Abstract
Muscle injuries are frequent, both in sports and work, and may be caused by stretching, distension, repetitive effort or bruising. Such lesions can lead to the generation of free radicals, triggering oxidative stress and the release of some inflammatory mediators. Therapeutic ultrasound (UST) is one of the most used electrotherapy resources in the physiotherapist’s clinical practice. Our aim was to evaluate the use of therapeutic ultrasound on oxidative stress and inflammatory process in an experimental model of single quadriceps muscle injury in Wistar rats. We used a total of 28 male rats, weighing between 250-300 grams, randomly divided into four groups. In the right quadriceps, a simple impact of contusion was induced by means of a press. The animals were submitted to a daily UST treatment for a total of seven consecutive applications for three minutes each, that started 24 hours after the trauma induction. The results in the Trauma + Therapeutic ultrasound group at TBARS levels and in the enzymatic activity of SOD and GPx presented a significant difference. In the histological analysis of the Trauma + Therapeutic ultrasound group presented a reorganization of the fiber’s structure and a reduction of the presence of inflammatory infiltrate. In the results of the immunohistochemistry of iNOS, TNF-α and NF-κB in muscle tissue, we observed that the group treated with ultrasound showed a reduction in the expression of the proteins. The use of UST was effective in protecting muscle tissue from oxidative stress, inflammatory process and in the rearrangement of muscle fibers.
Keywords: Free radicals, inflammatory process, muscle trauma, oxidative stress, ultrasound
Introduction
Muscle injuries are frequent, both in sports and work activities, and may be caused by stretching, distension, repetitive effort or bruising [1]. Muscle contusion usually occurs through direct trauma, as a result of external forces, common in contact sports. It is characterized by the presence of pain, edema, muscular rigidity and restriction of the range of motion. It can reach any muscle, the lower limb muscles being more affected, such as the quadriceps and gastrocnemius [2].
Contusion muscle lesions can lead to an inflammatory process with increased formation of reactive oxygen species (ROS) and free radicals in the injured tissue. The ROS generation can be considered physiological, as long as its values do not exceed the levels of toxicity. However, when its production exceeds its elimination, it establishes the oxidative stress. This condition triggers mitochondrial dysfunction, increasing the concentration of superoxide anion (O2 -) and hydroxyl radical (OH-), that can oxidize lipids, proteins and DNA [3-8].
The ways of treating muscle injuries can be surgical or conservative. The conservative form uses cryotherapy, thermotherapy, electrotherapy, compression, immobilization and use of drugs such as non-steroidal topical anti-inflammatory drugs (NSAIDs).
Among the various therapeutic modalities for treating muscle injuries, the use of therapeutic ultrasound (UST) is one of the major indications to help in tissue repair, however this technique depends on the area to be treated, the tissue and the depth of the lesion [9-13]. In a previous study on muscle tissue and therapeutic ultrasound observed a significant increase in the amount of intramuscular collagen was achieved when applied for 7 days, and the organization of collagen at the lesion site was significantly better at the beginning of the tissue repair process (4th day) [14]. On the other hand, other authors observed more alignment of the fibers than amount of collagen deposition in soft-tissue lesions [9-11]. An explanation for the differences in treatment results in muscle and tendon injuries may be due to the lack of description regarding the size of the area to be treated with UST, making it impossible to compare the data [15-20].
Recent studies have shown that NF-κB, an inflammatory marker, is involved in inflammatory responses that may result in muscle protein degradation. This indicates that NF-κB may play a crucial role in the regulation of inflammatory processes, protein turnover and degradation in skeletal striated muscle [5,21]. Another inflammatory marker is TNF-α, that is among the major cytokines involved in inflammatory processes and has some biological effects such as the activation of macrophages and neutrophils and the increase of adhesion molecules involved in leukocyte rolling, cell differentiation and apoptosis [22]. TNF-α is induced by a number of stimuli including microorganisms, lipid mediators, tumor cells and cytokines. Thus, the inflammatory environment promotes the activation of TNF-α, as well as the migration of macrophages, which are involved in the regulatory mechanisms of iNOS [23].
Therapeutic ultrasound is one of the electrotherapy resources most used in the physiotherapist’s clinical practice, in order to reduce pain, attenuate the effects of inflammation and help in tissue regeneration.
In view of the arguments expressed above, the aim of the present study was to evaluate the efficacy of therapeutic ultrasound in reducing oxidative stress, the inflammatory process and DNA damage in an experimental model of muscle injury caused by a single impact press.
Materials and methods
Animals
An injury to the quadriceps muscle was performed by trauma in Wistar rats. Twenty-eight male Wistar rats, weighing between 250 and 300 grams, from the vivarium of the Universidade Federal from Pelotas were used and kept on the vivarium of ULBRA from Canoas (RS). During the experiment, animals were kept in a light/dark cycle of 12 hours in a temperature between 18 and 22°C. Water and feed were distributed ad libitum. The procedures with the animals were carried out after the approval of the Comitê de Ética em Pesquisa no Uso de Animais (CEUA from Universidade Luterana do Brasil (ULBRA) (project 2016-159P) and were carried out obeying the legal ethical principles (Law 11.794/2008) (CONCEA, 2016) [24].
Experimental and procedures
The animals were randomized into four groups (n = 7), all groups being evaluated after nine days.
● Control Group (CO): The animals in this group were anesthetized and manipulated, but received no trauma or therapeutic ultrasound.
● Control Group + Therapeutic Ultrasound (CO + UST): The animals in this group were anesthetized and received therapeutic ultrasound twenty four hours after the start of the experiment. Daily, on the subsequent seven days, the animals in this group continued to receive therapeutic ultrasound.
● Trauma Group (T): The animals in this group were anesthetized and traumatized on the first day and received no treatment.
● Trauma Group + Therapeutic Ultrasound (T + UST): The animals in this group were anesthetized and suffered trauma on the first day. Twenty four hours after the start of the experiment, they received therapeutic ultrasound daily on the subsequent seven days.
In the right quadriceps of the T and T + UST rats a simple impact of contusion was induced by a press developed by the Centro Industrial de Equipamentos de Ensino e Pesquisa Ltda (CIDEP/RS, Brasil) and in partnership with the Laboratório de Estresse Oxidativo e Antioxidantes from Universidade Luterana do Brasil (Canoas/RS). The trauma was caused by a piece of metal (with a mass of 0.459 kg) falling through a metal rod, height of 18 cm, in the middle of the quadriceps. The kinetic energy derived from the impact was 0.811 J [7,8].
The animals were submitted to daily therapeutic ultrasound treatment of the brand KLD Biossistemas, AVATAR V model, with a 3 MHz frequency head, due to the small thickness of the treated area. The mechanical energy generated by the 3 MHz head is absorbed into the superficial tissues (1 cm). In the total of seven consecutive applications, in pulsed mode, pulse frequency of 48 Hz and intensity of 0.7 W/cm2, aiming at the non-thermal effects of the UST.
The treatment, at the same time of day, was of three minutes daily, and began 24 hours after the trauma induction, using as a medium a hydrosoluble gel and performing circular movements on the lesion site [25]. The animal was introduced into a mechanical contender to facilitate immobilization, without causing damage to the animal and the person handling. The right hind paw was exposed from the container to be performed the UST application.
On the ninth day, animals were euthanized by excess of anesthetics. The right quadriceps muscle was removed. A fragment was immersed in 10% formaldehyde solution for 24 hours for histological analysis and the remain tissue was immediately frozen in liquid nitrogen at -80°C for further analysis. The femur was collected to obtain the bone marrow for micronucleus analysis. The proximal end of the femur, after performing asepsis of the site, was cut to expose the spinal canal. A histological needle was inserted into the opening of the femur to extract the marrow to be analyzed.
Biochemical analysis
Homogenate
Muscle tissues were homogenized for 1 minute in an ULTRA-TURRAX® disperser (IKA-Werke GmbH & Co. KG) at 4°C using 1.15% KCl (5 ml/g of tissue) and phenylmethanesulfonylfluoride (PMSF) at a concentration of 100 mM. Next, the homogenates were centrifuged for 10 minutes at 3.000 rpm in a refrigerated centrifuge (SORVALL Super T21, Kendro Laboratory Products, USA). The supernatant was removed and stored in microtubes. The samples were stored at -80°C for later analyses [26].
Protein quantification
The Bradford method is the technique for the determination of total proteins using the “Coomassie brilliant blue” dye BG-250. This method is based on the interaction between BG-250 dye and macromolecules of proteins containing basic or aromatic side chain amino acids. At the reaction pH, the interaction between the high molecular weight protein and the BG-250 dye causes the equilibrium of the dye to shift to the anionic form, which absorbs strongly at 595 nm and can be read spectrophotometrically [27].
Lipoperoxidation
The amount of aldehydes generated by LPO is determined by the method that measures the amount of thiobarbituric acid reacting substances (TBARS). Thiobarbituric acid was added to the samples at 0.67%, whereas trichloroacetic acid was added at 10%. The samples were incubated at 100°C for 15 minutes and centrifuged at 3,000 rpm (1612.8 × g) for 10 minutes at 4°C. Absorbency was determined by spectrophotometry at 535 nm and the values expressed as nmol/mg prot [28].
Activity of antioxidant enzymes: SOD and GPx
The analysis of SOD activity was based on the inhibition of the reaction of epinephrine with superoxide radical, which could be detected spectrophotometrically at 480 nm with values expressed as USOD/mg prot [29]. GPx activity was determined based on the consumption of nicotinamide adenine dinucleotide phosphate (NADPH) in the reduction of oxidized glutathione, which could be detected spectrophotometrically at 340 nm within two minutes with the values expressed as nmol/min/mg prot [30].
Metabolites of nitric oxide (nitrites and nitrates)
Nitric oxide production was measured indirectly using the quantitative Griess colorimetric assay. This assay is based on enzymatic reduction of nitrates (NO3) to nitrites (NO2) in the presence of nitrate reductase and NADPH, with subsequent colorimetric determination of NO2 by the Griess reagent (a mixture of sulfanilamide and naphthylethylenediamine specific for NO2). Because excess NADPH inhibits the Griess reaction, it is necessary to oxidize all NADPH that was not used in the reduction of NO3. This is achieved by adding nitrate reductase. The reading was performed in a microplate reader at 540 nm and the results are expressed as mmol/L [31].
Micronucleus test
The micronucleus test was performed according to the US Environmental Protection Agency Gene-Tox Program. Bone marrow extracted from femurs of the rats was suspended in fetal calf serum and smeared on clean glass slides. Slides were air-dried, fixed in methanol, stained in 10% Giemsa and coded for a blind analysis. To avoid false negative results and to obtain a measure of toxicity on bone marrow, the polychromatic erythrocyte: normocromatic erythrocyte (PCE:NCE) ratio was scored in 1000 cells. The incidence of a micronucleus (MN) was observed in 2,000 PCEs for each animal [32,33].
Histological evaluation
For microscopic analysis, the muscle fragments were fixed in 10% formaldehyde for 24 hours and after they were embedded in paraffin. In the next step, the paraffin blocks were cut to the microtome (Leitz® 1512) for the cuts with three microns (3 μ) and the slides were dipped in hematoxylin-eosin dyes. The slides were evaluated by a blinded pathologist for the experiment through a microscope equipped with a digital camera for image capture using Image-Plus software (Media Cybernetics, Bethesda, USA) increasing by 200 ×.
Immunohistochemistry and quantification of the expression of iNOS, TNF-α and NF-κB
The expression of iNOS, TNF-α and NF-κB in muscle tissue was determined by immunohistochemical analysis. Antigen retrieval was performed using buffer at 60°C, and endogenous peroxidase activity was blocked by incubation in absolute methanol. The slides were incubated with rabbit polyclonal antibody iNOS (SC-7271), TNF-α (SC-52746) and NF-κB (p65) (SC-372) (Santa Cruz Biotechnology, Santa Cruz, CA, EUA) at 1:200 overnight at 4°C. The slides were washed with buffer and incubated with the secondary antibody (anti-mouse IgG-HRP, anti-goat IgG-HRP, Santa Cruz Biotechnology, Santa Cruz, CA, USA) at 1:300 for 30 minutes at room temperature. The slides were examined by a pathologist, who was unaware of the groups, using a microscope equipped with a digital analysis system including a Zeiss Axioskop 40 microscope (Oberkochen, Germany) connected by a Roper Scientific camera (Media Cybernetics, Rockville, USA) to a computer with an image capture software. The Image-Pro Plus version 4.5 software (Media Cybernetics, Rockville, USA) was used to analyze digital images. The expression was determined by multiplying the mean density of the image by the percentage of positively stained areas (brown-stained areas). All images were magnified 200 ×.
Statistical analysis
Data are expressed as means ± standard error. Statistical significance was calculated using Graphpad Instat, version 3.0 for Windows. We used one-way analysis of variance (ANOVA) followed by the Student-Newman-Keuls test for multiple analysis and Tukey’s test for micronucleus analysis. Results were considered statistically significant when P<0.05.
Results
Lipoperoxidation
Oxidative stress is involved in muscle trauma, thus, evaluated oxidative damage in the quadriceps muscle using the technique of thiobarbituric acid reactive substances (TBARS) (Figure 1). We observed that in the trauma group (T), there was a significant increase in oxidative damage when compared to the CO and CO + UST groups (P<0.05). In the group treated with therapeutic ultrasound (T + UST), a significant reduction was observed when compared to the T group (P<0.05).
Figure 1.
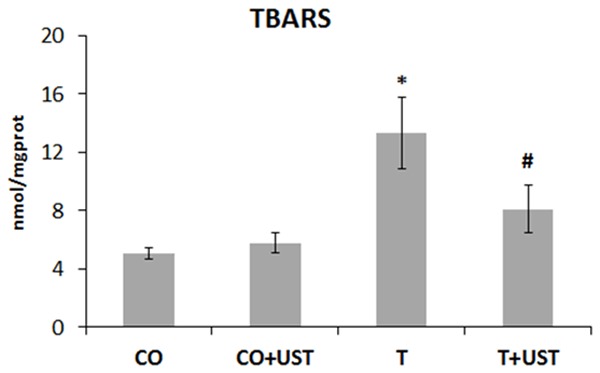
Mean values of the levels of the substances that react to thiobarbituric acid (TBARS) (nmol/mgprot) in the different experimental groups. Data are expressed as mean ± standard error. Legend: CO: control, CO + UST: control + therapeutic ultrasound, T: trauma, T + UST: trauma + therapeutic ultrasound. *Significant difference between the T group in relation to the CO and CO + UST groups (P<0.05). #Significant difference between the T + UST group and the T group (P<0.05).
Activity of antioxidant enzymes: SOD and GPx
Due to the increased oxidative damage in the quadriceps muscle of the trauma group (T) animals, we analyzed the activity of the antioxidant enzymes superoxide dismutase (SOD) (Figure 2A) and glutathione peroxidase (GPx) (Figure 2B).
Figure 2.
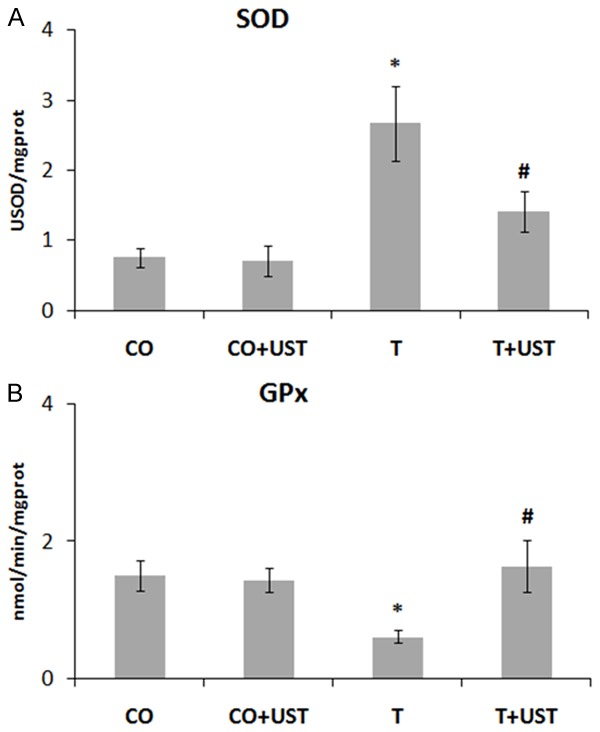
Mean values of the activity of the enzyme SOD (USOD/mgprot) and GPx (nmol/min/mgprot) in the different experimental groups. Data are expressed as mean ± standard error. Legend: CO: control, CO + UST: control + therapeutic ultrasound, T: trauma, T + UST: trauma + therapeutic ultrasound. SOD - *Significant difference between the T group in relation to the CO and CO + UST groups (P<0.01). #Significant difference between the T + UST group and the T group (P<0.05). GPx - *Significant difference between the T group in relation to the CO and CO + UST groups (P<0.05). #Significant difference between the T + UST group and the T group (P<0.05).
In the enzymatic activity of SOD presented in Figure 2A a significant increase was observed in the T group when compared to the CO and CO + UST groups (P<0.01). In the group that received treatment with a therapeutic ultrasound, a significant reduction of the enzymatic activity was observed when compared to the T group (P<0.05). The enzymatic activity of GPx demonstrated in Figure 2B showed a significant reduction in the T group when compared to CO and CO + UST groups (P<0.05). The group treated with therapeutic ultrasound showed a significant increase in the enzymatic activity in the group when compared to the T group (P<0.05). The results showed that oxidative stress decreased after treatment with UST due to its possible antioxidant effect.
Metabolites of nitric oxide (nitrites and nitrates)
Nitric oxide (nitrite and nitrate) metabolite levels were evaluated in the muscle (Figure 3), but no significant results were observed between the groups studied (P>0.05). The results obtained may be associated with the model time used in this experiment, as previously observed in other studies.
Figure 3.
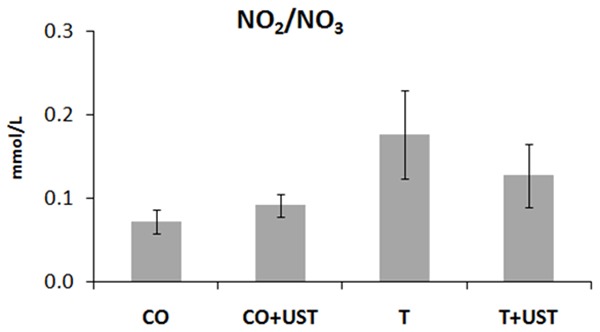
Mean values of nitrite and nitrate levels (mmol/L) in the different experimental groups. Data are expressed as mean ± standard error. Legend: CO: control, CO + UST: control + therapeutic ultrasound, T: trauma, T + UST: trauma + therapeutic ultrasound. There was no significant difference between groups (P>0.05).
Micronucleus test
Micronucleus test performed in bone marrow (Table 1) did not show a significant difference in micronuclei frequency in the studied groups (P>0.05).
Table 1.
Micronucleus test in bone marrow of the rats
| Group | MNPCEa in 2,000 PCE Mean ± SD | Ratio PCE/NCEb Mean ± SD |
|---|---|---|
| CO | 5.7 ± 1.0 | 1.7 ± 0.1 |
| CO + UST | 3.9 ± 2.4 | 1.8 ± 0.3 |
| T | 4.7 ± 1.6 | 1.5 ± 0.2 |
| T + UST | 3.0 ± 2.1 | 1.6 ± 0.4 |
MNPCE: micronucleus in polychromatic erythrocytes.
Ratio PCE/NCE: ratio polychromatic erythrocytes/normochromatic erytrocytes.
Histological evaluation
The histological evaluation observed in Figure 4 was performed by staining hematoxylin and eosin by evaluating the tissue lesions.
Figure 4.
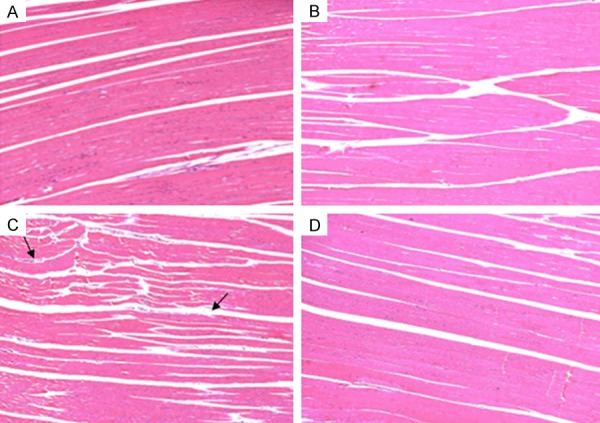
Histological analysis of the 200 × magnification quadriceps muscle in the different experimental groups. In the (A and B) we observed the normal structure of muscle tissue. In the (C) changes occurred in muscle tissue with the presence of inflammatory infiltrate (black arrow). In (D), we observed the reorganization of the tissue and reduction of the presence of inflammatory infiltrate.
After 7 days of experiment, it was observed that the animals of the groups that did not suffer the muscular trauma CO (Figure 4A) and CO + UST (Figure 4B) presented normal structural conditions of the muscle fiber. In the group that suffered muscle trauma (Figure 4C) showed changes in the structure of muscle fibers and the presence of inflammatory infiltrate (black arrows). In the group that was treated with therapeutic ultrasound (T + UST) (Figure 4D) the reorganization of the fibers structure and the reduction of the presence of inflammatory infiltrate occurred, being similar to the control groups.
Immunohistochemistry and quantification of the expression of iNOS, TNF-α and NF-κB
In muscular trauma the inflammatory process is involved and was evaluated by the expression of inducible nitric oxide synthase (iNOS) (Figure 5), tumor necrosis factor alpha (TNF-α) (Figure 6) and nuclear transcription factor kappa B (NF-κB) (Figure 7) in muscle tissue by immunohistochemistry technique. We observed that in the CO and CO + UST groups little or no labeling of this protein was detected. The T group showed a more intense marking in some areas (brown staining). The group that was treated with therapeutic ultrasound presented a significant reduction when compared to the T group.
Figure 5.
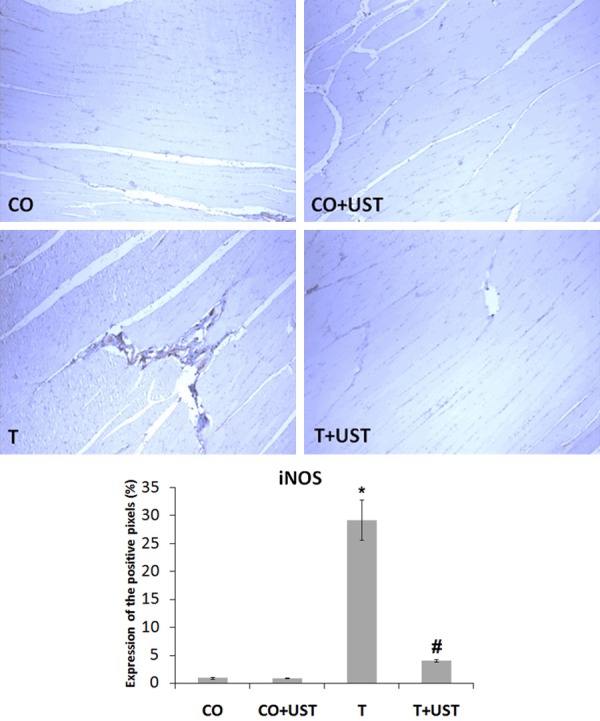
Expression of inducible nitric oxide synthase (iNOS) in muscle tissue of animals submitted to muscle trauma and treated with therapeutic ultrasound. In the quantification data are expressed as mean ± standard error. Increase of 200 x. Legend: CO: control, CO + UST: control + therapeutic ultrasound, T: trauma, T + UST: trauma + therapeutic ultrasound. *Significant difference between the T group in relation to the CO and CO + UST groups (P<0.001). #Significant difference between the T + UST group and the T group (P<0.001).
Figure 6.
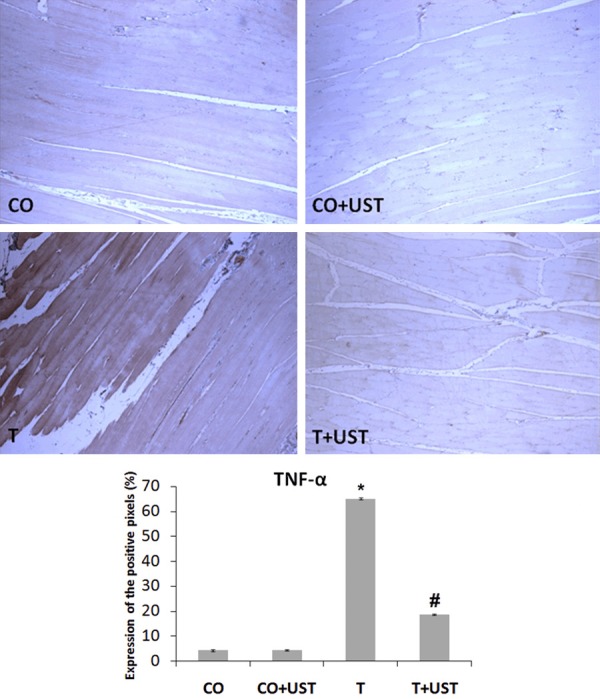
Expression of tumor necrosis factor alpha (TNF-α) in muscle tissue of animals submitted to muscle trauma and treated with therapeutic ultrasound. In the quantification data are expressed as mean ± standard error. Increase of 200 ×. Legend: CO: control, CO + UST: control + therapeutic ultrasound, T: trauma, T + UST: trauma + therapeutic ultrasound. *Significant difference between the T group in relation to the CO and CO + UST groups (P<0.001). #Significant difference between the T + UST group and the T group (P<0.001).
Figure 7.
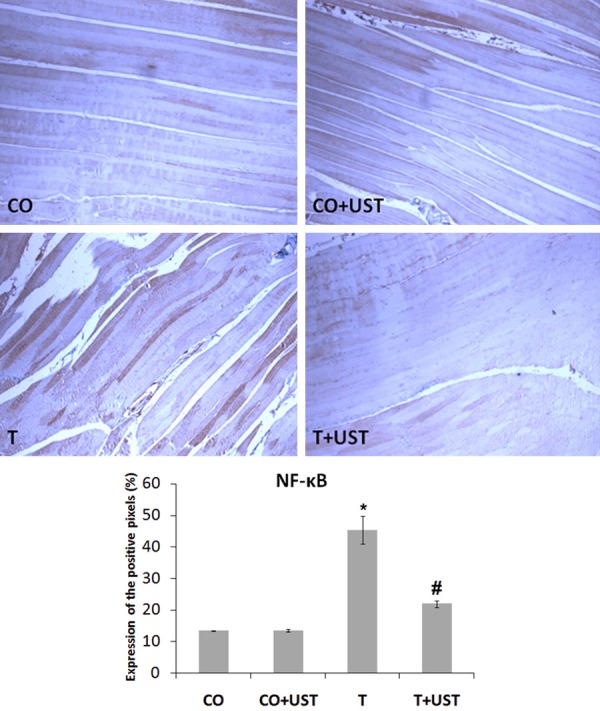
Expression of nuclear transcription factor kappa B (NF-κB) in muscle tissue of animals submitted to muscle trauma and treated with therapeutic ultrasound. In the quantification data are expressed as mean ± standard error. Increase of 200 ×. Legend: CO: control, CO + UST: control + therapeutic ultrasound, T: trauma, T + UST: trauma + therapeutic ultrasound. *Significant difference between the T group in relation to the CO and CO + UST groups (P<0.001). #Significant difference between the T + UST group and the T group (P<0.001).
Discussion
In this study, we analyzed the effect of therapeutic ultrasound treatment on the experimentally injured muscle of rats, assessing and identifying oxidative stress as well as the inflammatory process. It was possible to verify the anti-inflammatory effect of UST and the realignment capacity of post-trauma muscle fibers.
Figure 1 shows that the LPO levels detected by the TBARS technique are higher in the T group and significantly reduced in the T + UST group, which suggests lower lipoperoxidation, less oxidation of the long chain fatty acids, resulting in an effect protect the UST against biological membranes during the repair period. In experimental studies of muscle trauma by mechanical crushing, animals treated with cryotherapy had significantly lower LPO [34]. As well as, the Laser treatment presented similar results to the UST in the treatment of traumatic muscle injury [4].
The mechanical trauma of the press under the muscle initiated the inflammatory process, which worsened the lipoperoxidation in this tissue. This redox imbalance increases superoxide anion levels and by response increases the activity of the SOD enzyme. As shown in Figure 2A, higher SOD activity was detected in the T group and in the T + group UST revealed a reduction in its levels. A similar study on crush muscle injury model using cryotherapy as a therapeutic resource also showed increased concentration of SOD [34]. Similar results were found in the study [35] using telmisartan as a treatment for muscle injury in the ischemia and reperfusion model which demonstrated that the treatment resulted in positive regulation of the SOD enzyme. The biological effects triggered by UST are the result of a combination of three physical phenomena: stable cavitation, acoustic propagation and micromassage, which occur at interfaces and cell membranes [36].
As shown in Figure 2B, reduced levels of GPx were observed in T group. However, the application of UST in the T + UST group showed a significant increase in enzyme levels close to the levels found in the CO group and CO + UST group. In addition, another study observed similar results using UST associated with the use of anti-inflammatory substances in Wistar rats in the muscle contusion model, performed with the same press from that study [37].
The increase in GPx activity in the T + UST group can be explained by the sonication effect of small amounts of water of the OH and O2 - radicals. In the process of sonication, these species generate water vapor and bubbles forming hydrogen peroxide. Hydrogen peroxide is the most stable specie catalyzed by the enzyme catalase or by glutathione peroxidase. In our study, we verified the presence of H2O2 as we observed lower LPO and higher GPx activities after administration of UST in post-trauma tissue [38].
The present study did not identify differences in significant NO2/NO3 levels between groups. Previous study [8] in his press-induced mechanical work on gastrocnemius of rats after 7 and 14 days of treatment with low-intensity Ga-As laser (wavelength 904 nm, mean power 45 mW, for 35 seconds) observed cicatricial improvement and reduction of redox imbalance. Although laser therapy acts differently on muscle and tendon, these authors propose that less oxidative stress and lower iNOS expression contribute to better muscle healing [4]. Another study [39] evaluated the local and systemic formation of ROS and nitric oxide at 5, 45 and 180 minutes after induction of blunt trauma in the rat gastrocnemius muscle and demonstrated that local ROS formation in injured muscle began immediately after trauma induction as indicated by changes in the redox balance of glutathione. The levels of nitrite/nitrate, however, were not increased at these times.
The micronucleus test is used to evaluate DNA damage that could not be repaired, but to perform this test, the cell must be in the division phase. The micronuclei can be originated due to damages in the DNA, break of double tape, when we have a fragment of chromosome; or through damage in the mitotic spindle when we have an entire chromosome, both will not be included in the main nucleus, giving rise to a small nucleus [32,33]. The present study did not identify significant differences in the frequency of micronuclei, performed in bone marrow, in the studied groups, thus not providing mutagenic effects as shown in Table 1. This evidence suggests that the form of press trauma as well as the treatment with UST does not act as mutagenic factors.
We used the 3 MHz UST with pulse frequency of 48 Hz, similar to the previous study [20] that who worked with tendons of rats and demonstrated that the use of UST for 3 minutes per effective radius (ERA), presented the organization of muscle fibers in the repair process and hypothesized that longer times of application confer better organization of collagen fibers. In the T + UST group we can observe a significant reorganization of the muscle fibers, a result that is shown in Figure 4D. Alike the same, another study [40] observed an increase in the total number of fibroblast cells after the use of UST, which contributes to muscle healing. Moreover, a recent study [41] used resveratrol as a treatment in the muscle injury model in rats and demonstrated that in the model group had muscle fiber disruption and inflammatory infiltrate and in the treated group, these changes were significantly reduced with uniformly arranged muscle fibers, as observed in our study.
The inflammatory markers evaluated in this study (iNOS, TNF-α and NF-κB) were significantly reduced in the groups that received the treatment with UST in relation to the T group. The inflammatory markers evaluated in this study (iNOS, TNF-α and NF-κB) were significantly reduced in the groups that received the treatment with UST in relation to the T group. Similar results with the use of UST, demonstrated reduction of iNOS and NF-κB caused by the application of a phenolic compound isolated from propolis in rats in an eccentric exercise muscle injury protocol [42]. Our study demonstrated that UST was able to control the inflammatory process and reduce oxidative stress. We suggest that these effects are due to the action of the mechanical field and the low pulse frequency (48 HZ), because this pulse favors the movement of Ca2+, which helps in the resolution of the inflammatory process; and increases the protein synthesis that contributes to the quality of the tissue repair [43,44]. Studies suggest that the nonthermal effects of the UST act on the immune response by induced arteriolar vasodilation and activation of adhesion molecules. The mechanical effect of UST exhibits the ability to modulate the degradation of mast cells, and pulsed frequencies act on the Ca2+ flow in fibroblasts [44].
TNF-α in muscle restoration processes is a myogenic flag that prevents the phases of activation and differentiation of satellite cells (CS) and maintain them in the phases of duplication and self renewal. It is clear that the absence of this marker in the first two days after the injury would prevent important phases of the muscle restoration, as well as the delay in the removal of this marker from the lesion site (more than two days) would impair later stages of the restoration, such as differentiation, fusion and terminal differentiation and growth [45]. In this study, nine days after the induction of muscle trauma, UST treated animals showed a reduction in TNF-α expression when compared to the animals in the T group, showing that animals receiving UST treatment had an acceleration of the muscle restoration process.
Recent studies have revealed that NF-κB is involved in inflammatory responses that may result in muscle protein degradation. This indicates that NF-κB may play a crucial role in the regulation of inflammatory processes and protein turnover and degradation in skeletal striated muscle [5,21]. In addition, previous study showed results similar to ours, in a work with physical exercises in the muscular tissue, being studied the oxidative stress and the regulation of the NF-κB induced by TNF-α in muscle cells. The authors reported that there was a significant increase in the labeling of NF-κB in the trauma group compared to the control groups [3].
In conclusion, we suggest that therapeutic Ultrasound was effective in the treatment of muscle injury by contusion in rats demonstrated by the reduction of oxidative stress parameters, evaluated by lipid peroxidation by TBARS and the antioxidant enzymes SOD and GPx, by reorganization of muscle tissue evaluated by histology and reducing the expression of inflammatory markers. In the form of UST of 3 MHZ, with pulse frequency of 48 HZ, for 3 minutes, no DNA damage was evidenced, suggesting that therapeutic ultrasound does not present mutagenic effects.
Acknowledgements
Supported by donations from Brazilian agencies such as the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq-167453/2017-3), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Fundação de Amparo à Pesquisa do Estado do Rio Grande do Sul (FAPERGS), Laboratório de Hepatologia e Gastroenterologia Experimental (HCPA/UFRGS) from Universidade Federal do Rio Grande do Sul (UFRGS) and Laboratório de Estresse oxidativo e Antioxidantes from Universidade Luterana do Brasil (ULBRA).
Disclosure of conflict of interest
None.
References
- 1.Gundersen K, Bruusgaard JC. Nuclear domains during muscle atrophy: nuclei lost or paradigm lost? J Physiol. 2008;586:2675–2681. doi: 10.1113/jphysiol.2008.154369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ramos GA, Arliani GG, Astur DC, Pochini AC, Ejnisman B, Cohen M. Rehabilitation of hamstring muscle injuries: a literature review. Rev Bras Ortop. 2017;52:11–16. doi: 10.1016/j.rboe.2016.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bar-Shai M, Carmeli E, Ljubuncic P, Reznick AZ. Exercise and immobilization in aging animals: the involvement of oxidative stress and NF-kappaB activation. Free Radic Biol Med. 2008;44:202–214. doi: 10.1016/j.freeradbiomed.2007.03.019. [DOI] [PubMed] [Google Scholar]
- 4.Fillipin LI, Moreira AJ, Marroni NP, Xavier RM. Nitric Oxide and repair of skeletal muscle injury. Nitric Oxide. 2009;21:157–163. doi: 10.1016/j.niox.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 5.Lushchak VI. Free radicals, reactive oxygen species, oxidative stress and its classification. Chem Biol Interact. 2014;224:164–175. doi: 10.1016/j.cbi.2014.10.016. [DOI] [PubMed] [Google Scholar]
- 6.de Sousa JA, Pereira P, Allgayer MDC, Marroni NP, de Barros Falcão Ferraz A, Picada JN. Evaluation of DNA damage in wistar rat tissues with hyperlipidemia induced by tyloxapol. Exp Mol Pathol. 2017;103:51–55. doi: 10.1016/j.yexmp.2017.06.009. [DOI] [PubMed] [Google Scholar]
- 7.Fillipin LI, Mauriz JL, Vedovelli K, Moreira AJ, Zettler CG, Lech O, Marroni NP, González-Gallego J. Low-level laser therapy (LLLT) prevents oxidative stress and reduces fibrosis in rat traumatized Achilles tendon. Lasers Surg Med. 2005;37:293–300. doi: 10.1002/lsm.20225. [DOI] [PubMed] [Google Scholar]
- 8.Rizzi CF, Mauriz JL, Freitas Corrêa DS, Moreira AJ, Zettler CG, Filippin LI, Marroni NP, González-Gallego J. Effects of low-level laser therapy (LLLT) on the nuclear factor (NF)-kappaB signaling pathway in traumatized muscle. Lasers Surg Med. 2006;38:704–713. doi: 10.1002/lsm.20371. [DOI] [PubMed] [Google Scholar]
- 9.Xu J, Bigelow TA, Riesberg GM. Impact of preconditioning pulse on lesion formation during high-intensity focused ultrasound histotripsy. Ultrasound Med Biol. 2012;38:1918–1929. doi: 10.1016/j.ultrasmedbio.2012.06.009. [DOI] [PubMed] [Google Scholar]
- 10.Leinenga G, Langton C, Nisbet R, Götz J. Ultrasound treatment of neurological diseases-current and emerging applications. Nat Rev Neurol. 2016;12:161–174. doi: 10.1038/nrneurol.2016.13. [DOI] [PubMed] [Google Scholar]
- 11.Malliaropoulos N, Alaseirlis D, Konstantinidis G, Papalada A, Tsifountoudis I, Petras K, Maffulli N. Therapeutic ultrasound in navicular stress injuries in elite track and field athletes. Clin J Sport Med. 2017;27:278–282. doi: 10.1097/JSM.0000000000000356. [DOI] [PubMed] [Google Scholar]
- 12.Nicholson JA, Tsang STJ, MacGillivray TJ, Perks F, Simpson AHRW. What is the role of ultrasound in fracture management?: diagnosis and therapeutic potential for fractures, delayed unions, and fracture-related infection. Bone Joint Res. 2019;8:304–312. doi: 10.1302/2046-3758.87.BJR-2018-0215.R2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liang C, Yang T, Wu G, Li J, Geng W. Therapeutic effect of low-intensity pulsed ultrasound on temporomandibular joint injury induced by chronic sleep deprivation in rats. Am J Transl Res. 2019;11:3328–3340. [PMC free article] [PubMed] [Google Scholar]
- 14.Baldan CS. Tese (Doutorado em Fisiopatologia Experimental) - Universidade de São Paulo, Brasil. 2012. Influência do tempo de irradiação da terapia por ultrassom sobre o tecido conjuntivo no processo de reparação muscular de ratos; p. 80. [Google Scholar]
- 15.da Cunha A, Parizotto NA, Vidal Bde C. The effect of therapeutic ultrasound on repair of the achilles tendon (tendo calcaneus) of the rat. Ultrasound Med Biol. 2001;27:1691–1696. doi: 10.1016/s0301-5629(01)00477-x. [DOI] [PubMed] [Google Scholar]
- 16.Saini NS, Roy KS, Bansal PS, Singh B, Simran PS. A preliminary study on the effect of ultrasound therapy on the healing of surgically severed achilles tendons in five dogs. J Vet Med A Physiol Pathol Clin Med. 2002;49:321–328. doi: 10.1046/j.1439-0442.2002.00441.x. [DOI] [PubMed] [Google Scholar]
- 17.Koeke PU, Parizotto NA, Carrinho PM, Salate AC. Comparative study of the efficacy of the topical application of hydrocortisone, therapeutic ultrasound and phonophoresis on the tissue repair process in rat tendons. Ultrasound Med Biol. 2005;3:345–350. doi: 10.1016/j.ultrasmedbio.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 18.Carvalho Pde T, Silva IS, Reis FA, Belchior AC, Aydos RD, Facco GG, Dourado DM. Histological study of tendon healing in malnourished wistar rats treated with ultrasound therapy. Acta Cir Bras. 2006;21:13–17. doi: 10.1590/s0102-86502006001000004. [DOI] [PubMed] [Google Scholar]
- 19.Wood VT, Pinfildi CE, Neves MA, Parizoto NA, Hochman B, Ferreira LM. Collagen changes and realignment induced by low-level laser therapy and low-intensity ultrasound in the calcaneal tendon. Lasers Surg Med. 2010;42:559–565. doi: 10.1002/lsm.20932. [DOI] [PubMed] [Google Scholar]
- 20.Farcic TS, Baldan CS, Cattapan CG, Parizotto NA, João SM, Casarotto RA. Treatment time of ultrasound therapy interferes with the organization of collagen fibers in rat tendons. Braz J Phys Ther. 2013;17:263–271. doi: 10.1590/s1413-35552012005000090. [DOI] [PubMed] [Google Scholar]
- 21.Weidinger A, Kozlov AV. Biological activities of reactive oxygen and nitrogen species: oxidative stress versus signal transduction. Biomolecules. 2015;5:472–484. doi: 10.3390/biom5020472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Collins RA, Grounds MD. The role of tumor necrosis factor-alpha (TNF-alpha) in skeletal muscle regeneration. Studies in TNF-alpha (-/-) and TNF-alpha(-/-)/LT-alpha(-/-) mice. J Histochem Cytochem. 2001;49:989–1001. doi: 10.1177/002215540104900807. [DOI] [PubMed] [Google Scholar]
- 23.Rigamonti E, Touvier T, Clementi E, Manfredi AA, Brunelli S, Rovere-Querini P. Requirement of inducible nitric oxide synthase for skeletal muscle regeneration after acute damage. J Immunol. 2013;190:1767–1777. doi: 10.4049/jimmunol.1202903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.CONCEA. Diretriz Brasileira para o Cuidado e a Utilização de Animais para Fins Científicos e Didáticos - DBCA. Portaria nº 465, de 23 de maio de. 2013. Disponível em http://www.mct.gov.br/upd_blob/0226/226494.pdf. Acesso em: 18/08/2016.
- 25.Farcic TS. Tese (Doutorado em Ciências da Reabilitação) - Universidade de São Paulo, Brasil. 2016. Efeito da aplicação do ultrassom terapêutico durante 4 e 5 minutos por área do transdutor no processo de reparação de tendão de ratos; p. 51. [Google Scholar]
- 26.Llesuy SF, Milei J, Molina H, Boveris A, Milei S. Comparison of lipid peroxidation and myocardial damage induced by adriamycin and 4’-epiadriamycin in mice. Tumori. 1985;71:241–249. doi: 10.1177/030089168507100305. [DOI] [PubMed] [Google Scholar]
- 27.Bradford MM. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 28.Buege JA, Aust SD. Microsomal lipid peroxidation. Methods Enzymol. 1978;52:302–310. doi: 10.1016/s0076-6879(78)52032-6. [DOI] [PubMed] [Google Scholar]
- 29.Misra HP, Fridovich I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem. 1972;247:3170–3175. [PubMed] [Google Scholar]
- 30.Flohé L, Günzler WA. Assays of glutathione peroxidase. Methods Enzymol. 1984;105:114–121. doi: 10.1016/s0076-6879(84)05015-1. [DOI] [PubMed] [Google Scholar]
- 31.Granger DL, Anstey NM, Miller WC, Weinberg JB. Measuring nitric oxide production in human clinical studies. Methods Enzymol. 1999;301:49–61. doi: 10.1016/s0076-6879(99)01068-x. [DOI] [PubMed] [Google Scholar]
- 32.Mavournin KH, Blakey DH, Cimino MC, Salam-One MF, Heddle JA. The in vivo micronucleus assay in mammalian bone marrow and peripheral blood. Mutat Res. 1990;239:29–80. doi: 10.1016/0165-1110(90)90030-f. [DOI] [PubMed] [Google Scholar]
- 33.Picada JN, Da Silva KV, Erdtmann B, Henriques AT, Henriques JA. Genotoxic effects of structurally related beta-carboline alkaloids. Mutat Res. 1997;379:135–149. doi: 10.1016/s0027-5107(97)00116-4. [DOI] [PubMed] [Google Scholar]
- 34.Martins CN, Moraes MB, Hauck M, Guerreiro LF, Rossato DD, Varela AS Jr, da Rosa CE, Signori LU. Effects of cryotherapy combined with therapeutic ultrasoundon oxidative stress and tissue damage after musculoskeletalcontusion in rats. Physiotherapy. 2016;102:377–383. doi: 10.1016/j.physio.2015.10.013. [DOI] [PubMed] [Google Scholar]
- 35.Rodríguez-Lara SQ, Trujillo-Rangel WA, Castillo-Romero A, Totsuka-Sutto SE, Garcia-Cobián TA, Cardona-Muñoz EG, Miranda-Díaz AG, Ramírez-Lizardo EJ, García-Benavides L. Effect of telmisartan in the oxidative stress components induced by ischemia reperfusion in rats. Oxid Med Cell Longev. 2019;2019:1302985. doi: 10.1155/2019/1302985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.ter Haar G. Therapeutic applications of ultrasound. Prog Biophys Mol Biol. 2007;93:111–129. doi: 10.1016/j.pbiomolbio.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 37.Victor EG, Silveira PC, Possato JC, da Rosa GL, Munari UB, de Souza CT, Pinho RA, da Silva L, Streck EL, Paula MM. Pulsed ultrasound associated with gold nanoparticle gel reduces oxidative stress parameters and expression of pro-inflammatory molecules in an animal model of muscle injury. J Nanobiotechnology. 2012;10:11. doi: 10.1186/1477-3155-10-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Feng R, Zhao Y, Zhu C, Mason TJ. Enhancement of ultrasonic cavitation yield by multi-frequency sonication. Ultrason Sonochem. 2002;9:231–236. doi: 10.1016/s1350-4177(02)00083-4. [DOI] [PubMed] [Google Scholar]
- 39.Kerkweg U, Schmitz D, de Groot H. Screening for the formation of reactive oxygen species and of NO in muscle tissue and remote organs upon mechanical trauma to the mouse hind limb. Eur Surg Res. 2006;38:83–89. doi: 10.1159/000092609. [DOI] [PubMed] [Google Scholar]
- 40.Zhou S, Schmelz A, Seufferlein T, Li Y, Zhao J, Bachem MG. Molecular mechanisms of low intensity pulsed ultrasound in human skin fibroblasts. J Biol Chem. 2004;279:54463–54469. doi: 10.1074/jbc.M404786200. [DOI] [PubMed] [Google Scholar]
- 41.Feng Y, He Z, Mao C, Shui X, Cai L. Therapeutic effects of resveratrol liposome on muscle injury in rats. Med Sci Monit. 2019;25:2377–2385. doi: 10.12659/MSM.913409. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 42.Shen YC, Yen JC, Liou KT. Ameliorative effects of caffeic acid phenethyl ester on an eccentric exercise-induced skeletal muscle injury by down-regulating NF-κb mediated inflammation. Pharmacology. 2013;91:219–228. doi: 10.1159/000348412. [DOI] [PubMed] [Google Scholar]
- 43.Johns LD. Nonthermal effects of therapeutic ultrasound: the frequency resonance hypothesis. J Athl Train. 2002;37:293–299. [PMC free article] [PubMed] [Google Scholar]
- 44.Dyson M, Luke DA. Induction of mast cell degranulation in skin by ultrasound. IEEE Trans Untrason Ferroelectr Freq Control. 1986;33:194–201. doi: 10.1109/t-uffc.1986.26814. [DOI] [PubMed] [Google Scholar]
- 45.Tidball JG. Regulation of muscle growth and regeneration by the immune system. Nat Rev Immunol. 2017;17:165–178. doi: 10.1038/nri.2016.150. [DOI] [PMC free article] [PubMed] [Google Scholar]


