Abstract
Glioma is an aggressive nervous system tumor with poor prognosis. Although the therapeutic strategies to overcome glioma have been improved largely recent years, the potential mechanism of its carcinogenesis remains largely unclear. The present study aimed to investigate the role of long non-coding RNA HOMEOBOX A11 antisense RNA (lncRNA HOXA11-AS) in glioma, and further to explore the underlying mechanism. We forst detected the level of lncRNA HOXA11-AS and microRNA-125a (miR-125a) in glioma tissues and human glioma U251 cells using quantitative real time polymerase chain reaction (qRT-PCR). Then, effect of lncRNA HOXA11-AS silencing on U251 cell migration, invasion, proliferation, and apoptosis was determined. Meanwhile, the expression of caspase-3/8/9 and several tumor-related genes was measured by Western blotting and qRT-PCR. Dual luciferase activity assay was used to confirm the targeting relationship between lncRNA HOXA11-AS and miR-125a. Results indicated that lncRNA HOXA11-AS was significantly increased in U251 cells and positively correlated with glioma World Health Organization (WHO) grade in glioma tissues. lncRNA HOXA11-AS silencing could inhibit cell migration, invasion, proliferation, and promote apoptosis, while up-regulate the expression of caspase-3/8/9 and Bax, inhibit the expression of Bcl-2 and gab2 in U251 cells. miR-125a inhibitor could partially reverse these effects of lncRNA HOXA11-AS silencing on U251 cells. In vivo assays also indicated that lncRNA HOXA11-AS inhibitor could inhibit glioma growth in vivo by regulating the expression of miR-125a. In conclusion, we revealed that lncRNA HOXA11-AS acted as an oncogene in glioma via interacting with miR-125a and considered that lncRNA HOXA11-AS was a potential therapeutic target for glioma.
Keywords: Glioma, long non-coding RNA, lncRNA HOXA11-AS, microRNA-125a
Introduction
Glioma, which is characterized by aggressive growth and development, is the most common brain malignancy and can lead to an increasing number of cancer-related death every year [1,2]. Although the therapeutic strategies to overcome glioma have been improved largely recent years, it is still hard to control its aggressive invasion and malignant processes. Thus, it is still significant to discover novel targets for the early efficient treatment for glioma patients.
Long non-coding RNAs (LncRNAs) are a variety of non-coding RNAs with more than 200 nucleotides in length, which participate in regulating gene expression based on multiple levels, including epigenetic modification, transcriptional regulation, and post-transcriptional regulation [3]. Increasing evidence have revealed that lncRNAs act as competing endogenous RNAs (ceRNAs) that sponge miRNA to regulate expression of targeted genes. However, most latent functions of lncRNAs are still not known in glioma.
Long non-coding RNA HOMEOBOX A11 antisense RNA (lncRNA HOXA11-AS) has been reported to be up-regulated in multiple cancers, including gastric cancer [4], colorectal cancer [5], hepatocellular carcinoma [6], and osteosarcoma [7]. microRNA-125a (miR-125a), a member of endogenous small RNAs (approximately 22 nucleotides in length), has been revealed to function as a tumor suppressor in diverse cancers by down-regulating targeted oncogenes expression at post-transcriptional level [8-10]. Chen et al. indicated that lncRNA HOXA11-AS functions as a competing endogenous RNA to up-regulate peptidyl arginine deiminase 2 (PADI2) expression by sponging miR-125a-5p in liver-metastatic colorectal cancer [11]. However, the underlying mechanism of lncRNA HOXA11-AS/miR-125a axis in the carcinogenesis of glioma has not been fully understood.
This study aimed to investigate the role of lncRNA HOXA11-AS/miR-125a axis in glioma, and further to explore the underlying mechanism.
Materials and methods
Clinical information
A total 60 glioma patients (30 cases of I-II stage, 30 cases of III-IV stage) were recruited by Taizhou people’s Hospital from May 2012 to August 2017. All cases had been diagnosed with glioma by a pathologist on the basis of hematoxylin-eosin (HE) staining. Ethical approval for the study was obtained from the Ethics Committee of Taizhou people’s Hospital. All the participants voluntarily joined this study with informed contents.
Cell culture
Human glioma U251 cell line was purchased from the Cell Bank of Chinese Academy of Sciences (Shanghai, China) and cultured in Dulbecco’s modified Eagle’s medium (DMEM, high glucose) (Hyclone, Thermo Scientific, Waltham, MA) supplemented with 10% (v/v) fetal bovine serum (FBS) (Hyclone) at 37°C with 5% CO2.
Cell transfection
For gene knockdown, the si-HOXA11-AS and miR-125a inhibitor were purchased from GenePharma (Shanghai, China). The control of si-HOXA11-AS (si-control), si-HOXA11-AS, the negative control of miR-125a inhibitor (miR-125a inhibitor NC), miR-125a inhibitor, si-HOXA11-AS+miR-125a inhibitor NC, or si-HOXA11-AS+miR-125a inhibitor were transfected into U251 cells using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) based on the manufacturer’s instructions. Knockdown efficiency was evaluated 48 h after transfection by measuring mRNA levels in cell lysates using qRT-PCR.
Western blotting analysis
Cells were washed three times with phosphate buffer saline (PBS), and then the cellar proteins were harvested with Radio Immunoprecipitation Assay (RIPA) buffer (Beyotime Biotechnology, Shanghai, China). Sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) was performed as standard protocols. The primary antibodies (ProteinTech, Wuhan, China) used were as follows: anti-GAPDH, anti-Caspase3, anti-Caspase8, anti-Caspase9, anti-Bax, anti-Bcl2, anti-Gab2. Protein expression levels were normalized to GAPDH for each sample.
Transwell assay
The cell invasion assay was performed with transwell chamber (BD, San Diego, CA). After transfection, U251 cells were subjected to re-suspension in serum-free medium and digested with pancreatin. 200 μl cell suspension (containing 5×103 cells) was added to the top of polycarbonate Transwell filter pre-coated with Matrigel (BD, San Diego, CA), while 500 μl DMEM medium including 10% FBS was infused to the lower chamber. After incubation in a moist incubator for 24 h at 37°C, cells that invaded through the transwell chamber were washed and fixed with 4% formaldehyde and then dyed with 0.1% crystal violet at room temperature for 20 min. Cell number was counted in every five random fields under microscope to evaluate invasive capacity.
Wound healing assay
Cell migration ability was assessed using a wound healing assay. After transfection, U251 cells were maintained in 6-well plates. A small wound area was created using a 200 µl pipette tip when cells reached a confluence of 90%. Cells were then washed twice with PBS and were further incubated in serum-free DMEM at 37°C for 24 h. The migration distance of the cells was detected by a microscope.
Cell counting kit-8 (CCK-8) assay
Cell proliferation ability was tested using CCK-8 (Dojindo, Kumamoto, Japan) following the manufacturer’s specification. After cell transfection, U251 cells were plated in 96-well plates. Then, the CCK-8 solution (10% of the medium, 10 µl) was added to each well and incubated for 4 h prior to analysis. Then the absorbance at 450 nm was measured by a micro-plate reader (Bio-Rad, Hercules, CA, USA) according to the manufacturer’s instructions.
Apoptosis assay
U251 cells were placed into 6-well plates (6×105 cells/well). Forty-eight hours after the transfection, flow cytometry (BD Biosciences, Bedford, MA, USA) was employed to determinate the apoptosis of transfected cells by measuring the amount of AnnexinV-positive and PI-negative cells. Briefly, cells were stained with 5 μl fluorescein isothiocyanate (FITC)-Annexin V and 5 μl propidium iodide (PI) (BD Biosciences, San Diego, CA) at room temperature for 30 min in the dark. Cell apoptosis was analyzed by flow cytometer and the data were analyzed by applying the FlowJo7.6 analysis software (FlowJo LLC, Ashland, OR, USA).
Dual-luciferase activity assay
Starbase 2.0 (http://starbase.sysu.edu.cn) was used to predict the relationship between HOXA11-AS and miR-125a. The prediction was confirmed by dual-luciferase activity assay. The 3’-UTR of HOXA11-AS which contained the putative target sites of miR-125a was synthesized and ligated into pGL3 construct (Promega, Madison, WI). The constructs pGL3-HOXA11-AS-3’-UTR-WT or pGL3-HOXA11-AS-3’-UTR-mutant and pRL-TK were co-transfected with miR-125a mimic or the negative control of miRNA mimic (miR-NC mimics) by Lipofectamine 2000 (Invitrogen). Twenty-four hours after transfection, Dual-Luciferase Reporter Assay System (Promega) was performed to measure the luciferase activity following the manufacturer’s instructions.
qRT-PCR
Total RNA was extracted by using TRIzol (Invitrogen, Thermo Fisher Scientifc). The miScript Reverse Transcription Kit (Qiagen NV, Venlo, the Netherlands) was sued to perform reverse transcription experiment. And expression of HOXA11-AS and miR-125a was determined by using the miScript SYBR Green PCR kit (Qiagen NV). Primers were provided by Sangon Biotech (Shanghai, China) and the primer sequences were listed as follows: GAPDH, forward 5’-TGTTGCCATCAATGACCCCTT-3’; reverse 5’-CTCCACGACGTACTCAGCG-3’; U6, forward 5’-GCTTCGGCAGCACATATACTAAAAT-3’; reverse 5’-CGCTTCACGAATTTGCGTGTCAT-3’; HOXA11-AS, forward 5’-CGGCTAACAAGGAGATTTGG-3’; reverse 5’-AGGCTCAGGGATGGTAGTCC-3’; miR-125a, forward 5’-GCGACTCCCTGAGACCCTTTAA-3’; reverse, 5’-GCGAGCACAGAATTAATACGAC-3’; Caspase-3, forward 5’-TGTCGATGCAGCAAACCTCA-3’; reverse 5’-GACTTCTACAACGATCCCCTC-3’; Caspase-8, forward 5’-CAGCAGCCTTGAAGGAAGTC-3’; reverse 5’-CGAGATTGTCATTACCCCACA-3’; Caspase-9, forward 5’-GGGAGCAGAAAGACCATGGGT-3’; reverse 5’-ATTTGCGGCCGCTTATGATGTTTTAAAGAAAAGTT-3’; Bcl-2 forward, 5’-TTGGATCAGGGAGTTGGAAG-3’; reverse, 5’-TGTCCCTACCAACCAGAAGG-3’; Bax forward, 5’-CGTCCACCAAGAAGCTGAGCG-3’; reverse, 5’-CGTCCACCAAAGCTGAGCG3-3’; Gab2, forward 5’-CGAAGAGAACTATGTCCCTATGC-3’; reverse 5’-AGGGGCAGGACTGTTCGT-3’. GAPDH or U6 was used as the internal loading controls for mRNA and miRNA respectively, and all analysis was performed using the 2-∆∆Ct method.
Establishment of mouse xenografts in vivo
Nude mice were purchased from Slac (Shanghai, China) and feed in a SPF condition. The environment was maintained at a constant temperature (22-25°C) with 40-50% humidity and 12 h dark/light cycle conditions. All mice had access to food and water ad libitum. A total of 1×106 indicated U251 cells dissolved in 0.1 ml PBS were injected into the subcutaneous of mice. 15 days after tumor inoculation, mice were treated with si-control, si-HOXA11-AS, si-HOXA11-AS+inhibitor NC, or si-HOXA11-AS+miR-125a inhibitor through subcutaneous injection once a day for 20 days. 35 days after tumor inoculation, mice were executed and we measured the tumor size and weight. Tumor growth was monitored once every two days and the volume of tumors were calculated using the following formula: Volume = (length × width2)/2. Ethical approval for this animal study was obtained from The Ethics Committee of Taizhou People’s Hospital.
Statistical analysis
All statistical analyses were calculated using SPSS 25.0 software (Chicago, IL, USA). Difference between groups were analyzed by Student’s t-test or one-way analysis of variance (ANOVA) followed by Dunnett’s multiple posthoc tests. All data were presented as mean ± standard deviation (SD). P<0.05 was deemed to significantly different.
Results
Expression of lncRNA HOXA11-AS and miR-125a in glioma cells and tissues
First of all, we measured the expression of lncRNA HOXA11-AS and miR-125a in glioma cells and tissues using qRT-PCR. lncRNA HOXA11-AS expression was significantly up-regulated in U251 cells compared to the normal nerve cells (Figure 1A), while miR-125a expression significantly down-regulated (Figure 1B). In glioma tissues, we found that lncRNA HOXA11-AS expression was increased significantly (Figure 1C) and miR-125a expression was decreased significantly (Figure 1D) with WHO tumor grade increase.
Figure 1.
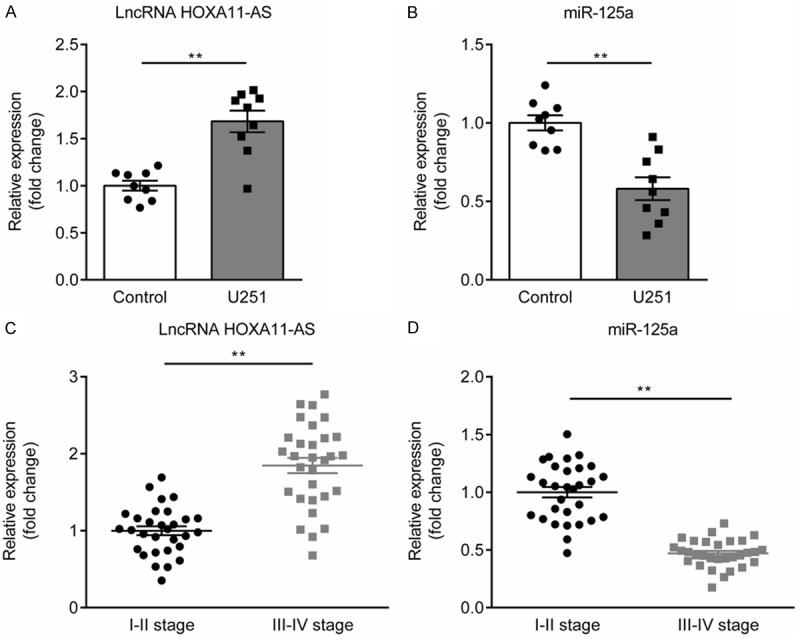
Expression of lncRNA HOXA11-AS and miR-125a in glioma cells and tissues. A. The expression of lncRNA HOXA11-AS was significantly increased in glioma U251 cells compared to normal nerve cell. B. The expression of miR-125a was significantly decreased in glioma U251 cells compared to normal nerve cell. C. The expression of lncRNA HOXA11-AS in glioma tissues of glioma patients with III-IV stage was higher than that in the glioma tissues of glioma patients with I-II stage. D. The expression of miR-125a in glioma tissues of glioma patients with III-IV stage was lower than that in the glioma tissues of glioma patients with I-II stage. **P<0.01.
Effect of lncRNA HOXA11-AS silencing on miR-125a expression in U251 cells
In this study, we selected the human glioma cell line U251 to evaluate the effect of lncRNA HOXA11-AS silencing on human glioma cells. U251 cells were transfected with miR-125a inhibitor NC, miR-125a inhibitor, si-HOXA11-AS, si-control, si-HOXA11-AS+miR-125a inhibitor NC, or si-HOXA11-AS+miR-125a inhibitor for 48 h, and the transfection efficiency was confirmed by using qRT-PCR. The results showed that compared with the control group, miR-125a inhibitor significantly reduced miR-125a expression in U251 cells (Figure 2A). Compared with the control group, si-HOXA11-AS significantly decreased HOXA11-AS expression in U251 cells (Figure 2B). Besides, miR-125a expression was significantly increased in si-HOXA11-AS transfection group, but miR-125a inhibitor indeed blocked si-HOXA11-AS-induced up-regulation of miR-125a in U251 cells (Figure 2C).
Figure 2.
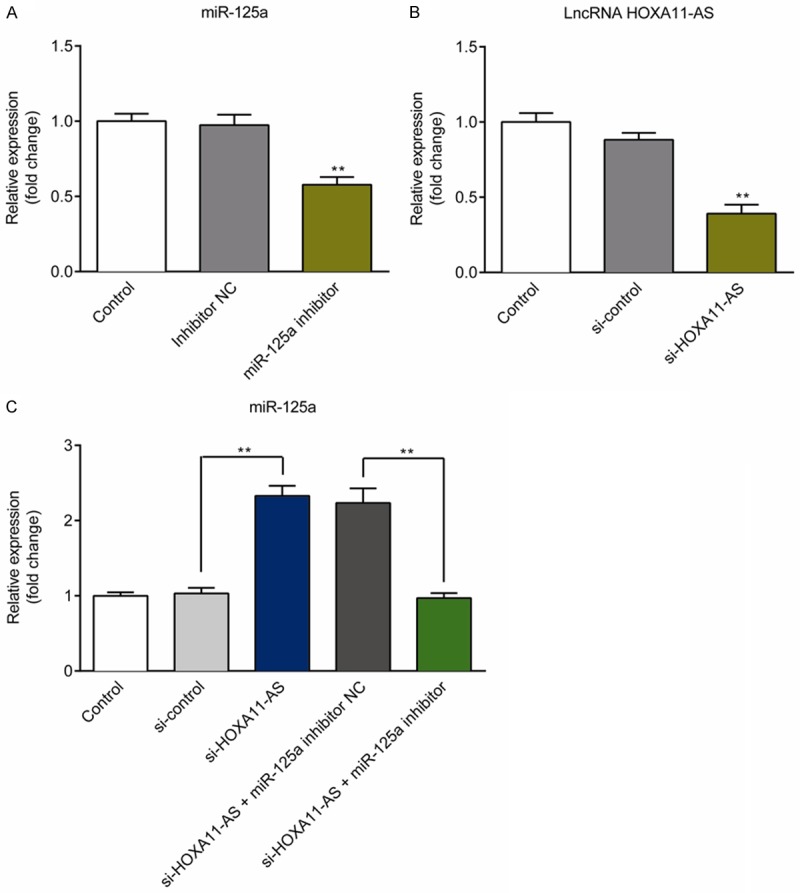
Confirmation of transfection efficiency in U251 cells. A. miR-125a expression in U251 cells transfected with miR-125a inhibitor NC or miR-125a inhibitor was detected using qRTPCR. B. lncRNA HOXA11-AS expression in U251 cells transfected with si-control or si-HOXA11-AS was detected using qRTPCR. C. Detection of miR-125a expression in U251 cells from the Control, si-control, si-HOXA11-AS, si-HOXA11-AS+miR-125a inhibitor NC, and si-HOXA11-AS+miR-125a inhibitor groups. **P<0.01.
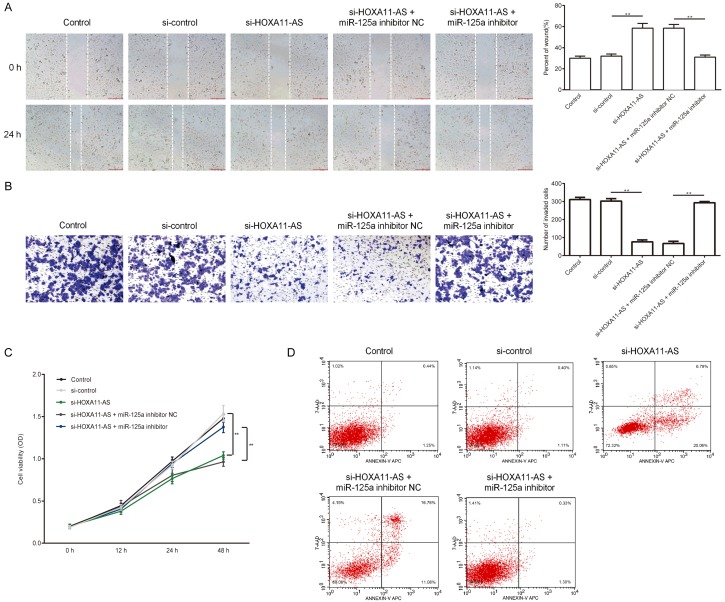
Effect of lncRNA HOXA11-AS silencing on cell migration, invasion, proliferation, and apoptosis in U251 cells
In the current study, U251 cells were divided into five group: control; si-control; si-HOXA11-AS, si-HOXA11-AS+miR-125a inhibitor NC, and si-HOXA11-AS+miR-125a inhibitor. 48 hours after transfection, wound heal assay and transwell assay were used to compare the migration and invasion ability of each group, MTT assay was used to compare the proliferation of cells in each group, and flow cytometry analysis was used to detect the apoptosis of each group. As we expected, lncRNA HOXA11-AS silencing inhibited U251 cell migration, invasion and proliferation, while promoted cell apoptosis (Figure 3A-D). Besides, inhibition of miR-125a expression at least partially eliminated the effects of lncRNA HOXA11-AS silencing on U251 cells. Taken together, lncRNA HOXA11-AS silencing inhibited human glioma cellular functions via improving the expression of miR-125a.
Figure 3.
Effect of lncRNA HOXA11-AS silencing on the migration, invasion, proliferation, and apoptosis of U251 cells. A, B. Migration and invasion ability detection of U251 cells transfected with si-control, si-HOXA11-AS, si-HOXA11-AS+miR-125a inhibitor NC, and si-HOXA11-AS+miR-125a inhibitor. C. Cell proliferation ability detection of U251 cells transfected with si-control, si-HOXA11-AS, si-HOXA11-AS+miR-125a inhibitor NC, and si-HOXA11-AS+miR-125a inhibitor. D. Cell apoptosis detection of U251 cells transfected with si-control, si-HOXA11-AS, si-HOXA11-AS+miR-125a inhibitor NC, and si-HOXA11-AS+miR-125a inhibitor. **P<0.01.
Effect of lncRNA HOXA11-AS silencing on the expression of caspase and cancer-related genes in U251 cells
We next explored whether lncRNA HOXA11-AS silencing affected the expression of caspase and several cancer-related genes. qRT-PCR and Western blotting were applied to measure the expression of cancer-related bio-markers in each group, including Caspase-3/8/9, Bcl-2, Bax, and Gab2. The results exhibited that lncRNA HOXA11-AS silencing could up-regulate the expression of caspase-3/8/9 in both mRNA and protein levels (Figures 4A-C, 5). Furthermore, lncRNA HOXA11-AS silencing could improve the expression of Bax while suppress the expression of Gab2 and Bcl-2 in U251 cells (Figures 4D-F, 5); However, miR-125a inhibitor could partially reverse the effect of lncRNA HOXA11-AS silencing on Caspase-3/8/9, Bcl-2, Bax, and Gab2 expression in U251 cells.
Figure 4.
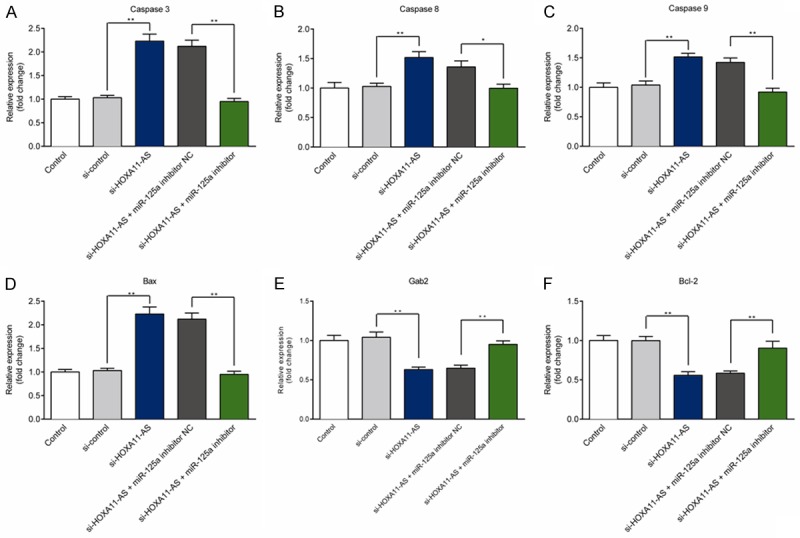
Effect of lncRNA HOXA11-AS silencing on the mRNA level of caspases and tumor-related genes in U251 cells. A-F. The mRNA levels of caspase-3/8/9, Bax, Gab2, and Bcl-2 in U251 cells transfected with si-control, si-HOXA11-AS, si-HOXA11-AS+miR-125a inhibitor NC, or si-HOXA11-AS+miR-125a inhibitor were detected using qRT-PCR. **P<0.01.
Figure 5.
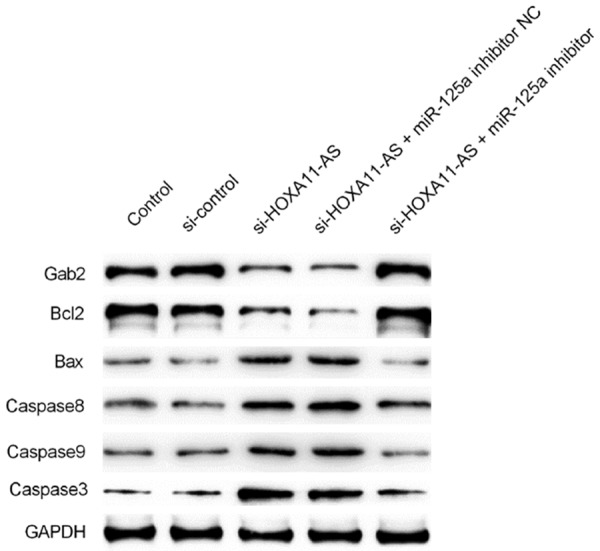
Effect of lncRNA HOXA11-AS silencing on the protein level of caspases and tumor-related genes in U251 cells. The protein levels of caspase-3/8/9, Bax, Gab2, and Bcl-2 in U251 cells transfected with si-control, si-HOXA11-AS, si-HOXA11-AS+miR-125a inhibitor NC, or si-HOXA11-AS+miR-125a inhibitor were detected using western blot assay.
Validation of the targeting relationship between lncRNA HOXA11-AS and miR-125a
We performed a bioinformatics analysis using Starbase 2.0 (http://starbase.sysu.edu.cn) to predict the relationship between lncRNA HOXA11-AS and miR-125a, and the results showed that lncRNA HOXA11-AS and miR-125a indeed contain complementary base pairs (Figure 6A). To further verity this observation, we performed dual-luciferase activity assay. miR-125a mimics significantly decreases the luciferase activity of U251 cells co-transfected with lncRNA HOXA11-AS-WT (Figure 6B), but this decrease did not show in lncRNA HOXA11-AS-MUT co-transfection group. Both results indicated that miR-125a directly bint to HOXA11-AS.
Figure 6.
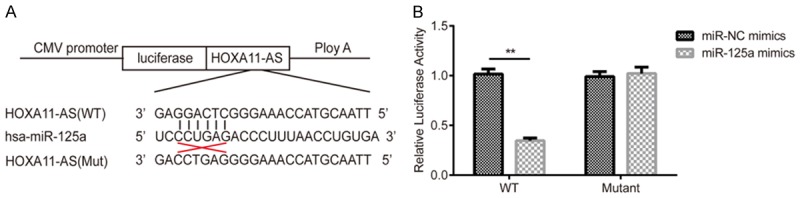
Validation of the targeting relationship between lncRNA HOXA11-AS and miR-125a. A. The putative miR-125a-binding sequence of lncRNA HOXA11-AS. A mutation was constructed in the lncRNA HOXA11-AS sequence in the complementary site for the seed region of miR-125a. B. Dual-luciferase reporter gene assay was used to confirm the binding sites between miR-125a and lncRNA HOXA11-AS. **P<0.01.
lncRNA HOXA11-AS silencing inhibited glioma tumor growth in vivo
We further validated the effect of lncRNA HOXA11-AS and miR-125a on glioma growth in vivo. Figure 7A showed the picture of the representative transplanted tumor mice. Figure 7B showed the representative pictures of tumors from each group of mice. As shown in Figure 7B and 7C, lncRNA HOXA11-AS silencing largely inhibited the tumor weight (Figure 7C) and tumor volume (Figure 7D), and this inhibition was rescued by the miR-125a inhibitor. Thus, both in vitro and in vivo assays indicated that lncRNA HOXA11-AS mediated the proliferation of human glioma cells via regulating miR-125a expression.
Figure 7.
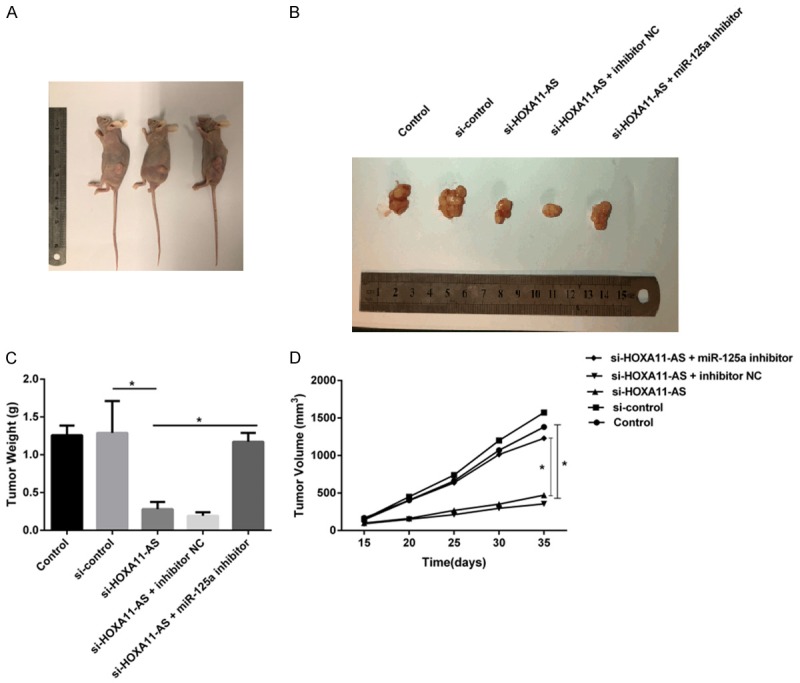
LncRNA HOXA11-AS silencing inhibits tumor growth in vivo. (A) The phenotype of mice injected with indicated human glioma cells. (B) Representative picture of the tumor of mouse from different groups. (C) The weight and (D) volume of tumors were measured after the execution of mice. *P<0.05.
Discussion
Dysregulation of lncRNAs is identified to play a critical role in the development and progression of numerous cancers. It has been revealed that lncRNA HOXA11-AS functions as a regulator in promoting cell proliferation and cell migration in breast cancer [11], gastric cancer [12], oral squamous cell carcinoma [13], and renal cell carcinoma [14], etc. The oncogene role of lncRNA HOXA11-AS in glioma has already been observed in limited number of researches. Wang Q et al. revealed that over-expressed lncRNA HOXA11-AS is positively associated with poor prognosis in glioma patients [15]. Xu C et al. suggested that lncRNA HOXA11-AS functions as an oncogene that promotes malignant process of glioma via mediating miR-214-3p/enhancer of zeste 2 polycomb repressive complex 2 subunit (EZH2) axis [16]. In our current study, we discovered a significantly up-regulation of lncRNA HOXA11-AS in glioma cells, and the expression of lncRNA HOXA11-AS was positively correlated with WHO tumor grade in glioma tissues, which suggested lncRNA HOXA11-AS acted as an oncogene in glioma.
miR-125a has been reported to suppress the carcinogenesis of various cancers by directly down-regulating cancer-related gene [8,9,17]. Yin F et al. reported that miR-125a-3p plays anti-oncogene role in glioma development by directly down-regulating the expression of neuregulin 1 (Nrg1) [18]. Yuan J et al. reported that miR-125a-5p suppresses cell proliferation and promotes cell differentiation via targeting tafazzin (TAZ) in glioma [19]. Interestingly, we found that lncRNA HOXA11-AS silencing could up-regulate miR-125a expression in glioma U251 cells, thus inhibiting cell migration, invasion, proliferation, and inducing cell apoptosis.
To explore the underlying mechanism of lncRNA HOXA11-AS in glioma U251 cells. We performed a bioinformatics analysis using Starbase 2.0 (http://starbase.sysu.edu.cn) to predict the relationship between lncRNA HOXA11-AS and miR-125a. Based on the prediction of lncRNA HOXA11-AS sponging miR-125a in glioma, dual-luciferase activity assay was carried to confirm the targeted relationship between lncRNA HOXA11-AS and miR-125a. Using dual-luciferase activity assay, we identified that lncRNA HOXA11-AS was a target lncRNA for miR-125a. The targeting relationship between lncRNA HOXA11-AS and miR-125a represented a key step in our previously unknown mechanism of the lncRNA HOXA11-AS silencing-induced effect on cellular behavior in glioma cells.
Finally, we investigated the effect of lncRNA HOXA11-AS and miR-125a on glioma growth in vivo. Consistent with our in vitro study, we found that lncRNA HOXA11-AS silencing significantly inhibited the tumor weight and tumor volume of glioma, and this inhibition was rescued by the miR-125a inhibitor.
In conclusion, lncRNA HOXA11-AS was up-regulated in glioma. Both in vitro and in vivo assays indicated that lncRNA HOXA11-AS silencing could inhibit the malignant biological behaviors of glioma by up-regulating the expression of miR-125a. The results indicated that lncRNA HOXA11-AS might be a novel and promising target for the treatment of glioma.
Acknowledgements
The work was supported by grants from the Natural Science Foundation of China (NSFC, grant 81601106), and Key Disciplines Group Construction Project of Pudong Health Bureau of Shanghai (Grant No. PWZxq2017-13).
Disclosure of conflict of interest
None.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7–30. doi: 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- 2.Diamandis P, Aldape KD. Insights from molecular profiling of adult glioma. J. Clin. Oncol. 2017;35:2386–2393. doi: 10.1200/JCO.2017.73.9516. [DOI] [PubMed] [Google Scholar]
- 3.Lorenzi L, Avila Cobos F, Decock A, Everaert C, Helsmoortel H, Lefever S, Verboom K, Volders PJ, Speleman F, Vandesompele J, Mestdagh P. Long non-coding RNA expression profiling in cancer: challenges and opportunities. Genes Chromosomes Cancer. 2019;58:191–199. doi: 10.1002/gcc.22709. [DOI] [PubMed] [Google Scholar]
- 4.Liu Z, Chen Z, Fan R, Jiang B, Chen X, Chen Q, Nie F, Lu K, Sun M. Over-expressed long noncoding RNA HOXA11-AS promotes cell cycle progression and metastasis in gastric cancer. Mol Cancer. 2017;16:82. doi: 10.1186/s12943-017-0651-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li T, Xu C, Cai B, Zhang M, Gao F, Gan J. Expression and clinicopathological significance of the lncRNA HOXA11-AS in colorectal cancer. Oncol Lett. 2016;12:4155–4160. doi: 10.3892/ol.2016.5129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhan M, He K, Xiao J, Liu F, Wang H, Xia Z, Duan X, Huang R, Li Y, He X, Yin H, Xiang G, Lu L. LncRNA HOXA11-AS promotes hepatocellular carcinoma progression by repressing miR-214-3p. J Cell Mol Med. 2018 doi: 10.1111/jcmm.13633. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cui M, Wang J, Li Q, Zhang J, Jia J, Zhan X. Long non-coding RNA HOXA11-AS functions as a competing endogenous RNA to regulate ROCK1 expression by sponging miR-124-3p in osteosarcoma. Biomed Pharmacother. 2017;92:437–444. doi: 10.1016/j.biopha.2017.05.081. [DOI] [PubMed] [Google Scholar]
- 8.Fan Z, Cui H, Xu X, Lin Z, Zhang X, Kang L, Han B, Meng J, Yan Z, Yan X, Jiao S. MiR-125a suppresses tumor growth, invasion and metastasis in cervical cancer by targeting STAT3. Oncotarget. 2015;6:25266–25280. doi: 10.18632/oncotarget.4457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang Y, Zhang D, Lv J, Wang S, Zhang Q. MiR-125a-5p suppresses bladder cancer progression through targeting FUT4. Biomed Pharmacother. 2018;108:1039–1047. doi: 10.1016/j.biopha.2018.09.100. [DOI] [PubMed] [Google Scholar]
- 10.Cai M, Chen Q, Shen J, Lv C, Cai L. Epigenetic silenced miR-125a-5p could be self-activated through targeting Suv39H1 in gastric cancer. J Cell Mol Med. 2018;22:4721–4731. doi: 10.1111/jcmm.13716. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 11.Chen D, Sun Q, Zhang L, Zhou X, Cheng X, Zhou D, Ye F, Lin J, Wang W. The lncRNA HOXA11-AS functions as a competing endogenous RNA to regulate PADI2 expression by sponging miR-125a-5p in liver metastasis of colorectal cancer. Oncotarget. 2017;8:70642–70652. doi: 10.18632/oncotarget.19956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li W, Jia G, Qu Y, Du Q, Liu B, Liu B. Long non-coding RNA (LncRNA) HOXA11-AS promotes breast cancer invasion and metastasis by regulating epithelial-mesenchymal transition. Med Sci Monit. 2017;23:3393–3403. doi: 10.12659/MSM.904892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yao Y, Chen X, Lu S, Zhou C, Xu G, Yan Z, Yang J, Yu T, Chen W, Qian Y, Ding S, Tang J, Chen Y, Zhang Y. Circulating long noncoding RNAs as biomarkers for predicting head and neck squamous cell carcinoma. Cell Physiol Biochem. 2018;50:1429–1440. doi: 10.1159/000494605. [DOI] [PubMed] [Google Scholar]
- 14.Yang FQ, Zhang JQ, Jin JJ, Yang CY, Zhang WJ, Zhang HM, Zheng JH, Weng ZM. HOXA11-AS promotes the growth and invasion of renal cancer by sponging miR-146b-5p to upregulate MMP16 expression. J Cell Physiol. 2018;233:9611–9619. doi: 10.1002/jcp.26864. [DOI] [PubMed] [Google Scholar]
- 15.Wang Q, Zhang J, Liu Y, Zhang W, Zhou J, Duan R, Pu P, Kang C, Han L. A novel cell cycle-associated lncRNA, HOXA11-AS, is transcribed from the 5-prime end of the HOXA transcript and is a biomarker of progression in glioma. Cancer Lett. 2016;373:251–259. doi: 10.1016/j.canlet.2016.01.039. [DOI] [PubMed] [Google Scholar]
- 16.Xu C, He T, Li Z, Liu H, Ding B. Regulation of HOXA11-AS/miR-214-3p/EZH2 axis on the growth, migration and invasion of glioma cells. Biomed Pharmacother. 2017;95:1504–1513. doi: 10.1016/j.biopha.2017.08.097. [DOI] [PubMed] [Google Scholar]
- 17.Liu Y, Li Z, Wu L, Wang Z, Wang X, Yu Y, Zhao Q, Luo F. MiRNA-125a-5p: a regulator and predictor of gefitinib’s effect on nasopharyngeal carcinoma. Cancer Cell Int. 2014;14:24. doi: 10.1186/1475-2867-14-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yin F, Zhang JN, Wang SW, Zhou CH, Zhao MM, Fan WH, Fan M, Liu S. MiR-125a-3p regulates glioma apoptosis and invasion by regulating Nrg1. PLoS One. 2015;10:e0116759. doi: 10.1371/journal.pone.0116759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yuan J, Xiao G, Peng G, Liu D, Wang Z, Liao Y, Liu Q, Wu M, Yuan X. MiRNA-125a-5p inhibits glioblastoma cell proliferation and promotes cell differentiation by targeting TAZ. Biochem Biophys Res Commun. 2015;457:171–176. doi: 10.1016/j.bbrc.2014.12.078. [DOI] [PubMed] [Google Scholar]