I. Soluble ULBP suppresses natural killer cell activity via down-regulating NKG2D expression. Cell Immunol. 2006;239:22–30. doi: 10.1016/j.cellimm.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 46.Kamei R, Yoshimura K, Yoshino S, Inoue M, Asao T, Fuse M, Wada S, Kuramasu A, Furuya-Kondo T, Oga A, Iizuka N, Suzuki N, Maeda N, Watanabe Y, Matsukuma S, Iida M, Takeda S, Ueno T, Yamamoto N, Fukagawa T, Katai H, Sasaki H, Hazama S, Oka M, Nagano H. Expression levels of UL16 binding protein 1 and natural killer group 2 member D affect overall survival in patients with gastric cancer following gastrectomy. Oncol Lett. 2018;15:747–754. doi: 10.3892/ol.2017.7354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Holdenrieder S, Stieber P, Peterfi A, Nagel D, Steinle A, Salih HR. Soluble MICA in malignant diseases. Int J Cancer. 2006;118:684–687. doi: 10.1002/ijc.21382. [DOI] [PubMed] [Google Scholar]
- 48.Tamaki S, Sanefuzi N, Kawakami M, Aoki K, Imai Y, Yamanaka Y, Yamamoto K, Ishitani A, Hatake K, Kirita T. Association between soluble MICA levels and disease stage IV oral squamous cell carcinoma in Japanese patients. Hum Immunol. 2008;69:88–93. doi: 10.1016/j.humimm.2008.01.010. [DOI] [PubMed] [Google Scholar]
- 49.Li K, Mandai M, Hamanishi J, Matsumura N, Suzuki A, Yagi H, Yamaguchi K, Baba T, Fujii S, Konishi I. Clinical significance of the NKG2D ligands, MICA/B and ULBP2 in ovarian cancer: high expression of ULBP2 is an indicator of poor prognosis. Cancer Immunol Immunother. 2009;58:641–652. doi: 10.1007/s00262-008-0585-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Arai J, Goto K, Tanoue Y, Ito S, Muroyama R, Matsubara Y, Nakagawa R, Kaise Y, Lim LA, Yoshida H, Kato N. Enzymatic inhibition of MICA sheddase ADAM17 by lomofungin in hepatocellular carcinoma cells. Int J Cancer. 2018;143:2575–2583. doi: 10.1002/ijc.31615. [DOI] [PubMed] [Google Scholar]
- 51.Maccalli C, Giannarelli D, Chiarucci C, Cutaia O, Giacobini G, Hendrickx W, Amato G, Annesi D, Bedognetti D, Altomonte M, Danielli R, Calabro L, Di Giacomo AM, Marincola FM, Parmiani G, Maio M. Soluble NKG2D ligands are biomarkers associated with the clinical outcome to immune checkpoint blockade therapy of metastatic melanoma patients. Oncoimmunology. 2017;6:e1323618. doi: 10.1080/2162402X.2017.1323618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yang FQ, Liu M, Yang FP, Zhang XL, Yang B, Guo CC, Huang JH, Che JP, Yan Y, Zheng JH. Matrix metallopeptidase 2 (MMP2) mediates MHC class I polypeptide-related sequence A (MICA) shedding in renal cell carcinoma. Actas Urol Esp. 2014;38:172–178. doi: 10.1016/j.acuro.2013.09.015. [DOI] [PubMed] [Google Scholar]
- 53.Lopez-Soto A, Gonzalez S, Galluzzi L. Soluble NKG2D ligands limit the efficacy of immune checkpoint blockade. Oncoimmunology. 2017;6:e1346766. doi: 10.1080/2162402X.2017.1346766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang J, Liu D, Li G, Staveley-O’Carroll KF, Graff JN, Li Z, Wu JD. Antibody-mediated neutralization of soluble MIC significantly enhances CTLA4 blockade therapy. Sci Adv. 2017;3:e1602133. doi: 10.1126/sciadv.1602133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schwinn N, Vokhminova D, Sucker A, Textor S, Striegel S, Moll I, Nausch N, Tuettenberg J, Steinle A, Cerwenka A, Schadendorf D, Paschen A. Interferon-gamma down-regulates NKG2D ligand expression and impairs the NKG2D-mediated cytolysis of MHC class I-deficient melanoma by natural killer cells. Int J Cancer. 2009;124:1594–1604. doi: 10.1002/ijc.24098. [DOI] [PubMed] [Google Scholar]
- 56.Lee JC, Lee KM, Kim DW, Heo DS. Elevated TGF-β1 secretion and down-modulation of NKG2D underlies impaired NK cytotoxicity in cancer patients. J Immunol. 2004;172:7335–40. doi: 10.4049/jimmunol.172.12.7335. [DOI] [PubMed] [Google Scholar]
- 57.Wischhusen J, Weller M, Eisele G, Friese MA, Mittelbronn M, Meyermann R, Steinle A, Waldhauer I. TGF-β and metalloproteinases differentially suppress NKG2D ligand surface expression on malignant glioma cells. Brain. 2006;129:2416–2425. doi: 10.1093/brain/awl205. [DOI] [PubMed] [Google Scholar]
- 58.Gorelik L, Flavell RA. Transforming growth factor-β in T-cell biology. Nat Rev Immunol. 2002;2:46–53. doi: 10.1038/nri704. [DOI] [PubMed] [Google Scholar]
- 59.Friese MA, Wischhusen J, Wick W, Weiler M, Eisele G, Steinle A, Weller M. RNA interference targeting transforming growth factor-β enhances NKG2D-mediated antiglioma immune response, inhibits glioma cell migration and invasiveness, and abrogates tumorigenicity in vivo. Cancer Res. 2004;64:7596–7603. doi: 10.1158/0008-5472.CAN-04-1627. [DOI] [PubMed] [Google Scholar]
- 60.Zhuang L, Fulton RJ, Rettman P, Sayan AE, Coad J, Al-Shamkhani A, Khakoo SI. Activity of IL-12/15/18 primed natural killer cells against hepatocellular carcinoma. Hepatol Int. 2019;13:75–83. doi: 10.1007/s12072-018-9909-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Konjevic G, Mirjacic Martinovic K, Vuletic A, Babovic N. In-vitro IL-2 or IFN-α-induced NKG2D and CD161 NK cell receptor expression indicates novel aspects of NK cell activation in metastatic melanoma patients. Melanoma Res. 2010;20:459–467. doi: 10.1097/CMR.0b013e32833e3286. [DOI] [PubMed] [Google Scholar]
- 62.Anderson TD, Hayes TJ, Gately MK, Bontempo JM, Stern LL, Truitt GA. Toxicity of human recombinant interleukin-2 in the mouse is mediated by interleukin-activated lymphocytes. Separation of efficacy and toxicity by selective lymphocyte subset depletion. Lab Invest. 1988;59:598–612. [PubMed] [Google Scholar]
- 63.Song H, Hur DY, Kim KE, Park H, Kim T, Kim CW, Bang S, Cho DH. IL-2/IL-18 prevent the down-modulation of NKG2D by TGF-β in NK cells via the c-Jun N-terminal kinase (JNK) pathway. Cell Immunol. 2006;242:39–45. doi: 10.1016/j.cellimm.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 64.Ghasemi R, Lazear E, Wang X, Arefanian S, Zheleznyak A, Carreno BM, Higashikubo R, Gelman AE, Kreisel D, Fremont DH, Krupnick AS. Selective targeting of IL-2 to NKG2D bearing cells for improved immunotherapy. Nat Commun. 2016;7:12878. doi: 10.1038/ncomms12878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Easom NJW, Stegmann KA, Swadling L, Pallett LJ, Burton AR, Odera D, Schmidt N, Huang WC, Fusai G, Davidson B, Maini MK. IL-15 overcomes hepatocellular carcinoma-induced NK cell dysfunction. Front Immunol. 2018;9:1009. doi: 10.3389/fimmu.2018.01009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hagemann-Jensen M, Uhlenbrock F, Kehlet S, Andresen L, Gabel-Jensen C, Ellgaard L, Gammelgaard B, Skov S. The selenium metabolite methylselenol regulates the expression of ligands that trigger immune activation through the lymphocyte receptor NKG2D. J Biol Chem. 2014;289:31576–31590. doi: 10.1074/jbc.M114.591537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vantourout P, Willcox C, Turner A, Swanson CM, Haque Y, Sobolev O, Grigoriadis A, Tutt A, Hayday A. Immunological visibility: posttranscriptional regulation of human NKG2D ligands by the EGF receptor pathway. Sci Transl Med. 2014;6:231ra249. doi: 10.1126/scitranslmed.3007579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jung H, Hsiung B, Pestal K, Procyk E, Raulet DH. RAE-1 ligands for the NKG2D receptor are regulated by E2F transcription factors, which control cell cycle entry. J Exp Med. 2012;209:2409–2422. doi: 10.1084/jem.20120565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cebo C, Da Rocha S, Wittnebel S, Turhan AG, Abdelali J, Caillat-Zucman S, Bourhis JH, Chouaib S, Caignard A. The decreased susceptibility of Bcr/Abl targets to NK cell-mediated lysis in response to imatinib mesylate involves modulation of NKG2D ligands, GM1 expression, and synapse formation. J Immunol. 2006;176:864–872. doi: 10.4049/jimmunol.176.2.864. [DOI] [PubMed] [Google Scholar]
- 70.Boissel N, Rea D, Tieng V, Dulphy N, Brun M, Cayuela JM, Rousselot P, Tamouza R, Le Bouteiller P, Mahon FX, Steinle A, Charron D, Dombret H, Toubert A. BCR/ABL oncogene directly controls MHC class I chain-related molecule A expression in chronic myelogenous leukemia. J Immunol. 2006;176:5108–5116. doi: 10.4049/jimmunol.176.8.5108. [DOI] [PubMed] [Google Scholar]
- 71.Lewis BP, Burge CB, Bartel DP. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell. 2005;120:15–20. doi: 10.1016/j.cell.2004.12.035. [DOI] [PubMed] [Google Scholar]
- 72.Xie J, Liu M, Li Y, Nie Y, Mi Q, Zhao S. Ovarian tumor-associated microRNA-20a decreases natural killer cell cytotoxicity by downregulating MICA/B expression. Cell Mol Immunol. 2014;11:495–502. doi: 10.1038/cmi.2014.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shen J, Pan J, Du C, Si W, Yao M, Xu L, Zheng H, Xu M, Chen D, Wang S, Fu P, Fan W. Silencing NKG2D ligand-targeting miRNAs enhances natural killer cell-mediated cytotoxicity in breast cancer. Cell Death Dis. 2017;8:e2740. doi: 10.1038/cddis.2017.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Breunig C, Pahl J, Kublbeck M, Miller M, Antonelli D, Erdem N, Wirth C, Will R, Bott A, Cerwenka A, Wiemann S. MicroRNA-519a-3p mediates apoptosis resistance in breast cancer cells and their escape from recognition by natural killer cells. Cell Death Dis. 2017;8:e2973. doi: 10.1038/cddis.2017.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Heinemann A, Zhao F, Pechlivanis S, Eberle J, Steinle A, Diederichs S, Schadendorf D, Paschen A. Tumor suppressive microRNAs miR-34a/c control cancer cell expression of ULBP2, a stress-induced ligand of the natural killer cell receptor NKG2D. Cancer Res. 2012;72:460–471. doi: 10.1158/0008-5472.CAN-11-1977. [DOI] [PubMed] [Google Scholar]
- 76.Li XJ, Ren ZJ, Tang JH. MicroRNA-34a: a potential therapeutic target in human cancer. Cell Death Dis. 2014;5:e1327. doi: 10.1038/cddis.2014.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhang L, Liao Y, Tang L. MicroRNA-34 family: a potential tumor suppressor and therapeutic candidate in cancer. J Exp Clin Cancer Res. 2019;38:53. doi: 10.1186/s13046-019-1059-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhang HC, Liu H, Zhou MT, Hu S, Yang WJ, Chen X, Tang KF, Zhao C, Wei LJ, Huang WJ, Li G, Lou H, Ou R, Zhang ZC, Li DW, Wu X, Xu Y. MicroRNA-34a promotes MICB expression in hepatocytes. Carcinogenesis. 2018;39:1477–1487. doi: 10.1093/carcin/bgy128. [DOI] [PubMed] [Google Scholar]
- 79.Ediriweera MK, Tennekoon KH, Samarakoon SR. Emerging role of histone deacetylase inhibitors as anti-breast-cancer agents. Drug Discov Today. 2019;24:685–702. doi: 10.1016/j.drudis.2019.02.003. [DOI] [PubMed] [Google Scholar]
- 80.Zhu S, Denman CJ, Cobanoglu ZS, Kiany S, Lau CC, Gottschalk SM, Hughes DP, Kleinerman ES, Lee DA. The narrow-spectrum HDAC inhibitor entinostat enhances NKG2D expression without NK cell toxicity, leading to enhanced recognition of cancer cells. Pharm Res. 2015;32:779–792. doi: 10.1007/s11095-013-1231-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Satwani P, Bavishi S, Saha A, Zhao F, Ayello J, van de Ven C, Chu Y, Cairo MS. Upregulation of NKG2D ligands in acute lymphoblastic leukemia and non-Hodgkin lymphoma cells by romidepsin and enhanced in vitro and in vivo natural killer cell cytotoxicity. Cytotherapy. 2014;16:1431–1440. doi: 10.1016/j.jcyt.2014.03.008. [DOI] [PubMed] [Google Scholar]
- 82.Chu Y, Yahr A, Huang B, Ayello J, Barth M, S Cairo M. Romidepsin alone or in combination with anti-CD20 chimeric antigen receptor expanded natural killer cells targeting Burkitt lymphoma in vitro and in immunodeficient mice. Oncoimmunology. 2017;6:e1341031. doi: 10.1080/2162402X.2017.1341031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Weiss T, Schneider H, Silginer M, Steinle A, Pruschy M, Polic B, Weller M, Roth P. NKG2D-dependent antitumor effects of chemotherapy and radiotherapy against glioblastoma. Clin Cancer Res. 2018;24:882–895. doi: 10.1158/1078-0432.CCR-17-1766. [DOI] [PubMed] [Google Scholar]
- 84.Okita R, Yukawa T, Nojima Y, Maeda A, Saisho S, Shimizu K, Nakata M. MHC class I chain-related molecule A and B expression is upregulated by cisplatin and associated with good prognosis in patients with non-small cell lung cancer. Cancer Immunol Immunother. 2016;65:499–509. doi: 10.1007/s00262-016-1814-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Luo D, Dong XW, Yan B, Liu M, Xue TH, Liu H, You JH, Li F, Wang ZL, Chen ZN. MG132 selectively upregulates MICB through the DNA damage response pathway in A549 cells. Mol Med Rep. 2019;19:213–220. doi: 10.3892/mmr.2018.9676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lee YS, Heo W, Nam J, Jeung YH, Bae J. The combination of ionizing radiation and proteasomal inhibition by bortezomib enhances the expression of NKG2D ligands in multiple myeloma cells. J Radiat Res. 2018;59:245–252. doi: 10.1093/jrr/rry005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Park MJ, Bae JH, Chung JS, Kim SH, Kang CD. Induction of NKG2D ligands and increased sensitivity of tumor cells to NK cell-mediated cytotoxicity by hematoporphyrin-based photodynamic therapy. Immunol Invest. 2011;40:367–382. doi: 10.3109/08820139.2010.551435. [DOI] [PubMed] [Google Scholar]
- 88.Belicha-Villanueva A, Riddell J, Bangia N, Gollnick SO. The effect of photodynamic therapy on tumor cell expression of major histocompatibility complex (MHC) class I and MHC class I-related molecules. Lasers Surg Med. 2012;44:60–68. doi: 10.1002/lsm.21160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Vihinen P, Kahari VM. Matrix metalloproteinases in cancer: prognostic markers and therapeutic targets. Int J Cancer. 2002;99:157–166. doi: 10.1002/ijc.10329. [DOI] [PubMed] [Google Scholar]
- 90.Alaseem A, Alhazzani K, Dondapati P, Alobid S, Bishayee A, Rathinavelu A. Matrix metalloproteinases: a challenging paradigm of cancer management. Semin Cancer Biol. 2019;56:100–115. doi: 10.1016/j.semcancer.2017.11.008. [DOI] [PubMed] [Google Scholar]
- 91.Sternlicht MD, Lochter A, Sympson CJ, Huey B, Rougier JP, Gray JW, Pinkel D, Bissell MJ, Werb Z. The stromal proteinase MMP3/stromelysin-1 promotes mammary carcinogenesis. Cell. 1999;98:137–146. doi: 10.1016/s0092-8674(00)81009-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Liu SC, Yang SF, Yeh KT, Yeh CM, Chiou HL, Lee CY, Chou MC, Hsieh YS. Relationships between the level of matrix metalloproteinase-2 and tumor size of breast cancer. Clin Chim Acta. 2006;371:92–96. doi: 10.1016/j.cca.2006.02.026. [DOI] [PubMed] [Google Scholar]
- 93.Shiraishi K, Mimura K, Kua LF, Koh V, Siang LK, Nakajima S, Fujii H, Shabbir A, Yong WP, So J, Takenoshita S, Kono K. Inhibition of MMP activity can restore NKG2D ligand expression in gastric cancer, leading to improved NK cell susceptibility. J Gastroenterol. 2016;51:1101–1111. doi: 10.1007/s00535-016-1197-x. [DOI] [PubMed] [Google Scholar]
- 94.Le Maux Chansac B, Misse D, Richon C, Vergnon I, Kubin M, Soria JC, Moretta A, Chouaib S, Mami-Chouaib F. Potentiation of NK cell-mediated cytotoxicity in human lung adenocarcinoma: role of NKG2D-dependent pathway. Int Immunol. 2008;20:801–810. doi: 10.1093/intimm/dxn038. [DOI] [PubMed] [Google Scholar]
- 95.Liu G, Atteridge CL, Wang X, Lundgren AD, Wu JD. The membrane type matrix metalloproteinase MMP14 mediates constitutive shedding of MHC class I chain-related molecule A independent of A disintegrin and metalloproteinases. J Immunol. 2010;184:3346–3350. doi: 10.4049/jimmunol.0903789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lisi S, D’Amore M, Sisto M. ADAM17 at the interface between inflammation and autoimmunity. Immunol Lett. 2014;162:159–169. doi: 10.1016/j.imlet.2014.08.008. [DOI] [PubMed] [Google Scholar]
- 97.Zheng X, Jiang F, Katakowski M, Lu Y, Chopp M. ADAM17 promotes glioma cell malignant phenotype. Mol Carcinog. 2012;51:150–164. doi: 10.1002/mc.20772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wolpert F, Tritschler I, Steinle A, Weller M, Eisele G. A disintegrin and metalloproteinases 10 and 17 modulate the immunogenicity of glioblastoma-initiating cells. Neuro Oncol. 2014;16:382–391. doi: 10.1093/neuonc/not232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zocchi MR, Camodeca C, Nuti E, Rossello A, Vene R, Tosetti F, Dapino I, Costa D, Musso A, Poggi A. ADAM10 new selective inhibitors reduce NKG2D ligand release sensitizing Hodgkin lymphoma cells to NKG2D-mediated killing. Oncoimmunology. 2016;5:e1123367. doi: 10.1080/2162402X.2015.1123367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wang X, Lundgren AD, Singh P, Goodlett DR, Plymate SR, Wu JD. An six-amino acid motif in the alpha3 domain of MICA is the cancer therapeutic target to inhibit shedding. Biochem Biophys Res Commun. 2009;387:476–481. doi: 10.1016/j.bbrc.2009.07.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ferrari de Andrade L, Tay RE, Pan D, Luoma AM, Ito Y, Badrinath S, Tsoucas D, Franz B, May KF Jr, Harvey CJ, Kobold S, Pyrdol JW, Yoon C, Yuan GC, Hodi FS, Dranoff G, Wucherpfennig KW. Antibody-mediated inhibition of MICA and MICB shedding promotes NK cell-driven tumor immunity. Science. 2018;359:1537–1542. doi: 10.1126/science.aao0505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Deng W, Gowen BG, Zhang L, Wang L, Lau S, Iannello A, Xu J, Rovis TL, Xiong N, Raulet DH. A shed NKG2D ligand that promotes natural killer cell activation and tumor rejection. Science. 2015;348:136–139. doi: 10.1126/science.1258867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Vetter CS, Groh V, thor Straten P, Spies T, Brocker EB, Becker JC. Expression of stress-induced MHC class I related chain molecules on human melanoma. J Invest Dermatol. 2002;118:600–605. doi: 10.1046/j.1523-1747.2002.01700.x. [DOI] [PubMed] [Google Scholar]
- 104.Liu G, Lu S, Wang X, Page ST, Higano CS, Plymate SR, Greenberg NM, Sun S, Li Z, Wu JD. Perturbation of NK cell peripheral homeostasis accelerates prostate carcinoma metastasis. J Clin Invest. 2013;123:4410–4422. doi: 10.1172/JCI69369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Pende D, Rivera P, Marcenaro S, Chang CC, Biassoni R, Conte R, Kubin M, Cosman D, Ferrone S, Moretta L, Moretta A. Major histocompatibility complex class I-related chain A and UL16-binding protein expression on tumor cell lines of different histotypes. Cancer Res. 2002;62:6178–86. [PubMed] [Google Scholar]


